Abstract
The present study was designed to compare the effects of lidocaine and ropivacaine in intravenous regional anaesthesia (IVRA) in dogs. Twelve adult male dogs were used. Under isoflurane anaesthesia, exsanguination was performed in the target forelimb. Then, a blood pressure cuff was encircled around the limb proximal to the elbow joint with a pressure of approximately 150 mmHg above the mean arterial blood pressure. The animals then received one of the two treatments of lidocaine (3 mg/kg) or ropivacaine (1.5 mg/kg) with a final volume of 0.6 mL/kg into the cephalic vein. After 60 min, the anaesthesia was disrupted and the tourniquet was removed using intermittent opening (30 s) and closing (5 min) manner for three times. The results revealed that at 20 and 30 min after the initiation of IVRA, the dogs in ROP showed higher analgesia than LID. A leakage under the tourniquet during IVRA was detected. Tremor and hypersalivation were observed after tourniquet removal in some dogs. It was concluded that ropivacaine might provide a higher quality of anaesthesia than lidocaine in IVRA in dogs. The development of local anaesthetic toxicity is a major concern and should be considered at the time of tourniquet removal.
Keywords: analgesia, canine, exsanguination, toxicity
The effects of lidocaine and ropivacaine in intravenous regional anaesthesia (IVRA) in dogs were investigated. The results showed that ropivacaine provided a better quality of analgesia than lidocaine. Leakage under the tourniquet during IVRA was detected. Tremor and hypersalivation were observed after tourniquet removal in some dogs.

1. INTRODUCTION
Intravenous regional anaesthesia (IVRA) or Bier's block is a technique employed for providing anaesthesia in surgical procedures involving distal extremities. In this method, after the closure of a tourniquet in the limb, a local anaesthetic is injected intravenously below the tourniquet to provide anaesthesia and/or analgesia to the structures below the tourniquet. This technique has been used in humans for more than 100 years (Staffieri, 2013), with an estimated success rate of 94%–98% (Hartmannsgruber et al., 1999). IVRA is relatively easy and safe to perform in humans and is frequently used for the surgery of extremities. A study in humans reported that 86% of North American anaesthesiologists routinely use this method for pain management in surgical patients (Henderson et al., 1997).
In veterinary medicine, although IVRA is commonly used in ruminants (Edmondson, 2016), limited studies are available employing this method in small animals (De Marzo et al., 2012; Kushner et al., 2002; Webb et al., 1999). Because of some benefits, including its simplicity and reliability, providing appropriate intraoperative anaesthesia and analgesia as well as reducing bleeding in the surgical site, IVRA seems to have the potential to be used more widely in dogs and cats. It has been suggested that IVRA, in combination with general anaesthesia or deep sedation, can be used in a variety of surgical procedures in these species, including amputation, fracture repair, arthrodeses and wound closure (Staffieri, 2013).
Lidocaine is the popularly used local anaesthetic for IVRA; however, maintaining analgesia after the tourniquet release is short (Chan et al., 1999). Further, lidocaine has been shown to have some adverse effects when used for IVRA in human volunteers (Hartmannsgruber et al., 1999). Ropivacaine is a long‐acting local anaesthetic with less central and cardiovascular neurotoxicity than bupivacaine (Chan et al., 1999; Feldman et al., 1989) and the strength of about three times as large as that of lidocaine (Reiz et al., 1989). It has been suggested that IVRA with ropivacaine compared to lidocaine can be associated with greater tolerance of tourniquet closure, better analgesia after tourniquet removal, and reduced postoperative analgesic use in human patients (Asik et al., 2009; Atanassoff et al., 1998). Some authors have suggested that ropivacaine may be a suitable alternative to lidocaine for IVRA (Asik et al., 2009; Hartmannsgruber et al., 1999).
Since the authors did not find any study comparing the anaesthetic and cardiorespiratory effects following IVRA with lidocaine and ropivacaine in small animals, the present study was designed to determine the efficacy, cardiorespiratory impacts, serum concentrations and potential complications of IVRA with lidocaine and ropivacaine in dogs. We hypothesized that both lidocaine and ropivacaine provide acceptable anaesthesia with minimal complications when used for IVRA in dogs and the anaesthesia after ropivacaine would last longer than lidocaine after removing the tourniquet.
2. MATERIALS AND METHODS
2.1. Animals
The present study was designed as a prospective experimental double‐blinded study. Twelve adult mongrel male dogs (belonging to a canine shelter intended to keep and provide supports for stray dogs) with the weight of 19.3 ± 2.7 kg and 1.5–2.5 years of age were used. The dogs were transferred to the Veterinary Hospital at least 2 weeks before the commencement of the experiments and were kept in separate cages. Animal health was confirmed by a thorough physical examination, CBC and TP measurements. During the study period, the animals were fed twice a day and had access to water ad libitum. Animals were given 12 h fasting and 2 h water limitation before each experiment. Before the initiation of the study, informed consent was obtained from the shelter owner. The Ethics Committee of our university approved this study (EE/96.24.3.88374/scu.ac.it). After completion of the experiments, the dogs were returned to their shelter.
