Abstract
Background
Tobacco use is highly prevalent amongst people living with HIV/AIDS (PLWHA) and has a substantial impact on morbidity and mortality.
Objectives
To assess the effectiveness of interventions to motivate and assist tobacco use cessation for people living with HIV/AIDS (PLWHA), and to evaluate the risks of any harms associated with those interventions.
Search methods
We searched the Cochrane Tobacco Addiction Group's Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and PsycINFO in June 2015. We also searched EThOS, ProQuest, four clinical trial registries, reference lists of articles, and searched for conference abstracts using Web of Science and handsearched speciality conference databases.
Selection criteria
Controlled trials of behavioural or pharmacological interventions for tobacco cessation for PLWHA.
Data collection and analysis
Two review authors independently extracted all data using a standardised electronic data collection form. They extracted data on the nature of the intervention, participants, and proportion achieving abstinence and they contacted study authors to obtain missing information. We collected data on long‐term (greater than or equal to six months) and short‐term (less than six months) outcomes. Where appropriate, we performed meta‐analysis and estimated the pooled effects using the Mantel‐Haenszel fixed‐effect method. Two authors independently assessed and reported the risk of bias according to prespecified criteria.
Main results
We identified 14 studies relevant to this review, of which we included 12 in a meta‐analysis (n = 2087). All studies provided an intervention combining behavioural support and pharmacotherapy, and in most studies this was compared to a less intensive control, typically comprising a brief behavioural intervention plus pharmacotherapy.
There was moderate quality evidence from six studies for the long‐term abstinence outcome, which showed no evidence of effect for more intense cessation interventions: (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.72 to 1.39) with no evidence of heterogeneity (I2 = 0%). The pooled long‐term abstinence was 8% in both intervention and control conditions. There was very low quality evidence from 11 studies that more intense tobacco cessation interventions were effective in achieving short‐term abstinence (RR 1.51, 95% CI 1.15 to 2.00); there was moderate heterogeneity (I2 = 42%). Abstinence in the control group at short‐term follow‐up was 8% (n = 67/848) and in the intervention group was 13% (n = 118/937). The effect of tailoring the intervention for PLWHA was unclear. We further investigated the effect of intensity of behavioural intervention via number of sessions and total duration of contact. We failed to detect evidence of a difference in effect according to either measure of intensity, although there were few studies in each subgroup. It was not possible to perform the planned analysis of adverse events or HIV outcomes since these were not reported in more than one study.
Authors' conclusions
There is moderate quality evidence that combined tobacco cessation interventions provide similar outcomes to controls in PLWHA in the long‐term. There is very low quality evidence that combined tobacco cessation interventions were effective in helping PLWHA achieve short‐term abstinence. Despite this, tobacco cessation interventions should be offered to PLWHA, since even non‐sustained periods of abstinence have proven benefits. Further large, well designed studies of cessation interventions for PLWHA are needed.
Plain language summary
Interventions to help people living with HIV and AIDS to stop using tobacco
Background: Tobacco use is common amongst people living with HIV and AIDS (PLWHA); it causes a range of health problems and accounts for many deaths. There is good evidence about interventions to help people quit tobacco use in the general population, however the effectiveness in PLWHA was not known.
Methods: We reviewed the available evidence from trials to help PLWHA stop using tobacco. This evidence is correct up to June 2015. We conducted analyses of whether people were able to successfully quit tobacco use in the long‐term (six months and over) and short‐term (measured at less than six months).
Results: We found 14 relevant studies including over 2000 participants. All studies, except one, were conducted in the United States (US). All studies compared a behavioural intervention with medication, to a control group. The behavioural intervention was delivered via a range of methods including face‐to‐face, telephones, computers, and text messages. Nicotine replacement therapy or varenicline (medications that help tobacco users quit) was also given. Control participants typically received a less intensive, brief behavioural intervention, and the same medication as the intervention group. Six studies of moderate quality evidence investigated long‐term abstinence; they did not show clear evidence of benefit of the more intense intervention. Eleven studies of very low quality evidence investigated short‐term abstinence. The evidence suggested that a more intense intervention combining behavioural support and medication might help people to quit in the short‐term.
Quality of the evidence: The quality of the evidence was judged to be moderate for the long‐term abstinence outcome and very low for the short‐term abstinence outcome, and so further research is needed to increase our confidence in our findings.
Summary of findings
Summary of findings for the main comparison. Tobacco use cessation in people living with HIV and AIDS.
| Tobacco use cessation in people living with HIV and AIDS | ||||||
| Patient or population: consumers of tobacco living with HIV and AIDS Setting: All included studies conducted in USA Intervention: combined pharmacotherapy and behavioural support for smoking cessation Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with combined cessation intervention | |||||
| Proportion of participants abstinent ‐ long‐term (> 6 months) assessed via self report +/‐ biochemical verification | Study population | RR 1.00 (0.72 to 1.39) | 1602 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 80 per 1000 | 80 per 1000 (58 to 112) | |||||
| Moderate | ||||||
| 63 per 1000 | 63 per 1000 (46 to 88) | |||||
| Proportion of participants abstinent ‐ short‐term (> 4 weeks to < 6 months) assessed via self report +/‐ biochemical verification | Study population | RR 1.51 (1.15 to 2.00) | 1785 (11 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | ||
| 79 per 1000 | 119 per 1000 (91 to 158) | |||||
| Moderate | ||||||
| 65 per 1000 | 98 per 1000 (75 to 130) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded due to risk of bias. One study had high risk of reporting bias (although impact on results mitigated by obtaining unpublished data from study authors). Allocation concealment and blinding poorly described. 2 Downgraded due to suspected publication bias indicated by asymmetrical funnel plot. 3 Downgraded due to inconsistency. The direction of effect was not always consistent and moderate heterogeneity was present (I2 = 42%).
Background
The introduction of combination anti‐retroviral therapy (ART) has transformed HIV into a chronic disease (Deeks 2013), comparable to other long‐term conditions such as diabetes (Nakagawa 2012). Once diagnosed, people living with HIV/AIDS (PLWHA) can have a near normal life expectancy (Nakagawa 2012). Causes of morbidity and mortality have changed; between 50% and 84% of deaths in PLWHA are now not AIDS‐related (Ehren 2014; May 2013; Weber 2013), and rates of opportunistic infections have declined substantially over the past two decades (Buchacz 2010). Non‐communicable diseases, particularly ischaemic heart disease and lung cancer, now represent a growing burden of disease in this population (May 2013).
The prevalence of tobacco consumption in PLWHA is substantial, and greater than that of the general population: between 47% and 65% of PLWHA smoke cigarettes (Friis‐Møller 2003; Helleberg 2013; Miguez‐Burbano 2005). Prevalence of tobacco use varies between countries, but there is evidence that PLWHA consume more tobacco than the general population in a range of contexts, from Zimbabwe to the United States (US) (Gritz 2004; Munyati 2006). Where ART is accessible, smoking results in greater loss of life years than the HIV infection itself in PLWHA who smoke (Helleberg 2013). In light of the high prevalence of smoking in combination with the changing trends in morbidity and mortality, smoking cessation has become highly relevant for this population.
Description of the condition
Tobacco may affect the immune system of PLWHA, resulting in increased viral replication in macrophages, microglial, and T cells (Abbud 1995, Valiathan 2014). Valiathan and colleagues demonstrated that PLWHA who smoked had higher levels of immune exhaustion and impaired T cell functioning compared to both PLWHA non‐smokers and HIV‐negative smokers (Valiathan 2014).
Untreated HIV destroys CD4 cells (T4 lymphocytes expressing CD4 proteins), which play a central role in the immune system (Naif 2013; Simon 2006). In untreated HIV infection the 'CD4 count' (number of CD4 cells) gradually falls, increasing the risk of opportunistic infections and other complications (Naif 2013; Simon 2006). Treatment with ART aims to increase the CD4 count and to achieve viral suppression ‐ to reduce the amount of HIV virus in the blood (the ‘viral load’) to an undetectable level.
Smoking tobacco may affect the immune response to ART. Some evidence indicates that tobacco use might be associated with poorer ART outcomes including a lower likelihood of achieving viral suppression, and a higher likelihood of immunological failure (when CD4 count falls below the lowest point it had been prior to ART initiation) (Feldman 2006). However, cohort study data showed no difference in CD4 and viral load between smokers and non‐smokers (Helleberg 2015).
Tobacco use causes substantial morbidity and mortality in PLWHA. The tobacco‐related harm is substantially higher in PLWHA than smokers in the general population. Smoking was found to be attributable for 24.3% of all‐cause mortality, 25.3% of major cardiovascular disease, 30.6% of non‐AIDS‐related cancer, and 25.4% of bacterial pneumonia amongst people living with HIV (Lifson 2010). This is partly due to a higher prevalence of tobacco use in PLWHA than the general population and partly due to their increased susceptibility to the impact of tobacco compared to other smokers. Lung cancer is the commonest non‐AIDS‐related cancer amongst PLWHA (May 2013), and compared to the general population, lung cancer occurs at a younger age and after shorter exposure to cigarettes (Winstone 2013). HIV has been identified as an independent factor for greater lung cancer risk (Sigel 2012). In addition, smoking is associated with increased incidence of a number of other cancers in PLWHA, including cancer of the anus and mouth (Bertisch 2013; Clifford 2005). Cardiovascular disease risk may be elevated in PLWHA, due to a combination of HIV viraemia, a pro‐inflammatory state, and the association of some ART regimens with high cholesterol and impaired glucose tolerance (Friis‐Møller 2003; Palella 2011). Tobacco use further increases cardiovascular risk, and cessation was found to be effective in significantly reducing this risk (Petoumenos 2011). Case‐control studies show that the impact of smoking on acute coronary syndrome is nearly doubled for PLWHA who smoked compared to HIV‐negative controls (Calvo‐Sánchez 2013).
Amongst PLWHA who consume tobacco, incidence of oral lesions such as oral candidiasis and oral hairy leukoplakia are increased compared to non‐users (Sroussi 2007). Current smokers are at significantly higher risk of bacterial pneumonia than non‐smokers (Gordin 2008). The outcome of chronic obstructive pulmonary disease may be worse in PLWHA compared to HIV‐negative people (Morris 2011). Smoking tobacco during pregnancy is an independent risk factor for adverse pregnancy outcomes for PLWHA, including small for gestational age, low birth weight and preterm birth (Aliyu 2013).
PLWHA who use tobacco are different from other tobacco users in several respects, which justifies a focused evidence review for smoking cessation in this population. PLWHA have been shown to have higher nicotine dependency levels than the general population and there is an increased prevalence of other co‐dependencies, such as alcohol and illicit drugs (Benard 2007). This makes them more vulnerable to withdrawal symptoms on stopping tobacco use, and means sustained abstinence could be difficult to achieve. The prevalence of mental illness, particularly depression, in PLWHA is higher than that in the general population (Nurutdinova 2012; Schadé 2013), and is associated with a lower likelihood of quitting smoking and an increased likelihood of relapse after quitting (Weinberger 2012). Tobacco use was reported as a coping mechanism for general HIV‐related symptoms and specifically for HIV‐related neuropathy, depression, anxiety, and ART‐associated lipodystrophy (Grover 2013; Reynolds 2004; Shuter 2012a). Despite the good prognosis of HIV, some PLWHA report fatalistic ideas and a pessimistic perception of their life expectancy, affecting their perceived susceptibility‐associated risks of tobacco; one participant said: “If I live long enough to get cancer that's great!” (Reynolds 2004).
Socioeconomic factors also have a substantial impact on tobacco use. Many PLWHA who use tobacco are members of one or more marginalised groups, including ethnic minorities, migrants, and men who have sex with men. Tobacco use was found to be consistently higher in lesbian, gay, bisexual, and transgender adults, compared to heterosexual adults in a range of countries (Marshal 2008), and they are also at higher risk of HIV. Additionally, in one study of PLWHA who use tobacco, two‐thirds were found to be unemployed, almost half had an income under USD 10,000 per annum and more than one‐third were in inadequate housing (Humfleet 2009). These factors may contribute to their continued tobacco use and reduce the likelihood of success in quitting. Social support networks are lacking for many PLWHA. In addition, some PLWHA who smoke, report that more than 40% of people in their social network are smokers, reinforcing their continued smoking (Humfleet 2009).
Description of the intervention
In the general population, combined pharmacotherapy and counselling interventions are effective in achieving tobacco cessation (Stead 2016). PLWHA who are engaged in care, come into frequent contact with health professionals for regular tests and clinic appointments. This presents an opportunity to discuss and support cessation, but currently this opportunity is underutilised. HIV clinicians report a lack of confidence in initiating cessation therapies and insufficient time, despite recognising the importance (Horvath 2012; Shuter 2012b). Despite previous unsuccessful attempts to quit by over 80% of PLWHA who smoke (Shuter 2012a), a high proportion remain motivated to quit (Benard 2007; Shuter 2012a). The high prevalence of smoking, despite a substantial proportion expressing a desire to quit, reflects an unmet need for effective tobacco cessation interventions in PLWHA. There is need for clarity in how best to support PLWHA in tobacco cessation.
Tobacco cessation interventions may be brief advice, behavioural, pharmacological, or a combination. Behavioural support interventions may include group or individual counselling, consisting of appointments following the quit attempt where the smokers receive information, advice, and encouragement. Pharmacological interventions may include use of nicotine replacement therapy (NRT) via a range of modalities, as well as bupropion or varenicline. The literature on tobacco cessation suggests that individual pharmacotherapies are effective for tobacco cessation; however, these in combination with behavioural support are found to be more effective in the general population (Stead 2016). There is evidence of effectiveness of these tobacco cessation interventions in the general population (Stead 2012; Stead 2016), but to our knowledge, there has not been an in‐depth systematic review into their effectiveness in PLWHA.
Why it is important to do this review
Tobacco use is highly prevalent and responsible for substantial morbidity and mortality amongst PLWHA (Helleberg 2013). It is therefore, important that health workers have the best available evidence to support PLWHA in their attempts to quit tobacco. A dedicated review of cessation interventions in PLWHA is justified as a number of relevant attributes of tobacco users with HIV/AIDS differ from those of other tobacco users. Furthermore, despite motivation to quit, PLWHA often find it difficult to achieve sustained abstinence.
Objectives
Primary objective:
To assess the effectiveness of interventions to motivate and assist tobacco use cessation for people living with HIV/AIDS (PLWHA), and to evaluate the risks of any harms associated with those interventions.
Secondary objectives:
To assess whether interventions combining pharmacotherapy and behavioural support are more effective than either type of support alone in PLWHA.
To assess whether in PLWHA, tobacco cessation or cessation induction interventions tailored to PLWHA are more effective than ‘usual care’ non‐tailored cessation interventions.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Cluster‐randomised controlled trials (cluster‐RCTs).
Quasi‐randomised controlled trials.
Other non‐randomised controlled trials.
We did not exclude studies on the basis of language or publication status.
Types of participants
We included trials of adults over 18 years who were HIV‐positive. We included studies of all stages of HIV infection, and studies of men only, women only, and all genders.
Trial participants were consumers of tobacco. Had we located studies which differentiated between different types of tobacco users, we would have considered subgroup analysis.
Types of interventions
We included interventions that targeted individuals. The interventions included behavioural and pharmacological elements. We did not locate any studies of cessation induction trials (typically brief advice by health professionals) that aimed to encourage future quit attempts by those tobacco users who were unwilling to give up at the time of recruitment.
We included interventions delivered via any format including telephone call, the Internet, and face‐to‐face. There was no restriction on the identity of the provider which included nurses, counsellors, and peers.
Types of outcome measures
Primary outcomes
The primary outcome measure is tobacco abstinence at a minimum of six months after the start of the intervention, referred to as long‐term cessation. We did include trials with a shorter follow‐up, but these did not contribute to the primary analysis. We recognise that measurement of cessation at six months or longer is optimal (West 2005); however we included a shorter‐term outcome measure due to the relative paucity of research in the area of tobacco cessation for PLWHA. We assessed short‐term abstinence as a secondary outcome measure and completed separate analyses for the short‐ and long‐term follow‐up periods.
We used the strictest definition of abstinence reported in the study, using sustained abstinence rates in preference to point prevalence, or floating prolonged abstinence. Definitions of sustained abstinence may allow for a small number of cigarettes during the period (West 2005). We preferred, but did not require, that abstinence was biochemically verified (for example, by exhaled carbon monoxide or serum, salivary, or urinary cotinine). We treated those participants lost to follow‐up as continuing users of tobacco. These outcome measures are guided by the Russell Standards for smoking cessation trials (West 2005).
Secondary outcomes
We assessed short‐term abstinence as a secondary outcome measure. We required that the assessment point was at least four weeks, but less than six months, from the target quit date, or start of the intervention for studies of cessation induction.
In addition to short‐term abstinence, we planned to look for data on the following secondary outcome measures: HIV viral load, CD4 count, and the incidence of opportunistic infections. We planned to extract data on and report any adverse effects.
Search methods for identification of studies
Electronic searches
The Tobacco Addiction Group's Trials Search Co‐ordinator searched the Cochrane Tobacco Addiction Group's Specialised Register using terms related to the topic of HIV/AIDS, and the Cochrane Central Register of Controlled Trials (CENTRAL) combining topic‐related and smoking cessation terms. We also searched MEDLINE, EMBASE, and PsycINFO, combining HIV topic terms with the smoking‐related terms and study design limits, as used for the Specialised Register. The MEDLINE search terms are included in Appendix 1. All searches were carried out on the 17th June 2015. Not other time period limitations were used.
Searching other resources
We searched the grey literature as follows: theses and dissertations via EThOS and ProQuest. We looked for conference abstracts by searching the Conference Proceedings database in Web of Science and by handsearching the databases of the Society for Research on Nicotine and Tobacco, International AIDS Conference, and British HIV Association. We reviewed reference lists of literature reviews and consulted experts via email.
We searched for clinical trials via the US National Institutes of Health registry at www.clinicaltrials.gov, the World Health Organization (WHO) trials registry platform at apps.who.int/trialsearch/, the European Union (EU) clinical trials register at www.clinicaltrialsregister.eu, and the Pan African Clinical Trials Registry at www.pactr.org. For unpublished trials identified via the registries, we attempted to contact authors and requested data for analysis.
Data collection and analysis
Selection of studies
Two review authors (EP reviewed all studies, RL and KS each reviewed a proportion) independently checked the title and abstracts of all retrieved records for relevance. Two authors (from EP, KS, and RL) then each reviewed the full‐text reports of all studies not excluded based on title or abstract, and which were potentially eligible for inclusion. We resolved any disagreements regarding study inclusion through discussion with a third party (OD). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (from EP, OD, and RL) extracted data using a standardised electronic data collection form. We then entered data into Review Manager 5 computer software for preparing Cochrane systematic reviews (RevMan 2014).
We extracted the following information, where available, for each study, in the Characteristics of included studies tables.
Methods: Study name (if applicable), study recruitment period, country, number of study centres, study setting, study recruitment procedure, study design.
Participants: N (intervention/control), definition of smoker used, specific demographic characteristics (e.g. mean age, age range, gender, ethnicity, sexuality), mean cigarettes per day, mean Fagerström Test for Nicotine Dependence (FTND) score, relevant inclusion criteria and exclusion criteria.
Interventions: Description of intervention(s) (treatment, dosage, regimen, behavioural support), description of control (treatment, dosage, regimen, behavioural support); what comparisons will be constructed between which groups, concomitant medications, and excluded medications.
Outcomes: Abstinence time points for long‐ and short‐term analyses, definition of abstinence (e.g. sustained or point prevalence) at each point, biochemical validation, proportion of participants with follow‐up data at each point. Other outcome reported (HIV viral load, CD4 count, incidence of opportunistic infections, adverse effects).
Notes: Source of funding for trial, and notable conflicts of interest of trial authors.
Assessment of risk of bias in included studies
As recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions, we utilised the 'Risk of bias' tool within Review Manager 5 to assess the risk of bias for each included study (Higgins 2011; RevMan 2014). Two review authors (from EP, OD, and RL) independently assessed and reported the following information in the 'Risk of bias' table.
Method of random sequence generation.
Method of allocation concealment.
Blinding of participants, providers, or outcome assessors.
Numbers lost to follow‐up or with unknown outcome, for each outcome used in the review, by intervention/control group.
Selective outcome reporting.
Any other threats to study quality.
We graded each trial as being at 'high', 'low', or 'unclear' risk of bias for each domain, and provided justification for our judgement in the table. We summarised the risk of bias judgements across different studies for each of the domains listed, and displayed the summary results in two 'Risk of bias' figures.
Measures of treatment effect
We calculated a risk ratio (RR) for each cessation outcome for each trial included in the meta‐analysis as follows: (number of participants abstinent from tobacco in the intervention group/number of participants in the intervention group) / (number of participants abstinent from tobacco in the control group/number of participants in the control group).
Unit of analysis issues
The unit of analysis is the individual level.
Dealing with missing data
Where we identified missing data, we contacted the study authors to request missing data. We also contacted study authors if aspects of trial design or conduct were unclear.
We treated participants who have dropped out, or who were lost to follow‐up, as continuing to use tobacco. We completed reanalysis, where possible, if study authors had not considered these participants as continuing to use tobacco. We noted the proportion of participants for whom data were missing in the 'Risk of bias' table.
Assessment of heterogeneity
We evaluated levels of heterogeneity (study characteristics, methods, outcomes) between included studies to decide whether or not it is appropriate to pool the data, in two ways; firstly by checking if the confidence intervals (CIs) overlap. We used the I² statistic to assess statistical heterogeneity, given by the formula [(Q ‐ df)/Q] x 100%, where Q is the Chi² statistic and df is its degrees of freedom (Higgins 2003). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than to sampling error (chance). A value greater than 50% may be considered to indicate substantial heterogeneity. The test has low power when used on a small number of studies (Higgins 2011).
Assessment of reporting biases
Searching multiple sources (as detailed in the search strategies above) should reduce reporting biases. We avoided language bias by not limiting the search terms by language and used translation services where required. We did not exclude on the basis of publication status and aimed to minimise publication bias by inclusion of grey literature, conference abstracts, and the inclusion of data from unpublished trials identified from trial registries (Higgins 2011). However, this is dependent on the data being obtained.
We created a funnel plot with pseudo‐95% confidence limits to identify possible publication bias for the secondary outcome of short‐term cessation. We did not create a funnel plot for the primary outcome (long‐term cessation) because we identified less than ten studies.
Data synthesis
We extracted data from individual studies, reported them in table form, and completed a meta‐analysis. We calculated quit rates based on numbers randomised to an intervention or control group. Where possible, we conducted intention‐to‐treat analyses, i.e. including all participants initially assigned to intervention or control in their original groups. We excluded from the denominators any deaths. We treated any other losses to follow‐up as continuing tobacco users, as described above. We noted adverse events, serious adverse events, and deaths in the Results section.
Incidence of adverse events were poorly reported. It was therefore not possible to conduct a meta‐analysis of the incidence of serious adverse events, taking those randomised as the denominator and including events up to thirty days after the end of treatment. It was also not possible to conduct a sensitivity analyses restricting the denominator to those known to have taken at least one dose of treatment/intervention as this figure was not reported.
For the meta‐analysis, we pooled RRs using a Mantel‐Haenszel fixed‐effect model ((number of events in intervention condition/intervention denominator) / (number of events in control condition/control denominator)) with a 95% CI. Where the event is defined as tobacco cessation, a RR greater than one indicates that more people successfully quit in the treatment group than in the control group.
'Summary of findings' table
We created a 'Summary of findings' table for long‐term abstinence outcomes (six months or longer) and short‐term outcomes (greater than or equal to four weeks, but less than six months). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence as it relates to the studies which contribute data to the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software (GRADEproGDT 2015). We justified all decisions to down‐ or upgrade the quality of the evidence using footnotes.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses, to explore the impact of different variables on the findings of the review. This allowed us to identify and investigate unexplained sources of heterogeneity.
We also completed subgroup analyses to compare the relative efficacy of the different interventions. We were unable to complete subgroup analysis to establish the effect of combined behavioural and pharmacological interventions versus single‐focus interventions in PLWHA since all identified studies used combination interventions. We therefore added a post hoc objective to investigate the effect of intensity of the intervention.
We conducted additional subgroup analyses for each intervention‐population‐outcome association and study characteristics to explore sources of any heterogeneity, using the I² statistic.
Only one study reported on the outcomes of HIV viral load and CD4 count. therefore. we did not calculate the RR or mean difference (MD).
We conducted subgroup analyses based on the provider of the behavioural intervention, mode of contact, participant selection, tailoring, number of sessions, and total duration of contact. The subgroups are as follows.
Provider of behavioural intervention
Healthcare professional
Researcher
Co‐facilitation by a PLWHA peer and a health professional
Mode of contact
Face‐to‐face
Telephone
Text message
Website/computer‐based
Where the intervention was undertaken via more than one mode, the most frequently used mode was coded.
Participant selection
Selected for willingness or motivation to quit
Not selected for willingness or motivation to quit
Tailoring
Intervention tailored for PLWHA
Intervention not tailored for PLWHA
Where no tailoring was described, it was assumed that the intervention was non‐tailored.
Intensity
A post hoc objective involved analysis according to intensity of behavioural intervention. This analysis was undertaken using the same categories defined in a previous Cochrane review (Stead 2016), adapted from the US Guidelines AHRQ 2008. This involved analysis according to number of sessions and total duration of contact time. We used planned contact time and number of sessions where possible, if this was not reported or not clear, we used the reported average.
We categorised total contact time as follows.
0 (where support was provided by text message or website use alone)
1 to 30 minutes
31 to 90 minutes
91 to 300 minutes
More than 300 minutes
We categorised number of person‐to‐person sessions as follows.
0 (where support was provided by text message or website use alone)
1 ‐ 3 sessions
4 ‐ 8 sessions
More than 8 sessions
Intensity subgroup analyses were not completed for short‐term outcomes because the planned contact may not have been completed at the time of short‐term outcome assessment.
Sensitivity analysis
We tested for small‐study effects on the results of the meta‐analysis performed.
Results
Description of studies
Results of the search
Figure 1 contains a flow diagram detailing the search results. We identified 881 potentially relevant records; we identified some studies from more than one record. After screening and duplicate removal, we assessed 48 studiesfor eligibility and included 14 in the qualitative synthesis, 12 of which we included in a meta‐analysis. The 22 studies that we excluded are listed in Characteristics of excluded studies, and we judged 12 studies to be ongoing, which are summarised in Characteristics of ongoing studies. We assigned the studies a study identifier (ID) based on the first author and year of the first major publication.
1.

