Abstract
Food handlers regardless of whether preparing or serving food, play key roles in the transmission of food‐borne infections. This study aimed to evaluate the prevalence of intestinal parasitic infections in food handlers in Iran. In the present study, a comprehensive literature search was carried out in electronic databases, including PubMed, Scopus, Google Scholar, Science Direct, Magiran, Scientific Information Database (SID), Iran Medex and Iran Doc, to identify all the published studies from 2000 to 31st April 2019. A total of 25 articles from different regions of Iran were identified and fulfilled our eligibility criteria. Totally, 140,447 cases were examined and 1163 cases were infected with intestinal parasites. Of all cases, 19,516 were male and 5901 were female with 1163 and 652 infected cases, respectively. The overall prevalence of intestinal parasitic infections was evaluated 14.0% [95% CI: 11.0‐17.0%]. It is revealed that protozoan, such as Giardia lamblia, with prevalence of 41.0% [95% CI: 25.0‐59.0%], Blastosystis hominis with 28.0% [95% CI: 15.0‐44.0%] and Entamoeba coli with 22.0% [95% CI: 16.0‐29.0%] had the highest prevalence while, Dientamoeba fragilis 5.0% [95% CI: 4.0‐7.0%], Iodamoeba bütschlii 5.0% [95% CI: 2.0‐8.0%], Chilomastix mesnili 5.0% [95% CI: 2.0‐9.0%] and Endolimax nana with 3.0% [95% CI: 1.0‐7.0%], were less prevalent. Infection with Ascaris lumbricoides7.0% [95% CI: 0.0‐29.0%] was more prevalent helminth followed with Enterobius vermicularis 3.0% [95% CI: 1.0‐5.0%], Hymenolepis nana 2.0% [95% CI: 1.0‐3.0%], Taenia spp. 2.0% [95% CI: 0.0‐7.0%] and Trichuris trichiura 1.0% [95% CI: 0.0‐1.0%]. The high prevalence of commensal parasites, such as Entamoeba coli, which does not need cure is indicating the importance of personal hygiene in food handlers.
Our results revealed the high prevalence of intestinal parasitic infection in food handlers in Iran. Monitoring programs to prevent and controlling of transmission to individuals are needed.
Keywords: food handlers, intestinal parasites, Iran, meta‐analysis, systematic review
Food handlers regardless of whether preparing or serving food, play key roles in transmission of food‐borne infections. This study aimed to evaluate the prevalence of intestinal parasitic infections in food handlers.

1. INTRODUCTION
Intestinal parasitic infections are widespread in the world and transmitting directly or indirectly among populations (FeizHadad et al., 2017). In some cases, carriers without any symptoms of the disease are the main source of infection especially if they work as food handlers. Given the high prevalence of 48.4 million cases of parasitic infections in the world, this fact is not reality. The importance of this issue emerges when those people work as food handlers and do not care about personal hygiene (Saki et al., 2012; Torgerson et al., 2015).
Although people are in constant contact with environmental pathogens, including parasites, they are not affected seriously since immunity is important in disease aetiology. Despite the good toleration of parasitic infection in healthiest individuals, some people are vulnerable to parasites (FeizHadad et al., 2017). The importance of parasitic infection is highlighted when the infected individual plays a major role in food handling or food industries.
Iran is a suitable region for most parasitesˊ growth and distribution due to the geographic, socioeconomic and behavioural conditions. Serious efforts to control parasitic infection have resulted in a burden decrease of parasitic infections, but contamination with intestinal parasites is still a concern for health‐care services (Kusolsuk et al., 2011). Using animal and human faeces as fertilizers for agriculture and vegetable gardens, climatic conditions, traditions, and customs are considered the main reasons for the incidence of parasitic infections in some parts of the country. Direct transmission from person to person is another factor that complicates the parasite control programs. This kind of parasite transmission is markedly important in food handlers and particularly in oral‐faecal parasites such as Giardia lamblia (G. lamblia), Hymenolepis nana (H. nana) and Enterobius vermicularis (E. vermicularis) (Kusolsuk et al., 2011; Kheirandish et al., 2014). If food handlers do not care about personal hygiene, they can contaminate dishes, salads and other food materials which finally results in the contamination of the customers (Koohsar et al., 2012).
Studies on transmitted parasites by food handlers indicate that Entamoeba coli (E. coli) is the most common non‐pathogenic protozoa indicating a contamination with faecal materials and poor hygiene (Kassani et al., 2015). Also, zoonotic nature of some parasites, such as Entamoeba histolytica (E. histolytica), Cryptosporidium parvum (C. parvum), H. nana, Taenia saginata (T. saginata), Giardia lamblia, Iodamoeba butschlii (I. butschlii), Chilomastix mesnili (C. mesnili), Endolimax nana (E. nana) and Entamoeba coli (E. coli), makes the control programs challengeable. Among all mentioned zoonotic parasites, some are more important and cause more morbidities, including E. histolytica, C. parvum, T. saginata and G. lamblia and need more attention from both humans and animals. Although, there was a doubt about the pathogenic nature of some protozoan, such as Blastocystis hominis (B. hominis), in humans at present it is proven that they are associated with diarrhoea (Motazedian et al., 2016). Several studies have been conducted in different parts of the world regarding the prevalence of intestinal parasites in food handlers (Acilel et al., 2008; Abd Al‐Muhsin AL‐Khayat et al., 2017; Esparar et al., 2004; Kusolsuk et al., 2011; Wali et al., 2017). In this study, we performed a systematic review and meta‐analysis to find out the pooled estimate of the prevalence of intestinal parasites, such as G. lamblia, E. coli, B. hominis and H. nana, in food handlers, so the health‐care officials discovered the routes to prevent and control the disease transmitted by parasites and also, the best and most practical method used in conducting experiments to achieve the best results.
2. MATERIALS AND METHODS
This systematic review and meta‐analysis was conducted based on the guidelines of Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. The PROSPERO registration number is: CRD42019123662
2.1. Literature search and search strategy
In this meta‐analysis, a comprehensive literature search was carried out in electronic databases, including PubMed, Scopus, Google Scholar, Science Direct, Magiran, Scientific Information Database (SID), Iran Medex, and Iran Doc, to identify all the published studies from 2000 to 31st April 2019. Duplicates and studies out of Iran were excluded. All original descriptive studies (designated as cross‐sectional) about intestinal parasites in food handlers were concerned. The process is shown in Figure 1. The search was performed using terms: ‘intestinal parasites’, ‘parasitic infection’, ‘parasitic diseases’, ‘parasite’, ‘food handlers’, ‘prevalence’, alone or in combination, both in Persian and English languages.
FIGURE 1.
