Abstract
The present study aimed to estimate the prevalence of zoonotic pathogens Giardia duodenalis, Cryptosporidium spp., Toxoplasma gondii and Erysipelothrix in muskoxen (Ovibos moschatus) and sheep (Ovis aries) from Greenland. In 2017 and 2018, faecal samples were collected from wild muskoxen from three distinct populations (Zackenberg, Kangerlussuaq, and Ivittuut) and from domestic sheep from southwest Greenland. Blood samples were collected from muskoxen from Kangerlussuaq and Ivittuut and from sheep. Faecal samples were tested for specific DNA of G. duodenalis and Cryptosporidium spp., and blood samples were tested for antibodies against T. gondii and Erysipelothrix. The estimated prevalence of G. duodenalis was 0% (0/58), 17% (7/41) and 0% (0/55) in muskoxen from Zackenberg, Kangerlussuaq and Ivittuut, respectively, and 37% (16/43) in sheep. The estimated prevalence of Cryptosporidium was 0% (0/58), 2% (1/41), 7% (4/55) in muskoxen from Zackenberg, Kangerlussuaq, Ivittuut, respectively, and 2% (1/43) in sheep. Neither Giardia nor Cryptosporidium were detected in winter samples (0/78). Of the positive samples, Giardia from one muskox sample only was successfully typed as G. duodenalis assemblage A, and Cryptosporidium from two muskoxen was successfully typed as C. parvum, subtype IIdA20G1e. The estimated T. gondii seroprevalence was 2% (1/44) and 0% (0/8) in muskoxen from Kangerlussuaq and Ivittuut, respectively, and 1% (1/155) in sheep. The estimated Erysipelothrix seroprevalence was 2% (1/45) and 13% (1/8) in muskoxen from Kangerlussuaq and Ivittuut, respectively, and 7% (10/150) in sheep. The results of this study add to the scarce knowledge on zoonotic pathogens in the Arctic.
Keywords: arctic regions, epidemiology, ruminants, serology, zoonoses
We estimated the prevalence of four zoonotic agents – Giardia duodenalis, Cryptosporidium, Toxoplasma gondii and Erysipelothrix – in muskoxen (Ovibos moschatus) and sheep (Ovis aries) from Greenland and reported the first molecular detection of G. duodenalis and C. parvum in Greenland. The results add to the scarce knowledge on zoonotic pathogens in the Arctic.
1. INTRODUCTION
Endemic and emerging zoonotic infectious diseases have received increasing attention during the last few decades (Cunningham et al., 2017). Climate warming and other environmental changes (AMAP, 2019) may alter the distribution and transmission patterns of a range of zoonotic agents in the Arctic (Davidson et al., 2011; MacPhee & Greenwood, 2013; Thompson et al., 2010), which in turn may impact the health of Arctic animals and Arctic subsistence hunters (Hoberg et al., 2012; Jenkins et al., 2013). However, data on the distribution of zoonotic pathogens in Arctic key subsistence animals are limited (AMAP, 2015), and studies on zoonotic agents in humans and terrestrial mammals from Greenland are few and scattered (e.g. Carlsson et al., 2019; Clausen & Hjort, 1986; Møller et al., 2010; Raundrup et al., 2015; Sonne et al., 2018). Specifically, no data are available on the prevalence of Giardia duodenalis, Cryptosporidium spp. and Erysipelothrix rhusiopathiae in muskoxen (Ovibos moschatus) and sheep (Ovis aries) in Greenland, while a few serological surveys for T. gondii have been performed (Bille, 1974; Clausen & Hjort, 1986). However, exposure to or shedding of these pathogens have been reported in muskoxen and sheep elsewhere within the Arctic and Subarctic range (Jenkins et al., 2013; Kutz et al., 2000, 2008, 2012; Mavrot et al., 2020; Olsen et al., 2019; Robertson et al., 2010).
The protozoan parasites Giardia and Cryptosporidium are transmitted via the faecal–oral route, for example, through the intake of food or water contaminated with faeces containing the cysts or oocysts (Smith et al., 2007) and may cause diarrheal disease in humans and domestic animals worldwide; however, their clinical impact on most wildlife hosts is largely unknown (Appelbee et al., 2005; Jenkins et al., 2013; Kutz et al., 2009; Savioli et al., 2006).
Toxoplasma gondii is a globally distributed protozoan parasite, with wild and domestic felids as the only known definitive hosts, and with a wide range of warm‐blooded animals, including humans, as intermediate hosts (Dubey, 2010). Infection typically occurs through ingestion of sporulated oocysts in the environment or viable tissue cysts in the tissues of infected animals, but transplacental transmission also occurs (Dubey, 2010). The infection is often subclinical in immunocompetent individuals but it may result in severe disease, especially in immunocompromised hosts and offspring (Dubey, 2010).
Erysipelothrix rhusiopathiae is a zoonotic bacterium with a global distribution and a wide range of hosts (Forde et al., 2016; Wang et al., 2010). It is commonly known as an opportunistic pathogen in pigs, poultry and humans, and a relatively common cause of polyarthritis in sheep (Ersdal et al., 2015). Additionally, it has been associated with abortions in ewes (Fthenakis et al., 2006). Transmission occurs through contact with contaminated materials or soil (Wang et al., 2010). Erysipelothrix rhusiopathiae can cause localised cutaneous infections and systemic infections in humans (Wang et al., 2010). Furthermore, E. rhusiopathiae has been associated with widespread mortality events in Arctic ungulates (Forde et al., 2016; Kutz et al., 2015; Mavrot et al., 2020) and its presence in Arctic wildlife has recently been recognised as a potential public health concern (Groeschel et al., 2019).
Impacts
We estimated the prevalence of four zoonotic agents—Giardia duodenalis, Cryptosporidium, Toxoplasma gondii, and Erysipelothrix—in muskoxen (Ovibos moschatus) and sheep (Ovis aries) in Greenland.
We report the first molecular detection of G. duodenalis and C. parvum in Greenland.
The results add to the scarce knowledge on zoonotic pathogens in the Arctic.
Muskoxen and sheep are known hosts to several zoonotic agents. In the 1960s, muskoxen were translocated from their native range in northeast Greenland (NEG) to mid‐west Greenland (MWG) (Cuyler et al., 2020). Subsequent translocations have distributed muskoxen along the west coast of Greenland, and there are now nine populations, some of which overlap the sheep‐farming area in southwest Greenland (SWG) (Cuyler et al., 2020). The majority of the muskox populations are regulated annually, and muskox meat is consumed without prior veterinary inspection or control (Naalakkersuisut, 2020).
The present study aimed to estimate the prevalence of G. duodenalis, Cryptosporidium spp., T. gondii and Erysipelothrix in wild muskoxen and domestic sheep in Greenland.
2. MATERIALS AND METHODS
2.1. Locations and animal host populations
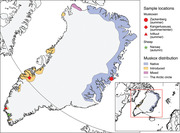
The study included muskox samples from three regions in Greenland, collected during two seasons (Figure 1): Samples collected during summer from Zackenberg (NEG), Kangerlussuaq (MWG) and Ivittuut (SWG), and winter samples from Kangerlussuaq. Muskoxen were divided into the following age groups: calves (<1 year), subadults (1 and <3 years) and adults (3 years). Calves are usually born in May or June; thus, they were 1–3 and 8–10 months old during summer and winter sampling, respectively. Sheep samples were collected in SWG during the autumn, the sheep were divided into two age groups: Lambs (<1 year) and adults (1).
FIGURE 1.

Map of Greenland showing the sampling locations and seasons for the study of zoonotic pathogens in muskoxen (Ovibos moschatus) and domestic sheep (Ovis aries). The map was created in QGIS software version 2.18 (QGIS.org, 2019)
Northeast Greenland, where Zackenberg (74°28′N) is located, has been populated by muskoxen for at least 4500 years (Campos et al., 2010). Human activity in this part of Greenland is limited.
The Kangerlussuaq (67°01′N) muskox population on the west coast was introduced from NEG in the 1960s and is the largest in Greenland (Cuyler et al., 2020). The area around Kangerlussuaq is used by tourists and locals for recreational activities and commercial hunting of muskoxen and caribou (Rangifer tarandus).
The Ivittuut muskox population was introduced from Kangerlussuaq in the 1980s (Cuyler et al., 2020) and is found around the Arsuk fiord (61°14′N), where it shares its range with caribou. Approximately 130 km south of Arsuk lies Greenland's northernmost sheep farms. The sheep, belonging to the 37 separate sheep farms in SWG (Grønlands Statistik, 2020), are typically free‐roaming in the mountain grazing areas until the slaughtering period in September/October. The area is used for commercial hunting, farming, and recreational activities.
2.2. Faecal sampling
Faecal samples were collected from wild muskoxen (NEG, MWG and SWG) and sheep (SWG) in 2017 and 2018 (Table 1). Faecal consistency was not recorded. Neither was weight or other indication of body condition of the sampled animals.
