Abstract
Antifungal drugs have already been established as an effective treatment option for Candida parapsilosis infections, but there is no universal consensus on the ideal target for clinical efficacy and safety of antifungal drugs for the treatment of C. parapsilosis infections. Few studies have directly compared the efficacies of antifungal drugs for the treatment of C. parapsilosis infections. We hypothesize that different antifungal drugs offer differing clinical efficacy and safety for the treatment of C. parapsilosis infections. We performed a comprehensive network meta-analysis on different strategies for C. parapsilosis infection treatment and compared the clinical efficacy and safety of antifungal drugs as interventions for C. parapsilosis infections. The Cochrane Database of Systematic Reviews, Medline, Embase, PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), Technology of Chongqing VIP database, Wan Fang Data, and SinoMed databases were searched to identify appropriate randomized trials. Among the extracted C. parapsilosis cases, the survival and death rates with treatment of C. parapsilosis infection were compared among groups treated with different antifungal drugs. According to the evidence-network analysis, echinocandins were a better choice than other drugs for treating C. parapsilosis infections, and more importantly, caspofungin showed a more preferable effect for decreasing the risk of 30 day mortality. In conclusion, this study systematically evaluated the effectiveness and safety of antifungal drugs for the purpose of helping clinicians choose the most appropriate antifungal drugs. Future studies with larger samples are needed to evaluate the effects of patient factors on the clinical efficacy and safety of antifungal drugs for C. parapsilosis infections.
Keywords: antifungal drugs, Candida parapsilosis, clinical efficacy, meta-analysis, safety
Introduction
Invasive fungal infection has become an important factor leading to increased mortality in hospitalized patients [1, 2]. Since the 1980s, with the widespread use of corticosteroids/antibacterial drugs and the development of organ transplantation and medical life support technology, the incidence of fungal bloodstream infections has increased significantly among hospitalized patients, especially those who are immunocompromised [3]. The SENTRY Antimicrobial Surveillance Programme reported that Candida albicans remains the most common clinical pathogenic fungal type among hospital-acquired bloodstream infections. Meanwhile, with the increase in the incidence of fungal infections, the pathogen spectrum has changed [4]. The proportion of Candida albicans infections is decreasing year by year, while infections with other opportunistic pathogens such as non-Candida albicans including Candida parapsilosis, Candida glabrata, and Candida tropicalis are showing an increasing tend in prevalence and often show resistance to multiple antifungal drugs. In China, a nationwide invasive fungal infection surveillance study revealed that Candida species accounted for 91.3 % of yeast pathogens from invasive fungal infections, followed by Cryptococcus neoformans (7.0 %) and other non-candidal yeasts (1.7 %) [5]. Among these, C. parapsilosis as a common human skin commensal fungus is non-albicans Candida species [6, 7]. It does not lead to severe disease under normal immune conditions as a typical opportunistic fungus [8]. However, when patients receive broad-spectrum antibiotics or immunosuppressive treatments in the hospital, the incidence of C. parapsilosis infection increases significantly [7]. In recent decades, it was reported that the incidence of C. parapsilosis infection is rising in North America, Europe and Asia, accounting for 8–10 % of all nosocomial bloodstream infections [9, 10]. Since the first antifungal drug griseofulvin was discovered in the 1930s, antifungal drugs such as polyenes, allylamines, azoles, and echinocandins have been used to clear fungal infections. In recent years, great progress has been made in the treatment of fungal infections. Antifungal drugs including polyenes, triazoles, and echinocandins have already become effective treatment options for C. parapsilosis infections, even though all have some advantages and certain limitations in terms of efficacy, safety, bioavailability, and drug–drug interactions [11, 12]. Therefore, there is no universal consensus on the ideal target for clinical efficacy and safety of antifungal drugs for the treatment of C. parapsilosis infections [13]. Also, few studies have directly compared the efficacies of antifungal drugs for the treatment of C. parapsilosis infections [14].
Therefore, a comprehensive review on different strategies for C. parapsilosis infection treatment and a comparison of their efficacies will be meaningful and helpful for the clinical application of antifungal drugs. Accordingly, we focused on antifungal inventions in the present study, highlighting the important aspects of this therapy and performing a network meta-analysis to determine the best therapeutic choice for C. parapsilosis infection patients. Most importantly, the objective of this research was to provide new treatment insight extending beyond the specific therapeutic strategy itself.
