Abstract
Transport disturbs birds’ welfare and health status which lead to oxidative stress and dietary ascorbic acid mitigates the adverse effects of transport stress. The present study was aimed to evaluate the impacts of ascorbic acid administration on oxidative stress indices, cortisol, H/L ratio, tonic immobility reaction and rectal temperature of pigeons exposed to road transport. A total of 80 clinically healthy pigeons were selected and randomly divided to eight equal groups as follow: (1) Ctrl–: fed by basal diet and no subjected to transport stress; (2) Ctrl+: fed by the basal diet and subjected to transport stress; (3, 4) 1DBS10 and 1DBS16: received ascorbic acid from 1 day before transport stress at doses of 10 g/100 L and 16 g/100 L of drinking water, respectively; (5, 6) 3DBS10 and 3DBS16: treated with ascorbic acid from 3 consecutive days before transport stress at doses of 10 g/100 L and 16 g/100 L, respectively and (7, 8) 7DBS10 and 7DBS16: received ascorbic acid from 7 consecutive days before the transport at doses 10 g/100 L and 16 g/100 L, respectively. Birds were transported for 3 h over a distance of about 200 km. The total antioxidant capacity, malondialdehyde and cortisol were measured before transport and at 6, 24 and 72 h post‐transportation. The rectal temperature and tunic immobility reactions were recorded. Dietary ascorbic acid led to a decrease in tonic immobility response, hetrophil to lymphocyte ratio, circulating cortisol and total antioxidant capacity, and an increase in circulating malondialdehyde in pigeons exposed to transport stress compare to Ctrl+ group. In conclusion, ascorbic acid administration at dose 16 g/100 L of drinking water from 3 and 7 days before exposure to stress helps attenuate undesirable effects of oxidative stress in pigeons.
Keywords: ascorbic acid, dietary management, pigeon, prevention, transport stress
Ascorbic acid administration at dose 16 g/100 L of drinking water from 3 and 7 days before exposure to rad transport stress helps attenuate undesirable effects of oxidative stress in pigeons.

1. INTRODUCTION
Stress is the nonspecific and adaptive responses of the body to any stressful conditions in birds (Etim et al., 2013). Stress activates the pituitary‐adrenal axis and leads to secrete adrenocorticotropin (ACTH) hormone, affecting the blood homeostasis system (Scanes, 2016). Stressful conditions affect birds’ physiological status, causing an increase in cortisol secretion, heart rate and body temperature. Furthermore, stressors increase susceptibility to infectious diseases and mortality in birds (Etim et al., 2013).
Numerous behavioural and physiological markers have to evaluate stress in avian (Edelaar et al., 2012). Tonic immobility (TI) reaction, serum level of cortisol and heterophil to lymphocyte (H/L) ratio used as reliable behavioural and physiological indicators for assessing welfare and stress status in birds (Alm et al., 2016). Stressful situations in birds lead to oxidative stress, resulting in an imbalance between reactive oxygen species (ROS) production and antioxidant defences. Hence, evaluating the levels of oxidation and the antioxidant components in the body could be beneficial for assessing the oxidative status (Vassalle, 2008). There are various oxidative stress‐related biomarkers, including malondialdehyde (MDA) and total antioxidant capacity (T‐AOC) to evaluate the oxidative status in avian (Lee et al., 2017). Birds are exposed to a variety of psychological, physical, chemical, nutritional or infectious stressors in their lifetime, which decreased their productive performance, immune response and welfare (Etim et al., 2013).
Transport stress is one of the stress conditions in birds that lead to disturbs their welfare and health status (Von Borell, 2001). Transport stress disturbs hormonal, physiological, biochemical and immunological responses in birds (Zhang et al., 2017). During transportation, birds are exposed to various potential stressors such as handling, crowding, feed withdrawal, insufficient ventilation, vibration, thermal extremes and noise that can multiply the unfavourable effects of the transport stress (Al‐Aqil et al., 2013). Road transport stress is more vital in sick birds referred to veterinary clinics, which leads to a change in the clinical symptoms of the disease, resulting in misdiagnosis and sudden death (Minka et al., 2012). Accordingly, attenuation of its adverse effects is valuable for maintaining the health and welfare of birds (Ghareeb et al., 2008). Transport stress leads to oxidative stress, which increases the body's demands for antioxidants for neutralising the ROS and protecting the body against oxidative stress (Pisoschi & Pop, 2015). Thus, administering the natural antioxidants could help ameliorate the oxidative stress induced by transport (Şenay et al., 2019).
Ascorbic acid (AA) is a potent natural antioxidant, which mitigates the adverse effects of stress and improves productive performance (Rao et al., 2011; Whitehead & Keller, 2019). AA intake has significant impacts on the incidence and severity of infectious diseases and stress (Khan et al., 2012). Studies have established the positive effects of dietary AA on improving immune response, growth performance and health status in birds under stressful conditions. As well as, supplements of AA modulated oxidative stress and increased the survival rate in broilers under stress (Gouda et al., 2020; Shojadoost et al., 2021). Various researches have used AA for attenuating the destructive effects of transport stress on chickens, ostriches, quail and laying hens (Minka and Ayo, 2008, 2010, 2013).
According to previous findings, it was hypothesised that the AA intake may reduce the negative effects of road transport in pigeons; therefore, the present study was aimed to evaluate the impacts of prophylactic administration of AA with low and high doses at three different short, moderate and long term on some oxidative stress indices of pigeons exposed to road transport. Furthermore, the effects of AA administration on circulating cortisol, H/L ratio, tonic immobility reaction and rectal temperature of pigeons were investigated. The results of the present study may also aid to the better management of pigeons under transport stress.
2. MATERIALS AND METHODS
2.1. Birds and experimental groups
The study was carried out on 80 clinically healthy pigeons weighing 398.10 ± 18.52 g. During the experiment, they were fed by commercial diet (Parsdan®, fars, Iran) ad libitum. After 2‐weeks adaptation period, the pigeons were randomly assigned to eight equal treatment groups (n = 10) as follow (Figure 1): Ctrl– (negative control): fed by basal diet and no subjected to transport stress; Ctrl+ (positive control): fed the basal diet and subjected to transport stress; 1DBS10 and 1DBS16: received AA from 1 day before transport stress at doses of 10 g/100 L and 16 g/100 L, respectively; 3DBS10 and 3DBS16: treated with AA from 3 consecutive days before transport stress at doses of 10 g/100 L and 16 g/100 L, respectively; 7DBS10 and 7DBS16: received AA from 7 consecutive days before the transport at doses 10 g/100 L and 16 g/100 L, respectively. AA (ascorbic acid 50%, Royan Darou Co., Tehran, Iran) was administrated in two different doses, including low (10 g/100 L) and high dose (16 g/100 L) for three different periods, such as 1, 3 and 7 days (short, moderate and long‐term, respectively) before exposure to transport stress via drinking water. The birds had 12–14 h of natural lighting daily.
FIGURE 1.
