Abstract
The aim of this study was to evaluate the impact of renal impairment on the pharmacokinetics (PKs), safety, and tolerability of daridorexant, a dual orexin receptor antagonist intended for the treatment of insomnia. A single‐center, open‐label study evaluated the PKs of daridorexant in patients with severe renal function impairment (SRFI; determined by creatinine clearance using the Cockcroft‐Gault equation; N = 8) not on dialysis, and in matched control subjects (based on sex, age, and body weight; N = 7). A single oral dose of daridorexant 25 mg was orally administered in the morning. Blood samples were collected up to 72 h postdose for PK assessments of daridorexant. In patients with SRFI, maximum plasma concentrations (Cmax; geometric mean ratio [GMR] and 90% confidence interval [CI]: 0.94 [0.60–1.46]), time to reach Cmax (T max; median difference [90% CI] of −0.25 h [−0.75 to 0.25]), and half‐life (GMR [90% CI] of 0.99 [0.66–1.48]), were virtually unchanged. Exposure (area under the plasma concentration‐time profile) to daridorexant was slightly higher in patients with SRFI than in control subjects with the GMR (90% CI) being 1.16 (0.63–2.12). No safety issue of concern was detected as all adverse events were transient and of mild or moderate intensity, and no treatment‐related effects on vital signs, clinical laboratory, or electrocardiogram variables were observed following daridorexant administration in patients with SRFI and control subjects. Based on these observations, PK alterations of daridorexant due to renal function impairment are not considered of clinical relevance and no dose adjustment is necessary in these patients.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Daridorexant, a potent and selective dual orexin receptor antagonist being developed to treat insomnia, has been shown to have significant effects on sleep onset and sleep maintenance, and improves the impaired daytime functioning of patients with insomnia. Daridorexant has recently been submitted for marketing authorization (in the United States and the European Union).
WHAT QUESTION DID THIS STUDY ADDRESS?
This study compared the pharmacokinetics (PKs), safety, and tolerability of daridorexant between patients with severe renal function impairment (SRFI) and control subjects.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Similar PK profiles and no tolerability issues were observed in patients with SRFI and control subjects following single‐dose administration of 25 mg daridorexant.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
In clinical practice, the same dose of daridorexant can be administered to patients with insomnia with or without any degree of renal function impairment.
INTRODUCTION
Throughout the brain, the orexin system, consisting of two hypothalamic neuropeptides, orexin A (OxA) and orexin B (OxB), and two G protein‐coupled receptors, orexin‐1 (OX1) and orexin‐2 (OX2), is widely expressed. 1 , 2 , 3 Transiently blocking the signaling of the orexin pathways, which play an important role in the regulation of sleep and wakefulness, has been shown to be a valid approach for the development of drugs intended to treat sleeping disorders. 4 , 5 , 6 Daridorexant (ACT‐541468) blocks the actions of the orexin neuropeptides at both OX1 and OX2 receptors (i.e., it is a dual orexin receptor antagonist), which has thus shown to promote sleep and is currently being developed for the treatment of insomnia. 5 , 7 , 8 , 9 , 10
In the anticipated clinical dose range of 25–50 mg, in control individuals, daridorexant’s pharmacokinetic (PK) profile is characterized by quick absorption, with time to maximum plasma concentration (T max) of 1–2 h, a terminal half‐life (t ½) of ~ 8 h, a clearance of 5.0 L/h, and volume of distribution at steady‐state of 31 L. 8 , 11 , 12 The absolute bioavailability is 62%. The PK parameters of daridorexant following multiple‐dose administration are similar to those observed following single‐dose administration, whereby no relevant accumulation has been observed. 13 In a previous absorption, distribution, metabolism, and elimination (ADME) study, 14C‐labeled daridorexant was shown to be extensively metabolized with only traces of parent drug excreted unchanged. 8 , 14 Most of the administered radioactive dose was recovered in feces (50–69%), followed by urine (24–35%). Most observed metabolic reactions of daridorexant are mediated by cytochrome P450 (CYP) 3A4 oxidative transformations, whereas the three major metabolites of daridorexant, namely M1, M3, and M10, identified in human plasma have considerably lower affinity to OX1 and OX2 receptors than the parent drug and, thus, do not appear to contribute to a significant extent to the pharmacological effect. 8 , 12 , 14
For drugs that are predominantly eliminated via nonrenal routes and likely to be used in patients with impaired renal function, as is the case for daridorexant, health authorities recommend a reduced study design to assess the PKs in patients with renal impairment to provide appropriate dosing recommendations as, especially in severe renal function impairment (SRFI), can adversely affect other pathways of metabolism (i.e., may also affect the disposition of hepatically cleared drugs). 15 , 16
Therefore, the intent of this conducted study was to represent a “worst‐case scenario” whereby the greatest impact altered renal function might have on the PKs, safety, and tolerability of daridorexant was evaluated.