2.2. Preparation, anaesthesia and instrumentation
On the day of the experiment, the dogs were transferred to the experiment room and kept in a quiet environment for 30 min. The animals were then placed onto a surgery table, and both forelimbs from the toe to the shoulder joint were clipped and aseptically prepared. Twenty‐gauge catheters were inserted into the cephalic veins of both forelimbs and fixed. The dogs were preoxygenated with 100% oxygen for 5 min using a flow‐by method. Anaesthesia was then induced with administration of propofol (6 mg/kg; Lipuro 10 mg/mL, Melsungen, Germany) into the cephalic vein. After the induction of anaesthesia, the animal's trachea was intubated with a suitable tracheal tube (7.5–8.5 ID), and anaesthesia was maintained using isoflurane (1.5%–2%; Isoflurane, USP, Terrel, USA) in 100% oxygen (100 mL/kg/min) via a circle breathing circuit with spontaneous breathing. Also, normal saline (10 mL/kg/h) was administered through the same cephalic vein used for propofol injection. During the anaesthesia period, it was attempted to keep the body temperature above 37˚C using blankets and warm water bags. The animals were then positioned in right lateral recumbency (the left forelimb placed uppermost). Next, the left jugular vein (for blood collection) and the right metatarsal artery [for measuring direct mean blood pressure (MBP)] were catheterized. To obliterate the effect of the limb position (i.e. uppermost vs. lowermost), IVRA was applied in three left forelimbs and three right forelimbs in each treatment.
2.3. IVRA
After 20 min of the initiation of the general anaesthesia, the target (test) forelimb was held up for 5 min for exsanguination. A compressive bandage was then applied around the limb from the toe proximally; caution was taken not to interfere with the intravenous catheter. A blood pressure cuff (6.5 cm width) was then encircled, closed approximately 10 cm above the elbow joint and secured by several twists of a leukoplast adhesive tape around it. The pressure was adjusted to be about 150 mmHg above the MBP measured by the pressure gauge attached to the metatarsal artery catheter. The applied bandage was then removed. To ensure no blood flow, a pulse oximeter probe (Vitapia 7000 kv, Trismed, South Korea) was attached to the interdigital area of the limbs, which had not to detect any signal indicating blood flow to the limb.
Then, a toe pinch response (TPR) at the two forelimbs (control and test) was applied using a Halsted mosquito haemostat closed at the first ratchet for 2 s to exert a positive response (i.e. limb withdrawal). In the absence of a positive response in either limb, the isoflurane concentration was reduced by approximately 0.25% via a vaporizer, with 10 min allowed to elapse. Next, the toe pinch response was re‐evaluated and the process was repeated until a positive response was elicited at both limbs. At this time, the dogs were randomly (www.randomizer.com) allocated to one of the treatments of 1‐LID: lidocaine (3 mg/kg; lidocaine hydrochloride 2%, 100 mg/5 mL, Caspian Tamin, Rast, Iran) and 2‐ROP: ropivacaine (1.5 mg/kg; Ropivacaine Molteni, 5 mg/mL, Molteni, Italy) into the cephalic vein. The final volume of the administered solution was adjusted to 0.6 mL/kg using normal saline. The solution was administered slowly during about 2 min. After completion of the injection, the TPR was evaluated every 30 s to be absent to confirm the success of the block. The depth of anaesthesia was maintained to the plan to allow to exert a positive TPR in the control limb. If TPR was negative in the control limb, the concentration of isoflurane was reduced by approximately 25%. If the depth of anaesthesia was going to be very shallow (indicated by nystagmus and/or limb and head movements), the isoflurane concentration rose by approximately 25%. Intraoperative and postoperative analgesia was assessed using the same methods as Costa et al. (2019). If pain scores were greater than 10 for each assessment scale, fentanyl (3 μg/kg) would be administered intravenously.
During isoflurane anaesthesia, the pressure of the applied cuff was maintained to be approximately 150 mmHg above the MBP. Animals were kept under general anaesthesia for 60 min after the administration of local anaesthetics. At this time, isoflurane was discontinued, but the animal received 100% oxygen. The cuff in the limb was deflated for 30 s and closed again with the same pressure for 5 min. The cuff was deflated and closed again in the same previous manner. In the third episode, the cuff was deflated and removed from the limb. After returning the swallowing reflex and/or tongue movement, oxygen was discontinued and the trachea was extubated. At the end of the procedures and monitoring, animals received ketoprofen at a dose of 1.1 mg/kg intramuscularly (IM). All the dogs received IM cefazolin (22 mg/kg) and tramadol (2 mg/kg) every 12 h for 3 days.
2.4. Assessments
Pain assessment was performed using TPR at 5 and 10 min after administration of local anaesthetic and then every 10–60 min after IVRA initiation followed by every 5 min after tourniquet removal. The animals' response to the pain test was graded according to the following scoring system: severe pain: sudden limb withdrawal (Staffieri, 2013), moderate pain: slight limb withdrawal (Hartmannsgruber et al., 1999), mild pain: limb trembling rather than withdrawal (Henderson et al., 1997) and no pain: no vibrational or withdrawal response (Edmondson, 2016).
Physiologic variables including heart rate (HR), respiratory rate (f R) and rectal temperature (RT) were determined and recorded at baseline (before induction) and every 10 min after initiation of IVRA and every 10 min after the end of IVRA up to 60 min later. HR and f R were counted via chest auscultation and extrusion, respectively. RT was measured via a thermometer attached to the rectal mucosa for 1 min. MBP was measured via the metatarsal artery catheter at baseline (10 min after isoflurane anaesthesia) and every 10 min after initiation to the end of IVRA.
Electrocardiography (ECG; Lead II, 50 mm/s, 1 cm/mV; Vitapia 7000 kv, Trismed, South Korea) was recorded at the induction time and every 10 min after beginning IVRA until 60 min later.
