Abstract
American Academy of Sleep Medicine practice parameters designate sodium oxybate (SXB) as a standard of care for cataplexy, excessive daytime sleepiness (EDS), and disrupted night‐time sleep in narcolepsy. Recently, a lower‐sodium oxybate (LXB) with 92% less sodium than SXB was approved in the United States for the treatment of cataplexy or EDS in patients 7 years of age and older with narcolepsy. Two phase I, open‐label, randomized, single‐dose crossover pharmacokinetic studies in healthy adults were conducted. Single 4.5‐g oral doses of LXB and SXB were administered in a fasted or fed state. In the fasted state at equivalent oxybate doses, LXB, compared with SXB, had a lower maximum plasma concentration (Cmax; study 1 [total aqueous volume, 240 ml]: 101.8 vs. 135.7 µg/ml; study 2 [60 ml]: 94.6 vs. 123.0 μg/ml), delayed time to Cmax (Tmax; study 1: 0.75 vs. 0.5 h; study 2: 1.0 vs. 0.5 h), but similar area under the curve (AUC; study 1: AUC0‐t, 235.4 vs. 263.9 μg∙h/ml; AUC0‐∞, 236.5 vs. 265.2 μg∙h/ml; study 2: AUC0‐t, 241.5 vs. 254.7 μg∙h/ml; AUC0‐∞, 243.1 vs. 256.3 μg∙h/ml). Bioequivalence criteria were met for AUC but not Cmax (both studies). Cmax and AUC were lower under fed than fasted conditions (LXB and SXB); differences between fed versus fasted were smaller for LXB than SXB. These pharmacokinetic differences between LXB and SXB are likely due to the lower sodium content in LXB. Pooled analyses demonstrated that a higher Cmax is associated with a higher incidence of nausea and vomiting.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Sodium oxybate (SXB) and lower‐sodium oxybate (LXB) are approved in the United States for the treatment of cataplexy or excessive daytime sleepiness in patients greater than or equal to 7 years of age with narcolepsy. The pharmacokinetics (PK) of SXB includes a negative food effect (reduced maximum plasma concentration [Cmax] and area under the curve [AUC]) and greater than dose‐proportional increase in exposure.
WHAT QUESTION DID THIS STUDY ADDRESS?
What are the relative bioavailability and bioequivalence of LXB and SXB in the fasted state, and how is the PK of LXB affected by food?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
At equivalent oxybate doses, in the fasted state, LXB had a lower Cmax, delayed time to Cmax, and similar AUC versus SXB (bioequivalence criteria met for AUC). Cmax and AUC were lower under fed conditions (LXB and SXB); reduction in Cmax with food was less for LXB compared with SXB. Lower oxybate Cmax was associated with lower incidence of nausea and vomiting.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
PK differences between LXB and SXB may stem from reduced sodium. LXB represents a novel oxybate treatment for narcolepsy.
INTRODUCTION
Narcolepsy is a lifelong neurologic disorder characterized by a pentad of symptoms: excessive daytime sleepiness (EDS), cataplexy, disrupted night‐time sleep, sleep‐related hallucinations (hypnagogic and hypnopompic), and sleep paralysis. 1 Symptom onset typically occurs in adolescence or early adulthood, but diagnosis may be delayed by 10 years or more due to lack of symptom recognition by healthcare professionals. 2
The American Academy of Sleep Medicine practice parameters designate sodium oxybate (SXB; Xyrem) as a standard of care for cataplexy, EDS, and disrupted night‐time sleep in narcolepsy. 3 SXB is approved in the United States for cataplexy or EDS in patients greater than or equal to 7 years of age with narcolepsy. 4 The safety and efficacy of SXB for the treatment of cataplexy and EDS in adults and children/adolescents with narcolepsy have been established in randomized controlled trials; commonly observed adverse events included nausea and vomiting. 5 , 6 , 7 , 8 , 9 The therapeutic effects of SXB in narcolepsy are hypothesized to be mediated through effects at gamma‐aminobutyric acid B (GABAB) receptors on noradrenergic, dopaminergic, and thalamocortical neurons. 4
SXB is administered orally as a solution, diluted before ingestion with 60 ml water, with the total nightly dose typically divided into two doses, the first at bedtime and the second 2.5–4 h later. The effective dosage range in adults is 6–9 g/night. 4 In pediatric patients, dosing is based on weight, with an effective dose range of 3–9 g/night. 4 , 5 The pharmacokinetics (PK) of SXB has been characterized in adult and pediatric populations. 6 SXB is rapidly absorbed (average time to reach maximum plasma concentration [Tmax], 0.5–1.25 h) and quickly metabolized and eliminated from the body (average half‐life, 0.5–1 h). 4 Dosing recommendations include the instruction to allow 2 h after eating before dosing due to the known effect of food, which causes a reduction in exposure with administration immediately following ingestion of a high‐fat meal, compared with the fasted state. 4
At the recommended adult dosage (6–9 g/night), SXB contributes 1100–1640 mg to daily sodium intake. 4 The American Heart Association recommends total daily sodium intake of less than 1500 mg as ideal and less than 2300 mg as the upper limit. 7 The National Academy of Sciences established 2300 mg/day as the sodium Chronic Disease Risk Reduction Intake. 8 Reductions in sodium intake have been shown to reduce blood pressure and risk of hypertension and cardiovascular disease. 9 , 10
Calcium, magnesium, potassium, and sodium oxybates (lower‐sodium oxybate [LXB]; Xywav; formerly designated JZP‐258) is a novel oxybate medication with a unique composition of cations resulting in 92% less sodium compared with SXB (a reduction of 1013–1509 mg at a dosage range of 6–9 g/night, from 1100–1640 mg with SXB to 87–131 mg with LXB). Like SXB, LXB is approved in the United States for the treatment of cataplexy or EDS in patients 7 years of age and older with narcolepsy. 11 In a phase III clinical trial in participants with narcolepsy with cataplexy, LXB demonstrated positive effects on cataplexy and EDS, compared with placebo. 12 The active moiety of LXB, oxybate (gamma‐hydroxybutyrate), is the same as for SXB, and the mechanism of action is considered to be the same. 4 , 13
The primary objectives of the studies described in this article were to compare the bioavailability and bioequivalence of LXB and SXB in the fasted state and assess the effect of food (high‐fat, high‐calorie meal) on the PK parameters of LXB and SXB. Additional objectives were to examine the effect of the volume of water taken with the medication on the PK parameters of LXB and to evaluate the safety and tolerability of LXB and SXB following a single dose in healthy volunteers. Post hoc analyses were conducted to compare the magnitude of the food effect between LXB and SXB and evaluate the relationship between oxybate PK parameters and adverse events (AEs) of nausea and vomiting.
