Abstract
Mannheimia haemolytica serotype A2 is the main bacterial causative agent of ovine mannheimiosis, a disease that leads to substantial economic losses for livestock farmers. Several virulence factors allow M. haemolytica to colonize the lungs and establish infection. Virulence factors can be directly secreted into the environment by bacteria but are also released through outer membrane vesicles (OMVs). In addition, due to the abuse of antibiotics in the treatment of this disease, multidrug-resistant bacterial strains of M. haemolytica have emerged. One therapeutic alternative to antibiotics or an adjuvant to be used in combination with antibiotics could be lactoferrin (Lf), a multifunctional cationic glycoprotein of the mammalian innate immune system to which no bacterial resistance has been reported. The aim of this work was to determine the effect of bovine iron-free Lf (apo-BLf) on the production and secretion of proteases into culture supernatant (CS) and on their release in OMVs. Zymography assays showed that addition of sub-MIC concentrations of apo-BLf to M. haemolytica cultures inhibited protease secretion without affecting culture growth. Biochemical characterization revealed that these proteases were mainly cysteine- and metalloproteases. The secretion of a 100 kDa metalloprotease was inhibited by sub-MIC concentrations of apo-BLf since this protease was present in the cytoplasm and OMVs but not in CS proteins, as corroborated by Western blotting. On the other hand, proteases produced by M. haemolytica caused cleavage of apo-BLf. However, when Lf is cleaved, peptides known as lactoferricins, which are more bactericidal than natural Lf, can be produced. M. haemolytica A2 protease-mediated degradation of host tissue proteins could be an important virulence factor during the infectious process of pneumonia in ovines. The mechanism of M. haemolytica protease secretion could be inhibited by treatment with apo-BLf in animals.
Keywords: lactoferrin, Mannheimia haemolytica, proteases, zymography
Introduction
Mannheimia haemolytica is a Gram-negative coccobacillus that is the causative agent of mannheimiosis in ruminants, leading to significant economic losses for livestock farmers [1, 2]. M. haemolytica possesses multiple virulence factors, including a secreted potent leukotoxin (Lkt) [3], outer membrane (OM) lipopolysaccharide (LPS) [4], a 60 kDa adhesin, capsule, the outer membrane proteins (OMPs) FbpA (35 kDa), TbpA (100 kDa) and TbpB (75 kDa) for binding host transferrin [5], a neuraminidase (200 kDa), and a 35 kDa zinc metalloglycoprotease [6, 7]. Serotypes A1 and A2 are the main types responsible for mannheimiosis in cattle and sheep, respectively. We have reported that M. haemolytica A2 releases outer membrane vesicles (OMVs), which contain an adhesin, LPS, Lkt, OMPs and plasmids [8].
The treatment of mannheimiosis is complicated because it is a multifactorial disease in which viruses and stress suffered by the animals are also implied. However, antimicrobial therapy is the method most frequently used. In addition, in most cases of pneumonia, treatment is started before samples are collected and sent to a diagnostic laboratory to identify the most effective antimicrobial for treatment. Elimination of the desired pathogen is not guaranteed with the administration of an incorrect treatment, which most likely results in the selection of antibiotic-resistant bacteria [9, 10]. In particular, there are studies of M. haemolytica strains in which resistance to streptomycin (81.6 %) and gentamicin (24.4 %) has been found [11]. In other studies, resistance to ampicillin, enrofloxacin, ofloxacin and penicillin has been reported [12, 13]. Due to the increase in frequency of multidrug-resistant strains of M. haemolytica in farms, new antimicrobial therapeutic alternatives are being explored. One remarkable option is the use of lactoferrin (Lf), a cationic glycoprotein of the mammalian innate immune system that is highly conserved among mammals. Lf has a molecular mass of 75–80 kDa [14], depending on the origin, and has a bilobal structure (N- and C-terminal lobes). The N-terminal region functions as a serine-protease domain [15]. Each lobe can be reversibly bound to a Fe3+ ion. The molecule without bound iron is called apo-Lf, and when it binds one or two iron atoms, it is called monoferric Lf and holo-Lf, respectively. Lf is present in mammalian exocrine secretions and secondary granules of neutrophils and is a multifunctional protein with microbiostatic, microbicidal, antiviral, immunomodulatory, and anticarcinogenic activities [16]. Lf limits the growth of bacteria, fungi and protozoa through the sequestration of iron and, together with transferrin, maintains a concentration of 10−18 M iron in body fluids. This concentration is lower than the iron requirements of microorganisms. Lf can destabilize and induce bactericidal activity in Gram-positive and Gram-negative bacteria either by binding to cell wall teichoic acids or by binding to LPS, porins, or other proteins present in the OM, respectively [17–19]. In addition, there are reports indicating that Lf affects the binding of bacteria to host cells and degrades bacterial proteins via its serine-protease activity [20–22]. Our group described two OMPs in M. haemolytica serotype A1 (mainly affects cattle) that bind bovine Lf (BLf), a heat-modifiable OmpA (32.9 kDa) and a porin (34.2 kDa) [23], and showed that apo-BLf is bactericidal for M. haemolytica serotypes 1 and 2 [23, 24]. Furthermore, we also reported that apo-BLf produced morphological changes in the OM of OMVs and whole bacteria, altering their permeability and increasing the number of OMVs. Apo-BLf increased Lkt secretion into culture supernatant (CS) but not the levels present in OMVs. On the other hand, the Lkt released from cells grown in medium supplemented with apo-BLf and that contained in OMVs presented cytotoxic activity against ovine macrophages [24]. The foremost secreted virulence factor reported for M. haemolytica is Lkt, and proteases are less studied in this bacterial species. We have reported cysteine- and metalloprotease activity against gelatin across a broad pH range, a standard substrate used to detect the activity of these enzymes, in M. haemolytica A2. Proteases showing different molecular masses were found to be secreted by this bacterium into CS and released through OMVs. In addition, M. haemolytica A2 produces a 100 kDa protease that cross-reacts with antibodies against a zinc-metalloprotease of Actinobacillus pleuropneumoniae , a species of the Pasteurellaceae family, which M. haemolytica also belongs to [25].
The aim of this work was to determine the effect of apo-BLf on the secretion of proteases into CS and their release in OMVs in M. haemolytica A2. Preincubation of bacterial cultures with sub-MIC concentrations of apo-BLf caused inhibition of cysteine- and metalloprotease secretion and activity against apo-BLf and gelatin, as determined by zymography of both CS and OMV proteins. In addition, M. haemolytica proteases from bacteria cultured in brain-hearth infusion (BHI) medium caused cleavage of apo-BLf. However, when Lf is cleaved, it can produce peptides named lactoferricins (Lfcins), which are also bactericidal and thus counterproductive to bacteria. Therefore, apo-BLf could be used as a prophylactic treatment or complement to antibiotics against M. haemolytica .