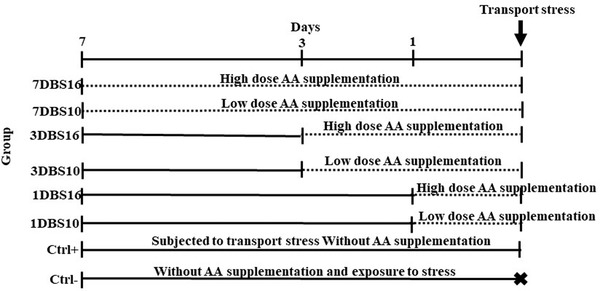
Experimental schedule of transport stress induction in pigeons. Ctrl–: negative control; Ctrl+: positive control; 1DBS16: AA treated at dose of 16 g/100 L from 1 day before transport stress; 1DBS10: AA treated at dose 10 g/100 L from 1 day before transport stress; 3DBS16: AA‐supplemented at dose 16 g/100 L from 3 days before transport stress; 3DBS10: AA‐supplemented at dose 10 g/100 L from 3 days before transport stress; 7DBS16: AA‐supplemented at dose 16 g/100 from 7 days before transport stress; 7DBS10: AA‐supplemented at dose 10 g/100 from 7 days before transport stress
2.2. Transport stress induction
Transport stress induction in pigeons was performed according to a study by Scope et al. (2002). On transport day, the various groups of pigeons were colour‐marked for easy identification. Then, all groups expect Ctrl– were loaded into carrier crates at a stocking density of about 245 cm2/bird that each basket contained five birds from each group. Then, birds were transported for 3 h over a distance of about 200 km with an average speed of 60 km/h (between 08:00 and 11:00 a.m.). All treatments were the same transportation conditions. Feed and water were withdrawn from the birds from 6 and 2 h before transit, respectively. The feed and water were not available for the pigeons during transit. The vehicle condition (temperature and humidity) were similar for all the pigeons, and near the experiment room condition to reduce the effect of heat or cold stress. After transport, all birds were returned to the experimental rooms and received the same conditions as before transit.
2.3. Blood sampling and serum biochemical measurement
Blood samples (3 ml) were taken from the wing vein immediately before transport (zero time) and at 6, 24 and 72 h post‐transportation from all birds in each group. About 2.5 ml of the blood samples were collected into the plain tube, centrifuged at 750 g for 10 min and separated sera were stored at −20°C until further assay. About 0.5 ml of the blood samples were collected in heparinised tubes for gathering whole blood. The whole blood samples were smeared on microscopic slides, air‐dried and then stained with Wright‐Giemsa stain. H/L ratio was determined under a compound microscope at a magnification of 100× with oil immersion in each slide. In each slide, lymphocyte and heterophil cells were counted to 100 cells using the straight edge method and the H/L ratio was obtained by dividing the number of heterophils by the number of lymphocytes (Jayaprakash et al., 2016).
The serum levels of T‐AOC and MDA as oxidative indices and cortisol as stress markers were measured. The T‐AOC was assayed by spectrophotometric method (Kiazist life science CO., Iran; sensitivity equal to 20 nmol/mL; intra‐assay and inter‐assay CV < 17% and 16%, respectively). MDA was analysed by colorimetric/fluorometric method (Kiazist life Science Co., Iran; sensitivity equal to 10 μM; intra‐assay and inter‐assay CV < 15% and 19%, respectively). The serum concentration of cortisol was evaluated by the ELISA technique using the commercial kits (DIMETRA, Italy) according to the instructions of the manufacturer of the kit.
2.4. Rectal temperature and TI reaction measurement
The rectal temperature (RT) was recorded as a physiological indicator in all the pigeons from each group at different times: before the transportation (BT), immediately post‐transport (PT) and 3, 6 and 24 h post‐transport. The RT was recorded using a digital thermometer by inserting about 2 cm into the cloacae of birds.
TI reaction was assessed as a fear response in all the pigeons per each treatment. The birds were tested individually for the duration of TI and the number of inductions to attain TI at three different times as follows: 3 (1st test), 24 (2nd test) and 72 (3rd test) h post‐transport. Immediately following the transport stress, the birds were transported to separate rooms (no visual contact with other birds), and the TI test was performed as described by Edelaar et al. (2012). Each bird was individually placed on its back for 15 s, fully covering the pigeon with one hand. After 15 s, the force of the hand was slowly removed. If the pigeon righted itself before 5 s, the TI was repeated immediately. If the bird remained immobile on its back for more than 5 s, the time until the bird righted itself was recorded as TI duration using a stopwatch. Furthermore, the number of efforts to induce TI was documented as the number of inductions of TI. The maximum test duration allowed was 10 min, and no more than five attempts were made to induce TI (Edelaar et al., 2012).
2.5. Statistical analysis
All data obtained were subjected to one‐way ANOVA followed by the Post‐Hoc Tukey's HSD test. Repeated measures ANOVA was employed for assessing the trend alteration of all the studied parameters. The effects of groups, time and their interactions on the changes of each parameter were also analysed. Results were expressed as mean ± standard error (mean ± SE) and the significance level was set at p < 0.05.
3. RESULTS
Table 1 shows the effects of AA administration on the H/L ratio and serum levels of cortisol, MDA, T‐AOC in different groups of pigeons subjected to transport stress. There were no significant differences in different parameters between various treatments at time zero (Tables 1, 2, 3; p > 0.05). At all times (6, 24 and 72 h post‐transport stress) the Ctrl+ group had significantly the lowest level of the T‐AOC and the highest levels of MDA and the cortisol compared to all other groups (Table 1; p < 0.05). At 6 h post‐transport, the serum concentration of MDA was significantly lower in the 1DBS16 group than all groups that received AA (Table 1; p < 0.05). As shown in Table 1, the Ctrl–, 3DBS16 and 7DBS16 groups had lower MDA levels compared to other treatments at 24 h post‐transport (p < 0.05). As shown in Table 1, the MDA level was lower in 7DBS16 and 7DBS10 groups than other treatments at 72 h post‐transport. At all times after transport stress, the 3DBS16 and 7DBS16 groups had higher T‐AOC amonge various treatments that received AA (Table 1; p < 0.05).
TABLE 1.