METHODS
Study design and participants
Data from two phase I, open‐label, randomized, single‐dose crossover studies (13–010 [study 1] and JZP258‐101 [study 2]) were included in these analyses. Eligible participants were healthy adults 18–50 years (study 1) or 18–45 years of age (study 2), with a body mass index (BMI) between 18 and 30 kg/m2 (study 1) or between 20 and 30 kg/m2 (study 2). The study protocol also specified that eligible participants were White and not Hispanic or Latino.
Study 1 comprised two parts. In part 1, the PK, relative bioavailability, bioequivalence, and food effect for LXB compared with SXB, administered in single doses under fasted and fed conditions, were evaluated (primary objective). In part 2, the PK of LXB at a lower dose (2.25 g) was evaluated under fasted conditions, in addition to evaluations of other oxybate formulations (not reported here). Separate cohorts of participants were enrolled for each part of the study.
In study 1, part 1, participants were randomized to receive the following treatments, on different days (with a minimum 1‐day washout period separating each crossover treatment), in one of four treatment sequences (Table S1): 4.5 g LXB (240 ml) under fasted conditions (treatment A), 4.5 g LXB (240 ml) under fed conditions (treatment B), 4.5 g SXB (240 ml) under fasted conditions (treatment C), and 4.5 g SXB (240 ml) under fed conditions (treatment D; Table S1). SXB and LXB were supplied as 500‐mg/ml solutions. Participants took a 9‐ml volume of drug solution (4.5 g SXB or LXB) diluted with 51 ml of water; this 60‐ml solution was taken with an additional 180 ml of water, for a total volume of 240 ml. Throughout this article, we will refer to the water volume in these treatments as 240 ml. In study 1, part 2, participants received 2.25 g LXB (240 ml; treatment E). For this treatment, a 4.5‐ml volume of LXB 500 mg/ml oral solution (2.25 g) was diluted with 51 ml of water, and then taken with 180 ml of water.
In study 2, the relative bioavailability and bioequivalence of LXB were compared with SXB (primary objective); the effects of food (secondary objective) and water volume (exploratory measure) on oxybate PK were also evaluated. Participants were randomized, stratified by sex, to receive the following treatments, on different days (with a minimum 1‐day washout period separating each crossover treatment), in one of six treatment sequences: 4.5 g LXB (60 ml) under fasted conditions (treatment F); 4.5 g SXB (60 ml) under fasted conditions (treatment G); 4.5 g LXB (60 ml) under fed conditions (treatment H); 4.5 g SXB (60 ml) under fed conditions (treatment I); 4.5 g SXB (240 ml) under fasted conditions (treatment J); and 4.5 g LXB (240 ml) under fasted conditions (treatment K; Table S1). Throughout this article, we will refer to the water volume in study 2, treatments F, G, H, and I as 60 ml and in treatments J and K as 240 ml.
In studies 1 and 2, for fasted conditions, participants received study drug in the morning following a 10‐hour fast and continued fasting for an additional 4 h after dosing. For fed conditions, participants fasted for at least 10 h before dosing, ate a standard high‐fat (~ 50% of total caloric content of the meal), high‐calorie (approximately 800–1000 calories) breakfast immediately before dosing, and then fasted until 4 h after dosing. This test meal was to derive ~ 150, ~ 250, and ~ 500–600 calories from protein, carbohydrate, and fat, respectively, as specified by the US Food and Drug Administration (FDA) guidance. 14 Participants completely consumed this meal in 30 min or less. Study drug was administered ~ 30 min after the start of the meal.
Assessments
Blood samples to determine oxybate PK profiles were collected predose; at 10, 20, 30, 45, and 60 minutes postdose; and at 1.25, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 7, and 8 h postdose following each treatment on days 1, 3, 5, and 7 (study 1, part 1 and part 2) or on days 1, 3, 5, 7, 9, and 11 (study 2). Blood samples for PK analysis were obtained within ±2 min of the specified time points for the first hour after each dose and within ±5 min of the specified time points after 1 h.
Safety was assessed throughout the studies, and follow‐up safety assessments occurred on the final day of the studies or early termination. Safety assessments included AE monitoring, clinical laboratory tests (chemistry, hematology, coagulation, and urinalysis), vital sign measurements, pulse oximetry, electrocardiograms, and physical examinations.
Analysis of oxybate concentrations in plasma samples
A validated liquid chromatography–tandem mass spectrometry method was used to determine oxybate concentrations in the plasma samples collected from the two studies. The assay was validated for use on human samples according to FDA guidance. 15 The linear range for the assay was between 0.75 and 192 µg/ml with the lower limit of quantitation (LLOQ) of 0.75 µg/ml. The intra‐assay accuracy was 1.5% to 12.5% at LLOQ and −3.4% to 5.7% above LLOQ; the intra‐assay precision was 6.4% to 13.2% at LLOQ and 0.9% to 4.7% above LLOQ. The interassay accuracy was 8.5% at LLOQ and −0.9% to 3.9% above LLOQ; the interassay precision was 11.5% at LLOQ and 2.9% to 3.8% above LLOQ. Oxybate was stable in plasma at ambient and refrigerated temperatures for 74 h and at −20°C for 92 days.