METHODS
Study design
This was a single‐center, open‐label, single‐dose, phase I study (NCT04024332). The study was conducted at APEX GmbH (Munich, Germany). The protocol was approved by the German National Health Authority and approved by the local ethics committee (Ethikkommission der Technischen Universität München, Germany). The study adhered to the Declaration of Helsinki and was conducted according to good clinical practice. Prior to any study procedure, written informed consent was obtained from each participant.
Study population
Male and female patients with SRFI (defined as creatinine clearance [CrCL] <30 ml/min, calculated using the Cockcroft‐Gault formula), and control subjects (defined through physical examination, 12‐lead electrocardiogram [ECG], clinical laboratory data, and age‐appropriate normal renal function: CrCL ≥60 ml/min for subjects aged 61–85 years, ≥70 ml/min for subjects aged 51–60 years, and ≥80 ml/min for subjects ≤50 years) were enrolled in the study. 17 , 18 Patients with SRFI were excluded if they required dialysis and were required to be between 18 and 85 years of age, with a body mass index (BMI) of 18–35 kg/m2 (body weight ≥50 kg). Control subjects were individually matched to renally impaired subjects regarding sex, age (±10 years), and body weight (±15%) at screening. Women of childbearing potential had to have a negative pregnancy test at screening on day −1, and had to use a highly effective method of contraception. Pregnant or breastfeeding women were ineligible, as were subjects with a history of renal and/or liver transplant. Subjects with a history or clinical evidence of any disease or the existence of any surgical or medical condition with a potential to interfere with the ADME of the study drug (except if related to renal impairment) were not enrolled in the study. Patients with renal impairment could continue taking their regularly prescribed medications unless they might reasonably influence results of the trial (e.g., CYP3A4 inhibitors and inducers). All subjects were not permitted to take any creatinine supplements from screening until the end‐of‐study visit (EOS).
Study conduct
Following screening assessments, subjects were admitted to the study center on day −1 and were administered a single dose of daridorexant 25 mg in the morning on day 1 in the fasted condition, which was followed by a 24‐h observation period. Selection and enrollment of individually matched control subjects (group A) was performed after the corresponding subject with SRFI (in group B) had completed the EOS. The EOS took place 72 h after dosing for patients with renal impairment (48 h postdose for control subjects). A dose of 25 mg daridorexant was selected as it represented the mid‐range dose investigated in the phase III studies (i.e., 10, 25, and 50 mg were evaluated).
PK assessments
Blood samples for the measurement of daridorexant were collected into potassium EDTA‐containing tubes from an indwelling catheter or by direct venipuncture predose and at scheduled intervals up to 48 h (further PK samples were collected at 60 and 72 h postdose to ensure appropriate characterization of daridorexant PKs in patients with SRFI). For determination of total and free (unbound) daridorexant concentration in plasma, samples were taken at 1 and 3 h postdose (i.e., range covering the expected T max).
Plasma concentrations of daridorexant were measured using a validated liquid chromatography coupled to tandem mass spectrometry (LC‐MS/MS) assay. Details thereof have previously been published. 8 The limit of quantification (LOQ) was 0.5 ng/ml with the method covering a range up to 2000 ng/ml. In the present study, the interbatch precision was less than or equal to 7.8%, whereas the accuracy was in the range from 0.1% to 6.3%.
The unbound fraction (C u/C) of daridorexant in plasma was determined using equilibrium dialysis followed by analysis of both compartments. 19 The slightly adapted validated LC‐MS/MS method was linear in the concentration range of 0.1–400 ng/ml with an LOQ of 0.1 ng/ml. In the present study, the performance of the method was characterized by interbatch precision less than or equal to 3.3% and interbatch accuracy in the range from −1.6% to 4.7%.