Methods
Literature search
The following electronic bibliographic databases were searched: Cochrane Database of Systematic Reviews), Medline, Embase, PubMed, and Web of Science (science and social science citation index) as well as the Chinese databases China National Knowledge Infrastructure (CNKI), Technology of Chongqing VIP database, Wan Fang Data, and SinoMed for related studies. The search time limit was from the establishment of each database until 1 June 2020. We also manually searched collections of relevant conferences and traced the reference documents included in the research. If the information was incomplete, we contacted the authors to obtain relevant data. The search strategy included terms relating to or describing clinical trials. The search terms were ‘Candida parapsilosis’, ‘randomized clinical trials’ ‘randomized controlled trail’, ‘antifungal agents’, ‘treatment’, ‘itraconazole’, ‘miconazole’, ‘fluconazole’, ‘econazole’, ‘terbinafine’ and ‘terconazole’.
Inclusion criteria
The eligible studies include randomized controlled trials (RCTs) that compared the protective effects of any antifungal drug with placebo or other antifungal drugs for C. parapsilosis infections. The inclusion criteria were: 1. RCT; 2. adult patients over 18 years of age, with no limit on gender or nationality; 3. interventions of antifungal drug treatment for C. parapsilosis infections including candidemia or invasive candidiasis; 4. outcomes included overall effectiveness, 30 day mortality, adverse drug reactions, etc.; and 5. published in English or Chinese. The exclusion criteria were: 1. non-RCT studies; 2. non-research studies such as reviews, case reports, communications, and pharmacokinetics papers; 3. duplicate publications; 4. studies with overlapping cohorts; and 5. data that could not be extracted, converted, or obtained.
Literature quality evaluation
Two researchers (J.Q, H.Y) independently performed the literature searches and screening. After excluding studies that did not meet the inclusion criteria, including reviews, case reports and meta-analyses, the abstracts and full texts were further reviewed to determine whether they met the inclusion criteria. The extracted data included the basic information of the RCT, baseline data for patients, interventions, primary and secondary outcomes, and follow-up data. Two authors (Z.S, L.J) used the RCT Quality Evaluation Standards recommended by the Cochrane Evaluation Manual Version 5.1 to assess the methodological quality. Quality assessment included determining whether the randomization was correct, whether the allocation was blind, whether there was loss to follow-up and withdrawal, whether there was selective reporting bias, and whether there were other biases. If the information in the clinical trials was incomplete, the authors were contacted to obtain the missing data to the extent possible. The two reviewers (J.Q, H.Y) independently extracted information. The decisions recorded by the reviewers were compared and discrepancies were resolved by a third reviewer (Q.Z).
Outcome measures
The primary outcome was the efficacy of antifungal agents (for treating invasive fungal infections). The secondary outcomes included 30 day mortality due to fungal infection, incidence of invasive candidiasis, and other probable adverse events.
Statistical analysis
The traditional and network meta-analyses were performed with R3.5 software according to the Bayesian framework. Both direct and indirect evidence was utilized to compare the efficacy of various treatments, as described by mean differences and 95 % confidence intervals (CIs) with a significance level of 0.05. Assessment of heterogeneity among eligible studies was carried out according to Cochran’s Q-statistic and I2 test. The χ2 test was used to analyse the clinical heterogeneity and method heterogeneity of the included studies with α set at 0.1. The surface under the cumulative ranking curve (SUCRA) was adopted to rank probabilities with respect to each clinical outcome. Funnel plots were generated to investigate publication bias.
