Abstract
Pharmacogenomics (PGx)‐based personalized medicine (PM) is increasingly utilized to guide treatment decisions for many drug‐disease combinations. Notably, London Health Sciences Centre (LHSC) has pioneered a PGx program that has become a staple for London‐based specialists. Although implementational studies have been conducted in other jurisdictions, the Canadian healthcare system is understudied. Herein, the multistakeholder perspectives on implementational drivers and barriers are elucidated. Using a mixed‐method qualitative model, key stakeholders, and patients from LHSC’s PGx‐based PM clinic were interviewed and surveyed, respectively. Interview transcripts were thematically analyzed in a stepwise process of customer profiling, value mapping, and business model canvasing. Value for LHSC located specialist users of PGx was driven by the quick turnaround time, independence of the PGx clinic, and the quality of information. Engagement of external specialists was only limited by access and awareness, whereas other healthcare nonusers were limited by education and applicability. The major determinant of successful adoption at novel sites were institutional champions. Patients valued and approved of the service, expressed a general willingness to pay, but often traveled far to receive genotyping. This paper discusses the critical pillars of education, awareness, advocacy, and efficiency required to address implementation barriers to healthcare service innovation in Canada. Further adoption of PGx practices into Canadian hospitals is an important factor for advancing system‐level changes in care delivery, patient experiences, and outcomes. The findings in this paper can help inform efforts to advance clinical PGx practices, but also the potential adoption and implementation of other innovative healthcare service solutions.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Evidence development for pharmacogenomic (PGx) biomarkers continues to grow, however, clinical implementation has lagged behind. Key stakeholder perspectives, barriers, and drivers of clinical implementation of PGx have been studied in the United States and Europe, and through siloed approached in Canada.
WHAT QUESTION DID THIS STUDY ADDRESS?
What are the clinical implementational barriers and drivers recognized by multiple key stakeholders for PGx services in a real‐world Canadian healthcare setting?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study on multiple stakeholders involved in PGx services, elucidates the different perspectives between major specialist users and major specialist nonusers of PGx services. As well, elicits the perspectives of other nonuser healthcare professionals and patients. In doing so, an appropriate framework for successful clinical implementation of PGx is understood.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Clearly understanding the core values of specialists whom PGx is largely applicable will confer greater adoption of the services. Moreover, understanding the perspectives of other key stakeholders will streamline the implementational process for future prospective sites, build primary care awareness, and continue to meet the needs of patients. Together, generating evidence for changes in PGx funding and governance in Canada.
INTRODUCTION
Pharmacogenomics (PGx), a component of personalized medicine (PM), is the study of how genetic factors can affect drug responses. 1 Used in concert, patient and disease‐specific biomarkers can inform a more precise stratification of patients into different response/risk groups, enabling more appropriate drug prescription and more precise dose selection. 2 , 3 PGx biomarkers can broadly be classified as prognostic, predictive, or both. 4 Prognostic biomarkers provide insight into the course of a disease through the evaluation of the disease‐specific genome. 4 These biomarkers, commonly seen in a cancer setting, determine the presence of therapeutically targetable driver mutations. 5 Alternatively, predictive biomarkers are determined by analyzing the patient‐specific genome and help discern whether a treatment will be helpful or harmful to that specific patient. 4
THE PROBLEM
Annually, adverse drug reactions (ADRs) kill about 15,000 Canadians and costs the healthcare system over CDN $13 billion. 6 Given the impact of ADRs and growing evidence for genome‐informed prescription, 7 , 8 , 9 current trial‐and‐error methods should be eager to give way for a more effective approach to therapeutic intervention. The dramatic reduction in sequencing costs and optimization of genetic testing tools have significantly improved the viability of PGx. 10 Although researchers continue to produce evidence to support gene‐drug relationships, 11 the clinical adoption of PGx‐based PM has significantly lagged behind. Implementational barriers and drivers have been well‐studied—issues regarding the validity, cost‐effectiveness, guideline consistency, and lack of physician or patient awareness, education, and acceptance in this rapidly emerging field are often cited. 12 , 13 , 14 In response, large implementational studies have been conducted in the United States through the National Institutes of Health‐funded Implementing GeNomics In pracTicE (IGNITE) program 15 and in Europe through the Ubiquitous Pharmacogenomics (U‐PGx) program. 16 Complementary large‐scale pan‐Canadian evaluations on PGx implementation in Canada are scarce. A review of the Canadian PGx implementation literature (Figure S1) yielded siloed approaches where only one stakeholder group’s perspectives—clinicians, patients, public, or pharmacists—were evaluated (Table 1). Although relevant, these singular stakeholder perspectives result in a Canadian healthcare reliant on robust implementation science literature from other jurisdictions—a context that is not always complementary. Herein, we present the first report of multistakeholder perspectives from a successful regional PGx program in Canada. Without broader adoption and deployment of PGx, the requisite critical mass necessary to affect widespread changes in Canadian PGx funding and governance, has been hindered. As such, this study highlights the various perspectives, implementational barriers, and drivers from a developed PGx program in the Canadian healthcare system.
TABLE 1.