The serum concentrations of local anaesthetics were determined using high performance liquid chromatography (HPLC). Two mL blood samples were taken via the metatarsal artery before anaesthesia and at 5, 15, 30 and 45 of local anaesthesia administration as well as at 5, 15, 30, 45, 90 and 120 min after tourniquet removal. First, a 1 mL blood sample was collected and reserved. Then, the blood sample was taken for analysis. Next, the first blood sample was injected into the artery and the catheter was flushed with 1 mL of saline solution containing heparin (2 U/mL). Sera were then separated through centrifugation (2000 rpm for 10 min) and stored at –20°C until measurement by HPLC. A modification of Imani et al. (2013) was used to determine lidocaine and ropivacaine serum concentrations. The diagrams obtained from the device measurements were analysed using standard curves and, after which the measured drug concentrations were determined. The detection limit for both drugs was 0.01 μg/mL.
2.5. Statistical analysis
Statistical analyses were performed using SPSS software (version 25, IBM Corporation, NY, USA). Since the comparison of data between the right and left forelimbs was not significant, data from both forelimbs were pooled for further analysis. The normal distribution of data was analysed by a Kolmogorov–Smirnov test. Parametric data were presented as mean (standard deviation) as well as nonparametric data as median (maximum–minimum). An Independent Sample t‐test was used to compare animal weights, the concentration of isoflurane, cuff pressure changes and physiologic variables between the two groups. Mann–Whitney and Wilcoxon tests were used to compare the pain scores between the two groups as well as between the test and control limb in each group, respectively. A repeated measure for ANOVA followed by Bonferroni's test was used for comparing the physiologic variables over time.
3. RESULTS
There was no difference in the weights of the studied dogs between the two treatments: 19.4 ± 2.8 kg in the LID versus 19.1 ± 2.7 kg in the ROP (p = 0.834). All animals tolerated the anaesthesia (including induction, catheterization, tracheal intubation and maintenance) plus the IVRA procedures well and recovered eventually. The concentration of isoflurane during the anaesthesia period was 1.8% ± 0.25% in the LID group and 1.75% ± 0.27% in the ROP group without significant differences between the two treatments (p = 0.787). The cuff pressure from the closure to the opening was 225.5 ± 47.2 mmHg in the LID and 222.5 ± 37.7 mmHg in the ROP (p = 0.582). In two dogs (one in each treatment), the pulse oximeter showed oxygen saturation after closure which disappeared with an increase of 10–20 mmHg in the cuff pressure. At the time of tourniquet removal, four and three out of six dogs in each group showed muscle tremor and/or hypersalivation. None of the dogs required rescue analgesia during and after the anaesthesia session. No complication was observed during and after the completion of the study (follow‐up of at least 2 weeks).
Figures 1, 2, 3 display changes in the pain scores in the studied dogs. Pain scores in the test limbs were significantly lower for LID than ROP at 20 and 30 min after IVRA initiation (p = 0.005). There was no significant difference in the control limbs in either the treatments (p ≥ 0.211). The comparison of the pain scores in the LID group showed significantly higher values at 20 and 40 min in the test limb compared to the control limb (p = 0.046 and 0.038, respectively). Also, in the ROP group, pain scores were significantly higher at 20, 30, 50 and 60 min after anaesthesia in the test limb than in the control (p = 0.038, 0.034, 0.038 and 0.034, respectively).
FIGURE 1.
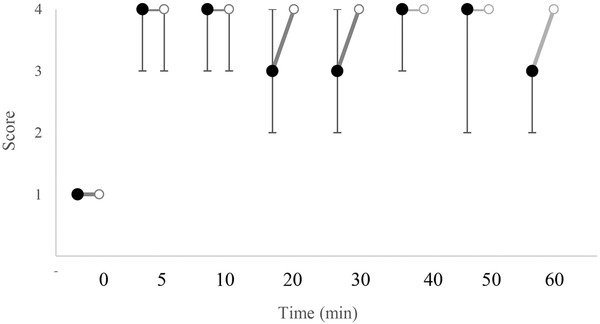
Median (range) of pain scores in the test limb in dogs receiving lidocaine (LID;•) or ropivacaine (ROP;o) for IVRA. †Significant different from other group (p < 0.05)
FIGURE 2.
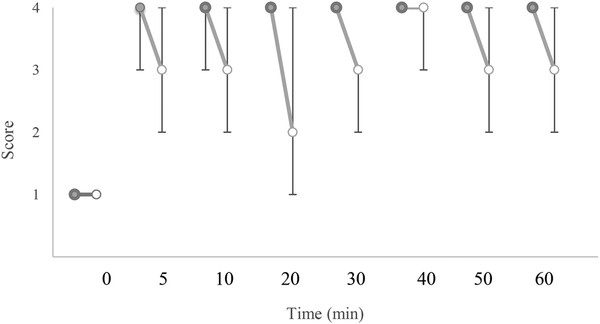
Median (range) of pain scores in the test (o) and control (•) limbs in dogs receiving lidocaine (LID) for IVRA. †Significant different from control (p < 0.05)
FIGURE 3.