Methods
Bacterial strain and culture
A field strain of M. haemolytica A2 that was obtained in a previous work was used in this study [25]. This strain was isolated from lamb lung tissue with pneumonic lesions characteristic of the disease (pulmonary consolidation of cranial lobes, presence of fibrin in interseptal spaces and pleura, and purulent exudate on the cutting surface). The strain was characterized by biochemical methods and identified by conventional culture and API 20E tests; subsequently, it was cultured on sheep blood agar at 37 °C for 24 h before experiments.
Viability of M. haemolytica A2 in the presence of bovine apo-lactoferrin
In previous work, we reported the viability and minimum inhibitory concentration (MIC) for apo-BLf (NutriScience, 97 % purity, containing 5 mg per 100 g iron) in M. haemolytica A2 cultures in vitro [24]. The viability and MIC in the presence of apo-BLf was confirmed in this work to standardize the sub-MIC doses to be used to study protease secretion. The effect of apo-BLf on the growth of M. haemolytica A2 was determined using different concentrations of apo-BLf (0, 3.5, 7.5, 11.5 and 15.5 µM) and incubating at 37 °C. A sample of culture (100 µl) was taken out at 3, 6, and 9 h and diluted in saline solution, the bacteria were plated on sheep blood agar, and the CFU were recorded. To determine the MIC of apo-BLf it was employed the method of microdilution in broth. Bacteria (106 CFU) were incubated with 7.5, 11.5, 15.5, 19.5 and 23.5 µM apo-BLf up to 24 h in agitation (200 r.p.m.) or static conditions in sterile 96-well plates. After incubation, growth or no growth was recorded by OD at 595 nm in a plate reader (Ultra Microplate Reader Elx808; Bio-Tek Instrumentals, Inc. USA). All experiments were repeated five times in triplicate [26, 27].
Proteins in culture supernatant (CS) and outer membrane vesicles (OMVs)
Brain hearth infusion (BHI) was the medium used for harvesting CS and OMVs from M. haemolytica . Bacterial growth was carried out by transferring a colony from a pure culture to 5 ml of BHI medium and incubating the culture for 24 h at 37 °C. Then, 5% (500 μl, 1.98×107 CFU) of the culture was transferred to 150 ml of BHI medium in the absence or presence of 1.0 or 3.5 µM apo-BLf (sub-MIC concentrations) for 24 h at 37 °C. Subsequently, the cultures were centrifuged (2600 g for 20 min), and the CS was filtered through a 0.22 µm mixed cellulose ester membrane (Millipore, Ireland). The filtered CS was ultracentrifuged (150 000 g for 3 h at 4 °C). The pellet containing the OMVs was suspended in 300 µl of 1× phosphate-buffered saline (PBS) and stored at −70 °C until use. Afterward, the CS obtained was mixed in a 1 : 3 ratio (sample:absolute ethanol) for 1 h at −20 °C. Then, it was subjected to centrifugation (7 500 g for 40 min at 4 °C). The supernatant was removed, and the resulting precipitate was resuspended in 300 µl of 1× PBS; this sample corresponded to precipitated proteins contained in the CS [8, 25]. The concentration of precipitated proteins in the CS and OMVs was determined by colorimetry using Bradford's method [28].
Extraction of cell fractions (proteins from the cytoplasm, inner membrane (IM), OM, and periplasm)
Bacterial culture was carried out as mentioned above. The supernatant was then discarded, and the last drops of liquid were carefully removed with a pipette. The pellet was gently suspended in 1 ml of Tris-sucrose-EDTA (TSE) buffer: 200 mM Tris–HCl (pH 8.0), 500 mM sucrose and 1 mM EDTA. The cells were incubated in TSE on ice for 30 min. Subsequently, the cell suspension was centrifuged at 16 000 g for 30 min at 4 °C. The obtained pellet corresponded to cytoplasmic proteins. The supernatant was centrifuged at 16 000 g for 30 min at 4 °C; the obtained pellet corresponded to the IM proteins, and the supernatant contained the periplasm and OM proteins. For separation of the OM proteins from the periplasmic proteins, the supernatant was centrifuged at 100 000 g for 1 h at 4 °C. The pellet from this step contained the OM proteins, and the supernatant contained the periplasmic proteins [29]. The concentration of proteins in the cytoplasm, IM, OM and periplasm was determined by colorimetry using Bradford's method [28].
Zymograms of CS and OMV proteins from M. haemolytica A2
To determine the effect of apo-BLf on the proteolytic activity of M. haemolytica A2, the zymography method was used. The protein amount was adjusted to 15 µg (CS and OMVs). Electrophoresis was performed on polyacrylamide gels copolymerized with 0.2 % apo-BLf as substrate, using the same concentration of gelatin as a control (100 V, 2 h, 4 °C). Afterward, the gels were stained with 0.5 % Coomassie Brilliant Blue R-250 (Bio-Rad, Germany) for 1 h at 37 °C and destained with a washing solution until the desired bands were visualized; proteolytic activity was identified as a clear band on a blue background. Finally, the gels were preserved in 10 % acetic acid [30].
To determine the effect of pH on the proteolytic activity of M. haemolytica against apo-BLf, after performing electrophoresis, the gels were incubated overnight at 37 °C with different buffers, all from Sigma Aldrich (USA): 100 mM sodium acetate (pH 5.0), 100 mM Tris-CaCl2 (pH 7.0) and 100 mM Na2CO3-NaHCO3 (pH 9.0). To identify the type of protease, different inhibitors were added and incubated with the CS or OMV proteins for 30 min at 22 °C. The following inhibitors (Sigma) were employed: 5 mM p-hydroxymercurybenzoate (pHMB) for cysteine proteases; 3 mM phenylmethylsulfonylfluoride (PMSF) for serine proteases; and 3 mM EGTA, 3 mM EDTA or 10 mM o-phenanthroline for metalloproteases. Finally, the gels were stained with Coomassie blue and destained with a washing solution until the desired bands were visualized [25]. All assays were performed in five independent experiments.