Canadian stakeholder perspectives on pharmacogenomics
| Stakeholder group | Jurisdiction | Year | Reference |
|---|---|---|---|
| Clinicians | Quebec | 2018 | Amara et al. 32 |
| Ontario | 2015 | Walden et al. 31 | |
| Ontario | 2019 | Chan et al. 29 | |
| Alberta | 2021 | Asgarpour et al. 30 | |
| Patients | Ontario | 2019 | Waldman et al. 39 |
| Ontario | 2013 | Loo et al. 38 | |
| Ontario | 2014 | Cuffe et al. 41 | |
| Public | Eastern Canada | 2020 | Etchegary et al. 37 |
| Ontario | 2020 | Bereza et al. 40 | |
| Students | Ontario | 2014 | Lanktree et al. 35 |
| Pharmacists | Quebec | 2013 | de Denus et al. 43 |
| Quebec | 2020 | Petis et al. 44 | |
| Quebec | 2020 | Meloche et al. 45 | |
| British Columbia | 2020 | Breaux et al. 46 |
DEVELOPMENT AND LAUNCH
The London Health Sciences Centre (LHSC) PGx program began in 2008, optimizing treatment using the blood thinner warfarin. Soon thereafter, the initiative expanded to offer PGx testing for tamoxifen, an anti‐estrogen used for the treatment of breast cancer, broader testing for oral anticoagulants, and cardiovascular medications. 17 Over the years, the focus has been on the systematic integration of additional evidence‐based clinically actionable common medications; namely Thiopurine Methyltransferase (TPMT) testing for gastroenterologists treating inflammatory bowel disease with Azathioprine, and Dihydropyrimidine Dehydrogenase (DPYD) testing for oncologists treating gastrointestinal‐related cancers with fluoropyrimidine‐based therapies (Figure S2). To date, over 3000 patients have been provided PGx‐guided recommendations.
Recently, a published analysis on the efficacy of pre‐emptive DPYD testing at LHSC (n = 1394), reinforced the known clinical efficacy of DPYD genotyping, but in the limited Canadian context. 18 In this study, a scholarly investigation is conducted on LHSC’s integration of PGx‐based PM into their clinical practice in an effort to better codify the various stakeholder perspectives on implementation considerations.
METHODS
Stakeholder identification and semi‐structured interviews
Initial interviews with the PGx program’s principal investigator (author R.K.) at LHSC were conducted to identify the key stakeholders involved in their care pathway and program development. These stakeholders could be categorized into four major groups: referring specialists, other nonreferring healthcare professionals, researchers, and hospital management. Referring specialists (primarily oncologists and gastroenterologists) snowball sampled 19 starting with those frequently referring to the PGx clinic at LHSC. Nonreferring healthcare professionals were convenience sampled through recommendations from specialist interviews or identified from the institution’s employee registry. Finally, researchers and hospital management were identified from the institution’s employee registry and purposefully sampled. To provide structure to interviews within the same stakeholder group, interview guides were developed. For each stakeholder group, literature review and informal discussions with PGx clinic leaders at LHSC guided identification of their general roles, responsibilities, and possible involvement in the PGx clinic. Based on these findings, interview guides were developed to elicit insights into the challenges and scaling potential of the PGx clinic, with respect to the day‐to‐day activities of each stakeholder group. Individuals were contacted via email and requested to participate in a 30–60 min teleconference interview to identify their perspectives on PGx‐based PM. With informed consent, interviews were recorded and subsequently transcribed (REB #112204). An a priori interview sample size of ~ 40 stakeholders was selected based on studies conducted in this field of research. 20 , 21 Interviews continued to be requested and conducted until information saturation 22 —totaling 42 stakeholder interviews: both locally around LHSC (n = 35) and externally (n = 7) at hospitals in three other regions (Table 2).
TABLE 2.
Characteristics of stakeholder interview participants
| Stakeholder group | Subgroup | Local (LHSC) | External |
|---|---|---|---|
| Referring Specialists | Oncologists | 4 | 4 |
| Gastroenterologists | 4 | 2 | |
| Nonreferring Healthcare Professionals | General Practitioners | 5 | |
| Nurses | 1 | ||
| Geriatricians | 3 | ||
| Pediatricians | 3 | ||
| Psychiatrists | 2 | ||
| Pharmacists | 1 | ||
| Cardiologists | 1 | ||
| Respirologists | 1 | ||
| Endocrinologists | 1 | ||
| Research | Pharmacology | 5 | 1 |
| Admin/management |
Southwest‐LHIN LHSC |
1 2 |
Abbreviations: LHIN, Local Health Integration Network; LHSC, London Health Sciences Centre.
Interview data analysis
Using a directed content analysis approach, 23 the transcripts were thematically analyzed, as suggested by the creators of the Business Model Canvas (BMC), 24 through an iterative and independent coding process by two of the project researchers. First, transcripts were coded to fit into customer (stakeholder) profiles and value maps. Stakeholder profiles intend to understand the background of the stakeholder group and their relation to PGx‐based PM. Within stakeholder profiles, subthemes included jobs/roles (day‐to‐day activities), gains (existing and possible from PGx‐based PM), and pains (existing and possible from PGx‐based PM). Value maps elucidated mechanisms to maintain gains or mitigate pains. Within value maps, subthemes included products and services (required for day‐to‐day activities), gain creators, and pain relivers. Within customer profile and value map subthemes, points were ranked based on how frequently they arose in interview transcripts. In addition to these subthemes, any other pertinent perspectives were noted.
Using the customer profiles and value maps, a BMC was created 24 , 25 through an iterative process between the two coding researchers. Interview transcripts were reviewed to ensure nothing was omitted, a visualization exercise of customer profile and value map subthemes was conducted, and finally subtheme points were integrated into the BMC to finalize customer profiles and value maps.
The BMC, which has previously been utilized to evaluate healthcare technologies and innovations, 26 , 27 is a tool used to identify the value creation logic and helps determine the quality of a business model. The benefit of using the BMC is the deconstruction of the business into nine essential interconnected building blocks graphically represented on the BMC framework. These nine building blocks can be clustered into four groups. The customer segments block, which defines the key stakeholders, is derived from customer profiles. The three blocks for key activities, key partners, and key resources, collectively, defines how value is generated from the products/services that are offered by the business. The value proposition, which defines the core value‐creating traits, is derived from the gain creators and pain relivers of the value map. The blocks for customer relationships and channels, which defines how the value‐creating product/service is delivered to key stakeholders, is derived from the fit between the customer profile and value map. Finally, are the cost structure and revenue streams, which in the case of healthcare this is often interpreted as cost‐savings (Figure 1).