Results
Characteristics of included studies
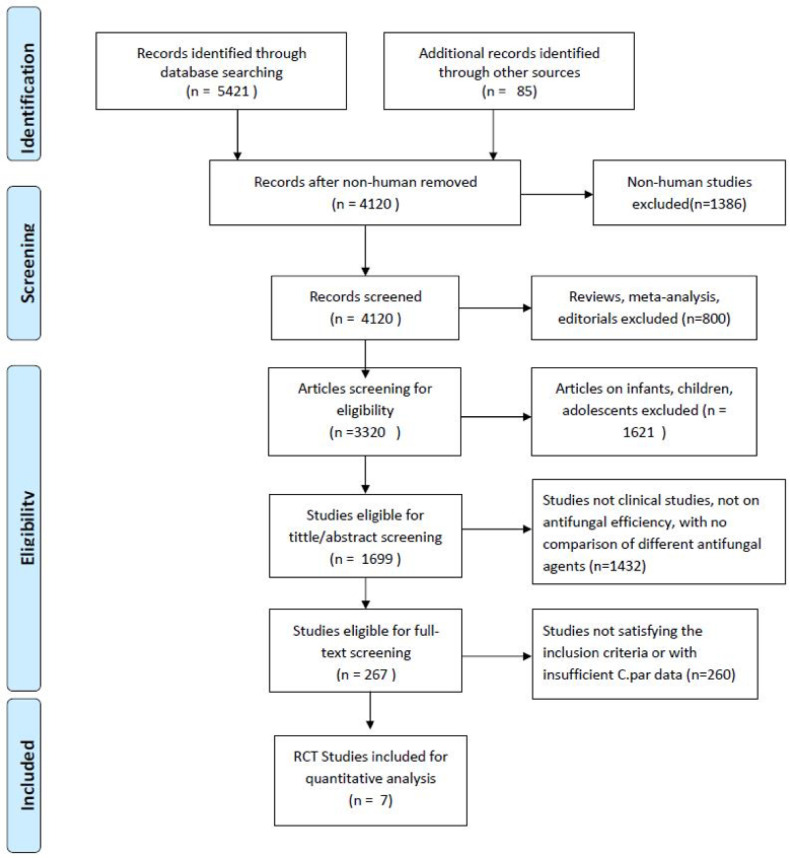
A total of 5506 records were identified in the primary literature search. As shown in Fig. 1, records were retrieved from the databases by searching relevant keywords, and after exclusion of case reports, editorials, comments, laboratory studies, trials involving children, and other irrelevant literature, 267 studies remained. According to the exclusion criteria, only seven out of the remaining studies were finally included in this network meta-analysis. Finally, our meta-analysis compared the clinical efficacy and safety of antifungal drugs including fluconazole (FLU), micafungin (MIC), isavuconazole (ISA), amphotericin B (AMP), caspofungin (CAS), and anidulafungin (AND) across seven studies with a total sample size of 2434 patients [15–21] (Table 1, Fig. 1).
Fig. 1.
Flow diagram of the study selection process.
Table 1.
Characteristics of included randomized controlled trials studies
|
Study (First author) |
Reported date |
Study location |
Total sample size |
Mean age (range), years |
Sex/male (%) |
Type of infection |
Interventions |
|---|---|---|---|---|---|---|---|
|
Rex et al. [15] |
1994 |
America |
206 |
59 (na) |
105 (56.0) |
candidemia |
FLU/AMP |
|
Mora-Duate et al. [16] |
2002 |
America |
224 |
56 (18–84) |
125 (55.8) |
invasive candidiasis |
CAS/AMP |
|
Colombo et al. [17] |
2003 |
America/Europe/Asia |
210 |
52.7 (18–97) |
120 (57.1) |
invasive candidiasis |
CAS/AMP |
|
Pappas et al. [18] |
2007 |
America/Europe/Asia |
578 |
55.8 (24–92) |
336 (58.1) |
candidemia or invasive candidiasis |
MIC/CAS |
|
Reboli et al. [19] |
2007 |
America |
245 |
58.1 (24–91) |
125 (51.0) |
invasive candidiasis |
AND/FLU |
|
Kuse et al. [20] |
2007 |
America/Europe/Asia/Africa |
531 |
55.3 (18–84) |
325 (61.2) |
candidemia or invasive candidiasis |
MIC/AMP |
|
Kullberg et al. [21] |
2019 |
Europe |
440 |
57.9 (na) |
269 (61.1) |
candidemia or invasive candidiasis |
CAS/ISA |
FLU, Fluconazole; AMP, Amphotericin B; VOR, Voriconazole; CAS, Caspofungin; AND, Anidulafungin; MIC, micafungin; na, data not available.
Risk of bias in the evidence base
All included studies were RCTs, and the quality evaluation was conducted using the criteria of the Cochrane Collaboration tool for assessing the risk of bias in RCTs (Fig. 2). All seven included RCTs satisfied the required items, including random sequence generation, allocation concealment, blinding of the study participants and personnel, and blinding of outcome assessments.
Fig. 2.