Statistical analyses
Baseline demographic and safety data were analyzed for the safety population, defined as participants who took greater than or equal to 1 dose of study drug, and summarized using descriptive statistics.
PK parameters calculated for plasma oxybate concentrations included area under the plasma concentration‐time curve (AUC), maximum plasma concentration (Cmax), and Tmax; PK parameters were estimated by noncompartmental methods and summarized by treatment period using descriptive statistics. The PK completer population, defined as all participants who received study drug and provided postdose PK data for at least one treatment regimen, did not vomit within two times the oxybate median Tmax, and completed all treatment periods, was used for the primary descriptive PK summaries.
An analysis of variance (ANOVA) for natural logarithm–transformed PK parameters (AUC0‐t, AUC0‐∞, and Cmax), with terms for sequence, period, and treatment as fixed effects and subject nested within sequence as a random effect, was performed. From these analyses, least squares (LS) means, LS mean treatment differences, and 90% confidence intervals (CIs) for the treatment differences on the log scale were estimated. The results were transformed back to the original scale by exponentiation to provide treatment geometric LS means, point estimates of the geometric LS mean ratios, and the 90% CIs for these ratios.
To assess bioequivalence between LXB and SXB, both under fasted conditions, the 90% CIs for the ratio of geometric LS means of log‐transformed AUC0‐t, AUC0‐∞, and Cmax were used. Bioequivalence was declared if the 90% CIs for the ratios of all three parameters were entirely contained within 80% and 125%. In a post hoc analysis, a similar statistical model was used to assess bioequivalence between LXB and SXB, both under fed conditions. In an exploratory analysis, relative bioavailability was evaluated to assess potential bioequivalence between different total volumes (60 compared with 240 ml; study 2) for the same formulations.
To assess if the impact of food on Cmax was similar for LXB and SXB, a similar statistical model to that used for the assessment of bioequivalence was used. The ratio of fed/fasted treatments for LXB was compared with the ratio of fed/fasted treatments for SXB. This comparison was assessed at the 0.005 significance level (Bonferroni adjusted for 10 comparisons).
AE summaries include only treatment‐emergent AEs (TEAEs). Events were considered to be treatment emergent if they had an onset date and time on or after the date and time of the first dose of study medication or if they were present before the first dose of study medication but increased in severity after dosing.
An exploratory analysis was conducted to evaluate the relationship between nausea and vomiting occurrences and PK parameters (Cmax and AUC), utilizing pooled data from both studies. A generalized linear mixed model (GLIMMIX) was utilized, with AE occurrence (nausea and vomiting) as the dependent variable, study number, period, and treatment as fixed effects, and participant as a random effect.
In study 1, SAS version 9.4 or later was used for all statistical analyses. In study 2, WinNonlin version 6.3 was used for noncompartmental analyses and SAS version 9.4 was used for all other statistical analyses.
Informed consent and ethics
Studies 1 and 2 were conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent in accordance with local institutional review board/independent ethics committee requirements before the performance of any study‐related procedures.
RESULTS
Demographics and participant disposition
Participant disposition and demographics are presented in Table 1. In study 1, part 1, 36 participants were enrolled and randomized to a treatment sequence; 35 (97.2%) participants completed the study; and 1 (2.8%) participant withdrew for personal reasons. In study 1, part 2, 24 participants were enrolled and randomized to a treatment sequence; 21 (87.5%) participants completed the study; and three (12.5%) participants withdrew because of AEs, including vomiting (two participants) and nausea, vomiting, and somnolence (one participant). In study 2, 48 participants were enrolled and randomized to a treatment sequence; 45 (93.8%) participants completed the study; and 3 (6.3%) participants withdrew because of AEs, including bradypnea, atrioventricular block second degree, and folliculitis. In both studies, about half of the participants were men, with a mean age of ~ 26 years and a mean BMI of ~ 24 kg/m. 2
TABLE 1.
Participant disposition and demographics
| Characteristic | Study 1 (part 1) | Study 1 (part 2) | Study 2 |
|---|---|---|---|
| Number of participants, n (%) | |||
| Enrolled and randomized a | 36 (100.0) | 24 (100.0) | 48 (100.0) |
| Discontinued | 1 (2.8) | 3 (12.5) | 3 (6.3) |
| Adverse event | 0 (0.0) | 3 (12.5) b | 3 (6.3) c |
| Withdrawal by participant | 1 (2.8) d | 0 | 0 (0.0) |
| Completed | 35 (97.2) | 21 (87.5) | 45 (93.8) |
| PK completer population e | 30 (83.3) f | 16 (66.7) g | 42 (87.5) h |
| Age (y), mean (SD) | 27.6 (5.5) | 26.7 (6.6) | 25.4 (5.6) |
| Sex, n (%) | |||
| Male | 19 (52.8) | 14 (58.3) | 24 (50.0) |
| Female | 17 (47.2) | 10 (41.7) | 24 (50.0) |
| BMI (kg/m2), mean (SD) | 22.9 (2.8) | 23.1 (2.8) | 24.9 (2.6) |
Abbreviations: BMI, body mass index; LXB, lower‐sodium oxybate; PK, pharmacokinetics; SD, standard deviation; SXB, sodium oxybate; Tmax, time to maximum plasma concentration.
Safety population. Defined as all participants who took greater than or equal to 1 dose of study drug.
Vomiting (moderate, related to study drug, fasted LXB 2.5 g and SXB 2 g); vomiting (severe, related to study drug, fasted SXB 4.5 g); and nausea (severe, related to study drug, fasted LXB 2.5 g and SXB 2 g), vomiting (severe, related to study drug, fasted SXB 4.5 g), and somnolence (severe, related to study drug, fasted SXB 4.5 g).