The effects of ascorbic acid on serum values of MDA, TAC and cortisol, and ratio of H/L (mean ± SE) in different groups of pigeons subjected to transport stress
| Parameters | Groups | Times | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 (BT) | 6 h PT | 24 h PT | 72 h PT | Group | Time | Group × Time | ||
| MDA (μmol/L) | Ctrl– | 171.03 ± 7.21a | 118.93 ± 6.54a | 115.13 ± 8.05a | 105.82 ± 9.73a | < 0.001 | < 0.001 | < 0.001 |
| Ctrl+ | 169.69 ± 5.62a | 214.85 ± 11.55b | 204.78 ± 8.22b | 198.45 ± 9.49b | ||||
| 1DBS16 | 172.67 ± 4.90a | 166.24 ± 5.16c | 176.11 ± 1.66c | 143.83 ± 7.96c | ||||
| 1DBS10 | 165.93 ± 5.22a | 199.13 ± 2.76d | 200.02 ± 3.93b | 162.17 ± 7.41c | ||||
| 3DBS16 | 176.38 ± 6.25a | 181.63 ± 1.62e | 114.52 ± 13.49a | 95.73 ± 4.15a,d | ||||
| 3DBS10 | 182.09 ± 7.12a | 182.31 ± 2.83e | 166.81 ± 8.05c | 114.58 ± 7.66a | ||||
| 7DBS16 | 196.72 ± 5.88a | 191.39 ± 2.58e,d | 127.81 ± 6.98a | 88.61 ± 5.93d | ||||
| 7DBS10 | 170.54 ± 6.74a | 202.70 ± 1.76b,d | 159.37 ± 7.74c | 83.91 ± 5.71d | ||||
| TAC (mmol/L) | Ctrl– | 580.98 ± 10.98a | 632.16 ± 11.11a | 624.62 ± 12.68a | 576.18 ± 13.54a | < 0.001 | < 0.001 | < 0.001 |
| Ctrl+ | 569.87 ± 19.43b | 348.09 ± 29.15b | 338.18 ± 19.54b | 428.41 ± 14.16b | ||||
| 1DBS16 | 573.73 ± 18.03a,b | 428.97 ± 31.63c | 445.83 ± 23.63c | 442.94 ± 20.16b | ||||
| 1DBS10 | 572.76 ± 19.01a,b | 273.96 ± 24.86d | 369.80 ± 18.82b | 428.26 ± 23.54b | ||||
| 3DBS16 | 587.93 ± 19.32a | 510.64 ± 10.75e | 531.26 ± 26.24d | 602.73 ± 35.15a | ||||
| 3DBS10 | 562.53 ± 18.32b | 345.24 ± 15.89b | 401.60 ± 23.03c | 463.94 ± 21.12b | ||||
| 7DBS16 | 582.09 ± 20.53a | 527.13 ± 14.54e | 558.07 ± 11.26d | 549.27 ± 30.27a | ||||
| 7DBS10 | 575.76 ± 22.53a,b | 396.32 ± 16.64b,c | 430.97 ± 21.53c | 548.99 ± 24.15a | ||||
| Cortisol (ng/ml) | Ctrl– | 0.73 ± 0.09 a | 0.84 ± 06a | 0.8072 ± 0.8a | 0.8340 ± 0.06a | < 0.001 | < 0.001 | < 0.001 |
| Ctrl+ | 0.98 ± 0.14a | 2.89 ± 0.14b | 0.8110 ± 0.12a | 0.8800 ± 0.04a | ||||
| 1DBS16 | 0.78 ± 0.09a | 1.99 ± 0.12c | 0.8700 ± 0.20a | 0.9840 ± 0.08a | ||||
| 1DBS10 | 0.93 ± 0.11a | 1.86 ± 0.20c | 0.9840 ± 0.16a | 0.8120 ± 0.09a | ||||
| 3DBS16 | 0.79 ± 0.05a | 1.05 ± 0.16d | 0.7880 ± 0.15a | 0.8730 ± 0.10a | ||||
| 3DBS10 | 0.85 ± 0.08a | 0.99 ± 0.09d | 0.7310 ± 0.10a | 0.8330 ± 0.04a | ||||
| 7DBS16 | 0.81 ± 0.12a | 0.81 ± 0.10d | 0.8380 ± 0.13a | 0.8190 ± 0.09a | ||||
| 7DBS10 | 0.94 ± 0.37a | 0.83 ± 0.13d | 0.8240 ± 0.05a | 0.7630 ± 0.10a | ||||
| H/L | Ctrl– | 0.60 ± 0.05a | 0.58 ± 0.03a | 0.66 ± 0.06a | 0.63 ± 0.72a | < 0.001 | < 0.001 | < 0.001 |
| Ctrl+ | 0.61 ± 0.03a | 1.89 ± 0.13b | 1.75 ± 0.10b | 1.63 ± 0.08b | ||||
| 1DBS16 | 0.60 ± 0.05a | 1.48 ± 0.14c | 1.38 ± 0.10c | 1.47 ± 0.06b | ||||
| 1DBS10 | 0.66 ± 0.04a | 1.59 ± 0.07b,c | 1.42 ± 0.12b,c | 1.21 ± 0.10b | ||||
| 3DBS16 | 0.70 ± 0.05a | 1.41 ± 0.10c | 1.18 ± 0.13c | 0.88 ± 0.08c | ||||
| 3DBS10 | 0.64 ± 0.03a | 1.68 ± 0.17c | 1.00 ± 0.08c | 0.82 ± 0.04a,c | ||||
| 7DBS16 | 0.57 ± 0.02a | 1.35 ± 0.16c | 0.74 ± 0.17a | 0.60 ± 0.11a | ||||
| 7DBS10 | 0.64 ± 0.04a | 1.47 ± 0.14c | 1.18 ± 0.12c | 0.70 ± 0.04a,c | ||||
MDA: malondialdehyde; T‐AOC: total antioxidant capacity; H/L: hetrophil to lymphocyte ratio; Ctrl–: negative control; Ctrl+: positive control; 1DBS16: AA treated at dose of 16 g/100 L from 1 day before transport stress; 1DBS10: AA treated at dose 10 g/100 L from 1 day before transport stress; 3DBS16: AA‐supplemented at dose 16 g/100 L from 3 days before transport stress; 3DBS10: AA‐supplemented at dose 10 g/100 L from 3 days before transport stress; 7DBS16: AA‐supplemented at dose 16 g/100 from 7 days before transport stress; 7DBS10: AA‐supplemented at dose 10 g/100 from 7 days before transport stress; BT: before transport; h PT: h post‐transport. Different superscript letters indicate significant differences in column among different groups (p < 0.05).
TABLE 2.
Effect of AA administration on rectal temperature at different pre‐ and post‐transport times at different groups of pigeons subjected to transport stress
| Parameter | Groups | Times | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BT | PT | 3 h PT | 6 h PT | 24 h PT | Group | Time | Group × Time | ||
| Rectal temperature | Ctrl– | 41.66 ± 0.12a | 40.21 ± 0.69a | 40.22 ± 1.02a | 40.33 ± 1.02a | 40.43 ± 0.34a | < 0.001 | < 0.001 | < 0.001 |
| Ctrl+ | 41.03 ± 0.54a | 41.57 ± 0.81b | 40.86 ± 1.03b | 40.64 ± 1.03b | 40.32 ± 0.58a | ||||
| 1DBS16 | 41.80 ± 0.76a | 41.42 ± 0.64b | 40.48 ± 0.98a | 40.44 ± 0.95a | 40.67 ± 0.75b | ||||
| 1DBS10 | 41.71 ± 0.86a | 41.77 ± 0.97b | 40.38 ± 1.43a | 40.32 ± 0.82a | 40.28 ± 0.93a | ||||
| 3DBS16 | 41.09 ± 0.97a | 40.96 ± 0.99c | 40.33 ± 0.91a | 40.33 ± 0.83a | 40.28 ± 0.78a | ||||
| 3DBS10 | 41.82 ± 1.09a | 40.78 ± 0.86c,d | 40.37 ± 0.75a | 40.46 ± 0.92a | 40.36 ± 0.76a | ||||
| 7DBS16 | 41.08 ± 1.05a | 40.61 ± 1.12d | 40.57 ± 0.75b | 40.27 ± 0.93a | 40.49 ± 0.97a | ||||
| 7DBS10 | 41.15 ± 0.76a | 40.39 ± 0.87a,d | 40.21 ± 0.94a | 40.21 ± 1.20a | 40.29 ± 0.93a | ||||
AA: ascorbic acid; Ctrl–: negative control; Ctrl+: positive control; 1DBS16: AA treated at dose of 16 g/100 L from 1 day before transport stress; 1DBS10: AA treated at dose 10 g/100 L from 1 day before transport stress; 3DBS16: AA‐supplemented at dose 16 g/100 L from 3 days before transport stress; 3DBS10: AA‐supplemented at dose 10 g/100 L from 3 days before transport stress; 7DBS16: AA‐supplemented at dose 16 g/100 from 7 days before transport stress; 7DBS10: AA‐supplemented at dose 10 g/100 from 7 days before transport stress; BT: before transport; h PT: h post‐transport. Different superscript letters indicate significant differences in column among different groups (p < 0.05).