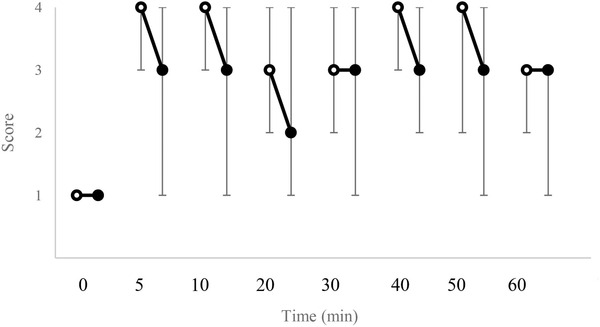
Median (range) of pain scores in the test (•) and control (o) limbs in dogs receiving ropivacaine (ROP) for IVRA. †Significant difference from control (p < 0.05)
Two dogs in LID showed some degree of anaesthesia up to 10 min after the tourniquet removal; however, the remaining dogs responded to noxious stimuli within 5 min after tourniquet removal (i.e. pain score 1). In the ROP, two dogs showed pain scores of 2 and one dog showed a score of 3 at 5 min after tourniquet removal. In this treatment, complete anaesthesia (i.e. pain score 4) was observed in two dogs up to 20 min and in one dog up to 30 min after tourniquet removal.
Table 1 reports the data related to physiologic parameters. Comparison of HR changes showed no significant difference between (p ≥ 0.156) and within LID (p ≥ 0.203). HR was significantly different in ROP at 60 min after initiation of IVRA (p = 0.013) and 10 min after tourniquet removal (p = 0.015) compared to the baseline. The comparison of changes in MBP showed no significant difference between the two treatments (p ≥ 0.197). MBP in the LID was significantly higher at 50 and 60 min after initiation of IVRA in comparison to the baseline value (p = 0.04 and 0.020, respectively). There was no significant difference in the ROP over time in comparison with the baseline (p ≥ 0.076). When comparing the two groups, f R was significantly higher in the ROP than the LID at 10, 20, 30, 40 and 60 min after IVRA initiation and 10 min after tourniquet removal (p ≤ 0.029). f R in the LID was significantly lower than baseline at 10 and 30 min after IVRA initiation (p = 0.041 and 0.039, respectively). In the ROP, f R was not significantly different over time in comparison with the baseline value (p > 0.05). A comparison of RT changes showed no significant difference between the two treatments (p > 0.148). RT in the LID was significantly lower than baseline at several time points after IVRA initiation (p ≤ 0.039). RT in the ROP was significantly lower at 10 min after induction of anaesthesia and 10 min after initiation of IVRA compared to the baseline (p = 0.013 and 0.007, respectively).
TABLE 1.
Mean ± SD heart rate (HR), mean arterial blood pressure (MBP), respiratory rate (f R) and rectal temperature (RT) in dogs (n = 6) receiving lidocaine (LID) or ropivacaine (ROP) for IVRA
| Group | Base | 10 | Initiation of IVRA | 10 | 20 | 30 | 40 | 50 | 60 | End of IVRA | 10 | 20 | 30 | 40 | 50 | 60 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (beats/min) | LID | 79 ± 14 | 103 ± 24 | 106 ± 29 | 122 ± 23 | 127 ± 18 | 121 ± 11 | 126 ± 12 | 129 ± 11 | 123 ± 27 | 137 ± 36 | 108 ± 23 | 89 ± 22 | 90 ± 11 | 82 ± 16 | ||
| ROP | 87 ± 18 | 116 ± 21 | 125 ± 37 | 131 ± 33 | 133 ± 31 | 133 ± 34 | 131 ± 38 | 133 ± 25 ‡ | 127 ± 13 ‡ | 117 ± 17 | 125 ± 26 | 112 ± 29 | 107 ± 25 | 105 ± 33 | |||
| MBP (mmHg) | LID | ND | 65 ± 5 | 71 ± 10 | 100 ± 31 | 104 ± 29 | 112 ± 32 | 119 ± 19 | 123 ± 27 | ND | ND | ND | ND | ND | ND | ||
| ROP | ND | 73 ± 15 | 79 ± 32 | 75 ± 25 | 87 ± 30 | 95 ± 31 | 98 ± 30 | 102 ± 25 | ND | ND | ND | ND | ND | ND | |||
| f R (breaths/min) | LID | 14 ± 2 | 8 ± 3 | 7 ± 3*, † | 9 ± 3* | 8 ± 2*, † | 9 ± 2* | 12 ± 4 | 14 ± 4* | 13 ± 4 | 14± 4 | 12 ± 3 | 13 ± 3 | 13 ± 2 | 13 ± 2 | ||
| ROP | 17 ± 4 | 12 ± 3 | 17 ± 4 | 17 ± 4 | 17 ± 5 | 17 ± 3 | 24 ± 5 | 23 ± 4 | 19 ± 3 | 15 ± 2 | 14 ± 2 | 15 ± 2 | 16 ± 3 | 15 ± 2 | |||
| RT (˚C) | LID | 38.6 ± 0.4 | 37.3 ± 0.3 | 37.3 ± 0.3 † | 37.4 ± 0.2 † | 37.4 ± 0.3 † | 37.2 ± 0.2 † | 37.3 ± 0.4 † | 37.3 ± 0.3 | 37.7 ± 0.3 | 37.3 ± 0.4 | 37.9 ± 0.4 | 38.2 ± 0.4 | 38.4 ± 0.5 | 38.5 ± 0.4 | ||
| ROP | 38.5 ± 0.2 | 37.5 ± 0.3 ‡ | 37.0 ± 0.3 ‡ | 37.6 ± 0.2 | 37.6 ± 0.3 | 37.5 ± 0.4 | 37.4 ± 0.5 | 37.4 ± 0.4 | 37.6 ± 0.5 | 37.2 ± 0.4 | 37.8 ± 0.4 | 38.0 ± 0.3 | 38.3 ± 0.3 | 38.2 ± 0.3 |
Significantly different from other group (p < 0.05).
