Abstract
Introduction
Global poliovirus eradication is a public health emergency of international concern. The acute flaccid paralysis (AFP) surveillance programme in South Africa has been instrumental in eliminating polioviruses and keeping the country poliovirus free.
Gap statement
The sensitivity of surveillance for polioviruses by every African country is of global interest in the effort to ensure global health security from poliovirus re-emergence.
Aim
To describe the epidemiology of polioviruses from AFP cases and environmental samples in South Africa and to report the performance of the AFP surveillance system for the years 2016–2019 against targets established by the World Health Organization (WHO).
Methods
Stool specimens from AFP or suspected AFP cases were received and tested as per WHO guidelines. Environmental samples were gathered from sites across the Gauteng province using the grab collection method. Concentration was effected by the two-phase polyethylene glycol method approved by the WHO. Suspected polioviruses were isolated in RD and/or L20B cell cultures through identification of typical cytopathic effects. The presence of polioviruses was confirmed by intratypic differentiation PCR. All polioviruses were sequenced using the Sanger method, and their VP1 gene analysed for mutations.
Results
Data from 4597 samples (2385 cases) were analysed from the years 2016–2019. Two cases of immunodeficiency-associated vaccine-derived poliovirus (iVDPV) type 3 were detected in 2017 and 2018. A further 24 Sabin type 1 or type 3 polioviruses were detected for the 4 years. The national surveillance programme detected an average of 3.1 cases of AFP/100 000 individuals under 15 years old (2.8/100 000–3.5/100 000). The stool adequacy of the samples received was 53.0 % (47.0–55.0%), well below the WHO target of 80 % adequacy. More than 90 % of results were released from the laboratory within the turnaround time (96.6 %) and non-polio enteroviruses were detected in 11.6 % of all samples. Environmental surveillance detected non-polio enterovirus in 87.5 % of sewage samples and Sabin polioviruses in 12.5 % of samples.
Conclusion
The AFP surveillance programme in South Africa is sensitive to detect polioviruses in South Africa and provided no evidence of wild poliovirus or VDPV circulation in the country.
Keywords: AFP, eradication, infectious, poliomyelitis, paralysis, surveillance, South Africa, viral, vaccine-derived
Introduction
Polio eradication has been a goal of the World Health Organization (WHO) and its member states since the launch of the Global Polio Eradication Initiative in 1988 [1]. While the initial target for eradication by 2000 was missed, as well as the 2005 and 2018 targets, the number of acute flaccid paralysis (AFP) cases due to wild polioviruses is exceedingly low [2] compared to the cases detected before the programme started [3]. Cases per year have been reduced from approximately 350 000 in the 1980s to 176 cases worldwide for 2019 [2].
Poliovirus types 1, 2 and 3 are members of the family Picornaviridae, genus Enterovirus, species Enterovirus C. Poliovirus transmission is via the faecal–oral route and infection can be asymptomatic, or may cause a variety of symptoms, including AFP [4]. AFP can affect proximal and/or distal muscles, causing weakness and loss of function. If the swallowing or breathing muscles are affected, death can occur. Asymptomatic cases pose a challenge to tracing transmission, as viruses can spread widely to other regions before clusters of paralysis are observed [5–7].
Globally and in South Africa the oral poliovirus vaccine (OPV) has been used extensively for decades to prevent wild poliovirus importation and outbreaks. In 2016, South Africa participated in the global switch from trivalent OPV, which comprised all three types of poliovirus, to bivalent OPV comprising only type 1 and type 3. The switch occurred in response to the global eradication of poliovirus type 2, declared in September 2015 [8]. Additionally, inactivated polio vaccine (IPV) was introduced in South Africa in 2009. IPV has since been used in combination with OPV in the routine vaccination schedule. OPV is administered at birth and 6 weeks, and IPV is administered as part of hexavalent vaccine at 6, 10 and 14 weeks, with a booster at 18 months [9]. In September 2019, poliovirus type 3 was declared eradicated [8]. Thus, the only wild poliovirus type in circulation is wild poliovirus type 1.
The Centre for Vaccines and Immunology at the National Institute for Communicable Diseases (NICD) in Johannesburg, a division of the National Health Laboratory Services, South Africa, supports the national AFP surveillance programme. The centre is the only laboratory in South Africa accredited by the WHO for polio isolation. South Africa’s last wild poliovirus case occurred in 1989 [10]. Prior to the reporting period, there was one case of immune-deficiency associated vaccine-derived polioviruses (iVDPV) in South Africa in 2011, with no evidence of transmission to anyone in the community [11].