PK parameters of daridorexant were obtained by noncompartmental analysis using Phoenix WinNonlin (version 8.0; Certara, Princeton, NJ, USA). The measured individual plasma concentration was used to directly obtain maximum plasma concentration (Cmax) and T max. Area under the plasma concentration‐time curve (AUC) from time zero to infinity (AUC0−inf) was calculated by combining AUC from zero to time of the last measured concentration above the LOQ (AUC0−t) according to the linear trapezoidal rule and AUC representing an extrapolated value obtained by C t/λ z (AUCextra), where C t was the last plasma concentration measured above the LOQ and λ z represented the terminal elimination rate constant determined by log‐linear regression analysis of the measured plasma concentrations of the terminal elimination phase. The t ½ was calculated as follows: t ½ = ln(2)/λz . Concentrations that were below the LOQ were entered as zero and included as such in the calculation of means. For plasma protein binding, C u/C was expressed as a percentage.
The PK parameters Cmax, AUC, apparent plasma clearance (CL/F), apparent volume of distribution (V z/F), and t ½ were summarized using geometric means (GMs) and their two‐sided 95% confidence interval (CI). Median and range values were used for T max. PK parameters were compared between both groups based on geometric mean ratios (GMRs) of group B/group A and their 90% CIs. Differences between treatments for T max were explored using the nonparametric Wilcoxon signed rank test and Hodges‐Lehmann estimates of the median of differences and their 90% CIs. Due to the exploratory nature of the study, the sample size of eight subjects per group was based on empirical considerations, which was also supported by a precision estimate approach based on the variables of AUC and Cmax from a former study. 12
Safety and tolerability assessments
The safety and tolerability of the study drug were evaluated throughout the study on the basis of reported adverse events (AEs), and the results of physical examination, and the assessment of body weight, vital signs (blood pressure and pulse rate), 12‐lead ECGs, and clinical laboratory tests (hematology, clinical chemistry, coagulation, and urinalysis) and were analyzed descriptively.
RESULTS
Disposition and demographics
Due to the coronavirus disease 2019 (COVID‐19) outbreak, and in line with the various measures and recommendations put in place to limit the spread of the virus and dedicate healthcare resources to managing patients with COVID‐19, only seven control subjects were recruited into the study. All 15 enrolled subjects (8 men and 7 women)—eight patients with SRFI and seven matched control subjects—completed the study as per protocol. Demographic variables were overall similar between control subjects and patients with SRFI based on mean (SD) age (63.0 years [10.0] vs. 63.5 years [13.3]) and BMI (24.0 kg/m2 [2.1] vs. 24.7 kg/m2 [3.0]). All patients with SRFI reported intake of concomitant medications to treat their renal condition and associated diseases, whereas one control subject reported intake of concomitant medication (levothyroxine).
Pharmacokinetics
The plasma concentration‐time profiles of daridorexant were virtually superimposable in control subjects and in patients with SRFI (Figure 1). After single‐dose administration of 25 mg daridorexant, plasma concentrations in control subjects (group A) were characterized by a GM (95% CI) Cmax of 794 ng/ml (445–1417), a median T max of 0.75 h (range 0.50–3.00), and an AUC0‐inf of 6223 ng·h/ml (2780–13,931; Table 1). A larger than anticipated variability in AUC0‐inf was observed in group A and was caused by one subject with an AUC0‐inf value of 20,613 ng·h/ml. In addition, t ½ was 11.4 h (7.6–17.1), CL/F was 4.01 L/h (1.79–8.99), and a Vz /F of 65.9 L (43.8–99.1) was observed. In patients with SRFI (group B) an almost unchanged Cmax (GMR [90% CI]: 0.94 [0.60–1.46]), T max (median difference [90% CI]: −0.25 h [−0.75 to 0.25]), and t ½ (0.99 [0.66–1.48]) was measured. An increase in AUC0‐inf by 1.16‐fold (0.63–2.12) was observed in group B compared to group A, whereas the corresponding CL/F and Vz /F decreased by 13% (0.47–1.59) and 15% (0.64–1.15), respectively.
FIGURE 1.
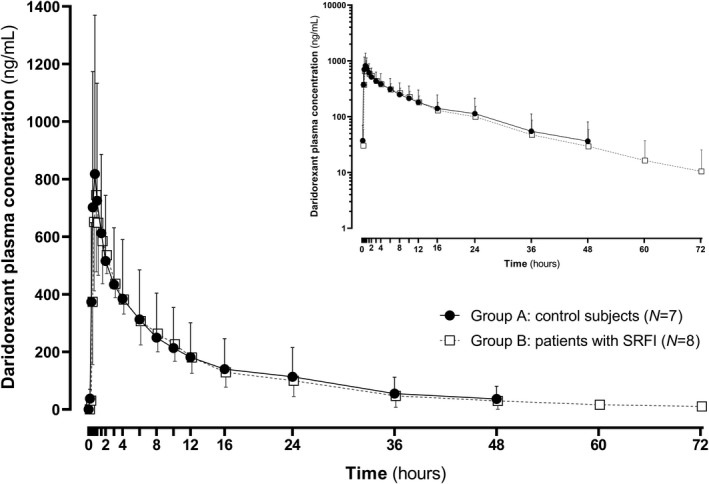
Plasma concentration‐time profile of daridorexant in control subjects (group A) and in patients with severe renal function impairment (SRFI) on linear scale (on semi‐logarithmic scale in inset). The concentration data were obtained after administration of a single oral dose of 25 mg and are presented as mean ± SD
TABLE 1.