Immunoblot analysis
To determine whether apo-BLf can affect the production and secretion of a previously identified 100 kDa protease in CS and OMVs of M. haemolytica [25], bacterial cultures to which a sub-MIC concentration of apo-BLf (1.0 or 3.5 µM) was added were used. In addition, bacteria grown in the presence of 3.5 µM apo-BLf were fractionated according to the methodology of Shu Quan et al. [29], and proteins from the cytoplasm, IM, OM and periplasm were obtained. After electrophoretic separation by 10 % SDS-PAGE, the proteins were transferred to a nitrocellulose membrane (Sigma) for 1 h at 400 mA according to Towbin et al. [31]. The membranes were blocked with skim milk for 2 h at 22 °C, and the samples were incubated overnight with a rabbit anti- A. pleuropneumoniae 100 kDa protease (1 : 2000) antiserum at 4 °C [32]. The samples were washed three times with PBS-Tween (0.05 %) and incubated with a secondary anti-rabbit-HRP antibody (1 : 3000) (Sigma) for 1 h at 22 °C. The membrane was washed seven times, and signal was revealed with a luminol kit reagent (Santa Cruz Biotechnology, Germany) using an Odyssey FC imaging system (LI-COR).
Statistical analysis
The experimental design was carried out with repeated observations. A comparison of models with regression analysis was used with a significance level of P=0.05. Differences were considered statistically significant when the P value was less than 0.05. The statistical analysis was performed using GraphPad Prism Software 9.2.0 (GraphPad, CA, USA).
Results
Effect of apo-BLf on the growth of M. haemolytica A2
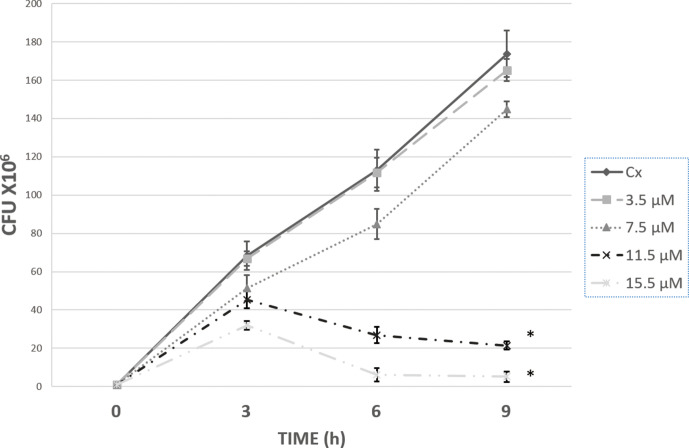
It is known that apo-BLf can be bacteriostatic and/or bactericidal to different pathogens. To determine the concentration of apo-BLf in which its effect on proteases could be studied without affecting culture viability, different concentrations of apo-BLf were first added to M. haemolytica A2 cultures growing in BHI medium. Fig. 1 shows that addition of 11.5 µM apo-BLf drastically diminished culture growth after 3 h of incubation and practically eliminated bacterial growth at 9 h.
Fig. 1.
Viability of M. haemolytica A2 cultures in the presence of apo-BLf. The BHI culture medium was supplemented with the apo-BLf concentration indicated. Cultures were started with 106 bacteria and incubated at 37 °C for up to 9 h. At the times indicated, a sample was obtained, diluted, and cultured on BHI agar, and the CFUs were determined. The graph shows the average of five independent experiments, each in triplicate; *P<0.05.
The MIC for apo-BLf was 11.5–15.5 µM (Table S1a, available in the online version of this article); in Table S1b the growth up to 24 h in agitation was recorded as a control. Therefore, it was confirmed that apo-BLf has a microbicidal effect on M. haemolytica A2, and concentrations lower than 11.5 µM were used as sub-MICs for the determination of protease production and secretion.
Proteases in CS and OMVs of M. haemolytica A2 degrade apo-BLf
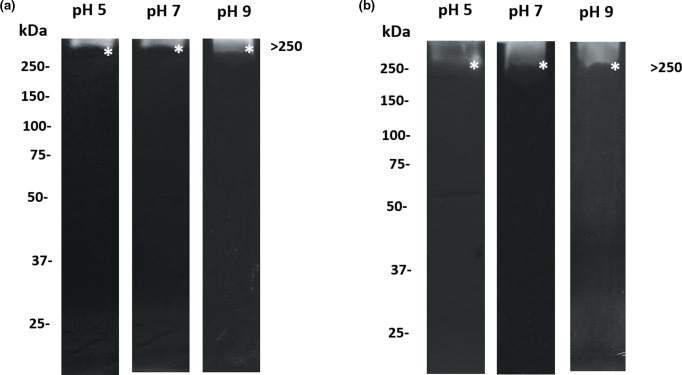
We previously identified different patterns of proteases in CS and OMVs of M. haemolytica A2 in gels copolymerized with gelatin, which is a general substrate used for testing proteolytic activity [25]. In this work, we used the specific substrate apo-BLf to identify and characterize the M. haemolytica A2 proteases that degrade this mammalian glycoprotein since, if apo-BLf is used in treatments for ovines, it could be cleaved by secreted cysteine- and metalloproteases that this bacterium produces and releases into the environment. Zymograms with apo-BLf and CS and OMV proteins are shown in Fig. 2(a, b), respectively. The main proteolytic activity that was consistently observed over a wide pH range in both cases was apparent at a molecular mass of >250 kDa.
Fig. 2.
Zymography with apo-BLf gels containing CS and OMV proteins of M. haemolytica A2. On the left side, the reference molecular masses are indicated; the protein amount was adjusted to 15 µg (CS and OMVs), and electrophoresis was performed on polyacrylamide gels copolymerized with 0.2 % apo-BLf as the substrate (a) CS (b) OMVs. Proteases are marked with an *. A result representative of five independent experiments is shown.
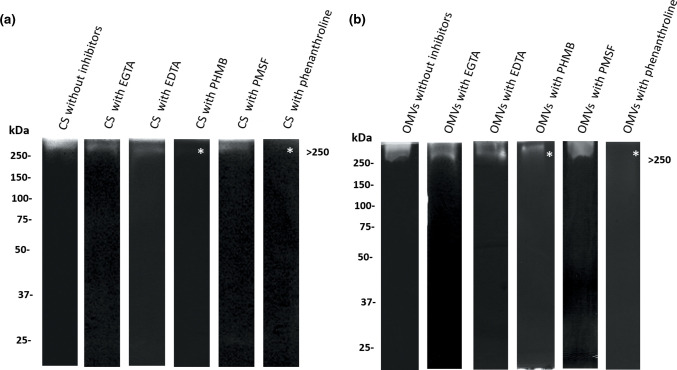
In addition, biochemical characterization of proteases from both samples revealed that inhibitors of cysteine proteases (pHMB) and of zinc metalloproteases (phenanthroline) suppressed the activity against apo-BLf in both CS and OMVs (Fig. 3a, b, respectively).