TABLE 3.
Effects of AA administration on TI duration and number of TI induction (mean ± SE) at different times in pigeons subjected to transport stress
| Indices | Groups | Times (h) | p Value | ||||
|---|---|---|---|---|---|---|---|
| 3 h PT | 24 h PT | 72 h PT | Group | Time | Group × Time | ||
| TI duration (s) | Ctrl– | 41.40 ± 5.69a | 31.30 ± 5.05a | 30.40 ± 5.71a | < 0.001 | < 0.001 | < 0.001 |
| Ctrl+ | 145.20 ± 7.83b | 84.70 ± 5.43b | 36.10 ± 5.64a | ||||
| 1DBS16 | 136.30 ± 12.38b | 68.30 ± 4.35c | 40.40 ± 5.53a | ||||
| 1DBS10 | 133.20 ± 7.21b | 68.10 ± 4.70c | 37.00 ± 2.08a | ||||
| 3DBS16 | 103.30 ± 4.60c | 69.30 ± 6.87c | 44.10 ± 4.19b | ||||
| 3DBS10 | 96.50 ± 7.07c,d | 68.80 ± 6.42c | 32.40 ± 2.51a | ||||
| 7DBS16 | 99.01 ± 9.54c,d | 48.20 ± 4.69d | 27.70 ± 2.08a | ||||
| 7DBS10 | 79.70 ± 4.44d | 54.10 ± 5.64c,d | 30.00 ± 3.14a | ||||
| Number of TI induction | Ctrl– | 0.80 ± 0.09a | 0.80 ± 0.13a | 0.80 ± 0.10a,b | < 0.001 | < 0.001 | < 0.001 |
| Ctrl+ | 2.30 ± 0.13b | 1.60 ± 0.14b | 1.00 ± 0.09a | ||||
| 1DBS16 | 2.00 ± 0.21b | 1.30 ± 0.12b | 1.00 ± 0.14a | ||||
| 1DBS10 | 2.10 ± 0.97b | 1.10 ± 0.15a | 0.90 ± 0.13a,b | ||||
| 3DBS16 | 1.80 ± 0.18b,c | 1.20 ± 0.17a | 0.90 ± 0.12a,b | ||||
| 3DBS10 | 1.70 ± 0.23c | 1.00 ± 0.10a | 0.90 ± 0.21a,b | ||||
| 7DBS16 | 1.40 ± 0.18c | 0.90 ± 0.11a | 0.80 ± 0.30a,b | ||||
| 7DBS10 | 1.30 ± 0.32c | 1.10 ± 0.13a | 0.60 ± 0.21b | ||||
AA: ascorbic acid; Ctrl–: negative control; Ctrl+: positive control; 1DBS16: AA treated at dose of 16 g/100 L from 1 day before transport stress; 1DBS10: AA treated at dose 10 g/100 L from 1 day before transport stress; 3DBS16: AA‐supplemented at dose 16 g/100 L from 3 days before transport stress; 3DBS10: AA‐supplemented at dose 10 g/100 L from 3 days before transport stress; 7DBS16: AA‐supplemented at dose 16 g/100 from 7 days before transport stress; 7DBS10: AA‐supplemented at dose 10 g/100 from 7 days before transport stress; BT: before transport; h PT: h post‐transport; TI: tunic immobility. Different superscript letters indicate significant differences in column among different groups (p < 0.05).
As seen in Table 1, the serum level of cortisol was significantly higher in the Ctrl+, 1DBS10 and 1DBS16 groups than others at 6 h post‐transport stress (p < 0.05). The 3DBS16, 3DBS10, 7DBS16 and 7DBS10 groups had significantly lower cortisol than other groups exposed to transport stress at 6 h after transit. There were no significant differences between various groups in term cortisol at 24 and 72 h post‐transport (Table 1; p > 0.05).
The H/L ratio was significantly higher in the Ctrl+ compared to the Ctrl– at all times of post‐transport stress (Table 1; p < 0.05). At 6 h post‐transport, all the AA‐supplemented groups had lower the H/L ratio compared to the Ctrl+ (Table 1; p < 0.05). There was no significant difference in the H/L ratio between various groups treated with AA (p > 0.05). At 24 and 72 h post‐transport, the 7DBS16 had decreased the ratio of H/L compared to other treatments (p < 0.05).
Table 2 presents the effects of AA administration on the RT in different groups subjected to transport stress. There were no significant differences in the RT between various groups before transport stress (BT time) (p > 0.05). At all times of post‐transport stress, the RT increased significantly in the Ctrl+ compared to the Ctrl–. At immediately and 3 h post‐transport, the 3DBS10 and 3DBS16 groups had lower the RT than other the AA‐treated groups (Table 2; p < 0.05). There was no significant difference between various treatments in the RT at 24 and 72 h post‐stress (p > 0.05).
Table 3 shows the variations in the TI duration and the number of TI inductions in response to AA administration in different groups. At 3 and 24 h after stress, the TI duration elevated in all groups subjected to stress compared to Ctrl– (p < 0.05). The 7DBS10 and 7DBS16 groups had significantly lower the TI duration and the number of TI inductions than other treatments received AA at 3 and 24 h post‐transport. At 72 h post‐transport, there was no significant difference in the TI duration between treatments (Table 3; p > 0.05).
4. DISCUSSION
Birds are transported by road for various purposes such as trading between different cities, attending exhibitions and visiting clinics (Zheng et al., 2020). Literature showed that road transport leads to oxidative stress and impairment in the health status of birds (Zhang et al., 2010). Numerous researchers have used electrolytes, amino acids and antioxidants to mitigate the deleterious effects of oxidative stress induced by transport stress (Salami et al., 2015; Zhang et al., 2019). The current experiment revealed that prophylactic administration of AA at dose 16 g/100 L of drinking water for 3 and 7 consecutive days before exposure to transport stress mitigated oxidative stress in pigeons (Table 1). Moreover, the current experiment indicated that AA intake could regulate physiological, fear and behavioural responses in pigeons exposed to transport stress (Tables 1, 2, 3).