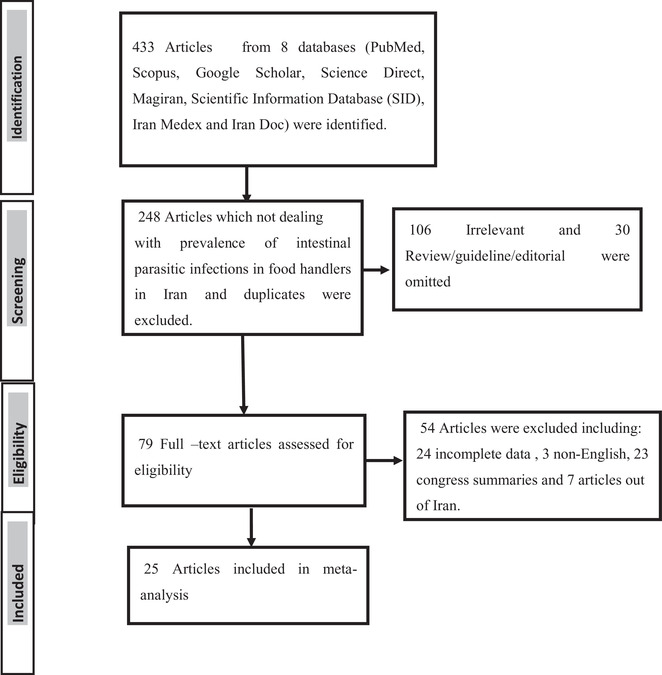
PRISMA flowchart describing the study design process
2.2. Data collection
In the initial search of collected bibliographic references, 433 articles were found. After removing duplicated, irrelevant studies and studies out of Iran, finally, 25 articles with epidemiological parameters of interest fulfilled the inclusion criteria. Those articles reporting the prevalence of intestinal parasitic infections in food handlers in Iran were included to our study (Table 1).
TABLE 1.
Baseline characteristics of included studies
| Ref | Author | Province | City | N. sample | N. positive | Infection rate (%) | Age group with highest infection | (%) Infection in age group | Male | Female | Laboratory diagnostic technique |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Balarak et al. (2016) | East Azarbaijan | Tabriz | 4612 | 156 | 3.73 | 20‐40 | 55.8 | 3966 | 646 | Formalin ether |
| 2 | Fallahizadeh et al. (2017) | Khozestan | Shush county | 15132 | 778 | 5.14 | 349 | 429 | Direct smear | ||
| 3 | Garedaghi et al. (2014) | East Azarbaijan | Tabriz | 100 | 72 | 72 | ND | ND | Direct smear, formalin ether | ||
| 4 | Hatami et al. (2018) | Tehran | Tehran | 4072 | 271 | 6.7 | 33.3 | 3122 | 950 | Direct smear, formalin ether | |
| 5 | Heydari Hengami et al. (2018) | Hormozgan | Bandarabbas | 800 | 279 | 34.9 | 40‐49 | 43.1 | 625 | 175 | Direct smear, formalin ether, staining |
| 6 | Saki et al. (2012) | Khuzestan | Khuzestan | 62007 | 20580 | 33.1 | ND | ND | Direct smear, formalin ether, staining | ||
| 7 | Kheirandish et al. (2014) | Lorestan | Khorramabad | 210 | 19 | 9 | 20‐40 | 31 | 184 | 26 | Direct smear, formalin ether, staining |
| 8 | Kheirandish et al. (2011) | Lorestan | Khorramabad | 816 | 96 | 11.9 | ND | ND | Direct smear, formalin ether, staining | ||
| 9 | Mohammadzadeh et al. (2018) | East Azarbaijan | Tabriz | 87 | 16 | 18.4 | >50 | 43.21 | 81 | 6 | Direct smear, formalin ether, staining |
| 10 | Motazedian et al. (2015) | Fars | shiraz | 1021 | 105 | 10.4 | 21‐30 | 48.1 | 577 | 444 | Direct smear, formalin ether |
| 11 | Neghab et al. (2006) | Shiraz | Shiraz | 39 | 23 | 59.4 | 37 | 2 | Direct smear, formalin ether | ||
| 12 | Sharif et al. (2015) | Mazandaran | Sari | 1041 | 161 | 30–39 | 84 | 620 | 421 | ||
| 13 | Amiri et al. (2013) | Khorasan | Shahroud | 801 | 75 | 16.2 | 35.7 | 535 | 266 | ||
| 14 | Khazan et al. (2014) | Mazandaran | Gonbad e kavus | 100 | 1 | ND | ND | Direct smear, formalin ether | |||
| 15 | Balarak et al. (2014) | Tehran | Qom | 2925 | 112 | 3.8 | 20‐40 | 50.8 | 2614 | 311 | Direct smear |
| 16 | Dargahi et al. (2016) | Tehran | Tehran | 109 | 69 | 63.3 | ND | ND | Direct smear, formalin ether | ||
| 17 | Asadi et al. (2011) | Khorasan | Neishabour | 8142 | 424 | 5.2 | ND | ND | Direct smear | ||
| 18 | Davami et al. (2006) | Markazi | Arak | 460 | 201 | 43.7 | 13‐50 | ND | 455 | 5 | Formalin ether |
| 19 | Salary et al. (2013) | Kerman | Kerman | 7748 | 5318 | 2430 | Direct smear | ||||
| 20 | Fallah et al. (2004) | Hamadan | Hamadan | 938 | 713 | 76 | ND | ND | Direct smear, formalin ether | ||
| 21 | Koohsar et al. (2012) | Golestan | Gorgan | 500 | 30 | 6 | 51‐60 | 11.8 | 398 | 102 | Direct smear, flotation |
| 22 | Haraty Nejad Torbati et al. (2011) | Khorasan | Rashtkhar | 9001 | 673 | 7.5 | ND | ND | ND | ND | Direct smear |
| 23 | Safi et al. (2012) | Ahvaz | Ahvaz | 14614 | 1693 | 10.1 | ND | ND | ND | ND | Direct smear, formalin ether |
| 24 | Safi et al. (2013) | Ahvaz | Ahvaz | 12444 | 632 | 4.5 | ND | ND | ND | ND | Direct smear, formalin ether |
| 25 | Babaei pouya et al. (2018) | Azarbaijan | Ardabil | 1000 | 26 | 3.1 | 31‐40 | 884 | 116 | Direct smear, formalin ether |
ND, Not defined.
2.3. Data extraction
Two authors screened the titles, abstracts and full text of literatures, independently. Any disagreements between two reviewers were resolved by discussion among researchers. Extracted data included first author name, the year of publication, prevalence rate, demographic information (age and gender), geographical region of study, diagnostic test, sample size (number of examined people), and the number of infected cases (Table 1).