Fig. 3.
Biochemical characterization of proteases from CS and OMVs of M. haemolytica A2 that degrade apo-BLf (zymography). Left side, reference molecular masses. (a) CS. (b) OMVs. In both CS and OMVs, the >250 kDa protease was inhibited by using PHMB or phenanthroline. Inhibited proteases are marked with an *. A result representative of five independent experiments is shown.
Moreover, protease cleavage prediction software was used (https://prosper.erc.monash.edu.au/) to estimate sites in the BLf molecule sequence susceptible to protease cleavage (https://www.uniprot.org/uniprot/P24627). This evaluation showed different sites where BLf could be degraded by M. haemolytica A2 cysteine- and metalloproteases; thus, it is possible that the bacterial proteases degrade the BLf molecule. However, this result is notably not detrimental to the use of apo-BLf as an antimicrobial against M. haemolytica ; it is beneficial because cleavage of the Lf molecule could promote the production of Lfcins and Lactoferrampin (Lfcin B - short sequence (FKCRRWQWRMKKLG), Lfcin B - long sequence (FKCRRWQWRMKKLGAPSITCVRRAF), Lactoferrampin (DLIWKLLSKAQEKFGKNKSR)), from the N-terminal end, which are known to often have a stronger antimicrobial effect than the native molecule; thus, this cleavage is counterproductive to the bacteria [33] (Fig. S1).
Effect of apo-BLf on protease secretion into the CS and OMVs of M. haemolytica A2
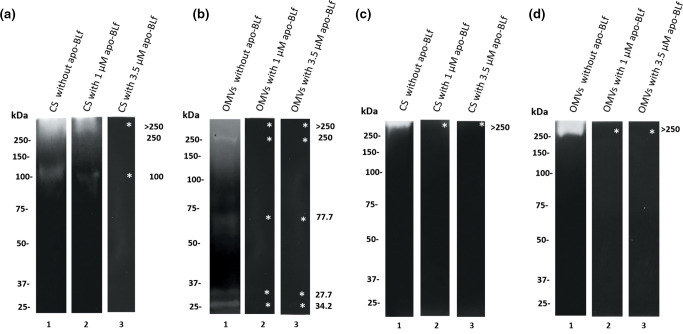
As shown previously, apo-BLf has a bactericidal effect towards M. haemolytica A2 in vitro, with an MIC of 11.5 µM. This effect is mainly produced by destabilization of the bacterial membrane [24]. To study whether apo-BLf affects the secretion of proteases, sub-MIC concentrations of apo-BLf (1 or 3.5 µM) were added to M. haemolytica A2 cultures, and then, the proteases in CS and OMVs were examined. Zymograms copolymerized with apo-BLf or with gelatin as a control were performed (Fig. 4). The protein profile is shown in the Fig. S2.
Fig. 4.
Effect of apo-BLf on the proteases from the CS and OMVs of M. haemolytica A2. Proteolytic activities of the CS and OMVs in zymograms copolymerized with gelatin (a, b) or apo-BLf (c, d), respectively. (a, c). Lane 1, CS proteins from bacteria not incubated with apo-BLf; lanes 2 and 3, CS proteins from bacteria incubated with 1 or 3.5 µM apo-BLf, respectively. (b, d). Lane 1, OMV proteins from bacteria not incubated with apo-BLf; lanes 2 and 3, OMV proteins from bacteria incubated with 1 or 3.5 µM apo-BLf, respectively. Inhibited proteases are marked with an *. A result representative of five independent experiments is shown.
Sub-MIC doses of apo-BLf were used since the effect could not be observed with higher lethal doses. Interestingly, no proteolytic activity was observed when bacteria were grown in the presence of sub-MIC concentrations of apo-BLf, as established by zymography. In CS, proteases that degrade gelatin were not active when tested after growing M. haemolytica with 3.5 µM apo-BLf (Fig. 4a, lane 3, gelatin), while in OMVs, the proteolytic activity was inhibited by 1 µM apo-BLf (Fig. 4b, lanes 2 and 3, gelatin). Remarkably, in the zymograms containing apo-BLf as the substrate, no protease activity was observed at any concentration used (Fig. 4c, d, lanes 2 and 3). This result suggests that apo-BLf affects protease production or secretion into the CS and OMVs in M. haemolytica A2.
Apo-BLf inhibits the secretion of a 100-kDa protease from M. haemolytica A2
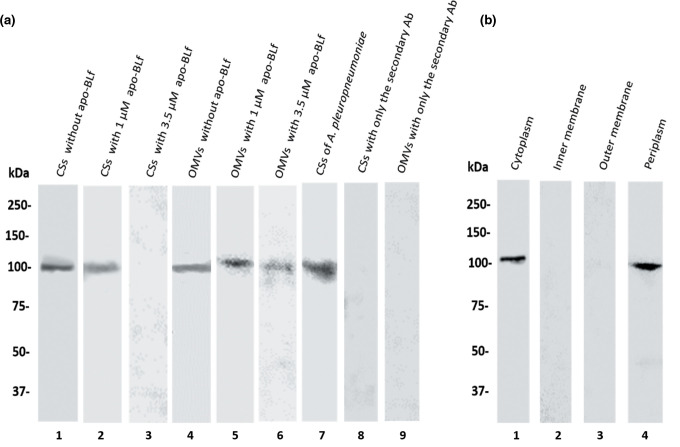
We previously demonstrated that a 100 kDa protease present in the CS and OMVs of M. haemolytica A2 shares homology with the 100 kDa protease of A. pleuropneumoniae serotype 1, which was affirmed based on cross-reactivity by Western blot [25]. Next, the effect of apo-BLf on the secretion of this protease was analysed by Western blotting in CS and OMV proteins (Fig. 5a). When sub-MIC concentrations of apo-BLf were added during the growth of M. haemolytica A2, the 100 kDa protease band in OMVs persisted, indicating that the enzyme was secreted inside the OMVs when these structures were released from the bacteria (Fig. 5a, lanes 4–6). However, 3.5 µM apo-BLf exerted an effect on the secretion of the 100 kDa protease into CS since, in zymograms, no proteolytic activity was found at that molecular mass (Fig. 5a, lane 3). Due to these results, an immunoblot was performed using different cell fractions of M. haemolytica A2. For the proteins of the cytoplasm and periplasm, a single band reacting with the anti-100 kDa protease antibodies was found, suggesting that the 100 kDa protease is produced (Fig. 5b, lanes 1 and 4). However, this protease was not secreted to the CS due to the effect of apo-BLf (Fig. 5a, lanes 4–6).