TABLE 1.
The number of samples included in the study of selected zoonotic organisms in wild muskoxen (Ovibos moschatus) and free‐roaming domestic sheep (Ovis aries) in Greenland by location, age group, a sampling method and year of sampling. Samples were collected in three regions of Greenland: northeast (Zackenberg), mid‐west (Kangerlussuaq) and southwest (Ivittuut and Narsaq)
| Species/season/ location/age group | No. of faecal samples | Sampling method (D/R) b | No. of serology samples | Sampling method (ST/FP) c | Year of sampling |
|---|---|---|---|---|---|
| Muskox | |||||
| Summer | |||||
| Zackenberg | |||||
| Calf | 10 | 10/0 | 0 | NA d | 2017 |
| Subadult | 2 | 2/0 | 0 | NA | |
| Adult | 44 | 44/0 | 0 | NA | |
| Unknown | 3 | 3/0 | 0 | NA | |
| Total | 59 | 59/0 | 0 | NA | |
| Kangerlussuaq | |||||
| Calf | 5 | 5/0 | 0 | 2018 | |
| Subadult | 11 | 11/0 | 1 | 0/1 | |
| Adult | 23 | 22/1 | 1 | 0/1 | |
| Unknown | 2 | 2/0 | 0 | NA | |
| Total | 41 | 40/1 | 2 | 0/2 | |
| Ivittuut | |||||
| Calf | 2 | 2/0 | 0 | NA | 2018 |
| Subadult | 12 | 12/0 | 0 | NA | |
| Adult | 33 | 25/5 | 8 | 5/3 | |
| Unknown | 8 | 8/0 | 0 | NA | |
| Total | 55 | 50/5 | 8 | 5/3 | |
| Winter | |||||
| Kangerlussuaq | |||||
| Calf | 4 | 0/4 | 4 | 4/0 | 2017 |
| Subadult | 17 | 0/24 | 16 | 16/0 | |
| Adult | 39 | 0/32 | 17 | 17/0 | |
| Unknown | 18 | 0/18 | 6 | 6/0 | |
| Total | 78 | 0/78 | 43 | 43/0 | |
| Total | 233 | 149/84 | 53 | 48/5 | |
| Sheep | |||||
| Autumn | |||||
| Narsaq | |||||
| Lamb | 29 | 0/29 | 30 | 30/0 | 2017 |
| Adult | 15 | 0/15 | 20 | 20/0 | |
| Total | 44 | 0/44 | 50 | 50/0 | |
| Narsaq | |||||
| Lamb | 0 | NA | 79 | 79/0 | 2018 |
| Adult | 0 | NA | 26 | 26/0 | |
| Total | 0 | NA | 105 | 105/0 | |
| Total | 44 | 0/44 | 155 | 155/0 |
Muskox: calf < 1 year, subadult 1 and < 3, adult 3; sheep: lamb: < 1, adult 1.
Dropping/rectal.
Serum tube/filter paper.
Not applicable.
Sampled muskoxen were identified and their sex and age group were determined from a distance by telescope or binoculars based on the field guide by Olesen and Thing (1989). Faecal samples not assigned to specific individuals were marked as ‘unknown’. To minimise the risk of re‐sampling, sampling was attempted to include different groups in different areas; however, because transportation was on foot, the reachable area was limited. Faecal samples were collected directly from the rectum of harvested muskoxen in MWG in winter 2017 and in SWG in summer 2018. Ages and sexes of the sampled animals were estimated and recorded by hunters.
Samples from sheep were collected in the autumn of 2017 at the abattoir in Narsaq from the distal colon or rectum of slaughtered sheep from at least 16 farms.
Each faecal sample consisted of approximately 25 g faeces and was immediately transferred to a 50‐ml tube containing 30 ml 96% ethanol, with the exception of sheep samples, which were stored at 5°C for up to three days before adding of ethanol. The Zackenberg samples and the Kangerlussuaq winter samples were stored at –18°C for up to 3 weeks and 1.5 years, respectively, prior to shipping. Samples were drained and stored wet in polystyrene boxes during transport to the University of Copenhagen, Denmark. Muskox samples collected during the summer in Kangerlussuaq and Ivittuut were stored cool under field conditions and sheep samples were stored at 5°C for up to 5 weeks until transport. Upon arrival at the University of Copenhagen all samples were stored in 96% ethanol at 5°C for up to 3 months until further processing.
2.3. Samples for serology
Samples for serology were collected from muskoxen (MWG and SWG) and sheep (SWG) in 2017 and 2018 (Table 1). Blood samples from the Kangerlussuaq population were collected at bleeding from the jugular vessels directly into 9‐ml serum tubes (VACUETTE, Serum Clot Activator, Greiner Bio‐One, Austria) 15–30 min after the animals were shot. Some of the samples collected during winter in MWG were obtained from carcasses 4–6 h post‐mortem, directly in a serum tube from the heart (if available), the thoracic cavity, or from the femoral blood vessels.
Blood samples were collected from harvested muskoxen in SWG and MWG during the summer of 2018. Samples were collected at bleeding in serum tubes or on Nobutu filter paper strips (Toyo Roshi Kaisha, Ltd., Tokyo, Japan) as described by Curry et al. (2011). The eluate retrieved from the filter paper samples were estimated to be 1:10 dilution of serum and serology protocols were adjusted accordingly (Curry et al., 2011).
At the abattoir in Narsaq, blood samples were collected from domestic sheep from at least 30 separate farms during the autumn of 2017 and 2018. All samples were collected in serum tubes from the jugular vessels during bleeding. Information on sex and age was noted.
All samples were kept from freezing during transport and stored at 5°C overnight or at room temperature for 2 h before centrifugation at approximately 1400 × g for 10 min. Filter paper samples were kept frozen for up to 4 months until further processing. All samples were stored at –18°C for up to 3.5 years before analysis.
2.4. PCR detection of Giardia and Cryptosporidium
A subsample of each faecal sample was transferred to a 2‐ml Eppendorf tube and washed three times to remove residual ethanol by adding of phosphate‐buffered saline (PBS) and subsequent centrifugation at 2000 × g for 5 min. The samples were subsequently subjected to DNA extraction. Briefly, approximately 100 mg of each washed sample was mixed with 260 μl Lysis Buffer in tubes containing 1.4‐mm Zirconium beads (OPS Diagnostics LLC, Lebanon, USA) and bead‐beaten in a TissueLyser II (QIAGEN, Germany) for 2 min at 30 Hz, followed by 8 min of incubation at room temperature, to release DNA from cysts and oocysts. Samples were then centrifuged at 1000 × g for a few seconds, and the entire supernatant was transferred with a 1000 μl pipette to a new Eppendorf tube and centrifuged for 5 min at 16,000 × g to sediment large particles. Subsequently, 100 μl of the supernatant from each sample was processed by the automated NucliSENS easyMAG (bioMérieux, France) platform according to the manufacturer's protocol. No inhibition controls were included in the DNA extraction. Extracted DNA was stored at ‐20°C until further processing.
For detection of G. duodenalis and Cryptosporidium spp. DNA, we used the diagnostic duplex real‐time PCR in place at Statens Serum Institut, Copehagen, Denmark (Thomas‐Lopez et al., 2020). Primers and probes used are listed in Table 2. The 25 μl reaction mixture contained 0.2 μl IMMOLASE™ DNA Polymerase (Bioline), 5 μl 10 × ImmoBuffer (Bioline), 1.25 μl (1 μM) of each primer, 0.125 μl (0.075 μM) of each probe, 5 μl of DNA eluate and water sufficient to reach the total volume of 25 μl. Negative (water) and positive (G. duodenalis and C. parvum DNA), controls and inhibition controls were included in each run on the Applied Biosystems 7500 Fast Real‐Time PCR Thermocycler (Thermo Fisher Scientific, Denmark). PCR cycling conditions were as follows: 10 min at 95°C (initial denaturation), 50 cycles of 15 s at 95°C, and 60 s at 60°C. PCR products were analysed with the Sequence Detection Software v.2.3 (Thermo Fisher Scientific). Samples were considered positive if they exhibited a sigmoid growth curve with a threshold cycle value (Ct value) ≤ 37. Samples with a Ct value > 37 and ≤ 42 (read at ΔRn = 0.1) were considered borderline‐positive.
TABLE 2.
