Abstract
Oxytocin regulates parturition, lactation, parental nurturing, and many other social behaviors in both sexes. The circuit mechanisms by which oxytocin modulates social behavior are receiving increasing attention. Here, we review recent studies on oxytocin modulation of neural circuit function and social behavior, largely enabled by new methods of monitoring and manipulating oxytocin or oxytocin receptor neurons in vivo. These studies indicate that oxytocin can enhance the salience of social stimuli and increase signal-to-noise ratios by modulating spiking and synaptic plasticity in the context of circuits and networks. We highlight oxytocin effects on social behavior in nontraditional organisms such as prairie voles and discuss opportunities to enhance the utility of these organisms for studying circuit-level modulation of social behaviors. We then discuss recent insights into oxytocin neuron activity during social interactions. We conclude by discussing some of the major questions and opportunities in the field ahead.
Keywords: hypothalamus, maternal care, neural circuits, neuromodulation, social bonding, social behavior
INTRODUCTION
Oxytocin is a hypothalamic neuropeptide known for peripheral actions regulating uterine contraction during parturition and milk letdown during nursing. However, oxytocin also acts within the brain to coordinate a suite of social behaviors, including maternal nurturing, mother-infant bonding, social recognition, and pair-bonding. Over the past decades, there has been a surge of research investigating the neural mechanisms of oxytocin regulation of social behaviors. New tools enabling circuit manipulation and monitoring of neural activity have greatly enhanced our understanding of these cellular and synaptic mechanisms.
Here, we review select recent studies of oxytocin neuromodulation largely in rodents, focusing on relating changes in cellular physiology to functional effects on synaptic transmission, plasticity, and social behavior. We highlight a common theme emerging from these studies: Oxytocin increases the salience of sensory information (analogous to turning up the volume) via a conserved set of cellular and circuit mechanisms shared with other neuromodulators. We also discuss other species that provide opportunities to explore the neural circuitry for oxytocin-mediated behaviors. We argue that there are specific advantages of the oxytocin system for investigating behaviorally relevant neuroplasticity, and we conclude by describing some major open questions in the field.
A SUMMARY OF THE OXYTOCIN SYSTEM
Oxytocin is a nine amino acid–long cyclical peptide that is synthesized in hypothalamic neurons, packaged into dense-core vesicles, and transported into dendrites and axons for release. Oxytocin is synthesized in the paraventricular nucleus (PVN), supraoptic nucleus (SON), and accessory magnocellular hypothalamic nuclei. Most oxytocin is secreted into the bloodstream via axonal release from the posterior pituitary. Oxytocin is also released somatodendritically to act within the SON and PVN to facilitate synchronous firing and pulsatile release during parturition and nursing (Burbach et al. 2006, Leng et al. 2008, Numan & Young 2016, Jurek & Neumann 2018, Valtcheva & Froemke 2019).
Magnocellular oxytocin neurons that project to the posterior pituitary have collaterals innervating numerous forebrain structures (Ross & Young 2009, Knobloch et al. 2012). Large-scale and whole-brain anatomical mapping (Figure 1a), enabled by oxytocin-Cre mice (Irani et al. 2010) and oxytocin neuron–specific viruses first used in rats and later in mice, revealed oxytocinergic axons throughout the brain, including in the thalamus, cortex, amygdala, striatum, and hippocampus (Knobloch et al. 2012, Mitre et al. 2016, Zhang et al. 2021). Conversely, a number of regions provide input to PVN oxytocin neurons (Figure 1b), including the brain stem and thalamic, hypothalamic, and cortical areas (Dobolyi et al. 2018, Tang et al. 2020).
Figure 1.
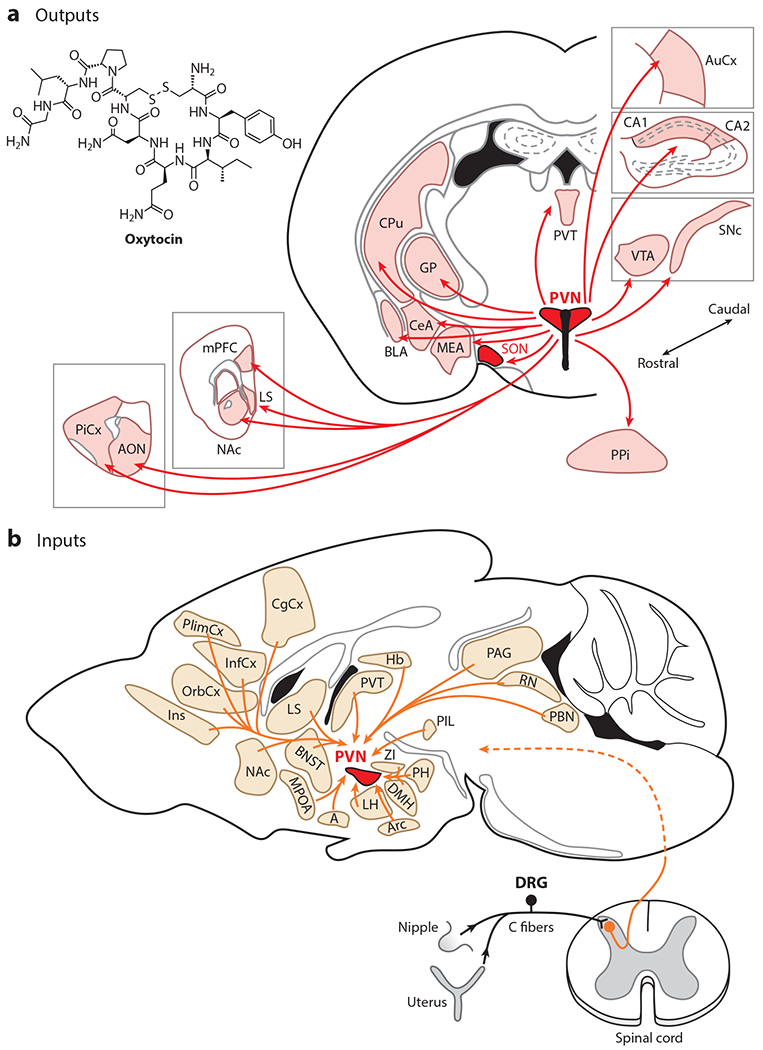
The mouse oxytocin system. (a) Axonal projections of PVN oxytocin neurons. Panel a adapted from Knobloch et al. (2012), Mitre et al. (2016), and Zhang et al. (2021). (b) Inputs to PVN oxytocin neurons. Panel b adapted from Tang et al. (2020). Abbreviations: A, amygdala; AON, anterior olfactory nucleus; Arc, arcuate nucleus; AuCx, auditory cortex; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; CPu, caudate putamen; DMH, dorsomedial hypothalamic nucleus; DRG, dorsal root ganglion; GP, globus pallidus; Hb, habenula; InfCx, infralimbic cortex; Ins, insular cortex; LH, lateral hypothalamus; LS, lateral septum; MEA, medial amygdala; mPFC, medial prefrontal cortex; MPOA, medial preoptic area; NAc, nucleus accumbens; OrbCx, orbitofrontal cortex; PAG, periaqueductal gray; PBN, parabrachial nucleus; PH, posterior hypothalamic nucleus; PiCx, piriform cortex; PIL, posterior intralaminar nucleus of the thalamus; PlimCx, prelimbic cortex; PPi, posterior pituitary; PVN, paraventricular nucleus; PVT, paraventricular thalamus; RN, raphe nuclei; SNc, substantia nigra pars compacta; SON, supraoptic nucleus; VTA, ventral tegmental area; ZI, zona incerta.
Oxytocin receptor distribution in the brain has traditionally been characterized using receptor autoradiography. Comparative studies have revealed remarkable species diversity in receptor distribution in the brain (Figure 2), likely contributing to species-specific social behaviors (Freeman & Young 2016, Johnson & Young 2017). Oxytocin receptors are also expressed pre- and postnatally, raising the possibility that developmental oxytocin signaling could have a lasting impact on brain organization and behavior (Mitre et al. 2016, Vaidyanathan & Hammock 2017, Kingsbury & Bilbo 2019, Newmaster et al. 2020).
Figure 2.
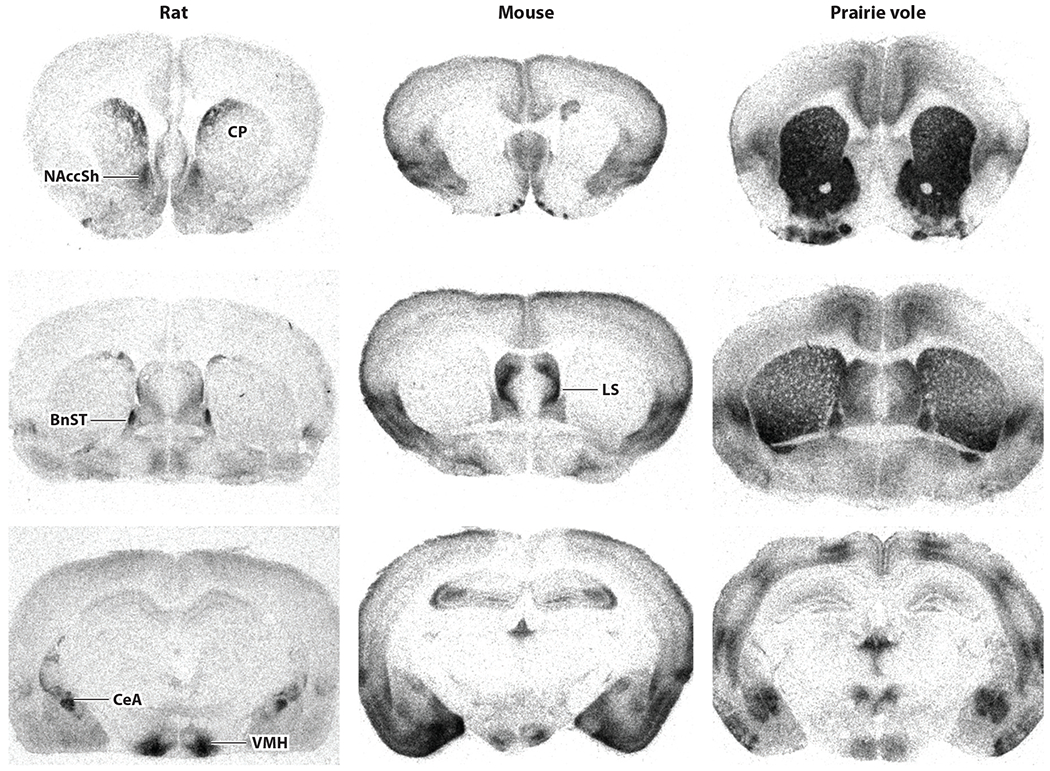
Receptor autoradiograms illustrating oxytocin receptor binding sites in the forebrain of rat, mouse, and prairie vole. Receptor distribution is conserved in some regions, including the lateral septum (LS), bed nucleus of the stria terminalis (BnST), central nucleus of the amygdala (CeA), and ventromedial nucleus of hypothalamus (VMH). There are species differences for other regions: In rat striatum, oxytocin receptors are restricted to dorsal caudate putamen (CP) and nucleus accumbens shell (NAccSh), whereas oxytocin receptors are abundant throughout prairie vole striatum and not detectable in mouse striatum. Figure adapted from Burbach et al. (2006).