Bradypnea (moderate, related to study drug, SXB fasted, 60 ml water); atrioventricular block second degree (mild, not related to study drug, SXB fasted, 240 ml water); and folliculitis (moderate, related to study drug, SXB fasted, 240 ml water).
Personal reasons.
Defined as all participants who received study drug and provided postdose PK data for at least one treatment regimen, who did not vomit within two times the oxybate median Tmax, and who completed all treatment periods, which was used for the primary descriptive PK summaries.
Of 35 participants who completed part 1 of study 1, five were excluded from PK analysis due to vomiting within two times the median Tmax.
Of 21 participants who completed part 2 of study 2, five were excluded from the overall PK analysis due to vomiting within two times the median Tmax.
Of 45 participants who completed study 2, three were excluded from PK analysis due to vomiting within two times the median Tmax.
Oxybate concentration profiles and PK parameters
The oxybate plasma concentration profile for study 1 is presented in Figure 1; data for study 2 are similar (Figure S1). A summary of oxybate PK parameters is presented in Table 2.
FIGURE 1.
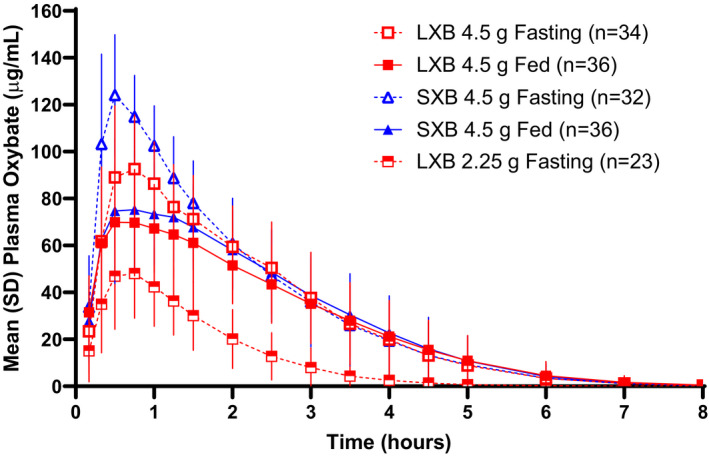
Concentration‐time profile of oxybate following oral administration of LXB and SXB in study 1.a LXB, lower‐sodium oxybate; SXB, sodium oxybate. aTreatments were A: LXB 4.5 g fasted, 240 ml water; B: LXB 4.5 g fed, 240 ml water; C: SXB 4.5 g fasted, 240 ml water; D: SXB 4.5 g fed, 240 ml water; E: LXB 2.25 g fasted, 235.5 ml water
TABLE 2.
Oxybate plasma PK parameters a
| Study | Treatment | Description | Arithmetic mean (coefficient of variation percentage) | |||
|---|---|---|---|---|---|---|
| Cmax (µg/ml) | Tmax b (h) | AUC0‐t (µg·h/ml) | AUC0‐∞ (µg·h/ml) | |||
| Study 1, part 1 | A | LXB 4.5 g fasted, 240 ml water | 101.8 (21.2) | 0.75 (0.33–1.50) | 235.4 (32.3) | 236.5 (32.3) |
| B | LXB 4.5 g fed, 240 ml water | 77.4 (25.0) | 0.75 (0.33–2.50) | 213.3 (33.9) | 214.8 (34.0) | |
| C | SXB 4.5 g fasted, 240 ml water | 135.7 (14.8) | 0.50 (0.33–1.00) | 263.9 (30.0) | 265.2 (30.1) | |
| D | SXB 4.5 g fed, 240 ml water | 84.3 (31.3) | 0.75 (0.33–2.50) | 228.0 (33.5) | 229.6 (33.5) | |
| Study 1, part 2 | E | LXB 2.25 g fasted, 235.5 ml water | 51.3 (40.1) | 0.75 (0.33–3.00) | 77.7 (49.4) | 81.0 (47.5) |
| Study 2 | F | LXB fasted, 60 ml water | 94.63 (20.5) | 1.000 (0.33–3.00) | 241.5 (38.8) | 243.1 (39.0) |
| G | SXB fasted, 60 ml water | 123.0 (21.5) | 0.520 (0.33–1.52) | 254.7 (36.4) | 256.3 (36.5) | |
| H | LXB fed, 60 ml water | 64.8 (27.4) | 1.000 (0.33–2.50) | 206.4 (43.1) | 208.6 (43.3) | |
| I | SXB fed, 60 ml water | 69.65 (26.4) | 0.875 (0.33–3.00) | 208.2 (41.3) | 209.8 (41.5) | |
| J | SXB fasted, 240 ml water | 130.5 (20.3) | 0.500 (0.33–1.50) | 262.1 (38.5) | 263.6 (38.6) | |
| K | LXB fasted, 240 ml water | 96.34 (20.3) | 0.750 (0.50–2.50) | 238.8 (39.8) | 240.5 (39.9) | |
Abbreviations: AUC0‐t, area under the plasma concentration‐time curve from time 0 to time t of the last quantifiable concentration; AUC0‐∞, area under the plasma concentration‐time curve from time 0 to infinity; CI, confidence interval; Cmax, maximum plasma concentration; LXB, lower‐sodium oxybate; PK, pharmacokinetics; Tmax, time to maximum plasma concentration; SXB, sodium oxybate.
PK completer population (study 1, part 1, n = 30; study 1, part 2, n = 16; study 2, n = 42).
Median (minimum–maximum).