2.4. Quality of study
To assess the quality of observational studies included in this meta‐analysis using a checklist as in Table 1. It contains 12 items with scores ‘Yes = 1’ and ‘No = 0’. The sum of scores is 0 to 12 and for including study in meta‐analysis a quality score of at least 8 is required.
2.5. Statistical analysis
After extracting the sample size and the number of positive infections for each study, the proportion of infection and standard error (SE) were computed. Before estimating pooled effect size, sensitivity analysis was used to explore the effect of each study on pooled effect size. Heterogeneity among studies assessed using both Q‐test which is suggested by the Cochrane Handbook (p < 0.1 as substantial heterogeneity) and I‐square index I 2 < 50%, as substantial heterogeneity). If we found substantial heterogeneity, sub‐group meta‐analysis (fixed or random effect model) was performed to compute the pooled prevalence of infection based on a characteristic such as sex, country, education, pathogenicity and parasite species. In addition to meta‐regression examined to find the source of heterogeneity. To detect sources of heterogeneity, we performed meta‐regression on publish year and sample size of studies.
To evaluate publication bias, we aided a funnel plot and egger's test as a statistical test (p < 0.1 as significant). If we detected a substantial publication bias, the trim and fill method was applied to estimate and adjust for the number of missing studies (due to publication bias) in a meta‐analysis (Ebrahim, 2006). All statistical analysis was performed by using Stata/MP software (version 14.0, College Station, TX, USA).
3. RESULTS
Among all searched databases (eight databases) and unpublished data from 2000 to 2019 (19 years), 25 articles were eligible to include in this systematic review and meta‐analysis. The literature searches and selection process are shown in Figure 1. Totally 1,40,447 cases were examined. As all studies did not define the gender of studied cases, in studies that defined the gender of participants, a number of 19,516 cases were male and 5901 cases were female with 1163 (13.0%) infected cases in males and 652 (8.0%) infected in females, respectively (Table 1). There was a significant difference between infection among males 13.0% (10.0‐15.0%) and females 8.0% (5.0‐11.0%) (p = 0.027) (Figure 5).
FIGURE 5.
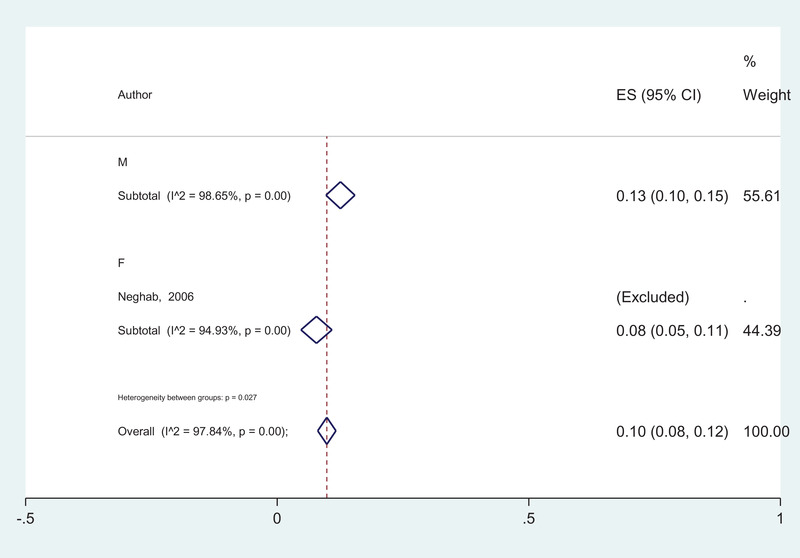
The forest plot of pooled prevalence of intestinal parasitic infection according to the gender
To evaluate the effect of each study on the pooled estimate of prevalence, by repeating the meta‐analysis after omitting each study, the sensitivity of studies was depicted in Figure 2. All effect sizes of 25 studies were located in 95% confidence interval (95% CI). Therefore, none of the studies substantially affected the pooled prevalence of intestinal infection and we can include all studies in the meta‐analysis (Figure 2).
FIGURE 2.
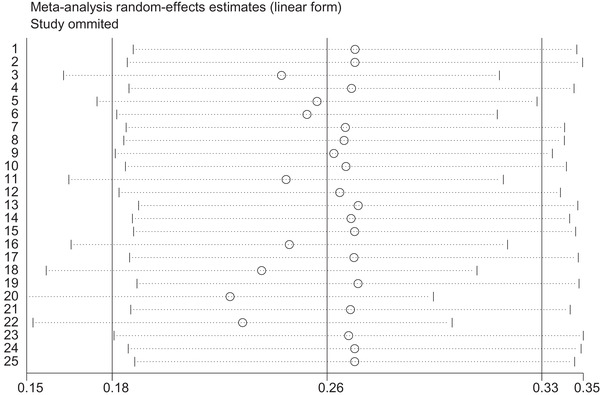
Sensitivity analysis to assess effect of each study on pooled effect size by omitting each study
The results of Egger's test showed that there is no evidence of publication bias among studies on species of the parasite (p > 0.1). Also, there were not enough studies for assessing publication bias for D. fragilis and T. trichiura (Table 2).
TABLE 2.
Comparison of the pooled frequency of infection among four parasite species
| Characteristics | Levels | Sample | Prevalence (95% CI) | I2 (%) | p |
|---|---|---|---|---|---|
| Gender | Male | 14 | 29.0 (9.0‐38.0) | 97.7 | 0.39 |
| Female | 11 | 24.0 (18.0‐42.0) | 89.2 | ||
| Age | 25 | 22.0 (14.0‐32.0) | 99.7 | 0.65 | |
| 7 | 29.0 (6.0‐60.0) | 99.2 | |||
| 13 | 24.0 (9.0‐43.0) | 99.1 |
*The sample size was small for estimated pooled prevalence.
The overall prevalence of intestinal parasitic infections in food handlers in Iran was evaluated 14.0% (95% CI: 11.0‐17.0%). According to the results of sub‐group analysis, G. lamblia, with prevalence of 41.0% (95% CI: 25.0‐59.0%), B. hominis with 28.0% (95% CI: 15.0‐44.0%) and E. coli with 22.0% (95% CI: 16.0‐29.0%), had the highest prevalence, respectively. Also, other species had the prevalence between 1.0% (T. trichiura) to 9.0% (E. histolytica/dispar) (Figure 3).
FIGURE 3.