Significantly different from baseline in LID treatment (p < 0.05).
Significantly different from baseline in ROP treatment (p < 0.05).
Sinus arrhythmia was seen in some of the cases in both treatments at the evaluation times. No other arrhythmia was observed in any of the animals during the evaluation period.
Figures 4 and 5 display the changes in lidocaine and ropivacaine concentrations in the LID and ROP, respectively. The maximum serum concentration (Cmax) of lidocaine and ropivacaine were 4.61 ± 2.15 and 1.36 ± 0.63 μg/mL, respectively. The time of reaching the maximum serum concentration (Tmax) of lidocaine and ropivacaine was 5 min after the tourniquet removal.
FIGURE 4.
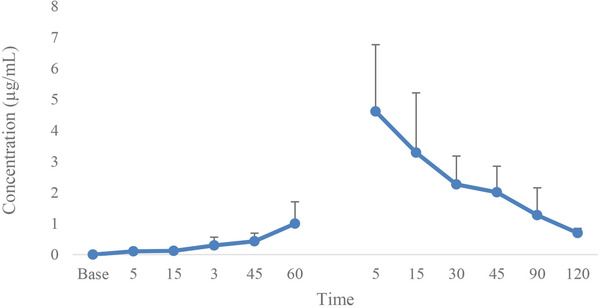
Mean and SD of lidocaine concentration in dogs receiving lidocaine (LID) for IVRA (60 min IVRA and 120 min after tourniquet removal)
FIGURE 5.
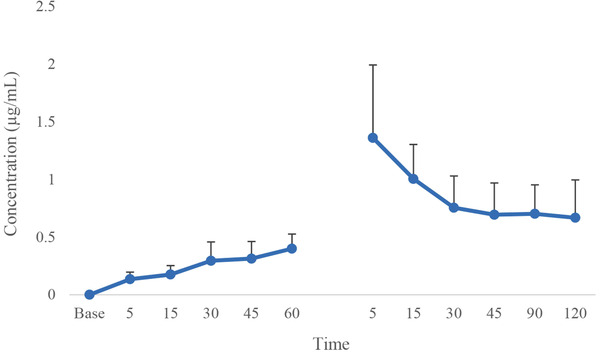
Mean and SD of ropivacaine concentration in dogs receiving ropivacaine (ROP) for IVRA (60 min IVRA and 120 min after tourniquet removal)
4. DISCUSSION
The results of the present study showed that the animals receiving IVRA with ropivacaine experienced less pain than those administered lidocaine at 20 and 30 min in the test limb after IVRA initiation. Leakage of the local anaesthetics was detected during tourniquet closure of IVRA according to the results of HPLC. Adverse effects, including muscle tremor and hypersalivation, were observed in both treatments after tourniquet removal.
In previous studies in dogs and cats, lidocaine was used at a dose of 3 mg/kg and a concentration of 0.5%–2% in IVRA (De Marzo et al., 2012; Kushner et al., 2002; Webb et al., 1999). In the present study, lidocaine was applied at a dose of 3 mg/kg and a final volume of 0.6 mL/kg (i.e. 0.4%) as recommended by Staffieri (2013). In human studies, ropivacaine was applied with a concentration of 0.2%–0.25% for IVRA (Asik et al., 2009; Atanassoff et al., 1998; Hartmannsgruber et al., 1999). One study also used 1.2 and 1.8 mg/kg ropivacaine doses for IVRA in human volunteers (Chan et al., 1999). Due to the lack of information regarding the use of ropivacaine for IVRA in veterinary medicine, in the present, study the dose rate of 1.5 mg/kg with the final volume of 0.6 mL/kg (i.e. 0.16%) was chosen for IVRA in dogs.
The drug leakage under the tourniquet is always a concern when IVRA is employed because in the event of drug leakage, the block will fail and a large amount of drug may enter into the systemic blood circulation resulting in local anaesthetic toxicity. Strategies that need to be done to avoid drug leakage in IVRA include exsanguination, slow injection of the drug into the vein and maintaining a tourniquet pressure of at least 100 mmHg above the systolic pressure (Hoffmann et al., 1995; Staffieri, 2013). Lack of arterial pulse at the distal extremity, no excessive pressure during the drug injection (Staffieri, 2013) and exceeding the cuff's width over 20% of the limb diameter have also been recommended for ensuring no drug leakage (Grice et al., 1986). In the present study, exsanguination was performed by holding the limb up and closing a pressure bandage. The drug was also injected into the vein within 2 min. With the MBP measured, it was attempted to keep the pressure always about 150 mmHg above the MBP when the tourniquet was in place. For this purpose, the pressure of the tourniquet was checked regularly and re‐set if necessary. In addition, a pulse oximeter probe was also placed at the interdigital area to ensure the absence of arterial flow. Although the pressure during drug injection was not measured, it was attempted to avoid excessive pressure. The width of the cuff was 6.5 cm which was greater than 20% of the limb diameter.