Study flow diagram.
Included studies
Fourteen studies met the inclusion criteria and contributed to the review. We included all 14 studies in the qualitative synthesis, while 12 studies contributed to the quantitative synthesis. Six studies contributed to the analysis of long‐term tobacco abstinence (the primary outcome) and 11 studies to short‐term abstinence (the secondary outcome). Four studies reported both long‐ and short‐term abstinence and we included them in both analyses.
The studies are described in detail in the Characteristics of included studies table. All studies were conducted after 2000, 13 of the 14 studies were conducted in the US, while one study, Elzi 2006, was conducted in Switzerland. All studies combined a behavioural intervention with pharmacotherapy, therefore, we could not investigate the objective of single versus combined intervention in this review. As an alternative, we investigated the effect of level of intensity of counselling.
We included two three‐arm studies (Humfleet 2013; Shelley 2015). In both cases, the two intervention groups were not similar enough to warrant combining them to create a single group. For these two studies, we divided the control groupsinto two equal groups and made independent comparisons as follows: intervention one versus half of the control and intervention two versus half of the control. For Humfleet 2013: computer‐based intervention versus control and individual counselling versus control. For Shelley 2015: text message versus control and text message + adherence‐based therapy versus control. In the meta‐analyes, the intervention groups are identified in the footnotes.
We did not include two studies in the meta‐analysis (Elzi 2006; Ferketich 2013). Elzi 2006 was nested in the Swiss HIV Cohort Study (SHCS), the intervention group was not randomised, and it comprised participants who expressed an interest in quitting. Whereas, the control group was formed of all other smokers participating in the SHCS at the study site; they were therefore systematically different from the intervention group. Inclusion of this study in the meta‐analysis resulted in substantial heterogeneity with an I2 > 80%. We did not include Ferketich 2013 in the meta‐analysis as this study compared two pharmacotherapies and was not in‐keeping with the comparison of behavioural intervention versus less intense behavioural intervention, and did not have a control arm. The study compared 12 weeks counselling and varenicline, with 12 weeks counselling and nicotine replacement therapy (NRT). It was also non‐randomised.
Sample size was variable with a high proportion of small studies; seven studies had a sample size of less than 150 participants (Cropsey 2013; Ingersoll 2009; Manuel 2013; Moadel 2012; Shuter 2014; Vidrine 2006; Wewers 2000).
Participant characteristics
More than 1600 participants contributed to the meta‐analysis for the primary outcome of long‐term abstinence (six months or greater). An additional 485 participants contributed to the meta‐analysis for the secondary outcome of short‐term abstinence (greater than or equal to four weeks, but less than six months).
Sexual orientation and ethnicity were recorded inconsistently in different studies. Only six out of 14 studies reporting any measure of sexual orientation, despite the relevance of sexual orientation in studies of PLWHA. Where sexual orientation was described, approximately 35% to 50% of participants were homosexual. The overall proportion of homosexual participants ranged from 3% in Manuel 2013 to 68% in Humfleet 2013. Notably, the participants in Manuel 2013 were all women.
Black or African American ethnicity was the modal category in eight studies. There were variations: from 95% of participants being Black in Ingersoll 2009 to 53% White in Humfleet 2013. The population of the only European study was 87% White (Elzi 2006). One study was specifically designed for the Latino community, and having a Latino/Hispanic ethnicity was an inclusion criterion of that study (Stanton 2015). The reported average age of study participants was approximately 35 to 50 years.
Description of the intervention: behavioural
Provider
Some degree of behavioural intervention was provided to the intervention group in all studies. We categorised the provider of the behavioural interventions as follows; healthcare professionals, researchers or co‐facilitated by a peer and a professional. The intervention was provided by healthcare professionals in nine studies (Elzi 2006; Ferketich 2013; Humfleet 2013; Ingersoll 2009; Lloyd‐Richardson 2009; Manuel 2013; Shelley 2015; Stanton 2015; Vidrine 2012), by a researcher or a graduate student in two studies (Cropsey 2013; Vidrine 2006), and co‐facilitated by a PLWHA ex‐smoker peer alongside a professional in two studies (Moadel 2012; Wewers 2000).
There was variation in the degree of detail to which the qualifications or experience of the individuals delivering the behavioural interventions was described. We therefore adopted a broad categorisation of 'healthcare professional’, which included nurses, motivational interviewing clinicians, counsellors, psychologists, and health educators, irrespective of the detail provided on their counselling skills, experience, or qualifications. Likewise, while some studies described the academic qualifications of the researchers providing interventions, little detail was provided on their training or counselling experience.
In addition to the website intervention, the participants in the computer‐based intervention group of Humfleet 2013 also received a 45‐60 minute face‐to‐face meeting, including discussion of a quit date. However, it is unclear if the provider was a clinician or a researcher, so this group was not included in the provider subgroup analysis. We attempted to contact the authors but were unable to clarify the intervention provider.
Mode of contact
In most studies the behavioural intervention was delivered face‐to‐face or via telephone, often in combination. Two studies investigated computer‐ or web‐based delivery, in which participants completed online modules (Humfleet 2013; Shuter 2014). One study delivered the intervention entirely via text message (Shelley 2015). Additional resources were provided in most studies, such as written materials.
Nearly half of the intervention groups (n = 7/16, 44%) offered between four and eight face‐to‐face or telephone sessions, and a quarter (n = 4/16, 25%) offered more than eight (Elzi 2006; Ferketich 2013; Vidrine 2012; Wewers 2000). Most of the studies with long‐term follow‐up planned to provide 91‐300 minutes of total contact time. Categorisations were made according to planned duration of contact; actual contact time per participant may have been lower. These categories also do not account for time spent using web‐based interventions or reviewing text messages.
Tailoring
Eleven of the interventions were specifically tailored to PLWHA. This was achieved through a range of methods, including emphasising impact of tobacco on the immune system and facilitation by a HIV‐positive ex‐smoker peer. Some authors did not describe how the intervention was tailored in detail. In the text message group of Shelley 2015, the text messages did not contain the words HIV or AIDS in order to ensure confidentiality, although the messages were designed to emphasise particular barriers faced by PLWHA, such as stress. We did consider this intervention tailored, but recognise that the degree of tailoring varies between studies. In the computer‐based intervention in Humfleet 2013, the authors did not explicitly state that the computer‐based intervention was tailored, although it was described as modelled on the individual counselling intervention. The counselling intervention was targeted to the needs of HIV‐positive smokers through focussing on the impact of smoking on HIV. We considered the computer‐based intervention group likely to be tailored, and therefore included it in the tailored versus generic control subgroup.
Description of interventions: pharmacotherapy
Nicotine replacement therapy (NRT) via patches and/or lozenges was offered or provided to the intervention groups in most studies. In Shelley 2015 and Ferketich 2013, varenicline was used instead. No studies included bupropion in their protocol, possibly due to the potential drug‐drug interactions with a number of anti‐retroviral therapy (ART) drugs. In Shuter 2014 only NRT administration was planned in the protocol, but some participants also received bupropion and varenicline off protocol, however, they were retained within their original groups for the ITT analysis.
Description of controls
In most studies the control group received ‘usual care’, which typically comprised NRT or varenicline, brief advice, and written materials. In two studies, the participants in the control group received no behavioural input or pharmacotherapy (Cropsey 2013; Elzi 2006).
In two studies, participants in the control group received enhanced standard care, with NRT alongside a counselling schedule involving multiple contacts (Lloyd‐Richardson 2009; Stanton 2015). Although in these studies the intervention was still more intense than the control.
Other study characteristics
The vast majority of included studies were randomised controlled trials (RCTs). The Ferketich 2013 study was not randomised; participants selected their own group (varenicline or NRT) but were encouraged by study staff to select varenicline, unless medically contraindicated. The Elzi 2006 study was also not randomised; all cohort study participants who expressed an interest in quitting were allocated to the intervention group; the control group included the remaining cohort study smokers.
Study inclusion criteria differed on one key point: whether willingness or motivation to quit was explicitly required for inclusion in the study. Five studies did not refer to motivation or willingness to quit in their inclusion criteria (Cropsey 2013; Humfleet 2013; Ingersoll 2009; Lloyd‐Richardson 2009; Stanton 2015). In Elzi 2006, interest in quitting was required for the intervention group, but not for the control.
The outcome of cessation was self reported in all studies, which was biochemically verified in all except one study (Elzi 2006). Biochemical verification was achieved mostly through expired carbon monoxide. Most studies used a cut‐off point of < 7 parts per million (ppm) or < 10 ppm, although the lowest cut‐off point used was < 3 ppm (Cropsey 2013). In this study no participants in the intervention or control groups achieved abstinence verified by this low cut‐off point, although 22% of the intervention group did achieve abstinence at the < 10 ppm cut‐off point (Cropsey 2013). In keeping with our protocol, we used the most conservative estimate of abstinence (Pool 2014). Two studies used a combination of methods for biochemical verification; expired carbon monoxide and urine cotinine, or urine cotinine and nicotine levels.
Only three studies used the outcome measure of sustained abstinence. The most commonly used measure was 7‐day point prevalence smoking abstinence (PPA). One study planned to measure both 7‐day PPA and sustained abstinence, but the data collected were insufficient to report sustained abstinence (Shuter 2014).
Excluded studies
We list 22 studies as excluded. Reasons for exclusion can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
Figure 2 and Figure 3 present the review authors’ judgements about each risk of bias item as percentages across all included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies reported non‐randomised allocation and were therefore at high risk of bias for allocation concealment (Elzi 2006; Ferketich 2013). All studies included in the meta‐analysis were randomised. The method of allocation concealment was generally not described; only one study explicitly reported method of allocation concealment, but this was not in sufficient detail to permit judgement of bias.
Blinding
Blinding of participants or personnel was not described in 12 out of 14 studies, and therefore the risk of these biases was unclear. In Ferketich 2013, participants chose their group; we therefore judged this study to be at high risk of performance bias. In Manuel 2013, a single provider delivered the counselling to both the intervention and control groups, therefore we judged that it was not feasible to blind the provider. Due to the nature of the behavioural interventions, it would have been difficult to blind providers to participant allocation in most studies.
Blinding of outcome assessment was only reported in detail in two studies (Lloyd‐Richardson 2009; Manuel 2013). In both studies, researchers blinded to the allocation status of the participant completed the outcome assessment and we therefore judged these studies to be at low risk of detection bias. In all other studies, risk of detection bias was unclear.
Incomplete outcome data
We judged one study to be at high risk of attrition bias (Manuel 2013). In this study, one participant was lost to follow‐up and no data were imputed for them (i.e. not considered missing = smoking). In addition, of the three participants who reported abstinence, biochemical testing for confirmation of abstinence was only performed on two specimens of urine, the reason for which was not explained. We considered the remaining studies to have low or unclear risk of attrition bias.
Selective reporting
No study authors posted full study results on the clinical trial registries. We judged five studies to be at high risk of reporting bias. The outcome measures were not reported as described in the protocol in three studies (Humfleet 2013; Moadel 2012; Shuter 2014). In each study, the authors stated in the protocol that sustained abstinence and PPA outcomes would be assessed, however only reported PPA outcomes without explanation. Sustained abstinence data were obtained via communication with the authors for Humfleet 2013, however PPA outcomes were used in the meta‐analysis since the authors definition of sustained abstinence (defining relapse as seven consecutive days of smoking) means that PPA is the strictest definition of abstinence.
In Ingersoll 2009, the design of the trial included two groups: intervention versus control. However, the study authors stated that there was no difference between the two groups and published only aggregated data, including baseline characteristics and outcome. We obtained per group data from the study author for inclusion in the analysis.
In Cropsey 2013, the authors report measuring expired carbon monoxide and urine cotinine, but did not report these results.
Other potential sources of bias
We did not judge any studies to be at risk of 'other’ bias.
Effects of interventions
See: Table 1
Long‐term cessation
For long‐term abstinence, a pooled estimate combining the six included studies showed no evidence of effect for the intervention (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.72 to 1.39; moderate quality evidence) with no evidence of heterogeneity (I2 = 0%) (Analysis 1.1; Figure 4; Table 1). Abstinence in the control group at long‐term follow‐up was 8% (n = 69/842) and in the intervention group was also 8% (n = 61/760).
1.1. Analysis.