Recently, fluorescent reporter mouse lines and specific antibodies for the mouse oxytocin receptor have been used to characterize oxytocin receptor distributions (Yoshida et al. 2009, Marlin et al. 2015, Mitre et al. 2016, Newmaster et al. 2020). Receptors are expressed in low-to-moderate levels in virtually every brain area examined, with some interesting differences between adult males and females. Olfactory piriform cortex, hippocampal area CA2, and female left auditory cortex had relatively higher numbers of oxytocin receptor–positive cells. Cortical oxytocin receptors are found primarily on parvalbumin-positive or somatostatin-positive inhibitory interneurons (Nakajima et al. 2014, Marlin et al. 2015), including at inhibitory synaptic terminals (Mitre et al. 2016).
Some actions of oxytocin are mediated via vasopressin receptors and vice versa (Chini et al. 2008, Schorscher-Petcu et al. 2010, Xiao et al. 2017, Song & Albers 2018). Oxytocin and vasopressin receptors can have spatially distinct distributions (Huber et al. 2005, Xiao et al. 2017). Despite this segregation, there are likely important physiological consequences of neurohypophysial peptide (oxytocin and vasopressin) receptor cross-reactivity. Xiao et al. (2017) showed that oxytocin modulated dopamine neuron excitability. A fraction of this effect was insensitive to oxytocin receptor antagonists (OTAs) and instead required vasopressin receptor antagonists for full blockade. Thus, expression of the cognate receptor in specific neurons may not strictly regulate neurohypophysial peptide modulation, depending on the presence and sensitivities of noncognate receptors.
Genomic evidence suggests that vertebrate oxytocin and vasopressin receptors likely arose from a single receptor shared with a common invertebrate ancestor via whole-genome and large segmental duplications. Oxytocin and vasopressin peptides likewise derive from a common ancestral gene via duplication (Gwee et al. 2009) and transposition (Theofanopoulou et al. 2021) around the same time. These evolutionary relationships, and the cross-talk between the systems, have led to the propositions that these paralogous peptides be referred to as oxytocin and vasopressin across species and that their receptors be referred to as oxytocin and vasopressin receptors, despite some sequence differences, or neurohypophysial receptors instead (Young & Flanagan-Cato 2012). Theofanopoulou et al. (2021) have proposed a universal nomenclature in which the names oxytocin and vasotocin would be used across vertebrates, with the common ending (-tocin) portraying the paralogy (common genetic origin) of the genes.
OXYTOCIN MODULATION OF SYNAPSES AND NEURONS
Oxytocin receptor activation has different functional effects depending on the target tissue or cell type. Oxytocin receptors are generally coupled to Gq/11α GTP-binding proteins, which stimulate phospholipase C (PLC) to produce inositol triphosphate and diacylglycerol (Figure 3a). This increases intracellular Ca2+, leading to depolarization, neurotransmitter release, and/or gene transcription and protein synthesis (Bakos et al. 2018, Jurek & Neumann 2018, Tirko et al. 2018).
Figure 3.
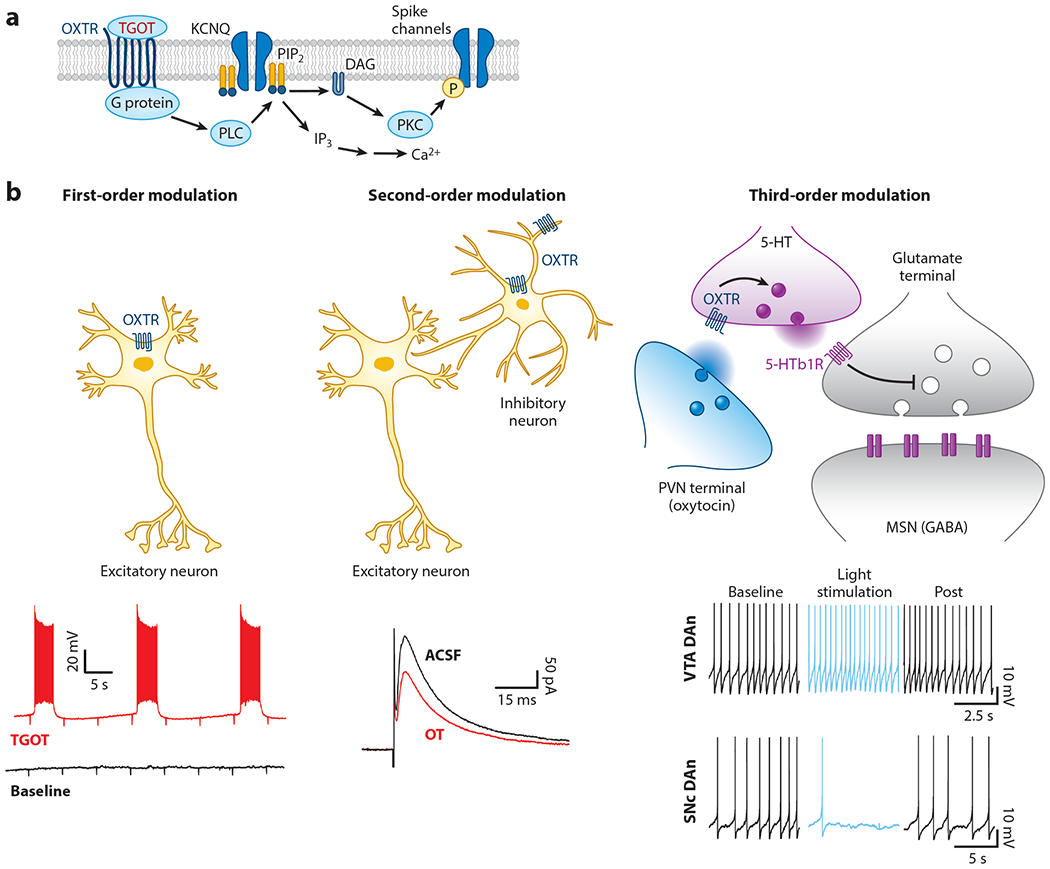
Different modes of oxytocinergic modulation. (a) Proposed mechanism of oxytocin receptor GPCR signaling. A G protein activates PLC to degrade PIP2 into IP3 and DAG. Depleting membrane PIP2 closes KCNQ channels, increasing resistance and depolarizing neurons. DAG activates PKC to phosphorylate spike channels (Tirko et al. 2018). (b, left) In first-order modulation, oxytocin directly depolarizes some principal excitatory cells such as CA2 pyramidal neurons (Tirko et al. 2018). (Middle) In second-order modulation, oxytocin reduces inhibitory transmission by either increasing spontaneous firing or impairing GABA release (Owen et al. 2013). (Right) In third-order modulation, or modulation of modulation, oxytocin receptors on serotoninergic terminals impact serotonin signaling in nucleus accumbens (top) (Dölen et al. 2013), and oxytocin increases VTA dopamine neuron firing but decreases SNc dopamine neuron firing (bottom) (Xiao et al. 2017). Abbreviations: 5-HT, 5-hydroxytryptamine; DAG, diacylglycerol; DAn, dopamine neuron; GABA, γ aminobutyric acid; GPCR, G protein–coupled receptor; IP3, inositol triphosphate; KCNQ, M-type K+ channel; MSN, medium spiny neuron; OXTR, oxytocin receptor; P, phosphorylation; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLC, phospholipase C; PVN, paraventricular nucleus of the hypothalamus; SNc, substantia nigra pars compacta; TGOT, [Thr4, Gly7]-oxytocin; VTA, ventral tegmental area.
Although peptidergic modulation is now appreciated to be commonplace throughout the central nervous system, including cortex (Smith et al. 2019), only a fraction of neurons seem to express oxytocin receptors (Mitre et al. 2016). This pattern of expression leads to various degrees of oxytocinergic modulation of principal neurons (i.e., excitatory projection neurons), which could be considered first-order modulation for cells directly expressing oxytocin (or vasopressin) receptors, second-order modulation for regulation of local circuit elements (e.g., inhibitory interneurons) that influence principal cell output, and third-order effects such as neuromodulation of other modulatory signals (Figure 3b).
First-Order Neuromodulation
A prime example of first-order neuromodulation and depolarization of principal neurons has been identified in rodent hippocampal area CA2, which is important for social recognition memory (Hitti & Siegelbaum 2014, Raam et al. 2017, Leroy et al. 2018, Lin et al. 2018, Cymerblit-Sabba et al. 2020, Donegan et al. 2020). Tirko et al. (2018) made whole-cell recordings from CA2 pyramidal neurons in hippocampal brain slices and found that oxytocin receptor activation depolarized these cells, which led to spike bursts (Figure 3a,b) due to M-type K+ channel (KCNQ) closure via Gαq and Gαi G protein coupling through PLC. Thus, brief episodes of oxytocin modulation can dramatically change the CA2 firing mode and alter information processing through the hippocampal circuit, possibly enhancing social recognition memory.
