Abstract
Background
Autoimmune polyendocrine syndrome, also called polyglandular autoimmune syndrome, is a rare immune‐mediated disorder that involves various endocrine glands.
Purpose
To report autoimmune polyendocrine syndrome in a dog.
Methods
A 9‐year‐old spayed female miniature poodle diagnosed with insulin‐dependent diabetes mellitus emergently visited our clinic for anorexia, severe depression, and vomiting. Hyponatremia, hypochloridemia, and recurrent hypoglycaemia were found. Hypoadrenocorticism was diagnosed based on consistent clinical signs and repeated adrenocorticotropic hormone stimulation tests.
Results
After injecting deoxycorticosterone pivalate and increasing the oral prednisolone dose, the patient's systemic condition improved.
Conclusions
To the best of our knowledge, this is the first case report of hypoadrenocorticism concurrent with diabetes mellitus in a dog. Furthermore, we would like to present the probability of an immune‐mediated disorder with multiple organs involved, like type IV autoimmune polyendocrine syndrome in humans.
Keywords: glomerular nephropathy, hyperadrenocorticism, inflammatory bowel disease, insulin‐dependent diabetes mellitus, polyglandular autoimmune syndrome
This is the first reported case of hypoadrenocorticism concurrent with diabetes mellitus in a dog. Considering that it has not only the characteristics of type II APS, but also characteristics of IBD, GNP, and IMP, the patient is considered for type IV APS diagnosis. This case highlights the importance of being vigilant about new components of a syndrome.
1. INTRODUCTION
Autoimmune polyendocrine syndrome (APS), also called polyglandular autoimmune syndrome, is an immune‐mediated disorder involving various endocrine glands (Cryer, 2008; Idowu, 2018). In this article, we describe a dog with diabetes mellitus (DM) that had recurrent hypoglycaemic episodes due to the sudden onset of hypoadrenocorticism (HOAC). This dog also had inflammatory bowel disease (IBD) with possible glomerular nephropathy (GNP) and immune‐mediated pneumonitis (IMP), resembling type IV APS in humans (Blois, 2011).
2. CASE DESCRIPTION
A 9‐year‐old spayed female toy poodle with a past medical history of IBD, IMP, and insulin‐dependent DM (IDDM) emergently visited our clinic for anorexia, severe depression, and vomiting. The medications prescribed to manage the patient's disease were as follows: prednisolone (0.2 mg/kg, BID, PO, Yuhan, Seoul, Korea) and cyclosporine (7 mg/kg, SID, PO, ChongKunDang, Seoul, Korea). Steroids have been under management at low doses for the past year, and the patient is doing well with no clinical symptoms. Clinical examination showed the following: temperature, 38℃ (reference range, 37.4–39.2℃); heart rate, 120 bpm; respiratory rate, 24 breaths/min; and systolic blood pressure, 100 mm Hg. There were no other significant findings. Serum chemistry, urinalysis, and abdominal ultrasonography were performed, and azotemia (blood urea nitrogen 106 mg/dl, reference range, 9.6–31.4 mg/dl; and creatinine 2.9 mg/dl, 0.4–1.3 mg/dl), severe hyponatremia (119 mEq/L; reference range, 145.1–152.6 mEq/L), and hypochloridemia (93 mEq/L; reference range, 113.2–122.9 mEq/L) were found, with an Na:K ratio of 22.04 (potassium 5.4 mEq/L; reference range, 3.6–5.5 mEq/L). C‐reactive protein (45.1 mg/dl, reference range, 0–20 mg/dl) and symmetric dimethylarginine (45 μg/dl; reference range –18 μg/dl) levels were elevated. Ketosis was not confirmed. In addition, a urine dipstick test indicated proteinuria (2+) with a protein:creatinine ratio of 6.05. Ultrasonography was unremarkable. After fluid therapy to improve azotemia and electrolyte imbalance, urine output was normal, and the patient's condition resolved slowly during the hospital period.