FIGURE 1.
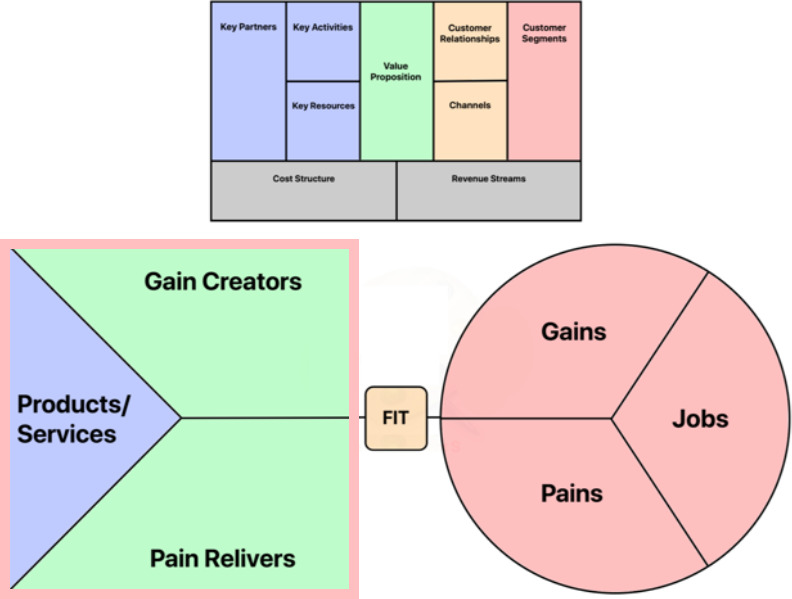
Integration of customer profiles, value maps and the business canvas model. Customer profiles (bottom right) defines the pains and gains associated with each stakeholders’ respective jobs. This information is leveraged to create the value map (bottom left), which defines the products and services (i.e., key partners, activities, and resources) that will drive the creation of gains and relief of pains (i.e., value). The delivery of gain creators and pain relivers is derived from the fit between the customer profile and value map, in other words the relationship and channels to the business offering. Together, these integrate into the business canvas model (top), which defines the value proposition and outlines the necessary components for value creation
Survey instrument and patient recruitment
In addition to the four major stakeholder groups identified for interviews, patients were also highlighted as a relevant stakeholder group. In order to capture patient perspectives, a cross‐sectional survey design, with some open‐ended questions was developed. Questions were created through discussions with the LHSC PGx clinic staff, and focused on patients’ prior expectations, experience during, and future recommendations for the clinic’s operations. Patients surveyed were either patients with cancer that underwent DPYD testing prior to fluoropyrimidine‐based (5‐FU) therapy or patients with inflammatory bowel disease that underwent TPMT testing prior to Azathioprine (AZA) therapy. Genotyped patients were provided a letter of information and, if consented, received a survey during their routine clinic visit with respective specialists (REB #114822). The survey took ~ 15 min to complete, and consisted of multiple choice, Likert‐scaled, and open‐ended questions. A total of 18 of 40 surveys distributed were returned, a response rate of 45%.
RESULTS
Recurring and relevant themes, with respect to implementational barriers and drivers of PGx services, within each stakeholder group are outlined below. Selected quotes from interviews are presented to highlight these significant themes.
Major considerations from local referring specialists
Oncologist’s and gastroenterologist’s relationship with the PGx clinic was long‐term and self‐service in that patients identified for pre‐emptive genetic testing would be referred. The PGx clinic would set up an appointment, coinciding with their specialist visit, to obtain a blood sample. Specialists gained an awareness of the PGx clinic services and benefits through professional relationships, internal and external meetings, and field‐specific scientific literature. Buy‐in was also developed through advocacy from “champions”—those who turn advocacy into action. From the PGx clinic’s perspective, their success was incumbent on allowing specialists to maintain standard of care, handling the patient education on PGx, and providing expert genotyping interpretations. The financial value generation was appraised through cost savings generated from more precise drug/dose selection and the reduction of ADR‐related expenditures. For example, 1:20 patients potentially receiving 5‐FU and 1:300 patients potentially receiving AZA at LHSC would have severe side effects without pre‐emptive genotyping. Finally, what these specialists valued most was the reliability, quality, and speed of responses. Alternative PGx service offerings through third‐party vendors or similar programs in other jurisdictions had significantly longer turnaround times—weeks, compared to days at LHSC. By integrating an internal, independent, and trusted provider of PGx services, specialists experienced minimal impositions on their practices, rapid diagnostics that were concise, accurate, and comprehensive, and enhanced patient and provider satisfaction (Figure 2).
FIGURE 2.