Fig. 5.
Effect of apo-BLf on the 100 kDa protease of M. haemolytica A2 secreted into CS and released in OMVs. (a) CS and OMVs of M. haemolytica A2. Lane 1, proteins derived from a culture without incubation with apo-BLf; lanes 2 and 3, CS proteins from bacteria cultured with 1 or 3.5 µM apo-BLf, respectively; lane 4, proteins from OMVs of bacteria without incubation with apo-BLf; lanes 5 and 6, OMVs from bacteria cultured with 1 or 3.5 µM apo-BLf, respectively; lane 7, CS proteins of A. pleuropneumoniae as a positive control; 8 and 9, CS and OMVs proteins with only the secondary Ab as a negative control, respectively. (b). Cellular fractions of M. haemolytica A2 from bacteria cultured in the presence of 3.5 µM apo-BLf. Lane 1, cytoplasm; lane 2, inner membrane; lane 3, outer membrane; lane 4, periplasm. A result representative of three independent experiments is shown.
Discussion
M. haemolytica is the main bacterial species involved in the respiratory complex of ruminants, triggering secondary infection after a viral primary infection and causing high economic losses worldwide. Unfortunately, high antimicrobial resistance and low vaccine efficiency contribute to disease progression. Therefore, it is important to study therapeutic alternatives against this microorganism and the different virulence factors that it possesses. The objective of this work was to determine the effect of apo-BLf on the secretion of proteases into CS and on their release in OMVs in M. haemolytica A2. In our study, we confirmed that apo-BLf showed a bactericidal effect. In addition to M. haemolytica , A. pleuropneumoniae [26] and Aggregatibacter actinomycetemcomitans [34, 35] are among the pathogens of the Pasteurellaceae family for which apo-BLf has a bactericidal effect. Our research group reported two proteins that are able to bind both apo-BLf and holo-BLf in M. haemolytica A1, and we established by MALDI-TOF that they correspond to a 32.9 kDa heat-modifiable OMP (OmpA) and a 34.2 kDa porin [23]. The binding of apo-BLf to LPS and these proteins could mediate the destabilization of the OM and consequently cause bacterial death. In the case of a sub-MIC concentration, the bacterial OM could also be damaged, although possibly to a lesser extent. Interestingly, the MIC found in M. haemolytica A2 (11.5 µM) was lower than that reported in other bacteria, such as Escherichia coli K88 (104 µM), E. coli FT28 (21.7 µM), Pseudomonas intermedia 25 611 (104 μM) and Staphylococcus aureus (34.7 µM). It should be noted that there are bacteria for which apo-BLf has only a bacteriostatic effect, such as Helicobacter pylori and enteroaggregative E. coli 042 [22, 36–39].
Proteases are an integral component of existing life on Earth, including animals, plants and microbes. Proteases are an essential virulence factor, and their secretion into tissues is an important pathogenicity mechanism. Inside a host, one of the main functions of pathogen protease secretion is to destroy the extracellular matrix (ECM), resulting in the dispersal of collagen fibrils and weakening of the stroma of different tissues [40, 41]. In addition, proteases secreted by bacteria can degrade other host components, such as those of the immune response, and contribute particularly to colonization and subsequently to disease progression [42, 43]. In this study, we found a constant proteolytic activity with a molecular weight of >250 kDa that maintained cleavage activity over a wide pH range in both CS and OMV samples. This protease could be a multimeric protein, such as the protease secreted by A. pleuropneumoniae reported by Negrete et al. in 1998 [44].
The characterization and identification of proteases that degrade mammalian Lf have important significance regarding the design of novel strategies to inhibit their activity, such as antibodies or synthetic peptides for vaccines coupled or not with Lf to generate protective immune responses. This type of strategy has been reported in an in vivo model of pulmonary tuberculosis, where the administration of Bacillus Calmette-Guerin (BCG) with emulsified BLf and Freund’s adjuvant reduced the mycobacterial load in the lungs after challenge with Mycobacterium tuberculosis in aerosols more efficiently than a mixture of BCG and Freund’s adjuvant. The authors found that the expression of the proinflammatory cytokines TNF-α and IL-1β was downregulated due to BLf and the adjuvant. Furthermore, the oral administration of this mixture in water reduced lung inflammation, with an increase in IFN levels, compared with that in animals vaccinated with BCG alone [45].
Interestingly, we found that sub-MIC levels of apo-BLf in M. haemolytica A2 cultures inhibited proteolytic activities in CS and OMVs in zymograms using either gelatin or apo-BLf. At least two mechanisms could explain these results: 1, due to the activity of apo-BLf on the bacterial surface, protease secretion could be impaired; and/or 2, direct binding of apo-BLf to bacterial proteases could occur, preventing the degradation of this substrate. In this study, an inoculum of 106 bacteria was used, and a minimum of 11.5 µM apo-BLf was necessary for the bactericidal effect; however, in the disease process involving M. haemolytica , the amount of bacteria could be greater, and the endogenous Lf concentration might not be sufficient for sheep to combat the disease. Furthermore, mannheimiosis development is a complex process in which other factors, including stress from animal handling, age, weaning time, immunological factors, and mycoplasma and viral infections (syncytial virus, parainfluenza 3, rhinotracheitis and adenovirus), enhance the problem [46]. In addition to infection and inflammation, a disbalance of iron homeostasis has been observed in pneumonia, and high levels of iron (up to >100 µM) in airway secretions have been recorded. Excess iron can result from altered expression of the main proteins involved in iron homeostasis, such as ferritin, transferrin and ferroportin. Overall, all the changes in these proteins influence the rate of iron accumulation in the lower respiratory tract. Iron overload in airways increases bacterial growth, thus worsening the inflammatory status and host damage. Therefore, infection, inflammation, and iron disorders reinforce each other, beginning a dangerous vicious cycle that is difficult to counteract and solve in vivo [47].
In the literature, there are reports suggesting that Lf has effects on different virulence factors of Gram-negative bacteria. Several observations have shown that Lf causes LPS release, suggesting that Lf has membrane-permeabilizing activity. Our group described the release of LPS from M. haemolytica A2 grown in medium supplemented with apo-BLf, thereby destabilizing LPS, inducing OM damage and causing increased cell permeability [24]. In the enteropathogenic pathotype of E. coli (EPEC), which causes disease of the digestive tract in humans, it was reported that Lf damages the type III secretion system, the main secretion system of virulence factors of this bacterium [48]. It is important to mention that until now, there has been no available evidence of the mechanism of protease secretion in M. haemolytica .