Risk of bias for all individual studies included in the analysis. The risk of bias graph shows the reviewers’ assessment of the risk of bias shown as percentages for all included studies.
Evaluation of antifungal treatment
Using a pair-wise meta-analysis to compare the overall efficacy of antifungal drugs, the sizes of the nodes reflect the numbers of participants, and the widths of the lines indicate the numbers of included trials. The results showed a significant benefit in favour of antifungal inventions for C. parapsilosis infections (Fig. 3). We next performed a meta-analysis comparing primary outcomes achieved with various antifungal agents. The forest plot results showed that antifungal treatment promoted good outcomes with an overall pooled relative risk (RR) of 0.97 (95 % CI :0.86–1.09). Based on the chi-square and I2 analyses, small differences in heterogeneity were observed between the treatment groups [I2=34.2 %] (Fig. 4).
Fig. 3.
Network graph of included studies reporting outcomes. Each node represents a therapy with the thickness of the line and size of the circle proportional to the number of studies and number of participants, respectively, in the head-to-head comparison. Abbreviations: CAS, Caspofungin; AND, Anidulafungin; AMP, Amphotericin B; MIC, Micafungin; ISA, Isavuconazole; FLU, Fluconazole.
Fig. 4.
Forest plot of included studies for comparing the overall efficacy of antifungal drugs in the included studies.
Network meta-analysis of antifungal treatment
To compare the different effects of antifungal treatments for C. parapsilosis infections, we then used the network meta-analysis to explore whether there were differences among different antifungal drugs in patients with C. parapsilosis infections. First, we performed an evidence-network analysis for different treatments and generated a forest plot for every included study with direct and indirect analysis (Fig. 5). The results demonstrated that CAS presented a better efficacy than AMP or ISA against C. parapsilosis infections. Furthermore, all of the drugs, including FLU, MIC, ISA, AMP, CAS and AND were compared separately with each other, and Fig. 6 shows the contribution plot for the included publications in the network. The most informative direct evidence in the network was CAS vs. ISA with an overall contribution of 28.9 % to the network estimates.
Fig. 5.
Evidence-network analysis for paired-comparison of the efficacy of antifungal drugs in the included studies.
Fig. 6.

Contribution plot for the included studies.
To explore the risks of death with antifungal agents, we analysed the included studies reporting 30 day mortality for patients with C. parapsilosis infection. In the subgroup analysis, compared with patients who received AMP, patients treated with CAS (RR 0.60, 95 % CI 0.43–0.84) presented with a significantly lower risk of 30 day mortality than those who received other treatments (Fig. 7).
Fig. 7.
Comparisons of 30 day mortality. Summarized RR and corresponding 95 % CI for 30 day mortality comparing multiple treatments.
Consistency and publication bias assessment
To assess the inconsistency among the included studies, node-splitting models were applied by testing the difference between the direct and indirect comparisons (Fig. 8). The results of the consistency model were reliable, which demonstrated good convergence and efficiency. The comparison-adjusted funnel plot for the efficacy of these seven treatments showed that there was no publication bias based on Begg’s test (P=0.61) among the included studies.
Fig. 8.

Comparison-adjusted funnel plot for the network meta-analysis.
Discussion
Systemic fungal infections caused by conditioned pathogens such as non-albicans Candida including C. parapsilosis have emerged along with a gradual increase in blood-stream infections in healthcare settings with the widespread application of broad-spectrum antibiotics, immunosuppressants, and anti-malignant drugs, increased performance of organ transplantation, advances in medical support technology, the extension of human life, as well as the increase in the prevalence of acquired immune deficiency syndrome (AIDS) [22–24]. Research on the pathogen C. parapsilosis and exploration of its pathogenic mechanism are underway [25].
Antifungal drugs are currently the most effective method for the treatment of Candida infections [26, 27]. AMP serves as a representative of polyene antifungal drugs and has been widely used in the treatment of fungal infections, especially severe fungal infections [28]. It has been reported that AMP is more than 70 % effective in treating fungal infections. However, it has several obvious side effects, especially nephrotoxicity. The first-generation azoles such as FLU, itraconazole, and voriconazole show relatively good efficacy for reducing the death rate among haematological stem cell transplant recipients [29]. However, the bioavailability of itraconazole varies greatly, and drug resistance to FLU develops readily [30]. In contrast, the newer triazoles such as voriconazole and posaconazole show a broader antibacterial spectrum, higher bioavailability, and significantly fewer adverse reactions than the first-generation triazole drugs [31]. Echinocandins such as micafungin target 1,3-β-D glucan synthase, inhibit the synthesis of glucan synthase, and interrupt formation of the cell wall, ultimately leading to cell death [32]. Caspofungin was the first echinocandin drug approved by the US Food and Drug Administration (FDA) and proven to be comparatively safe and efficacious against Candida species, including C. albicans, and non-C. albicans [33].