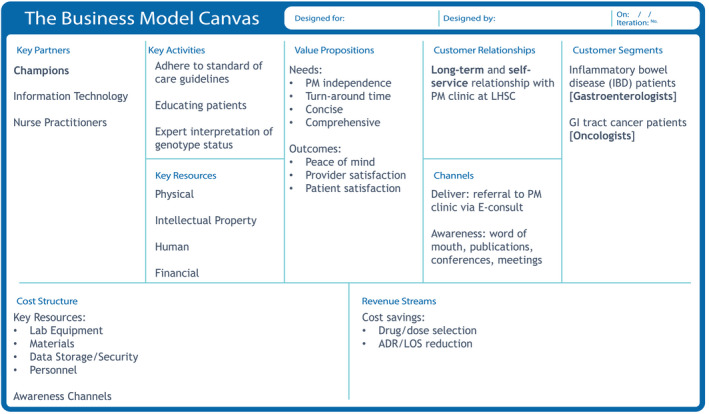
Business canvas model for LHSC’s major referring specialists to the PGx clinic. Starting with interview transcripts, the customer profiles (customer jobs, gain, and pains) and value maps (products/services, gain creators, and pain relivers) were developed. From here, the nine quadrants of the business canvas model were completed. The value of the PGx clinic for major specialists was driven by the independence of the clinic, the rapid turnaround time, and how concise yet comprehensive the information provided was. ADR, adverse drug reaction; GI, gastrointestinal; LHSC, London Health Sciences Centre; LOS, length of stay; PM, personalized medicine
Major considerations from external gastroenterologists
The external gastroenterologists (n = 2) were not users of the LHSC PGx clinic and fell into two categories; those with another network for genetic testing and those without. Those without networks either offered TPMT testing through private companies or started low‐dose AZA therapy, incrementally increasing while monitoring for ADRs. Most patients declined the option to pay out of pocket to receive genetic testing from private laboratory services. In contrast, those with a network for genetic testing would send a requisition to their network’s institution, however, their treatment plan was often delayed by long turnaround times.
I think it’s more of how quickly can the information be relayed, because it is obvious that if I see a patient in clinic, I do want to get them started on something today. I won’t have the TPMT results today. It might take a couple of weeks depending on how fast the patient gets the blood work done and how fast the lab actually sends it and so forth. So that certainly is a potential limitation, which if it is more mainstream, or if I could get quicker access to the results and therefore decide whether or not I could put the patient on it, it would be more ideal. (External Gastroenterologist #1).
When asked what the major barriers were to utilize the LHSC PGx clinic, the two major themes were access and awareness. For example, when asked if TMPT testing would be beneficial to their practice.
I would like to have that just because you know, Azathioprine is at risk of low white blood cell count or neutropenia, which can lead to infection, and it’s nice to be able to be more comfortable that that’s not going to happen for your patient. So, if I had access to it, at least for my patients in my population. (External Gastroenterologist #2).
Major considerations from external oncologists
Among the external oncologists (n = 5), four were users of the LHSC PGx clinic whereas one was not. The nonuser acknowledged the increasing use of prognostic biomarkers for precision therapies in the field but noted in their personal experience the frequency of DPYD deficiency was rare, occurring in one in 10,000–20,000 patients. However, this frequency is inconsistent with observations at LHSC or in the literature. 28
The four users of the LHSC PGx clinic discovered the service through professional relationships, word of mouth, or at patient case discussions. They agreed that dissemination of information regarding innovative services would be successful during journal clubs, round meetings, or an accredited organization, such as Cancer Care Ontario (CCO). Champions at their respective institutions appreciated the value of the pre‐emptive DPYD testing.
I think part of it too is that I was in [another city] where we didn’t have access to it at all, and you’d see so much more toxicity from these drugs, like as an oncology trainee, you know, you’re dreading prescribing [5‐FU] in particular because everyone got sick on it, but you know, coming back [near LHSC] I was like yes! I have access to this so I can more comfortably prescribe it, and certainly the toxicity that I see is much lower here than my residency experience, and I think that’s partly related to the fact that we do pick up on these intermediate metabolizers and can adjust things appropriately. (External Oncologist #1).
When I first came into a practice, we had a patient who we suspected was DPYD, but there was really, there was no way of testing, and so you know, we’ve all had at least one patient that’s had a very extreme severe toxicity. I think [my colleague] lost a patient from a 5‐FU toxicity. So, you know, when you have that happen, even though there’s a relatively low likelihood, if there’s something that you can do to screen for that deficiency or relative deficient in the treatment plans accordingly, I think it’s very valuable. (External Oncologist #2).
Prior to the LHSC PGx clinic there were no other feasible options, so testing was not offered. The barrier of long turnaround times for private or international testing were exacerbated in the context of a cancer diagnosis. Patients from these external sites had to travel, on average, 1–2 h to receive testing at LHSC. When asked how patients have appreciated the service despite inconveniences, such as travel, specialists cited that ~ 95% of patients were content and compliant. The unwilling patients typically had a physical ailment that precluded them from traveling such distances. Probing on the potential integration of DPYD testing into their respective institutions; external specialists all noted the infrastructure existed but simple disinterest or concerns that deployment would be inherently challenging caused institutional inertia. A purposed decentralized service model that facilitated local blood collection and subsequent sample delivery to LHSC for PGx testing was cited to be more amenable. Extending from this model could be other major institutions, like LHSC, that facilitate the testing and interpretation for neighboring specialist practices who draw and deliver the blood samples.
If there was some sort of roll out of trying to expand this program to other centers and show, for example, a couple hospitals could cover most of the province. (External Oncologist #3).
Finally, wheres pre‐emptive DPYD testing is not defined as standard of care they believed it should be. When asked, outside of colleague recommendations, what threshold of evidence would be required to integrate a novel genetic test into their practices, the responses included: (a) understanding the nature of the test; (b) comprehension of incremental risks and benefits; (c) some preliminary clinical or retrospective evidence to show clinical benefit; (d) how long has the test been around; (e) an acceptable margin of error; and (f) short and defined turnaround time.
Other considerations
Among the nonreferring healthcare professionals, the specialists were ambivalent toward the PGx clinic. They felt their practices had minimal relevance to the clinic but concurred that more evidence and research into predictive biomarkers was necessary. Similarly, general practitioners were largely unaware of the service, primarily learning about it when reviewing specialist referrals. However, if pre‐emptive PGx testing became standard‐of‐care, these physicians expressed a positive attitude toward directly referring to the PGx clinic, if adequately educated.