Bioavailability and bioequivalence of LXB and SXB in the fasted state
In study 1, part 1, under fasted conditions with treatments administered in a total volume of 240 ml, Cmax of LXB (test) was lower compared with SXB (reference), and Tmax of LXB was delayed compared with SXB (Cmax, 101.8 vs. 135.7 μg/ml; Tmax, 0.75 vs. 0.5 h; Table 2). AUC was comparable between LXB and SXB (AUC0‐t, 235.4 vs. 263.9 μg∙h/ml; AUC0‐∞, 236.5 vs. 265.2 μg∙h/ml). The ratios of geometric mean oxybate PK parameters Cmax and AUC0‐∞ for LXB and SXB were 74.2% (90% CI: 67.8% to 81.2%) and 88.3% (84.4% to 92.4%), respectively (Table 3). LXB and SXB met bioequivalence criteria under fasted conditions for oxybate plasma AUC but not for oxybate plasma Cmax.
TABLE 3.
Statistical analysis of bioequivalence for oxybate plasma PK parameters following a single dose of LXB or SXB under fasted conditions a
| Treatment | Description | Parameter | Percentage of ratio of geometric means | 90% CI b | |
|---|---|---|---|---|---|
| Study 1 | A vs. C | LXB vs SXB fasted, 240 ml water | Cmax | 74.21 | 67.803, 81.222 |
| AUC0‐t | 88.29 | 84.343, 92.423 | |||
| AUC0‐∞ | 88.31 | 84.389, 92.415 | |||
| Study 2 | F vs. G | LXB vs SXB fasted, 60 ml water | Cmax | 77.05 | 71.89, 82.58 |
| AUC0‐t | 94.24 | 90.42, 98.22 | |||
| AUC0‐∞ | 94.27 | 90.49, 98.20 |
Abbreviations: AUC0‐t, area under the plasma concentration‐time curve from time 0 to time t of the last quantifiable concentration; AUC0‐∞, area under the plasma concentration‐time curve from time 0 to infinity; CI, confidence interval; Cmax, maximum plasma concentration; LXB, lower‐sodium oxybate; PK, pharmacokinetics; SXB, sodium oxybate.
PK completer population (study 1, n = 30; study 2, n = 42).
The bold numbers denote the geometric mean 90% CIs that are not contained within 80% and 125% and, therefore, do not meet criteria for bioequivalence.
In study 2, under fasted conditions with treatments administered in a total volume of 60 ml of water, Cmax of LXB was lower compared with SXB, and Tmax of LXB was delayed compared with SXB (Cmax, 94.6 vs. 123.0 μg/ml; Tmax, 1.0 vs. 0.5 h; Table 2). AUC was comparable between LXB and SXB (AUC0‐t, 241.5 vs. 254.7 μg∙h/ml; AUC0‐∞, 243.1 vs. 256.3 μg∙h/ml). The ratios of geometric mean oxybate PK parameters Cmax and AUC0‐∞ for LXB and SXB were 77.1% (90% CI: 71.9% to 82.6%) and 94.3% (90.5% to 98.2%), respectively (Table 3). LXB and SXB met bioequivalence criteria under fasted conditions for AUC but not for Cmax.
In part 2 of study 1, when LXB was administered at a dose of 2.25 g in a total volume of 240 ml, mean Cmax was approximately half that for a 4.5‐g dose (51.3 vs. 101.8 μg/ml; Table 2). Tmax was the same for both doses (0.75 h). AUC at a dose of 2.25 g was approximately one‐third that for a 4.5‐g dose (AUC0‐t, 77.7 vs. 235.4 μg∙h/ml; AUC0‐∞, 81.0 vs. 236.5 μg∙h/ml).
Effect of food on PK parameters of LXB and SXB
In both studies, plasma oxybate Cmax and AUC were lower under fed versus fasted conditions for LXB and SXB (Table 2). However, the PK parameters were similar between LXB and SXB under the fed state. In study 1, in the fed state, Cmax values were 77.4 and 84.3 μg/ml for LXB and SXB, respectively, and AUC0‐∞ values were 214.8 and 229.6 μg·h/ml, respectively; median Tmax values were 0.75 h for both treatments. In study 2, in the fed state, Cmax values were 64.8 and 69.7 μg/ml for LXB and SXB, respectively, and AUC0‐∞ values were 208.6 and 209.8 μg·h/ml, respectively; median Tmax values were 1.0 h for LXB and 0.875 for SXB.
Fed conditions reduced Cmax by ~ 25% and ~ 41% for LXB and SXB, respectively, in study 1, and by ~ 33% and ~ 44% for LXB and SXB, respectively, in study 2 (Table S2). Fed conditions reduced AUC by ~ 10% and ~ 16% for LXB and SXB, respectively, in study 1, and by ~ 16% and ~ 19% for LXB and SXB, respectively, in study 2.
The post hoc analysis assessing whether the impact of food on oxybate PK was similar for LXB and SXB indicated Cmax was reduced less for LXB compared with SXB in study 1 (nominal p = 0.0034) and study 2 (nominal p = 0.0016), whereas reduction in AUC was similar for LXB and SXB (Table S3).
Effect of water volume on PK parameters of SXB and LXB
In study 2, there appeared to be no effect of water volume on the PK of oxybate for either LXB or SXB (Table S4). Oxybate exposure as measured by Cmax, AUC0‐t, and AUC0‐∞ demonstrated bioequivalence between the two dosing conditions: 60 and 240 ml water.
Safety
The overall incidences of TEAEs, and TEAEs with an incidence greater than or equal to 10% in any group, are presented in Table 4 (study 1) and Table 5 (study 2). Overall incidence of TEAEs was comparable across treatments in study 1 (91.7% to 100%) and study 2 (87.0% to 97.9%). Somnolence was the most frequently reported TEAE in both studies (71.4% to 88.9% across treatments in study 1 and 71.7% to 87.2% across treatments in study 2), followed by dizziness (36.1% to 58.3% across treatments in study 1 and 39.1% to 51.1% across treatments in study 2), nausea (11.1% to 47.2% across treatments in study 1 and 6.5% to 44.7% across treatments in study 2), and headache (8.3% to 22.9% across treatments in study 1 and 12.8% to 21.3% across treatments in study 2).
TABLE 4.