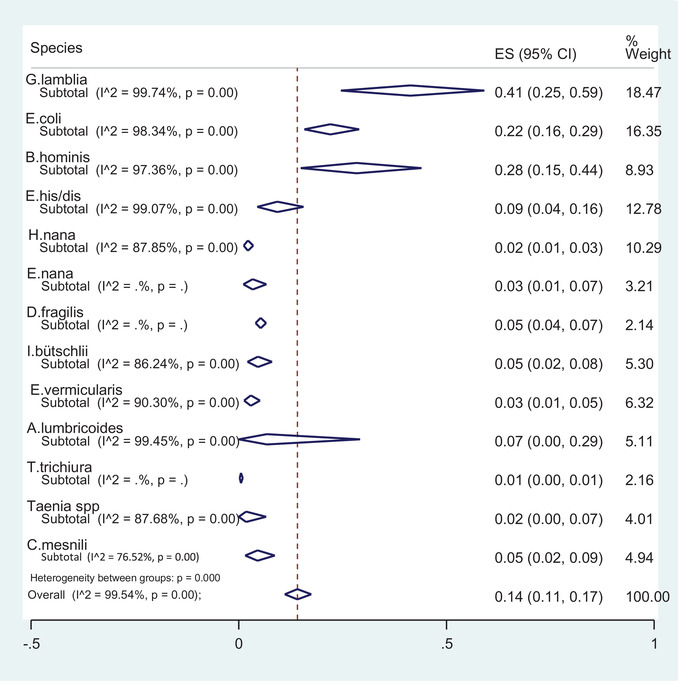
The forest plot of Intestinal parasites in food handlers in Iran
The sub‐group analysis for intestinal protozoan parasites revealed the prevalence of D. fragilis 5.0% [95% CI: 4.0‐7.0%], I. bütschlii 5.0% [95% CI: 2.0%‐8.0%], C. mesnili 5.0% (95% CI: 2.0‐9.0%) and E. nana 3.0% (95% CI: 1.0‐7.0%). The results for intestinal helminthic infections showed that A. lumbricoides with prevalence of 7.0% (95% CI: 0.0‐29.0%) had the highest prevalence and then E. vermicularis with infection rate of 3.0% (95% CI: 1.0‐5.0%), H. nana with 2.0% (95% CI:1.0‐3.0%), Taenia spp. with 2.0% (95% CI: 0.0‐7.0%] and T. trichiura 1.0% [95% CI: 0.0‐1.0%] were the most prevalent intestinal helminthic infections (Figure 3). In this review, some of the parasites were non‐pathogenic (Tables 3,4).
TABLE 3.
The results of examine publication bias for each parasite species
| Species | N | bias | p * |
|---|---|---|---|
| Giardia lamblia | 20 | 0.99 | 0.11 |
| Entamoeba coli | 17 | −0.8 | 0.37 |
| Blastocystise hominis | 8 | 0.97 | 0.68 |
| Entamoeba hitolytica/dispar | 12 | 1.29 | 0.194 |
| Hymenolepis nana | 12 | −0.15 | 0.37 |
| Endolimax nana | 3 | −0.53 | 0.76 |
| Dientamoeba fragilis | 2 | 3.14 | SS |
| Iodamoeba butschlii | 5 | −1.03 | 0.22 |
| Enterobius vermicularis | 5 | 0.35 | 0.78 |
| Ascaris lumbricoides | 7 | −3.07 | 0.37 |
| Trichuris trichiura | 2 | −1.86 | SS |
| Taenia saginata | 4 | 0.31 | 0.39 |
| Chilomastix mesnili | 5 | 0.69 | 0.15 |
SS, Small sample size.
Results of Egger’ test.
TABLE 4.
Intestinal parasitic infections in food handlers
| Type of Parasite: No(%) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | No of Cases/No of infected Cases (%) | Job title | Most infected group No (%) | E. coli | G. lambia | Blasto | Chilo | E. his | E. hart | Ioda | E. nana | D. fra | H. nana | As | Oxy | Trich | Others | |
| 1 | Fallah et al. (2004) |
938/713 (76) |
Food industry worker | ND |
422 (45) |
84 (9) |
32 (3.4) |
136 (14.5) |
94 (10) |
84 (9) |
40 (4.3) |
35 (3.7) |
11 (1.1) |
363 (38.7) |
38 (20.3) |
2 (0.2) |
||
| 2 | Neghab et al. (2006) |
39/24 (61.5) |
Catering staff | ND |
6 (2.3) |
3 (1.2) |
10 (3.9) |
3 (1.2) |
0 | |||||||||
| 3 | Davami et al. (2006) |
460/201 (43.7) |
Food industry worker | Bakery workers |
79 (17.2) |
29 (6.3) |
5 (1.1) |
|||||||||||
| 4 | Kheirandish et al. (2011) |
816/97 (11.9) |
Bakery workers |
Bakery workers 97 (11.9%) |
45 (5.5) |
35 (3.7) |
17 (2.1) |
1 (0.1) |
||||||||||
| 5 | Kohsar et al. (2011) |
500/30 (6) |
Food deliverers |
Butchers 8(25%) |
5 (1) |
17 (3.4) |
3 (0.6) |
|||||||||||
| 6 | Haraty Nejad Torbati et al. (2011) |
729/55 (7.5) |
Food industry worker | ND |
47 (6.7) |
376 (55.9) |
3 (0.4) |
47 (7) |
201 (30) |
|||||||||
| 7 | Asadi et al. (2011) |
8142/423 (5.2) |
Food industry worker | ND |
263 (3.2) |
81 (1) |
2 (0.25) |
151 (1.9) |
||||||||||
| 8 | saki et al. (2012) |
62007/4830 (7.8) |
Food handlers | Food handlers |
5643 (9.1) |
2804 (4.52) |
7019 (11.32) |
˂(0.5) |
865 (1.39) |
3100 (5) |
802 (1.29) |
359 (0.57) |
˂(0.5) | ˂(0.5) | ||||
| 9 | Safi et al. (2012) |
14614/1693 (11.6) |
Food industry worker | ND |
128 (7.58) |
1445 (85.35) |
31 (1.83) |
60 (3.54) |
20 (1.71) |
|||||||||
| 10 | Safi et al. (2013) |
12444/632 (5.1) |
Food industry worker | ND |
33 (5.86) |
510 (80.69) |
20 (3.17) |
46 (7.28) |
19 (3.01) |
|||||||||
| 11 | Salary et al. (2013) |
7748/93 (1.2) |
Food industry worker |
Supermarket Owners (1.2) |
96 (1.2) |
|||||||||||||
| 12 | Garedaghi et al. (2014) |
100 1 (1) |
Restaurant workers | ND | (16.66) | (36.11) | (47.22) | |||||||||||
| 13 | Amiri et al. (2014) |
801/141 (17.6) |
Food industry worker | ND |
74 (9.2) |
35 (4.4) |
7(0.9) |
12 (1.5) |
1 (0.1) |
1 (0.1) |
1 (0.1) |
1 (0.1) |
||||||
| 14 | Khazan et al. (2014) |
100/1 (1) |
Food sellerts | ND | 1 (1) | |||||||||||||
| 15 | Kheirandish et al. (2014) |
210/19 (9) |
Food industry worker | ND |
8 (4.3) |
7 (2.9) |
3 (1.4) |
1 (0.5) | ||||||||||
| 16 | Motazedian et al. (2015) |
1021/106 (10.4) |
Food handlers |
Herbal sellers 25 (16) |
40 (37.7) |
27 (25.5) |
40 (37.7) |
5 (4.7) |
7 (6.6) |
1 (0.9) |
||||||||
| 17 | Balarak et al. (2015) |
2925/112 (3.8) |
Food industry worker |
Restaurant 16 (14.5) Supermarket Workers 25 (22.3) |
22 (19.6) |
74 (66) |
4 (3.6) |
7 (6.3) |
||||||||||
| 18 | Sharif et al. (2015) |
1041 161/(15.5) |
Food industry worker |
Restaurant 38 (19.2) Fast food worker 33 (17.8) |
25 (15.5) |
86 (53.4) |
29 (18) |
9 (5.6) |
5 (3.1) |
30 (18.6) |
5 (3.1) |
|||||||
| 19 | Dargahi et al. (2016) |
109/69 (63.3) |
Restaurant workers | ND | 69 | |||||||||||||
| 20 | Balarak et al. (2016) |
4612/172 (3.7) |
Food industry worker |
Supermarket workers 38 (22.1) Fast food worker 33 (19.2) |
38 (22) |
109 (63) |
9 (5.2) |
6 (3.5) |
10 (5.8) |
|||||||||
| 22 | Hatami et al. (2018) |
4072 271 (6.6) |
Food handlers | ND |
72 (26.6) |
148 (54.6) |
21 (7.7) |
19 (7) |
1 (0.3) |
11 (3.7) |
||||||||
| 23 | Heydari Hengami et al. (2018) |
800/279 (34.9) |
Food handlers |
Restaurant worker 58 (33.9) Supermarket worker 52 (34.7) |
64 (22.9) |
54 (19.4) |
194 (69.5) |
7 (2.2) |
8 (2.9) |
2 (0.4) |
34 (12.2) |
2 (0.4) |
||||||
| 24 | Mohammadzadeh et al. (2018) |
87/16 (18.4) |
Food handlers | Chef 2 (66.7) |