Oxidative stress is one of the most important biological harmful mechanisms that impair the growth and function of birds (Surai et al., 2019). Oxidative stress causes cellular damages by protein oxidation, nucleic acid damage and lipid peroxidation (Moloney & Cotter, 2018). Many oxidative stress‐related biomarkers can be measured to evaluate the oxidative status in avian (Emami et al., 2021). MDA is the most commonly used biomarker to estimate lipid peroxidation in oxidative stress (Rehman et al., 2018). Evaluation of the activity of antioxidant components is another reliable marker for evaluating oxidative status. In birds exposed to stress, antioxidant status is measured individually as non‐enzymatic and enzymatic antioxidants or the T‐AOC (El‐Senousey et al., 2018). Measuring the various antioxidant molecules one by one would be excessively time‐consuming, expensive and required numerous analytical instruments and assays. Consequently, T‐AOC is commonly measured as a reliable index for estimating the status of antioxidant components in the body (Lee et al., 2017). Numerous Researches have suggested that antioxidant compounds could control oxidative stress by neutralising free radicals and increasing the activities of the antioxidant enzymes. For this reason, antioxidants improve health status and immune function in birds (Guo et al., 2020).
According to Table 2, there was no significant difference in circulating MDA and T‐AOC in various treatments at time zero (before exposure to transport stress), indicating birds had no exposure to any stressors before transit. At all times of post‐transport (6, 24 and 72 h), the all transport‐stressed groups had a significantly higher MDA and lower T‐AOC levels in serum compared to the Ctrl– (Table 1; p < 0.05). Elevated serum level of MDA in pigeons exposed to transport stress (Table 1) is in line with previous studies that suggested increased MDA in biological fluids are associated with various environmental stressors (Ito et al., 2019). In line with previous literature, transport‐stressed pigeons had a higher T‐AOC level in serum (Table 1). In agreement with our findings, Lee et al. (2017) reported that the enzymatic and non‐enzymatic antioxidant activities changed under stress for neutralising ROS. The results of this study showed that road transport led to oxidative stress in pigeons by increasing MDA production and decreasing T‐AOC level in serum.
Vitamin C or AA has potent antioxidant impacts in defence system by free radicals neutralisation, dehydrocarboxyl production and elevation of the activity of antioxidant enzymes in birds subjected to stressors (Shojadoost et al., 2021). Furthermore, AA protects birds against stress by decreasing cortisol secretion, lipid peroxidation and increasing the activity of antioxidant enzymes such as glutathione in the body (Macan et al., 2019). The birds can synthesise AA in their body to meet their requirements under normal conditions (Gouda et al., 2020). Studies have shown that the AA demands of birds elevated following exposure to stress (Abidin & Khatoon, 2013). In this respect, various studies showed that the positive effects of AA on improvement of health and oxidative status in birds exposed to stressors (Gouda et al., 2020; Shojadoost et al., 2021). In the present study, at 6 h post‐transport, the 1DBS16 group had a lower serum concentration of MDA among the groups that received AA (Table 1; p < 0.05). At 24 and 72 h after transit, 3DBS16 and 7DBS16 groups had higher T‐AOC and lower MDA levels among groups exposed to transport stress (Table 1; p < 0.05), indicating these groups had potent responses against transport stress. Although receiving a high dose of ascorbic acid for one day before transport in the 1DBS16 group had a better response at first hours post‐transport. However, as seen in Table 2, the trend of decreasing serum MDA level in 3DBS16 and 7DBS16 groups was more significant at different days post‐stress. These findings indicated that AA intake at dose 16 g/100 L for 3 and 7 consecutive days before transit had more potent effects on improving oxidative status in stressed pigeons. AA as a natural antioxidant inhibits lipid peroxidation and MDA production during stress conditions. Furthermore, AA supplementation leads to an increase in the antioxidant capacity and antioxidant enzymes activity in the bird's body (Leskovec et al., 2018). Saiz del Barrio et al. (2020) reported that AA increased the antioxidant capacity of the broiler's body. Horváth and Babinszky (2018) stated that AA intake led to decrease MDA and increase T‐AOC in broilers exposed to stress conditions. The results of the current research indicate that AA receiving at dose 16 g/100 L of drinking water from 3 and 7 days before exposure to transport stress significantly depressed MDA value and increased T‐AOC level in serum of pigeons, indicating prophylactic administration with AA had positive effects on attenuating the oxidative stress in pigeons.
Assaying the H/L ratio and the blood cortisol concentration are valuable biomarkers in avian exposed to stressors (Bale et al., 2020; Vicuña et al., 2015). Stress increases the number of heterophils and decreases the number of lymphocytes, leading to elevating the relative proportion of neutrophils to lymphocytes (Davis et al., 2008). Previous literature revealed that transportation increased fearfulness in birds, leading to an increase in the H/L ratio and serum level of cortisol in their body (Jayaprakash et al., 2016). The H/L ratio reflects of body's reaction to various stressful conditions, which is a reliable marker for assessing the well‐being and health status of birds (Huth & Archer, 2015).
The literature revealed that the ratio of H/L increase in chickens under various stressors, including feed restriction, heat stress, transportation stress and infectious diseases (Al‐Aqil et al., 2013; Najafi et al., 2015). According to Table 1, there was no significant difference in the ratio of H/L in various treatments at time zero (before exposure to transport stress), indicating birds did no expose to any stressful conditions before transit. The H/L ratio was higher in pigeons subjected to transport stress than Ctrl– group at 6, 24 and 72 h post‐transport, indicating that transport stress had unfavourable effects on pigeon's physiology system for three days after transit (Table 1; p < 0.05). These results confirmed that road transport stress increases the ratio of H/L in pigeons, which is consistent with previous studies stating the stress increases the ratio of H/L in birds (Minka & Ayo, 2008). AA supplementing with a dose of 16 g/100 L for 7 consecutive days led to a decrease in the H/L ratio of pigeons exposed to transport stress (Table 1; p < 0.05). In agreement with these results, Minka & Ayo (2010) showed that AA receiving reduced the H/L ratio in pullets exposed to transport stress. The reducing effect of AA on the ratio of H/L in pigeons may be related to its antioxidant properties (Minka & Ayo, 2010). Furthermore, Selvam et al. (2017) demonstrated that antioxidant compounds reduced the H/L ratio in broilers subjected to stress. Based on Table 1, transport stress increased the serum levels of cortisol in Ctrl+, 1DBS10 and 1DBS16 groups compared to other treatments at 6 h post‐transport stress. AA administration in the 3DBS16, 3DBS10, 7DBS16 and 7DBS10 groups led to a decrease in circulating level of cortisol at 6 h after transit. There were no significant differences between various groups in term cortisol at 24 and 72 h post‐transport (Table 1; p > 0.05). These findings are in line with results by Gou et al. (2020) that indicated transport stress increased significantly circulating cortisol in broilers. Saiz del Barrio et al. (2020) showed that AA supplementation reduces the plasma cortisol level in broiler chickens exposed to heat stress. Based on the current investigation, long‐term AA administration at dose 16 g/100 L reduced the ratio of H/L and circulating cortisol, indicating that AA could be attenuate the negative effects of stress induced by road transport in pigeons.
