Abstract
Individuals who identify as Black, indigenous, and people of color face well‐documented health disparities. A root cause is the lack of empiric evidence for or against the use of various treatments in their medical management. This communication proposes a new benchmarking strategy for evaluating racial and ethnic representation in clinical research that can be compared across institutions with the intent of increasing accountability for diversity and inclusion among organizations that conduct clinical research.
COMMENTARY
“The true neighbor will risk his position, his prestige, and even his life for the welfare of others.” [Dr. Martin Luther King, Jr., On Being a Good Neighbor 1962]
The transformational issues that surfaced in 2020 included a resounding call for equity. Several events spotlighted long‐standing issues faced by Black, indigenous, and people of color (BIPOC) triggering profound reflection and an immediate call to action. The opportunity to enact meaningful change impacting health inequities may be fleeting if we fail to leverage this opportunity. The clinical research community is in a unique position to address systemic inequities by tackling a root cause of health disparities that exacerbates the burden faced by BIPOC communities—inadequate representation in trials which leaves us unable to identify the safest and most effective course of treatment for those who are under‐represented. Researchers bear the responsibility for establishing the utility of medical interventions in minority groups, and legislation enacted and reauthorized over the past 30 years validates this responsibility; explicitly mandating the reporting of racial and ethnic diversity in federally funded clinical research with periodic evaluation of racial and ethnic underrepresentation by the Comptroller General. 1 , 2 However, the nature of accountability beyond reporting remains unclear.
Recently, 3 one of us examined racial and ethnic representation among children enrolled in clinical studies funded under the Best Pharmaceuticals for Children Act (BPCA). Diversity was explored using Simpson’s diversity index (D), a quantitative metric representing the probability that two individuals selected at random from a population would belong to different racial and ethnic groups (i.e., 0 = no diversity; 1 = maximal diversity; Equation 1). 4 In preparing this commentary, we applied the same calculation to enrollment data extracted from the triennial reports of the 10 largest institutes at the National Institutes of Health (NIH), 5 and observed an enormous degree of variability in representation across studies funded by these institutes (Figure 1). The importance of the data published by the NIH cannot be overemphasized; however, they lay out a number of caveats; (1) NIH “policy is not to endorse or enforce quotas,” (2) racial and ethnic representation “depends upon a number of factors,” and (3) “enrollment figures should not be compared directly to national census figures,” leaving limited avenue for accountability. In this commentary, we are proposing that accountability begin at the local level in the communities we serve with the patients we treat and we have constructed a simple, singular, scalable metric, the “representation quotient” (RQ).
FIGURE 1.
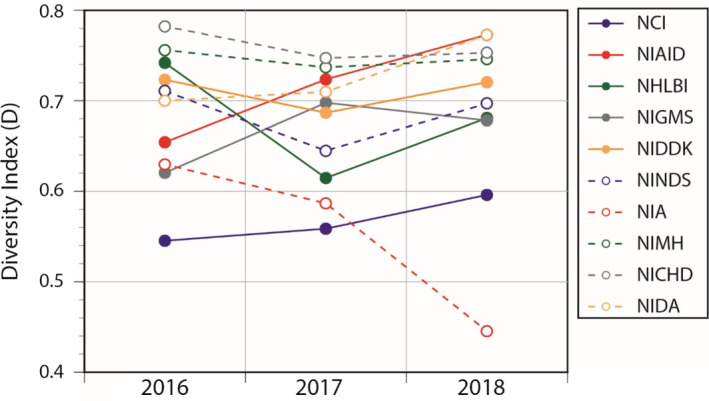
Enrollment diversity for the top 10 NIH institutes over the most recent 3 reporting years. NCI, National Cancer Institute; NHLBI, National Heart, Lung, and Blood Institute; NIA, National Institute on Aging; NIAID, National Institute of Allergy and Infectious Diseases; NICHD, Eunice Kennedy Shriver National Institute of Child Health and Human Development ; NIDA, National Institute on Drug Abuse; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIGMS, National Institute of General Medical Sciences; NIH, National Institutes of Health; NIMH, National Institute of Mental Health; NINDS, National Institute of Neurological Disorders and Stroke
As a measure of representation, the diversity index consolidates individual subgroup statistics into a singular value. By way of example, our BPCA review considered 14 racial and ethnic subgroups derived from the combination of seven racial groups (American Indian or Alaskan Native, Asian American, Black, Hawaiian or Pacific Islander, multiracial, not reported or unknown, and White) with two ethnic groups (Hispanic and non‐Hispanic). However, the number of subgroups available for diversity index calculation will vary depending on the level of detail reported and the index itself lacks a standard for comparison. The proposed RQ is obtained by calculating the diversity index of the research participant population (Drp) and dividing the value by the diversity index of the reference population (DRP) determined by sampling the general population at the same geographic frequency as the research participant population. Estimates of variance (σ²) are calculated to define bounds for the value (Equation 2). 4 , 6
An RQ greater than 1 signals diversity in the clinical research population that exceeds that of the reference population while an RQ less than 1 suggests that the clinical research population is less diverse than the reference population. In the case of an individual organization, such as Children’s Mercy, both the numerator and denominator reflect the population being served by that specific institution. Accordingly, the RQ is agnostic to the overarching population composition allowing insight into representation efforts for clinical studies irrespective of population diversity in the communities where organizations are located. This enables an apples‐to‐apples comparison between organizations and circumvents the challenge of quotas.