South Africa is at risk of importation of vaccine-derived and wild polioviruses due to the economic, tourist and travel hubs present in the country. These risks make it essential that AFP surveillance remains optimal as per the WHO recommendations for poliovirus eradication. Performance indicators are used to monitor the success and sensitivity of the surveillance programme.
The WHO guidelines state that countries should investigate at least 1 case of AFP for 100 000 individuals under the age of 15 years, or 2/100 000 in a WHO region still endemic for wild poliovirus for a calendar year, which is applicable to Africa [12]. The South African National Department of Health has set a national target of 4 cases/100 000 per annum [12].
The WHO target for stool adequacy is for at least 80 % of AFP cases to have two stool specimens collected 24–48 h apart and within 14 days of the onset of paralysis. Specimens should arrive at the laboratory within 3 days, be of adequate volume (approximately 8–10 g), have appropriate documentation (a laboratory request form) and be in good condition, with no leakage or desiccation and with evidence that the reverse cold chain had been maintained (presence of ice or temperature indicator) [13].
The WHO requires that 80.0 % of virus isolation result reports be sent within 14 days after receipt of the specimens by the laboratory [14, 15].
The WHO target for detection of non-polio enteroviruses (NPENT) is to detect NPENT in at least 10.0 % of AFP cases inoculated in cell cultures. While a good tool for confirming the sensitivity of the laboratory testing, this indicator varies from country to country and is a guideline for the laboratory and field processes to investigate their processes if the detection rate drops too low [13].
In addition to AFP surveillance, the NICD conducts surveillance on wastewater. Testing sewage samples can supplement the AFP surveillance programme by detecting polioviruses shed into wastewater [14, 15].
This descriptive study illustrates the epidemiological distribution of AFP cases in South Africa between 2016 and 2019, demonstrating the performance of the AFP surveillance programme in the country.
Methods
Country and demographics
South Africa, the southernmost country on the African continent, spans an area of 1 220 813 km2. There are nine provinces and there was an estimated population of 58.78 million people in 2019 with 28.8 % being <15 years old [16].
Specimens
The case definition used for a suspected case of AFP was as any child below 15 years of age with weakness or floppiness of one or more limbs, or any person of any age in whom a clinician suspects polio. An adequate stool specimen consists of two stool specimens collected 24–48 h apart, obtained within 14 days of onset of paralysis and transported in the cold chain (at 4 °C) [12]. Stool specimens were received at the NICD together with standardized case investigation forms collected through routine surveillance. Information for stools arriving at the laboratory within 72 h is only available for 2019.
Environmental surveillance samples were collected as grab samples from three wastewater treatment plant inlets in Gauteng province. Samples were concentrated using the WHO-approved two-phase polyethylene glycol method.
Virus detection and identification
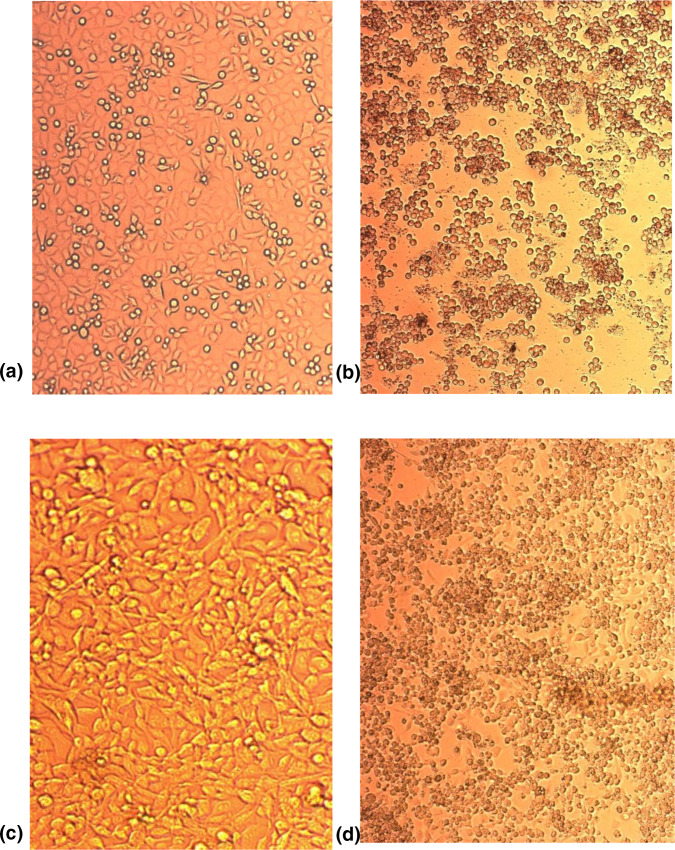
Virus detection and identification were carried out as per the WHO Polio Laboratory Manual and supplement [17, 18]. Briefly, stool specimens or environmental concentrates were prepared and inoculated into cell cultures of RD (human rhabdomyosarcoma) and L20B (mouse L cells transfected with the human cellular receptor gene for poliovirus). Cells were monitored via light microscopy for typical enteroviral cytopathic effect (Fig. 1), and any specimens with suspected poliovirus were tested by polymerase chain reaction (PCR) (Poliovirus rRT-PCR ITD 5.0 and Poliovirus rRT-PCR VDPV 5.0 kits, Centers for Disease Control, Atlanta, GA, USA) to confirm the presence and classification of polioviruses as non-enterovirus, non-polio enterovirus or vaccine-like (Sabin-like) poliovirus type 1, 2 or 3. Sanger sequencing of the VP1 gene of the poliovirus genome (primers and protocol obtained from Centers for Disease Control, Atlanta, GA, USA) was used to confirm the strains as Sabin vaccine poliovirus.