5 (19.2) |
5 (19.2) |
5 (19.2) |
9 (34.6) |
1 (3.8) |
1 (3.8) |
||||||||
| 25 | Babaei pouya et al. (2018) |
1000/31 (3.1) |
Food handlers | Restaurant workers 12 (38.7) |
8 (0.8) |
14 (1.4) |
6 (0.6) |
2 (0.2) |
1 (0.1) |
|||||||||
ND, not defined; H. nana, Hymenolepis nana; E.coli, Entamoeba coli; G.lamb, Giardia lambelia; Blasto, Blastocystis hominis; Chilo, Chilomastix mesnili; E.his, Enatamoeba histolytica/dispar; E.hart, Entamoeba hartmani; Ioda, Iodamoeba butschlii; E.nana, Endolimax nana; D.fra, Dientamoeba fragilis.
AS, Ascaris lumbericoides; Oxy, Oxyuris vermicularis; Trich, Trichuris trichiura.
The highest rate of infection was found in owners of the school cafeterias with 28.0% followed by 11.50% in butchers and 10.20% among bakeries. The lowest infection rate was 1.70% in confectioners (Tables 3, 5). The results of meta‐regression showed that the prevalence of intestinal parasitic infection in food handlers has significantly decreased in recent years (p = 0.01). Also, our analysis revealed that sample size did not affect the prevalence of intestinal parasitic infection in food handlers (p = 0.68). To evaluate the effect of each study on the pooled prevalence, by meta‐analysis, the sensitivity of studies is shown in Figure 2. At the first level, a fixed‐effect meta‐analysis was performed on 25 included studies and results revealed considerable heterogeneity (Iˆ 2 = 99.40%, p < 0.001). In sub‐group analysis, a random effect model was performed on parasite species (Figure 3). All effect sizes of 25 studies were located with 95% interval confidence. Therefore, studies did not affect the pooled prevalence of intestinal infections in food handlers and we can include all studies in the meta‐analysis (Figure 2).
TABLE 5.
Distribution of intestinal parasitic infection in different jobs
| Job title | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Bakery No cases/Inf | Supermarket owner No cases/Inf | Restaurant/fast food workers No cases/Inf | Butcher No cases/Inf | Coffee shop owner No cases/Inf | Office servant No cases/Inf | Food Factory workers No cases/Inf | School cafeteria No cases/Inf | Confectioner No cases/Inf | |
| 1 | Balarak et al. (2016) | 274/9 | 880/38 | 821/24 | 95/4 | 229/33 | 889/13 | |||
| 2 | Fallahizadeh et al. (2017) | |||||||||
| 3 | Garedaghi et al. (2014) | |||||||||
| 4 | Hatami et al. (2018) | |||||||||
| 5 | Heydari Hengami et al. (2018) | 81/44 | 150/52 | 233/80 | 33/9 | 86/23 | 161/48 | 56/23 | ||
| 6 | Saki et al. (2012) | |||||||||
| 7 | Kheirandish et al. (2014) | |||||||||
| 8 | Kheirandish et al. (2011) | 816/97 | ||||||||
| 9 | Mohammadzadeh et al. (2018) | 87/16 | ||||||||
| 10 | Motazedian et al. (2015) | 28/3 | 125/15 | 244/27 | 48/6 | 80/3 | 163/21 | 46/2 | ||
| 11 | Neghab et al. (2006) | |||||||||
| 12 | Sharif et al. (2015) | 112/9 | 383/71 | 204/27 | 36/3 | 18/5 | ||||
| 13 | Amiri (2014) | |||||||||
| 14 | Khazan et al. (2014) | |||||||||
| 15 | Balarak (2015) | 172/6 | 533/25 | 954/36 | 48/3 | 207/14 | 623/9 | 130/5 | ||
| 16 | Dargahi et al. (2016) | |||||||||
| 17 | Asadi (2011) | |||||||||
| 18 | Davami et al. (2006) | |||||||||
| 19 | Salary et al. (2013) | 2256/35 | 1709/28 | 2161/21 | 673/7 | |||||
| 20 | Fallah (2005) | |||||||||
| 21 | Kohsar (2011) | 123/3 | 181/12 | 92/8 | 8/6 | 20/3 | 13/0 | 63/2 | ||
| 22 | Haraty Nejad Torbati et al. (2011) | |||||||||
| 23 | Safi (2012) | |||||||||
| 24 | Safi (2013) | |||||||||
| 25 | Babaei pouya (2018) | 144/7 | 125/2 | 136/12 | 68/3 | 66/1 | ||||
4. DISCUSSION
Food‐borne parasitic diseases are one of the main public‐health concerns all around the world which may lead to morbidity and mortality in developing countries (Simsek et al., 2009). The importance of hygienic food preparation and delivery reveals the importance of personal sanitation and education in food handlers. This group of people is involved in handling, storage, transportation, process and preparation of food on several levels for other peoples. This systematic review and meta‐analysis aimed to evaluate the prevalence of intestinal parasitic infections in food handlers in Iran during 19 years (from 2000 to 2019). The results of the meta‐analysis revealed the overall prevalence of intestinal parasitic infections was 14.0% [95% CI: 11.0‐17.0%] in food handlers in Iran. The results indicated poor health and inadequate personal hygiene in food handlers who are involved in food‐producing and food‐serving processes in Iran. The highest rate (72.0%) of infection was reported in a study carried out in East Azarbaijan by Garedaghi et al. (2014); Dargahi et al. (2016) who reported the rate of 59.4% in Tehran province. The lowest prevalence of infection (1.0%) was reported from Mazandaran province by Khazan et al. (2014) (Table 1). The sub‐group analysis revealed that G. lamblia with the prevalence of 41.0% [95% CI: 25.0‐59.0%], B. hominis, with 28% [95% CI: 15.0‐44.0%] and E. coli with 22.0% [95% CI: 16.0‐29.0%], had the highest prevalence among all intestinal parasites in food handlers in Iran. Although we know that E. coli is a non‐pathogenic parasite and the infection only reflects personal and public health condition but, it is considerable in persons who are working as food handlers.