Other first-order types of oxytocinergic modulation include changes to spike shape and/or threshold of principal cells in the olfactory bulb (Oettl et al. 2016), CA2 (Tirko et al. 2018), and rat insular cortex (Rogers-Carter et al. 2018). Oxytocin in insular cortex brain slices depolarized pyramidal neurons, increased input resistance, decreased spike amplitude, and increased spike number evoked by depolarizing current injection. These changes were prevented by blocking protein kinase C (PKC) (Rogers-Carter et al. 2018), implicating a conserved mechanism of G protein–coupled oxytocin receptor activation of PLC, downstream kinases, and changes to ion channels in principal neurons (Figure 3a). In this manner, first-order modulation is effective at enhancing all inputs to a neuron or network, thereby increasing sensitivity to any signal but potentially at the cost of discriminating between similar signals with different behavioral significance.
Second-Order Neuromodulation
In contrast to hippocampal CA2, oxytocin does not depolarize CA1 pyramidal cells but instead activates parvalbumin-positive fast-spiking interneurons (Owen et al. 2013, Tirko et al. 2018, Maniezzi et al. 2019). This increase in spontaneous firing of γ aminobutyric acid (GABA)ergic cells leads to dual second-order mechanisms for enhancing the signal-to-noise ratio of Schaffer collateral input into CA1 by modulating local interneuron function. First, increased tonic GABA release in the absence of external input suppresses spontaneous firing of CA1 pyramidal neurons, reducing background noise in the circuit. Second, this weakens evoked (phasic) disynaptic inhibitory postsynaptic currents (IPSCs) but not excitatory postsynaptic currents (EPSCs) when Schaffer collaterals are stimulated, likely through the depletion of GABAergic vesicle pools from enhanced spontaneous interneuron activity (Figure 3b). We refer to this reduction of evoked IPSCs as disinhibition, which leads to a heightened net excitatory response to incoming stimulation, thus increasing the signal strength. Together, these changes lead to a greater signal-to-noise ratio, increasing the salience of social information received during periods of oxytocin modulation.
Regulation of synaptic inhibition seems to be a major, if not the most common, mode of oxytocinergic modulation in the brain, although specific effects vary across regions. Oxytocin activates inhibitory cell types in other cortical, subcortical, and peripheral regions (Eliava et al. 2016, Harden & Frazier 2016, Crane et al. 2020, Francesconi et al. 2020), and oxytocinergic disinhibition has been observed in auditory cortex, piriform cortex, and PVN (Mitre et al. 2016). Correspondingly, oxytocin receptors in mouse auditory cortex are found on many cell types, including glia and pyramidal cells, although mainly on somatostatin-positive and parvalbumin-positive interneurons (Mitre et al. 2016). Disinhibition in auditory cortical layer V pyramidal neurons occurs with increased spontaneous IPSC frequency, increased spiking, and higher temporal precision of evoked action potentials throughout cortical layers (Marlin et al. 2015, Schiavo et al. 2020). It is unclear whether cortical disinhibition is due to changes of inhibitory neuron firing patterns (as in CA1) and/or regulation of GABA release at presynaptic terminals, which could be mediated by oxytocin receptors detected on GABAergic synapses onto excitatory neurons (Mitre et al. 2016).
In mouse somatosensory, but not prefrontal, cortex, oxytocin instead increased miniature EPSC frequency and evoked excitatory transmission (Zheng et al. 2014). Instead, somatostatin-positive interneurons are the main cell type expressing oxytocin receptors in medial prefrontal cortex (Nakajima et al. 2014). These prefrontal inhibitory neurons are strongly connected to local pyramidal neurons in the superficial and deep layers of both sexes, with IPSCs evoked in over 90% of principal cells after optogenetic stimulation of oxytocin receptor–expressing interneurons (Li et al. 2016).
Thus, oxytocin acts in many brain areas to recruit local inhibitory circuits, and second-order forms of oxytocin neuromodulation seem more widespread than first-order modulation. Modulation of inhibitory circuits enables more selective signal enhancement for specific cell types, synapses, and sensory inputs than does the direct excitation of principal cells (Froemke & Schreiner 2015). These observations underscore the importance of examining spontaneous and evoked patterns of synaptic transmission to determine local circuit effects of neuromodulation. The various mechanisms by which oxytocin affects excitability or synaptic transmission afford different levels of control, from individual inputs or spines (which could provide highly stimulus-specific behavioral sensitivities) to entire neurons or circuits as a whole (increasing perceptual/behavioral salience of an entire class or modality of stimulus, although perhaps compromising discriminability).
Third-Order Neuromodulation
There are potentially many biological processes by which oxytocin receptor signaling can indirectly influence gene expression, excitability, synaptic transmission, and neural computations (Jurek & Neumann 2018) beyond the scope of this review. In the remainder of this section, we highlight newer studies implicating third-order modulation, or the modulation of modulation, as a set of mechanisms by which oxytocin regulates circuit function. Dölen et al. (2013) observed PVN oxytocin fibers innervating mouse nucleus accumbens, where oxytocin release was important for social-conditioned place preference and serotonin-dependent long-term depression (LTD). The relevant oxytocin receptors are believed to reside on serotoninergic terminals of raphe inputs to nucleus accumbens (Figure 3b), such that presynaptic oxytocin receptor activation enhances serotoninergic modulation of nucleus accumbens medium spiny neurons. This interaction indicates that neuromodulatory systems need not act in isolation but instead can directly activate each other, perhaps accounting for the wide range of effects ascribed to any one neuromodulator.
Oxytocin fibers also innervate midbrain dopaminergic centers, with axons projecting to mouse ventral tegmental area (VTA) and substantia nigra. The selective oxytocin agonist [Thr4,Gly7]-oxytocin (TGOT) reduced evoked IPSC amplitudes (as in CA1 and auditory cortex) together with increased spontaneous spiking and enhanced evoked EPSCs (Tang et al. 2014, Hung et al. 2017, Xiao et al. 2017, Hörnberg et al. 2020). Removing oxytocin receptors from postsynaptic VTA neurons prevented the reduction of EPSCs, whereas IPSCs were still reduced by TGOT, indicating that disinhibition is due to presynaptic oxytocinergic modulation of GABAergic terminals. In contrast to the increase of spontaneous firing of VTA dopamine neurons, oxytocin suppressed the spontaneous firing of substantia nigra dopamine neurons (Xiao et al. 2017). Spontaneous IPSC rates increased onto VTA and substantia nigra neurons, leading to the development of a model in which GABAergic neurons in both areas are directly modulated by oxytocin and increase firing rates onto dopamine neurons. Increased inhibitory tone is counteracted by oxytocin receptor expression on VTA but not substantia nigra dopamine neurons. This leads to differential consequences on firing rate (and downstream dopamine release), with the net effect of oxytocin in the midbrain dopamine system biasing overall activity away from substantia nigra and toward VTA. An additional level of input selection and control of circuit function in mouse VTA is provided by oxytocinergic downregulation of excitation via presynaptic endocannabinoid receptors (Xiao et al. 2018). The effects of optogenetic stimulation of oxytocin-positive but vasopressin-negative fibers in VTA were not entirely reduced by selective OTAs but also required antagonists to vasopressin receptors to be fully prevented (Xiao et al. 2017).
Another form of third-order neuromodulation is oxytocin-induced oxytocin release. Magnocellular oxytocin neurons have a range of spontaneous firing patterns maintaining a low level of oxytocin tone. However, during parturition and lactation, oxytocin neurons fire more synchronously in bursts, in part due to changes to intrinsic excitability properties and incoming excitatory inputs (Teruyama & Armstrong 2002, Sabatier et al. 2004, Rossoni et al. 2008). Burst firing results in pulsatile oxytocin secretion for uterine contractions and milk ejection during nursing (Belin et al. 1984, Brown et al. 2013). There seems to be an interesting type of positive feedback that regulates this system (Neumann et al. 1996): Injection of exogenous oxytocin into the third ventricle increases spontaneous firing of rat oxytocin neurons and can produce milk ejection when pups are suckling (Freund-Mercier & Richard 1984). Conversely, injection of an OTA into SON decreased hypothalamic oxytocin concentrations and milk intake by suckling pups (Neumann et al. 1994). Pharmacologically evoked somatodendritic oxytocin release in PVN (e.g., via melanocortin receptor agonists) may prime PVN neurons to respond to social stimuli and enhance oxytocin release in the brain, with translational implications for treating disorders such as autism (Modi et al. 2015, Young & Barrett 2015).
OXYTOCIN AND LONG-TERM NEURAL PLASTICITY
These three types of neuromodulation (first, second, and third order) acutely amplify input processing across different spatiotemporal scales. Each mechanism can also potentially influence and induce long-term synaptic modifications via changes in excitability and recruitment of intracellular signaling. For example, oxytocin lowers the threshold for depolarizing inputs to induce long-term potentiation (LTP) in hippocampal CA2 (Pagani et al. 2015, Carstens & Dudek 2019) and olfactory bulb (Fang et al. 2008). CA2 activity also enables CA1 plasticity after oxytocin-triggered burst firing of CA2 neurons increases collateral drive onto CA1 pyramidal neurons (Eyring et al. 2020). Rapid and lasting modifications to synaptic transmission would then consolidate changes in neural responses important for parental care or other social behaviors in an enduring manner that is less dependent on continued neuromodulation (Froemke 2015, Rajamani et al. 2018, Pekarek et al. 2020).
Disinhibition is an effective and physiological mechanism for permitting long-term synaptic plasticity (Froemke 2015), strengthening repetitively active synaptic inputs during periods of reduced inhibition during/after oxytocin release. The reduction of inhibition by oxytocin can facilitate LTP induction in auditory cortical brain slices, presumably by boosting NMDA receptor responses and/or promoting dendritic spikes. Pairing oxytocin with synaptic stimulation persistently increases EPSCs and spiking (Mitre et al. 2016, Schiavo et al. 2020). Paired inhibitory inputs also increase in amplitude to balance synapse-specific excitatory increases (Field et al. 2020), ensuring reliable and precise spiking to incoming input.