Comparison 1 Tobacco cessation intervention versus control, Outcome 1 Long‐term abstinence (≥ 6 months).
4.

Forest plot of comparison: 1 Tobacco cessation intervention versus control, outcome: 1.1 Proportion of participants abstinent.
We did not create a funnel plot for the long‐term outcome because fewer than 10 studies were included.
Short‐term cessation
For short‐term abstinence, a pooled estimate of the 11 included studies showed benefit of intervention (RR 1.51, 95% CI 1.15 to 2.00; very low quality evidence) with moderate heterogeneity (I2 = 42%) (Analysis 2.1; Figure 5; Table 1). Abstinence in the control group at short‐term follow‐up was 8% (n = 67/848) and in the intervention group was 13% (n = 118/937).
2.1. Analysis.

Comparison 2 Tobacco cessation intervention versus control, Outcome 1 Short‐term abstinence (4 weeks to < 6 months).
5.

For the short‐term outcome, a funnel plot showed some evidence of asymmetry, suggesting the possibility of publication bias (Figure 6).
6.

Funnel plot of comparison: 2 Tobacco cessation intervention versus control, outcome: 2.1 Short‐term abstinence (4 weeks to < 6 months).
We undertook a sensitivity analysis, removing smaller studies, and this did not significantly change the pooled estimate. We undertook a sensitivity analysis according to whether NRT or varenicline was given; it did not significantly change the pooled estimates for long‐term or short‐term abstinence (Analysis 3.1; Analysis 3.2). Only one study included in the meta‐analysis used varenicline (Shelley 2015). We undertook a further sensitivity analysis according to the degree of intervention provided to the control: no input, standard care, or enhanced standard care. Two studies provided enhanced standard care (Lloyd‐Richardson 2009; Stanton 2015); as may be expected the quit rate observed amongst controls was higher in these studies compared to controls receiving standard care or no input. For short‐term outcomes, in the sensitivity analysis excluding these studies, the pooled estimate for the effect of the intervention increased slightly (Analysis 4.2). For long‐term outcomes, the pooled estimate did not markedly change (Analysis 4.1).
3.1. Analysis.
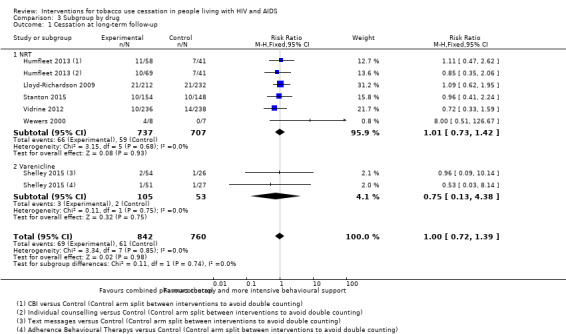
Comparison 3 Subgroup by drug, Outcome 1 Cessation at long‐term follow‐up.
3.2. Analysis.

Comparison 3 Subgroup by drug, Outcome 2 Cessation at short‐term follow‐up.
4.2. Analysis.
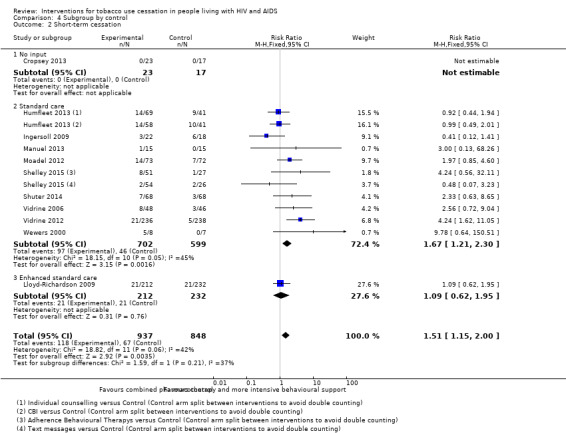
Comparison 4 Subgroup by control, Outcome 2 Short‐term cessation.
4.1. Analysis.

Comparison 4 Subgroup by control, Outcome 1 Long‐term cessation.
Subgroup analyses
Effect of provider
We categorised the provider of the behavioural intervention as follows: healthcare professional, researcher, or co‐facilitation by a peer and professional (Analysis 5.1). We failed to detect evidence of a difference in the effect according to provider in the analysis for long‐term abstinence. For short‐term outcomes, the pooled estimate for interventions that were co‐facilitated by a peer and professional (RR 2.52, 95% CI 1.14 to 5.56) were slightly larger than for other providers (healthcare professional alone: RR 1.41, 95% CI 0.98 to 2.03; and researcher: RR 2.56, 95% CI 0.72 to 9.04). However, the CIs overlapped.
5.1. Analysis.
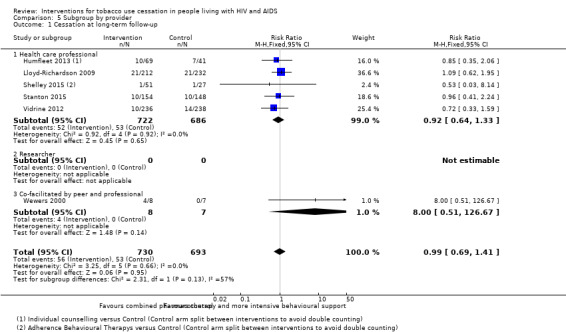
Comparison 5 Subgroup by provider, Outcome 1 Cessation at long‐term follow‐up.
Effect of mode of contact
We failed to detect evidence of an effect of subgroup categorisation by mode of contact for long‐term abstinence (Analysis 6.1). For short‐term outcomes, the pooled estimate was highest for interventions delivered via telephone (RR 3.69, 95% CI 1.80 to 7.53; Analysis 6.2). This was predominantly driven by the effect of one, large study (Vidrine 2012). There were few studies in some subgroups, which limits the conclusions possible from this subgroup analysis; only one intervention was delivered via text message (Shelley 2015), and two were delivered via computers (Humfleet 2013; Shuter 2014).
6.1. Analysis.

Comparison 6 Subgroup by mode of contact, Outcome 1 Cessation at long‐term follow‐up.
6.2. Analysis.

Comparison 6 Subgroup by mode of contact, Outcome 2 Cessation at short‐term follow‐up.
Participant selection
We failed to detect evidence of an effect of participant selection for long‐term abstinence (Analysis 7.1). For short‐term outcomes there is evidence of a difference in effect according to whether willingness or motivation to quit was required for participant inclusion (Analysis 7.2). The subgroup of participants who were selected for their willingness or motivation to quit had a higher pooled estimate of effect (RR 2.74, 95% CI 1.73 to 4.34, I² = 0%) compared to the subgroup for whom this was not required (RR 0.94, 95% CI 0.65 to 1.35, I² = 0%).
7.1. Analysis.

Comparison 7 Subgroup by selection, Outcome 1 Cessation at long‐term follow‐up.
7.2. Analysis.

Comparison 7 Subgroup by selection, Outcome 2 Cessation at short‐term follow‐up.
Tailoring
All studies which reported long‐term outcomes provided a tailored intervention, and one of these studies even tailored the control (Shelley 2015); therefore it was not possible to compare tailored to generic interventions for long‐term abstinence (Analysis 8.1). For short‐term outcomes, there was no strong evidence of a difference in effect for interventions tailored for PLWHA compared to generic interventions (Analysis 8.2). The comparison was limited due to the small number of studies providing a generic intervention. Only two studies provided a generic intervention, and in one of these, no participants in the intervention or control arm achieved abstinence at the level of biochemical verification set by the authors (Cropsey 2013).
8.1. Analysis.

Comparison 8 Subgroup by tailoring, Outcome 1 Cessation at long‐term follow‐up.
8.2. Analysis.

Comparison 8 Subgroup by tailoring, Outcome 2 Cessation at short‐term follow‐up.
Intensity
The investigation of intensity of behavioural intervention, via number of sessions and total duration of contact for long‐term abstinence, was a post hoc objective; we failed to detect evidence of a difference in effect according to either number of sessions or duration of total intervention (Analysis 9.1; Analysis 10.1). There were few studies in each subgroup. Intensity subgroup analyses were not completed for short‐term outcomes because the intervention may not have been completed at the time of short‐term outcome assessment.
9.1. Analysis.
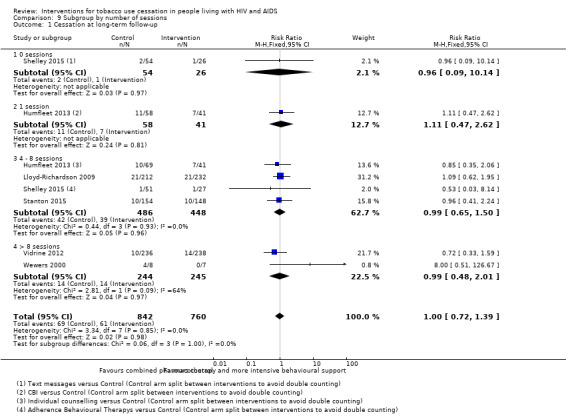
Comparison 9 Subgroup by number of sessions, Outcome 1 Cessation at long‐term follow‐up.
10.1. Analysis.