Fig. 1.
Photographs of poliovirus permissive cell lines L20B (a, b) and RD (c, d) showing uninoculated cells (a, c) and inoculated cells with cytopathic effect indicative of enteroviral infection (b, d).
Results
Polioviruses detected
For 2016–2019, the Centre for Vaccines and Immunology at the NICD tested a total of 4597 stool specimens from 2385 cases of AFP in South Africa for 2016–2019. No wild-type polioviruses were detected in any of the specimens tested. Polioviruses were isolated from 58 specimens (1.26 %, 58/4597), of which 24 (0.52 %, 24/4597) were Sabin vaccine strains type 1 or 3.
Two cases of iVDPV were detected, which yielded 34 specimens on follow-up. One iVDPV case had a date of onset of paralysis of 28 December 2017 [19], and the second case had a date of onset of paralysis of 2 October 2018 (unpublished). The two iVDPV cases are described briefly below.
In December 2017, a 5 to 15 week-old child from Cape Town presented with asymmetric AFP. He had been vaccinated at birth and at 6 weeks with OPV, and at 6 weeks with a hexavalent combination vaccine containing IPV. The patient was subsequently diagnosed with X-linked agammaglobulinaemia, a genetic immune deficiency syndrome. Immunoglobulin replacement therapy was attempted for 6 months, but VDPV type 3 was still detected in the child’s stool. With the failure of this therapy, an investigational drug, Pocapavir (Virodefense, Inc., MD, USA) was used. The treatment was considered to be a success after no poliovirus was detected in the child’s stool from 2 days post-treatment. This case was described previously [19].
In October 2018, a second child from Johannesburg presented with iVDPV type 3. Investigation revealed the child to have a rare immunodeficiency disorder, MHC class II deficiency (known as bare lymphocyte syndrome), with complete absence of HLA-DR expression on lymphocytes. Pocapavir treatment was not effective to clear the virus. The child died in March 2019.
In both situations, several activities were conducted as part of a multi-stakeholder public health response. The activities included case investigation, household and community contact investigation, including stool sampling, a vaccine coverage survey and active case finding. No circulation of the virus was detected in any community or close contacts of either case.
Surveillance indicators
Non-polio acute flaccid paralysis detection rate
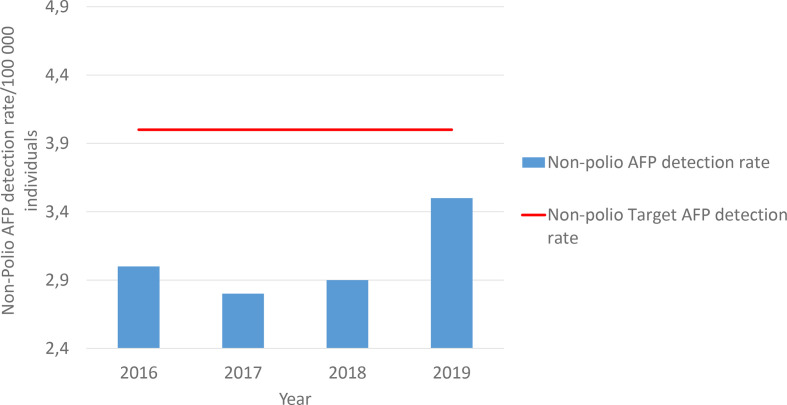
The national programme has consistently reached the global non-polio AFP target of more than 2 per 100 000 but has not met the national target of 4 per 100 000. The mean non-polio AFP detection rate from 2016 to 2019 has been 3.1/100 000, with a minimum of 2.8/100 000 in 2017 and a maximum of 3.5/100 000 in 2019 (Fig. 2). Performance for each district has varied from year to year, although consistency can be seen in well-performing districts from 2016 to 2019 (Table 1). A shortcoming with the surveillance programme is the ability to transport the stool samples from the medical facility to the national laboratory within 72 h. In 2019, even within Gauteng province, the province in which the laboratory is situated, only 73 % of specimens reached the laboratory on time (Table 1).