Trialing the RQ at our organization, we collected metrics on clinical research participation and recorded corresponding challenges in data acquisition to illuminate deficiencies and propose system improvements for enhancing the quality of these measures. Data from enrollment in federally funded studies were extracted from all research performance progress reports (RPPR) submitted to the NIH through the Research & Grants Administration office over the most recent 5‐year period. The geographic distribution of patients we serve was pulled from patient encounter data maintained by the organization and the DRP determined by extracting county level data from the US Census Bureau. 7 Importantly, senior leaders were approached before undertaking this analysis to ensure support at the highest level of leadership regardless of what the data would subsequently reveal.
Our findings indicated that diversity among our clinical research participants exceeded that of the population we serve in 3 of the last 5 years, but fell significantly short in the other 2 years, giving Children’s Mercy a 5‐year rolling average of 0·89 (Figure 2). Because the RQ is calculated using all of the data one typically uses to examine representation, we can perform a more detailed interrogation of the RQ inputs to identify the extent to which population subgroups are over‐ or under‐represented in our trials. In doing so, we observed consistent under‐representation of Hispanic, and to a lesser extent Asian American, children in our studies (data not shown). We also discovered that several individual studies recorded high rates of unknown race and ethnicity, despite the reporting requirements mandated by the NIH, contributing to year‐over‐year fluctuations.
FIGURE 2.
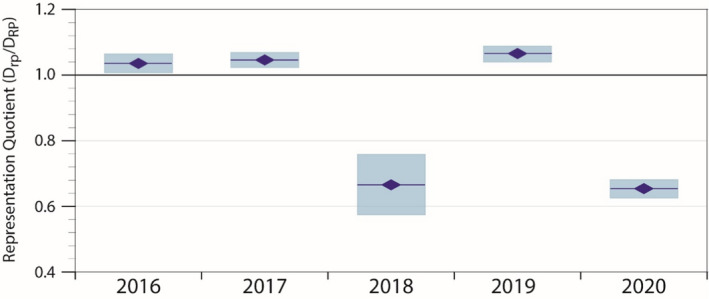
Children’s Mercy Hospital annual RQ for the preceding 5 years. Drp‐ diversity index of the research participant population, DRP‐ diversity index of the reference population
There are limitations to the conclusions we can draw from the RQ. For example, we cannot discriminate whether under‐represented populations are approached at different frequencies or elect to participate at different rates. We also do not know the extent to which the observations are influenced by the inclusion of studies for conditions which have inherent imbalances with respect to disease epidemiology, healthcare utilization patterns, medication utilization, access to care, or prescriber treatment bias. Nevertheless, it represents a current snapshot of clinical research participation at our organization.
In the process of conducting this analysis, we uncovered several information gaps that we believe are important enough to initiate system‐wide changes in our organization. An unbiased snapshot of research participation requires an objective data source that is independent of a willingness to disclose. Although the RPPR reflect the desired data, they do not represent the entirety of clinical research activities at our institution. The availability of a clinical trial management system (CTMS), which we are currently deploying, can supplement RPPR reports but it will not entirely resolve the data challenge as CTMS will only contribute data from studies incurring patient care costs. Thus, participation in other types of research (e.g., community‐based studies, psychosocial research, quality‐of‐life studies, epidemiologic research, genetic studies, etc.) remains unaccounted for in our current system resulting in an incomplete picture of racial and ethnic outreach in our clinical research program. The changes we are considering to address these challenges include mandated institutional reporting across all studies, a thorough examination of the barriers that investigators encounter in obtaining this information, creation of a dashboard that provides real‐time metrics for our organization, and refinement of educational strategies to emphasize the importance of representation in clinical research.