Comparison 10 Subgroup by total contact time, Outcome 1 Cessation at long‐term follow‐up.
Adverse events
It was not possible to perform a quantitative synthesis of adverse events, since only one study reported them in detail (Ferketich 2013).
HIV outcomes
It was not possible to perform the planned quantitative synthesis of HIV outcomes ‐ CD4 count, viral load, and incidence of opportunistic infections ‐ since only one study reported them (Elzi 2006).
Discussion
Summary of main results
This systematic review provides evidence from 14 studies. More than 1600 participants from 12 studies contributed to the meta‐analysis for the primary outcome of long‐term abstinence.
In this review we found that more intense combined interventions of pharmacotherapy and behavioural support were effective in increasing the chance of achieving abstinence in the short‐term (four weeks to less than six months) compared to a control group that typically included a single brief intervention and pharmacotherapy. The pooled estimate for short‐term outcomes (risk ratio (RR) 1.51, 95% confidence interval (CI) 1.15 to 2.00) indicates that a combined intervention might typically increase cessation success by 15% to 100%. However, this effect was not observed for long‐term abstinence (greater than six months).
There are differences between studies ‐ in particular whether participants were selected for their willingness or motivation to quit. Those studies including only motivated or willing participants showed a larger pooled estimate of the effect of the intervention for short‐term outcomes, although this was not observed at long‐term follow‐up.
As noted, we were unable to assess one of the original objectives ‐ whether interventions combining pharmacotherapy and behavioural support were more effective than either type of support alone. This was because all included studies assessed a combined intervention compared to control. We added a post hoc objective assessing the effect of intensity of behavioural intervention. This analysis did not find any evidence of a difference in the effect of intensity. Although this subgroup analysis was limited by the small number of studies and does not definitively indicate that increasing the intensity would not result in an increased effect. In the general population, Stead 2016 also found no evidence that more intensive support increased the effect of treatment. Our analysis according to intensity only included personal contact time via telephone or in person. This may have underestimated the impact of interventions delivered via computers or text messages.
Subgroup analysis was undertaken according to whether the intervention was tailored for people living with HIV/AIDS (PLWHA). We failed to detect evidence of a difference in effect for tailored interventions for short‐term abstinence, although this analysis was limited by a small number of studies providing a generic intervention. Subgroup analysis by tailoring was not possible for long‐term outcomes because all studies provided a tailored intervention.
Overall completeness and applicability of evidence
All studies included in the meta‐analysis were undertaken in the US, although they do encompass a range of demographic profiles. There are health system and socioeconomic differences between the US and Europe, as well as between the US and sub‐Saharan Africa, where the majority of PLWHA live (UNAIDS 2015). This limits the generalisability of these results. Among ongoing studies, most are based in North America, although NCT01484340 is based in South Africa.
There remains a lack of studies which have large sample sizes and that assess long‐term abstinence (greater than six months).
Quality of the evidence
We evaluated the overall quality of the evidence according to GRADE criteria. For the primary outcome of long‐term cessation, we judged the quality of evidence to be moderate. The quality of the evidence was downgraded due to risk of bias; one study was judged to be at high risk of reporting bias, and allocation concealment and blinding were poorly described.
For the secondary outcome of short‐term cessation, we judgedthe quality of evidence to be very low due to inconsistency, in addition to reporting and detection biases.
We judged five studies to be at high risk of reporting biases where outcomes described in the protocol were not accounted for in the published reports. Although, in most cases communication with the study authors resulted in additional data being made available, or the study authors provided an explanation as to why they were unable to report the proposed outcomes. Allocation concealment and blinding were poorly described and therefore we assessed most studies to have 'unclear' judgements in these areas. There was also a high proportion of small studies, which reflects that this is a relatively new area of research. The rationale for upgrading or downgrading the quality of the body of evidence is presented in Table 1.
Potential biases in the review process
There is evidence of possible publication bias in the funnel plot for short‐term outcomes, despite substantial effort being made to locate studies within the grey literature. Although we did identify some attrition bias and reporting bias, the impact of these were reduced through correspondence with authors to clarify points or request additional data. Additional data were kindly provided in most of these cases.
We added one objective post hoc ‐ assessing the impact of intensity of the behavioural intervention on abstinence, and therefore potentially introducing some bias. However, we felt it was logical and consistent with the ethos of the original objective ‐ to assess whether combined interventions are more effective than pharmacotherapy and behavioural support alone.
As is standard for Cochrane Reviews, all reports were reviewed and all data were extracted in duplicate in order to reduce bias.
Agreements and disagreements with other studies or reviews
We believe that this is the first systematic review of tobacco cessation interventions for PLWHA to include meta‐analysis. A previous systematic review provided a narrative overview and included some studies excluded by our stricter inclusion criteria (Moscou‐Jackson 2014).
In the general population, interventions that combine behavioural and pharmacotherapy components have been shown to be effective for long‐term abstinence when compared to a brief intervention without pharmacotherapy (Stead 2016), the reason for this effect not being observed in PLWHA is not clear. In the general population, tailoring cessation interventions has been shown to increase their effect (Hartmann‐Boyce 2014). This was not shown in this review, but our comparison was limited by a small number of studies with a non‐tailored generic intervention. It is important to consider that the evidence presented here is limited and has been judged to be of very low to moderate quality. There is much more and higher quality evidence assessing smoking cessation interventions and finding them effective in the general public. Therefore, it would be too premature to say that interventions aimed at the general population will not benefit PLWHA.
However, the following could explain a less pronounced effect. People living with mental illnesses have some similarities to PLWHA; they use more tobacco than the general population, often consume tobacco alongside drugs or alcohol, and may use tobacco to cope with their illness or treatment side effects (Tsoi 2013). In Tsoi and colleagues’ review of smoking cessation interventions for people with schizophrenia they found that at short‐term follow‐up there was evidence in favour of a combined intervention (counselling and nicotine replacement therapy (NRT)) (Tsoi 2013), but long‐term follow‐up failed to detect evidence of a difference between the intervention and control. Their results echo the results of this meta‐analysis and could reflect a higher potential for relapse in people with complex chronic diseases and multiple challenges. However, the comparison is limited, as the two populations have distinct differences, both medically and psychosocially.
Authors' conclusions
Implications for practice.
The results of this review must be considered in the context of the small number of studies included and the low to moderate quality of the evidence, and it therefore should not be ruled out that smoking cessation interventions that have been found to work in the general population will not have an effect in PLWHA. More intense combined interventions of pharmacotherapy and behavioural support were shown to be effective in assisting PLWHA to achieve short‐term abstinence, when compared to controls in this review; however this effect was not observed at long‐term follow‐up. This could be a function of study biases. Therefore, evidence suggests that clinicians should offer a combined intervention to PLWHA who use tobacco, at least comprising a brief behavioural intervention with pharmacotherapy, since even non‐sustained periods of abstinence have proven health benefits. The effects of tailoring, number of contacts and total duration of contact of behavioural support remain unclear.
Implications for research.
Further randomised controlled trials (RCTs) of tobacco cessation interventions in PLWHA are needed; they should include a large sample size and ensure that follow‐up continues for at least six months, and preferably 12 months.
In this review, there was evidence of effectiveness of the intervention at short‐term follow‐up; however, we rated the quality of this evidenceas 'very low' according to GRADE, and we did not observe any effect at long‐term follow‐up. Further research is needed to address the potential biases in the existing literature, including investigating relapse prevention in PLWHA who achieve short‐term abstinence. This would maximise the probability of short‐term success being translated into long‐term abstinence.
Trials assessing the impact of tailoring and intensity of interventions are also needed. Data on sexual orientation should be routinely collected and reported in studies of PLWHA. Tobacco consumption may effect treatment response, as such, future studies measuring HIV outcomes ‐ CD4 count, viral load, and incidence of opportunistic infections ‐ would be highly informative. The fact that almost all studies are based in the US limits generalisability due to population and health system differences. Further studies should be based in a range of contexts, particularly in low‐ and middle‐income countries with a high burden of HIV and tobacco consumption.
Future studies should ensure that reporting is in line with CONSORT criteria (Schulz 2010), particularly with regard to blinding and allocation concealment. None of the included studies reported their study in line with these criteria and as such, we judged many to be of 'unclear' risk of bias.
Acknowledgements
We acknowledge the support of all at the Cochrane Tobacco Addiction group, particularly Lindsay Stead, Nicola Lindson‐Hawley and Monaz Mehta. We would like to thank Lindsay Stead and Claire Duddy for assistance with the search strategy, and Gonçalo Figueiredo Augusto at the London School of Hygiene and Tropical Medicine (LSHTM) who translated an article from Spanish to English for the review.
Appendices
Appendix 1. MEDLINE search strategy
Search terms for MEDLINE search:
1. RANDOMIZED‐CONTROLLED‐TRIAL.pt.
2. CONTROLLED‐CLINICAL‐TRIAL.pt.
3. CLINICAL‐TRIAL.pt.
4. Meta analysis.pt.
5. exp Clinical Trial/
6. Random‐Allocation/
7. randomized‐controlled trials/
8. double‐blind‐method/
9. single‐blind‐method/
10. placebos/
11. Research‐Design/
12. ((clin$ adj5 trial$) or placebo$ or random$).ti,ab.
13. ((singl$ or doubl$ or trebl$ or tripl$) adj5 (blind$ or mask$)).ti,ab.
14. (volunteer$ or prospectiv$).ti,ab.
15. exp Follow‐Up‐Studies/
16. exp Retrospective‐Studies/
17. exp Prospective‐Studies/
18. exp Evaluation‐Studies/ or Program‐Evaluation.mp.
19. exp Cross‐Sectional‐Studies/
20. exp Behavior‐therapy/
21. exp Health‐Promotion/
22. exp Community‐Health‐Services/
23. exp Health‐Education/
24. exp Health‐Behavior/
25. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24
26. smoking cessation.mp. or exp Smoking Cessation/
27. "Tobacco‐Use‐Cessation"/
28. "Tobacco‐Use‐Disorder"/
29. Tobacco‐Smokeless/
30. exp Tobacco‐Smoke‐Pollution/
31. exp Tobacco‐/
32. exp Nicotine‐/
33. ((quit$ or stop$ or ceas$ or giv$) adj5 smoking).ti,ab.
34. exp Smoking/pc, th [Prevention & Control, Therapy]
35. 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 [A category smoking terms]
36. exp Smoking/ not 35 [B category smoking terms]
37. 1 or 2 or 3 [Likely CT design terms; RCTs, CCTs, Clinical trials]
38. 35 and 25 [A category smoking+all design terms]
39. 35 and 37 [A category smoking terms+likely CT design terms]
40. (animals not humans).sh. [used with 'not' to exclude animal studies for each subset]
41. ((26 or 27 or 28 or 29) and REVIEW.pt.) not 38 [Set 4: Core smoking related reviews only]
42. 36 and 25 [B category smoking+all design terms]
43. (42 and 37) not 40 [Set 3: B smoking terms, likely CT design terms, human only]
44. 38 not 39 not 40 [Set 2: A smoking terms, not core CT terms, human only]
45. (35 and 37) not 40 [Set 1: A smoking terms, likely CT design terms, human only]
46. exp Smoking Cessation/ not (44 or 45) [Smoking cessation only, no design terms]
47. exp hiv infections/ or exp acquired immunodeficiency syndrome/
48. hiv/ or hiv‐1/ or hiv‐2/
49. ("acquired immunodeficiency syndrome" or "acquired immunedeficiency syndrome" or "acquired immuno‐deficiency syndrome" or "acquired immune‐deficiency syndrome").mp.
50. "HIV/AIDS".mp.
51. HIV.mp.
52. PLWHA.mp
53. 47 or 48 or 49 or 50 or 51 or 52 [Any topic term]
54. 45 and 53 [Set 1 plus topic]
55. 44 and 53 [Set 2 plus topic]
56. 43 and 53 [Set 3 plus topic]
57. 54 or 55 or 56 [All sets]
Data and analyses
Comparison 1. Tobacco cessation intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term abstinence (≥ 6 months) | 6 | 1602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
Comparison 2. Tobacco cessation intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term abstinence (4 weeks to < 6 months) | 11 | 1785 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.15, 2.00] |
Comparison 3. Subgroup by drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at long‐term follow‐up | 6 | 1602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 1.1 NRT | 5 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.42] |
| 1.2 Varenicline | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.13, 4.38] |
| 2 Cessation at short‐term follow‐up | 11 | 1785 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.15, 2.00] |
| 2.1 NRT | 10 | 1627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.13, 1.99] |
| 2.2 Varenicline | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.49, 5.92] |
Comparison 4. Subgroup by control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term cessation | 6 | 1602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 1.1 Standard care | 4 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.61, 1.51] |
| 1.2 Enhanced standard care | 2 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.69] |
| 2 Short‐term cessation | 11 | 1785 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.15, 2.00] |
| 2.1 No input | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Standard care | 9 | 1301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.21, 2.30] |
| 2.3 Enhanced standard care | 1 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.62, 1.95] |
Comparison 5. Subgroup by provider.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at long‐term follow‐up | 6 | 1423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.41] |
| 1.1 Health care professional | 5 | 1408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.33] |
| 1.2 Researcher | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Co‐facilitated by peer and professional | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [0.51, 126.67] |
| 2 Cessation at short‐term follow‐up | 10 | 1470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.19, 2.23] |
| 2.1 Healthcare professional | 6 | 1176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.98, 2.03] |
| 2.2 Researcher | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [0.72, 9.04] |
| 2.3 Co‐facilitated by peer and professional | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.14, 5.56] |
5.2. Analysis.