Primers and probes used for real‐time PCR for Giardia duodenalis and Cryptosporidium spp
| Target organism/oligo name | Sequences (5′–3′ direction) | Reference |
|---|---|---|
| Giardia duodenalis | ||
| Giardia‐80F | GACGGCTCAGGACAACGGTT | Verweij et al. (2004) |
| Giardia‐127R | TTGCCAGCGGTGTCCG | Verweij et al. (2004) |
| Probe Giardia‐105T | FAM‐CCCGCGGCGGTCCCTGCTAG‐BHQ‐1 | Verweij et al. (2004) |
| Cryptosporidium spp. | ||
| CRY F3 | CTACACTGATGCATCCATCRAGT | This study |
| CRY R3 | CCCATCACGATGCATAYTCAAAA | This study |
| Probe CRY P | VIC‐TCCTGTTTCGAAGGAAATGGGTAATC‐MGB | This study |
2.5. Subtyping of giardia and cryptosporidium
DNA samples identified as positive for Cryptosporidium by real‐time PCR were subjected to species identification and genotyping. Species identification was performed by nested PCR targeting the SSU rRNA gene with outer primers SSU‐F2 and SSU‐R2 and the inner primers SSU‐F3 and SSU‐R3 after Xiao et al. (2019). Genotyping relied on the protocol originally described by 2003 targeting the gp60 gene. Briefly, the nested PCR used AL3531 and AL3535 as outer primers and AL3532 and AL3534 as inner primers.
DNA samples identified as positive for Giardia were subjected to species identification and assemblage typing of Giardia by PCR of three loci: SSU rRNA, ‐giardin and tpi. Briefly, species identification (SSU rDNA analysis) was performed by single PCR with primers RH 11 and RH 4, while assemblage typing was conducted by nested PCR of the ‐giardin gene with the outer primers BG‐G7‐F and BG‐G759‐R and inner primers BF‐G376‐F and BG‐G759‐R according to Cacciò et al (2002). Likewise, a nested PCR of the tpi gene was conducted with AL3543 and AL3546 as outer primers and AL3544 and AL3545 as inner primers (Sulaiman et al., 2003).
If no samples were successfully genotyped based on the originally extracted DNA further isolation of cysts/oocysts was attempted using a sucrose gradient protocol modified from Faridi et al. (2020) and Lebbad et al. (2008) and subsequent subtyping. In short, 3 g of faeces were homogenised with 8 ml PBS, and the solution was filtered through a double layer of gauze into a 50‐ml tube. The tube was centrifuged at 400 × g for 5 min, and the supernatant was discarded. The sediment was washed in 10 ml PBS with centrifugation at 1500 × g for 5 min and the supernatant was discarded. After addition of 7 ml PBS, the suspension was vortexed, and slowly—drop by drop—added on top of a 5°C sucrose solution (1 M) in a 15‐ml tube so that two distinct phases were formed. The tube was centrifuged at 600 × g for 5 min. The cloudy cyst‐containing layer between the sucrose and the faecal solution was transferred to a new 15‐ml tube with a Pasteur pipette. The transferred material was washed four times in PBS with centrifugation at 1500 × g for 5 min in order to remove the sucrose from the cysts. The supernatant was removed, and the remaining 1 ml sediment was transferred to a 2‐ml Eppendorf tube and stored at –20°C until DNA extraction, as previously described.
Sequences were obtained by bidirectional Sanger sequencing (Eurofins Genomics, Germany) and submitted to GenBank with the following accession numbers: MW980720–MW980724. Forward and reverse sequences were assembled using Staden Package (https://sourceforge.net/projects/staden/), manually edited and consensus sequences compared with reference sequences in the GenBank database using the Basic Local Alignment Search Tool (BLAST). All chromatograms were manually inspected for the presence of double peaks indicating mixed species/subtypes. For Cryptosporidium, the CryptoGenotyper (Yanta et al., 2021), available on the web‐based platform Galaxy (Jalili et al., 2021), was used for initial Cryptosporidium genotyping and gp60 subtype were confirmed by manual identification of trinucleotide repeats as previously described (Chalmers et al., 2019).
If sequencing of DNA extracted from original faecal material or from cysts/oocysts isolated by the sucrose gradient method was not successful, no further attempts for characterisation were performed.
2.6. Serology for Toxoplasma gondii
The sera were screened for T. gondii‐specific IgG antibodies using a commercially available modified direct agglutination test (DAT; Toxo‐Screen DA, bioMérieux, France), following the manufacturer's instructions, except that only 1:40 dilutions were used. Animals that tested positive at the 1:40 dilution were considered seropositive and tested further at 12 threefold dilutions, from 1:6 to 1:162,000, to determine the antibody titres. Positive and negative controls provided in the kit were included on each plate, and the antigen was controlled for autoagglutination.
2.7. Serology for Erysipelothrix
Samples were screened for antibodies against Erysipelothrix according to Mavrot et al. (2020). We used a conservative cut‐off value with only samples above the upper limit of the cut‐off confidence intervals (CI) considered positive. For muskox samples we used the cut‐off values of 0.25% positivity (95% CI: 0.23%–0.28%) and 0.49% positivity (CI: 0.36%–0.59%) for serum and filter paper samples, respectively (Mavrot et al., 2020). For sheep samples, we used a cut‐off value of 0.087% positivity (CI: 0.056%–0.162%) of a pig positive control. The cut‐off values for sheep were established by a mixture‐distribution modelling approach (see Mavrot et al., 2020), using the sheep samples included in this study. All plates included high‐, medium‐, and low‐positive controls and a negative control.
2.8. Statistical analyses
The open‐source software for epidemiological statistics OpenEpi (Dean et al., 2013) was used to calculate prevalence estimates and Wilson score 95% CI for binomial data (Wallis, 2013). Fisher's exact test (Kim, 2017) implemented in the statistical programming software R (v. 4.0.0) (R Core Team, 2019) was used to compare prevalence estimates by location, season and age groups. p Values < 0.05 were considered to indicate statistical significance.
3. RESULTS
3.1. Copro‐prevalence of Giardia duodenalis and Cryptosporidium spp
The overall prevalence estimate of G. duodenalis was 3.0% (CI: 1.5%–6.1%) in muskoxen and 37.2% (CI: 24.4%–52.1%) in sheep. Two samples—an adult muskox from Zackenberg and a lamb—were inconclusive due to inhibition in the real‐time PCR assay and were excluded from further analyses. For muskoxen, DNA specific for G. duodenalis was identified in summer samples from Kangerlussuaq, but not in samples from Zackenberg, Ivittuut or winter samples (Table 3). Among the summer samples from Kangerlussuaq, seven tested positive for G. duodenalis, resulting in an estimated prevalence of 17.1%. Four samples tested borderline‐positive for G. duodenalis. There was a significantly higher prevalence of G. duodenalis in summer than in winter (p < 0.001). Considering summer samples, the estimated prevalence of G. duodenalis in Kangerlussuaq was significantly higher than in Zackenberg (p = 0.002) and Ivittuut (p = 0.002). Giardia duodenalis‐positive muskoxen were found across all age groups.
TABLE 3.
Prevalence of Giardia duodenalis and Cryptosporidium spp. in muskoxen (Ovibos moschatus) and sheep (Ovis aries) from Greenland by location, season and age group a
| Giardia duodenalis | Cryptosporidium spp. | ||||
|---|---|---|---|---|---|
| Species/season/location/age group | No. of animals tested | No. positive | % positive (CI b ) | No. positive | % positive (CI) |
| Muskox | |||||
| Summer | |||||
| Zackenberg | |||||
| Calf | 10 | 0 | 0.0 (0.0–25.9) | 0 | 0.0 (0.0–25.9) |
| Subadult | 2 | 0 | 0.0 (0.0–77.6) | 0 | 0.0 (0.0–77.6) |
| Adult | 43 | 0 | 0.0 (0.0–8.2) | 0 | 0.0 (0.0–8.2) |
| Unknown | 3 | 0 | 0.0 (0.0–56.2) | 0 | 0.0 (0.0–56.2) |
| Total | 58 | 0 | 0.0 (0.0–6.2) | 0 | 0.0 (0.0–6.2) |
| Kangerlussuaq | |||||
| Calf | 5 | 1 | 20.0 (3.6–62.4) | 1 | 20.0 (3.6–62.4) |
| Subadult | 11 | 3 | 27.3 (9.7–56.6) | 0 | 0.0 (0.0–25.9) |
| Adult | 23 | 2 | 26.1 (12.6–46.5) | 0 | 0.0 (0.0–14.3) |
| Unknown | 2 | 1 | 50.0 (9.5–90.6) | 0 | 0.0 (0.0–65.8) |
| Total | 41 | 7 | 26.8 (15.7–41.9) | 1 | 2.4 (0.4–12.6) |
| Ivittuut | |||||
| Calf | 2 | 0 | 0.0 (0.0–65.8) | 1 | 50.0 (9.5–90.6) |
| Subadult | 12 | 0 | 0.0 (0.0–24.3) | 0 | 0.0 (0.0–24.3) |
| Adult | 33 | 0 | 0.0 (0.0–10.4) | 3 | 9.1 (3.1–23.6) |
| Unknown | 8 | 0 | 0.0 (0.0–32.4) | 0 | 0.0 (0.0–32.4) |
| Total | 55 | 0 | 0.0 (0.0–6.5) | 4 | 7.3 (2.9–17.3) |
| Winter | |||||
| Kangerlussuaq | |||||
| Calf | 4 | 0 | 0.0 (0.0–49.0) | 0 | 0.0 (0.0–49.0) |
| Subadult | 17 | 0 | 0.0 (0.0–18.4) | 0 | 0.0 (0.0–18.4) |
| Adult | 39 | 0 | 0.0 (0.0–9.0) | 0 | 0.0 (0.0–9.0) |
| Unknown | 18 | 0 | 0.0 (0.0–17.6) | 0 | 0.0 (0.0–17.6) |
| Total | 78 | 0 | 0.0 (0–4.7) | 0 | 0.0 (0–4.7) |
| Sheep | |||||
| Autumn | |||||
| Narsaq | |||||
| Lamb | 28 | 16 | 57.1 (39.1–73.5) | 1 | 3.6 (0.6–17.7) |
| Adult | 15 | 0 | 0.0 (0.0–20.4) | 0 | 0.0 (0.0–20.4) |
| Total | 43 | 16 | 37.2 (24.4–52.1) | 1 | 2.3 (0.4–12.1) |
Muskox: calf < 1 year, subadult 1 and < 3, adult 3; sheep: lamb: < 1, adult 1.