Here, we provide evidence for the first time that OMVs could participate in the secretion of proteases that cause damage to an important protein from the innate immune system. Preliminary results showed that the proteases of this bacterium also cleave bovine haemoglobin (data not shown). Interestingly, it has been reported that Lf blocks actin polymerization mediated by EPEC in HEp2 cells and blocks the hemolysis induced by this microorganism. The mechanism of this inhibition was shown to be the Lf-mediated degradation of secreted proteins necessary for bacterial contact and pore formation, particularly EspB [48]. In Shigella flexneri , the effect of Lf on the secreted proteins was similar to that in EPEC. Lf induced secretion and caused the degradation of two key virulence proteins, the B and C antigens (IpaB and IpaC), encoded by the invasion plasmid, which are necessary for the invasion of mammalian cells by this pathogen [49]. Likewise, in Haemophilus influenzae , BLf attenuated its pathogenic potential by selectively inactivating the IgA1 protease and Hap adhesin at sites rich in arginine. These two self-transported proteins are presumed to facilitate colonization. Lf, with its serine protease character, cleaved the IgA1 protease and Hap adhesin from the bacterial cell wall; both actions were inhibited by serine protease inhibitors [50, 51]. In this work, we did not find serine protease activity of apo-BLf against proteases secreted from M. haemolytica (data not shown).
Lactoferrin represents a nonspecific defence mechanism since it is an innate immune system protein. As M. haemolytica is susceptible to BLf, it could be used as a therapeutic alternative against mannheimiosis; a benefit for its use is that Lf is an immunomodulatory protein [52]. The treatment could be affordable since BLf is commercially available at low cost, and there are no reports of resistance to Lf, in contrast to the various antimicrobials experimentally tested in vitro against M. haemolytica and the high resistance found to most of the antibiotics used in the field [13, 53]. Additionally, it is important to consider that one aspect of the regulation of antibacterial activity is related to the iron saturation of Lf, showing selective activity against bacterial families. Fortunately, holo-BLf is not used as a sole iron source by M. haemolytica [23]. On the other hand, Lf can display divergent effects on the intestinal microbiota, increasing, decreasing, or not affecting the viability and proliferation of bacteria [54]. However, it has been reported that Lf causes direct damage to pathogenic bacteria; some in vitro and in vivo studies using sepsis models showed that recombinant Lf induced immunomodulation of macrophages via proinflammatory cytokine elicitation, promoting their bacterial killing activity [54–56]; thus, Lf is a possible alternative in the treatment of mannheimiosis. It is important to perform in vivo assays to determine the optimal concentration of apo-BLf to be used in ovines and other ruminants. The susceptibility of M. haemolytica A2 and A1 to apo-BLf suggests its use for elimination of this and other pathogenic members of the Pasteurellaceae family. Moreover, since M. haemolytica is involved in a multifactorial disease in which associated viruses are involved, Lf might also show antiviral properties in mannheimiosis. In other viruses, the antiviral properties are related to the ability of Lf to block the cellular attachment or replication of a virus by inducing type I interferons (α/β) with antiviral action [57].
Conclusion
Apo-BLf has a bactericidal effect and affects the production/secretion of proteases in M. haemolytica ; the effect of BLf is on proteases that are secreted and those that are released in vesicles. This result is important since proteases degrade tissue and immune proteins helping bacteria to advance inside the host. It is necessary to carry out studies in ovine to establish the dose of apo-BLf required to neutralize the pneumonic process caused by M. haemolytica . In acute cases of the disease, apo-BLf could be used as an adjuvant to antibiotic treatment and probably has a better and more rapid effect, diminishing the dose of antibiotics needed. In this case, it has been shown that Lf helps to the membrane permeabilization, and after that the antibiotic can penetrate the membrane. The use of BLf could also be as a preventive alternative in clinically healthy ruminants. It is important to study the effect that apo-BLf could have on other virulence factors and mechanisms.
Supplementary Data
Funding information
The first author received scholarships from Conacyt No. 581720/599200 and 581720/786869. This work was partly supported by Conacyt, Mexico, grant CB-A1-S-8989 (Mireya de la Garza).
Acknowledgements
We thank Dr Magda Reyes López and Carlos Villasana for their technical assistance.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: apo-BLf, bovine apo-lactoferrin (iron-free bovine Lf); BHI, brain hearth infusion; CFU, colony-forming units; CS, culture supernatant; Lf, lactoferrin; Lfcins, lactoferricins; Lkt, leukotoxin; LPS, lipopolysaccharide; MIC, minimum inhibitory concentration; OD, optical density; OM, outer membrane; OMPs, outer membrane proteins; OMVs, outer membrane vesicles; PBS, phosphate buffered saline.
One supplementary table and two supplementary figures are available with the online version of this article.