Among researchers and hospital management, the general consensus was funding required a larger body of evidence. “Champions” of the program are those that are motivated to utilize and advocate for the service and are essential to success. These were not restricted to specialists but also throughout the institution.
I guess on the topic of champions, so I think within our own environment we had clinical champions within the medical oncology division who would be actually using the technology. I think we also had champions at sort of my level, department leaders saying okay I think this is important for our division and I’m supportive of it, but then we also had administrative leaders who were supportive as well, so the VP of the cancer program sort of understood the potential value of this and was supportive of it, and so you kind of need champions at multiple levels for this to happen. (Hospital Management #1).
In some cases, there was resistance to the adoption of PGx services.
I think the biggest resistance, even though it’s just a psychological problem that our institution has, is space. There’s this perception that if someone moves into your space, they are somehow going to take it over or, interfere with other patient care issues, and so that’s the biggest issue, but we are able to overcome that. (Researcher #1).
This resistance was resolved by developing strong working relationships with referring specialists—importantly, developing an independent service that minimally imposed on their clinics. Strengthening bonds, such as joint fellowship training between specialist groups and PGx was another tangible method to reinforce interdisciplinary cohesion.
Patient survey findings
Survey respondents age ranged from 35 to 84 with the most common age range being 55–64 (TMPT genotyped patients; n = 4) and 65–74 (DPYD genotyped patients; n = 14). There were three major takeaways from the patient stakeholder group. First, all patients were extremely satisfied with the service (Figure 3). Patients citied thoroughness in explanations, professionalism, appointment brevity, and overall knowledge as defining traits (Table 3). Second, only four of 18 patients were driving under 30 min to attend their appointment (Table 3). Finally, whereas the willingness to pay (WTP) dollar amount varied from under $100 (n = 4), between $100 and $200 (n = 6), and to over $200 (n = 3), no patients cited their unwillingness to pay for the PGx services (Table 3). This contrasted the experiences of external gastroenterologists, who stated patients were unwilling to pay out‐of‐pocket for PGx testing.
FIGURE 3.
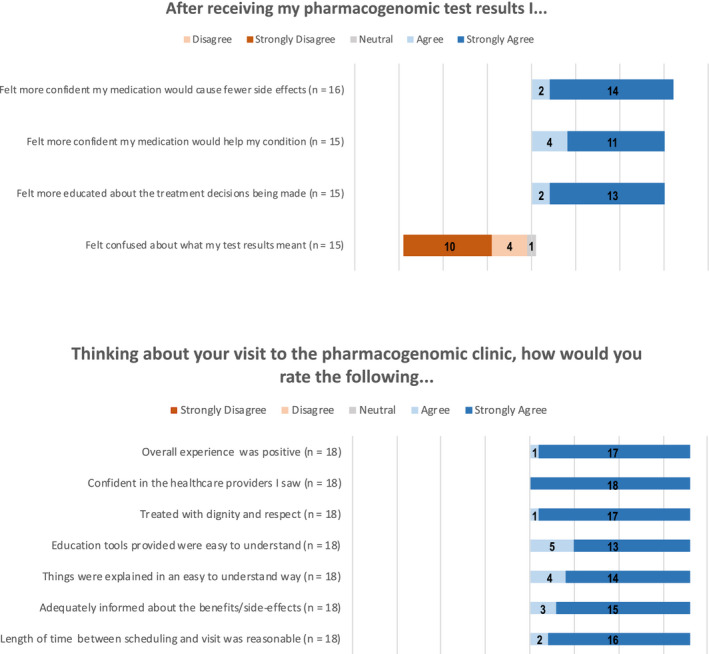
Additional patient responses to survey questions. Questions were scaled on a 5‐point Likert scale. Some patients did not answer every question (n = 15–18). All patients had a positive experience, and one patient felt neutral about their level of confusion after receiving their test results
TABLE 3.
Survey results from patients genotyped at LHSC PGx clinic
| Test | Age range | Sex | Travel time | Clinic expectations | Amount willing to pay | Summarized comments |
|---|---|---|---|---|---|---|
| DPYD | 35–44 | F | >2 h | Greatly exceeded | >$200 | Kind, friendly, informative team, efficient. Thorough understanding of benefits. |
| 35–44 | M | 30–45 min | Greatly exceeded | Prefer not to say | Switched from blood to saliva sample; explained the legalities – I was overjoyed! | |
| 45–54 | N/A | 1.5–2 h | Greatly exceeded | $100–$200 | On time, minimal waiting, very professional doctor and staff, explained things really well. | |
| 55–64 | M | 45 min–1 h | Greatly exceeded | $100–$200 | Got in quickly. Things were explained well by doctor and nurse, who were truly kind. | |
| 55–64 | F | >2 h | Greatly exceeded | $100–$200 | Very quick from referral to appointment, answered every question I had. | |
| 55–64 | M | >2 h | Exceeded | >$200 | Very knowledgeable, answered all questions. Could testing be done at same time as blood draw? | |
| 65–74 | M | >2 h | Exceeded | $100–$200 | I was treated so well, appointment was so fast. | |
| 65–74 | F | 1.5–2 h | Exceeded | >$200 | Staff was very pleasant and helpful. Explained the nature of his study. | |
| 65–74 | M | 30–45 min | Greatly exceeded | <$100 | Appointment on time with no waiting, very knowledgeable staff. | |
| 65–74 | F | <30 min | Exceeded | $100–$200 | So well explained. Quick appointment and painless. | |
| 65–74 | M | 1–1.5 h | Greatly exceeded | Prefer not to say | Spent time to explain and felt better once I understood the concept and how it helps me. | |
| 65–74 | M | <30 min | N/A | Prefer not to say | Very quick appointment and explained everything well. | |
| 65–74 | F | 1.5–2 h | Matched | Prefer not to say | Very informative, pleasant and positive. | |
| 75–84 | F | <30 min | N/A | <$100 | Very informative and easy to understand. | |
| TMPT | 18–24 | F | <30 min | Matched | <$100 | Explained research and process being done, passionate, caring, informative team. |
| 55–64 | F | 45 min–1 h | Greatly exceeded | N/A | Kind, explained the research, explained the procedure. | |
| 55–64 | N/A | 30–45 min | Exceeded | <$100 | Fast appointment, on time, efficient staff. Impressed to find out drug and dosage can be optimized. | |
| 55–64 | M | 1–1.5 h | Greatly exceeded | $100–$200 | Staff were wonderful at explaining the testing and answered questions in full. |
Abbreviations: DPYD, Dihydropyrimidine Dehydrogenase; LHSC, London Health Sciences Centre; N/A, not available; PGx, pharmacogenomics.