One day, this patient had hypoglycaemia (glucose 34 mg/dl; reference range, 74.5–120 mg/dl) when fasting. Despite an abrupt reduction of insulin dose (NPH insulin, 0.81–0.16 IU/kg/dose, gradually for 8 days), the hypoglycaemic episodes kept recurring. There were no specific findings in serum chemistry, urinalysis, or ultrasonography. When measuring thyroid hormone levels, total thyroxine (T4) (<0.5 μg/dl; reference range, 1.0–4.0 μg/dl) and free T4 (<0.3 μg/dl; reference range, 0.77–3.49) levels were low with a normal thyroid stimulating hormone (0.065 μg/dl; reference range, 0.05–0.42 μg/dl) concentration. Based on these results, the patient was diagnosed with non‐thyroidal illness syndrome, which is a condition with abnormal thyroid panel with normal thyroid function. As this patient's Na:K ratio was decreased at emergency visit, hypoadrenocorticism (HOAC) was considerable. Interestingly, after an adrenocorticotropic hormone (ACTH) stimulation test, both pre‐ (<1.0 μg/dl; reference range, 1–6 μg/dl) and post‐test (<1.0 μg/dl; reference range, 5.5‐18 μg/dl) cortisol concentrations were very low, and blood aldosterone (<1.5 μg/dl; reference range, 3.5–4.0 μg/dl) was also below the reference range.
Deoxycorticosterone pivalate (DOCP, 2.2 mg/kg SC every 22 days) and prednisolone (PDS, 1 mg/kg, PO daily) were administered for HOAC. The patient's general condition improved with no hypoglycaemia at fasting (fasting glucose, 232 mg/dl). After that, IBD, DM, HOAC, and chronic kidney disease were well managed. However, a month later, she died of symptoms of dyspnoea.
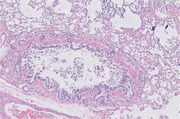
A full necropsy was conducted for the histopathologic evaluation of four organs: the lungs, kidneys, ileum, and adrenal glands. Histopathological analysis of lung tissue confirmed mineralization of the alveolar interstitium, compatible with uremic pneumopathy. There was also histologic evidence of mild neutrophilic bronchopneumonia, primarily within the large airways, which was likely secondary or incipient (Figure 1; IDEXX Laboratories, Westbrook, ME). A primarily glomerular lesion was found in a section of the kidney, with glomeruli expanded by material that could be sclerotic collagen (glomerulosclerosis, as the chronic stage of immune‐mediated glomerulonephritis). There is some mild evidence of chronic tubular damage, presumably secondary to glomerular disease (Figure 2; IDEXX Laboratories). In histopathology analysis of the adrenal gland, the cortex was histologically unremarkable with appropriate architecture, including a small area of the medulla. However, some degree of cortical atrophy was suggested (Figure 3; IDEXX Laboratories). In an ilium section, mild lymphoplasmacytic and eosinophilic enteritis was diagnosed (Figure 4; IDEXX Laboratories).
FIGURE 1.

Histopathology of lung diagnosed with uremic pneumopathy and neutrophilic bronchopneumonia. Sections of lung have marked mineralization of the alveolar interstitial, compatible with uremic pneumopathy. This represents a form of metastatic calcification, and we can suspect some clinical correlation with renal failure. Regionally, alveolar septa are also thickened by fibrosis, and there is smooth muscle hypertrophy. There is also histologic evidence of a mild neutrophilic bronchopneumonia, primarily within large airways, and likely secondary or incipient
FIGURE 2.

Histopathology of kidney diagnosed with chronic tubular damage, presumably secondary to glomerular disease. Almost all glomeruli have mesangium expanded by eosinophilic hyalinized material, with thickened capillary loops, occasionally obliterating the glomerular tuft. Renal tubules are occasionally ecstatic, lined by attenuated epithelium. Tubules occasionally contain protein casts or, more rarely, red blood cell casts. They are occasionally small mineralized tubules within the medulla. The lesion appears primarily glomerular, with glomeruli expanded by material that could be sclerotic collagen (glomerulosclerosis, as the chronic stage of immune mediated glomerulonephritis). There is evidence of chronic tubular damage, presumably secondary to glomerular disease
FIGURE 3.

Histopathology of adrenal gland diagnosed with cortical atrophy. The specimen consists of histologically unremarkable adrenal cortex, with appropriate architecture, and including a small area of medulla. However, some degree of cortical atrophy was suggested
FIGURE 4.