Comparison 5 Subgroup by provider, Outcome 2 Cessation at short‐term follow‐up.
Comparison 6. Subgroup by mode of contact.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at long‐term follow‐up | 6 | 1602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 1.1 Face‐to‐face | 2 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.63, 1.65] |
| 1.2 Telephone | 2 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.33, 1.50] |
| 1.3 Computer | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.47, 2.62] |
| 1.4 Text message | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.09, 10.14] |
| 1.5 Equal face‐to‐face and telephone | 2 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.61, 2.82] |
| 2 Cessation at short‐term follow‐up | 11 | 1785 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.15, 2.00] |
| 2.1 Face‐to‐face | 6 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.77, 1.61] |
| 2.2 Telephone | 3 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.69 [1.80, 7.53] |
| 2.3 Computer | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.68, 2.34] |
| 2.4 Text message | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.07, 3.23] |
| 2.5 Equal face‐to‐face and telephone | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.78 [0.64, 150.51] |
Comparison 7. Subgroup by selection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at long‐term follow‐up | 6 | 1602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 1.1 Selected for motivation/willingness to quit | 3 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.84] |
| 1.2 Motivation/willingness to quit not required for inclusion | 3 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.70, 1.49] |
| 2 Cessation at short‐term follow‐up | 11 | 1785 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.15, 2.00] |
| 2.1 Selected for motivation/willingness to quit | 7 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [1.73, 4.34] |
| 2.2 Motivation/willingness to quit not required for inclusion | 4 | 733 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.65, 1.35] |
Comparison 8. Subgroup by tailoring.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at long‐term follow‐up | 6 | 1602 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.31] |
| 1.1 Tailored intervention versus generic control | 5 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.68, 1.33] |
| 1.2 Tailored intervention versus tailored control | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.13, 4.38] |
| 1.3 Generic intervention versus generic control | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Cessation at short‐term follow‐up | 11 | 1785 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.10, 1.91] |
| 2.1 Tailored intervention versus generic control | 7 | 1463 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.02, 1.83] |
| 2.2 Tailored intervention versus tailored control | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.86, 5.01] |
| 2.3 Generic intervention versus generic control | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
Comparison 9. Subgroup by number of sessions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at long‐term follow‐up | 6 | 1602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 1.1 0 sessions | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.09, 10.14] |
| 1.2 1 session | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.47, 2.62] |
| 1.3 4 ‐ 8 sessions | 4 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.65, 1.50] |
| 1.4 > 8 sessions | 2 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.48, 2.01] |
Comparison 10. Subgroup by total contact time.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at long‐term follow‐up | 5 | 1128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.75, 1.55] |
| 1.1 0 minutes | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.09, 10.14] |
| 1.2 1 ‐ 30 minutes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 31 ‐ 90 minutes | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.47, 2.62] |
| 1.4 91 ‐ 300 minutes | 4 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.73, 1.80] |
| 1.5 > 300 minutes | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.35, 2.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cropsey 2013.
| Methods | Country: US
Design: paralell, pilot RCT
Number of centres: 1
Selection: invited to participate during appointments Definition of tobacco user/smoker: self report ≥ 5 cpd |
|
| Participants | Number: 40 participants
Average age: 44.5 years Gender: 48% male Sexuality: not reported Race/ethnicity: 58% Black cpd: 19 cpd in intervention group, 15.5 cpd in control group Mean FTND score: 6.0 in intervention group, 6.5 in control group |
|
| Interventions | 1. Intervention: one informational session for 20 minutes, delivered by a research assistant face‐to‐face. NRT, 14 mg patches and 2 mg lozenges for four weeks
2. Control: no intervention by research team Provider: research assistant (Bachelors level) Tailoring: neither intervention nor control were tailored for PLWHA |
|
| Outcomes | Abstinence: self reported PPA at 2, 4, and 8 weeks post‐enrolment Validation: exhaled CO < or equal to 2 ppm | |
| Notes | Cessation was not a primary outcome of the study. The cessation outcomes were not published but were obtained following correspondence with the author Funding: Centers for AIDS Research grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Particpants were randomised using a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants were asked questions and given questionnaires, the process is insufficiently described to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 participants were excluded. The distribution of missing participants was balanced between groups (3:2) The reasons for missing data are insufficiently described to permit judgement |
| Selective reporting (reporting bias) | High risk | Biochemical verification of abstinence (by eCO and urine cotinine) was planned but not reported These data were obtained via communication with the author |
Elzi 2006.
| Methods | Country: Switzerland
Design: parallel, pilot controlled trial within Swiss HIV Cohort Study
Number of centres: 1
Selection: participants who expressed an interest in quitting smoking were invited to participate; control group participants were smokers not interested in quitting Definition of tobacco user/smoker: self report ≥ 1 cpd for > 12 months |
|
| Participants | Number: 417 (33 died, therefore excluded from final analysis) Average age: 43 years in intervention group, 40 years in control group Gender: 69% male Sexuality: not reported Race/ethnicity: 87% White cpd: 28 cpd in intervention group, 21 cpd in control group FTND score: > 7 for 74% in intervention group, not reported in control group | |
| Interventions | 1. Intervention: 15 individual face‐to‐face counselling sessions based on CBT. Sessions were 30 minutes duration and took place weekly during the first month, and monthly thereafter for 12 months. NRT patches, tablets, chewing gum, and sprays were offered to all participants; dose and duration not stated
2. Control: no intervention Provider: nurse trained in smoking cessation counselling Tailoring: the intervention was not described as tailored to PLWHA |
|
| Outcomes | Abstinence: self reported sustained abstinence at 12 months or greater Validation: no biochemical validation | |
| Notes | This study was not included in the meta‐analysis due to study design and heterogeneity Funding: the Swiss HIV Cohort Study is supported by the Swiss National Science Foundation Deaths: in the intervention group, 1/34 (3%) participants died of lung cancer. In the control group, 32/383 (8%) died during the study period. Of these deaths, 11 were HIV‐related, including one related to lung cancer and one related to cardiovascular disease |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised "participation in the programme [intervention group] was offered to those individuals who expressed an interest to quit smoking" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described in sufficient detail to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Smoking status extracted from routine clinic data, but clinician blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The distribution of missing participants was balanced between groups. Explanations were provided |
| Selective reporting (reporting bias) | Low risk | Primary outcome was reported in full |
Ferketich 2013.
| Methods | Country: US
Design: parallel, non‐randomised controlled study, safety study
Number of centres: 1
Selection: participants were recruited from within the Lung HIV study, interest in quitting was an inclusion criterion Definition of tobacco user/smoker: self report ≥5 cpd |
|
| Participants | Number: 228 Average age: 43 years in intervention group, 43 years in control group Gender: 86% male in intervention group, 85% male in control group Sexuality: not reported Race/ethnicity: 61% White in intervention group, 50% White in control group cpd: 19.7 cpd in intervention group, 20 cpd in control group Mean FTND score: 4.9 in intervention group, 5.4 in control group | |
| Interventions | 1. Intervention: varenicline, titrated up to 1 mg twice daily. 12 weekly individual counselling sessions, first session face‐to‐face, all following sessions via telephone
2. Control: NRT 21 mg patch per day plus 4 mg gum as required to a maximum of 24 pieces per day. Counselling as per intervention group Provider: advanced practice nurse "experienced in delivering tobacco dependence treatment" Tailoring: neither intervention nor control were tailored for PLWHA |
|
| Outcomes | Abstinence: self reported 7‐day PPA at 12 weeks post‐initiation of treatment, TQD was set at approximately week 3, therefore abstinence assessment is approximately 9 weeks post‐TQD Validation: eCO < 10 ppm for participants not using NRT, saliva cotinine < 15 ng/ml for participants using NRT |
|
| Notes | Funding: National Institutes of Health grant This study was not included in the meta‐analysis as it was designed to compare pharmacotherapy and was not in keeping with the comparison of intervention versus less intense control |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised, "participants were encouraged to select varenicline" unless it was contraindicated in which case they were assigned to the control NRT group |
| Allocation concealment (selection bias) | High risk | Allocation was according to participant selection and not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessed by face‐to‐face interview, insufficiently described to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants missing was approximately balanced between groups. Missing data imputed as missing = smoking |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported in full |
Humfleet 2013.
| Methods | Country: US Design: parallel, three‐group RCT Number of centres: 3 Selection: participant self referral or clinician referral. Postcards and flyers at clinics, letters sent to patients Definition of tobacco user/smoker: self report smoking most days in a month | |
| Participants | Number: 209 Average age: 45 years Gender: 82% male Sexuality: 62% Gay/Lesbian, 24.3% straight, 7.4% bisexual Race/ethnicity: 53% White Average cpd: 19.8 Mean FTND score: 4.9 | |
| Interventions | 1. Computer‐based intervention: one face‐to‐face orientation meeting of 45‐60 minutes. Six counselling sessions based on CBT, delivered via a website, in addition to message board and ‘ask the experts’ options on the website. Mean duration on website 30‐45 minutes. 10 weeks of NRT, patch or gum, available to participants who smoked ≥ 5 cpd; dose not stated
2. Individual counselling: six individual, face‐to‐face sessions based on CBT over 12 weeks. Session duration 40‐60 minutes. NRT as per Computer‐based intervention group 3. Control: one‐off, brief, face‐to‐face meeting with research staff and written reference guide provided. NRT as per the intervention groups Provider of individual counselling: "clinicians with a master’s or doctoral degree in social work or psychology and had previous experience in smoking cessation treatment" Tailoring: individual counselling was tailored via focus on impact of smoking on HIV, stress, depression, low social support and HIV‐related health issues. CBI was described as modelled on individual counselling and therefore assumed to be tailored. Control was not described as tailored |
|
| Outcomes | Abstinence: 7‐day PPA and sustained abstinence at 12, 24, 26, and 52 weeks following intervention. In individual counselling group TQD was in week 2. TQD is not clearly described for CBI or control groups Validation: PPA outcomes were verified by eCO ≤ 10 ppm, the sustained abstinence outcomes were not biochemically verified | |
| Notes | PPA and sustained abstinence outcomes were measured but sustained outcomes were not described in detail in the published report. The sustained outcome data for meta‐analysis were obtained via email communication with the authors Funding: NIDA grants and California TRDRP grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were "randomized via computer algorithm to one of three conditions in 1:1:1 fashion into a parallel group design" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in sufficient detail to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing participants was evenly balanced between groups |
| Selective reporting (reporting bias) | High risk | The study was registered on clinicaltrials.gov NCT00297453. The primary outcome measure of smoking cessation was measured by PPA and sustained abstinence, but sustained abstinence results were not reported in full |
Ingersoll 2009.
| Methods | Country: US Design: parallel RCT Number of centres: 1 Selection: flyers and self referral or direct contact with researcher Definition of tobacco user/smoker: self report smoking daily | |
| Participants | Number: 40 Average age: 42 years Gender: 55% male Sexuality: not reported Race/ethnicity: 95% Black Average cpd: 17.3 Mean FTND score: 5.0 | |
| Interventions | 1. Intervention: one individual face‐to‐face counselling session, based on MI. NRT provided according to volume of tobacco consumed. If participants smoked > 10 cpd; 21 mg NRT patch for 1 month, 14 mg patch for 2 weeks and 7 mg patch for two weeks. If participants smoked < 10 cpd; 14 mg patch for 2 weeks and 7 mg patch for two weeks
2. Control: written materials to facilitate self assessment and tips for cessation. One‐off face‐to‐face session where the materials were reviewed, but no counselling was provided. NRT as per intervention group Provider: post‐doctoral fellow or counsellor Tailoring: the intervention was tailored through a focus on risk of smoking in the context of HIV. Control was not tailored for PLWHA |
|
| Outcomes | Abstinence: self report PPA at 1 and 3 month follow‐up Validation: eCO < 3 ppm | |
| Notes | Additional data were obtained following correspondence with the author. In the published report the data from the two groups were added and presented as one data set Funding: NIH K01MH10688, NIH K23DA15774, and VCU’s Institute for Drug and Alcohol Studies Conflicts of interest: Glaxo Smith Kline provided nicotine patches for the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...participants were randomly assigned to one of two treatment conditions” but sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome was assessed by self report and eCO, process not described in sufficient detail to permit judgement of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants (5) is reported but distribution is not described and explanations not given |
| Selective reporting (reporting bias) | High risk | The design was a two‐group trial but the results were aggregated and only reported as a whole |
Lloyd‐Richardson 2009.
| Methods | Country: US Design: parallel RCT Number of centres: 8 (6 outpatient HIV clinics and 2 primary care centres) Selection: participants were recruited at their clinic Definition of tobacco user/smoker: self report ≥5 cpd | |
| Participants | Number: 444 Average age: 42 years Gender: 63% male Sexuality: not reported Race/ethnicity: 52% White cpd: 18.2 cpd Mean FTND score: 5.9 | |
| Interventions | 1. Intervention: 1 brief advice session, plus 4 face‐to‐face individual counselling sessions based on MI and a quit day phone call. Duration of each counselling session was 30 minutes. Participants willing to set a TQD were provided with NRT patches, 8 weeks duration; dose not described. 2. Control: 2 brief advice sessions, delivered face‐to‐face and self help written materials. In addition, participants willing to set a TQD received biweekly brief sessions (5 minutes duration) to reinforce quit effort, check patch side effects and distribute NRT patches. NRT provided as per intervention group Provider: health educator trained in smoking cessation Tailoring: Intervention was tailored via an emphasis on the impact on infections and immunity. Control was not tailored for PLWHA |
|
| Outcomes | Abstinence: 7‐day PPA at 2, 4, and 6 months post‐enrolment Validation: eCO < 10 ppm | |
| Notes | The published report included percentage abstinence rate only; the number of participants abstinent were calculated as we were unable to be obtain these via email correspondence with the authors Funding: NIDA grant, the National Heart, Lung, and Blood Institute grant, National Cancer Institute grant, an NIH‐funded Transdisciplinary Tobacco Use Research Center Award grant, NIH‐funded Lifespan/Tufts/Brown Center for AIDS Research Award, and by the Robert Wood Johnson Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were then randomized (using block randomization to ensure stratification by gender and level of motivation to quit smoking)" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described in sufficient detail to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Follow‐up assessments were administered by research staff blinded to participant intervention assignment” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing participants is balanced between groups. A missing = smoking assumption was used |
| Selective reporting (reporting bias) | Low risk | All of the primary outcomes were reported in full. A study protocol was published on clinicaltrials.gov (NCT00551720), the published method and outcomes correlate with the protocol methods and outcomes |
Manuel 2013.
| Methods | Country: US Design: parallel RCT Number of centres: 1 Selection: participants were recruited at their clinic through flyers and clinician referrals. Participant interest in quitting was an inclusion criterion Definition of tobacco user/smoker: self reports of smoking daily for at least five of the last seven days | |
| Participants | Number: 30 Average age: 49 years Gender: 100% female (being female was an inclusion criterion) Sexuality: 67% heterosexual Race/ethnicity: 47% Black cpd: 15.5 in intervention, 16.7 in control Mean FTND score: 4.1 in intervention, 5.0 for control | |
| Interventions | 1. Intervention: 1 face‐to‐face counselling session, based on MI. Average duration 27 minutes. Participants were referred to NRT programmes, but dose, duration and frequency of NRT not described
2. Control: 1 face‐to‐face session and written materials. Written materials were reviewed and strategies discussed. Duration not described. NRT as per the intervention Therapist: "highly experienced MI clinician" Tailoring: neither intervention nor control were tailored for PLWHA |
|
| Outcomes | Abstinence: 7‐day PPA at 1 month follow‐up Validation: urine nicotine and cotinine, scale not described | |