DISCUSSION
Other studies conducted in the Canadian context have focused on a single stakeholder group. With respect to clinician perspectives, four studies have been previously conducted. Two of these studies highlighted the lack of physician awareness, despite guideline recommendations, for human leukocyte antigen genotyping prior to carbamazepine and allopurinol treatment—which in some haplotypes can result in fatal dermatological complications. 29 , 30 Another study focused on psychotropic medications and found the majority of Canadian physicians who previously ordered at least one PGx test, to understand the PGx report. 31 However, this understanding and satisfaction declined from clinician scientists, to psychiatrists, to general practitioners. Similarly, we found these primary care physicians to be unaware of PGx services, yet confident in utilizing them if adequately educated. Finally, a survey of over 400 physicians in Quebec corroborated these findings, citing family physicians were less likely to adopt PGx testing due to the lack of information and clinical guidelines. 32 They also found that for many physicians, adoption required regulatory approval from Health Canada, scientific literature demonstrating the clinical benefit, recommendations experts and peers, and clinical guidelines. 32 Nonusers in our study also cited the requirement of scientific evidence, but in general focused less on regulations of guidelines and more on their individual understanding to sway future use of PGx services. With respect to the other jurisdictions, both IGNITE and U‐PGx have conducted evaluations on physician readiness for PGx implementation. 33 , 34 In the first instance, IGNITE surveyed 285 physicians across five sites and found two‐thirds felt their PGx‐related training was inadequate. 33 Similarly, U‐PGx surveyed 70 physicians across seven European countries and found that lack of PGx‐related knowledge was a significant barrier if the application of testing in clinical practise. 34 Resolving the lack of awareness and understanding in Canada will likely require a coordinated effort between health authorities for current physicians and medical schools for future physicians. In fact, despite students’ positive attitude towards PGx, 35 Canadian medical schools have historically lagged behind in PGx curriculum compared to pharmacy schools. 36
Among Canadians, past studies have found general agreeability with PGx technology, 37 increased amenability to less invasive sampling, 38 and that many patients utilizing private services do not disclose findings to their healthcare providers. 39 With respect to our work, Bereza et al. assessed the WTP of general Ontarians in Canada and found WTP to increase for faster turnaround time. 40 Moreover, Sinead et al. showed in the same geography that patients with cancer were willing to pay a median $1000 to $2000, and wait upwards of 2 weeks for their results if it meant a clinical benefit. 41 Although our study limited the upper threshold of WTP at >$200 CAD, almost all our surveyed patients stated some WTP. In contrast, a study in the United States surveying 869 patients found close to half (42%) were unwilling to incur costs of PGx testing. 42 From those who were willing to pay, most would pay a maximum of $250 (87%) or $100 (58%). 42 An important difference to note is, about half of the patients surveyed had no previous exposure to the clinical benefit of PGx testing, whereas, in our study, all patients had undergone testing. Therefore, the WTP may depend on the understanding and exposure to the potential benefits of PGx.
Other Canadian studies—three in Quebec and one in British Columbia—assessed the overall feasibility of disseminating PGx services through pharmacists. 43 , 44 , 45 , 46 Although our study evaluated the deployment of PGx services through a large outpatient hospital, the broader adoption of PGx may hinge on leveraging primary care physicians and pharmacists.
Many factors played into the successful implementation and continued growth of LHSC’s PGx clinic. Education, awareness, advocacy, and efficiency were crucial pillars to its success. Implementation was incumbent on champions of the service and tied closely into education and advocacy. Those who do not understand, or are unaware of, the benefit of pre‐emptive PGx testing, will not be inclined to participate. Therefore, effective knowledge translation and provisioning of education on the evidence surrounding PGx needs to be disseminated through appropriate channels within the institution. These channels should reach not only the relevant specialists, but also others involved in the care pathway, including hospital administration and nurses. 20 , 47 Once a substantive support network has been established, a suitable workflow can be developed to simultaneously maximize efficiency and minimize resistance. Specialist users of the PGx service were highly motivated because of the clinic’s independence, clear and comprehensive interpretation, and quick turnaround time. Utilizing identified awareness channels, such as hospital rounds or through agencies, like CCO, to promote the benefits of PGx services will be important for attracting champions and continuing to expand the geographic scope.