Long-term synaptic plasticity can serve as a mechanism for enhancing and stabilizing neural representations of important sensory stimuli related to social interactions or maternal care. As a consequence, responses to incoming signals like infant cries or other social stimuli would be detected and recognized more readily without the need for additional oxytocin. One form of maternal behavior shown to initially require oxytocin signaling leading to long-term neural plasticity is pup retrieval, in which caretakers respond to ultrasonic distress calls made by isolated pups and return them to safety (Noirot 1972, Ehret 2005). Pup-naïve virgin females do not initially retrieve pups, but experience with pups leads to concaveation: maternal behaviors expressed by virgin mice (Numan & Insel 2003). Cohousing virgin females with experienced dams and litters accelerates the onset of maternal behavior (Koch & Ehret 1989), and oxytocin further enables this process (Marlin et al. 2015).
The experience-dependent onset of maternal behavior is at least partially due to rapid plasticity sensitizing left auditory cortex to pup call sounds. Pup calls evoke more robust cortical responses in experienced maternal mice compared to inexperienced or naïve virgins (Liu & Schreiner 2007, Cohen et al. 2011), with an unusual hemispheric overrepresentation on the left side of auditory cortex paralleled by a left hemisphere advantage for behavioral responses to pup vocalizations as well as left-lateralized oxytocin receptor expression in female auditory cortex (Ehret 1987, Marlin et al. 2015, Schiavo et al. 2020). Untuned neurons in deep layers of left female auditory cortex and narrowly tuned neurons in superficial layers increased responses to a range of pup calls, eventually representing the full statistical distribution of exposed pup call sounds (Marlin et al. 2015, Schiavo et al. 2020). More precise spiking responses arise from the temporal patterning and reliability of synaptic responses in experienced mice. These responses can emerge within minutes to hours during initial pup experience, forming a neural category of pup call sounds. This process elaborates on an initial sensitivity in virgin female cortex to the most common feature of pup calls (~5 Hz tempo or intersyllable interval). Suppressing oxytocin neuron firing during initial cohousing prevented plasticity, indicating that social and parental experience activates the oxytocin system even in nonlactating females (Schiavo et al. 2020). Once plasticity has occurred, blocking cortical oxytocin receptors did not substantially affect pup retrieval abilities (Marlin et al. 2015, Carcea et al. 2019).
Pairing oxytocin for a few minutes with pup call presentation in vivo also induced long-lasting changes in excitatory/inhibitory inputs to enhance spike responses to pup calls (Figure 4a). Oxytocin transiently decreased call-evoked IPSCs in virgin auditory cortex, leading to excitatory LTP within minutes. In this way, oxytocin modulation acts as a social salience signal to almost immediately boost neural responses, which are then consolidated via mechanisms of long-term plasticity (Marlin et al. 2015) so that auditory cortex remains responsive to pup calls perhaps for the lifetime of the animal. Together, these studies of infant distress calls have been useful for connecting cellular and synaptic properties (e.g., localization of oxytocin receptors) to physiological effects of oxytocin (e.g., disinhibition and LTP induction) and behavioral changes (e.g., pup retrieval onset).
Figure 4.
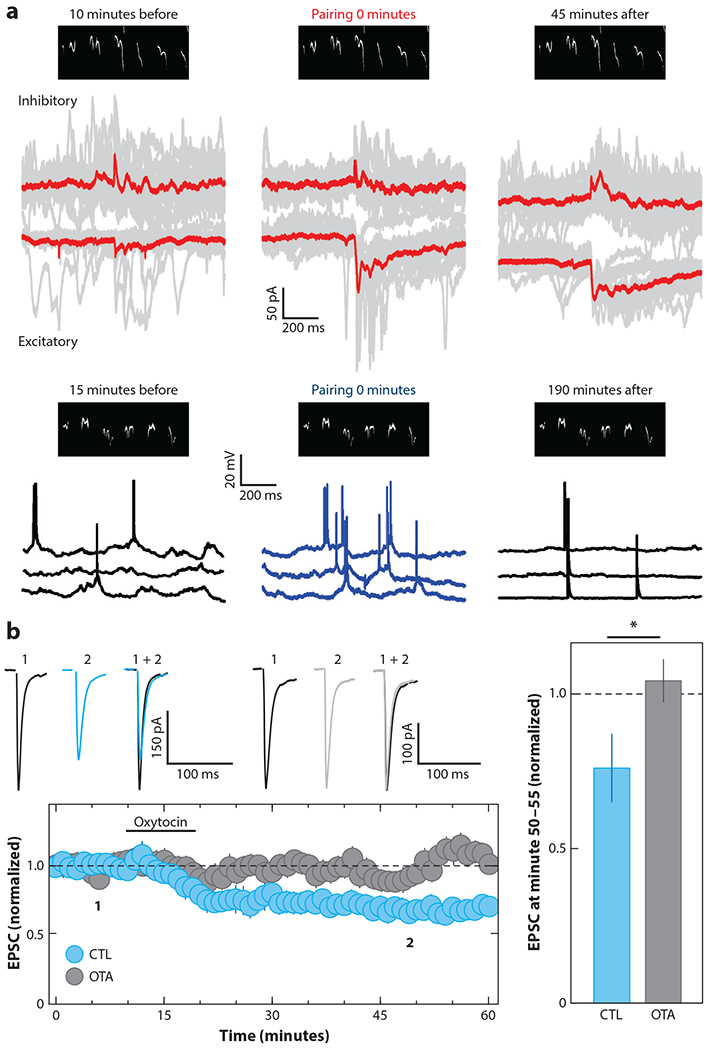
Mechanisms of oxytocin modulation enable long-term plasticity. (a) Long-term plasticity of synaptic and spiking responses to mouse infant distress calls is induced in vivo in female virgin left auditory cortex. (Top) Voltage-clamp recordings from cortical neurons are shown before, during, and after pairing oxytocin (red) with pup call (black spectrogram above traces). Excitatory-inhibitory correlation improved over time, and responses to the pup call became more reliable. (Bottom) Two consecutive current-clamp recordings of spiking responses are shown before, during, and after oxytocin pairing via optogenetic stimulation (blue). After pairing, pup calls evoked more spikes with higher temporal precision (Marlin et al. 2015). (b) Oxytocin induces LTD in vitro in mouse nucleus accumbens (blue) unless slices were preincubated with OTA (black) (Dölen et al. 2013). Abbreviations: CTL, control; EPSC, excitatory postsynaptic current; LTD, long-term depression; OTA, oxytocin receptor antagonist.
Third-order modulation, or modulation of modulation, is critical for long-term synaptic modifications related to social reward in mouse nucleus accumbens. Oxytocin induced LTD in nucleus accumbens medium spiny neurons (Figure 4b), as presynaptic oxytocin receptors on serotoninergic terminals lead to increased serotonin release onto medium spiny neurons and consequent LTD (Dölen et al. 2013). This LTD has a prolonged critical period and is induced by either serotonin or oxytocin at postnatal day (P)40 but not P90. LTD in adult nucleus accumbens was reinstated by stimulation of oxytocin fibers in nucleus accumbens, as well as by a single dose of MDMA, which might stimulate oxytocin neurons and bind to serotonin transporters. This time period and pharmacological sensitivity matched a critical behavioral time window during which mice expressed a form of social place preference (Nardou et al. 2019). These studies emphasize a few important points: the interrelatedness and dynamism of neuromodulatory systems; the potential for some acute therapeutic approaches to have enduring effects; and the need to understand mechanisms of modulation and plasticity in humans, especially for treatment of neuropsychiatric conditions.
CENTRAL EFFECTS OF OXYTOCIN ON NEURAL RESPONSES AND BEHAVIOR
Through widespread direct and indirect effects on neural excitability, oxytocin also affects perception and cognition. We restrict our review to maternal care and some other social interactions, including sexual behavior and pair-bond formation. It is worth noting that although oxytocin enhances prosocial behavior and maternal care, there is nothing intrinsically social about a nine amino acid–long peptide. Rather, the data indicate that oxytocin acts as a neuromodulator to amplify incoming signals, increasing the salience of sensory input and leading to refinement of subsequent behaviors via mechanisms of long-term plasticity, as described above. As oxytocin is released during social interactions, the contexts of specific experiences provide oxytocin with a maternal or social phenotype.
Maternal Care
Survival of many species relies on close and reliable attachment relationships across ages, ensuring that the young are nurtured and protected. Parental behaviors generally involve oxytocin (Rilling & Young 2014), although some aspects such as nest building instead require vasopressin (Bendesky et al. 2017).
In sheep, parturition triggers maternal behavior in ewes by stimulating brain oxytocin release. Hours after delivery, ewes become exclusively maternal to their own lambs. Vaginocervical stimulation in sheep induces release of oxytocin and other neuromodulators into the cerebrospinal fluid and the olfactory bulb (Keverne & Kendrick 1994). This heightened modulatory tone increases mitral cell responses specifically to offspring lamb odor. Central oxytocin seems fundamental for this mother-infant bonding, as cerebral injections of oxytocin induce acceptance of an unfamiliar lamb by ewes even after initial bonding (Kendrick et al. 1991, 1992).
Prairie voles are highly nurturing, and virgin females can display spontaneous levels of maternal care depending on density of nucleus accumbens oxytocin receptors (Olazábal & Young 2006, Keebaugh & Young 2011). In most other rodent species, however, males and nulliparous/virgin females are not usually parental and instead avoid or attack pups until after parturition or concaveation (Numan & Insel 2003, Wu et al. 2014, Scott et al. 2015). Pedersen et al. (1982) showed that oxytocin injection into the lateral cerebral ventricles of nulliparous/virgin rats rapidly induced maternal behaviors. More recent studies have used optogenetic stimulation to release endogenous oxytocin. Scott et al. (2015) found that optogenetic stimulation of tyrosine hydroxylase–positive (TH+) neurons of virgin female mouse anteroventral periventricular nucleus promoted maternal behaviors, including pup retrieval. These TH+ cells provide monosynaptic excitatory input to PVN oxytocin neurons, and stimulation of these cells increased plasma oxytocin levels.