| Notes | Funding: NIDA grant, the NIDA San Francisco Treatment Research Center grant, and University of California’s Center for AIDS Prevention grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A “permuted block randomization” technique was used |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were used but safeguards not described in sufficient detail to permit judgement: “the interviewer opened a sealed envelope indicating which condition the participant had been randomized to receive” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not clearly described, but a single therapist delivered both control and intervention treatments therefore blinding is unfeasible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “RA was blind to the participants’ treatment condition” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant lost to follow‐up and no data imputed. Of the 3 people who reported abstinence, only two urine samples were taken, no explanation provided |
| Selective reporting (reporting bias) | Low risk | Primary outcomes are all reported |
Moadel 2012.
| Methods | Country: US Design: parallel RCT Number of centres: 1 Selection: Participants were referred via clinicians or recruited in the waiting room, motivation to quit (> 6 on the readiness ladder) was required for inclusion Definition of tobacco user/smoker: self reported use of any product containing nicotine (cigarettes, pipes or cigars) in the past 5 days | |
| Participants | Number: 145
Average age: 49 years
Gender: 49% male
Sexuality: not directly reported, HIV risk group was 'same sex contact' for 14.5% and 'heterosexual contact' for 57.9% Race/ethnicity: 86% Black cpd: 12.0 cpd Mean FTND score: 5.0 |
|
| Interventions | 1. Intervention: eight group counselling sessions of six to eight participants. Sessions were based on social cognitive theory principles and occurred weekly (90 minutes duration). Participants were offered three months of NRT; dose and mode of delivery not described
2. Control: one‐off, face‐to‐face brief advice (< 5 minutes) to quit and written materials were provided. NRT as per intervention Provider: co‐facilitated by one professional (psychology graduate student and ex‐smoker) and one peer (PLWHA ex‐smoker). Both "completed certified courses in tobacco treatment" Tailoring: intervention was tailored through use of a HIV‐positive peer and highlighting the specific risks of smoking for PLWHA. The control was not tailored for PLWHA |
|
| Outcomes | Abstinence: 7‐day PPA at days 0, 42, and 132; assessment at 3 months from TQD Validation: eCO < 10 ppm | |
| Notes | NRT was the only cessation medication offered to trial participants, although the authors report that some participants also obtained bupropion or varenicline outside of the study protocol Funding: NIH/NIDA grant, Clinical Core of the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center funded by a NIH grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects who provided consent were randomized in a 1:1 schedule to the two study conditions" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in sufficient detail to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The distribution of missing participants was balanced between groups (4 from intervention and 2 from control). Missing data imputed as missing = smoking |
| Selective reporting (reporting bias) | High risk | The study was registered on clinicaltrials.gov NCT01106638. A secondary outcome measure of 'continuous abstinence' was described in the protocol but not reported |
Shelley 2015.
| Methods | Country: US
Design: paralell RCT
Number of centres: 3
Selection: participants were recruited from HIV care centres, willingness to quit within the next two weeks was required for inclusion Definition of tobacco user/smoker: at least 5 cpd |
|
| Participants | Number: 158 Participant characteristics are for 127 participants (outcome data are for all 158 participants) Average age: 50 years Gender: 84% male Sexuality: not reported Race/Ethnicity: 48% Black cpd: 15 cpd Mean FTND score: not reported |
|
| Interventions | Intervention 1. Text messages : adherence‐focused twice daily text messages. 12 weeks of varenicline. Based on Information Motivation Behaviour model Intervention 2. Adherence Behavioural Therapy : adherence‐focused twice daily text messages, plus 7 sessions of telephone counselling. Planned duration of call: 20‐30 minutes. Based on Information Motivation Behaviour model. 12 weeks of varenicline 3. Control/standard care: 12 weeks of varenicline, dose and frequency not reported. Information sheet and State Quitline number provided. Participants in intervention groups also received control Provider: trained counsellors (Masters level) Tailoring:text messages were tailored through conveying information considered particularly relevant to PLWH, but did not use the terms HIV/AIDS to ensure confidentiality. Adherence Behavioural Therapy intervention discussed the effects of smoking on HIV and focused on specific barriers for PLWH. The self help information sheet given as standard care was tailored to PLWH |
|
| Outcomes | Abstinence: 7‐day PPA at weeks 1, 4, 8, 12, and 24 from start of treatment Validation: eCO < 8 ppm |
|
| Notes | The study was registered on clinicaltrials.gov NCT01898195 In published reports, 1 month per protocol outcomes were reported. 24 week intention‐to‐treat outcomes were obtained following correspondence with the author. Funding: NIDA and Centre for Drug and HIV Research Conflicts of interest: Pfizer provided study medication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...participants were randomly assigned to one of two treatment conditions" but sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcome assessed by self report and eCO. Proportion of missing participants not described per group. Overall, insufficiently described to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Outcome assessed by self report and eCO. Proportion of missing participants not described per group. Overall, insufficiently described to permit judgement |
Shuter 2014.
| Methods | Country: US Design: parallel RCT Number of centres: one Selection: recruited at HIV clinic, interest in quitting was required for inclusion Definition of tobacco user/smoker: self reported use of cigarettes, pipes or cigars in the past five days | |
| Participants | Number: 138 (2 died, therefore excluded from final analysis)
Average age: 46 years
Gender: 60% male
Sexuality: not directly reported, HIV risk group was 'same sex contact' for 37% and 'heterosexual contact' for 44.2%
Race/ethnicity: 60% Black
cpd: 10.2 cpd in intervention, 11.5 cpd for control Mean FTND score: 4.8 in intervention, 4.9 in control |
|
| Interventions | 1. Intervention: eight interactive web‐based sessions, independently completed once a week, based on social cognitive theory. Mean time spent logged into the website; 59.8 minutes. NRT patches for three months were offered, dose and frequency not described
2. Control: brief advice (< 5 minutes duration) to quit and written materials. NRT as per intervention Provider: intervention was web‐based Tailoring: the intervention was tailored to PLWHA through comparison of changes in HIV risk behaviour and smoking behaviour. Control was not tailored |
|
| Outcomes | Abstinence: 7‐day PPA at 6 weeks and 3 months post‐TQD Validation: eCO < 10 ppm | |
| Notes | Draft report made available by authors prior to publication date Deaths: 2 participants died during the study period, 1 in control and 1 in intervention. Their deaths were not considered related to the study They were excluded in the reanalysis. Number abstinent were not reported (percentage abstinent were reported) in the publication, they were obtained via email communication with the author Funding: NIH/NCI grants, Clinical Core of the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center funded by the NIH grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... eligible subjects were randomized by study staff 1:1 into 2 conditions using a random number table and an even/odd allocation strategy" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described in sufficient detail to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment was by self administered questionnaire and eCO. Not described in sufficient detail to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The distribution of missing participants was balanced between groups (1 in intervention group, 3 in control group). Explanations were provided. Missing data imputed as missing = smoking |
| Selective reporting (reporting bias) | High risk | The methods in the report do not correlate with the protocol. The study was registered on clinicaltrials.gov NCT01570595. Primary and secondary outcomes differed in the published report compared to the protocol |
Stanton 2015.
| Methods | Country: US Design: parallel RCT Number of centres: 9 Selection: clinician referral from immunology clinics Definition of tobacco user/smoker: self report smoking cigarettes in the past 7 days | |
| Participants | Number: 302 Average age: 45 years Gender: 64% male Sexuality: not reported Race/ethnicity: 100% Latino (being Latino was required for inclusion) cpd and mean FTND score were not reported | |
| Interventions | 1. Intervention: as per control plus two additional face‐to‐face individual counselling sessions (average duration of session 1: 62 minutes) and two additional ten minute phone calls, provided by health educator. All sessions culturally tailored to Latino. Option to bring a ‘support buddy’, culturally sensitive written materials and videos. If willing to set a TQD received NRT for 8 weeks, dose according to smoking level
2. Control: physician brief advice, plus two face‐to‐face individual counselling sessions and one quit day phone call (ten minutes), and written materials. NRT as per intervention Provider: health educator "at least Masters level professionals (or had equivalent years of clinical research experience) and were trained on the implementation of the manual driven interventions" Tailoring: the intervention was tailored being both a PLWHA and a Latino, emphasis on the specific health consequences of smoking on HIV. Control not tailored to PLWHA or Latino |
|
| Outcomes | Abstinence: 7‐day PPA at 6 and 12 months post‐intervention Validation: eCO < 10 ppm | |
| Notes | Additional data and study design details were obtained via email communication with the authors ahead of full report publication. Funding: NIDA grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised “using an urn randomisation procedure” and stratified by gender |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in sufficient detail to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The distribution of missing participants was balanced between groups (54 from control group, 68 from intervention group). Missing data imputed as missing = smoking |
| Selective reporting (reporting bias) | Low risk | The study was registered on clinicaltrials.gov NCT00503230 and all primary outcomes were reported in full |
Vidrine 2006.
| Methods | Country: US Design: parallel RCT Number of centres: 1 Selection: all patients screened at clinic appointments and invited if eligible. Willingess to set a quit date within 7 days was required for inclusion Definition of tobacco user/smoker: self report ≥5 cpd and eCO > 7 ppm | |
| Participants | Number: 94 (1 participant died, therefore excluded in reanalysis) Average age: 43 years in intervention group, 43 years in control Gender: 78% male Sexuality: not directly reported, HIV risk group was 'same sex contact' for 37.9% and 'heterosexual contact' for 35.8% Race/ethnicity: 72% Black cpd: 20.6 cpd in intervention group, 19.5 in control Mean FTND score: 5.5 in intervention group, 5.7 in control | |
| Interventions | 1. Intervention: eight counselling sessions over two months, plus access to a hotline. Delivered via cell phone and based on CBT. NRT patches provided for ten weeks. All intervention participants also received control
2. Control: one session of brief advice, face‐to‐face. NRT patches provided for ten weeks. Written materials; personal plan, self‐help guide and tip sheet Provider: "research assistant (with a master’s level qualification) trained in smoking cessation counselling" Tailoring: the intervention was tailored to PLWHA through emphasising the impact of smoking on the immune system and HIV‐related diseases. Control tip sheet was tailored to PLWHA |
|
| Outcomes | Abstinence: self reported PPA and sustained abstinence Validation: eCO < 7 ppm | |
| Notes | Funding: National Cancer Institute grant and the Margaret and James A. Elkins, Jr Faculty Achievement Award in Cancer Prevention 1 death reported in the control group. Reanalysis of data undertaken excluding this participant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised via "adaptive randomization designed to minimize imbalances in the distribution of prognostic factors (i.e. depression, number of cigarettes smoked per day, and level of nicotine dependence” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessed by face‐to‐face interview and eCO, the process was insufficiently described to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The distribution of missing participants was balanced between groups (10 from intervention group, 8 from control group). Explanations were provided. Missing data imputed as missing = smoking |
| Selective reporting (reporting bias) | Low risk | Protocol not published, but all primary outcomes were reported in full |
Vidrine 2012.
| Methods | Country: US Design: parallel RCT Number of centres: 1 Selection: patients screened at clinic appointments and invited if eligible, willingness to set a quit date within 1 week was required for inclusion Definition of tobacco user/smoker: self report ≥5 cpd and eCO > 7 ppm | |
| Participants | Number: 474 Average age: 45 years Gender: 70% male Sexuality: not directly reported, HIV risk group was 'men who have sex with men' for 46%% and 'heterosexual contact' for 25% Race/ethnicity: 77% Black cpd: 19.2 cpd Mean FTND score: 5.73 in intervention group, 5.82 in control group | |
| Interventions | 1. Intervention: 11 counselling sessions, based on CBT, delivered via cell phone, over 3 months. Plus access to a hotline and self help written materials. Instructions to obtain NRT patches, details of dose, frequency and duration not described. All intervention participants also received the control interventions
2. Control: 1 brief counselling session delivered face‐to‐face and self help written materials. NRT as per intervention Provider: "counsellors were trained and supervised by a licensed clinical psychologist" Tailoring: intervention was tailored to PLWHA through reinforcing the HIV specific benefits of abstinence. Control was not tailored |
|
| Outcomes | Abstinence: 7‐day PPA and continuous abstinence at 3, 6, and 12 months post‐enrolment Validation: eCO < 7 ppm | |
| Notes | Only PPA outcomes were included in the published report, continuous abstinence outcomes were obtained via email communication with the authors Funding: NCI grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...participants were randomized to 1 of 2 treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome was assessed by self report using a computer and eCO. Process was not described in sufficient detail to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The distribution of missing participants was balanced between groups (retention was 75.8% for the intervention group, and 78.2% for the control group). Missing data imputed as missing = smoking |
| Selective reporting (reporting bias) | Low risk | The study was registered on clinicaltrials.gov NCT00502827, all primary outcomes were reported in full |
Wewers 2000.
| Methods | Country: US Design: parallel "quasi‐experimental" controlled trial, pilot study Number of centres: 1 Selection: the study was advertised to clinic patients, interest in quitting smoking was required for inclusion Definition of tobacco user/smoker: self report ≥ 10 cpd, for ≥ 1 year | |
| Participants | Number: 15
Average age: 40 years in intervention group, 37 years in control
Gender: 100% male
Sexuality: not reported Race/ethnicity: not reported cpd: 27 cpd in intervention group, 28 cpd in control |
|
| Interventions | 1. Intervention: three face‐to‐face individual counselling sessions (duration 30 minutes) and weekly phone calls (duration 10‐15 minutes) over 8 weeks. Additional calls as required and written materials. NRT patches 21 mg for 6 weeks
2. Control: written materials and a letter with a strong quit smoking message Provider: peer educator (PLWHA ex‐smoker) with a nurse as case manager. Peer was "trained by a nurse in smoking cessation treatment" Tailoring: the intervention was tailored through use of a HIV‐positive peer educator, control was not tailored |
|
| Outcomes | Abstinence: PPA and continuous abstinence at 8 weeks and 8 months post‐enrolment Validation: eCO < 8 ppm | |
| Notes | Pilot study. Poor retention of control participants, retention rate at 8 months was 43% in control group and 88% in intervention group Funding: National Institutes of Health, National Institute of Allergy and Infectious Diseases, Adult AIDS Clinical Trials Group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" but sequence generation not further described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not sufficiently described to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The distribution of missing participants was unbalanced between groups, with high loss to follow‐up in the control group at 8 months. However, since no control participants reported abstinence at 8 weeks, this would not have effected the outcome |
| Selective reporting (reporting bias) | Low risk | No study protocol was published. But all primary outcomes were reported in full |
CBT: cognitive behavioural therapy CO: carbon monoxide cpd: cigarettes per day eCO: expired carbon monoxide FTND score: Fagerström Test for Nicotine Dependence score MI: motivational interviewing NRT: nicotine replacement therapy PPA: point prevalence smoking abstinence ppm: parts per million PLWHA: people living with HIV/AIDS RCT: randomised controlled trial TQD: target quit date
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berg 2014 | Design: observational |
| Browning 2013 | Design: review, not primary research |
| Burkhalter 2013 | Outcome: not abstinence |
| Chefitz 2014 | Outcome: not abstinence |
| Cui 2012 | No control group |
| Cummins 2005 | No control group |
| Fuster 2009 | No control group |
| Huber 2012 | Study design: observational |
| Lazev 2004 | Follow‐up: under 30 days |
| Lima 2009 | No control group |
| Matthews 2013 | No control group |
| McKie 1985 | Not primary research |
| Mercie 2014 | Study complete but unpublished. Author contacted, results not available |
| NCT01393301 | Study complete but unpublished. Author contacted, results not available |
| NCT01436136 | Multifactorial intervention. Primary outcome: not abstinence |
| NCT02029612 | Study withdrawn |
| Pedrol‐Clotet 2006 | No control group |
| Reynolds 2009 | Not primary research |
| Shadel 2014 | Outcome: not abstinence |
| Shelley 2014 | Outcome: not abstinence |
| Tornero 2009 | No control group |
| Zwiebel 2008 | Design: observational service review |
Characteristics of ongoing studies [ordered by study ID]
NCT00701896.
| Trial name or title | Smoking cessation using motivational therapy and varenicline |
| Methods | Open‐label, non‐randomised study |
| Participants | HIV‐negative smokers and non‐smokers, and HIV‐positive smokers, in Ohio, US |
| Interventions | 'Healthy control' HIV‐negative non‐smokers (no intervention) versus 'Healthy control' HIV‐negative smokers (no intervention) versus 'Active comparator' HIV‐positive smokers (varenicline, NRT) versus HIV‐positive smokers (one motivational interview session) |
| Outcomes | "Develop and evaluate a specialized smoking cessation intervention" (outcome measure for evaluation not clearly documented) Lung function decline, the prevalence of respiratory symptoms and the occurrence/progression of emphysema |
| Starting date | June 2008 |
| Contact information | Philip T. Diaz, Ohio State University |