References
- 1.Dereck AM, Simons KR, Confer AW, Panciera RJ, Clinkenberd KD. Pasteurella haemolytica antigens associated with resistence to pneumonic pasteurelosis. Infect Immun. 1989;57:711–716. doi: 10.1128/iai.57.3.711-716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain R, Mahmood F, Ali HM, Bacterial SAB. PCR and clinico-pathological diagnosis of naturally occurring pneumonic pasturellosis (mannheimiosis) during subtropical climate in sheep. Microb Pathog. 2017;112:176–181. doi: 10.1016/j.micpath.2017.09.061. [DOI] [PubMed] [Google Scholar]
- 3.Jeyaseelan S, Sreevatsan S, Maheswaran SK. Role of Mannheimia haemolytica leukotoxin in the pathogenesis of bovine pneumonic pasteurellosis. Anim Health Res Rev. 2002;3:69–82. doi: 10.1079/ahrr200242. [DOI] [PubMed] [Google Scholar]
- 4.Paulsen DB, Confer AW, Clinkenbeard KD, Mosier DA. Pasteurella haemolytica lipopolysaccharide-induced cytotoxicity in bovine pulmonary artery endothelial monolayers: inhibition by indomethacin. Vet Pathol. 1995;32:173–183. doi: 10.1177/030098589503200211. [DOI] [PubMed] [Google Scholar]
- 5.Belzer CA. Characterization and identification of the immunoreactive 35 kilodalton periplasmic iron-regulated protein of Mannheimia (Pasteurella) haemolvtica . Vet Méx. 2001;40:293–314. [Google Scholar]
- 6.Straus DC, Jolley WL, Purdy CW. Characterization of neuraminidases produced by various serotypes of pasteurella-haemolytica. Infect Immun. 1993;61:4669–4674. doi: 10.1128/iai.61.11.4669-4674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdullah KM, Lo RY, Mellors A. Distribution of glycoprotease activity and the glycoprotease gene among serotypes of Pasteurella haemolytica . Biochem Soc Trans. 1990;18:901–903. doi: 10.1042/bst0180901. [DOI] [PubMed] [Google Scholar]
- 8.González RC, Trigo TF, Reyes LM, León SN, Godínez VD, et al. Characterization of microvesicles of Mannheimia haemolytica serotype A1 (Reference strain) and serotype A2 (field Isolate) J ANim Vet Adv. 2007;6:1172–1182. [Google Scholar]
- 9.Pijoan A, Aguilar RF. Resistencia y sensibilidad a antimicrobianos en cepas de Pasteurella haemolytica, P. multocida y Haemophilus somnus, aisladas de becerras lecheras en establos de Tijuana. Vet Méx. 2000;2:153–156. [Google Scholar]
- 10.Watts JL, Yancey RJ, Salmon SA, Case CA. A 4-year survey of antimicrobial susceptibility trends for isolates from cattle with bovine respiratory disease in North America. J Clin Microbiol. 1994;32:725–731. doi: 10.1128/jcm.32.3.725-731.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samaniego Barrón ML, Contreras JJL, Jaramillo–Arango CJ, Aguilar–Romero F, Vázquez Navarrete J, et al. Resistencia a antimicrobianos en cepas de Mannheimia haemolytica aisladas de exudado nasal de bovinos productores de leche. Vet Méx. 2012;43:123–132. [Google Scholar]
- 12.Olsen AS, Warrass R, Douthwaite S. Macrolide resistance conferred by rRNA mutations in field isolates of Mannheimia haemolytica and Pasteurella multocida . J Antimicrob Chemother. 2015;70:420–423. doi: 10.1093/jac/dku385. [DOI] [PubMed] [Google Scholar]
- 13.Lubbers BV, Hanzlicek GA. Antimicrobial multidrug resistance and coresistance patterns of Mannheimia haemolytica isolated from bovine respiratory disease cases--a three-year (2009-2011) retrospective analysis. J Vet Diagn Invest. 2013;25:413–417. doi: 10.1177/1040638713485227. [DOI] [PubMed] [Google Scholar]
- 14.Orsi N. The antimicrobial activity of lactoferrin: current status and perspectives. Biometals. 2004;17:189–196. doi: 10.1023/b:biom.0000027691.86757.e2. [DOI] [PubMed] [Google Scholar]
- 15.Vogel HJ. Lactoferrin, a bird’s eye view. Biochem Cell Biol. 2012;90:233–244. doi: 10.1139/o2012-016. [DOI] [PubMed] [Google Scholar]
- 16.Baker HM, Baker EN. Lactoferrin and iron: structural and dynamic aspects of binding and release. Biometals. 2004;17:209–216. doi: 10.1023/b:biom.0000027694.40260.70. [DOI] [PubMed] [Google Scholar]
- 17.Ellison RT, Giehl TJ, LaForce FM. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun. 1988;56 doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appelmelk BJ, An YQ, Geerts M, Thijs BG, de Boer HA, et al. Lactoferrin is a lipid a-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drago-Serrano ME, de la Garza-Amaya M, Luna JS, Campos-Rodríguez R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int Immunopharmacol. 2012;12:1–9. doi: 10.1016/j.intimp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Ammendolia MG, Bertuccini L, Iosi F, Minelli F, Berlutti F. Bovine lactoferrin interacts with cable pili of Burkholderia cenocepacia . Biometals. 2010;23:531–542. doi: 10.1007/s10534-010-9333-1. [DOI] [PubMed] [Google Scholar]
- 21.Arslan SY, Leung KP, Wu CD. The effect of lactoferrin on oral bacterial attachment. Oral Microbiol Immunol. 2009;24:411–416. doi: 10.1111/j.1399-302X.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 22.Ochoa TJ, Brown EL, Guion CE, Chen JZ, McMahon RJ, et al. Effect of lactoferrin on enteroaggregative E. coli (EAEC) Biochem Cell Biol. 2006;84:369–376. doi: 10.1139/o06-053. [DOI] [PubMed] [Google Scholar]
- 23.Samaniego-Barron L, Luna-Castro S, Pina-Vazquez C, Suarez-Guemes F, de la Garza M. Two outer membrane proteins are bovine lactoferrin-binding proteins in Mannheimia haemolytica A1. Vet Res. 2016;47:93. doi: 10.1186/s13567-016-0378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avalos-Gómez Christian R-L, Gerardo R-R, Efrén D-A, Edgar Z, Cynthia G-R, et al. Effect of apo-lactoferrin on leukotoxin and outer membrane vesicles of Mannheimia haemolytica A2. Vet Res. 2020;1:2–13. doi: 10.1186/s13567-020-00759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez Rico G, Martinez-Castillo M, Gonzalez-Ruiz C, Luna-Castro S, de la Garza M. Mannheimia haemolytica A2 secretes different proteases into the culture medium and in outer membrane vesicles. Microb Pathog. 2017;113:276–281. doi: 10.1016/j.micpath.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Luna-Castro S, Aguilar-Romero F, Samaniego-Barron L, Godinez-Vargas D, de la Garza M. Effect of bovine apo-lactoferrin on the growth and virulence of Actinobacillus pleuropneumoniae . Biometals. 2014;27:891–903. doi: 10.1007/s10534-014-9752-5. [DOI] [PubMed] [Google Scholar]