TEAEs in study 1
| Part 1 a | Part 2 a | ||||
|---|---|---|---|---|---|
| TEAE, n (%) |
Treatment A LXB Fasted (n = 35) |
Treatment B LXB Fed (n = 36) |
Treatment C SXB Fasted (n = 36) |
Treatment D SXB Fed (n = 36) |
Treatment E LXB Fasted (n = 23) |
| Any TEAE | 34 (97.1) | 33 (91.7) | 36 (100) | 33 (91.7) | 36 (100.0) |
| TEAEs in ≥10% participants in any group | |||||
| Somnolence | 25 (71.4) | 27 (75.0) | 32 (88.9) | 27 (75.0) | 11 (47.8) |
| Dizziness | 17 (48.6) | 13 (36.1) | 21 (58.3) | 15 (41.7) | 9 (39.1) |
| Nausea | 11 (31.4) | 4 (11.1) | 17 (47.2) | 4 (11.1) | 2 (8.7) |
| Headache | 8 (22.9) | 3 (8.3) | 5 (13.9) | 6 (16.7) | 3 (13.0) |
| Fatigue | 6 (17.1) | 4 (11.1) | 4 (11.1) | 3 (8.3) | 0 |
| Euphoric mood | 5 (14.3) | 2 (5.6) | 4 (11.1) | 1 (2.8) | 0 |
| Vomiting | 1 (2.9) | 1 (2.8) | 5 (13.9) | 0 | 0 |
| Feeling hot | 4 (11.4) | 4 (11.1) | 4 (11.1) | 3 (8.3) | 1 (4.3) |
| Abdominal pain | 4 (11.4) | 1 (2.8) | 3 (8.3) | 0 | 0 |
| Hypotonia | 4 (11.4) | 2 (5.6) | 2 (5.6) | 1 (2.8) | 1 (4.3) |
| Hyperhidrosis | 2 (5.7) | 1 (2.8) | 4 (11.1) | 0 | 0 |
| Back pain | 0 | 2 (5.6) | 4 (11.1) | 0 | 1 (4.3) |
Abbreviations: LXB, lower‐sodium oxybate; SXB, sodium oxybate; TEAE, treatment‐emergent adverse event.
A: LXB 4.5 g fasted, 240 ml water; B: LXB 4.5 g fed, 240 ml water; C: SXB 4.5 g fasted, 240 ml water; D: SXB 4.5 g fed, 240 ml water; E: LXB 2.25 g fasted, 235.5 ml water.
TABLE 5.
TEAEs in study 2 a
| TEAE, number (%) of participants |
Treatment F LXB (n = 47) |
Treatment G SXB (n = 47) |
Treatment H LXB (n = 46) |
Treatment I SXB (n = 46) |
Treatment J SXB (n = 47) |
Treatment K LXB (n = 47) |
|---|---|---|---|---|---|---|
| 60 ml water, fasted | 60 ml water, fed | 240 ml water, fasted | ||||
| Any TEAE | 44 (93.6) | 46 (97.9) | 40 (87.0) | 41 (89.1) | 45 (95.7) | 45 (95.7) |
| TEAEs in ≥10% participants in any group | ||||||
| Somnolence | 39 (83.0) | 41 (87.2) | 34 (73.9) | 33 (71.7) | 38 (80.9) | 37 (78.7) |
| Dizziness | 22 (46.8) | 23 (48.9) | 21 (45.7) | 18 (39.1) | 24 (51.1) | 19 (40.4) |
| Nausea | 13 (27.7) | 21 (44.7) | 5 (10.9) | 3 (6.5) | 16 (34.0) | 16 (34.0) |
| Headache | 9 (19.1) | 10 (21.3) | 7 (15.2) | 10 (21.7) | 6 (12.8) | 10 (21.3) |
| Fatigue | 5 (10.6) | 5 (10.6) | 4 (8.7) | 3 (6.5) | 1 (2.1) | 2 (4.3) |
| Paresthesia | 5 (10.6) | 11 (23.4) | 6 (13.0) | 5 (10.9) | 5 (10.6) | 3 (6.4) |
| Vertigo | 4 (8.5) | 8 (17.0) | 2 (4.3) | 3 (6.5) | 6 (12.8) | 5 (10.6) |
| Euphoric mood | 6 (12.8) | 2 (4.3) | 3 (6.5) | 2 (4.3) | 1 (2.1) | 1 (2.1) |
| Abdominal pain | 3 (6.4) | 5 (10.6) | 2 (4.3) | 0 | 1 (2.1) | 2 (4.3) |
| Vision blurred | 3 (6.4) | 5 (10.6) | 0 | 0 | 3 (6.4) | 3 (6.4) |
Abbreviations: LXB, lower‐sodium oxybate; SXB, sodium oxybate; TEAE, treatment‐emergent adverse event.
F: LXB 4.5 g fasted, 60 ml water; G: SXB 4.5 fasted, 60 ml water; H: LXB 4.5 g fed, 60 ml water; I: SXB 4.5 g fed, 60 ml water; J: SXB 4.5 g fasted, 240 ml water; K: LXB 4.5 g fasted, 240 ml water.
Exploratory analyses using pooled data from studies 1 and 2 indicated that a higher Cmax is associated with a higher incidence of nausea and vomiting for both LXB and SXB (Figure 2); no such relationship was found for AUC.
FIGURE 2.