Histopathology of ileum suggested with lymphoplasmacytic and eosinophilic enteritis. Ileum is examined with a full‐thickness section. The lamina propria has small to moderate numbers of lymphocytes and plasma cells, with fewer eosinophils. This mild lymphoplasmacytic and eosinophilic enteritis was consistent with a chronic enteropathy
3. DISCUSSION
Autoimmune polyendocrine syndrome (APS), also called polyglandular autoimmune syndrome (PAS), is an immune‐mediated disorder involving various endocrine glands (Kooistra, 1995). APS should be considered in every patient in the case of the co‐existence of two or more autoimmune endocrinopathies. According to the Neufeld and Blizzard Classification of 1980, there are four main types of APS; their most relevant features are described in Table 1 (Blois, 2011; Idowu, 2018). The combination of HOAC and type I DM (T1DM) is known as Schmidt syndrome, and is sometimes used interchangeably with type II APS (Kooistra, 1995; Solomon, 1965). Although less than 1% of people with T1DM have HOAC in human medicine, there are fewer reports of these cases in veterinary medicine (Cryer, 2008; Neufeld, 1981; Weiler, 2012).
TABLE 1.
Characteristics of the autoimmune polyendocrine syndromes (APS)
| APS type 1 | Chronic candidiasis, hypoparathyroidism, autoimmune adrenal insufficiency (at least two of them should be present) |
| APS type 2 | Autoimmune adrenal insufficiency (must always be present) + autoimmune thyroid disease and/or type 1 diabetes mellitus |
| APS type 3 | Autoimmune thyroid disease + other autoimmune disease (excluding autoimmune adrenal insufficiency, hypoparathyroidism, chronic candidiasis) |
| APS type 4 | Two or more organ‐specific autoimmune disease (which do not fall into type 1, 2, or 3) |
In this case, the histological examination of the pancreatic tissue and blood C‐peptide concentration evaluation were not performed, so the diagnosis was limited, but this patient needed exogenous insulin for blood glucose control. Additionally, according to Nelson et al., all diabetic dogs have type 1 insulin‐dependent diabetes related to immune mediation (Nelson, 2019). Therefore, this case was strongly considered type 1 diabetes.
During hospitalization, when hypoglycaemia persists despite repeatedly reducing insulin levels, another underlying cause must be considered (McAulay, 2000). Excessive activity, reduced carbohydrate intake, severe infection, and liver disease were not suspected in this patient. Certain tumours associated with hypoglycaemia, such as insulinoma, seem to be less likely (Goutal et al., 2012). In blood analysis, the Na:K ratio was found to be 22.04. Therefore, hypoadrenocorticism was considered first. Although hypoglycaemia is not an ordinary feature of HOAC, it may be related to increased insulin sensitivity caused by glucocorticoid deficiency (MaAulay, 2000). Based on this and repeated ACTH stimulation tests, HOAC was diagnosed in this case.
Since the patient has been taking PDS for 2 years owing to an immune‐mediated disease, secondary hypoadrenocorticism may be suspected owing to long‐term steroid administration. However, based on the low Na:K ratio, primary HOAC was more reasonable (Nelson, 2019). In addition, some degree of cortical atrophy was suggested in adrenal gland histopathology analysis.
After supplementing with increasing PDS dosage and subcutaneous DOCP injection, the patient's glucose curve stabilized with no remarkable hypoglycaemic events, and the patient's condition improved. Furthermore, after initiating HOAC management, this patient did not experience any hypoglycaemic event with the same dose that caused it before, and we could increase the definitive insulin dose stably (NPH insulin, 0.49 IU/kg/dose).
It is also noteworthy that this patient had many accompanying immune‐mediated diseases in multiple organs. There is a limit to the inability to distinguish between hypothyroidism and normal thyroid gland because no additional TSH stimulation tests have been performed besides the thyroid hormone test. However, this is considered a non‐thyroid disease syndrome, which is a change in the concentration of thyroid hormones due to acute/chronic disease. Although electron and immunofluorescence microscopy could not be performed, this patient was diagnosed with IBD and GNP that can be related to immune‐mediated glomerulonephritis. Moreover, as this patient had respiratory signs that improved following immunosuppressant therapy, we cannot exclude the possibility of IMP, which can be related to idiopathic lung fibrosis. Considering that the patient had not only the characteristics of type II APS, but also characteristics of IBD, GNP, and IMP, the diagnosis of type IV APS was considered.