Various behavioural and fear responses are widely assessed as re‐label indicators of stress in birds, and tonic immobility reaction is one of the most common tests for stress assessment in birds (Mutibvu et al., 2017). Tonic immobility (TI) reaction is a useful behavioural measurement to estimate the levels of fearfulness and stress in birds (Edelaar et al., 2012). Jayaprakash et al. (2016) stated that transport stress led to a change in the TI reaction in broiler chickens. Several studies reported that tonic immobility reaction enhanced in birds exposed to fear and stressors events (Ghareeb et al., 2008). Stressful conditions activate the hypothalamic–pituitary–adrenal axis, causing the secretion of cortisol and finally lead to an increase in tonic immobility reactions (Myers et al., 2017). Circulating cortisol correlates positively with the duration of tonic immobility in chickens during stressors (Bedanova et al., 2007). Previous literature stated that AA controlled the activity of the fear centre in the bird's brain (Egbuniwe et al., 2015). Furthermore, Fletcher et al. (2021) showed that AA intake during stress situations increased the production of some neurotransmitters and norepinephrine, thereby controlling many activities of brain. According to the results in Table 3, all birds exposed to transport stress had a significantly higher TI duration than the Ctrl– at 3 and 24 h post‐transport. Among the groups receiving AA, the lowest TI duration was observed in the 7DBS10 and 7DBS16 groups at 3 and 24 h post‐transport (p < 0.05). At 72 h after transit, the 3HBS16 group had the highest significant TI time (p < 0.05), and no significant difference was observed between the other treatment and control groups in terms of TI duration (Table 3; p > 0.05). At 3 h after transportation, the lowest number of TI inductions was significantly observed in the Ctrl–. At 3 h post‐transit, groups that received low‐dose and high‐dose AA supplementation for 3 and 7 days had a decreased number of TI inductions among groups under stress (p < 0.05). At 72 h after transit, the Ctrl+ and 1DBS16 groups had the highest frequency of TI induction. The results of the current experiment are in line with Ghareeb et al.’s research (2008) showing transport increases the duration of TI reaction in broilers. Minka & Ayo (2010) also showed that intake of vitamins C and E reduced fear behaviours and TI duration in pullet exposed to transport stress in hot weather. Accordingly, it can be concluded that the AA administration at a dose of 16 g/100 L of drinking water for 3 days may decrease TI reaction by reducing oxidative stress and boosting the body's antioxidant systems in pigeon.
The rectal temperature (RT) as a physiological marker is another index for assessing stress in birds. Body temperature increased in birds under stressful conditions (Edelaar et al., 2012). Studies have shown that transport stress increases body temperature in birds (Minka & Ayo, 2008, 2010). Many studies reported that hyperthermia during transit is one of the most important causes of death in various birds (Minka & Ayo, 2008, 2010). So, controlling the body temperature in stressed birds is very important and vital. Previous researches showed that antioxidants decreased the body temperature in broilers subjected to stressors (Ghazi Harsini et al., 2012). According to Table 2, the RT had no significant difference between treatments before exposure to transit. The RT increased in pigeons exposed to transport stress compared to the Ctrl– at different times (immediately, 3 and 6 h) post‐transport. The short‐term and long‐term AA administration with two different doses significantly decreased the RT in pigeons subjected to stress (Table 2). In the present study, pigeons were transported during cool hours of the day (between 8 and 11 a.m.), indicating ambient temperature had no effect on increasing the pigeons’ body temperature. These findings are in agreement with the results of Gonzalez et al. (2007) reporting the body temperature increases in quails exposed to a 95 min of transport stress. Minka and Ayo (2013) showed an increase in body temperature in quails exposed to transport stress compared to non‐stressed birds. Moreover, they showed that administrating alpha‐tocopherol and AA reduced the body temperature of stressed birds. AA decreases the body temperature in stressed birds by inhibiting the secretion of prostaglandins (especially PGE) and cytokines (IL‐1 and IL‐6) (Hemilä, 2017). Minka et al. (2012) reported that AA is involved in maintaining body homeostasis and reducing body temperature in bird exposure to stressors. It may be suggested that prophylaxis administration with AA reduces the body temperature, possibly due to the enhanced antioxidative status in pigeons.
5. CONCLUSION
Transport stress in pigeons led to a decrease in serum levels of T‐AOC and an increase in the ratio of H/L and serum concentration of MDA and cortisol in pigeons subjected to transport stress. Furthermore, transport stress results in a rise in body temperature and TI reaction in Ctrl+. Prophylactic administration of AA at dose 16 g/100 L of drinking water from 3 and 7 days before exposure to transport stress improved oxidative stress in pigeons by decreasing MDA production and elevating serum levels of T‐AOC in 3DBS16 and 7DBS16 groups compared to Ctrl+. Furthermore, AA administration led to reducing the ratio of H/L, circulating cortisol, body temperature and TI reaction in pigeons in 3DBS16 and 7DBS16 groups compared to transported stressed‐birds without AA administration. Therefore, it could be suggested that AA administration at dose 16 g/100 L of drinking water from 3 and 7 days before exposure to stress helps attenuate undesirable effects of transport stress in pigeons.
ANIMAL ETHICAL STATEMENT
All protocols used in the study were approved by the Iranian Animal Ethics Standards under the supervision of the Iranian Society for the prevention of cruelty to animals and Shiraz University Research Council (IACUC no: 4687/63).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENT
The authors would like to appreciate Shiraz University, for financial support (grant number: 97GCU1M340866).
Hosseinian, S. A. , & Ansari, S. (2021). Prophylactic effects of dietary ascorbic acid on oxidative stress indices, physiological and behavioural responses of domestic pigeons exposed to road transport stress. Veterinary Medicine and Science, 7, 2389–2398. 10.1002/vms3.609
REFERENCES
- Abidin, Z , Khatoon, A. (2013). Heat stress in poultry and the beneficial effects of ascorbic acid (vitamin C) supplementation during periods of heat stress. World's Poultr Sci J. 69(1), 135–152. 10.1017/S0043933913000123 [DOI] [Google Scholar]