The incomplete nature of our data led to debate among the authors regarding the merit in reporting our findings and the prematurity of a purposeful call‐to‐action for others to follow suit; however, we concluded that the utility in doing so is multifaceted:
First and foremost is elevated awareness. By adopting the RQ as a starting point and including it as part of our common metrics, we reinforce the principle that diversity and inclusion are expected to be part of every conversation related to research—in essence establishing a new normal.
Publicly releasing our data serves to promote transparency, underscore our commitment to accountability within the communities who partner with us in doing this research, and drive institutional changes to address the inadequacies we uncover.
These data offer a quantitative baseline on which to measure improvement efforts. The RQ provides an objective metric that accounts for regional population differences thereby laying the foundation for benchmarking initiatives that define racial and ethnic research representation against a national average in the same way that has been adopted for interventional procedures and disease outcomes. 8 , 9
We realize that resource limitations invariably restrict the scope and magnitude of clinical research projects. Moreover, simply committing to balanced racial and ethnic representation does not ensure that studies are powered to answer important questions related to effectiveness and impactfulness in every population subgroup. However, tolerating racial and ethnic imbalance in studies (apart from those of diseases affecting racial subgroups) requires an open and honest acknowledgment that the generalizability of the resultant research findings is severely restricted, and we hope that these frequent admissions will lead the clinical research community to reassess the current research enterprise.
We conclude with a challenge to our colleagues. If racial and ethnic representation in research is a publicly stated goal of your organization, please join us by submitting your unbiased, de‐identified research participation data for representation on a national benchmarking website we are hereby committing to create and support. The platform will display RQ on the homepage as the primary metric allowing organizations to benchmark against others of similar size and scope. Subpages will offer a deeper dive into subpopulation specific details so that prospective research partners (e.g., public and private funding agencies and research collaborators) can identify sites that (1) alone, or in combination, offer the greatest likelihood of meeting clinical trial needs and (2) provide objective evidence of having made a public commitment to ensuring racial and ethnic representation. Until we stand‐up the public facing website with details on data submission, please contact the corresponding author for the requirements for the inclusion of your organizations data.
We are committed to making transparent the successes and failures in our journey, no matter how uncomfortable it makes us, or how damaging to our prestige, and we believe that if enough organizations make the same commitment by standing together, we can achieve meaningful change on behalf of the patients we serve.
| (1) |
| (2) |
where, s is the individual racial and ethnic subgroup, n is the total number of children in each subgroup, N is the total number of children across all subgroups.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
Abdel‐Rahman SM, Wimes MP, Curran T. A call to action: Issuing a diversity and inclusion challenge to research organizations. Clin Transl Sci. 2021;14:2095–2098. 10.1111/cts.13105
Funding information
Funded, in part, by the Marion Merrell Dow/Missouri Chair in Pediatric Clinical Pharmacology held by Dr. Abdel‐Rahman.
REFERENCES
- 1. National Institutes of Health Revitalization Act, Pub L No. 103–43, 107 Stat 102 (1993). https://www.gpo.gov/fdsys/pkg/STATUTE‐107/pdf/STATUTE‐107‐Pg122.pdf.
- 2. Best Pharmaceuticals for Children Act (2002), United States Public Law No. 107–109.
- 3. Abdel‐Rahman SM, Paul IM, Hornik C, et al. Minority representation in pediatric studies funded under the best pharmaceuticals for children’s act. Pediatrics. 2021;147(5):e2020042903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simpson EH. Measurement of diversity. Nature. 1949;163:688. [Google Scholar]
- 5. NIH Research Portfolio Online Reporting Tools (RePORT) . Inclusion of women and minorities in clinical research. https://report.nih.gov/research/inclusion‐women‐and‐minorities‐clinical‐research
- 6. Hurlbert SH. The non‐concept of species diversity: a critique and alternative parameters. Ecology. 1971;52:577‐586. [DOI] [PubMed] [Google Scholar]
- 7. U.S. census data (2019). https://www.census.gov/quickfacts/fact/table/US/PST045219
- 8. Cystic Fibrosis Foundation Care Center Data . 2017. https://www.cff.org/Research/Researcher‐Resources/Patient‐Registry/Care‐Center‐Data/
- 9. HRSA Blood Stem Cell Transplant activity report. 2020. https://bloodstemcell.hrsa.gov/data/donation‐and‐transplantation‐statistics/transplant‐activity‐report#center