Maternal defensive aggression toward intruders also involves oxytocin (Bosch 2013). While rodents often freeze in response to threat-related cues (e.g., odors paired with foot shock), mothers must inhibit freezing to defend pups and the nest (Pinel et al. 1990). Blocking oxytocin receptors in central amygdala leads to differential effects depending on testing context and maternal state. Rickenbacher et al. (2017) observed increased freezing in maternal rats when presented with threat-related cues in the presence of their pups after administering OTA into the central amygdala. Similarly, Knobloch et al. (2012) showed that oxytocin in central amygdala decreased freezing induced by classical fear conditioning (instead of a maternal context), while OTA infusion into the amygdala increased freezing. In contrast, in a different context in which smaller male intruder rats were introduced to dams and litters, amygdala OTA infusion instead increased maternal aggression and attack (Lubin et al. 2003). Thus, rather than just decreasing (or increasing) the tendency to freeze or engage, oxytocin in the amygdala might flexibly switch circuit function depending on situational relevance (Dulac et al. 2014).
Assessing Social Cues and Contexts
Oxytocin is important for responding appropriately to social contexts. Rogers-Carter et al. (2018) showed that adult male rats approach distressed, stressed juveniles but avoid distressed adults. Oxytocin signaling in insular cortex is necessary for this social affect preference behavior and renders insular cortex pyramidal neurons more responsive to excitatory inputs via activation of PKC cascades. Some components of social recognition require oxytocin receptor signaling in hippocampus (Raam et al. 2017) and also in other brain areas depending on context. Mice can discriminate positive and negative emotional states of other mice (Ferretti et al. 2019), and chemogenetic inhibition of PVN oxytocin projections to central amygdala abolishes this ability. Adult mice visually observing other mice receiving auditory-conditioned foot shocks can experience emotion contagion—developing an aversion to conditioned stimuli. This interesting observational form of social transmission depends on anterior cingulate input to amygdala (Allsop et al. 2018). Oxytocin acting in anterior cingulate cortex also mediates empathy-based consoling behaviors in prairie voles (Burkett et al. 2016).
Recent advances in miniature wireless headstages and machine learning are revolutionizing studies of complex multiscale behaviors such as social interactions (Mathis et al. 2018, Ebbesen & Froemke 2020). Anpilov et al. (2020) used a wireless system to examine consequences of prolonged PVN oxytocin neuron stimulation in various social conditions. Activating PVN oxytocin neurons in an enriched social environment increased both prosocial and agonistic behaviors depending on context, consistent with the hypothesis that oxytocin is an attentional modulator acting to enhance social salience rather than being inherently prosocial.
Oxytocin is a candidate for neuropsychiatric disorders in terms of pathological disruption and use as a treatment, particularly for autism spectrum disorders (ASDs) (Wagner & Harony-Nicolas 2018, DeMayo et al. 2019). Harony-Nicolas et al. (2017) developed Shank3-deficient rats and found that these animals had impaired social recognition memory, visual spatial attention, and hippocampal/cortical long-term synaptic plasticity. These biobehavioral phenotypes were rescued by exogenous oxytocin, indicating that oxytocin receptor signaling itself was not impacted but rather that some aspect of endogenous oxytocin production and/or release may be disrupted in Shank3 mutants (Harony-Nicolas et al. 2017). Shank3b- and Cntnap2-knockout mice also had impaired social interactions rescued by oxytocin (Peñagarikano et al. 2015, Resendez et al. 2020). Somewhat differently, neuroligin3 (Nlgn3)-knockout mice also showed impaired social memory, but this seemed due to the insensitivity of VTA dopamine neurons to oxytocin even when applied exogenously (Hörnberg et al. 2020). Knocking out Nlgn3 in VTA dopamine neurons recapitulated these phenotypes, and replacing Nlgn3 in constitutive knockouts restored functionality. Loss of Nlgn3 disrupted translational programs of gene expression homeostasis, and translational, neural, and behavioral phenotypes were rescued by a brain-penetrant pharmacological compound that interfered with MAP kinase–interacting kinases. Thus, the therapeutic potential of drugs targeting the oxytocin system for treating social deficits in heterogeneous conditions such as ASDs will differ depending on the underlying genetic etiology of the disorder. Consequently, oxytocin-based pharmacotherapies for psychiatric disorders will require a precision medicine approach informed by context-dependent and circuit-based neuromodulation mechanisms (Ford & Young 2021).
Mate Preferences and Pair-Bonding
Oxytocin signaling in medial prefrontal cortex and amygdala is important for mating behavior in mice, perhaps via sex-specific modulation of each area. When somatostatin-positive interneurons expressing oxytocin receptors in prefrontal cortex are silenced (or when oxytocin receptors are selectively deleted from those cell types), female mice reduce time spent interacting with males (Nakajima et al. 2014). Much of sexual selection and partner preference in rodents comes from olfactory signals such as pheromones (Dulac et al. 2014), and mouse medial amygdala contains a distributed population code for olfactory responses to female, male, and predator odors. Sexual experience reorganizes this representation, increasing neural and behavioral discrimination between male and female odors. This discrimination is impaired in males either if oxytocin signaling is systemically blocked (Li et al. 2017) or by selectively deleting oxytocin receptors from aromatase-positive neurons of the medial amygdala (Yao et al. 2017). Together, these results indicate that a substantial amount of social information processing occurs in medial amygdala, with distinct behaviors arising from these oxytocin-dependent computations.
In monogamous prairie voles, oxytocin facilitates mating-induced bonding and long-term partner preference (Walum & Young 2018). In contrast to nonmonogamous species, prairie voles have high densities of oxytocin receptors in nucleus accumbens, and oxytocin signaling in the accumbens during mating is essential for partner preference formation (Keebaugh et al. 2015). During mating, prelimbic cortical projections modulate gamma frequency oscillations in nucleus accumbens, and optogenetically recapitulating that activity facilitates partner preference formation in the absence of mating (Amadei et al. 2017). Oxytocin facilitates mating-induced coordinated activity of several brain areas in the social salience network, suggesting that oxytocin facilitates the flow of social information across this network (Johnson et al. 2016, Johnson et al. 2017). Oxytocin interacts with dopamine to facilitate pair-bonding, possibly by facilitating synaptic plasticity to link neural representations of partner cues to the reward system (Walum & Young 2018). Loss of oxytocin signaling following partner loss also mediates depressive-like behavior reminiscent of grieving (Bosch et al. 2016). Early-life experiences interact with the oxytocin system to influence later-life social preferences in voles. For example, there is robust individual genetically determined variation in oxytocin receptor density in prairie vole nucleus accumbens (King et al. 2016). Repeated neonatal social isolations (modeling neglect) disrupt adult pair-bonding in voles with low accumbens receptor density but not in those with high receptor density, suggesting that accumbens oxytocin signaling promotes resilience to early-life neglect (Barrett et al. 2015). In Mandarin voles, loss of the father at two weeks of age leads to decreased social preference and reduced oxytocin receptor levels in prefrontal cortex. Optogenetic stimulation of PVN oxytocin terminals in prelimbic cortex restores social preferences in paternally deprived animals (He et al. 2019).
Studies in voles have yielded insights into the regulation of complex social behaviors, but tools for circuit- and cell type–specific manipulations have been limited in these species. Clustered regularly interspaced short palindromic repeats (CRISPR)-mediated genome editing provides exciting opportunities to extend circuit-level investigations to a wide range of species (Boender & Young 2020). CRISPR-generated oxytocin receptor knockout and oxytocin receptor–Cre prairie voles provide a means for elucidating the function of oxytocin signaling and social bonding (Horie et al. 2019, 2020).
Oxytocin also mediates mating preferences in polygamous species. In medaka fish, females preferentially mate with familiar males who successfully competed for proximity to the female the previous day (Yokoi et al. 2015, 2016). By contrast, males typically mate indiscriminately and with no preference for familiarity. However, mutating oxytocin receptors using CRISPR reduces mating preference in females and results in the emergence of preference for familiar females in males (Yokoi et al. 2020). Thus, in both monogamous and promiscuous species, oxytocin can promote adaptive mating preferences. Species such as voles and medaka fish provide opportunities to explore oxytocin modulation of complex social behaviors beyond those typical of mice (Boender & Young 2020).
RESPONSES OF OXYTOCIN NEURONS TO SOCIAL STIMULI
Other modulators can have effects similar to those of oxytocin in terms of changes in excitability, disinhibition, enabling long-term plasticity, and enhancing attention and behavioral responses toward external stimuli, social or otherwise (Froemke 2015). For example, oxytocin can shift auditory cortical frequency–tuning profiles for pure tones, similar to cholinergic modulation (Mitre et al. 2016). The oxytocin peptide is not intrinsically social, and so the maternal/social phenotype of oxytocin must instead come from the inputs that activate these cells, that is, the (social) receptive fields of oxytocin neurons.
Performing recordings from oxytocin neurons in vivo can be technically challenging, due to the deep and periventricular location of oxytocin neurons and the heterogeneity of PVN/SON cell types. Initial in vivo recordings relied on identifying oxytocin neurons based on spiking statistics together with measurements of milk ejection and detection of oxytocin from microdialysis (Lincoln & Wakerley 1974, Neumann & Landgraf 1989, Leng et al. 2005). These and other studies were essential for relating PVN and SON spiking activity to oxytocin release in nursing mother rodents, but it remained unclear when oxytocin was released in nonlactating females and males.
Newer studies rely on monitoring activity from identified cells in transgenic mice. Hung et al. (2017) performed fiber photometry from PVN oxytocin neurons expressing GCaMP6m in adult animals and observed substantial signals during interactions with conspecific juveniles but not toy mice. Using two-photon imaging in head-fixed adult males, Resendez et al. (2020) observed heterogeneous Ca2+ signals in PVN oxytocin neurons when the mice were presented with anesthetized juveniles. In contrast, sucrose (a nonsocial appetitive stimulus) did not activate PVN oxytocin neurons.
Tang et al. (2020) made single-unit recordings from identified oxytocin neurons in virgin female rats using a custom viral vector to express channelrhodopsin-2 under control of the oxytocin promoter. This is a major technical advance, enabling selective recordings from optically identified neurons not just in wild-type rodents but in other species as well. In virgin females, parvocellular PVN oxytocin neurons responded to gentle touch (Figure 5a). These neurons made monosynaptic excitatory connections onto magnocellular PVN oxytocin neurons. This direct connection might help ensure that sensory signals received by the smaller fraction of parvocellular neurons are transmitted to the larger magnocellular population to ensure synchronous firing and oxytocin release.
Figure 5.