Fig. 2.
Non-polio acute flaccid paralysis (AFP) detection rate for South Africa 2016–2019. Detection rate varied from 3.0/100 000 in 2016 to 3.5/100 000 in 2019. Average detection rate was 3.1/100 000 for 2016–2019. Target is 4/100 000.
Table 1.
Non-polio AFP detection rate and stool adequacy rate per district for South Africa 2016–2019
|
Districts |
Non-Polio AFP detection rate (under 15 years/100 000) |
Proportion of samples arriving at the lab within 72 h from collection (%) |
|||
|---|---|---|---|---|---|
|
2016 |
2017 |
2018 |
2019 |
2019 |
|
|
Alfred Nzo DM |
2 |
1 |
1.8 |
1.5 |
40.0 |
|
Amathole DM |
3.7 |
2.2 |
1.4 |
2.0 |
42.9 |
|
Buffalo City MM |
2.2 |
2.2 |
1.4 |
1.4 |
50.0 |
|
Chris Hani DM |
1.4 |
2.5 |
3.3 |
5.5 |
40.0 |
|
Joe Gqabi DM |
0.8 |
2.5 |
3.2 |
3.2 |
25.0 |
|
Nelson Mandela Bay MM |
2.1 |
1.8 |
1.5 |
1.5 |
50.0 |
|
Oliver Tambo DM |
2.4 |
2.4 |
3.2 |
4.4 |
16.0 |
|
Sarah Baartman DM |
3.1 |
2.3 |
0.6 |
2.4 |
25.0 |
|
Eastern Cape |
2.2 |
2.1 |
2.1 |
2.7 |
36.1 |
|
Fezile Dabi DM |
2.9 |
2.2 |
5.1 |
10.1 |
28.6 |
|
Lejweleputswa DM |
2.9 |
0.8 |
3.2 |
9.2 |
23.5 |
|
Mangaung MM |
1.3 |
3 |
3.2 |
5.5 |
66.7 |
|
T Mofutsanyane DM |
4.1 |
2.6 |
5.0 |
4.6 |
36.4 |
|
Xhariep DM |
3.2 |
0 |
5.9 |
8.9 |
33.3 |
|
Free State |
2.9 |
1.7 |
4.5 |
7.7 |
38 |
|
Ekurhuleni MM |
3.1 |
2.8 |
2.6 |
1.7 |
73.3 |
|
Johannesburg MM |
3.2 |
3.1 |
2.8 |
2.2 |
69.0 |
|
Sedibeng DM |
4.7 |
5.2 |
6.5 |
6.5 |
82.4 |
|
Tshwane MM |
2 |
1.6 |
1.9 |
2.8 |
52.0 |
|
West Rand DM |
4.7 |
3.7 |
5.7 |
4.3 |
90.0 |
|
Gauteng |
3.5 |
3.3 |
3.9 |
3.5 |
73 |
|
Amajuba DM |
4 |
2.8 |
3.2 |
2.7 |
0.0 |
|
eThekwini MM |
1.3 |
1.4 |
2.1 |
2.6 |
24.1 |
|
iLembe DM |
5 |
1 |
2.5 |
3.4 |
14.3 |
|
Harry Gwala DM |
4.1 |
1 |
2.6 |
3.8 |
22.2 |
|
Ugu DM |
3 |
3.1 |
1.8 |
2.0 |
0.0 |
|
uMgungundlovu DM |
5.9 |
5.3 |
4.3 |
3.2 |
33.3 |
|
Umkhanyakude DM |
0.8 |
3.4 |
2.6 |
2.9 |
36.4 |
|
Umzinyathi DM |
2.7 |
1.2 |
2.6 |
3.3 |
22.2 |
|
Uthukela DM |
3.3 |
2.7 |
2.8 |
4.5 |
20.0 |
|
King Cetshwayo DM |
2.5 |
3.8 |
3.3 |
3.0 |
0.0 |
|
Zululand DM |
2 |
1.3 |
0.6 |
3.3 |
9.1 |
|
KwaZulu-Natal |
3.1 |
2.5 |
2.6 |
3.2 |
17 |
|
Capricorn DM |
2.1 |
4 |
4.6 |
4.1 |
52.9 |
|
Greater Sekhukhune DM |
4.2 |
4.5 |
4.6 |
6.1 |
20.8 |
|
Mopani DM |
4 |
6 |
3.8 |
3.8 |
25.0 |
|
Vhembe DM |
0.9 |
5.8 |
4.1 |
3.6 |
50.0 |
|
Waterberg DM |
0.8 |
2.9 |
1.4 |
7.9 |
70.6 |
|
Limpopo |
2.4 |
4.6 |
3.7 |
5.1 |
44 |
|
Ehlanzeni DM |
7.9 |
4.4 |
5.6 |
5.1 |
55.2 |
|
Gert Sibande DM |
5.3 |
6.1 |