- Al‐Aqil, A. , Zulkifli, I. , Hair Bejo, M. , Sazili, A. Q. , Rajion, M. A. , Somchit, M. N. (2013). Changes in heat shock protein 70, blood parameters, and fear‐related behavior in broiler chickens as affected by pleasant and unpleasant human contact. Poultr Sci. 92(2013), 33–40. [DOI] [PubMed] [Google Scholar]
- Alm, M. , Tauson, R. , Holm, L. , Wichman, A. , Kalliokoski, O. , & Wall, H. (2016). Welfare indicators in laying hens in relation to nest exclusion. Poultr Sci. 95(6), 1238–1247. 10.3382/ps/pew100 [DOI] [PubMed] [Google Scholar]
- Bale, N.M. , Leon, A. E. , & Hawley, D. M. (2020). Differential house finch leukocyte profiles during experimental infection with Mycoplasma gallisepticum isolates of varying virulence. Avian Pathol. 49(4), 342–354. 10.1080/03079457.2020.1753652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedanova, I. , Voslarova, E. , Chloupek, P. , Pistekova, V. , Suchy, P. , Blahova, J. , Dobsikova, R. , & Vecerek, V. (2007). Stress in broilers resulting from shackling. Poultr Sci. 86(6), 1065–1069. 10.1093/ps/86.6.1065 [DOI] [PubMed] [Google Scholar]
- Davis, A. K. , Maney, D. L. , & Maerz, J. C. (2008). The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol. 22, 760–772. [Google Scholar]
- Edelaar, P. , Serrano, D. , Carrete, M. , Blas, J. , Potti, J. , & Tella, J. L. (2012). Tonic immobility is a measure of boldness toward predators: an application of Bayesian structural equation modeling. Behav Ecol. 23, 619–626. 10.1093/beheco/ars006 [DOI] [Google Scholar]
- Egbuniwe, I. , Ayo, J. , Kawu, M. , & Mohammed, A. (2015). Effects of betaine and ascorbic acid on tonic immobility, superoxide dismutase and glutathione peroxidase in broiler chickens during the hot‐dry season. J Veterin Behav: Clin Appl Res. 12, 60–65. 10.1016/j.jveb.2015.11.001 [DOI] [Google Scholar]
- El‐Senousey, H. K. , Chen, B. , Wang, J. Y. , Atta, A. M. , Mohamed, F. R. , & Nie, Q. H. (2018). Effects of dietary vitamin C, vitamin E, and alpha‐lipoic acid supplementation on the antioxidant defense system and immune‐related gene expression in broilers exposed to oxidative stress by dexamethasone. Poultr Sci. 97(1), 30–38, ISSN 0032‐5791. 10.3382/ps/pex298 [DOI] [PubMed] [Google Scholar]
- Emami, N. K. , Jung, U. , Voy, B. , & Dridi, S. (2021). Radical response: effects of heat stress‐induced oxidative stress on lipid metabolism in the avian liver. Antioxidants 10, 35. 10.3390/antiox10010035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etim, N. N. , Williams, M. E. , Evans, E. I. , & Offiong, E. E. A. (2013). Physiological and behavioural responses of farm animals to stress: implications to animal productivity. Am J Adv Agric Res. 1(2), 53–61. [Google Scholar]
- Fletcher, B. D. , Flett, J. A. M. , Wickham, S. , Pullar, J. M. , Vissers, M. C. M. , & Conner, T. S. (2021). Initial evidence of variation by ethnicity in the relationship between vitamin C status and mental states in young adults. Nutrients 13(3), 792. 10.3390/nu13030792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareeb, K. , Awod, W. A. , Nitsch, S. , Abdel‐Rahim, S. , & Bohm, J. (2008). Effect of transport on stress and fear responses of growing broilers supplemented with pre‐biotic and probiotic. Int J Poultr Sci. 7, 678–685. [Google Scholar]
- Ghazi Harsini, S. , Habibian, M. , Moeini, M. , & Abdolmohammadi, A. (2012). Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol Trace Elem Res. 148, 322–330. 10.1007/s12011-012-9374-0 [DOI] [PubMed] [Google Scholar]
- Gonzalez, V. A. , Rojas, G. E. , Aguilera, A. E. , Flores‐Peinado, S. S. , Lemus‐Flores, C. , Olmos‐Hernández, A. , Herrera, M. B. , Cardona‐Leija, A. , Alonso‐Spilsbury, M. , Ramírez, R. , & Mota‐Rojas, D. (2007). Effect of heat stress during transport and rest before slaughter, on the metabolic profile, blood gases and meat quality of quail. Int J Poultr Sci. 6(6), 397–402. 10.3923/ijps.2007.397.402 [DOI] [Google Scholar]
- Gou, Z. , Abouelezz, K. F. M. , Fan, Q. , Li, L. , Lin, X. , Wang, Y. , Cui, X. , Ye, J. , Masoud, M. A. , Jiang, S. , & Ma, X. (2020). Physiological effects of transport duration on stress biomarkers and meat quality of medium‐growing Yellow broiler chickens. Animal. 15(2), 100079. 10.1016/j.animal.2020.100079 [DOI] [PubMed] [Google Scholar]
- Gouda, A. , Amer, S. A. , Gabr, S. , & Tolba, S. A. (2020). Effect of dietary supplemental ascorbic acid and folic acid on the growth performance, redox status, and immune status of broiler chickens under heat stress. Trop Anim Health Prod. 52, 2987–2996. 10.1007/s11250-020-02316-4 [DOI] [PubMed] [Google Scholar]
- Guo, Q. , Li, F. , Duan, Y. , Wen, C. , Wang, W. , Zhang, L. , Huang, R. , & Yin, Y. (2020). Oxidative stress, nutritional antioxidants and beyond. Sci China Life Sci. 63, 866–874. 10.1007/s11427-019-9591-5 [DOI] [PubMed] [Google Scholar]
- Hemilä, H. (2017). Vitamin C and infections. Nutrients 9(4), 339. 10.3390/nu9040339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth, M. , & Babinszky, L. (2018). Impact of selected antioxidant vitamins (Vitamin A, E and C) and micro minerals (Zn, Se) on the antioxidant status and performance under high environmental temperature in poultry. A review. Acta Agriculturae Scandinavica, Section A—Anim Sci. 68(3), 152–160. 10.1080/09064702.2019.1611913 [DOI] [Google Scholar]
- Huth, J. C. , & Archer, G. S. (2015). Comparison of two LED light bulbs to a dimmable CFL and their effects on broiler chicken growth, stress, and fear. Poultr Sci. 94, 2027–2036. [DOI] [PubMed] [Google Scholar]
- Ito, F. , Sono, Y. , & Ito, T. (2019). Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants (Basel) 8(3), 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaprakash, G. , Sathiyabarathi, M. , Arokia Robert, M. , & Tamilmani, T. (2016).Transportation stress in broiler chickens. Int J Sci Environ Technol. 5, 806–809 [Google Scholar]
- Khan, R. , Naz, S. , Nikousefat, Z. , Selvaggi, M. , Laudadio, V. , & Tufarelli, V. (2012). Effect of ascorbic acid in heat‐stressed poultry. World's Poultr Sci J. 68(3), 477–490. 10.1017/S004393391200058x [DOI] [Google Scholar]
- Lee, S. G. , Wang, T. , Vance, T. M. , Hubert, P. , Kim, D. O. , Koo SI, & Chun, O. K. (2017). Validation of analytical methods for plasma total antioxidant capacity by comparing with urinary 8‐isoprostane level. J Microbiol Biotechnol. 27(2), 388–394. [DOI] [PubMed] [Google Scholar]
- Leskovec, J. , Levart, A. , Nemec Svete, A. , Perić, L. , Đukić Stojčić, M. , Žikić, D. , Salobir, J. , & Rezar, V. (2018). Effects of supplementation with α‐tocopherol, ascorbic acid, selenium, or their combination in linseed oil‐enriched diets on the oxidative status in broilers. Poultr Sci. 97(5), 1641–1650. 10.3382/ps/pey004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macan, M. , Kraljević, T. G. , & Raić‐Malić, S. (2019). Therapeutic perspective of vitamin C and its derivatives. Antioxidants 8(8), 247. 10.3390/antiox8080247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minka, N. S. , Adieza, A. A. , Hassan, F. B. , & Ayo, J. O. (2012). Effects of melatonin and transportation on rectal temperature, heterophil/lymphocyte ratio and behavior of Japanese male quails (Coturnix japonica). NY Sci J. 5: 52–59. [Google Scholar]