Summary of pharmacokinetic variables of daridorexant administered as single oral dose of 25 mg to control subjects (N = 7) and patients with SRFI (N = 8)
|
Control subjects (Group A, N = 7) a |
Patients with SRFI (Group B, N = 8) a |
Ratio of geometric means (90% CI), B/A b | |
|---|---|---|---|
| Cmax, ng/ml | 794 (445–1417) | 744 (573–965) | 0.94 (0.60–1.46) |
| T max, h | 0.75 (0.50–3.00) | 0.75 (0.50–1.50) | −0.25 (−0.75–0.25) |
| AUC0‐t, ng·h/ml | 5783 (2741–12,199) | 7000 (5136–9541) | 1.21 (0.69–2.12) |
| AUC0‐inf, ng·h/ml | 6223 (2780–13,931) | 7192 (5138–10,068) | 1.16 (0.63–2.12) |
| t ½, h | 11.4 (7.57–17.1) | 11.2 (7.74–16.3) | 0.99 (0.66–1.48) |
| CL/F, L/h | 4.01 (1.79–8.99) | 3.48 (2.48–4.87) | 0.87 (0.47–1.59) |
| Vz/F, L | 65.9 (43.8–99.1) | 56.2 (49.1–64.3) | 0.85 (0.64–1.15) |
Abbreviations: AUC0‐inf, area under the plasma concentration‐time curve from zero to infinity; AUC0‐t, area under the plasma concentration‐time curve from zero to time t of the last measured concentration above the limit of quantification; CI, confidence interval; CL/F, apparent total plasma clearance; Cmax, maximum plasma concentration; SRFI, severe renal function impairment; t ½, terminal half‐life; T max, time to reach maximum plasma concentration; V z/F, apparent volume of distribution.
Data are presented as geometric mean (95% CI) except for T max: median (range).
Data are presented as geometric mean ratios (B/A; 90% CI) except for T max: median difference (B‐A; 90% CI).
No difference in daridorexant plasma protein binding was measured between healthy subjects and patients with SRFI with Cu /C ranging from 0.20% to 0.24%.
Safety and tolerability
The most common AE was fatigue, which was reported in three patients with SRFI (3/8 [37.5%]) and two control subjects (2/7 [28.6%]), all mild in intensity. Further AEs of somnolence (moderate intensity) and dry mouth (mild intensity) were reported in control subjects on one occasion each (1/7 [14.3%]). All AEs were considered by the investigator to be related to the pharmacological activity of the study treatment and resolved without sequelae at the EOS. There were no deaths, serious AEs, or study discontinuations. Comparison of baseline values of hematology, clinical chemistry, coagulation, urinalysis, ECG, and vital sign assessments with those following daridorexant administration showed no treatment effect on these variables.
DISCUSSION
The objectives of this study were to evaluate the PKs, tolerability, and safety of daridorexant in patients with severe renal impairment in comparison to control subjects to be able to judge whether dose adjustments might be necessary in patients with renal impairment. Although the final individually matching control subject was not recruited, the study is, nonetheless, from a statistical view considered conclusive and valid, as the number of subjects enrolled in both groups was sufficient to ensure precise estimation of the relevant PK parameters of daridorexant. 16
PK results in control subjects in this study were within the range of variability observed in other studies, in which a single oral dose of 25 mg daridorexant was administered to a similarly aged population. 11 , 12 , 20 An apparent explanation for the outlier in group A could not be determined as nothing out of the ordinary in terms of demographic characteristics, medical history, and clinical laboratory variables was evident, whereas there was no concomitant intake of other drugs. The inherent variability of expression and function of CYP3A4, both intra‐ and interindividually, is considered a possible explanation. 21 , 22
In patients with SRFI, Cmax and t ½ were virtually identical compared with control subjects, whereas median T max was 0.75 h in both groups. A slightly lower CL/F (by 13%) and Vz /F (by 15%) in patients with SRFI was evident, and AUC0‐inf was increased 1.16‐fold compared to control subjects. Based on the results of the ADME study, which showed excretion of daridorexant and its major metabolites mainly via the liver, it was not unexpected that the effects of renal impairment on exposure to daridorexant were limited. 8 , 14
Renal impairment has been shown to impact the extent of plasma protein binding of a multitude of different drugs. 15 , 23 In accordance with previous in vitro and clinical studies, daridorexant was confirmed to be highly bound to plasma proteins (>99%). Herein, no effect of SRFI on concentrations of unbound daridorexant could be determined.