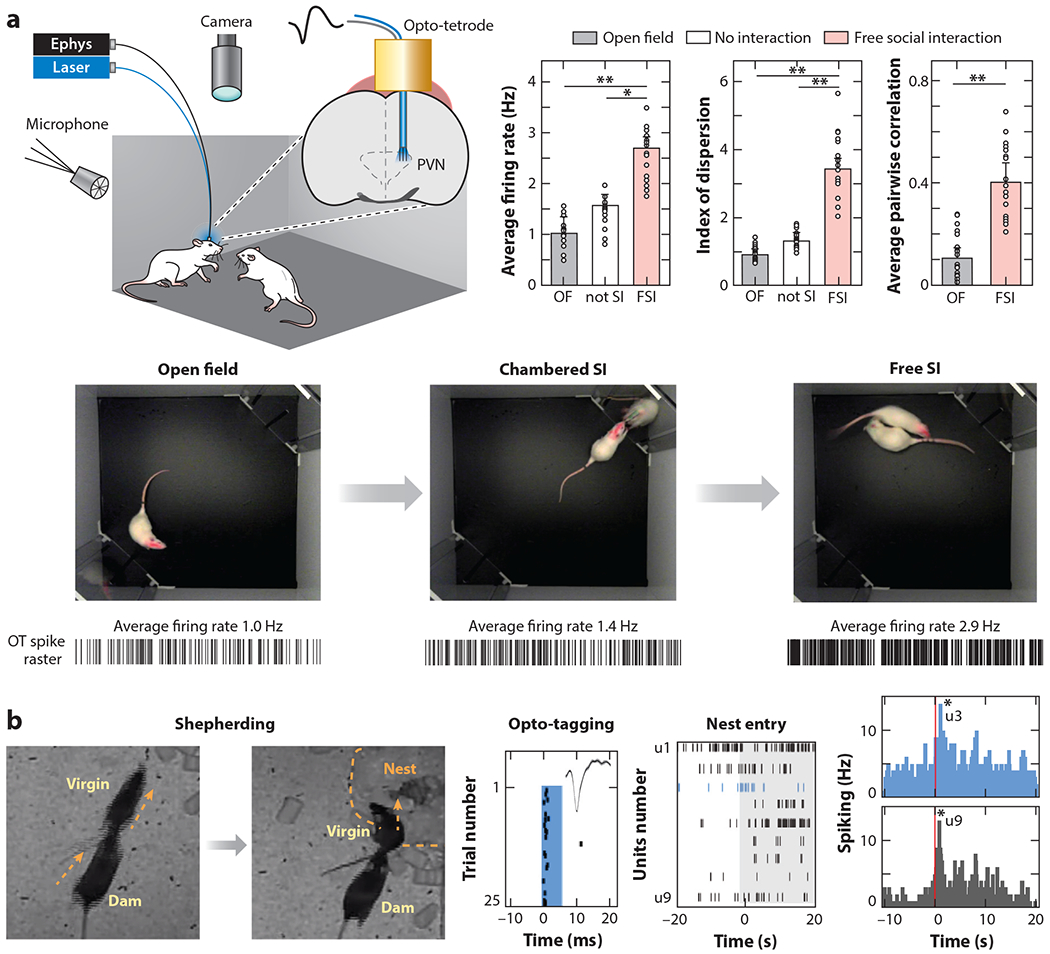
Single-unit recordings from optically identified PVN oxytocin neurons. (a) Combined recordings of behavior, ultrasonic vocalizations, and neural activity in virgin female rats measure responses of oxytocin neurons in vivo. FSI led to the greatest change in firing rate and synchronous activity in simultaneously recorded oxytocin neurons (Tang et al. 2020). (b) Social interactions between dams and virgins activate virgin PVN oxytocin neurons. (Left) Continuous videography of virgins cohoused for days with dams and litters revealed previously undescribed behavior such as dams shepherding virgins to nests and pups. (Right) Simultaneous in vivo recordings of nine PVN units, including a photo-tagged oxytocin PVN cell (u3), show bursting patterns during a single shepherding episode (middle raster plot) and over dozens of shepherding events (right). Many oxytocin neurons and unidentified cells (e.g., u9) reliably burst during virgin head nest entry (Carcea et al. 2019). Asterisks denote significance level: *p < .05, **p < .01. Abbreviations: CSI, chambered social interaction; FSI, free social interaction; OF, open field; PVN, paraventricular nucleus.
It has long been appreciated that some forms of nonsomatosensory stimulation can activate oxytocin neurons; for example, infant crying can release oxytocin and trigger milk ejection in human mothers (McNeilly et al. 1983). To understand when oxytocin neurons in virgin female mice might be activated during cohousing with an experienced dam and pups, Carcea et al. (2019) continuously monitored homecage behavior, combined with optically tagged recordings from virgin PVN oxytocin neurons. PVN neurons did not fire substantially during spontaneous interactions with pups, and initially, virgin animals stayed away from the dam and the nest. Over hours, mother animals began shepherding virgins toward the nest, agonistically chasing them toward the pups, and this shepherding behavior and virgin nest entry activated a fraction of PVN and the identified oxytocin neurons (Figure 5b). Virgins then began staying in the nest with dam and pups, and mother animals would spontaneously retrieve pups back to the nest if pups fell off of her when she left the nest to forage. Observation of maternal retrievals activated virgin oxytocin neurons, and some of these same neurons were reactivated when the virgin retrieved pups. Recordings in PVN taken simultaneously with fiber photometry in auditory cortex showed that bursts of activity in the hypothalamus produced bigger cortical responses, which increased just prior to individual virgins beginning to retrieve pups. Thus, inexperienced females can learn aspects of maternal behavior from interactions with and observations of experienced mothers, leading to cortical plasticity that builds on an intrinsic initial sensitivity to some pup call features (Carcea et al. 2019, Schiavo et al. 2020).
CONCLUSIONS AND OPEN QUESTIONS
Growing evidence, mainly from studies in rodents, has shown that oxytocin has a large number of effects on neurons in the central nervous system. A consistent finding is that oxytocin increases excitability and enables synaptic plasticity, although the specific mechanisms vary by cell type and brain region. Rather than unilaterally increasing prosocial behavior and initiating maternal care, oxytocin seems to act as an attentional modulator, increasing the salience of social information such that different circuits (e.g., amygdala, frontal cortex, auditory cortex, dopaminergic neurons) can respond selectively to social stimuli in a behaviorally appropriate manner.
While the use of genetic tools has been helpful for determining previously unknown aspects of oxytocin signaling and the functional anatomy of the rodent oxytocin system, much remains unknown about oxytocin release and the functions of oxytocinergic modulation. New types of molecular sensors promise to provide qualitative and quantitative measurements of oxytocin release in specific brain regions in real time (Wu et al. 2019, Mignocchi et al. 2020). Single-cell RNA-Seq strategies will provide more insights into the molecular phenotypes of oxytocin receptor neurons in diverse brain regions to reveal novel circuit-based modulatory mechanisms. Lewis et al. (2020) recently used RNA-Seq to characterize the transcriptome of magno- and parvocellular PVN oxytocin neurons to examine common pathways linking oxytocin and ASD-related genes. These data could potentially be used to identify drug targets for evoking endogenous oxytocin release from specific subpopulations of neurons, perhaps in a projection-specific manner. Continued research into the mechanisms of oxytocinergic modulation of neural circuits, plasticity, and behavior is needed to realize the maximum therapeutic potential of this peptide system (Ford & Young 2021). We outline three major questions or opportunities in relation to oxytocin, neuromodulation, and social behavior.
First, it is unlikely that neuromodulatory systems act in isolation. How then do multiple systems interact, either synergistically or in opposition, to regulate circuit function and behavior? While it is likely that at a more macroscopic level a neural circuit or brain region can be modulated by many different modulatory substances, at the level of single neurons, dendritic branches, or single spines, how is specificity in terms of modulatory control over excitatory and inhibitory synaptic transmission achieved?
Second, is oxytocin release coordinated throughout the brain to ensure neuromodulation and plasticity in relevant areas? To what degree can oxytocin release be regulated at the level of individual processes in specific brain areas? Anatomical mapping has revealed a large number of different input sources, and neural recordings demonstrated substantial heterogeneity of oxytocin responses to various stimuli. When and how would release be synchronized, and when might target-specific modulation occur? Furthermore, given that many areas receive projections from PVN oxytocin neurons, and given that essentially all brain regions express oxytocin receptors to some degree, what constitutes a neural circuit for complex oxytocin-dependent behaviors involved in parenting, and how many various neural systems are involved?
Third, how does context alter oxytocin function? Oxytocin induces nurturing behavior in a dam in response to pups but aggression in response to a male intruder. Animals respond differently to a distressed juvenile compared to a distressed adult. How does circuit-level modulation influence network activity and computations for differential responses to distinct stimuli? When might long-term and possibly life-long changes in synaptic and circuit function be engaged (e.g., after pair-bonding), and when would the system instead be transiently modulated or temporarily switched into a different operating state? Next-generation molecular oxytocin sensors might permit direct determination of oxytocin release or local concentration, which would be useful for a large number of studies of the physiology and pathology of this remarkable hormone system.
Addressing these questions will advance the therapeutic potential of oxytocin for psychiatric disorders, particularly those characterized by deficits in the social domain.