95% confidence interval.
For sheep, 16 samples were positive for G. duodenalis‐specific DNA (all positive samples were from lambs, Table 3) and one lamb was borderline‐positive. The prevalence in lambs was significantly higher than in adults (p < 0.001).
The attempt to genotype the Giardia parasites from the original faecal DNA extraction failed. Therefore, the four samples with lowest Ct‐values (Ct‐value < 30; two muskoxen from Kangerlussuaq and two sheep) were subjected to the sucrose gradient protocol and subsequent DNA extraction. The parasites in one of these samples (a muskox from Kangerlussuaq) were successfully genotyped as Giardia duodenalis assemblage A based on results for all three loci (GenBank accession no.: MW980722–MW980724). However, no information on sub‐assemblage was available for the identical sequences in GenBank. The ‐giardin PCR of the remaining samples was negative, and the tpi sequencing results were of mixed quality. We were not able to characterise the three remaining samples using either gene.
The estimated prevalence of Cryptosporidium spp. was 2.2% (CI: 0.9%–4.9%) in muskoxen and 2.3% (CI: 0.4%–12.1%) in sheep. In addition, two samples—an adult muskox from Ivittuut and a lamb—were borderline‐positive. For muskoxen, Cryptosporidium‐specific DNA was detected in samples collected during summer in Kangerlussuaq (2.4%) and Ivittuut (7.3%), but not in samples from Zackenberg nor in winter samples (Table 3). Within samples from Kangerlussuaq, there was no statistically significant difference between the prevalence in summer and winter (p = 0.34). Considering summer samples, no significant difference in the prevalence of Cryptosporidium was observed among the three populations; however, the prevalence tended to be higher in the Ivittuut population than in the Zackenberg population (p = 0.05). Cryptosporidium‐positive samples were found in two calves and three adults, but not in subadult animals.
For sheep, one lamb was positive for Cryptosporidium DNA. There was no significant statistical difference between the prevalence of Cryptosporidium in adults and lambs (p = 1).
The parasites in two of the eight Cryptosporidium‐positive samples, both from the Ivittuut muskox population, were identified as C. parvum IIdA20G1 by use of the CryptoGenotyper followed by manual analysis of the repeat region. Manual evaluation of chromatograms and analysis successfully identified the parasites in both samples as C. parvum IIdA20G1 (GenBank accession no.: MW980720–MW980721). Following BLAST, the sequences were found identical to C. parvum IIdA20G1e reference sequences (GenBank accession no.: KU852714 and FJ917375). We were not able to identify the species or genotype of the remaining samples positive by real‐time PCR.
3.2. Seroprevalence of Toxoplasma gondii
The overall seroprevalence of T. gondii was 1.9% (CI: 0.3%–10.1%) in muskoxen and 0.6% (CI: 0.1%–3.6%) in sheep (Table 4). In total, we found one antibody‐positive muskox (a yearling from Kangerlussuaq that was sampled in 2017), and one seropositive sheep (a lamb from SWG that was sampled in 2018) (Table 4). Both of these samples had been collected in serum tubes during bleeding of the animals. We did not detect antibodies against T. gondii in any of the tested samples from the Ivittuut muskox population (Table 4).
TABLE 4.
Toxoplasma gondii seroprevalence and Erysipelothrix seroprevalence in muskoxen (Ovibos moschatus) and sheep (Ovis aries) from Greenland by location and age group a
| Toxoplasma gondii | Erysipelothrix | |||||
|---|---|---|---|---|---|---|
| Species/location /age group | No. of animals tested (ST/FP) b | Positive (titre) | % positive (CI c ) | No. of animals tested (ST/FP) | Positive | % positive (CI) |
| Muskox | ||||||
| Total | 52 (47/5) | 1 | 1.9 (0.3–10.1) | 53 (48/5) | 2 | 3.8 (1.0–12.8) |
| Kangerlussuaq | ||||||
| Calf | 4 (4/0) | 0 | 0.0 (0.0–49.0) | 4 (4/0) | 0 | 0.0 (0.0–49.0) |
| Subadult | 17 (16/1) | 1 (180) | 5.9 (1.0–27.0) | 17 (16/1) | 0 | 0.0 (0.0–18.4) |
| Adult | 18 (17/1) | 0 | 0.0 (0.0–17.6) | 18 (17/1) | 1 | 5.6 (1.0–25.8) |
| Unknown | 5 (5/0) | 0 | 0.0 (0.0–43.5) | 6 (6/0) | 0 | 0.0 (0.0–39) |
| Total | 44 (42/2) | 1 | 2.3 (0.4–11.8) | 45 (43/2) | 1 | 2.2 (0.4–11.6) |
| Ivittuut | ||||||
| Calf | 0 | NA d | NA | 0 | NA | NA |
| Subadult | 0 | NA | NA | 0 | NA | NA |
| Adult | 8 (5/3) | 0 | 0.0 (0.0–32.4) | 8 (5/3) | 1 | 12.5 (2.2–47.1) |
| Unknown | 0 | NA | NA | 0 | NA | NA |
| Total | 8 (5/3) | 0 | 0.0 (0.0–32.4) | 8 (5/3) | 1 | 12.5 (2.2–47.1) |
| Sheep | ||||||
| Total | 155 (155/0) | 1 | 0.6 (0.1–3.6) | 150 (150/0) | 10 | 6.7 (3.7–11.8) |
| Narsaq | ||||||
| Lamb | 109 (109/0) | 1 (162) | 0.9 (0.2–5.0) | 107 (107/0) | 5 | 4.7 (2.0–10.5) |
| Adult | 46 (46/0) | 0 | 0.0 (0.0–7.7) | 43 (43/0) | 5 | 11.6 (5.1–24.5) |
Muskox: calf < 1 year, subadult 1 and < 3, adult 3; sheep: lamb: < 1, adult 1.
Sampling method (serum tube/filter paper).
95% confidence interval.
Not applicable.
3.3. Seroprevalence of Erysipelothrix
The overall seroprevalence of Erysipelothrix was 3.8% (1.0%–12.8%) in muskoxen and 6.7% (3.7%–11.8%) in sheep (Table 4). A total of two adult muskoxen were seropositive—one from Kangerlussuaq sampled in a serum tube in winter 2017, and one from Ivittuut sampled on filter paper in summer 2018. Among the sheep samples, no statistically significant difference between the estimated prevalence in adults and lambs was observed (p = 0.1).
4. DISCUSSION
Our study demonstrated that Cryptosporidium and Giardia duodenalis are circulating in muskox and sheep populations in west Greenland and that potentially zoonotic strains of G. duodenalis and C. parvum are present in muskoxen.
Several species and genotypes of Giardia and Cryptosporidium have been recognised, but only some, as G. duodenalis assemblage A and C. parvum, appear to have zoonotic potential (Monis & Thompson, 2003; Ryan & Zahedi, 2019; Sprong et al., 2009; Thompson et al., 2008). The clinical impact of Giardia and Cryptosporidium on free‐ranging muskox populations is unknown (Kutz et al., 2012), but infections in domestic sheep have been associated with diarrhoea and reduced slaughter weight (Ryan & Zahedi, 2019; Sweeny et al., 2011). During sample collection, the body condition of the animals was not recorded, and the potential health effect of infections to the host animal could, therefore, not be investigated in this study.
Our difficulties related to genotyping may be related to the way in which the samples were handled and stored. The use of ethanol as a fixative for long‐time storage of incompletely homogenised ruminant faecal pellets may have been insufficient for preventing the DNA from degrading (Kuk et al., 2012), leaving the DNA of sufficient quality for real‐time PCR (< 100 base pair [bp] products) but unsuitable for longer PCR products used for genotyping (e.g. 700 bp). Another reason for the challenges in the genotyping work may be the high Ct‐values observed for many of the samples, indicating low levels of DNA present. However, genotyping failed even for some of the samples with Ct‐values < 25.