4. CONCLUSION
This is the first reported case of hypoadrenocorticism concurrent with diabetes mellitus in a dog. Considering that the patient had not only the characteristics of type II APS, but also the characteristics of IBD, GNP, and IMP, the diagnosis of type IV APS was considered. This case highlights the importance of being vigilant about new components of a syndrome.
CONFLICT OF INTEREST
The authors declared no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Seo‐Young Hwang and Ju‐Hyun An: Conceptualization, investigation, resources, and writing‐original draft. Jeong‐Hwa Lee, Su‐Min Park, Hyung‐Kyu Chae, and Kyung‐Bo Kim: Investigation and validation. Woo‐Jin Song and Hwa‐Young Youn: Investigation, validation, and writing‐review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.625
Hwang, S.‐Y. , An, J.‐H. , Lee, J.‐H. , Park, S.‐M. , Kyu Chae, H. , Kim, K.‐B. , Song, W.‐J. , & Youn, H.‐Y. (2021). Autoimmune polyendocrine syndrome with hypoadrenocorticism and diabetes mellitus in a dog: A rare case. Veterinary Medicine and Science, 7, 2120–2123. 10.1002/vms3.625
Seo‐Young Hwang and Ju‐Hyun An are co‐first authors and contributed equally.
Contributor Information
Woo‐Jin Song, Email: ssong@jejunu.ac.kr.
Hwa‐Young Youn, Email: hyyoun@snu.ac.kr.
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs.
REFERENCES
- Blois, S. L. , Dickie, E. , Kruth, S. A. , & Allen, D. G. (2011). Multiple endocrine diseases in dogs: 35 Cases (1996–2009). Journal of the American Veterinary Medical Association, 238, 1616–1621. [DOI] [PubMed] [Google Scholar]
- Cryer, P. (2008). Hypoglycemia: Still the limiting factor in the glycemic management of diabetes. Endocrine Practice, 14, 750–756. [DOI] [PubMed] [Google Scholar]
- Goutal, C. M. , Brugmann, B. L. , & Ryan, K. A. , (2012). Insulinoma in dogs: A review. Journal of the American Animal Hospital Association, 48, 151–163. [DOI] [PubMed] [Google Scholar]
- Idowu, O. , & Heading, K. (2018). Hypoglycemia in dogs: Causes, management, and diagnosis. Canadian Veterinary Journal, 59, 642. [PMC free article] [PubMed] [Google Scholar]
- Kooistra, H. , Rijnberk, A. , & van den Ingh, T. S. (1995). Polyglandular deficiency syndrome in a boxer dog: Thyroid hormone and glucocorticoid deficiency. Veterinary Quarterly, 17, 59–63. [DOI] [PubMed] [Google Scholar]
- McAulay, V. , & Frier, B. M. (2000). Addison's disease in type 1 diabetes presenting with recurrent hypoglycaemia. Postgraduate Medical Journal, 76, 230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalache, L. , Arhire, L. , Gherasim, A. , Graur, M. , & Preda, C. (2016). A rare case of severe type 4 polyglandular autoimmune syndrome in a young adult. Acta Endocrinologica, 12, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, R. W. , & Couto, C. G. (2019). Small animal internal medicine‐E‐book. Elsevier Health Sciences. [Google Scholar]
- Neufeld, M. , Maclaren, N. K. , & Blizzard, R. M. (1981). Two types of autoimmune Addison's disease associated with different polyglandular autoimmune (PGA) syndromes. Medicine, 60, 355–362. [DOI] [PubMed] [Google Scholar]
- Solomon, N. , Carpenter, C. C. , Bennett, I. L. , & Harvey, A. M. (1965). Schmidt's syndrome (thyroid and adrenal insufficiency) and coexistent diabetes mellitus. Diabetes, 14, 300–304. [DOI] [PubMed] [Google Scholar]
- Weiler, F. G. , Dias‐da‐Silva, M. R. , & Lazaretti‐Castro, M. (2012). Autoimmune polyendocrine syndrome type 1: Case report and review of literature. Arquivos Brasileiros de Endocrinologia, 56, 54–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs.