To determine the ideal antifungal drugs for the treatment of Candida infections, many studies have compared the clinical outcomes of different antifungal drugs. The outcomes of different therapies in whole Candida infections have been widely reported; however, reports specifically on Candida parapsilosis infections are rare, especially with direct comparison. If differences in outcome were observed among those different therapies, the related studies are very limited and only several studies have reported a difference in treatment outcomes. In the present study, a network meta-analysis was performed including seven RCTs with a total of 2434 patients, and most studies were compared different clinical outcomes. Therefore, we analysed the clinical efficacy of treatments for C. parapsilosis infections. The main results of our study showed the efficacy of antifungal inventions. Among different target outcomes, with the evidence-network analysis, the efficacy of CAS was better than that of other drugs for treating C. parapsilosis infections from both the direct and indirect analyses of the included studies. These findings were slightly inconsistent with those of a previous prospective cohort study exploring the effectiveness of echinocandins including micafungin, caspofungin, and anidulafungin as empirical antifungal drugs for the treatment of invasive Candida infections, which found no significant difference. However, in the present study focused on the treatment efficacy of antifungal drugs, we revealed that the efficacy of antifungal treatment in the echinocandin group was significantly lower than that in the other groups.
CAS is an echinocandin that inhibits fungal growth by the inhibition of 1,3-β-glucan synthase and preventing fungal cell wall synthesis. CAS is being increasingly used as first-line therapy for invasive candidiasis. Clinical studies and in vitro susceptibility data have indicated that CAS is at least as active as AMB and FLU in the treatment of invasive candidiasis, and according to empirical therapy, caspofungin was superior to AMP and voriconazole for the outcome of survival [34]. Additionally, previous studies demonstrated that CAS is effective against C. parapsilosis and active in experimental systemic candidiasis caused by C. parapsilosis. More importantly, our study further confirmed that CAS showed a preferable effect on decreasing the risk of 30 day mortality.
This study has certain limitations. First, there is still a lack of similar high-quality clinical trials, and the number of included studies and the sample sizes were small. Second, the criteria for determining the effectiveness outcomes could not determine whether they were uniform. Third, the sample size for CAS treatment in the included studies was much larger than those for other treatments; therefore, the results may lead to a conclusion in favour of CAS treatment. Fourthly, the included studies did not cover all primary and secondary outcomes or the dosage of antifungal drugs. Finally, there may be sampling bias from using studies published before June, 2020 and new trials relevant to this topic may be published or updated later. The meta-analysis could be updated in the future with enough time to check for enough new evidence.
In summary, this study systematically evaluated the effectiveness and safety of antifungal drugs, analysed and summarized the status and problems of fungal drugs in clinical treatment, and aimed to help clinicians choose the most appropriate antifungal drugs and improve the level of clinical treatment. Additionally, the study provides useful data for the development of standardized dosing regimens and standardized diagnostic criteria, and high-quality, multi-centred and real-word studies with a long-term follow-up are needed to further explore and verify the efficacy of antifungal drugs for the prevention and treatment of C. parapsilosis infection.
Availability of data and material
The datasets generated and analysed in the present study are available from the corresponding author upon reasonable request.
Funding information
The authors received no specific grant from any funding agency.
Acknowledgements
Editorial assistance was provided by Medjaden Bioscience Limited. This assistance was funded by MSD China.
Author contributions
Q.Z., conceived and designed study. Z.Q., H.Y., Z.S. and L.J., collected and assembled the data. Z.Q., H.Y. and Q.Z., performed or supervised analyses, interpreted the results. Z.Q. and H.Y., wrote sections of the initial draft. Z.S., L.J. and Q.Z., provided substantive suggestions for revision. All authors reviewed and approved final version of the paper.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: AIDS, acquired immune deficiency syndrome; AMP, amphotericin B; AND, anidulafungin; CAS, caspofungin; CIs, confidence intervals; CNKI, China National Knowledge Infrastructure; C. parapsilosis, Candida parapsilosis; FLU, fluconazole; ISA, isavuconazole; MIC, micafungin; RCTs, randomized controlled trials; SUCRA, surface under the cumulative ranking curve.