The finding of G. duodenalis assemblage A in one Greenland muskox is consistent with typing studies of G. duodenalis in muskoxen from Canada and Norway (Davidson et al., 2014; Kutz et al., 2008). Babbott et al. (1961) microscopically identified G. duodenalis (cited as G. lamblia) in humans from Aasiaat in west Greenland, which indicates that it may have been circulating there at least since the 1950s.
Cryptosporidium appears to be relatively uncommon in Arctic wildlife, though reported in low prevalence in caribou from Canada (Jenkins et al., 2013; Johnson et al., 2010; Kutz et al., 2012) and Norwegian muskoxen (Davidson et al., 2014). Reports on C. parvum in free‐ranging wild ungulates are limited (Sturdee et al., 1999; Duszynski & Upton, 2001) and the IIdA20G1e subtype was hitherto only reported from Sweden in young cattle and in a food‐borne outbreak of cryptosporidiosis (Gherasim et al., 2012; Silverlås et al., 2010; SVA, 2019). Our DNA sequences were identical to reference sequences from Sweden (Silverlås et al., 2010), while differing by one bp from DNA sequences obtained from, for example humans from Israel (GenBank accession no.: MK095329, MK095314) and Egypt (GenBank accession no.: KX397563, KX443783), and from Chinese cattle (GenBank accession no.: KU248815).
The prevalence of anti‐T. gondii antibodies observed in this study was relatively low compared with prevalence estimates from Canadian muskoxen (6%) (Kutz et al., 2000) and pooled seroprevalence estimates from sheep from the Nordic‐Baltic region (23%) (Olsen et al., 2019). Earlier seroepidemiological studies on T. gondii in wild ungulates from Greenland failed to document seropositive animals (Carlsson et al., 2019; Clausen & Hjort, 1986; Kutz et al., 2012). For sheep, the only available reference is a conference abstract (Bille, 1974) mentioning the possible findings of antibodies against T. gondii in sheep from SWG, however, a later source (Clausen & Hjort, 1986) states that the animals were seronegative. The method used in this study has been widely used for different animal species (Dubey 2010); however, the use of a confirmatory method could have reduced the risk of generating false positive results. Still, the cut‐off for seropositivity we used can be considered relatively high, while the determination of titres supported the positive findings. To reduce the risk of false negatives due to the prozone phenomenon, a 1:4000 dilution of serum could have been included in addition to the 1:40 dilution used.
Adding to the findings of T. gondii seropositive polar bears (Ursus maritimus) from NEG (Oksanen et al., 2009), our results suggest that animals in Greenland are exposed to T. gondii, albeit at a relatively low rate. However, the source of the exposure remains unclear. In the absence of wild felids and because of the low number of domestic cats (Felis catus domesticus), migratory birds and marine mammals have been suggested as plausible reservoirs and sources of T. gondii in the Arctic (Jenkins et al., 2013; Kutz et al., 2012; Prestrud et al., 2008). Domestic cats are used for rodent control on sheep farms in SWG, but the current number of cats and the T. gondii prevalence in the cat population in Greenland is unknown.
Toxoplasma gondii can cause clinical disease and abortions in domestic sheep (Dubey, 2009). Furthermore, T. gondii has been isolated from the placenta of a captive muskox following abortion (Crawford et al., 2000), and T. gondii has, therefore, been considered a potential factor contributing to population declines in North American muskox populations (Kutz et al., 2000). However, the low prevalence observed in this study indicates that the population health impact of T. gondii in ungulates from Greenland is presently not a concern.
In recent years, E. rhusiopathiae has been associated with mass mortality events in North American muskox populations (Forde et al., 2016; Kutz et al., 2015; Mavrot et al., 2020). The seroprevalence study by Mavrot et al. (2020) indicates that E. rhusiopathiae has been circulating in the North American muskox populations, with fluctuating seroprevalence, at least since the 1970s. The importance of E. rhusiopathiae to muskox population health in Greenland remains unknown, although the observed prevalence of Erysipelothrix is low compared to that observed during years of high muskox mortality in North America. This suggests that Erysipelothrix may not currently be a health concern for the muskox population, but that it should be monitored, as studies in North America have shown considerable inter‐annual variability. Erysipelothrix sp. has previously been reported in faecal microbiome studies from NEG and the Canadian Arctic (Andersen‐Ranberg et al., 2018; Bird et al., 2019).
In sheep, especially lambs, E. rhusiopathiae can lead to significant disease with impact on animal welfare and economic loss from abortions, reduced growth rate, and condemnation of products at the abattoir (Ersdal et al., 2015; Fthenakis et al., 2006).
Our study demonstrates that muskoxen and sheep from Greenland are infected with or exposed to several zoonotic pathogens with possible seasonal, geographical, and interspecies differences. The observed relatively low prevalence of the selected zoonotic pathogens in muskoxen and sheep is reassuring but their presence in Greenland may have relevance to animal health and public health.
The limitations of this study include the limited sample size and that part of the samples were collected from hunted and slaughtered animals and may be biased by age, sex, and fitness. Regarding the sheep samples, the findings may not be representative for all sheep farms in Greenland, because samples from only a limited number of farms were included.
Our results expand our knowledge of the diversity of zoonotic pathogens in wildlife and domestic sheep in Greenland. Muskoxen are known to be susceptible to pathogens mainly recognised in other host species (Kutz et al., 2004, 2008; Samuelsson et al., 2013; Tomaselli et al., 2016) and are relatively sedentary animals (Schmidt et al., 2016). Muskoxen could serve as an indicator of the presence of zoonotic pathogens in the environment in Greenland. Muskoxen are hunted annually and samples from the harvested individuals could be tested to monitor the emergence, presence and prevalence of zoonotic pathogens that can infect both animals and humans.
We identified potential geographic and seasonal differences that may inform future monitoring and research efforts that seek to further define the circulation of these pathogens in wildlife and domestic animals in the Greenland environment and characterise the risks of pathogen exchange among people, wildlife, and domestic animals. The relative simplicity of the Arctic ecosystem allows the study of separate aspects of pathogen epidemiology and could thereby be a useful model for understanding the transmission patterns also in other, more complex, regions.
AUTHOR CONTRIBUTIONS
Rebecca P. K. D. Berg: Conceptualisation, data curation, formal analysis, funding acquisition, investigation, project administration, resources, visualisation, writing‐original draft.
C. Rune Stensvold: Data curation, funding acquisition, investigation, resources, validation, writing‐review and editing.
Pikka Jokelainen: Data curation, funding acquisition, investigation, resources, validation, writing‐review and editing.
Anna K. Grønlund: Investigation, writing‐review and editing.
Henrik V. Nielsen: Data curation, funding acquisition, resources, validation, writing‐review & editing.
Susan Kutz: Data curation, funding acquisition, resources, validation, writing‐review and editing.
Christian M. O. Kapel: Conceptualisation, funding acquisition, project administration, supervision, writing‐review and editing.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.599
ACKNOWLEDGEMENTS
We gratefully acknowledge the Greenland Research Council and the Danish Government for funding through ‘Danish State funding for Arctic Research’ (grant no.: 80.19). The project was also partly supported by ‘Den Grønlandske Fond’ (J. no 2016–557). Laboratory work and analyses conducted at the University of Calgary, Canada, were supported by grants from ‘Polar Knowledge Canada’ and ‘NSERC Discovery’ to SK. PJ, CRS and HVN are part of the TOXOSOURCES and PARADISE consortia, supported by funding from the European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement No. 773830: One Health European Joint Programme. We are grateful for the field assistance from Bolethe Skifte Egede, Larissa Beumer, Sascha Schiøtt, Maia Olsen and local hunting officers and hunters from Arsuk and Kangerlussuaq. Furthermore, we thank the Neqi A/S abattoir in Narsaq and the veterinarians for support with sample collection from sheep. We thank Angela Schneider and Fabien Mavrot from the University of Calgary for their invaluable help with Erysipelothrix laboratory work and analysis, Karl Zinglersen from the Greenland Institute of Natural Resources for input to graphics, and Ellinor Marving for assistance with implementation of the Cryptosporidium real‐time PCR. We are also grateful to the staff at Zackenberg research station for logistic support during the fieldwork.