ACKNOWLEDGMENTS
We thank G. Dölen, K. Eyring, V. Grinevich, H. Harony-Nicolas, E. Kozorovitskiy, R. Nardou, and C. Theofanopoulou for feedback and comments on the manuscript. S. Valtcheva created the artwork in Figures 1 and 3. The contribution by R.C.F. was supported by the National Institutes of Health (NIH) National Institute on Deafness and Other Communication Disorders (NIDCD) grant DC12557, NICHD grant HD088411, and the BRAIN Initiative grant NS107616 to R.C.F. The contribution by L.J.Y. was supported by NIH National Institute of Mental Health grants R01MH112788 and P50MH100023 to L.J.Y. and P51OD11132 to Yerkes National Primate Research Center.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
The Annual Review of Neuroscience is online at neuro.annualreviews.org
LITERATURE CITED
- Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang CJ, et al. 2018. Corticoamygdala transfer of socially derived information gates observational learning. Cell 173:1329–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadei EA, Johnson ZV, Kwon YJ, Shpiner AC, Saravanan V, et al. 2017. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anpilov S, Shemesh Y, Eren N, Harony-Nicolas H, Benjamin A, et al. 2020. Wireless optogenetic stimulation of oxytocin neurons in a semi-natural setup dynamically elevates both pro-social and agonistic behaviors. Neuron 107:644–55.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakos J, Srancikova A, Havranek T, Bacova Z. 2018. Molecular mechanisms of oxytocin signaling at the synaptic connection. Neural Plast. 2018:4864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, Young LJ. 2015. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl. Psychiatry 5:e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin V, Moos F, Richard P. 1984. Synchronization of oxytocin cells in the hypothalamic paraventricular and supraoptic nuclei in suckled rats: direct proof with paired extracellular recordings. Exp. Brain Res 57:201–3 [DOI] [PubMed] [Google Scholar]
- Bendesky A, Kwon YM, Lassance JM, Lewarch CL, Yao S, et al. 2017. The genetic basis of parental care evolution in monogamous mice. Nature 544:434–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boender AJ, Young LJ. 2020. Oxytocin, vasopressin and social behavior in the age of genome editing: a comparative perspective. Horm. Behav 124:104780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ. 2013. Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defense. Philos. Trans. R. Soc. Lond. BBiol. Sci 368:20130085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, et al. 2016. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 64:66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Bains JS, Ludwig M, Stern JE. 2013. Physiological regulation of magnocellular neurosecretory cell activity: integration of intrinsic, local and afferent mechanisms. J. Neuroendocrinol 25:678–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach JPH, Young LJ, Russell JA. 2006. Oxytocin: synthesis, secretion, and reproductive functions. In Knobil and Neill’s Physiology of Reproduction, ed. Neill JD, pp. 3055–127. London: Elsevier. 3rd ed. [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FBM, Young LJ. 2016. Oxytocin-dependent consolation behavior in rodents. Science 351:375–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcea I, Lopez Caraballo N, Marlin BJ, Ooyama R, Mendoza Navarro JM, et al. 2019. Oxytocin neurons enable social transmission of maternal behavior. bioRxiv 845495. 10.1101/845495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens KE, Dudek SM. 2019. Regulation of synaptic plasticity in hippocampal area CA2. Curr. Opin. Neurobiol 54:194–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Manning M, Guillon G. 2008. Affinity and efficacy of selective agonists and antagonists for vasopressin and oxytocin receptors: an “easy guide” to receptor pharmacology. Prog. Brain Res 170:513–17 [DOI] [PubMed] [Google Scholar]
- Cohen L, Rothschild G,Mizrahi A. 2011.Multisensory integration of natural odors and sounds in the auditory cortex. Neuron 72:357–69 [DOI] [PubMed] [Google Scholar]
- Crane JW, Holmes NM, Fam J, Westbrook RF, Delaney AJ. 2020. Oxytocin increases inhibitory synaptic transmission and blocks development of long-term potentiation in the lateral amygdala. J. Neurophysiol 123:587–99 [DOI] [PubMed] [Google Scholar]
- Cymerblit-Sabba A, Smith AS, Avram SKW, Stackmann M, Korgan AC, et al. 2020. Inducing partner preference in mice by chemogenetic stimulation of CA2 hippocampal subfield. Front. Mol. Neurosci 13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMayo MM, Young LJ, Hickie IB, Song YJC, Guastella AJ. 2019. Circuits for social learning: a unified model and application to Autism Spectrum Disorder. Neurosci. Biobehav. Rev 107:388–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Cservenak M, Young LJ. 2018. Thalamic integration of social stimuli regulating parental behavior and the oxytocin system. Front. Neuroendocrinol 51:102–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. 2013. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501:179–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan ML, Stefanini F,Meira T, Gordon JA, Fusi S, Siegelbaum SA. 2020. Coding of social novelty in the hippocampal CA2 region and its disruption and rescue in a 22q11.2 microdeletion mouse model. Nat. Neurosci 23:1365–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA, Wu Z. 2014. Neural control of maternal and paternal behaviors. Science 345:765–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbesen CL, Froemke RC. 2020. Automatic tracking of mouse social posture dynamics by 3D videography, deep learning and GPU-accelerated robust optimization. bioRxiv 2020.05.21.109629. 10.1101/2020.05.21.109629 [DOI] [Google Scholar]
- Ehret G 1987. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature 325:249–51 [DOI] [PubMed] [Google Scholar]
- Ehret G 2005. Infant rodent ultrasounds—a gate to the understanding of sound communication. Behav. Genet 35:19–29 [DOI] [PubMed] [Google Scholar]
- Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, et al. 2016. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron 89:1291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyring KW, Liu J, König GM, Hidema S, Nishimori K, et al. 2020. Oxytocin signals via Gi and Gq to drive persistent CA2 pyramidal cell firing and strengthen CA3-CA1 neurotransmission. bioRxiv 2020.05.07.082727. 10.1101/2020.05.07.082727 [DOI] [Google Scholar]
- Fang LY, Quan RD, Kaba H. 2008. Oxytocin facilitates the induction of long-term potentiation in the accessory olfactory bulb. Neurosci. Lett 438:133–37 [DOI] [PubMed] [Google Scholar]
- Ferretti V,Maltese F, Contarini G, Nigro M, Bonavia A, et al. 2019. Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr. Biol 29:1938–53 [DOI] [PubMed] [Google Scholar]
- Field RE, D’amour JA, Tremblay R, Miehl C, Rudy B, et al. 2020. Heterosynaptic plasticity determines the set point for cortical excitatory-inhibitory balance. Neuron 106:842–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CL, Young LJ. 2021. Translational opportunities for circuit-based social neuroscience: advancing 21st century psychiatry. Curr. Opin. Neurobiol 68:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Olivera-Pasilio V, Dabrowska J. 2020. Oxytocin selectively excites interneurons and inhibits output neurons of the bed nucleus of the stria terminalis (BNST). bioRxiv 2020.06.24.169466. 10.1101/2020.06.24.169466 [DOI] [Google Scholar]
- Freeman SM, Young LJ. 2016. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J. Neuroendocrinol 28: 10.1111/jne.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Richard P. 1984. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J. Physiol 352:447–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC. 2015. Plasticity of excitatory-inhibitory balance. Annu. Rev. Neurosci 38:195–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Schreiner CE. 2015. Synaptic plasticity as a cortical coding scheme. Curr. Opin. Neurobiol 35:185–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwee PC, Tay BH, Brenner S, Venkatesh B. 2009. Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: origin of the vertebrate neurohypophysial hormone genes. BMC Evol. Biol 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden SW, Frazier CJ. 2016. Oxytocin depolarizes fast-spiking hilar interneurons and induces GABA release onto mossy cells of the rat dentate gyrus. Hippocampus 26:1124–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harony-Nicolas H, Kay M, du Hoffmann J, Klein ME, Bozdagi-Gunal O, et al. 2017. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. eLife 6:e18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Young L, Ma XM, Guo Q, Wang L, et al. 2019. Increased anxiety and decreased sociability induced by paternal deprivation involve the PVN-PrL OTergic pathway. eLife 8:e44026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA. 2014. The hippocampal CA2 region is essential for social memory. Nature 508:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K, Inoue K, Nishimori K, Young LJ. 2020. Investigation of Oxtr-expressing neurons projecting to nucleus accumbens using Oxtr-ires-Cre knock-in prairie voles (Microtus ochrogaster). Neuroscience 448:312–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K, Inoue K, Suzuki S, Adachi S, Yada S, et al. 2019. Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Horm. Behav 111:60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörnberg H, Pérez-Garci E, Schreiner D, Hatstatt-Burklé L, Magara F, et al. 2020. Rescue of oxytocin response and social behaviour in a mouse model of autism. Nature 584:252–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. 2005. Vassopressin and oxytocin excite different neuronal populations in the central amygdala. Science 308:245–48 [DOI] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, et al. 2017. Gating of social reward by oxytocin in the ventral tegmental area. Science 357:1406–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani BG, Donato J Jr., Olson DP, Lowell BB, Sacktor TC, et al. 2010. Distribution and neurochemical characterization of protein kinase C-theta and -delta in the rodent hypothalamus. Neuroscience 170:1065–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, et al. 2016. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm. Behav 79:8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Xiao Y, Riefkohl PC, Young LJ. 2017. Oxytocin receptors modulate a social salience neural network in male prairie voles. Horm. Behav 87:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. 2017. Oxytocin and vasopressin neural networks: implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev 76:87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B, Neumann ID. 2018. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev 98:1805–908 [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. 2015. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc. Neurosci 10:561–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. 2011. Increasing oxytocin receptor expression in the nucleus accumbens of prepubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm. Behav 60:498–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Hinton MR, Goode JA. 1991. Cerebrospinal fluid and plasma concentrations of oxytocin and vasopressin during parturition and vaginocervical stimulation in the sheep. Brain Res. Bull 26:803–7 [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Lévy F, Keverne EB. 1992. Changes in the sensory processing of olfactory signals induced by birth in sheep. Science 256:833–36 [DOI] [PubMed] [Google Scholar]
- Keverne EB, Kendrick KM. 1994. Maternal behaviour in sheep and its neuroendocrine regulation. Acta Paediatr. Suppl 397:47–56 [DOI] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ. 2016. Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol. Psychiatry 80:160–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Bilbo SD. 2019. The inflammatory event of birth: how oxytocin signaling may guide the development of the brain and gastrointestinal system. Front. Neuroendocrinol 55:100794. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, et al. 2012. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73:553–66 [DOI] [PubMed] [Google Scholar]
- Koch M, Ehret G. 1989. Estradiol and parental experience, but not prolactin are necessary for ultrasound recognition and pup-retrieving in the mouse. Physiol. Behav 45:771–76 [DOI] [PubMed] [Google Scholar]