| Notes |
NCT01363245.
| Trial name or title | Effectiveness of smoking‐cessation interventions for urban hospital patients |
| Methods | Single‐blind (outcome assessment) RCT |
| Participants | Hospitalised HIV‐positive and HIV‐negative adults in New York, US. Current smokers (smoked tobacco in last 30 days) |
| Interventions | Hospital telephone counselling versus faxed referral to Quitline |
| Outcomes | Abstinence at 6 and 12 months post‐discharge, biochemically verified. Comparison of cessation outcomes between HIV‐positive and HIV‐negative participants |
| Starting date | July 2011 |
| Contact information | Scott E. Sherman, NYU School of Medicine |
| Notes |
NCT01484340.
| Trial name or title | A smoking cessation trial in HIV‐infected patients in South Africa |
| Methods | Open‐label RCT |
| Participants | HIV‐positive, current daily smokers (positive SmokeScreen test) in South Africa. Willing to set a quit date |
| Interventions | Intensive counselling versus intensive counselling + NRT |
| Outcomes | Abstinence at 2, 6, and 12 months. Biochemically verified by eCO < 8 ppm and urine cotinine |
| Starting date | March 2014 |
| Contact information | Sandy Chon, schon2@jhmi.edu |
| Notes |
NCT01710137.
| Trial name or title | A placebo controlled trial of varenicline for smoking among those with HIV/AIDS |
| Methods | Placebo controlled, double‐blind, RCT |
| Participants | HIV‐positive adults in Pennsylvania, USA. Current smokers (average at least 5 cigarettes per day) |
| Interventions | Varenicline + smoking cessation counselling versus placebo + smoking cessation counselling |
| Outcomes | Abstinence at 12 and 24 weeks, biochemically verified by urine cotinine |
| Starting date | October 2012 |
| Contact information | Sonja Blazekovic, sonjab@mail.med.upenn.edu |
| Notes |
NCT01800019.
| Trial name or title | The Canadian HIV Quit Smoking Trial: tackling the co‐morbidities of depression and cardiovascular disease in HIV+ smokers (CANQUIT) |
| Methods | Four‐group RCT |
| Participants | HIV‐positive, adults in Ontario, Canada. Current smokers (more than 5 cigarettes per day) and on anti‐retroviral therapy with an undetectable HIV viral load |
| Interventions | NRT alone versus NRT and HIV tailored quit smoking counseling versus varenicline alone versus varenicline and HIV tailored quit smoking counseling |
| Outcomes | Abstinence at 48 weeks, biochemically verified by eCO < 10 ppm |
| Starting date | January 2014 |
| Contact information | Louise Balfour, PhD. Ottawa Hospital Research Institute |
| Notes |
NCT01886924.
| Trial name or title | Computer‐based MI to engage smokers living with HIV in tobacco quitline treatment |
| Methods | 1) A pilot study to develop computer‐based intervention; interviews 2) Preliminary RCT |
| Participants | HIV‐positive, adults in Rhode Island, US. Current smokers (at least 10 cigarettes per day) |
| Interventions | Brief computer MI intervention to motivate tobacco quitline use versus computer‐delivered nutrition education (control) |
| Outcomes | Outcomes of pilot study: feasibility and acceptability Outcomes of RCT:engagement in cessation treatment, number of quit attempts, PPA at 6 months. |
| Starting date | February 2014 |
| Contact information | Jacki Hecht, jhecht@butler.org |
| Notes |
NCT01965405.
| Trial name or title | Behavioral smoking cessation treatment for people living with HIV/AIDS |
| Methods | Open‐label RCT |
| Participants | HIV‐positive adult in Michigan, USA. Current smoker (more than 10 cigarettes per day) |
| Interventions | Phase 1: Brief counselling + bupropion versus brief counselling + buproprion + prize contingency management. Phase 2 a: allocation dependent on non‐response in phase 1. Bupropion, continued counselling, monitored support to quit smoking versus bupropion, monitored support to quit smoking, prize contingency management for abstinence. Phase 2 b: allocation dependent on response in Phase 1. No additional treatment versus bupropion, continued monitoring and low intensity prize contingency management |
| Outcomes | Abstinence at 6 and 12 months, biochemically verified by urine cotinine and eCO |
| Starting date | August 2013 |
| Contact information | Lisa Sulkowski, lsulkows@med.wayne.edu |
| Notes |
NCT02072772.
| Trial name or title | A trial of Positively Smoke Free group therapy for HIV‐infected smokers |
| Methods | Open‐label RCT |
| Participants | HIV‐positive adult at one of two clinics in District of Columbia and New York, US. Current smoker and motivated to quit |
| Interventions | Positively Smoke Free (eight group therapy sessions led by a professional and a peer) + three months NRT versus standard care (brief advice and self help brochure) + three months NRT |
| Outcomes | Abstinence at 6 months, biochemically verified |
| Starting date | May 2014 |
| Contact information | Jonathan Shuter, jshuter@montefiore.org |
| Notes | Linked to Moadel 2012 NCT01106638 |
NCT02190643.
| Trial name or title | Improving nicotine patch adherence among Latino HIV‐positive smokers |
| Methods | RCT |
| Participants | HIV‐positive, Latino smokers in US. Ready to set quit date |
| Interventions | Standard care: brief counselling + eight weeks NRT versus includes a module focused on brief counselling including a module focused on improving adherence + eight weeks NRT |
| Outcomes | Abstinence at 3 months biochemically verified |
| Starting date | August 2014 |
| Contact information | Joan Tucker, jtucker@rand.org |
| Notes |
NCT02302859.
| Trial name or title | Mobile Media‐Rich Interactive Guideline System (MMRIGS) pilot study |
| Methods | Open‐label RCT |
| Participants | HIV‐positive patients at one clinic in Texas, US. Smokers (at least 5 per day and at least 100 lifetime cigarettes). Willing to make quit attempt within 1 week |
| Interventions | Standard care (brief advice, 8 sessions telephone counselling + 8 weeks NRT) versus automated treatment (brief advice + tailored video clips + 8 weeks of interactive text and graphical message via smartphone + 8 weeks NRT) |
| Outcomes | Abstinence at 3 months, biochemically verified by urine cotinine |
| Starting date | January 2015 |
| Contact information | Alex Prokhorov, M.D. Anderson Cancer Center |
| Notes |
NCT02432482.
| Trial name or title | A mobile intervention to promote cessation in HIV‐infected smokers |
| Methods | Open‐label RCT |
| Participants | HIV‐positive adults at one clinic in New York, US. Current smokers who are interested in quitting and own a smartphone |
| Interventions | Standard care: brief advice, written materials and offer of NRT versus mobile Positively Smoke Free (mPSF) providing 8 weekly sessions of audio/video messages, daily text messages + offer or NRT |
| Outcomes | Abstinence at 3 months, biochemically verified by eCO |
| Starting date | August 2015 |
| Contact information | Jonathan Shuter, jshuter@montefiore.org |
| Notes | Linked to Shuter 2014 NCT01570595 |
NCT02460900.
| Trial name or title | Optimizing smoking cessation for people with HIV/AIDS who smoke |
| Methods | Double‐blind, randomised placebo controlled trial |
| Participants | HIV‐positive adults in US |
| Interventions | Varenicline + standard behavioural care versus placebo + standard behavioural care versus varenicline + Positively Smoke Free; tailored behavioural intervention versus placebo + Positively Smoke Free; tailored behavioural intervention |
| Outcomes | Abstinence at 24 weeks |
| Starting date | December 2015 |
| Contact information | Seth S Himelhoch, shimelho@psych.umaryland.edu |
| Notes |
eCO: expired carbon monoxide MI: motivational interviewing NRT: nicotine replacement therapy ppm: parts per million RCT: randomised controlled trial
Differences between protocol and review
We could not address one of the secondary objectives ‐ to assess whether interventions combining pharmacotherapy and behavioural support are more effective than either type of support alone in PLWHA ‐ due to all included studies providing a combined intervention. We added a post hoc objective ‐ to assess whether more intense behavioural support is more effective. This involved analysis according to number of sessions and total duration of contact time.
We were unable to perform the planned synthesis of adverse events or HIV outcomes (CD4 count, viral load or incidence of opportunistic infections), since they were not reported in more than one study.
Two new authors (RL and PW) joined the review team following the protocol.
Contributions of authors
Erica Pool (EP) and Kamran Siddiqi (KS) conceived the review. EP wrote the protocol, with input from KS and Omara Dogar (OD). EP, KS and Ryan Lindsay (RL) screened the reports and agreed on inclusion of the studies. EP, RL, and OD extracted data. EP drafted the text. EP undertook the analyses, with input from OD. KS and Peter Weatherburn (PW) provided overall supervision of the project. All authors reviewed the text and are responsible for the analyses and conclusions.
Sources of support
Internal sources
-
London School of Hygiene and Tropical Medicine, UK.
This review was partly undertaken during Erica Pool's study at the London School of Hygiene and Tropical Medicine, which was supported by the LSHTM UK/EU Studentship Award.
-
NIHR Academic Clinical Fellowship, UK.
Erica Pool is funded by a National Institute for Health Research (NIHR) Academic Clinical Fellowship. This report presents independent research funded by the NIHR. The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health.
External sources
No sources of support supplied
Declarations of interest
Erica Pool (EP), Omara Dogar (OD), Ryan Lindsay (RL), and Peter Weatherburn (PW) have no conflicts of interest to declare. Kamran Siddiqi (KS) has received a research grant (GRAND 2014) from Pfizer, a pharmaceutical company that makes drugs for tobacco cessation.
New
References
References to studies included in this review
Cropsey 2013 {published and unpublished data}
- Cropsey KL, Hendricks PS, Jardin B, Clark CB, Katiyar N, Willig J, et al. A pilot study of screening, brief intervention, and referral for treatment (SBIRT) in non‐treatment seeking smokers with HIV. Addictive Behaviors 2013;38(10):2541‐6. [DOI: 10.1016/j.addbeh.2013.05.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elzi 2006 {published and unpublished data}
- Elzi L, Spoerl D, Voggensperger J, Nicca D, Simcock M, Bucher HC, et al. A smoking cessation programme in HIV‐infected individuals: A pilot study. Antiviral Therapy 2006;11(6):787‐95. [PubMed] [Google Scholar]
Ferketich 2013 {published and unpublished data}
- Browning KK, Wewers ME, Ferketich AK, Diaz P, Koletar SL, Reynolds NR. Adherence to tobacco dependence treatment among HIV‐infected smokers. AIDS and Behaviour 2016;20(3):608‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferketich AK, Diaz P, Browning KK, Lu B, Koletar S, Reynolds N, et al. Safety of varenicline among HIV‐infected smokers (POS3‐115). Proceedings of the Society for Research on Nicotine and Tobacco's 18th Annual Meeting; 2012 Mar 13‐16; Texas. 2012.
- Ferketich AK, Diaz P, Browning KK, Lu B, Koletar SL, Reynolds NR, et al. Safety of varenicline among smokers enrolled in the lung HIV study. Nicotine & Tobacco Research 2013;15(1):247‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00933595. Longitudinal studies of HIV‐associated lung infections and complications (Lung HIV). clinicaltrials.gov/show/NCT00933595 2009.
Humfleet 2013 {published and unpublished data}
- Humfleet G, Dilley J, Hall S, Delucchi K. Smoking cessation in HIV+ clinical care settings (SYM10D). Proceedings of the Society for Research on Nicotine and Tobacco's 17th Annual Meeting; 2011 Feb 16‐19; Toronto (ON). 2011.
- Humfleet GL, Hall SM, Delucchi KL, Dilley JW. A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine & Tobacco Research 2013;15(8):1436‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humfleet, G, Hall, S, Delucchi. K. Smoking cessation in HIV+ clinical care settings: participant characteristics. POS2‐17. Society for Research on Nicotine and Tobacco's 14th Annual Meeting, USA.. 2008. [DOI] [PMC free article] [PubMed]
- Humfleet, G, Hall, S, Delucchi, K, Dilley. J. Smoking cessation in HIV+ clinical care settings: preliminary findings. POS2‐17. Society for Research on Nicotine and Tobacco's 15th Annual Meeting, Ireland. 2008. [DOI] [PMC free article] [PubMed]
- NCT00297453. Smoking treatment in HIV clinical care settings. clinicaltrials.gov/ct2/show/NCT00297453 2006.
Ingersoll 2009 {published and unpublished data}
- Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS & Behavior 2009;13(3):545‐54. [DOI: 10.1007/s10461-007-9334-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloyd‐Richardson 2009 {published data only (unpublished sought but not used)}
- Lloyd‐Richardson EE, Stanton CA, Papandonatos GD, Betancourt RM, Stein M, Tashima K, et al. HIV‐positive smokers considering quitting: differences by race/ethnicity. American Journal of Health Behavior 2008;32(1):3‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd‐Richardson EE, Stanton CA, Papandonatos GD, Shadel WG, Stein M, Tashima K, et al. Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction 2009;104(11):1891‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Stanton C, Dios M, Tashima K. Socialization influences response to motivational enhancement for smoking cessation among HIV+ smokers (SYM10C). Proceedings of the Society For Research On Nicotine and Tobacco 17th Annual Meeting; 2011 Feb 16‐19; Toronto (ON).
- Niaura, R. Motivation and Patch Treatment for HIV‐Positive Smokers (Positive PATHS). NCT00551720. ClinicalTrials.gov 2007.
- Stanton C.A, Lloyd‐Richardson E.E, Papandonatos G.D, de Dios, M. A, Niaura R. [Mediators of the relationship between nicotine replacement therapy and smoking abstinence among people living with HIV/AIDS. POS2‐72]. Society for Research on Nicotine and Tobacco's 15th Annual Meeting, Ireland.. 2009. [DOI] [PMC free article] [PubMed]
- Stanton CA, Lloyd‐Richardson EE, Papandonatos GD, Niaura R. Smoking reduction among people living with HIV/AIDS: are there associations with short‐ term health outcomes? (POS5‐111). Proceedings of the Society For Research On Nicotine and Tobacco 16th Annual Meeting; 2010 Feb 24‐27; Baltimore (MD).
Manuel 2013 {published data only}
- Manuel JK, Lum PJ, Hengl NS, Sorensen JL. Smoking cessation interventions with female smokers living with HIV/AIDS: a randomized pilot study of motivational interviewing. AIDS Care 2013;25(7):820‐7. [DOI: 10.1080/09540121.2012.733331] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moadel 2012 {published and unpublished data}
- Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, Shuter J. A randomized controlled trial of a tailored group smoking cessation intervention for HIV‐infected smokers. Journal of Acquired Immune Deficiency Syndromes: JAIDS 2012;61(2):208‐15. [DOI: 10.1097/QAI.0b013e3182645679] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01106638. A trial to test the efficacy of a tailored intensive smoking cessation intervention in persons with HIV (PWHs). clinicaltrials.gov/ct2/show/NCT01106638 2010.
Shelley 2015 {published and unpublished data}
- NCT01898195. Improving adherence to smoking cessation medication among PLWHA (HIV). clinicaltrials.gov/ct2/show/NCT01898195 2013.
- Shelley D, Tseng TY, Gonzalez M, Krebs P, Wong S, Furberg R, et al. Correlates of adherence to varenicline among hiv+ smokers: a path analysis. Nicotine & Tobacco Research 2015;17(8):968‐74. [DOI: 10.1093/ntr/ntv068] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shuter 2014 {published and unpublished data}
- NCT01570595. Positively smoke free on the Web (PSFW) for smokers living with HIV. clinicaltrials.gov/ct2/show/NCT01570595 2012.
- Shuter J, Morales DA, Considine‐Dunn SE, An LC, Stanton CA. Feasibility and preliminary efficacy of a web‐based smoking cessation intervention for HIV‐infected smokers: a randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes: JAIDS 2014;67(1):59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanton 2015 {published and unpublished data}
- NCT00503230. Reducing ethnic health disparities: motivating HIV+ Latinos to quit smoking (AURORA). clinicaltrials.gov/ct2/show/NCT00503230.
- Stanton CA, Bicki A, Gould H, Makgoeng S, Papandonatos GD, Shuter J, et al. Does a preference for menthol cigarettes impact smoking patterns and sustained abstinence among Latino smokers living with HIV? (POS3‐75). Proceedings of the Society For Research On Nicotine and Tobacco 20th Annual Meeting; 2014 Feb 5‐8; Seattle.
- Stanton CA, Papandonatos GD, Shuter J, Bicki A, Lloyd‐Richardson EE, Dios MA, et al. Outcomes of a tailored intervention for cigarette smoking cessation among Latinos living with HIV/AIDS. Nicotine & Tobacco Research 2015;17(8):975‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton CA, Papandonatos GD, Shuter J, Makgoeng S, Lloyd‐Richardson EE, Dios MA, et al. Project aurora: a culturally tailored intervention for cigarette smoking cessation among Latinos living with HIV/ AIDS (POS1‐170). Proceedings of the Society for Research on Nicotine and Tobacco's 19th Annual Meeting; 2013 13‐16; Boston.
Vidrine 2006 {published data only}
- Vidrine DJ, Arduino RC, Gritz ER. Impact of a cell phone intervention on mediating mechanisms of smoking cessation in individuals living with HIV/AIDS. Nicotine & Tobacco Research 2006;8(Suppl 1):S103‐8. [DOI] [PubMed] [Google Scholar]
- Vidrine DJ, Arduino RC, Gritz ER. The effects of smoking abstinence on symptom burden and quality of life among persons living with HIV/AIDS. AIDS Patient Care and STDS 2007;21(9):659‐66. [DOI] [PubMed] [Google Scholar]
- Vidrine DJ, Arduino RC, Lazev AB, Gritz ER. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS 2006;20(2):253‐60. [DOI: 10.1097/01.aids.0000198094.23691.58] [DOI] [PubMed] [Google Scholar]
- Vidrine DJ, Mehta NV, Gritz ER, Arduino RC. Effects of smoking cessation on symptom status among persons living with HIV/AIDS (POS1‐104) Proceedings of the Society for Research on Nicotine and Tobacco's 13th Annual Meeting; 2007 Feb 21‐24; Texas.
Vidrine 2012 {published and unpublished data}
- Gritz ER, Danysh HE, Fletcher FE, Tami‐Maury I, Fingeret MC, King RM, et al. Long‐term outcomes of a cell phone‐delivered intervention for smokers living with HIV/AIDS. Clinical Infectious Diseases 2013;57(4):608‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz ER, Vidrine DJ, Marks RM, Arduino RC. A randomized trial of an innovative cell phone intervention for smokers living with HIV/AIDS (SYM10A). Proceedings of the Society for Research on Nicotine and Tobacco's 17th Annual Meeting; 2011 Feb 16‐19; Toronto.
- Gritz ER, Vidrine DJ, Marks RM, Danysh HE, Arduino RC. A cell phone‐delivered intervention for HIV‐positive smokers: project reach out (POS3‐113). Proceedings of the Society for Research on Nicotine and Tobacco's 18th Annual Meeting; 2012 Mar 13‐16; Houston. Society For Research On Nicotine and Tobacco's 18th Annual Meeting, USA.
- NCT00502827. Smoking cessation for HIV/AIDS patients. clinicaltrials.gov/ct2/show/NCT00502827 2007.
- Tamí‐Maury I, Vidrine D, Fletcher FE, Danysh H, Arduino R, Gritz ER. Impact of innovative smoking cessation interventions for hiv+ smokers who are multiple tobacco products users (POS2‐113). Proceedings of the Society for Research on Nicotine and Tobacco's 19th Annual Meeting; 2013 Mar 13‐16; Boston.
- Tamí‐Maury I, Vidrine DJ, Fletcher FE, Danysh H, Arduino R, Gritz ER. Poly‐tobacco use among HIV‐positive smokers: implications for smoking cessation efforts. Nicotine & Tobacco Research 2013;15(12):2100‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine DJ, Kypriotakis G, Li L, Arduino RC, Fletcher FE, Tamí‐Maury I, et al. Mediators of a smoking cessation intervention for persons living with HIV/AIDS. Drug and Alcohol Dependence 2015;1(147):76‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine DJ, Marks RM, Arduino RC, Gritz ER. Efficacy of cell phone‐delivered smoking cessation counseling for persons living with HIV/AIDS: 3‐month outcomes. Nicotine & Tobacco Research 2012;14(1):106‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wewers 2000 {published data only}