References
- 1.Almirante B, Rodríguez D, Cuenca-Estrella M, Almela M, Sanchez F, et al. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2006;44:1681–1685. doi: 10.1128/jcm.44.5.1681-1685.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang MR, Correia FP, Costa LC, Xavier PC, Palhares DB, et al. Candida bloodstream infection: data from a teaching hospital in Mato Grosso do Sul. Rev Inst Med Trop Sao Paulo. 2008;50:265–268.:S0036-46652008000500003. doi: 10.1590/s0036-46652008000500003. [DOI] [PubMed] [Google Scholar]
- 3.Saxen H, Virtanen M, Carlson P, Hoppu K, Pohjavuori M, et al. Neonatal Candida parapsilosis outbreak with a high case fatality rate. Pediatr Infect Dis J. 1995;14:776–781. doi: 10.1097/00006454-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Quindós G. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev Iberoam Micol. 2014;31:42–48.:S1130-1406(13)00101-0. doi: 10.1016/j.riam.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Xiao M, Chen SC, Kong F, Sun ZY, et al. In vitro susceptibilities of yeast species to fluconazole and voriconazole as determined by the 2010 National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J Clin Microbiol. 2012;50:3952–3959. doi: 10.1128/jcm.01130-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy I, Rubin LG, Vasishtha S, Tucci V, Sood SK. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin Infect Dis. 1998;26:1086–1088. doi: 10.1086/520277. [DOI] [PubMed] [Google Scholar]
- 7.Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21:606–625. doi: 10.1128/cmr.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonassoli LA, Bertoli M, Svidzinski TI. High frequency of Candida parapsilosis on the hands of healthy hosts. J Hosp Infect. 2005;59:159–162. doi: 10.1016/j.jhin.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Clark TA, Slavinski SA, Morgan J, Lott T, Arthington-Skaggs BA, et al. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J Clin Microbiol. 2004;42:4468–4472. doi: 10.1128/jcm.42.10.4468-4472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosun I, Akyuz Z, Guler NC, Gulmez D, Bayramoglu G, et al. Distribution, virulence attributes and antifungal susceptibility patterns of Candida parapsilosis complex strains isolated from clinical samples. Med Mycol. 2013;51:483–492. doi: 10.3109/13693786.2012.745953. [DOI] [PubMed] [Google Scholar]
- 11.Jebabli N, Salouage I, Gaies E, Trabelsi S, Charfi R, et al. Interaction between tacrolimus and azole antifugal drugs. Drug Saf. 2011 [Google Scholar]
- 12.Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med. 2012;125:S3–13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Chen JF, Miao CY, Xu P. Advance in research for clinical pharmacokinetics on antifugal drug of voriconazole. Chinese Journal of Clinical Pharmacology and Therapeutics. 2015;9 [Google Scholar]
- 14.Hua S, Huang J, Wu Z, Feng Z. A comparison study between Candida parapsilosis sepsis and Candida albicans sepsis in preterm infants. Turk J Pediatr. 2012;54:502–508. [PubMed] [Google Scholar]
- 15.Rex JH, Bennett JE, Sugar AM, Pappas PG, van der Horst CM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med. 1994;331:1325–1330. doi: 10.1056/nejm199411173312001. [DOI] [PubMed] [Google Scholar]
- 16.Mora-Duarte J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347:2020–2029. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 17.Colombo AL, Perfect J, DiNubile M, Bartizal K, Motyl M, et al. Global distribution and outcomes for Candida species causing invasive candidiasis: results from an international randomized double-blind study of caspofungin versus amphotericin B for the treatment of invasive candidiasis. Eur J Clin Microbiol Infect Dis. 2003;22:470–474. doi: 10.1007/s10096-003-0973-8. [DOI] [PubMed] [Google Scholar]
- 18.Pappas PG, Rotstein CM, Betts RF, Nucci M, Talwar D, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45:883–893. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 19.Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356:2472–2482. doi: 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]