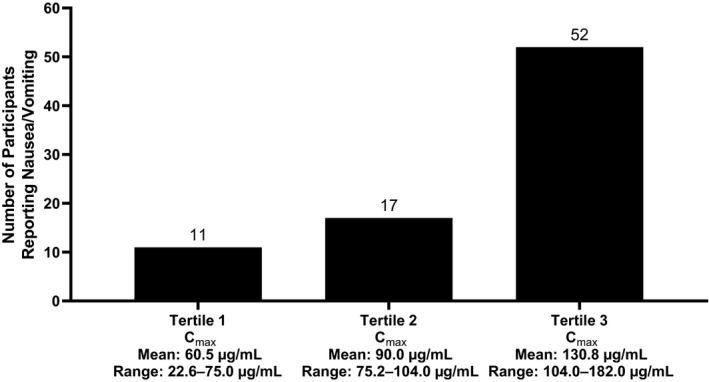
Number of incidences of nausea and vomiting by tertiles of oxybate Cmax in studies 1 and 2. Cmax, maximum plasma concentration
DISCUSSION
LXB is a novel formulation of oxybate with 92% less sodium than SXB at an equivalent dose, which is approved in the United States for the treatment of cataplexy or EDS in patients 7 years of age and older with narcolepsy. 11 Reduction of sodium intake has been shown to reduce risk of hypertension, stroke, and cardiovascular disease in the general population. 16 , 17 The primary goals of the studies described here were to assess the relative bioavailability and bioequivalence of LXB and SXB in the fasted state and evaluate the effect of food on oxybate PK following oral administration of LXB and SXB. In both studies, bioequivalence criteria were met for AUC but not Cmax when comparing LXB and SXB; LXB had a lower Cmax compared with SXB.
The presence of a certain level of sodium in the LXB oral solution appears to be critical to achieve a Cmax similar to that of SXB, as oxybate absorption has been reported to be mediated by both sodium‐ and proton‐dependent monocarboxylate transporters (MCTs), which are expressed throughout the intestine. 18 In vitro experiments with a human breast cancer cell line (MDA‐MB‐231 19 ), which expresses several MCTs, showed that uptake of oxybate at pH 7.5 was significantly reduced in the absence of sodium. 20 Based on these in vitro studies, the lower sodium content of LXB relative to SXB is projected to result in a lower rate of oxybate transport via sodium‐dependent MCTs (SMCTs), and thus the lower Cmax and delayed Tmax with LXB compared with SXB. 21
The effect of dosing water volume on Cmax was also considered after the results of study 1 showed a lower Cmax for LXB than SXB using a standard 240‐ml water volume, per FDA guidance on bioavailability and bioequivalence studies. 14 The lower Cmax and delayed Tmax could be related to slower gastric emptying arising from the different cation composition/osmolarity of LXB compared with SXB. 22 Therefore, study 2 was designed to include two total water volumes: 60 ml (as recommended in the SXB prescribing information) 4 and 240 ml (as in study 1). It was hypothesized that reducing the total water volume from 240 ml to 60 ml might increase the osmolarity of both SXB and LXB so that, if gastric emptying became the rate‐limiting step for oxybate absorption, it would result in a more comparable Cmax. However, there appeared to be no effect of water volume on the relative bioavailability and PK of oxybate for either LXB or SXB; both met bioequivalence criteria comparing exposure between 240‐ml and 60‐ml water dosing conditions.
In the two studies, the incidence of AEs of nausea and vomiting was higher after SXB (fasted) than after LXB (fasted); in addition, in a post hoc analysis of pooled data, lower oxybate Cmax was associated with fewer AEs of nausea and vomiting. Further study is needed to determine whether the lower Cmax of LXB compared with SXB will confer improved tolerability with respect to nausea and vomiting; if demonstrated, this may be an important factor for physicians and their patients, given that nausea is among the most common AEs in patients treated with LXB or SXB in clinical trials. 4 , 11
Oxybate has been shown to slow intestinal transit in mice, thus potentially delaying absorption of food in the gut. 23 A previous clinical study demonstrated a significant reduction in Cmax (59%) and AUC (37%) with administration of SXB immediately after a high‐fat, high‐calorie meal, compared with the fasted state. 4 The studies described here also demonstrated a significant effect of food on PK parameters, with a larger effect on Cmax compared with AUC. Moreover, compared with SXB, LXB had a smaller reduction in Cmax between the fasted and fed states. The hypothesized mechanistic reason for reduced Cmax and AUC after food intake is reduction in gut absorption of oxybate in the presence of food. The oxybate molecule is a butyric acid, a short‐chain fatty acid (SCFA). MCTs represent a major determinant in the absorption of oxybate. 24 SCFAs are transported across the gut membrane via MCTs and SMCTs. 25 The hypothesized mechanism for the food effect is that under fed conditions, dietary‐ingested SCFAs compete with oxybate for transport at MCTs and SMCTs, thereby reducing Cmax and AUC for both SXB and LXB. The hypothesized mechanistic reason for the higher Cmax with SXB is that the higher sodium in SXB allows for faster transport across the gut membrane by recruiting more SMCTs than LXB, which has 92% lower sodium. As the PK results from this study demonstrated a food effect with both SXB and LXB, dosing recommendations have been made to simplify labeling between LXB and SXB. The Xywav prescribing information recommends dosage administration 2 h after eating. 11
Certain limitations of these studies should be noted. First, both explored single 4.5‐g doses without titration in healthy, White, adult volunteers. In clinical practice, treatment is long term, dosing is typically twice nightly (recommended total in adults, 6–9 g/night 4 ), and patients are adults or children/adolescents (≥7 years of age) of any race with narcolepsy and possible comorbidities. Second, administration of study drug occurred in the morning, whereas actual dosing in patients with narcolepsy would occur at night (at bedtime and 2.5–4 h later), which may explain the large number of TEAEs of somnolence reported.
In conclusion, at equivalent oxybate doses, in the fasted state, LXB has a lower Cmax, delayed Tmax, and similar AUC, compared with SXB. The reduction in Cmax in the fed state was more pronounced than the reduction in AUC for both LXB and SXB, but Cmax was reduced less with LXB compared with SXB. There appeared to be no effect of water volume on the PK of oxybate for either LXB or SXB. A lower Cmax for oxybate was associated with lower incidence of nausea and vomiting. The observed differences between LXB and SXB may stem from the reduction of sodium content in LXB.