The highest rate of infection (28.0%) was achieved in owners of school snack bars, where children took cooked food and snacks. The results may have a bias for a small sample size, but the important point in this regard is that 5 of 18 different school cafeteria owners were infected with intestinal parasites which are significant. This may have resulted from weak health controlling programs in schools. In a study carried out by Costa‐ Cruz et al. in Brazil, the researchers studied 20 schools for the evaluation of intestinal parasitic infections in school food handlers. They found that 49 of 104 (47.10%) of school food handlers were infected (Khazan et al., 2013). Comparing their findings with ours indicates the higher rate of infection in their studied subjects. The meta‐analysis revealed the high prevalence of intestinal parasitic infection in butchers (11.50%) and backers (10.20%). These two groups play an important role in public food health. Interestingly, the lowest prevalence of intestinal parasitic infection rate was observed among confectioners (1.70%). Although the sample size comprised 978 cases and relatively big, the results indicate appropriate personal hygiene in this group which is regularly monitored by the health‐care system.
Also, our meta‐analysis revealed the infection rate in males (13%) was significantly higher than females (8%) which may be resulted from a smaller sample size in females and less involvement of females in food‐handling processes than males in Iran. In some countries, the ratio of male to female was different from ours. In a study in Thailand in 2011, Kusolsuk et al., studied 219 females and 47 males. This has resulted from the great role of females in food preparing and handling in Thailand. The result of their study revealed that the infection rate in 273 food handlers was 10.30% which is higher than our results when compared with the infection rate of 14.0% in 1,40,447 subjects in our study. In contrast with our results, the most infecting cases were found with hookworms (70%) while our most prevalent helminthic infection was with H. nana worms (Kusolsuk et al., 2013). Their results revealed insufficient hygiene in food preparation and our results indicated inappropriate personal hygiene. Our meta‐analysis showed that the highest intestinal infection in food handlers was caused by protozoan parasites and the most frequent parasite (41.0%) was G. lamblia (Figure 3). These protozoa are among the most pathogenic parasites (Arora, 2015) which can cause acute or chronic diarrhoea with or without clinical signs. The parasite can be transmitted directly from infected persons to healthy individuals. Therefore, eradication and controlling this parasite is very difficult. It is estimated that 200 million people in Asia, Latin America and Africa suffering from giardiasis (Abd Al‐Muhsin AL‐Khayat et al., 2017). In a study carried out by Simsek et al. in 2009 in Turkey, intestinal parasitic infection was evaluated in 299 food handlers from Sanliurfa, Southeastern Anatolia. The results showed that 52.20% of food handlers were infected with intestinal parasites and most of them (26.80%) were infected with G. lamblia, followed by A. lumbricoides (10.70%) and T. saginata (10.0%). Also, 13.30% of them were infected with both Staphylococcus aureus and intestinal parasites. Unlike our results, the infection rate with G. lamblia in their study was higher.
The meta‐analysis elucidated that the prevalence of intestinal parasitic infection in individuals with education level lower than high school, was 20.0% [95% CI:9.0‐34.0%] while in individuals with education level between high school to the bachelor of science level, was 16.0% [95% CI:7.0‐28.0%] and in cases with education higher than bachelor of science level was reduced to 12.0% [95% CI: 2.0‐28.0%] but, there found no statistically significant difference (Z = 0.41, p = 0.82) (Figure 4). Although the results indicated no association between intestinal parasitic infection and educational levels but, it seems that the infection rate in individuals with lower levels is higher than those with higher educational levels. It seems that food hygiene knowledge, attitudes and practices in food handlers play an important role in the prevention of food contamination with intestinal parasites. In a study designed by Acikel et al. in 2008, a total of 83 food handlers in the kitchen were evaluated with questionnaires for their information and behaviours before and after training. The results indicated a significant difference in behavioural practices, and the researchers concluded that education has an important impact on decreasing the infection rate in food handlers. Although the researchers studied the decreased bacterial density, it can be extended in parasitic infections too as the way of transmission is almost the same (Acikel et al., 2008). In a study by Kheirandish et al., in 2011, out of 816 bakery workers with health certificates, 630 individuals knew about intestinal parasitic infections and the ways of transmission but, 78 (12.30%) of them were infected with intestinal parasites. Also, 186 (22.80%) of this population had no knowledge in this regard and 19 (10.20%) individuals were infected among them. These researchers declared that 85% of intestinal parasitic infections were observed in people who did not attend hygiene training programs. This shows that training to upgrade personal information in parasite transmission is necessary for all food handlers. Also, training hygiene can affect the improvement of society's health (Kheirandish et al., 2011).
FIGURE 4.