- Minka, N. S. , & Ayo, J. O. (2008). Haematology and behaviour of pullets transported by road and administered with ascorbic acid during the hot‐dry season. Res Veterin Sci. 85, 389–393. [DOI] [PubMed] [Google Scholar]
- Minka, N. S. , & Ayo, J. O. (2010). Behavioural and rectal temperature responses of Black Harco pullets administered vitamin C and E and transported by road during the hot‐dry season. J Vet Behav: Clin Appl Res. 5, 134–144. [Google Scholar]
- Minka, N. S. , & Ayo, J. O. (2013). Effects of antioxidants Vitamin E and C on ErythrocyteFragility, HaemoglobinIndex and colonic temperature of transported Japanese quails (Coturnixcoturnix japonica). J Veterinar Sci Technol. 4, 149. 10.4172/2157-7579.1000149 [DOI] [Google Scholar]
- Moloney, J. N. , & Cotter, T. G. (2018). ROS signalling in the biology of cancer. Semin Cell Dev Biol. 80, 50–64. [DOI] [PubMed] [Google Scholar]
- Mutibvu, T. , Chimonyo, M. , & Halimani, T. E. (2017). Tonic immobility, heterophil to lymphocyte ratio, and organ weights in slow‐growing chickens. J Appl Poultr Res. 26(2), 226–235. 10.3382/japr/pfw066 [DOI] [Google Scholar]
- Myers, B. , Scheimann, J. R. , Franco‐Villanueva, A. , & Herman, J. P. (2017). Ascending mechanisms of stress integration: implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci Biobehav Rev. 74(Pt B), 366–375. 10.1016/j.neubiorev.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi, P. , Zulkifli, I. , Soleimani, A. F. , & Kashiani, P. (2015). The effect of different degrees of feed restriction on heat shock protein 70, acute phase proteins, and other blood parameters in female broiler breeders. Poultr Sci. 94(2015), 2322–2329. [DOI] [PubMed] [Google Scholar]
- Pisoschi, M. , & Pop, A. (2015). The role of antioxidants in the chemistry of oxidative stress: a review. Euro J Med Chem. 97, 55–74. [DOI] [PubMed] [Google Scholar]
- Rao, P. S. , Kalva, S. , Yerramilli, A. , & Mamidi, S. (2011). Free radicals and tissue damage: role of antioxidants. Free Rad Antioxid. 1(4), 2–7. [Google Scholar]
- Rehman, Z. U. , Meng, C. , Sun, Y. , Safdar, A. , Pasha, R. H. , Munir, M. , & Ding, C. (2018). Oxidative stress in poultry: lessons from the viral infections. Oxidat Med Cell Longev. 2018, 14. 10.1155/2018/5123147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz del Barrio, A. , Mansilla, W. D. , Navarro‐Villa, A. , Mica, J. H. , Smeets, J. H. , den Hartog, L. A. , & García‐Ruiz, A. I. (2020). Effect of mineral and vitamin C mix on growth performance and blood corticosterone concentrations in heat‐stressed broilers. J Appl Poultr Res. 29(1), 23–33, ISSN 1056–6171. 10.1016/j.japr.2019.11.001 [DOI] [Google Scholar]
- Salami, S. A. , Majoka, M. A. , Saha, S. , Garber, A. , & Gabarrou, J‐F. (2015). Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: science and market. Avian Biol Res. 8(2), 65–78. 10.3184/175815515x14291701859483 [DOI] [Google Scholar]
- Scanes, C. G. (2016). Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poultr Sci. 95, 2208–2215. 10.3382/ps/pew137 [DOI] [PubMed] [Google Scholar]
- Scope, A. , Filip, T. , Gabler, C. , & Resch, F. (2002). The influence of stress from transport and handling on hematologic and clinical chemistry blood parameters of racing pigeons (Columba livia domestica). Avian Dis. 46(1), 224–229. [DOI] [PubMed] [Google Scholar]
- Selvam, R. , Saravanakumar, M. , Suresh, S. , Sureshbabu, G. , Sasikumar, M. , & Prashanth, D. (2017). Effect of vitamin E supplementation and high stocking density on the performance and stress parameters of broilers. Braz J Poultr Sci. 19(4), 587–594. 10.1590/1806-9061-2016-0417 [DOI] [Google Scholar]
- Şenay, S. , Islim, P. , & Tugay, A. (2019). Supplementation Of natural antioxidants to reduced crude protein diets for japanese quails exposed to heat stress. Braz J Poult Sci, Campinas 21(1), Erbca‐2019‐0694. 10.1590/1806-9061-2017-0694 [DOI] [Google Scholar]
- Shojadoost, B. , Yitbarek, A. , Alizadeh, M. , Kulkarni, R. R. , Astill, J. , Boodhoo, N. , & Sharif, S. (2021). Centennial review: effects of vitamins A, D, E, and C on the chicken immune system. Poultr Sci. 100(4), 100930. 10.1016/j.psj.2020.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai, P. F. , Kochish, I. I. , Fisinin, V. I. , & Kidd, M. T. (2019). Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants 8, 235. 10.3390/antiox8070235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalle, C. (2008). An easy and reliable automated method to estimate oxidative stress in the clinical setting. Meth Mol Biol. 477, 31–39. [DOI] [PubMed] [Google Scholar]
- Vicuña, E. A. , Kuttappan, V. A. , Galarza‐Seeber, R. , Latorre, J. D. , Faulkner, O. B. , Hargis, B. M. , Tellez, G. , & Bielke, L. R. (2015). Effect of dexamethasone in feed on intestinal permeability, differential white blood cell counts, and immune organs in broiler chicks. Poultr Sci. 94, 2075–2080 [DOI] [PubMed] [Google Scholar]
- Von Borell, E. H. (2001). The biology of stress and its application to livestock housing and transportation assessment. J Anim Sci. 79, E260–E267. 10.2527/jas2001.79E-SupplE260x [DOI] [Google Scholar]
- Whitehead, C. C. , & Keller, T. (2019). An update on ascorbic acid in poultry. World's Poultr Sci J. 59(2), 161–184. 10.1079/Wps20030010 [DOI] [Google Scholar]
- Zhang, C. , Wang, L. , Zhao, X. H. , Chen, X. Y. , Yang, L. , & Geng, Z. Y. (2017). Dietary resveratrol supplementation prevents transport‐stress‐impaired meat quality of broilers through maintaining muscle energy metabolism and antioxidant status. Poultr Sci. 96(7), 2219–2225. 10.3382/ps/pex004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Li, J. L. , Wang, X. F. , Zhu, X. D. , Gao, F. , & Zhou, G. H. (2019). Attenuating effects of guanidinoacetic acid on preslaughter transport‐induced muscle energy expenditure and rapid glycolysis of broilers. Poultr Sci. 98(8), 3223–3232, 10.3382/ps/pez052 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Yue, H. Y. , Wu, S. G. , Xu, L. , Zhang, H. J. , Yan, H. J. , Cao, Y. L. , Gong, Y. S. , & Qi, G. H. (2010). Transport stress in broilers. II. Superoxide production, adenosine phosphate concentrations, and mRNA levels of avian uncoupling protein, avian adenine nucleotide translocator, and avian peroxisome proliferator‐activated receptor‐gamma coactivator‐1alpha in skeletal muscles. Poultr Sci. 89, 393–400. [DOI] [PubMed] [Google Scholar]
- Zheng, A. , Lin, S. , Pirzado, S. A. , Chen, Z. , Chang, W. , Cai, H. , & Liu, G. (2020). Stress associated with simulated transport, changes serum biochemistry, postmortem muscle metabolism, and meat quality of broilers. Animals 10, 1442. 10.3390/ani10081442 [DOI] [PMC free article] [PubMed] [Google Scholar]