In the present study, the safety profile of daridorexant was similar to previous observations. 5 , 8 , 11 , 12 , 13 , 20 Administration of daridorexant was well tolerated in all individuals and no safety concern related to the administration of daridorexant was raised.
In conclusion, although limited by the small sample size and by the fact that the enrolled individuals were not patients with sleep disorders, these results show that daridorexant can be used to treat patients suffering from insomnia independently of their renal function without the need for dose adjustment. Based on the observed dose‐proportional increase of Cmax and AUC in the anticipated clinical dose range of 25–50 mg, the conclusions regarding dosing recommendations from this renal PK study conducted with 25 mg daridorexant are also applicable to the administration of daridorexant in the specified dose range. 8 Furthermore, dialysis is not expected to influence the PKs of daridorexant in view of the drug’s high plasma protein binding.
CONFLICT OF INTEREST
B.B., C.M., and J.D. were full time employees of Idorsia Pharmaceuticals Ltd. at the time of study conduct. C.M. and J.D. own stocks (options) of Idorsia Pharmaceuticals Ltd. G.K. was employed by APEX GmbH at the time of study conduct. APEX GmbH received financial compensation for the clinical conduct. There are no other relationships, competing interests, or activities that could appear to have influenced this work.
AUTHOR CONTRIBUTIONS
B.B., C.M., and J.D. wrote the manuscript. C.M., G.K., and J.D. designed the research. G.K. performed the research. B.B., C.M., and J.D. analyzed the data.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study prior to any study‐mandated procedure.
ACKNOWLEDGEMENTS
The authors thank the study team at APEX GmbH with special thanks to Karin Schmid, Claudia Lüders, Stephanie Pucci Pegler, Barbara Wenzel, Veronica Rey Berutti, Susanne Globig, Giancarlo Sabbatini, and Stephane Delahaye (Department of Preclinical Pharmacokinetics and Metabolism, Idorsia Pharmaceuticals Ltd., Allschwil, Switzerland) and Mark Enzler (Swiss BioQuant AG, Reinach, Switzerland) for the bioanalytical conduct. Last but not least, the authors thank the clinical research team (i.e., Alexandre Mathis, István Kerekes, Antonella Santilli, Roberta Renai, Anna Kaufmann, Vincent Lemoine, and Pascale Gasser; Department of Clinical Pharmacology, Idorsia Pharmaceuticals Ltd., Allschwil, Switzerland).
Berger B, Muehlan C, Klein G, Dingemanse J. Pharmacokinetics of daridorexant, a dual orexin receptor antagonist, are not affected by renal impairment. Clin Transl Sci. 2021;14:2132–2138. 10.1111/cts.13079
Funding information
The study was sponsored by Idorsia Pharmaceuticals Ltd., Allschwil, Switzerland.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
REFERENCES
- 1. de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus‐specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322‐327. 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171‐181. [DOI] [PubMed] [Google Scholar]
- 3. Inutsuka A, Yamanaka A. The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front Endocrinol (Lausanne). 2013;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoever P, Dorffner G, Beneš H, et al. Orexin receptor antagonism, a new sleep‐enabling paradigm: a proof‐of‐concept clinical trial. Clin Pharmacol Ther. 2012;91:975‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muehlan C, Vaillant C, Zenklusen I, Kraehenbuehl S, Dingemanse J. Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders. Expert Opin Drug Metab Toxicol. 2020;16:1063‐1078. 10.1080/17425255.2020.1817380. [DOI] [PubMed] [Google Scholar]
- 6. Brisbare‐Roch C, Dingemanse J, Koberstein R, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150‐155. [DOI] [PubMed] [Google Scholar]
- 7. Treiber A, de Kanter R, Roch C, et al. The use of physiology‐based pharmacokinetic and pharmacodynamic modeling in the discovery of the dual orexin receptor antagonist ACT‐541468. J Pharmacol Exp Ther. 2017;362:489‐503. 10.1124/jpet.117.241596. [DOI] [PubMed] [Google Scholar]