CONFLICT OF INTEREST
C.C., K.Z., and R.S. are full‐time employees of Jazz Pharmaceuticals who, in the course of this employment, have received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. Jack Jenkins is a non‐employee consultant paid by Jazz Pharmaceuticals.
AUTHOR CONTRIBUTIONS
C.C., J.J., K.Z., and R.S. wrote the manuscript. C.C., K.Z., and R.S. designed the research. C.C., K.Z., and R.S. performed the research. J.J. analyzed the data. C.C. and K.Z. contributed new reagents/analytical tools.
Supporting information
Figure S1
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGEMENTS
The authors would like to thank the study team and participants for their participation in this research. Under the direction of the authors, Karyn Liu, PhD, and Michael J. Theisen, PhD, of Peloton Advantage, LLC, an OPEN Health company, provided medical writing and editorial assistance for this publication, which was funded by Jazz Pharmaceuticals.
Chen C, Jenkins J, Zomorodi K, Skowronski R. Pharmacokinetics, bioavailability, and bioequivalence of lower‐sodium oxybate in healthy participants in two open‐label, randomized, crossover studies. Clin Transl Sci. 2021;14:2278–2287. 10.1111/cts.13087
Funding information
This study was sponsored by Jazz Pharmaceuticals.
DATA AVAILABILITY STATEMENT
All relevant data are provided within the manuscript and supporting files.
REFERENCES
- 1. American Academy of Sleep Medicine . International Classification of Sleep Disorders, 3rd edn. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2. Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15:502‐507. [DOI] [PubMed] [Google Scholar]
- 3. Morgenthaler TI, Kapur VK, Brown TM, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xyrem® (sodium oxybate) oral solution Prescribing Information. Palo Alto, CA: Jazz Pharmaceuticals; 2018. [Google Scholar]
- 5. Plazzi G, Ruoff C, Lecendreux M, et al. Treatment of paediatric narcolepsy with sodium oxybate: a double‐blind, placebo‐controlled, randomised‐withdrawal multicentre study and open‐label investigation. Lancet Child Adolesc Health. 2018;2:483‐494. [DOI] [PubMed] [Google Scholar]
- 6. Chen C, Rosen CL, Ruoff C, et al. Population and noncompartmental pharmacokinetics of sodium oxybate support weight‐based dosing in children and adolescents with narcolepsy with cataplexy. Clin Transl Sci. 2020;13:932‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Why should I limit sodium?. American Heart Association; 2017. https://www.heart.org/‐/media/data‐import/downloadables/pe‐abh‐why‐should‐i‐limit‐sodium‐ucm_300625.pdf. Accessed July 18, 2019. [Google Scholar]
- 8. National Academies of Sciences, Engineering, and Medicine . Dietary Reference Intakes for Sodium and Potassium. Washington, DC: The National Academies Press; 2019. [PubMed] [Google Scholar]
- 9. Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624‐634. [DOI] [PubMed] [Google Scholar]
- 10. Bibbins‐Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xywav™ (calcium, magnesium, potassium, and sodium oxybates) oral solution, CIII Prescribing Information. Palo Alto, CA: Jazz Pharmaceuticals; 2020. [Google Scholar]
- 12. Bogan RK, Thorpy MJ, Dauvilliers Y, et al. Efficacy and safety of calcium, magnesium, potassium, and sodium oxybates (lower‐sodium oxybate [LXB]; JZP‐258) in a placebo‐controlled, double‐blind, randomized withdrawal study in adults with narcolepsy with cataplexy. Sleep. 2020;44:zsaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thorpy MJ. Recently approved and upcoming treatments for narcolepsy. CNS Drugs. 2020;34:9‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guidance for Industry: Food‐Effect Bioavailability and Fed Bioequivalence Studies. US Food and Drug Administration; 2002. https://www.fda.gov/files/drugs/published/Food‐Effect‐Bioavailability‐and‐Fed‐Bioequivalence‐Studies.pdf. Accessed February 17, 2020. [Google Scholar]
- 15. Bioanalytical Method Validation: Guidance for Industry. US Food and Drug Administration; 2018. https://www.fda.gov/media/70858/download. Accessed July 29, 2020. [Google Scholar]
- 16. Gardener H, Rundek T, Wright CB, Elkind MS, Sacco RL. Dietary sodium and risk of stroke in the Northern Manhattan study. Stroke. 2012;43:1200‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow‐up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gill RK, Saksena S, Alrefai WA, et al. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol. 2005;289:C846‐852. [DOI] [PubMed] [Google Scholar]
- 19. Cailleau R, Young R, Olivé M, Reeves WJ Jr. Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974;53:661‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Q, Morris ME. The role of monocarboxylate transporter 2 and 4 in the transport of gamma‐hydroxybutyric acid in mammalian cells. Drug Metab Dispos. 2007;35:1393‐1399. [DOI] [PubMed] [Google Scholar]
- 21. Wang Q, Lin T, Allphin C, van Osdol WW , Bolger MB, Chen C. Physiologically based pharmacokinetic modeling of oxybate: the role of counter‐ions in gastrointestinal absorption of oxybate [poster]. Presented at: Annual AAPS PharmSci 360; October 26‐November 5, 2020.
- 22. Hunt JN, Pathak JD. The osmotic effects of some simple molecules and ions on gastric emptying. J Physiol. 1960;154:254‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carai MA, Agabio R, Lobina C, et al. GABA(B)‐receptor mediation of the inhibitory effect of gamma‐hydroxybutyric acid on intestinal motility in mice. Life Sci. 2002;70:3059‐3067. [DOI] [PubMed] [Google Scholar]
- 24. Felmlee MA, Morse BL, Morris ME. γ‐Hydroxybutyric acid: pharmacokinetics, pharmacodynamics, and toxicology. AAPS J. 2021;23:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaji I, Iwanaga T, Watanabe M, et al. SCFA transport in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2015;308:G188‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
All relevant data are provided within the manuscript and supporting files.