3.8 |
6.3 |
54.5 |
|
Nkangala DM |
6.9 |
6.6 |
4.9 |
4.9 |
25.0 |
|
Mpumalanga |
6.7 |
5.7 |
4.8 |
5.4 |
45 |
|
Bojanala Platinum DM |
1.2 |
0 |
1.4 |
2.4 |
33.3 |
|
Dr K Kaunda DM |
2 |
2.5 |
1.3 |
2.2 |
40.0 |
|
Ngaka Modiri Molema DM |
2.7 |
1.8 |
1.0 |
3.7 |
27.3 |
|
Ruth Segomotsi Mompati DM |
5.7 |
2.5 |
2.8 |
4.0 |
71.4 |
|
North West |
2.9 |
1.7 |
1.7 |
3.1 |
43 |
|
Frances Baard DM |
2.7 |
3.6 |
2.9 |
6.9 |
71.4 |
|
John Taolo Gaetsewe DM |
6.4 |
3.9 |
2.6 |
1.3 |
0.0 |
|
Namakwa DM |
0 |
7.1 |
0.0 |
3.5 |
100.0 |
|
Pixley ka Seme DM |
3.6 |
7.2 |
10.6 |
3.5 |
50.0 |
|
ZF Mgcawu DM |
2.9 |
0 |
0.0 |
1.6 |
100.0 |
|
Northern Cape |
3.1 |
4.4 |
3.2 |
3.4 |
64 |
|
Cape Town MM |
2.1 |
1.9 |
3.7 |
3.8 |
44.7 |
|
Cape Winelands DM |
2.3 |
2.7 |
2.9 |
3.3 |
37.5 |
|
Central Karoo DM |
0 |
0 |
4.7 |
4.7 |
100.0 |
|
Eden DM |
3.3 |
1 |
5.8 |
4.5 |
71.4 |
|
Overberg DM |
2.8 |
0 |
2.8 |
4.2 |
100.0 |
|
West Coast DM |
0.9 |
1.9 |
2.5 |
2.5 |
33.3 |
|
Western Cape |
1.9 |
1.3 |
3.8 |
3.8 |
64 |
|
South Africa |
3.0 |
2.8 |
3.2 |
4.0 |
47 |
Red indicates a non-polio AFP detection rate of 0–1.99 or a stool adequacy rate of <50 %. Yellow indicates a non-polio AFP detection rate of 2–3.99 or a stool adequacy rate of 50–79.99 %. Green indicates a non-polio AFP detection rate of >4.0 or a stool adequacy rate of >80 %. Blue indicates a silent district not reporting any AFP cases for the year. DM, District Municipality; MM, Metropolitan Municipality.
Stool adequacy rate
The South African stool adequacy rate averaged 53 % over the 4 years surveyed. The best performance was in 2018, when 59 % of specimens were deemed adequate, and the lowest performance was in 2019 at 47 % (Fig. 3).
Fig. 3.
Stool adequacy rate for South Africa 2016–2019. Stool adequacy varied from 55.0 % in 2016 to 47.0 % in 2019. Average detection rate was 53.0 % for 2016–2019. Target is 80 %.
The reasons for stool specimens not being deemed adequate were collated for 2019 (Table 2). Gauteng, Kwa-Zulu Natal and Eastern Cape provinces had the highest number of inadequate stool specimens. Northern Cape and Mpumalanga provinces performed the best. Stools not collected with 14 days of onset of paralysis, a second stool not being collected, or the second stool collected after an interval of more than 48 h were the most common reasons that stools were declared inadequate. These results indicate that improvements are needed in some provinces to ensure stool specimens are collected correctly.
Table 2.