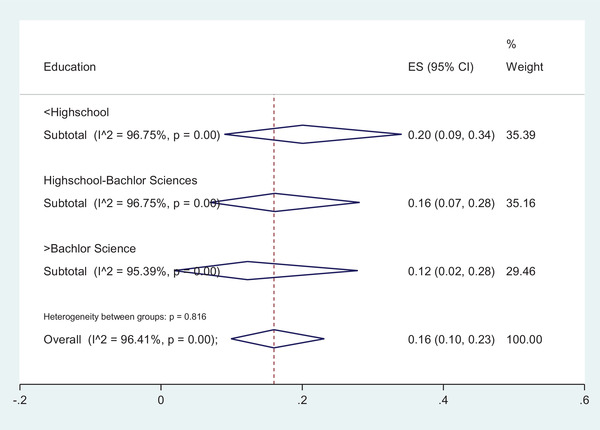
The forest plot of pooled prevalence of intestinal parasitic infection according to the educational levels
5. CONCLUSIONS
Our results revealed the high prevalence of intestinal parasitic infection in food handlers in Iran. This high prevalence is largely due to poor personal hygiene practice, poverty, lack of knowledge, insufficient environmental sanitation and inadequate health controlling services. Although the food industry workers, food handlers, and anyone who is connected with the production, handling, storage, transportation, preparation, or else, is obliged to undergo routine medical examinations including stool microscopy for intestinal parasitic infections (once every 6 months) but, it seems that they are not sufficient. It is advised that some strict rules such as obligation in filling the stool container in the lab should be added. Also, if infected food handler cases are identified, immediate decisions for the exclusion of the career up the resolving all symptoms or completion of further investigations should be made. Additional programs, including education for changing attitude about infectious diseases requires more consideration.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ETHICS APPROVAL
No ethical approval was required as this is a review article with no original research data.
AUTHOR CONTRIBUTION
K. S.S. and M. H.H. were involved in data gathering. A. S. was Project administration and Supervisor; involved in writing‐review & editing data and critical revise. S. H. T. was involved in methodology; data validation; formal data analysis and critical revise.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.590
Sharifi‐Sarasiabi, K. , Heydari‐Hengami, M. , Shokri, A. , & HosseyniTeshnizi, S. (2021). Prevalence of intestinal parasitic infection in food handlers of Iran: A systematic review and meta‐analysis. Veterinary Medicine and Science, 7, 2450–2462. 10.1002/vms3.590
Contributor Information
Azar Shokri, Email: azar_sh1969@yahoo.com.
Saeed HosseyniTeshnizi, Email: saeed.teshnizi@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Acikel, C. H. , Ogur, R. , Yaren, H. , Gocgeldi, E. , Ucar, M. , & Kir, T. (2008). The hygiene training of food handlers at a teaching hospital. Food Control, 19, 186–190. [Google Scholar]
- Abd Al‐Muhsin AL‐Khayat, F. , Jameel, S. K. , & Wali, M. H. (2017). Public health importance of some common intestinal protozoa in food handler in Baghdad. Intestinal Protozoa in Food Handler in Baghdad, 8 (1), 26–34. [Google Scholar]
- Amiri, M. , Nazemi, S. , Raei, M. , Chaman, R. , & Norouzi, P. (2013). A comparison of direct technique and formalin‐ether method in determining parasitic infection among health‐card applicants in Shahroud City. Medical Laboratory Journal, 7(3), 69–74. [paper in Persian]. [Google Scholar]
- Arora, D. R. (2015). Medical Parasitology, 2nd ed. India, New Delhi: CBS Publishers & Distributors Pvt Ltd, ISBN‐10: 8123911874 [Google Scholar]
- Asadi, B. , Ahi, A. (2011). Survey of these diseases ‐ intestinal Artisans and food preparation and distribution centers and public places in the city Neishabour during year 1389. Congress, paper, Yazd, Iran [paper in Persian].
- Babaei Pouya, N. , Akhlaghi, L. , & Razmjou, E. (2018). Epidemiology of intestinal parasites among applicants receiving Health Card of Ardabil City in 2014. Journal of Health, 9(1), 115–123. [Google Scholar]
- Balarak, D. , Jafari Modrek, M. , & Ansari, H. (2014). Prevalence of intestinal parasites among the food handlers in the city of Qom, 2014. Salamat Jamea, 8 (4), 20–28. [Google Scholar]
- Balarak, D. , Modrek, M. J. , Bazrafshan, E. , Ansari, H. , & Kord Mostafapour, F. (2016). Prevalence of intestinal parasitic infection among food handlers in Northwest Iran. Journal of Parasitology Research. 10.1155/2016/8461965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargahi, D. , Zarebavani, M. , Einollahi, N. , Dashti, N. , Rezaian, M. , & Abbasi, S. (2016). Prevalence of Giardia lamblia among food handlers and day‐care workers in Tehran. Payavard‐Salamat, 10(7), 402–408. [Article in Persian] [Google Scholar]
- Davami, M. H. , Kazaii, M. R. , Rfii, M. , & Milanii, M. (2006). An investigation on the prevalence of intestinal parasitic infections in food handlers in Arak (Iran) during 2002–2003. Journal of Jahrom University of Medical Sciences, 3(3), 1–15. [Article in Persian] [Google Scholar]
- Ebrahim, G. (2006). In H. R. R, Sutton A. J., Borenstein M., (eds). Publication bias in Meta‐Analysis: Prevention, assessment and adjustments. Chichester, John Wiley & Sons Ltd, ISBN 0–470–870–141£ 55, Oxford University Press [Google Scholar]
- Esparar, D. , Belizario, V. Y. , & Relos, J. (2004). Prevalence of intestinal parasitic infections among food‐handlers of a tertiary hospital in Manila using direct fecal smear and formalin ether concentration technique. Philippine Journal of Microbiology and Infectious Diseases, 33(3), 99–103. [Google Scholar]
- Fallahizadeh, S. , Feiz‐Haddad, M. H. , Kazemi, F. , & Afrisham, R. (2017). Prevalence of intestinal parasitic infections in Shush County, Southwest of Iran during 2014–2016. International Journal of Infection, 4(3), e14588. [Google Scholar]
- Fallah, M. , Sadeghyan, S. , Therkhani, H. , Habibi, F. , & Heydar Barghy, Z. (2004). An investigation on the prevalence of intestinal parasitic infections in food preparation and distribution centers and public places in the city Hamedan. Journal of Research in Health Sciences, 1(7), 3–10. [Article in Persian] [Google Scholar]
- Feiz Haddad, M. H. , Maraghi, S. , Ali, S. A. , Feiz Haddad, R. , & Nasser Zadeh, R. (2017). Intestinal parasitic infections frequency in referredpatients to a large teaching hospital, Khuzestan, Southwest, Iran. Tropical Biomedicine, 35(4), 915–925. [PubMed] [Google Scholar]