- 20.Kuse ER, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369:1519–1527.:S0140-6736(07)60605-9. doi: 10.1016/s0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 21.Kullberg BJ, Viscoli C, Pappas PG, Vazquez J, Ostrosky-Zeichner L, et al. Isavuconazole Versus Caspofungin in the Treatment of Candidemia and Other Invasive Candida Infections. Clin Infect Dis. 2019;68:1981–1989. doi: 10.1093/cid/ciy827. [DOI] [PubMed] [Google Scholar]
- 22.Gauna TT, Oshiro E, Luzio YC, Paniago AM, Pontes ER, et al. Bloodstream infection in patients with end-stage renal disease in a teaching hospital in central-western Brazil. Rev Soc Bras Med Trop. 2013;46:426–432. doi: 10.1590/0037-8682-0060-2013. [DOI] [PubMed] [Google Scholar]
- 23.Girmenia C, Martino P, De Bernardis F, Gentile G, Boccanera M, et al. Rising incidence of Candida parapsilosis fungemia in patients with hematologic malignancies: clinical aspects, predisposing factors, and differential pathogenicity of the causative strains. Clin Infect Dis. 1996;23:506–514. doi: 10.1093/clinids/23.3.506. [DOI] [PubMed] [Google Scholar]
- 24.Huang YC, Lin TY, Leu HS, Peng HL, Wu JH, et al. Outbreak of Candida parapsilosis fungemia in neonatal intensive care units: clinical implications and genotyping analysis. Infection. 1999;27:97–102. doi: 10.1007/bf02560505. [DOI] [PubMed] [Google Scholar]
- 25.Borghi E, Sciota R, Iatta R, Biassoni C, Montagna MT, et al. Characterization of Candida parapsilosis complex strains isolated from invasive fungal infections. Eur J Clin Microbiol Infect Dis. 2011;30:1437–1441. doi: 10.1007/s10096-011-1242-x. [DOI] [PubMed] [Google Scholar]
- 26.Cisneros Herreros JM, Cordero Matía E. Therapeutic armamentarium against systemic fungal infections. Clin Microbiol Infect. 2006;12:53–64. doi: 10.1111/j.1469-0691.2006.01606.x. [DOI] [Google Scholar]
- 27.Kohno S, Izumikawa K, Yoshida M, Takesue Y, Oka S, et al. A double-blind comparative study of the safety and efficacy of caspofungin versus micafungin in the treatment of candidiasis and aspergillosis. Eur J Clin Microbiol Infect Dis. 2013;32:387–397. doi: 10.1007/s10096-012-1754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai A, Yamagishi Y, Mikamo H. In vitro efficacy of liposomal amphotericin B, micafungin and fluconazole against non-albicans Candida species biofilms. J Infect Chemother. 2015;21:647–653.:S1341-321X(15)00132-4. doi: 10.1016/j.jiac.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Wang F, Wang LU. Analysis of the utilization of antifungal agents in our hospital during 2007-2009. Chinese General Practice. 2012;15:324–327. [Google Scholar]
- 30.Feng JW. Clinical analysis of candidal vaginitis patients and drug resistance. China Tropical Medicine. 2007;39:574–580. [Google Scholar]
- 31.Yamaguchi H. Basal studies and preclinical evaluations of newly developed antifungal agents. Nippon Ishinkin Gakkai Zasshi. 2009;26:116–125. doi: 10.3314/jjmm.45.83. [DOI] [PubMed] [Google Scholar]
- 32.Zeng R, Li M, Chen Q, Wang L, GX LV, et al. Dynamic study on the susceptibilities of caspofungin and micafungin to Candida species in vitro . Chinese Journal of Mycology. 2011;5 [Google Scholar]
- 33.Nawaz A, Pärnänen P, Kari K, Meurman JH. Proteolytic activity and cytokine up-regulation by non-albicans Candida albicans . Arch Microbiol. 2015;197:533–537. doi: 10.1007/s00203-015-1083-6. [DOI] [PubMed] [Google Scholar]
- 34.Freemantle N, Tharmanathan P, Herbrecht R. Systematic review and mixed treatment comparison of randomized evidence for empirical, pre-emptive and directed treatment strategies for invasive mould disease. J Antimicrob Chemother. 2011;66 Suppl 1:i25–35. doi: 10.1093/jac/dkq439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed in the present study are available from the corresponding author upon reasonable request.