Reasons for low stool adequacy per province, 2019
|
Province |
Reasons for inadequately investigated cases |
||||||
|---|---|---|---|---|---|---|---|
|
Second stool not collected |
Interval between stool is 0 days |
Interval between stools is >48 h |
No stool collected |
Specimen not collected within 14 days of onset |
Stool not on ice |
Total inadequate stools per province (percentages shown represent totals) |
|
|
Eastern Cape |
11 |
0 |
10 |
13 |
4 |
2 |
40 (19.1 %) |
|
Free State |
4 |
1 |
6 |
0 |
0 |
4 |
15 (7.2 %) |
|
Gauteng |
14 |
3 |
16 |
9 |
5 |
0 |
47 (22.5 %) |
|
KwaZulu-Natal |
5 |
2 |
11 |
4 |
19 |
3 |
44 (21.1 %) |
|
Limpopo |
2 |
3 |
2 |
0 |
8 |
1 |
16 (7.7 %) |
|
Mpumalanga |
4 |
1 |
1 |
1 |
3 |
0 |
10 (4.8 %) |
|
North West |
7 |
1 |
3 |
2 |
1 |
0 |
14 (6.7 %) |
|
Northern Cape |
1 |
0 |
2 |
0 |
1 |
0 |
4 (1.9 %) |
|
Western Cape |
1 |
0 |
10 |
2 |
5 |
1 |
19 (9.1 %) |
|
Total |
49 (23.4 %) |
11 (5.3 %) |
61 (29.2 %) |
31 (14.8 %) |
46 (22.0 %) |
11 (5.3 %) |
209 (100 %) |
Laboratory indicators
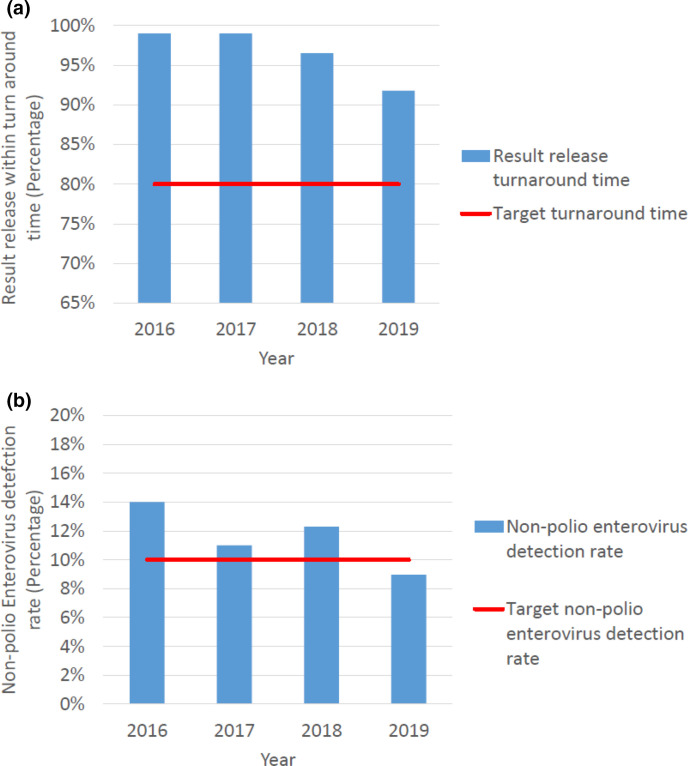
The laboratory consistently exceeded the target, with a minimum of 91.8 % of results released on time and an average of 96.6 % over the 4 years (Fig. 4a). The national NPENT rate averaged 11.57 % from 2016 to 2019. Only 1 year recorded a value below 10.0%, namely 9.0 % in 2019 (Fig. 4b).
Fig. 4.
(a) Percentage of virus isolation results released within the target turnaround time and (b) the non-polio enterovirus isolation rate in specimens received in South Africa 2016–2019. Turnaround time for results varied from 99.0 % in 2016 to 91.8 % in 2019. Average turnaround time was 97.0 % for 2016–2019. Target is 90 %. The non-polio enterovirus isolation rate varied from 14.0 % in 2016 to 9.0 % in 2019. The average detection rate was 12.0 % for 2016–2019. Target is 10 %.
Environmental surveillance
In 2019, NICD began testing South African wastewater samples. In 2019, 32 samples were collected and tested, and 4 samples (12.5 %) were positive for polioviruses. All were Sabin vaccine strains type 1 or type 3. Non-polio enteroviruses were detected in 28(87.5 %) samples. These findings indicate an excellent sensitivity of the test method to detect polioviruses if they were present in the sample.