This study is not without limitations. First, conducting interviews rather than surveys with healthcare stakeholders resulted in a small sample size. Although the findings are consistent with other large survey‐based studies, an enhancement to the study would be to gather perspectives from across Canada. Second, self‐reported bias may have played a factor. Eliciting response at multiple timepoints (i.e., before and after genotyping) and from a large sample sizes can identify and reduce the impact of these biases. Nonetheless, understanding these key implementational drivers and barriers faced by LHSC’s PGx clinic will help guide the implementation of similar offerings elsewhere—together, building a proof of concept for standard of care pre‐emptive PGx testing and governmental reimbursement.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
M.S. wrote the manuscript. M.S., D.B., J.S., L.B., and R.B.K. designed the research. M.S., and J.S., performed the research. M.S., and J.S., analyzed the data. D.B., L.B., and R.B.K. contributed new reagents/analytical tools.
Supporting information
Figures S1–S2
ACKNOWLEDGEMENTS
The authors would like to acknowledge the stakeholder interviewees, patient participants, and the LHSC PGx clinic staff for their cooperation and involvement in this study.
Subasri M, Barrett D, Sibalija J, Bitacola L, Kim RB. Pharmacogenomic‐based personalized medicine: Multistakeholder perspectives on implementational drivers and barriers in the Canadian healthcare system. Clin Transl Sci. 2021;14:2231–2241. 10.1111/cts.13083
Funding information
Ontario Research Fund Round 8 (RE08‐063)—“Pharmacogenomics technologies and patient‐centered approaches for enhancing drug safety and effectiveness”: Ministry of Research and Innovation (MRI).
REFERENCES
- 1. Mini E, Nobili S. Pharmacogenetics: implementing personalized medicine. Clin Cases Miner Bone Metab. 2009;6(1):17‐24. [PMC free article] [PubMed] [Google Scholar]
- 2. Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011;364(12):1144‐1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathur S, Sutton J. Personalized medicine could transform healthcare. Biomed Rep. 2017;7(1):3‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nalejska E, Mączyńska E, Lewandowska MA. Prognostic and predictive biomarkers: tools in personalized oncology. Mol Diagn Ther. 2014;18(3):273‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel JN. Application of genotype‐guided cancer therapy in solid tumors. Pharmacogenomics. 2014;15(1):79‐93. [DOI] [PubMed] [Google Scholar]
- 6. Bosch TM, Meijerman I, Beijnen JH, Schellens JH. Genetic polymorphisms of drug‐metabolising enzymes and drug transporters in the chemotherapeutic treatment of cancer. Clin Pharmacokinet. 2006;45(3):253‐285. [DOI] [PubMed] [Google Scholar]
- 7. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442‐473. [DOI] [PubMed] [Google Scholar]
- 8. Hayden M, Carleton B. Genotype specific approaches to therapy in childhood (GATC). Genome British Columbia. https://www.genomebc.ca/projects/genotype‐specific‐approaches‐to‐therapy‐in‐childhood‐gatc. Accessed January 19, 2021.
- 9. Johnson JA, Cavallari LH. Pharmacogenetics and cardiovascular disease–implications for personalized medicine. Pharmacol Rev. 2013;65(3):987‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wetterstrand KA. DNA sequencing costs: data from the NHGRI genome sequencing program (GSP). www.genome.gov/sequencingcostsdata. Accessed January 19, 2021.
- 11. Gameiro GR, Sinkunas V, Liguori GR, Auler‐Júnior JOC. Precision medicine: Changing the way we think about healthcare. Clinics. 2018;73:e723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hippman C, Nislow C. Pharmacogenomic testing: clinical evidence and implementation challenges. J Personal Med. 2019;9(3):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Unertl KM, Jaffa H, Field JR, Price L, Peterson JF. Clinician perspectives on using pharmacogenomics in clinical practice [published correction appears in Per Med. 2019 Mar;16(2):185]. Per Med. 2015;12(4):339‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stanek EJ, Sanders CL, Taber KA, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharmacol Ther. 2012;91(3):450‐458. [DOI] [PubMed] [Google Scholar]
- 15. Sperber NR, Carpenter JS, Cavallari LH, et al. Challenges and strategies for implementing genomic services in diverse settings: experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med Genomics. 2017;10(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Wouden CH, Cambon‐Thomsen A, Cecchin E, et al. Implementing pharmacogenomics in Europe: Design and Implementation Strategy of the Ubiquitous Pharmacogenomics Consortium [published correction appears in Clin Pharmacol Ther. 2017 Jul;102(1):152]. Clin Pharmacol Ther. 2017;101(3):341‐358. [DOI] [PubMed] [Google Scholar]
- 17. Kim RB. Precision medicine: Lessons learned from implementation of a pharmacogenetics‐based patient care program in a real‐world setting. Clin Pharmacol Ther. 2019;106:933‐935. [DOI] [PubMed] [Google Scholar]
- 18. Wigle TJ, Povitz BL, Medwid S, et al. Impact of pretreatment dihydropyrimidine dehydrogenase genotype‐guided fluoropyrimidine dosing on chemotherapy associated adverse events [published online ahead of print, February 23, 2021]. Clin Transl Sci. 2021. 10.1111/cts.12981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42:533‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rigter T, Jansen ME, Groot JM, Janssen SW, Rodenburg W, Cornel MC. Implementation of pharmacogenetics in primary care: a multi‐stakeholder perspective. Front. Genet. 2020;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel HN, Ursan ID, Zueger PM, Cavallari LH, Pickard AS. Stakeholder views on pharmacogenomic testing. Pharmacotherapy. 2014;34:151‐165. [DOI] [PubMed] [Google Scholar]