In spite of the aforementioned arrangements, the data on serum concentrations of the drugs indicated that the drug leaked under the tourniquet. It has been shown that in human patients, even if adequate tourniquet pressure is applied to close the vascular pathway, there is still a possibility of leakage under the tourniquet in IVRA (Coleman et al., 1999; Hoffmann et al., 1995; Rosenberg et al., 1983). Kushner et al. (2002) in a study of IVRA in cats also found lidocaine leakage under the tourniquet and high plasma concentrations of the drug, despite using two tourniquets in the limb with the maintenance of tourniquet pressure at 100 mmHg above the systolic pressure. Leakage of the local anaesthetics under the tourniquet in the present study can be attributed to the lack of sufficient tourniquet pressure due to technical issues (e.g., inadequate sealing of the tourniquet) or the inability of the tourniquet to prevent drug leakage. The pressure about 150 mmHg above the MBP may not be high enough to close the arteries; however, increasing the pressure might be associated with some adverse consequences, including tourniquet pain and ischemia. It seems that a pulse oximeter may not be an effective method in dogs. An ultrasonic Doppler probe was recommended to be used instead of pulse oximetry (Staffieri, 2013). Some researchers believe that despite sealing the superficial vessels in the proper application of the tourniquet, the drug may still leak into the systemic circulatory system via intraosseous pathways (Coleman et al., 1999).
In the present study, only one out of six dogs in the lidocaine group showed complete block (i.e. pain score 4) by the end of the 60 min tourniquet closure, while in the other dogs, some degrees of pain (i.e. pain score 2 and 3) was detected. In the ropivacaine group, in all six dogs, the complete block remained until the tourniquet closure. Accordingly, it seems that block's quality was higher for ropivacaine than for lidocaine during IVRA in dogs; nevertheless, a significant difference was observed at 20 and 30 min after injection of drugs. In human studies following lidocaine and ropivacaine use for IVRA, the complete block remained until the tourniquet was closed (Asik et al., 2009; Atanassoff et al., 1998; Chan et al., 1999; Hartmannsgruber et al., 1999). In a study in dogs, during IVRA with lidocaine used for pancarpal arthrodesis, the block was satisfactory and required no rescue analgesic administration (De Marzo et al., 2012). The lack of complete block in the 60 min duration of IVRA can be attributed to the leakage of the drug under the tourniquet resulting in insufficiency of lidocaine to provide complete anaesthesia and/or relatively lower concentration of lidocaine. Partial blockage appears not to be uncommon following lidocaine in IVRA. In this regard, Staffieri (2013) recommends that if the IVRA block is inadequate and pain response to stimulation is observed, short‐acting analgesics can be employed to improve the quality of analgesia.
One of the major limitations of IVRA with lidocaine is the lack or short duration of anaesthesia after the tourniquet removal (Chan et al., 1999; Staffieri, 2013). For this reason, ropivacaine has been suggested instead of lidocaine to maintain anaesthesia and analgesia after the tourniquet removal for a more extended period. Studies in human patients receiving IVRA indicated that anaesthesia remains longer in ropivacaine recipients than in lidocaine‐receiving patients (Asik et al., 2009; Atanassoff et al., 1998; Chan et al., 1999; Hartmannsgruber et al., 1999). In the present study, three out of six dogs in the ropivacaine group showed some reductions in pain response after tourniquet removal (in the two dogs up to 20 min and in one dog up to 30 min). In the lidocaine group, except for two dogs, which showed some degree of anaesthesia up to 10 min after the tourniquet removal, the rest of the dogs responded vigorously to noxious stimuli in 5 min after tourniquet opening (i.e. pain score 1). A study in cats reported that IVRA with lidocaine resulted in anaesthesia of the fingers up to 20 min after the tourniquet removal (Kushner et al., 2002). Leakage under the tourniquet and employing lower concentrations of the local anaesthetics can be attributed to the incomplete and short duration of anaesthesia after tourniquet removal in the current study. Further studies are required to rule out or confirm these findings and to elucidate the exact reason.
Local anaesthetic toxicity is always one of the concerns regarding the use of these agents, particularly in IVRA, as in this block, local anaesthetics are injected directly into the vessels. Symptoms of toxicity may be observed if high doses of local anaesthetics enter the general circulatory system. Studies on the toxicity of local anaesthetics in dogs have shown muscle tremors or seizures as the first signs of intoxication (Feldman et al., 1989; Feldman et al., 1991; Lemo et al., 2007; Wilcke et al., 1983). In a study in alert dogs, tremors, salivation, sedation and muscle stiffness have been reported as the symptoms of toxicity with local anaesthetics before the occurrence of seizures (Liu et al., 1983). In the current study, muscle tremor (4 dogs in each group) and hypersalivation (3 dogs in each group) were observed after tourniquet removal. In human studies, some slight and transient signs of discomfort have been documented after tourniquet removal in IVRA (Asik et al., 2009; Atanassoff et al., 1998; Chan et al., 1999; Hartmannsgruber et al., 1999).