Berg, R. P. K. D. , Stensvold, C. R. , Jokelainen, P. , Grønlund, A. K. , Nielsen, H. V. , Kutz, S. , & Kapel, C. M.O. (2021). Zoonotic pathogens in wild muskoxen (Ovibos moschatus) and domestic sheep (Ovis aries) from Greenland. Veterinary Medicine and Science, 7, 2290–2302. 10.1002/vms3.599
REFERENCES
- Alves M., Xiao L., Sulaiman I., Lal A. A., Matos O., Antunes F. (2003). Subgenotype Analysis of Cryptosporidium Isolates from Humans, Cattle, and Zoo Ruminants in Portugal. Journal of Clinical Microbiology, 41(6), 2744–2747. 10.1128/jcm.41.6.2744-2747.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMAP . (2015). AMAP assessment 2015: Human health in the Arctic. (p. vii + 165). Arctic Monitoring and Assessment Programme (AMAP). https://www.amap.no/documents/doc/amap‐assessment‐2015‐human‐health‐in‐the‐arctic/1346
- AMAP . (2019). Arctic climate change update 2019: An update to key findings of snow, water, ice, and permafrost in the Arctic (SWIPA) 2017 (pp. 1–12). Arctic Monitoring and Assessment Programme (AMAP). https://www.amap.no/documents/doc/amap‐climate‐change‐update‐2019/1761
- Andersen‐Ranberg E., Barnes C., Rasmussen L., Salgado‐Flores A., Grøndahl C., Mosbacher J., Hansen A., Sundset M., Schmidt N., Sonne C. (2018). A Comparative Study on the Faecal Bacterial Community and Potential Zoonotic Bacteria of Muskoxen (Ovibos moschatus) in Northeast Greenland, Northwest Greenland and Norway. Microorganisms, 6(3), 76. 10.3390/microorganisms6030076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbee, A. J. , Thompson, R. C. A. , & Olson, M. E. (2005). Giardia and Cryptosporidium in mammalian wildlife – Current status and future needs. Trends in Parasitology, 21(8), 370–376. 10.1016/j.pt.2005.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbott, F. L. , Frye, W. W. , & Gordon, J. E. (1961). Intestinal parasites of man in Arctic Greenland. American Journal of Tropical Medicine and Hygiene, 10(2), 185–190. 10.4269/ajtmh.1961.10.185 [DOI] [PubMed] [Google Scholar]
- Bille, T. (1974). Antibodies against Toxoplasma gondii in sheep in Greenland. In Proceedings of the Third International Congress of Parasitology: Third International Congress of Parasitology , Munich, p. 307.
- Bird, S. , Prewer, E. , Kutz, S. , Leclerc, L. M. , Vilaça, S. T. , & Kyle, C. J. (2019). Geography, seasonality, and host‐associated population structure influence the fecal microbiome of a genetically depauparate Arctic mammal. Ecology and Evolution, 9, 13202–13217. 10.1002/ece3.5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciò, S. M. , De Giacomo, M. , & Pozio, E. (2002). Sequence analysis of the β‐giardin gene and development of a polymerase chain reaction‐restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. International Journal for Parasitology, 32, 1023–1030. 10.1016/S0020-7519(02)00068-1 [DOI] [PubMed] [Google Scholar]
- Campos, P. F. , Willerslev, E. , Sher, A. , Orlando, L. , Axelsson, E. , Tikhonov, A. , Aaris‐Sørensen, K. , Greenwood, A. D. , Kahlke, R.‐D. , Kosintsev, P. , Krakhmalnaya, T. , Kuznetsova, T. , Lemey, P. , MacPhee, R. , Norris, C. A , Shepherd, K. , Suchard, M. A. , Zazula, G. D. , Shapiro, B. , & Gilbert, M. T. P. (2010). Ancient DNA analyses exclude humans as the driving force behind late Pleistocene musk ox (Ovibos moschatus) population dynamics. PNAS, 107(12), 5675–5680. 10.1073/pnas.0907189107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson, A. M. , Curry, P. , Elkin, B. , Russell, D. , Veitch, A. , Branigan, M. , Campbell, M. , Croft, B. , Cuyler, C. , Côté, S. D. , Leclerc, L. M. , Tryland, M. , Nymo, I. H. , & Kutz, S. J. (2019). Multi‐pathogen serological survey of migratory caribou herds: A snapshot in time. PLoS ONE, 14(7), 1–22. 10.1371/journal.pone.0219838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers R. M., Robinson G., Elwin K., Elson R. (2019). Analysis of the Cryptosporidium spp. and gp60 subtypes linked to human outbreaks of cryptosporidiosis in England and Wales, 2009 to 2017. Parasites & Vectors, 12(1), 10.1186/s13071-019-3354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen, B. , & Hjort, P. (1986). Survey for antibodies against various infectious disease agents in muskoxen (Ovibos moschatus) from Jamsonland, northeast Greenland. Journal of Wildlife Disease, 22(2), 264–266. 10.7589/0090-3558-22.2.264 [DOI] [PubMed] [Google Scholar]
- Crawford, G. C. , Dunker, F. H. , & Dubey, J. P. (2000). TOXOPLASMOSIS AS A SUSPECTED CAUSE OF ABORTION IN A GREENLAND MUSKOX (OVIBOS MOSHATUS WARDI). Journal of Zoo and Wildlife Medicine, 31(2), 247–250. 10.1638/1042-7260(2000)031[0247:taasco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cunningham, A. A. , Daszak, P. , & Wood, J. L. N. (2017). One health, emerging infectious diseases and wildlife: Two decades of progress? Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 20160167. 10.1098/rstb.2016.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry, P. S. , Elkin, B. T. , Campbell, M. , Nielsen, K. , Hutchins, W. , Ribble, C. , & Kutz, S. J. (2011). Filter‐paper blood samples for ELISA detection of Brucella antibodies in caribou. Journal of Wildlife Diseases, 47(1), 12–20. 10.7589/0090-3558-47.1.12 [DOI] [PubMed] [Google Scholar]
- Cuyler, C. , Rowell, J. , Adamczewski, J. , Anderson, M. , Blake, J. , Bretten, T. , Brodeur, V. , Campbell, M. , Checkley, S. L. , Cluff, H. D. , Côté, S. D. , Davison, T. , Dumond, M. , Ford, B. , Gruzdev, A. , Gunn, A. , Jones, P. , Kutz, S. , Leclerc, L. M. , … Ytrehus, B. (2020). Muskox status, recent variation, and uncertain future. Ambio, 49, 805–819. 10.1007/s13280-019-01205-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, R. K. , Amundsen, H. , Lie, N. O. , Luyckx, K. , Robertson, L. J. , Verocai, G. G. , Kutz, S. J. , & Ytrehus, B. (2014). Sentinels in a climatic outpost: Endoparasites in the introduced muskox (Ovibos moschatus wardi) population of Dovrefjell, Norway. International Journal for Parasitology: Parasites and Wildlife, 3, 154–160. 10.1016/j.ijppaw.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, R. K. , Simard, M. , Kutz, S. J. , Kapel, C. M. O. , Hamnes, I. S. , & Robertson, L. J. (2011). Arctic parasitology: Why should we care? Trends in Parasitology, 27(6), 238–244. 10.1016/j.pt.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Dean, A. G. , Sullivan, K. M. & Soe, M. M. (2013). OpenEpi: Open source epidemiologic statistics for public health, version 3.01. www.OpenEpi.com, updated 2013/04/06, accessed 2020/12/10
- Dubey, J. P. (2009). Toxoplasmosis in sheep – The last 20 years. Veterinary Parasitology, 163, 1–14. 10.1016/j.vetpar.2009.02.026 [DOI] [PubMed] [Google Scholar]
- Dubey, J. P. (2010). Toxoplasmosis of animals and humans. In Dubey J. P. (Ed.) (2 edn.). CRC Press. [Google Scholar]
- Duszynski, D. W. , & Upton, S. J. (2001). Cyclospora, Eimeria, Isospora, and Cryptosporidium spp. In Samuel W. M., Pybus M. J., & Kocan A. A. (Eds.), Parasitic diseases of wild mammals (2nd ed., pp. 416–459). Iowa State University Press. 10.1002/9780470377000 [DOI] [Google Scholar]
- Ersdal, C. , Jørgensen, H. J. , & Lie, K. I. (2015). Acute and chronic Erysipelothrix rhusiopathiae infection in lambs. Veterinary Pathology, 52(4), 635–643. 10.1177/0300985814556187 [DOI] [PubMed] [Google Scholar]