- 8. Muehlan C, Heuberger J, Juif P‐E, et al. Accelerated development of the dual orexin receptor antagonist ACT‐541468: integration of a microtracer in a first‐in‐human study. Clin Pharmacol Ther. 2018;104:1022‐1029. [DOI] [PubMed] [Google Scholar]
- 9. Zammit G, Dauvilliers Y, Pain S, et al. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. 2020;94:e2222‐e2232. [DOI] [PubMed] [Google Scholar]
- 10. Dauvilliers Y, Zammit G, Fietze I, et al. Daridorexant, a new dual orexin receptor antagonist to treat insomnia disorder. Ann Neurol. 2020;87:347‐356. [DOI] [PubMed] [Google Scholar]
- 11. Muehlan C, Zuiker R, Peeters P, Rowles R, Dingemanse J. Pharmacokinetics and pharmacodynamics of the dual orexin receptor antagonist daridorexant in Japanese and Caucasian subjects. J Clin Psychopharmacol. 2020;40:157‐166. [DOI] [PubMed] [Google Scholar]
- 12. Boof ML, Alatrach A, Ufer M, Dingemanse J. Interaction potential of the dual orexin receptor antagonist ACT‐541468 with CYP3A4 and food: results from two interaction studies. Eur J Clin Pharmacol. 2019;75:195‐205. [DOI] [PubMed] [Google Scholar]
- 13. Muehlan C, Brooks S, Zuiker R, van Gerven J , Dingemanse J. Multiple‐dose clinical pharmacology of ACT‐541468, a novel dual orexin receptor antagonist, following repeated‐dose morning and evening administration. Eur Neuropsychopharmacol. 2019;29:847‐857. [DOI] [PubMed] [Google Scholar]
- 14. Muehlan C, Fischer H, Zimmer D, et al. Metabolism of the dual orexin receptor antagonist ACT‐541468, based on microtracer/accelerator mass spectrometry. Curr Drug Metab. 2019;20:254‐265. 10.2174/1389200220666190206141814. [DOI] [PubMed] [Google Scholar]
- 15. Yuan R, Venitz J. Effect of chronic renal failure on the disposition of highly hepatically metabolized drugs. Int J Clin Pharmacol Ther. 2000;38:245‐253. 10.5414/cpp38245. [DOI] [PubMed] [Google Scholar]
- 16. FDA) . Draft Guidance for Industry: Pharmacokinetics in patients with impaired renal function – Study design, data analysis, and impact on dosing and labeling, US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). September 2020. Available from https://www.fda.gov/media/78573/download
- 17. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31‐41. 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 18. Gault MH, Longerich LL, Harnett JD, Wesolowski C. Predicting glomerular function from adjusted serum creatinine. Nephron. 1992;62:249‐256. 10.1159/000187054. [DOI] [PubMed] [Google Scholar]
- 19. Kaufmann P, Cruz HG, Krause A, et al. Pharmacokinetics of the novel oral prostacyclin receptor agonist selexipag in subjects with hepatic or renal impairment. Br J Clin Pharmacol. 2016;82:369‐379. 10.1111/bcp.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muehlan C, Boehler M, Brooks S, et al. Clinical pharmacology of the dual orexin receptor antagonist ACT‐541468 in elderly subjects: Exploration of pharmacokinetics, pharmacodynamics and tolerability following single‐dose morning and repeated‐dose evening administration. J Psychopharmacol. 2020;34:326‐335. [DOI] [PubMed] [Google Scholar]
- 21. Westlind A, Löfberg L, Tindberg N, Andersson TB, Ingelman‐Sundberg M. Interindividual differences in hepatic expression of CYP3A4: relationship to genetic polymorphism in the 5′‐upstream regulatory region. Biochem Biophys Res Commun. 1999;259:201‐205. [DOI] [PubMed] [Google Scholar]
- 22. Dorne JL, Walton K, Renwick AG. Human variability in CYP3A4 metabolism and CYP3A4‐related uncertainty factors for risk assessment. Food Chem Toxicol. 2003;41:201‐224. 10.1016/s0278-6915(02)00209-0. [DOI] [PubMed] [Google Scholar]
- 23. Vanholder R, Van Landschoot N, De Smet R, Schoots A, Ringoir S. Drug protein binding in chronic renal failure: evaluation of nine drugs. Kidney Int. 1988;33:996‐1004. 10.1038/ki.1988.99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