- Garedaghi, Y. , & Firouzivand, Y. (2014). Protozoan Infections of Restaurant Workers in Tabriz, Iran. Crescent Journal of Medical and Biological Sciences, 1(2), 46–48.Available at https://www.cjmb.org [Google Scholar]
- Haraty Nejad Torbati, A. R. & Khanjani, N. (2011). Epidemiological study of intestinal parasites in managers of food preparation, distribution and sales centers and public places from the beginning of 2008 to the end of 2010 in Rashtkhar city. https://www.civilica.com/Paper‐NATURE01‐NATURE01131.html, Article code: COI, NATURE01_131. Congress in Iran, [Article in Persian]
- Hatami, H. , & Azarbarzin, V. (2018). The prevalence of intestinal parasitic infections in food handlers health applicants in areas covered by Shomal Health Center in Tehran in 2016. Community Health, 5(1). 22–29. ISSN 2423–4702 [Google Scholar]
- Heydari Hengami, M. , Hamedi, Y. , Najafi‐Asl, M. , & Sharifi‐Sarasiabi, K. (2018). Prevalence of intestinal parasites in food handlers of Bandar Abbas, Southern Iran. Iranian Journal of Public Health, 47(1), 111–118. https://www.prisma‐statement.org [PMC free article] [PubMed] [Google Scholar]
- Kassani, A. , Shaterian, M. , Sharifirad, G. , Menati, R. , Abbastabar, H. , Ebrahimipour, M. , & Rezaianzadeh, A. , (2015). The prevalence of some intestinal parasites in food‐handlers of Asian and African countries: A meta‐analysis. Archives of Hygiene Sciences, 4(1), 49–56. [Google Scholar]
- Khazan, H. , & Halakou, A. (2014). Prevalence of intestinal parasitic infections among food vendors referred to Gonbad‐e‐kavus health central laboratories in 2013. Journal of Torbat Heydariyeh University of Medical Sciences, 2(3), 57–61. [Article in Persian]. [Google Scholar]
- Kheirandish, F. , Tarahi, M. J. , Haghighi, A. , Nazemalhosseini‐ Mojarad, E. , & Kheirandish, M. (2011). Prevalence of intestinal parasites in bakery workers in Khorramabad, Lorestan Iran. Iranian Journal of Parasitology, 6(4):76–83. [PMC free article] [PubMed] [Google Scholar]
- Kheirandish, F. , Tarahi, M. J. , & Ezatpour, B. (2014). Prevalence of intestinal parasites among food handlers in Western Iran. Revista do Instituto de Medicina Tropical de Sao Paulo, 56(2), 111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koohsar, F. , Amini, A. , Ayatollahi, A. , Noshak, G. , Hedayat Mofidi, H. , & Namjoo, M. (2012). The prevalence of intestinal parasitic infections in food handlers in Gorgan, Iran. Medical Laboratory Journal, 6(1), 26–34. [Article in Persian] [Google Scholar]
- Kusolsuk, T. , Maipanich, W. , Nuamtanong, S. , Pubampen, S. , Sa‐nguankiat, S. , Rojekittikhun, W. , Lekkla, A. , Tunyong, W. , Chettanadee, S. , & Komalamisra, C. (2011). Parasitic and enteric bacterial infections among food handlers in tourist‐area restaurants and educational‐institution cafeterias, Sai‐Yok district, Kanchanaburi province, Thailand. Journal of Tropical Medicine Parasitology, 34(2), 49–53. [Google Scholar]
- Motazedian, M. H. , Najjari, M. , Ebrahimipour, M. , Asgari, Q. , Mojtabavi, S. , & Mansouri, M. (2015). Prevalence of intestinal parasites among food‐handlers in Shiraz, Iran. Iranian Journal of Parasitology, 10(4), 652–657. [PMC free article] [PubMed] [Google Scholar]
- Mohammadzadeh, A. , Spotin, A. , Mikaeili Galeh, T. , & Fadaee, M. (2018). The prevalence of intestinal parasites in staff working at the restaurants of Tabriz city. Medical Journal of Tabriz University of Medical Sciences and Health Services, 40(4), 60–66. [Google Scholar]
- Neghab, M. , Moosavi, S. , & Moemenbellah‐Fard, M. D. (2006). Prevalence of intestinal parasitic infections among catering staff of students’ canteens at Shiraz, Southern Iran. Pakistan Journal of Biological Sciences, 9 (14), 2699–2703. [Google Scholar]
- Salary, S. , & Safizadeh, H. (2013). Prevalence of intestinal parasite infestation in the food suppliers of Kerman City, Iran, in 2010. Journal of Health & Development, 1(4), 315–322. [Article in Persian] [Google Scholar]
- Safi, M. , Tavalla, M. , Mardani, M. , & Afrisham, R. (2016). Prevalence of intestinal parasitic infections among applicants for health cards attending Ahvaz East Health Center during 2012–2013. Asian Pacific Journal of Tropical Disease, 6(2), 151–154. [Google Scholar]
- Sharif, M. , Daryani, A. , Kia, E. , Rezaei, F. , Nasiri, M. , & Nasrolahei, M. (2015). Prevalence of intestinal parasites among food handlers of Sari, Northern Iran. Revista do Instituto de Medicina Tropical de São Paulo, 57(2), 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saki, J. , Khademvatan, S. , Masoumi, K. , & Chafghani, M. (2012). Prevalence of intestinal parasitic infections among food handlers in Khuzestan, Southwest of Iran: A 10‐year retrospective study. African Journal of Microbiology Research, 6(10), 2475–2480. [Google Scholar]
- Simsek, Z. , Koruk, I. , Copur, A. C. , & Gürses, G. (2009). Prevalence of Staphylococcus aureus and intestinal parasites among food handlers in Sanliurfa, Southeastern Anatolia. Journal of Public Health Management and Practice, 15(6), 518–523. [DOI] [PubMed] [Google Scholar]
- Torgerson, P. R. , Devleesschauwer, B. , Praet, N. , Speybroeck, N. , Willingham, A. L. , Kasuga, F. , Rokni, M. B. , X. ‐ N., Zhou , Fèvre, E. M. , Sripa, B. , Gargouri, N. , Fürst, T. , Budke, C. M. , Carabin, H. , Kirk, M. D. , Angulo, F. J. , Havelaar, A. , & de Silva, N. (2015). World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med, 12(12), e1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali, K. , Noor‐un, N. , & Aly, K. (2017). Prevalence and risk factors associated with intestinal parasitic infections among food handlers of Swat, Khyber Pakhtunkhwa, Pakistan. Journal of Food and Nutrition Research, 5(5), 331–336. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