- Leng G, Caquineau C, Sabatier N. 2005. Regulation of oxytocin secretion. Vitam. Horm 71:27–58 [DOI] [PubMed] [Google Scholar]
- Leng G,Meddle SL, Douglas AJ. 2008. Oxytocin and the maternal brain. Curr. Opin. Pharmacol 8:731–34 [DOI] [PubMed] [Google Scholar]
- Leroy F, Park J, Asok A, Brann DH, Meira T, et al. 2018.A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature 564:213–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EM, Stein-O’Brien GL, Patino AV, Nardou R, Grossman CD, et al. 2020. Parallel social information processing circuits are differentially impacted in autism. Neuron 108:659–75.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Nakajima M, Ibañez-Tallon I, Heintz N. 2016. A cortical circuit for sexually dimorphic oxytocin-dependent anxiety behaviors. Cell 167:60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, et al. 2017. Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell 171:1176–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-T, Hsieh T-Y, Tsai T-C, Chen C-C, Huang C-C, Hsu K-S. 2018. Conditional deletion of hippocampal CA2/CA3a oxytocin receptors impairs the persistence of long-term social recognition memory in mice. J. Neurosci 38:1218–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln DW, Wakerley JB. 1974. Electrophysiological evidence for the activation of supraoptic neurones during the release of oxytocin. J. Physiol 242:533–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. 2007. Auditory cortical detection and discrimination correlates with communicative significance. PLOS Biol. 5:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. 2003. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav. Neurosci 117:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniezzi C, Talpo F, Spaiardi P, Toselli M, Biella G. 2019. Oxytocin increases phasic and tonic GABAergic transmission in CA1 region of mouse hippocampus. Front. Cell. Neurosci 13:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC. 2015. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520:499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, et al. 2018. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci 21:1281–89 [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Robinson IC, Houston MJ, Howie PW. 1983. Release of oxytocin and prolactin in response to suckling. Br. Med. J. (Clin. Res. Ed.) 286:257–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignocchi N, Krüssel S, Jung K, Lee D, Kwon HB. 2020. Development of a genetically-encoded oxytocin sensor. bioRxiv 2020.07.14.202598. 10.1101/2020.07.14.202598 [DOI] [Google Scholar]
- Mitre M, Marlin BJ, Schiavo JK,Morina E, Norden S, et al. 2016. A distributed network for social cognition enriched for oxytocin receptors. J. Neurosci 36:2517–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Inoue K, Barrett CE, Kittelberger KA, Smith DG, et al. 2015. Melanocortin receptor agonists facilitate oxytocin-dependent partner preference formation in the prairie vole. Neuropsychopharmacology 40:1856–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Görlich A, Heintz N. 2014. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell 159:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardou R, Lewis EM, Rothhaas R,Xu R,Yang A, et al.2019. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature 569:116–20 [DOI] [PubMed] [Google Scholar]
- Neumann I, Douglas AJ, Pittman QJ, Russell JA, Landgraf R. 1996. Oxytocin released within the supraoptic nucleus of the rat brain by positive feedback action is involved in parturition-related events. J. Neuroendocrinol 8:227–33 [DOI] [PubMed] [Google Scholar]
- Neumann I, Koehler E, Landgraf R, Summy-Long J. 1994. An oxytocin receptor antagonist infused into the supraoptic nucleus attenuates intranuclear and peripheral release of oxytocin during suckling in conscious rats. Endocrinology 134:141–48 [DOI] [PubMed] [Google Scholar]
- Neumann I, Landgraf R. 1989. Septal and hippocampal release of oxytocin, but not vasopressin, in the conscious lactating rat during suckling. J. Neuroendocrinol 1:305–8 [DOI] [PubMed] [Google Scholar]
- Newmaster KT, Nolan ZT, Chon U, Vanselow DJ, Weit AR, et al. 2020. Quantitative cellular-resolution map of the oxytocin receptor in postnatally developing mouse brains. Nat. Commun 11:1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noirot E 1972. Ultrasounds and maternal behavior in small rodents. Dev. Psychobiol 5:371–87 [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. 2003. The Neurobiology of Parental Behavior. New York: Springer-Verlag [Google Scholar]
- Numan M, Young LJ. 2016. Neural mechanisms of mother-infant bonding and pair bonding: similarities, differences, and broader implications. Horm. Behav 77:98–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, et al. 2016. Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron 90:609–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazábal DE, Young LJ. 2006. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience 141:559–68 [DOI] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. 2013. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 500:458–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani JH, Zhao M, Cui Z, Williams Avram SK, Caruana DA, et al. 2015. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol. Psychiatry 20:490–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ. 1982. Oxytocin induces maternal behavior in virgin female rats. Science 216:648–50 [DOI] [PubMed] [Google Scholar]
- Pekarek BT, Hunt PJ, Arenkiel BR. 2020. Oxytocin and sensory network plasticity. Front. Neurosci 14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Lázaro MT, Lu XH, Gordon A, Dong H,et al. 2015. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci. Transl. Med 7:271ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel JP, Petrovic DM, Jones CH. 1990. Defensive burying, nest relocation, and pup transport in lactating female rats. Q. J. Exp. Psychol. B 42:401–11 [PubMed] [Google Scholar]
- Raam T,McAvoy KM, Besnard A, Veenema AH, Sahay A. 2017. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Commun. 8:2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamani KT, Wagner S, Grinevich V, Harony-Nicolas H. 2018. Oxytocin as a modulator of synaptic plasticity: implications for neurodevelopmental disorders. Front. Synaptic Neurosci 10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Namboodiri VMK, Otis JM, Eckman LEH, Rodriguez-Romaguera J, et al. 2020. Social stimuli induce activation of oxytocin neurons within the paraventricular nucleus of the hypothalamus to promote social behavior in male mice. J. Neurosci 40:2282–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenbacher E, Perry RE, Sullivan RM, Moita MA. 2017. Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviours and mother-pup interactions. eLife 6:e24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. 2014. The biology of mammalian parenting and its effect on offspring social development. Science 345:771–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, et al. 2018. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat. Neurosci 21:404–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. 2009. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol 30:534–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. 2008. Emergent synchronous bursting of oxytocin neuronal network. PLOS Comput. Biol 4:e1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Brown CH, Ludwig M, Leng G. 2004. Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J. Physiol 558:161–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo JK, Valtcheva S, Bair-Marshall CJ, Song SC, Martin KA, Froemke RC. 2020. Innate and plastic mechanisms for maternal behaviour in auditory cortex. Nature 587:426–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupré A, Ritchie J, et al. 2010. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J. Neurosci 30:8274–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott N, Prigge M, Yizhar O, Kimchi T. 2015. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525:519–22 [DOI] [PubMed] [Google Scholar]
- Smith SJ, Sümbül U, Graybuck LT, Collman F, Seshamani S, et al. 2019. Single-cell transcriptomic evidence for dense intracortical neuropeptide networks. eLife 8:e47889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Albers HE. 2018. Cross-talk among oxytocin and arginine-vasopressin receptors: relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol 51:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Benusiglio D, Lefevre A, Hilfiger L, Althammer F, et al. 2020. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat. Neurosci 23:1125–37 [DOI] [PubMed] [Google Scholar]
- Tang Y, Chen Z, Tao H, Li C, Zhang X, et al. 2014. Oxytocin activation of neurons in ventral tegmental area and interfascicular nucleus of mouse midbrain. Neuro-pharmacology 77:277–84 [DOI] [PubMed] [Google Scholar]
- Teruyama R, Armstrong WE. 2002. Changes in the active membrane properties of rat supraoptic neurones during pregnancy and lactation. J. Neuroendocrinol 14:933–44 [DOI] [PubMed] [Google Scholar]
- Theofanopoulou C, Gedman G, Cahill JA, Boeckx C,Jarvis ED. 2021. Universal nomenclature for oxytocin–vasotocin ligand and receptor families. Nature 592:747–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirko NN, Eyring KW, Carcea I, Mitre M, Chao MV, et al. 2018. Oxytocin transforms firing mode of CA2 hippocampal neurons. Neuron 100:593–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan R, Hammock EA. 2017. Oxytocin receptor dynamics in the brain across development and species. Dev. Neurobiol 77:143–57 [DOI] [PubMed] [Google Scholar]
- Valtcheva S, Froemke RC. 2019. Neuromodulation of maternal circuits by oxytocin. Cell Tissue Res. 375:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Harony-Nicolas H. 2018. Oxytocin and animal models for autism spectrum disorder. Curr. Top. Behav. Neurosci 35:213–37 [DOI] [PubMed] [Google Scholar]
- Walum H, Young LJ. 2018. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci 19:643–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. 2014. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509:325–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Feng J,Jing M, Li Y. 2019. G protein-assisted optimization of GPCR-activation based (GRAB) sensors. Proc. SPIE 10865:12.2514631 [Google Scholar]
- Xiao L, Priest MF, Kozorovitskiy Y. 2018. Oxytocin functions as a spatiotemporal filter for excitatory synaptic inputs to VTA dopamine neurons. eLife 7:e33892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Priest MF, Nasenbeny J, Lu T, Kozorovitskiy Y. 2017. Biased oxytocinergic modulation of midbrain dopamine systems. Neuron 95:368–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Bergan J, Lanjuin A, Dulac C. 2017. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. eLife 6:e31373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S, Ansai S, Kinoshita M, Naruse K, Kamei Y, et al. 2016. Mate-guarding behavior enhances male reproductive success via familiarization with mating partners in medaka fish. Front. Zool 13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S, Naruse K, Kamei Y, Ansai S, Kinoshita M, et al. 2020. Sexually dimorphic role of oxytocin in medaka mate choice. PNAS 117:4802–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S, Okuyama T, Kamei Y, Naruse K, Taniguchi Y, et al. 2015. An essential role of the arginine vasotocin system in mate-guarding behaviors in triadic relationships of medaka fish (Oryzias latipes). PLOS Genet. 11:e1005009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, et al. 2009. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci 29:2259–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Barrett CE. 2015. Can oxytocin treat autism? Science 347:825–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Flanagan-Cato LM. 2012. Oxytocin, vasopressin, and social behavior. Horm. Behav 61:227–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Qiu L, Xiao W, Ni H, Chen L, et al. 2021. Reconstruction of the hypothalamo-neurohypophysial system and functional dissection of magnocellular oxytocin neurons in the brain. Neuron 109:331–46.e7 [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Li SJ, Zhang XD, Miao WY, Zhang D, et al. 2014. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat. Neurosci 17:391–99 [DOI] [PubMed] [Google Scholar]