Discussion
National polio vaccination coverage, according to the South African Department of Health, has reached 82–85 % of the population, though estimates from the United Nations Children’s Fund (UNICEF) show a vaccination coverage of 76–77 % [20], below the 90 % target set by the WHO [21]. Due to low coverage estimates and suboptimal stool adequacy, the African Regional Certification Committee (ARCC) revoked South Africa’s polio-free status in 2017. The ARCC subsequently renewed South Africa’s polio-free designation in September 2019 following improved vaccination coverage and stool adequacy indicators [22]. The maintenance of herd immunity may be due to the sustained use of multiple doses of oral polio vaccine in combination of multiple doses of inactivated polio vaccine in South Africa’s extended programme for immunization since 2009 [9].
A functional surveillance system is an essential tool to assist in the eradication of wild poliovirus. Despite having no wild poliovirus in the country in three decades, the NICD has processed samples from over 2000 AFP cases in the last 4 years, of which iVDPV was detected in 2. It is a tribute to the sensitivity of the AFP surveillance programme in South Africa that two iVDPVs were detected [19]. iVDPV does not arise in countries using an IPV-only regimen and may be misdiagnosed in countries without strong diagnostic capacity for primary immune deficiencies. South Africa’s capacity for identification of rare immune disorders within an OPV-using region is the likely reason for the multiple iVDPV detections.
Sabin vaccine strains type 1 and 3 were detected in 24 (0.5 %) specimens. Detection of Sabin strains in stools, as opposed to cerebrospinal fluid specimens, from AFP cases is usually a co-incidental finding in OPV-using countries, as OPV is shed in stools of both healthy and ill children for a few weeks following vaccination. The National Polio Expert Committee classified no cases as vaccine-associated paralysis. No poliovirus type 2 was detected in any specimens, as expected following the 2016 global switch from trivalent OPV use to bivalent OPV use.
The ability of the programme to obtain adequate stool specimens is a concern. Stool specimens are the only source for the national laboratory to test for the presence of poliovirus in patients with AFP. Missing specimens, receipt of only one specimen, specimens collected late in disease course and the long periods of time for the specimen to reach the national laboratory [13] compromise the integrity of the specimen, and can introduce uncertainty of measurement, but reflect logistical constraints of centralized national testing.
Conversely, there was adequate notification of non-polio AFP cases. The high national target set of 4.0 cases/100 000 individuals has increased the sensitivity of the AFP surveillance programme since prior years. While there are districts that did not meet the WHO target of 2.0/100 000 annually (Table 2), the national average non-polio AFP detection rate has been consistently above 2.0/100 000. In 2019, there was not a single district that failed to report at least one AFP case for the year.
Environmental surveillance has strengthened the overall capacity of the programme, although the number of sites is limited by the need for labour-intensive viral isolation prior to molecular testing.
In conclusion, the AFP surveillance programme in South Africa has provided evidence of no importation of wild PV or cVDPV into the country and provided reassurance that there is no endemic circulating VDPV. The sensitivity of the programme resulted in the detection two immune deficiency-linked VDPV in individuals with hereditary immune disorders.
Continuous poliovirus surveillance will support the global goal of poliovirus eradication and provide lessons for intensifying surveillance for other infectious diseases, and in particular emphasize the importance of surveillance adequacy indicators for programme monitoring.
Funding information
This work was funded by the National Institute for Communicable Diseases, the South African National and Provincial Departments of Health and the World Health Organization, South Africa.
Conflicts of interest
Dr Melinda Suchard, Wayne Howard, Shelina Moonsamy, Lerato Seakamela, Dr Sabelle Jallow, Faith Modiko, Heleen du Plessis and Rosinah Sibiya are employees of the NICD; Elizabeth Maseti is an employee of the Department of Health; and Dr Mercy Kamupira is an employee of the WHO, South Africa. Dr Melinda Suchard declares speaker honoraria from Sanofi-Pasteur and Pfizer.
Ethical statement
The University of Witwatersrand, Human Research Ethics Committee (M160667), approved publication from AFP surveillance by the NICD.
Footnotes
Abbreviations: AFP, acute flaccid paralysis; ARCC, African Regional Certification Committee; cVDPV, circulating vaccine-derived poliovirus; DM, district municipality; IPV, inactivated polio vaccine; ITD, intratypic differentiation; iVDPV, immune-deficiency associated vaccine-derived poliovirus; MM, metropolitan municipality; NICD, National Institute for Communicable Diseases; NPENT, non-polio enterovirus; OPV, oral polio vaccine; PCR, polymerase chain reation; RT-PCR, reverse trancription polymerase chain reaction; UNICEF, United Nations Childreds Fund; VDPV, vaccine-derived poliovirus; WHO, World Health Organisation.
References
- 1.Centers for Disease Control and Prevention (CDC) Progress toward global eradication of poliomyelitis, 1988-1991. MMWR Morb Mortal Wkly Rep. 1993;42:493–495. [PubMed] [Google Scholar]
- 2.Global polio eradication initiative This week. [ May 25; 2020 ]. http://polioeradication.org/polio-today/polio-now/this-week/ accessed.