- 22. Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893‐1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsieh H‐F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277‐1288. [DOI] [PubMed] [Google Scholar]
- 24. Osterwalder A, Pigneur Y. Business Model Generation: A Handbook for Visionaries, Game Changers and Challengers. Hoboken, NJ: John Wiley & Sons Inc; 2010. [Google Scholar]
- 25. Osterwalder A, Pigneur Y, Bernarda G, Smith A. Value Proposition Design: How to Create Products and Services Customers Want. Hoboken, NJ: John Wiley & Sons Inc; 2014. [Google Scholar]
- 26. van Rensen A, Voogdt‐Pruis HR, Vroonland E. The launch of the European patients’ academy on therapeutic innovation in the Netherlands: A qualitative multi‐stakeholder analysis. Front Med. 2020;7:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Limburg M, Wentzel J, Sanderman R, van Gemert‐Pijnen L. Business modeling to implement an eHealth portal for infection control: a reflection on co‐creation with stakeholders. JMIR Res Protoc. 2015;4:e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mattison LK, Fourie J, Desmond RA, Modak A, Saif MW, Diasio RB. Increased prevalence of dihydropyrimidine dehydrogenase deficiency in African‐Americans compared with Caucasians. Clin Cancer Res. 2006;12(18):5491‐5495. [DOI] [PubMed] [Google Scholar]
- 29. Chan FL, Shear NH, Maharaj A, et al. Knowledge and opinions among Canadian academic physicians regarding genetic screening to prevent severe cutaneous adverse drug reactions. J Am Acad Dermatol. 2019;81(6):1401‐1405. [DOI] [PubMed] [Google Scholar]
- 30. Asgarpour JMS, Lam LM, Vogel TK, Goez HR, Fiorillo L. Human leukocyte antigen gene testing and carbamazepine‐induced toxic epidermal necrolysis: a study of pediatric practice. J Cutan Med Surg. 2021;25(1):25‐29. [DOI] [PubMed] [Google Scholar]
- 31. Walden LM, Brandl EJ, Changasi A, et al. Physicians’ opinions following pharmacogenetic testing for psychotropic medication. Psychiatry Res. 2015;229(3):913‐918. [DOI] [PubMed] [Google Scholar]
- 32. Amara N, Blouin‐Bougie J, Bouthillier D, Simard J. On the readiness of physicians for pharmacogenomics testing: an empirical assessment. Pharmacogenomics J. 2018;18(2):308‐318. [DOI] [PubMed] [Google Scholar]
- 33. Owusu Obeng A, Fei K, Levy KD, et al. Physician‐reported benefits and barriers to clinical implementation of genomic medicine: a multi‐site IGNITE‐network survey. J Pers Med. 2018;8(3):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Just KS, Steffens M, Swen JJ, Patrinos GP, Guchelaar HJ, Stingl JC. Medical education in pharmacogenomics‐results from a survey on pharmacogenetic knowledge in healthcare professionals within the European pharmacogenomics clinical implementation project Ubiquitous Pharmacogenomics (U‐PGx). Eur J Clin Pharmacol. 2017;73(10):1247‐1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lanktree MB, Zai G, Vanderbeek LE, et al. Positive perception of pharmacogenetic testing for psychotropic medications. Hum Psychopharmacol. 2014;29(3):287‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Green JS, O'Brien TJ, Chiappinelli VA, Harralson AF. Pharmacogenomics instruction in US and Canadian medical schools: implications for personalized medicine. Pharmacogenomics. 2010;11(9):1331‐1340. [DOI] [PubMed] [Google Scholar]
- 37. Etchegary H, Wilson B, Rahman P, Simmonds C, Pullman D. Public interest in whole genome sequencing and information needs: an online survey study. Per Med. 2020;17(4):283‐293. [DOI] [PubMed] [Google Scholar]
- 38. Loo TT, Cuffe S, Hon H, et al. Willingness of patients to provide biologic samples for pharmacogenomic testing [abstract]. J Clin Oncol [serial online]. 2013;31(15):6543. [Google Scholar]
- 39. Waldman L, Shuman C, Cohn I, et al. Perplexed by PGx? Exploring the impact of pharmacogenomic results on medical management, disclosures and patient behavior. Pharmacogenomics. 2019;20(5):319‐329. [DOI] [PubMed] [Google Scholar]
- 40. Bereza BG, Coyle D, So DY, et al. Stated preferences for attributes of a CYP2C19 pharmacogenetic test among the general population presented with a hypothetical acute coronary syndrome scenario. Clinicoecon Outcomes Res. 2020;12:167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cuffe S, Hon H, Qiu X, et al. Cancer patients acceptance, understanding, and willingness‐to‐pay for pharmacogenomic testing. Pharmacogenet Genomics. 2014;24(7):348‐355. [DOI] [PubMed] [Google Scholar]
- 42. Bielinski SJ, St Sauver JL, Olson JE, et al. Are patients willing to incur out‐of‐pocket costs for pharmacogenomic testing? Pharmacogenomics J. 2017;17(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Denus S, Letarte N, Hurlimann T, et al. An evaluation of pharmacists’ expectations towards pharmacogenomics. Pharmacogenomics. 2013;14(2):165‐175. [DOI] [PubMed] [Google Scholar]
- 44. Petit C, Croisetière A, Chen F, Laverdière I. Are pharmacists from the province of Quebec ready to integrate pharmacogenetics into their practice. Pharmacogenomics. 2020;21(4):247‐256. [DOI] [PubMed] [Google Scholar]
- 45. Meloche M, Kwon HJ, Letarte N, et al. Opinion, experience and educational preferences concerning pharmacogenomics: an exploratory study of Quebec pharmacists. Pharmacogenomics. 2020;21(4):235‐245. [DOI] [PubMed] [Google Scholar]
- 46. Breaux S, Desrosiers FAD, Neira M, Sinha S, Nislow C. Pharmacogenomics at the point of care: a community pharmacy project in British Columbia. J Pers Med. 2020;11(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Levy KD, Decker BS, Carpenter JS, et al. Prerequisites to implementing a pharmacogenomics program in a large health‐care system. Clin Pharmacol Ther. 2014;96:307‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S2