- Faridi, A. , Tavakoli Kareshk, A. , Sadooghian, S. , & Firouzeh, N. (2020). Frequency of different genotypes of Giardia duodenalis in slaughtered sheep and goat in east of Iran. Journal of Parasitic Diseases, 44(3), 618–624. 10.1007/s12639-020-01237-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde, T. L. , Orsel, K. , Zadoks, R. N. , Biek, R. , Adams, L. G. , Checkley, S. L. , Davison, T. , De Buck, J. , Dumond, M. , Elkin, B. T. , Finnegan, L. , Macbeth, B. J. , Nelson, C. , Niptanatiak, A. , Sather, S. , Schwantje, H. M. , van der Meer, F. , & Kutz, S. J. (2016). Bacterial genomics reveal the complex epidemiology of an emerging pathogen in arctic and boreal ungulates. Frontiers in Microbiology, 7, 1759. 10.3389/fmicb.2016.01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fthenakis, G. C. , Christodoulopoulos, G. , Leontides, L. , & Tzora, A. (2006). Abortion in ewes associated with Erysipelothrix rhusiopathiae . Small Ruminant Research, 63, 183–188. 10.1016/j.smallrumres.2005.01.017 [DOI] [Google Scholar]
- Gherasim, A. , Lebbad, M. , Insulander, M. , Decraene, V. , Kling, A. , Hjertqvist, M. , & Wallensten, A. (2012). Two geographically separated food‐borne outbreaks in Sweden linked by an unusual Cryptosporidium parvum subtype, October 2010. Eurosurveillance, 17(46), 1–8. 10.2807/ese.17.46.20318-en [DOI] [PubMed] [Google Scholar]
- Groeschel, M. , Forde, T. , Turvey, S. , Joffe, A. M. , Hui, C. , Naidu, P. , Mavrot, F. , Kutz, S. , & Singh, A. E. (2019). An unusual case of Erysipelothrix rhusiopathiae prosthetic joint infection from the Canadian Arctic: Whole genome sequencing unable to identify a zoonotic source. BMC Infectious Diseases, 19, 282. 10.1186/s12879-019-3913-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrønlandsStatistik . (2020). Grønlands Statistik. Statistikbanken. https://bank.stat.gl/pxweb/en/Greenland/Greenland__FI__FI80/FIXHEKBED.px/?rxid=BEXST422‐05‐202005%3A45%3A39, accessed 2020/12/10
- Hoberg, E. P. , Galbreath, K. E. , Cook, J. A. , Kutz, S. J. , & Polley, L. (2012). Northern host‐parasite assemblages: History and biogeography on the borderlands of episodic climate and environmental transition. Advances in Parasitology, 79, 1–97. 10.1016/B978-0-12-398457-9.00001-9 [DOI] [PubMed] [Google Scholar]
- Jalili, V. , Afgan, E. , Gu, Q. , Clements, D. , Blankenberg, D. , Goecks, J. , Taylor, J. , & Nekrutenko, A. (2021). The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Research, 48 (Web Server issue), W395–W402. 10.1093/NAR/GKAA434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins, E. J. , Castrodale, L. J. , de Rosemond, S. J. C. , Dixon, B. R. , Elmore, S. A. , Gesy, K. M. , Hoberg, E. P. , Polley, L. , Schurer, J. M. , Simard, M. , & Thompson, R. C. A. (2013). Tradition and transition: Parasitic zoonoses of people and animals in Alaska, Northern Canada, and Greenland. Advances in Parasitology, 82, 33–204. 10.1016/B978-0-12-407706-5.00002-2 [DOI] [PubMed] [Google Scholar]
- Johnson, D. , Harms, N. J. , Latter, N. C. , Elkin, B. T. , Tabel, H. , & Wei, G. (2010). Serum biochemistry, serology, and parasitology of boreal caribou (Rangifer tarandus caribou) in the Northwest Territories, Canada. Journal of Wildlife Diseases, 46(4), 1096–1107. 10.7589/0090-3558-46.4.1096 [DOI] [PubMed] [Google Scholar]
- Kim, H.‐Y. (2017). Statistical notes for clinical researchers: Chi‐squared test and Fisher's exact test. Restorative Dentistry & Endodontics, 42(2), 152–155. 10.5395/rde.2017.42.2.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk, S. , Yazar, S. , & Cetinkaya, U. (2012). Stool sample storage conditions for the preservation of Giardia intestinalis DNA. Memórias Do Instituto Oswaldo Cruz, 107(8), 965–968. 10.1590/s0074-02762012000800001 [DOI] [PubMed] [Google Scholar]
- Kutz, S. , Bollinger, T. , Branigan, M. , Checkley, S. , Davison, T. , Dumond, M. , Elkin, B. , Forde, T. , Hutchins, W. , Niptanatiak, A. , & Orsel, K. (2015). Erysipelothrix rhusiopathiae associated with recent widespread muskox mortalities in the Canadian Arctic. Canadian Veterinary Journal. La Revue Vétérinaire Canadienne, 56, 560–563. [PMC free article] [PubMed] [Google Scholar]
- Kutz, S. J. , Ducrocq, J. , Verocai, G. G. , Hoar, B. M. , Colwell, D. D. , Beckmen, K. B. , Polley, L. , Elkin, B. T. , & Hoberg, E. P. (2012). Parasites in ungulates of Arctic North America and Greenland: A view of contemporary diversity, ecology, and impact in a world under change. Advances in Parasitology, 79, 99–252. 10.1016/B978-0-12-398457-9.00002-0 [DOI] [PubMed] [Google Scholar]
- Kutz, S. J. , Elkin, B. , Gunn, A. , & Dubey, J. P. (2000). Prevalence of Toxoplasma gondii antibodies in muskox (Ovibos moschatus) sera from Northern Canada. The Journal of Parasitology, 86(4), 879–882. h ttps://doi.org/10.1645/0022‐3395(2000)086[0879:POTGAI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kutz, S. J. , Hoberg, E. P. , Nagy, J. , Polley, L. , & Elkin, B. (2004). “Emerging” parasitic infections in Arctic ungulates. Integrative and Comparative Biology, 44, 109–118. 10.1093/icb/44.2.109 [DOI] [PubMed] [Google Scholar]
- Kutz, S. J. , Thompson, R. A. , Polley, L. , Kandola, K. , Nagy, J. , Wielinga, C. M. , & Elkin, B. T. (2008). Giardia assemblage A: human genotype in muskoxen in the Canadian Arctic. Parasites & Vectors, 1, 32. 10.1186/1756-3305-1-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz, S. J. , Thompson, R. C. A. , & Polley, L. (2009). Wildlife with Giardia: Villain, or victim and vector? In Ortega‐Pierres G., Cacciò S. M., Fayer R., Mank T. G., Smith H. V., & Thomson R. C. A. (Eds.), Giardia and cryptosporidium: From molecules to disease (pp. 94–106). CAB International. 10.1079/9781845933913.0000 [DOI] [Google Scholar]
- Lebbad, M. , Ankarklev, J. , Tellez, A. , Leiva, B. , Andersson, J. O. , & Svärd, S. (2008). Dominance of Giardia assemblage B in León, Nicaragua. Acta Tropica, 106, 44–53. 10.1016/j.actatropica.2008.01.004 [DOI] [PubMed] [Google Scholar]
- MacPhee, R. D. E. , & Greenwood, A. D. (2013). Infectious disease, endangerment, and extinction. International Journal of Evolutionary Biology, 2013, 571939. 10.1155/2013/571939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrot, F. , Orsel, K. , Hutchins, W. , Adams, L. G. , Beckmen, K. , Blake, J. E. , Checkley, S. L. , Davison, T. , Di Francesco, J. , Elkin, B. , Leclerc, L. M. , Schneider, A. , Tomaselli, M. , & Kutz, S. J. (2020). Novel insights into serodiagnosis and epidemiology of Erysipelothrix rhusiopathiae, a newly recognized pathogen in muskoxen (Ovibos moschatus). PLoS ONE, 15(4), e0231724. 10.1371/journal.pone.0231724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller, L. N. , Koch, A. , Petersen, E. , Hjuler, T. , Kapel, C. M. O. , Andersen, A. , & Melbye, M. (2010). Trichinella infection in a hunting community in East Greenland. Epidemiology and Infection, 138, 1252–1256. 10.1017/S0950268810000282 [DOI] [PubMed] [Google Scholar]
- Monis, P. T. , & Thompson, R. C. A. (2003). Cryptosporidium and Giardia‐zoonoses: Fact or fiction? Infection, Genetics and Evolution, 3, 233–244. 10.1016/j.meegid.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Naalakkersuisut . (2020). The veterinary and food authority. Government of Greenland. https://naalakkersuisut.gl/en/Naalakkersuisut/Departments/Fiskeri‐Fangst‐og‐Landbrug/Veterinaer‐og‐Foedevare‐Myndigheden‐i‐Groenland, accessed 2020/12/10 [Google Scholar]
- Oksanen, A. , Åsbakk, K. , Prestrud, K. W. , Aars, J. , Derocher, A. E. , Tryland, M. , Wiig, Ø. , Dubey, J. P. , Sonne, C. , Dietz, R. , Andersen, M. , & Born, E. W. (2009). Prevalence of antibodies against Toxoplasma gondii in polar bears (Ursus maritimus) from Svalbard and East Greenland. The Journal of Parasitology, 95(1), 89–94. 10.1645/GE-1590.1 [DOI] [PubMed] [Google Scholar]
- Olesen, C. R. , & Thing, H. (1989). Guide to field classification by sex and age of the muskox. Canadian Journal of Zoology, 67, 1116–1119. 10.1139/z89-159 [DOI] [Google Scholar]