- 3.Global polio eradication initiative History of Polio. [ May 25; 2020 ]. http://polioeradication.org/polio-today/history-of-polio/ accessed.
- 4.Minor P. Poliovirus biology. Structure. 1996:775–778. doi: 10.1016/s0969-2126(96)00084-6. [DOI] [PubMed] [Google Scholar]
- 5.Sousa IP, Burlandy FM, Oliveira SS, Nunes AM, Sousa C, et al. Acute flaccid paralysis laboratorial surveillance in a polio-free country: Brazil, 2005–2014. Hum Vaccines Immunother. 2017;13:717–723. doi: 10.1080/21645515.2016.1236164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins RE, Martin PAJ, Kelly H, Madin B, Watson C. An evaluation of the sensitivity of acute flaccid paralysis surveillance for poliovirus infection in Australia. BMC Infect Dis. 2009;9:162. doi: 10.1186/1471-2334-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostol LN, Suzuki A, Bautista A, Galang H, Paladin FJ, et al. Detection of non-polio enteroviruses from 17 years of virological surveillance of acute flaccid paralysis in the Philippines. J Med Virol. 2012;84:624–631. doi: 10.1002/jmv.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poliomyelitis (POLIO) [ Nov 04; 2020 ]. https://www.who.int/health-topics/poliomyelitis accessed.
- 9.Schoub BD. Introduction of inactivated polio vaccine (IPV) into the routine immunization schedule of South Africa. Vaccine. 2012;30 Suppl 3:C35–7. doi: 10.1016/j.vaccine.2012.02.056. [DOI] [PubMed] [Google Scholar]
- 10.Chezzi C, Blackburn NK, Schoub BD. Molecular characterisation of type 1 polioviruses associated with epidemics in South Africa. J Med Virol. 1997;52:42–49. doi: 10.1002/(sici)1096-9071(199705)52:1<42::aid-jmv8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Gumede N, Muthambi V, Schoub BD. Immunodeficiency-associated vaccine-derived poliovirus type 3 in Infant, South Africa, 2011. Emerg Infect Dis. 2012:992–994. doi: 10.3201/eid1806.120037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization WHO | WHO-recommended surveillance standard of poliomyelitis. [ Jul 16; 2020 ]. https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/poliomyelitis_standards/en/ accessed.
- 13.Global polio eradication initiative Surveillance indicators. [ May 26; 2020 ]. http://polioeradication.org/polio-today/polio-now/surveillance-indicators/ accessed.
- 14.Gardner TJ, Diop OM, Jorba J, Chavan S, Ahmed J, et al. Surveillance to Track Progress Toward Polio Eradication — Worldwide, 2016–2017. MMWR Morb Mortal Wkly Rep. 2018;67:418–423. doi: 10.15585/mmwr.mm6714a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel JC, Diop OM, Gardner T, Chavan S, Jorba J, et al. Surveillance to Track Progress Toward Polio Eradication — Worldwide, 2017–2018. MMWR Morb Mortal Wkly Rep. 2019;68:312–318. doi: 10.15585/mmwr.mm6813a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mid-year population estimate 2019.PDF. [ Nov 04; 2020 ]. https://www.statssa.gov.za/publications/P0302/P03022019.pdf accessed.
- 17.World Health Organisation Polio Laboratory Manual. 4th ed. Geneva, Switzerland: 2004. [Google Scholar]
- 18.Polio Laboratory Manual Supplement1.Pdf. [ Nov 04; 2020 ]. http://polioeradication.org/wp-content/uploads/2017/05/NewAlgorithmForPoliovirusIsolationSupplement1.pdf accessed.
- 19.Copelyn J, Hincks JR, Wilmshurst JM, Petersen W, Howard W, et al. Clearance of immunodeficiency-associated vaccine-derived poliovirus infection with pocapavir. Pediatr Infect Dis J. 2020;39:435–437. doi: 10.1097/INF.0000000000002584. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization South Africa: Who and UNICEF estimates of immunization coverage: 2019 revision. [ Jul 17; 2020 ]. https://www.who.int/immunization/monitoring_surveillance/data/zaf.pdf accessed.
- 21.World Health Organisation Global vaccine action Plan 2011–2020. [ Feb 09; 2021 ]. https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/global-vaccine-action-plan accessed.
- 22.National Institute for Communicable Diseases South Africa certified polio-free; 2019. [ Nov 04; 2020 ]. https://www.nicd.ac.za/south-africa-certified-polio-free/ accessed.