In the current investigation, the tremor and hypersalivation were seen in some dogs; however, the animals recovered eventually. As these manifestations were observed after tourniquet removal, they can be considered as the first signs of local anaesthetic toxicity in the studied dogs. There were no differences in the occurrence of the toxicity signs between the two treatments. In a study in dogs, the serum concentration of lidocaine at the beginning of toxicity presented at muscle tremor was 2.7 ± 1.1 μg/mL (Lemo et al., 2007). In another study, the plasma concentration of lidocaine at the time of seizure onset was 8.21 ± 1.69 μg/mL (Wilcke et al., 1983). Feldman et al. (1989) who evaluated the toxic effects of various local anaesthetics in dogs reported concentrations of 47.2 ± 5.6 μg/mL for lidocaine and 11.4 ± 0.9 μg/mL for ropivacaine at the onset of seizures following administration of high doses. In the current study, the average maximum concentration was 4.61 ± 2.15 μg/mL for lidocaine and 1.36 ± 0.63 μg/mL for ropivacaine observed at 5 min following tourniquet removal. It has been proposed that with a high infusion rate of local anaesthetics, due to lack of equilibration and minimizing the drug's redistribution and hepatic metabolism, even low doses can result in high plasma concentrations and thus toxicity (Chadwick, 1985; Malagodi et al., 1977). The same mechanism very likely to have occurred in the present study. By opening the tourniquet, entrance of local anaesthetics into the blood circulation resulted in high plasma concentration and hence toxicity. As such, the time of 30 s opening for tourniquet removal seems long. On the other hand, the 5 min interval between opening times may be not enough to permit the drug metabolism to proceed. Shortening the opening times and/or increasing the closing times might prevent the occurrence of the toxicity signs.
The present study had some limitations. First, an attempt should have been made to manage the depth of anaesthesia to such an extent that the evaluation of the TPR response in the control limb would show the pain response. As anaesthesia could be too shallow to achieve this goal, we had to increase the depth of anaesthesia, thereby losing the TPR response in both limbs. Nevertheless, the use of test and control limbs partly resolved the problem. The second was the drug leakage under the tourniquet during IVRA. Although the leakage appeared low, this might lead to the failure of anaesthesia in the post‐IVRA period in the lidocaine‐recipient dogs as well as some of the ropivacaine‐recipient ones. Third, the criteria employed for IVRA evaluation in the current paper are different from clinical situations in which surgery is performed in the limb, which probably has some impacts on pharmacologic properties and potential consequences of local anaesthetics. With all the issues, it seems that the results of the present study are acceptable and significant and can be considered as a practical guide for employing IVRA in dogs.
In conclusion, lidocaine (3 mg/kg) and ropivacaine (1.5 mg/kg) with the final volume of 0.6 mL/kg using normal saline can be used for IVRA in dogs. Despite all the necessary arrangements, the leakage of the drug under the tourniquet was observed in dogs. It seems anaesthesia with ropivacaine was of higher quality during IVRA and can be considered as an appropriate alternative for lidocaine. Some signs of toxicity, including tremor and hypersalivation were observed in both studied groups after tourniquet removal. At the time of tourniquet removal, reducing the opening times (i.e. less than 30 s) and increasing the closing times (i.e. more than 5 min) are recommended to avoid the occurrence of toxicity. Further studies are required to confirm and improve the results of the current study.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ANIMAL WELFARE AND ETHICS APPROVAL
All study procedures and dog care activities were approved by our Institutional Animal Care and Use Committee which covered the guidelines of ARRIVE on using animals in research.
AUTHOR CONTRIBUTIONS
HIR: conceptualization, designing, performing the experiment, data analysis and revision the manuscript; RM: designing supervision and revision the manuscript; MEG: conceptualization, supervision and revision the manuscript; MM: performing the experiment, data collection and drafting the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.608
ACKNOWLEDGEMENT
This study was supported by grant No. 96/3/02/16670 from the Research Council of Shahid Chamran University of Ahvaz, Ahvaz, Iran.
Imani Rastabi, H. , Mirzajani, R. , Givi, M. E. , & Mohammadpoor, M. (2021). Comparison of intravenous regional anaesthesia with lidocaine and ropivacaine in dogs. Veterinary Medicine and Science, 7, 2135–2143. 10.1002/vms3.608
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Asik, I. , Kocum, A. I. , Goktug, A. , Turhan, K. S. C. , & Alkis, N. (2009). Comparison of ropivacaine 0.2% and 0.25% with lidocaine 0.5% for intravenous regional anesthesia. Journal of Clinical Anesthesia, 21(6), 401–407. [DOI] [PubMed] [Google Scholar]
- Atanassoff, P. , Hartmannsgruber, M. , Bobart, V. , Silverman, D. , & Brull, S. (1998). Comparison of ropivacaine 0.2% and lidocaine 0.5% for intravenous regional anesthesia: A volunteer study. Anesthesia and Analgesia, 86(2S), 255S. [DOI] [PubMed] [Google Scholar]