- Olsen, A. , Berg, R. , Tagel, M. , Must, K. , Deksne, G. , Enemark, H. L. , Alban, L. , Johansen, M. V. , Nielsen, H. V. , Sandberg, M. , Lundén, A. , Stensvold, C. R. , Pires, S. M. , & Jokelainen, P. (2019). Seroprevalence of Toxoplasma gondii in domestic pigs, sheep, cattle, wild boars, and moose in the Nordic‐Baltic region: A systematic review and meta‐analysis. Parasite Epidemiology and Control, 3, e00100. 10.1016/j.parepi.2019.e00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestrud, K. W. , Dubey, J. P. , Åsbakk, K. , Fuglei, E. , & Su, C. (2008). First isolate of Toxoplasma gondii from arctic fox (Vulpes lagopus) from Svalbard. Veterinary Parasitology, 151, 110–114. 10.1016/j.vetpar.2007.11.011 [DOI] [PubMed] [Google Scholar]
- QGIS.org . (2019). QGIS Geographical Information System (v. 2.18). QGIS Association. https://www.qgis.org/en/site/index.html
- R Core Team . (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r‐project.org/ [Google Scholar]
- Raundrup, K. , Moshøj, C. M. , Wennerberg, S. E. , & Kapel, C. M. O. (2015). Spatiotemporal distribution of rabies in Arctic foxes in Greenland. European Journal of Wildlife Research, 61(3), 457–465. 10.1007/s10344-015-0917-5 [DOI] [Google Scholar]
- Robertson L.J., Gjerde B.K., Furuseth Hansen E. (2010). The zoonotic potential of Giardia and Cryptosporidium in Norwegian sheep: A longitudinal investigation of 6 flocks of lambs. Veterinary Parasitology, 171(1‐2), 140–145. 10.1016/j.vetpar.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Ryan, U. , & Zahedi, A. (2019). Molecular epidemiology of giardiasis from a veterinary perspective. Advances in Parasitology, 106, 209–254. 10.1016/bs.apar.2019.07.002 [DOI] [PubMed] [Google Scholar]
- Samuelsson, F. , Nejsum, P. , Raundrup, K. , Hansen, T. V. A. , & Kapel, C. M. O. (2013). Warble infestations by Hypoderma tarandi (Diptera; Oestridae) recorded for the first time in West Greenland muskoxen. International Journal for Parasitology: Parasites and Wildlife, 2, 214–216. 10.1016/j.ijppaw.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savioli, L. , Smith, H. , & Thompson, A. (2006). Giardia and Cryptosporidium join the “Neglected Diseases Initiative.” Trends in Parasitology, 22(5), 203–208. 10.1016/j.pt.2006.02.015 [DOI] [PubMed] [Google Scholar]
- Schmidt, N. M. , Van Beest, F. M. , Mosbacher, J. B. , Stelvig, M. , Hansen, L. H. , Nabe‐Nielsen, J. , & Grøndahl, C. (2016). Ungulate movement in an extreme seasonal environment: Year‐round movement patterns of high‐arctic muskoxen. Wildlife Biology, 22, 253–267. 10.2981/wlb.00219 [DOI] [Google Scholar]
- Silverlås, C. , Näslund, K. , Björkman, C. , & Mattsson, J. G. (2010). Molecular characterisation of Cryptosporidium isolates from Swedish dairy cattle in relation to age, diarrhoea and region. Veterinary Parasitology, 169, 289–295. 10.1016/j.vetpar.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Smith, H. V. , Cacciò, S. M. , Cook, N. , Nichols, R. A. B. , & Tait, A. (2007). Cryptosporidium and Giardia as foodborne zoonoses. Veterinary Parasitology, 149, 29–40. 10.1016/j.vetpar.2007.07.015 [DOI] [PubMed] [Google Scholar]
- Sonne, C. , Andersen‐Ranberg, E. , Rajala, E. L. , Agerholm, J. S. , Bonefeld‐Jørgensen, E. , Desforges, J. P. , Eulaers, I. , Gustavson, K. , Jenssen, B. M. , Koch, A. , Rosing‐Asvid, A. , Schmidt, N. M. , Grøndahl, C. , Mosbacher, J. B. , Siebert, U. , Tryland, M. , Mulvad, G. , Born, E. W. , Laidre, K. , … Magnusson, U. (2018). Prevalence of antibodies against Brucella spp. in West Greenland polar bears (Ursus maritimus) and East Greenland muskoxen (Ovibos moschatus). Polar Biology, 41, 1671–1680. 10.1007/s00300-018-2307-4 [DOI] [Google Scholar]
- Sprong, H. , Cacciò, S. M. , Van Der Giessen, J. W. B. , & ZOOPNET network and partners. (2009). Identification of zoonotic genotypes of Giardia duodenalis . PLoS Neglected Tropical Diseases, 3(12), e558. 10.1371/journal.pntd.0000558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturdee, A. P. , Chalmers, R. M. , & Bull, S. A. (1999). Detection of Cryptosporidium oocysts in wild mammals of mainland Britain. Veterinary Parasitology, 80, 273–280. 10.1016/S0304-4017(98)00226-X [DOI] [PubMed] [Google Scholar]
- Sulaiman, I. M. , Fayer, R. , Bern, C. , Gilman, R. H. , Trout, J. M. , Schantz, P. M. , Das, P. , Lal, A. A. , & Xiao, L. (2003). Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis . Emerging Infectious Diseases, 9(11), 1444–1452. 10.3201/eid0911.030084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVA . (2019). Surveillance of infectious diseases in animals and humans in Sweden 2019. SVA:S Rapportserie, 64, 1654–7098. National Veterinary Institute (SVA), Uppsala, Sweden. www.sva.se [Google Scholar]
- Sweeny, J. P. A. , Ryan, U. M. , Robertson, I. D. , & Jacobson, C. (2011). Cryptosporidium and Giardia associated with reduced lamb carcase productivity. Veterinary Parasitology, 182, 127–139. 10.1016/j.vetpar.2011.05.050 [DOI] [PubMed] [Google Scholar]
- Thomas‐Lopez, D. , Müller, L. , Vestergaard, L. S. , Christoffersen, M. , Andersen, A. M. , Jokelainen, P. , Agerholm, J. S. , & Stensvold, C. R. (2020). Veterinary students have a higher risk of contracting cryptosporidiosis when calves with high fecal Cryptosporidium loads are used for fetotomy exercises. Applied and Environmental Microbiology, 86, e01250–20. 10.1128/AEM.01250-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, R. C. A. , Lymbery, A. J. , & Smith, A. (2010). Parasites, emerging disease and wildlife conservation. International Journal for Parasitology, 40, 1163–1170. 10.1016/j.ijpara.2010.04.009 [DOI] [PubMed] [Google Scholar]
- Thompson, R. C. A. , Palmer, C. S. , & O'Handley, R. (2008). The public health and clinical significance of Giardia and Cryptosporidium in domestic animals. Veterinary Journal, 177(1), 18–25. 10.1016/j.tvjl.2007.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli, M. , Dalton, C. , Duignan, P. J. , Kutz, S. , van der Meer, F. , Kafle, P. , Surujballi, O. , Turcotte, C. , & Checkley, S. (2016). Contagious ecthyma, Rangiferine brucellosis, and lungworm infection in a Muskox (Ovibos moschatus) from the Canadian Arctic, 2014. Journal of Wildlife Diseases, 52(3), 719–724. 10.7589/2015-12-327 [DOI] [PubMed] [Google Scholar]
- Verweij, J. J. , Blange, R. A. , Templeton, K. , Schinkel, J. , Brienen, E. A. T. , Van Rooyen, M. A. A , Van Lieshout, L. , & Polderman, A. M. (2004). Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real‐time PCR. Journal of Clinical Microbiology, 42(3), 1220–1223. 10.1128/JCM.42.3.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis, S. (2013). Binomial confidence intervals and contingency tests: Mathematical fundamentals and the evaluation of alternative methods. Journal of Quantitative Linguistics, 20(3), 178–208. 10.1080/09296174.2013.799918 [DOI] [Google Scholar]
- Wang, Q. , Chang, B. J. , & Riley, T. V. (2010). Erysipelothrix rhusiopathiae. Veterinary Microbiology, 140, 405–417. 10.1016/j.vetmic.2009.08.012 [DOI] [PubMed] [Google Scholar]
- Xiao, X. , Qi, R. , Han, H. J. , Liu, J. W. , Qin, X. R. , Fang, L. Z. , Zhou, C. M. , Gong, X. Q. , Lei, S. C. , & Yu, X. J. (2019). Molecular identification and phylogenetic analysis of Cryptosporidium, Hepatozoon and Spirometra in snakes from central China. International Journal for Parasitology: Parasites and Wildlife, 10, 274–280. 10.1016/j.ijppaw.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanta, C. A. , Bessonov, K. , Robinson, G. , Troell, K. , & Guy, R. A. (2021). CryptoGenotyper: A new bioinformatics tool for rapid Cryptosporidium identification. Food and Waterborne Parasitology, 23, e00115. 10.1016/j.fawpar.2021.e00115 [DOI] [PMC free article] [PubMed] [Google Scholar]