- 27.Luber P, Bartelt E, Genschow E, Wagner J, Hahn H. Comparison of broth microdilution, E Test, and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni and Campylobacter coli . J Clin Microbiol. 2003;41:1062–1068. doi: 10.1128/JCM.41.3.1062-1068.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Quan S, Hiniker A, Collet JF, Bardwell JC. Isolation of bacteria envelope proteins. Methods Mol Biol. 2013;966:359–366. doi: 10.1007/978-1-62703-245-2_22. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Castillo M, Ramírez-Rico G, Serrano-Luna J, Shibayama M. Iron-binding protein degradation by cysteine proteases of Naegleria fowleri . Biomed Res Int. 2015;2015:1–8. doi: 10.1155/2015/416712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Towbin H, Staehelin Ty, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;74:4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia Gonzalez O, Garcia RM, de la Garza M, Vaca S, Paniagua GL, et al. Actinobacillus pleuropneumoniae metalloprotease: cloning and in vivo expression. FEMS Microbiol Lett. 2004;234:81–86. doi: 10.1016/j.femsle.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Haney EF, Nazmi K, Bolscher JG, Vogel HJ. Structural and biophysical characterization of an antimicrobial peptide chimera comprised of lactoferricin and lactoferrampin. Biochim Biophys Acta. 2012;1818:762–775. doi: 10.1016/j.bbamem.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Kalmar J, Arnold R. Killing of Actinobacillus actinomycetemcomitans by human lactoferrin. Infect Immun. 1988;56:2552–2557. doi: 10.1128/iai.56.10.2552-2557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roseanu A, Damian M, EVANS RW. Mechanisms of the antibacterial activity of lactoferrin and lactoferrin-derived peptides. J Biol Chem. 2010;47:203–209. [Google Scholar]
- 36.Ramos-Clamont GR-FD, Guzmán-Partida AM, Acedo-Félix EY, Vázquez-Moreno L. Actividad antibacteriana de lactoferrina bovina y lactoferrina porcina sobre Escherichia coli K88. Rev cient (Maracaibo) 2010;20:473–479. [Google Scholar]
- 37.Dial EJ, Hall LR, Serna H, Romero JJ, Fox JG, et al. Antibiotic properties of bovine lactoferrin on Helicobacter pylori. Dig Dis Sci. 1998;43:2750–2756. doi: 10.1023/a:1026675916421. [DOI] [PubMed] [Google Scholar]
- 38.Kutila T, Pyorala S, Saloniemi H, Kaartinen L. Antibacterial effect of bovine lactoferrin against udder pathogens. Acta Vet Scand. 2003;44:35–42. doi: 10.1186/1751-0147-44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakabayashi H, Yamauchi K, Kobayashi T, Yaeshima T, Iwatsuki K, et al. Inhibitory effects of lactoferrin on growth and biofilm formation of Porphyromonas gingivalis and Prevotella intermedia. Antimicrob Agents Chemother. 2009;53:3308–3316. doi: 10.1128/AAC.01688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez Rico G, Martinez-Castillo M, de la Garza M, Shibayama M, Serrano-Luna J. Acanthamoeba castellanii proteases are capable of degrading iron-binding proteins as a possible mechanism of pathogenicity. J Eukaryot Microbiol. 2015;62:614–622. doi: 10.1111/jeu.12215. [DOI] [PubMed] [Google Scholar]
- 41.Piña-Vázquez C, Reyes-López M, Ortíz-Estrada G, De la Garza M, Serrano-Luna J. Host-parasite interaction: parasite-derived and -induced proteases that degrade human extracellular matrix. J Parasitol Res. 2012;2012:26. doi: 10.1155/2012/748206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Culp E, Wright GD. Bacterial proteases, untapped antimicrobial drug targets. J Antibiot. 2017;70:366–377. doi: 10.1038/ja.2016.138. [DOI] [PubMed] [Google Scholar]
- 43.Mentlein R. Cell-surface peptidases. Int Rev Cytol. 2004;235:165–213. doi: 10.1016/S0074-7696(04)35004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Negrete-Abascal E, Tenorio VR, Guerrero AL, García RM, Reyes ME, et al. Purification and characterization of a protease from Actinobacillus pleuropneumoniae serotype 1, an antigen common to all the serotypes. Can J Vet Res. 1998;62:183–190. [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang SA, Wilk KM, Budnicka M, Olsen M, Bangale YA, et al. Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis. Vaccine. 2007;25:6730–6743. doi: 10.1016/j.vaccine.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaramillo ACJ, Trigo TFJ, Suárez GF. Mannheimiosis bovina: etiología, prevención y control. Vet Méx. 2009;40:293–314. [Google Scholar]
- 47.Cutone A, Lepanto MS, Rosa L, Scotti MJ, Rossi A, et al. Aerosolized bovine lactoferrin counteracts infection, inflammation and iron dysbalance in a cystic fibrosis mouse model of Pseudomonas aeruginosa chronic lung infection. Int J Mol Sci. 2019;20:14. doi: 10.3390/ijms20092128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochoa TJ, Noguera-Obenza M, Ebel F, Guzman CA, Gomez HF, et al. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect Immun. 2003;71:5149–5155. doi: 10.1128/IAI.71.9.5149-5155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez HF, Ochoa TJ, Carlin LG, Cleary TG. Human lactoferrin impairs virulence of Shigella flexneri . J Infect Dis. 2003;187:87–95. doi: 10.1086/345875. [DOI] [PubMed] [Google Scholar]
- 50.Qiu QJ, Hendrixson DR, Baker EN, Murphy TF, St. Geme III JW, et al. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc Natl Acad Sci USA. 1998;95:12641–12646. doi: 10.1073/pnas.95.21.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plaut AG, Qiu J. Human lactoferrin proteolytic activity: Analysis of the cleaved region in the IGA protease of Haemophilus influenzae. Vaccine. 2000;19 Suppl 1:S148–52. doi: 10.1016/S0264-410X(00)00296-6. [DOI] [PubMed] [Google Scholar]
- 52.Tomita M, Wakabayashi H, Shin K, Yamauchi K, Yaeshima T, et al. Twenty-five years of research on bovine lactoferrin applications. Biochimie. 2009;91:52–57. doi: 10.1016/j.biochi.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 53.Coetzee Johann F, Magstadt Drew R, Sidhu Pritam K, Lendie F, Schuler Adlai M, et al. Association between antimicrobial drug class for treatment and retreatment of bovine respiratory disease (BRD) and frequency of resistant BRD pathogen isolation from veterinary diagnostic laboratory samples. PloS one. 2019;14:1–24. doi: 10.1371/journal.pone.0219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vega-Bautista A, De la Garza M, César-Carrero J, Campos-Rodríguez R, Godínez-Victoria M, et al. The impact of lactoferrin on the growth of intestinal inhabitant bacteria. Int J Mol Sci. 2019;20:1–24. doi: 10.3390/ijms20194707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahman MM, Kim WS, Ito T, Kumura H, Shimazaki K. Growth promotion and cell binding ability of bovine lactoferrin to Bifidobacterium longum . Anaerobe. 2009;15:133–137. doi: 10.1016/j.anaerobe.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Tian H, Maddox IS, Ferguson LR, Shu Q. Influence of bovine lactoferrin on selected probiotic bacteria and intestinal pathogens. Biometals. 2010;23:593–596. doi: 10.1007/s10534-010-9318-0. [DOI] [PubMed] [Google Scholar]
- 57.Zarzosa-Moreno D, Avalos-Gomez C, Ramirez-Texcalco LS, Torres-Lopez E, Ramirez-Mondragon R, et al. Lactoferrin and its derived peptides: an alternative for combating virulence mechanisms developed by pathogens. Molecules. 2020;25:24. doi: 10.3390/molecules25245763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.