- Wewers ME, Neidig JL, Kihm KE. The feasibility of a nurse‐managed, peer‐led tobacco cessation intervention among HIV‐positive smokers. Journal of the Association of Nurses in AIDS Care 2000;11(6):37‐44. [DOI: 10.1016/S1055-3290(06)60353-1] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berg 2014 {published data only}
- Berg CJ, Nehl EJ, Wang X, Ding Y, He N, Johnson BA, et al. Healthcare provider intervention on smoking and quit attempts among HIV‐positive versus HIV‐negative MSM smokers in Chengdu, China. AIDS Care 2014;26(9):1201‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Browning 2013 {published data only}
- Browning KK, Wewers ME, Ferketich AK, Diaz P. Tobacco use and cessation in HIV‐infected individuals. Clinics in Chest Medicine 2013;34(2):181‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burkhalter 2013 {published data only}
- Burkhalter JE, Corner G, Noy A, Lubetkin E, Guidry J, Kornegay M. Motivational respiratory biomarker feedback cessation intervention for low‐income persons living with HIV/AIDS who smoke: intervention pre‐testing results (POS2‐164). Proceedings of the Society for Research on Nicotine and Tobacco's 19th Annual Meeting; 2013 Mar 13‐16; Boston.
- Burkhalter, J. Feasibility of Tobacco Assessment and Intervention With Low‐Income Persons Living With HIV‐AIDS (PLWHA) in Community‐Based AIDS Service Organizations. NCT01482923.. ClinicalTrials.gov.
Chefitz 2014 {published data only}
- Chefitz AC. Literacy among HIV‐infected persons referred to a web‐based tobacco treatment trial (POS1‐153). Proceedings of the Society for Research on Nicotine and Tobacco's 20th Annual Meeting; 2014 Feb 5‐8; Seattle.
Cui 2012 {published data only}
- Cui Q, Robinson L, Elston D, Smaill F, Cohen J, Quan C, et al. Safety and tolerability of varenicline tartrate (Champix(®)/Chantix(®)) for smoking cessation in HIV‐infected subjects: a pilot open‐label study. AIDS Patient Care and STDS 2012;26(1):12‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummins 2005 {published data only}
- Cummins D, Trotter G, Moussa M, Turham G. Smoking cessation for clients who are HIV‐positive. Nursing Standard 2005;20(12):41‐7. [DOI] [PubMed] [Google Scholar]
Fuster 2009 {published data only}
- Fuster M, Estrada V, Fernandez‐Pinilla MC, Fuentes‐Ferrer ME, Tellez MJ, Vergas J, et al. Smoking cessation in HIV patients: rate of success and associated factors. HIV Medicine 2009;10(10):614‐9. [DOI] [PubMed] [Google Scholar]
Huber 2012 {published data only}
- Huber M1, Ledergerber B, Sauter R, Young J, Fehr J, Cusini A, et al. Swiss HIV Cohort Study Group. Outcome of smoking cessation counselling of HIV‐positive persons by HIV care physicians. HIV Medicine 2012;13(7):387‐97. [DOI] [PubMed] [Google Scholar]
Lazev 2004 {published data only}
- Lazev A, Vidrine D, Arduino R, Gritz E. Increasing access to smoking cessation treatment in a low‐income, HIV‐positive population: The feasibility of using cellular telephones. Nicotine & Tobacco Research 2004;6(2):281‐6. [DOI] [PubMed] [Google Scholar]
Lima 2009 {published data only}
- Lima EM, Gualandro DM, Yu PC, Giuliano Ide C, Marques AC, Calderaro D, et al. Cardiovascular prevention in HIV patients: results from a successful intervention program. Atherosclerosis 2009;204(1):229‐32. [DOI] [PubMed] [Google Scholar]
Matthews 2013 {published data only}
- Matthews AK, Conrad M, Kuhns L, Vargas M, King AC. Project Exhale: preliminary evaluation of a tailored smoking cessation treatment for HIV‐positive African American smokers. AIDS Patient Care and STDS 2013;27(1):22‐32. [DOI] [PubMed] [Google Scholar]
McKie 1985 {published data only}
- McKie D. Assault on AIDS and smoking. Lancet 1985;14(8468):1371. [DOI] [PubMed] [Google Scholar]
Mercie 2014 {published data only (unpublished sought but not used)}
- NCT00918307. Efficacy and safety of varenicline among HIV‐infected patients (Inter‐ACTIV). clinicaltrials.gov/ct2/show/NCT00918307 2014.
NCT01393301 {published data only (unpublished sought but not used)}
- NCT01393301. Integrated treatment for smoking cessation & anxiety in people with HIV phase 1 (Project Quit). clinicaltrials.gov/ct2/show/NCT01393301.
NCT01436136 {published data only}
- NCT01436136. Reducing cardiovascular disease (CVD) risk in HIV on antiretroviral therapy over 12 months. clinicaltrials.gov/ct2/show/NCT01436136.
NCT02029612 {published data only}
- NCT02029612. An evidence based smoking cessation program for persons living with HIV/AIDS. clinicaltrials.gov/ct2/show/NCT02029612 2014.
Pedrol‐Clotet 2006 {published data only}
- Pedrol‐Clotet E, Deig‐Comerma E, Ribell‐Bachs M, Vidal‐Castell I, García‐Rodríguez P, Soler A. Bupropion use for smoking cessation in HIV‐infected patients receiving antiretroviral therapy [Uso de bupropión en la deshabituación tabáquica en pacientes infectados por el VIH en tratamiento antirretroviral]. Enfermedades Infecciosas y Microbiología Clínica 2006;24(8):509‐11. [DOI] [PubMed] [Google Scholar]
Reynolds 2009 {published data only}
- Reynolds NR. Cigarette smoking and HIV: more evidence for action. AIDS Education and Prevention 2009;21(3 Suppl):106‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shadel 2014 {published data only}
- Shadel WG, Galvan F, Tucker JS. Improving nicotine patch adherence among HIV‐positive Latino smokers (SYM14B). Proceedings of the Society for Research on Nicotine & Tobacco's 20th Annual Meeting; 2014 Feb 5‐8; Seattle.
Shelley 2014 {published data only}
- Shelley D, Krebs P, Tseng T‐Y, Wong S, Wright D, Wolfe H. Formative evaluation of a text messaging intervention to promote cessation medication (POS3‐174). Proceedings of the Society for Research on Nicotine and Tobacco's 20th Annual Meeting; 2014 Feb 5‐8; Seattle.
Tornero 2009 {published data only}
- Tornero C, Mafé C. Varenicline and antiretroviral therapy in patients with HIV. Journal of Acquired Immune Deficiency Syndromes 2009;52(2):656. [DOI] [PubMed] [Google Scholar]
Zwiebel 2008 {published data only}
- Zweibel MA, Hughes V. Smoking cessation program in one New York City clinic for people with HIV infection. Proceedings of the XVII International AIDS Conference; 2008 Aug 3‐8; Mexico City. www.who.int/mediacentre/events/meetings/intl_aids_conference/en/.
- Zwiebel MA, Hughes V. Smoking cessation efforts in one New York city HIV clinic. Journal of the Association of Nurses in AIDS Care 2010;21(1):11‐5. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00701896 {published data only}
- NCT00701896. Smoking cessation using motivational therapy and varenicline. clinicaltrials.gov/ct2/show/results/NCT00701896 2008.
NCT01363245 {published data only}
- Grossman E, Shelley D, Braithwaite RS, Lobach I, Goffin A, Rogers E, et al. Effectiveness of smoking‐cessation interventions for urban hospital patients: study protocol for a randomized controlled trial. Trials 2012;1(13):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01484340 {published data only}
- NCT01484340. A smoking cessation trial in HIV‐infected patients in South Africa. clinicaltrials.gov/ct2/show/NCT01484340 2011.
NCT01710137 {published data only}
- NCT01710137. Varenicline for nicotene dependence among those living with HIV/AIDS. clinicaltrials.gov/ct2/show/NCT01710137 2012.
NCT01800019 {published data only}
- NCT01800019. The Canadian HIV Quit Smoking Trial: tackling the co‐morbidities of depression and cardiovascular disease in HIV+ smokers. clinicaltrials.gov/ct2/show/NCT01800019 2013.
NCT01886924 {published data only}
- NCT01886924. Computer MI for Tobacco Quitline engagement in smokers living with HIV (MI‐HIV). clinicaltrials.gov/ct2/show/NCT01886924 2013.
NCT01965405 {published data only}
- NCT01965405. Smoking cessation people living with HIV/AIDS. clinicaltrials.gov/ct2/show/NCT01965405 2013.
NCT02072772 {published data only}
- NCT02072772. A trial of positively smoke free group therapy for HIV‐infected smokers. clinicaltrials.gov/ct2/show/NCT02072772 2014.
NCT02190643 {published data only}
- NCT02190643. Improving nicotine patch adherence among Latino HIV‐positive smokers. clinicaltrials.gov/ct2/show/NCT02190643 2014.
NCT02302859 {published data only}
- NCT02302859. Mobile Media‐Rich Interactive Guideline System (MMRIGS) pilot study. clinicaltrials.gov/ct2/show/NCT02302859 2014.
NCT02432482 {published data only}
- NCT02432482. A mobile intervention to promote cessation in HIV‐infected smokers. clinicaltrials.gov/ct2/show/NCT02432482 2015.
NCT02460900 {published data only}
- NCT02460900. Optimizing smoking cessation for people with HIV/AIDS who smoke. clinicaltrials.gov/ct2/show/NCT02460900 2015.
Additional references
Abbud 1995
- Abbud RA, Finegan CK, Guay LA, Rich EA. Enhanced production of human immunodeficiency virus type 1 by in vitro‐infected alveolar macrophages from otherwise healthy cigarette smokers. The Journal of Infectious Diseases 1995;172(3):859‐63. [DOI] [PubMed] [Google Scholar]
AHRQ 2008
- Agency for Healthcare Research and Quality. Treating Tobacco Use and Dependence: 2008 Update. www.ahrq.gov/professionals/clinicians‐providers/guidelines‐recommendations/tobacco/index.html May 2008.
Aliyu 2013
- Aliyu MH, Weldeselasse H, August EM, Keith LG, Salihu HM. Cigarette smoking and fetal morbidity outcomes in a large cohort of HIV‐infected mothers. Nicotine & Tobacco Research 2013;15(1):177‐84. [DOI] [PubMed] [Google Scholar]
Benard 2007
- Benard A, Bonnet F, Tessier JF, Fossoux H, Dupon M, Mercie P, et al. Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care and STDs 2007;21(7):458‐68. [DOI] [PubMed] [Google Scholar]
Bertisch 2013
- Bertisch B, Franceschi S, Lise M, Vernazza P, Keiser O, Schoni‐Affolter F, et al. Risk factors for anal cancer in persons infected with HIV: a nested case‐control study in the Swiss HIV Cohort Study. American Journal of Epidemiology 2013;178(6):877‐84. [DOI] [PubMed] [Google Scholar]
Buchacz 2010
- Buchacz K, Baker RK, Palella FJ Jr, Chmiel JS, Lichtenstein KA, Novak RM, et al. HOPS Investigators. AIDS‐defining opportunistic illnesses in US patients, 1994‐2007: a cohort study. AIDS 2010;24(10):1549‐59. [DOI] [PubMed] [Google Scholar]
Calvo‐Sánchez 2013
- Calvo‐Sánchez M, Perelló R, Pérez I, Mateo MG, Junyent M, Laguno M, et al. Differences between HIV‐infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: two parallel case‐control studies. HIV Medicine 2013;14(1):40‐8. [DOI] [PubMed] [Google Scholar]
Clifford 2005
- Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. Journal of the National Cancer Institute 2005;97(6):425‐32. [DOI] [PubMed] [Google Scholar]
Deeks 2013
- Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013;382(9903):1525‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehren 2014
- Ehren K, Hertenstein C, Kümmerle T, Vehreschild JJ, Fischer J, Gillor D, et al. Causes of death in HIV‐infected patients from the Cologne‐Bonn cohort. Infection 2014;42(1):135‐40. [DOI] [PubMed] [Google Scholar]
Feldman 2006
- Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts DH, et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the women's interagency HIV study. American Journal of Public Health 2006;96(6):1060‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friis‐Møller 2003
- Friis‐Møller N, Weber R, Reiss P, Thiébaut R, Kirk O, D'Arminio Monforte A, et al. Cardiovascular disease risk factors in HIV patients‐association with antiretroviral therapy. Results from the DAD study. AIDS 2003;17(8):1179‐93. [DOI] [PubMed] [Google Scholar]
Gordin 2008
- Gordin FM, Roediger MP, Girard PM, Lundgren JD, Miro JM, Palfreeman A, et al. Pneumonia in HIV‐infected persons: increased risk with cigarette smoking and treatment interruption. American Journal of Respiratory and Critical Care Medicine 2008;178(6):630‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Gritz 2004
- Gritz ER, Vidrine DJ, Lazev AB, Amick BC 3rd, Arduino RC. Smoking behavior in a low‐income multiethnic HIV/AIDS population. Nicotine & Tobacco Research 2004;6(1):71‐7. [DOI] [PubMed] [Google Scholar]
Grover 2013
- Grover KW, Gonzalez A, Zvolensky MJ. HIV symptom distress and smoking outcome expectancies among HIV+ smokers: a pilot test. AIDS Patient Care and STDS 2013;27(1):17‐21. [DOI] [PubMed] [Google Scholar]
Hartmann‐Boyce 2014
- Hartmann‐Boyce J, Lancaster T, Stead L. Print‐based self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001118.pub3] [DOI] [PubMed] [Google Scholar]
Helleberg 2013
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV‐1‐infected Individuals: a nationwide, population‐based cohort study. Clinical Infectious Diseases 2013;56(5):727‐34. [DOI] [PubMed] [Google Scholar]
Helleberg 2015
- Helleberg M, May MT, Ingle SM, Dabis F, Reiss P, Fätkenheuer G, et al. Smoking and life expectancy among HIV‐infected individuals on antiretroviral therapy in Europe and North America. AIDS 2015;29(2):221‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; Vol. 327, issue 7414:557‐60. [DOI] [PMC free article] [PubMed]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horvath 2012
- Horvath KJ, Eastman M, Prosser R, Goodroad B, Worthington L. Addressing smoking during medical visits: patients with human immunodeficiency virus. American Journal of Preventive Medicine 2012;43(5 Suppl 3):S214‐21. [DOI] [PubMed] [Google Scholar]
Humfleet 2009
- Humfleet GL, Delucchi K, Kelley K, Hall SM, Dilley J, Harrison G. Characteristics of HIV‐positive cigarette smokers: a sample of smokers facing multiple challenges. AIDS Education and Prevention 2009;21(3 Suppl):54‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lifson 2010
- Lifson AR, Neuhaus J, Arribas JR, Berg‐Wolf M, Labriola AM, Read TR, et al. INSIGHT SMART Study Group. Smoking‐related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. American Journal of Public Health 2010;100(10):1896‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshal 2008
- Marshal MP, Friedman MS, Stall R, King KM, Miles J, Gold MA, et al. Sexual orientation and adolescent substance use: a meta‐analysis and methodological review. Addiction 2008;103(4):546‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
May 2013
- May MT, Ingle SM, Costagliola D, Justice AC, Wolf F, Cavassini M, et al. Cohort profile: Antiretroviral Therapy Cohort Collaboration (ART‐CC). International Journal of Epidemiology 2013 April 18 [Epub ahead of print]. [DOI: 10.1093/ije/dyt010] [DOI] [PMC free article] [PubMed]
Miguez‐Burbano 2005
- Miguez‐Burbano MJ, Ashkin D, Rodriguez A, Duncan R, Pitchenik A, Quintero N, et al. Increased risk of Pneumocystis carinii and community‐acquired pneumonia with tobacco use in HIV disease. International Journal of Infectious Diseases 2005;9(4):208‐17. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement.. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2011
- Morris A, George MP, Crothers K, Huang L, Lucht L, Kessinger C, et al. HIV and chronic obstructive pulmonary disease: is it worse and why?. Proceedings of the American Thoracic Society 2011;8(3):320‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moscou‐Jackson 2014
- Moscou‐Jackson G, Commodore‐Mensah Y, Farley J, DiGiacomo M. Smoking‐cessation interventions in people living with HIV infection: a systematic review. Journal of the Association of Nurses in AIDS Care 2014;25(1):32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munyati 2006
- Munyati SS, Redzo N, Dauya E, Matambo R, Makamure B, Bandason T, et al. Human immunodeficiency virus, smoking and self‐rated health in Harare, Zimbabwe. International Journal of Tuberculosis and Lung Disease 2006;10(11):1279‐85. [PubMed] [Google Scholar]
Naif 2013
- Naif HM. Pathogenesis of HIV Infection. Infectious Disease Reports 2013;5(Suppl 1):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakagawa 2012
- Nakagawa F, Lodwick RK, Smith CJ, Smith R, Cambiano V, Lundgren JD, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS 2012;26(3):335‐43. [DOI] [PubMed] [Google Scholar]
Nurutdinova 2012
- Nurutdinova D, Chrusciel T, Zeringue A, Scherrer JF, Al‐Aly Z, McDonald JR, et al. Mental health disorders and the risk of AIDS‐defining illness and death in HIV‐infected veterans. AIDS 2012;26(2):229‐34. [DOI] [PubMed] [Google Scholar]
Palella 2011
- Palella FJ Jr, Phair JP. Cardiovascular disease in HIV infection. Current Opinion in HIV and AIDS 2011;6(4):266‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petoumenos 2011
- Petoumenos K, Worm S, Reiss P, Wit S, D'Arminio Monforte A, Sabin C, et al. D:A:D Study Group. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*). HIV Medicine 2011;12(7):412‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynolds 2004
- Reynolds NR, Neidig JL, Wewers ME. Illness representation and smoking behavior: a focus group study of HIV‐positive men. Journal of the Association of Nurses in AIDS Care 2004;15(4):37‐47. [DOI] [PubMed] [Google Scholar]
Schadé 2013
- Schadé A, Grootheest G, Smit JH. HIV‐infected mental health patients: characteristics and comparison with HIV‐infected patients from the general population and non‐infected mental health patients. BMC Psychiatry 2013;13:35. [DOI: 10.1186/1471-244X-13-35] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials.. PLoS Med 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shuter 2012a
- Shuter J, Bernstein SL, Moadel AB. Cigarette smoking behaviors and beliefs in persons living with HIV/AIDS. American Journal of Health Behavior 2012;36(1):75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shuter 2012b
- Shuter J, Salmo LN, Shuter AD, Nivasch EC, Fazzari M, Moadel AB. Provider beliefs and practices relating to tobacco use in patients living with HIV/AIDS: a national survey. AIDS and Behavior 2012;16(2):288‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sigel 2012
- Sigel K, Wisnivesky J, Gordon K, Dubrow R, Justice A, Brown ST, et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012;26(8):1017‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2006
- Simon V, Ho DD, Karim QA. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet 2006;368(9534):489‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sroussi 2007
- Sroussi HY, Villines D, Epstein J, Alves MC, Alves ME. Oral lesions in HIV‐positive dental patients ‐ one more argument for tobacco smoking cessation. Oral Diseases 2007;13(3):324‐8. [DOI] [PubMed] [Google Scholar]
Stead 2012
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
Stead 2016
- Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD008286.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsoi 2013
- Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD007253.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
UNAIDS 2015
- UNAIDS. Global Statistics. www.unaids.org/ 2015.
Valiathan 2014
- Valiathan R, Miguez MJ, Patel B, Arheart KL, Asthana D. Tobacco smoking increases immune activation and impairs T‐cell function in HIV infected patients on antiretrovirals: a cross‐sectional pilot study. PLoS ONE 2014;9(5):e97698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 2013
- Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Medicine 2013;14(4):195‐207. [DOI] [PubMed] [Google Scholar]
Weinberger 2012
- Weinberger AH, Pilver CE, Desai RA, Mazure CM, McKee SA. The relationship of major depressive disorder and gender to changes in smoking for current and former smokers: longitudinal evaluation in the US population.. Addiction 2012;107(10):1847‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2005
Winstone 2013
- Winstone TA, Man SF, Hull M, Montaner JS, Sin DD. Epidemic of lung cancer in patients with HIV infection. Chest 2013;143(2):305‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Pool 2014
- Pool ERM, Dogar O, Siddiqi K. Interventions for tobacco use cessation in people living with HIV and AIDS. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD011120] [DOI] [PMC free article] [PubMed] [Google Scholar]