- Chadwick, H. (1985). Toxicity and resuscitation in lidocaine or bupivacaine‐infused cats. Anesthesiology, 63(4), 385–390. [DOI] [PubMed] [Google Scholar]
- Chan, V. W. , Weisbrod, M. J. , Kaszas, Z. , & Dragomir, C. (1999). Comparison of ropivacaine and lidocaine for intravenous regional anesthesia in volunteers: A preliminary study on anesthetic efficacy and blood level. Ansthesiology, 90(6), 1602–1608. [DOI] [PubMed] [Google Scholar]
- Coleman, M. M. , Peng, P. W. , Regan, J. M. , Chan, V. W. , & Hendler, A. L. (1999). Quantitative comparison of leakage under the tourniquet in forearm versus conventional intravenous regional anesthesia. Anesthesia and Analgesia, 87(6), 1482–1486. [DOI] [PubMed] [Google Scholar]
- Costa, G. L. , Nastasi, B. , Spadola, F. , Leonardi, F. , & Interlandi, C. (2019). Effect of levobupivacaine, administered intraperitoneally, on physiological variables and on intrasurgery and postsurgery pain in dogs undergoing ovariohysterectomy. Journal of Veterinary Behavior, 30, 33–36. [Google Scholar]
- De Marzo, C. , Crovace, A. , De Monte, V. , Grimaldi, D. , Iarussi, F. , & Staffieri, F. (2012). Comparison of intra‐operative analgesia provided by intravenous regional anesthesia or brachial plexus block for pancarpal arthrodesis in dogs. Research in Veterinary Science, 93(3), 1493–1497. [DOI] [PubMed] [Google Scholar]
- Edmondson, M. A. (2016). Local, regional, and spinal anesthesia in ruminants. Veterinary Clinics of North America. Food Animal Practice, 32(3), 535–552. [DOI] [PubMed] [Google Scholar]
- Feldman, H. S. , Arthur, G. R. , & Covino, B. G. (1989). Comparative systemic toxicity of convulsant and supraconvulsant doses of intravenous ropivacaine, bupivacaine, and lidocaine in the conscious dog. Anesthesia and Analgesia, 69(6), 794–801. [PubMed] [Google Scholar]
- Feldman, H. S. , Arthur, G. R. , Pitkanen, M. , Hurley, R. , Doucette, A. M. , & Covino, B. G. (1991). Treatment of acute systemic toxicity after the rapid intravenous injection of ropivacaine and bupivacaine in the conscious dog. Anesthesia and Analgesia, 73(4), 373–384. [PubMed] [Google Scholar]
- Grice, S. C. , Morell, R. C. , Balestrieri, F. J. , Stump, D. A. , & Howard, G. (1986). Intravenous regional anesthesia: Evaluation and prevention of leakage under the tourniquet. Anesthesiology, 65(3), 316–320. [PubMed] [Google Scholar]
- Hartmannsgruber, M. W. , Silverman, D. G. , Halaszynski, T. M. , Bobart, V. , Brull, S. J. , Wilkerson, C. , Loepke, A. W. , & Atanassoff, P. G. , (1999). Comparison of ropivacaine 0.2% and lidocaine 0.5% for intravenous regional anesthesia in volunteers. Anesthesia and Analgesia, 89(3), 727–731. [DOI] [PubMed] [Google Scholar]
- Henderson, C. L. , Warriner, C. B. , McEwen, J. A. , & Merrick, P. M. (1997). A North American survey of intravenous regional anesthesia. Anesthesia and Analgesia, 85(4), 858–863. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. , Van Gessel, E. , Gamulin, Z. , Ryser, J. , & Forster, A. (1995). Quantitative evaluation of tourniquet leak during iv regional anaesthesia of the upper and lower limbs in human volunteers. British Journal of Anaesthesia, 75(3), 269–273. [DOI] [PubMed] [Google Scholar]
- Imani, H. , Vesal, N. , & Mohammadi‐Samani, S. (2013). Evaluation of intravenous lidocaine overdose in chickens (Gallus domesticus). Iranian Journal of Veterinary Surgery, 8(1), 9–16. [Google Scholar]
- Kushner, L. I. , Fan, B. , & Shofer, F. S. (2002). Intravenous regional anesthesia in isoflurane anesthetized cats: Lidocaine plasma concentrations and cardiovascular effects. Veterinary Anaesthesia and Analgesia, 29(3), 140–149. [DOI] [PubMed] [Google Scholar]
- Lemo, N. , Vnuk, D. , Radisic, B. , Skender, L. , Karacic, V. , & Brcic, I. (2007). Determination of the toxic dose of lidocaine in dogs and its corresponding serum concentration. Veterinary Record, 160(11), 374–375. [DOI] [PubMed] [Google Scholar]
- Liu, P. L. , Feldman, H. S. , Giasi, R. , Patterson, M. K. , & Covino, B. G. (1983). Comparative CNS toxicity of lidocaine, etidocaine, bupivacaine, and tetracaine in awake dogs following rapid intravenous administration. Anesthesia and Analgesia, 62(4), 375–379. [PubMed] [Google Scholar]
- Malagodi, M. , Munson, E. , & Embro, W. (1977). Relation of etidocaine and bupivacaine toxicity to rate of infusion in rhesus monkeys. British Journal of Anaesthesia, 49(2), 121–125. [DOI] [PubMed] [Google Scholar]
- Reiz, S. , Häggmark, S. , Johansson, G. , & Nath, S. (1989). Cardiotoxicity of ropivacaine—A new amide local anaesthetic agent. Acta Anaesthesiologica Scandinavica, 33(2), 93–98. [DOI] [PubMed] [Google Scholar]
- Rosenberg, P. H. , Kalso, E. A. , Tuominen, M. K. , & Linden, H. B. (1983). Acute bupivacaine toxicity as a result of venous leakage under the tourniquet cuff during a Bier block. Anesthesiology, 58(1), 95–98. [DOI] [PubMed] [Google Scholar]
- Staffieri, F. (2013). Intravenous regional anesthesia. In Campoy L., Read M. R. (Eds.), Small animal regional anesthesia and analgesia (261–271). India: John Wiley & Sons. [Google Scholar]
- Webb, A. A. , Cantwell, S. L. , Duke, T. , & Adkins, E. (1999). Intravenous regional anesthesia (Bier block) in a dog. Canadian Veterinary Journal, 40(6), 419–421. [PMC free article] [PubMed] [Google Scholar]
- Wilcke, J. , Davis, L. , & Neff‐Davis, C. (1983). Determination of lidocaine concentrations producing therapeutic and toxic effects in dogs. Journal of Veterinary Pharmacology and Therapeutics, 6(2), 105–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


