Sniffing a molecule emitted from baby heads makes men less aggressive yet women more aggressive.
Abstract
In terrestrial mammals, body volatiles can effectively trigger or block conspecific aggression. Here, we tested whether hexadecanal (HEX), a human body volatile implicated as a mammalian-wide social chemosignal, affects human aggression. Using validated behavioral paradigms, we observed a marked dissociation: Sniffing HEX blocked aggression in men but triggered aggression in women. Next, using functional brain imaging, we uncovered a pattern of brain activity mirroring behavior: In both men and women, HEX increased activity in the left angular gyrus, an area implicated in perception of social cues. HEX then modulated functional connectivity between the angular gyrus and a brain network implicated in social appraisal (temporal pole) and aggressive execution (amygdala and orbitofrontal cortex) in a sex-dependent manner consistent with behavior: increasing connectivity in men but decreasing connectivity in women. These findings implicate sex-specific social chemosignaling at the mechanistic heart of human aggressive behavior.
INTRODUCTION
Terrestrial mammalian aggressive behavior can be triggered or blocked by social odors. For example, a rabbit mother will attack and even kill her pups if they are tainted with the body odor of a stranger female (1), and two specific volatile components in mouse urine trigger fighting between males (2). These aggression-triggering chemosignals are not all volatile: Specific major urinary proteins can also trigger aggression, an effect mediated by the accessory olfactory system (3). In turn, chemosignals not only trigger aggression but also can block it. For example, tainting a pig with the known volatile reproductive pheromone androstenone (5a-androst-16-en-3-one) blocks conspecific aggression toward the tainted pig (4). Also, a mouse tear-bound peptide blocks male mouse aggression toward mouse pups tainted with the peptide (5). These tear-bound aggression-blocking chemosignals may be a mammalian-wide phenomenon: Male mole rats cover themselves with their own harderian secretions, and this blocks aggression toward them from dominant male conspecifics (6). Relatedly, sniffing human tears reduces testosterone in men (7, 8), but the impact of this on aggressive behavior has yet to be investigated. To conclude, both volatile and nonvolatile chemosignals can either trigger or block mammalian aggression.
Like all mammals, humans engage in reactive aggression from a very early age (9) and throughout life (10). Although aggression is a major factor in the human condition, the mechanistic neural substrates of human aggression are not well understood (11, 12). Could human aggression mechanisms be tied to chemosignaling mechanisms as they are in all other terrestrial mammals? Two studies have suggested that humans emit aggression-specific body odors (13, 14), and an electroencephalography (EEG) study suggested that these may be processed differently in the brains of men and women (15), but whether and how human aggressive behavior is then affected by social chemosignals remains unknown. To test the hypothesis that a social chemosignal can modulate human aggression through modulation of neural activity, we used standard behavioral and neuroimaging aggression paradigms with and without a concurrent social chemosignal. As a social chemosignal, we tested hexadecanal (HEX), a volatile long-chain aliphatic aldehyde, first identified as a social-buffering agent in mice (16). Although species specificity has been considered a hallmark of social chemosignaling (17), some chemosignaling molecules may be conserved across species, with either similar or different meaning (18, 19). Because the mouse receptor for HEX (OR37B) is highly conserved across mammals, it has been suggested that HEX may be one such cross-species conserved signaling molecule (20). There are several reasons for selecting HEX: First, humans express an OR37 receptor ortholog (21). Second, although pentadecanal and heptadecanal also bind to OR37, only HEX is emitted from human feces, skin, and breath (22, 23). Heptadecanal is emitted only in feces (22), and there was no previous report of pentadecanal emitted from the human body. Last, exposure to HEX reduced startle responses in humans (24), implicating impact of HEX on arousal. With all this in mind, we tested whether and how HEX affects human aggression.
RESULTS
HEX blocked aggression in men but triggered aggression in women
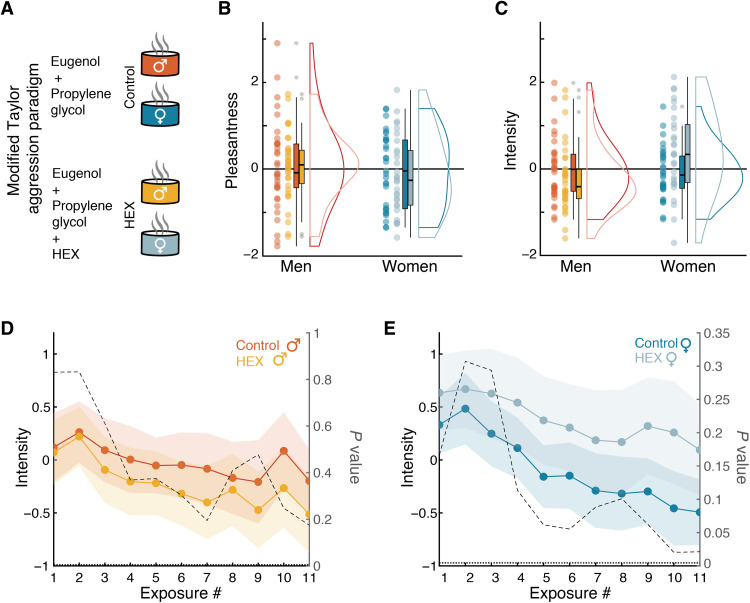
To gauge aggression, we used a modified Taylor aggression paradigm (TAP), a well-established measure of human aggressive behavior (25, 26). A total of 127 participants (67 men; mean age, 25.48 ± 3.46; range, 21 to 34) participated in a double-blind between-subjects design, half concurrent with exposure to HEX obscured in the carrier eugenol and half concurrent with exposure to the carrier eugenol alone (Control) (Fig. 1A). We used a carrier because we wanted to mask any possible perceptual impact of HEX alone, and we selected eugenol as the obscuring carrier because it was used for this purpose in social chemosignaling studies conducted by others (27). To ask whether the addition of HEX was associated with an explicit percept, participants used a visual analog scale (VAS) to rate the stimuli along the primary dimensions of human olfactory perception, namely, intensity and pleasantness (Fig. 1, B and C) (28). Odor ratings were Z-scored, i.e., standardized with respect to the entire sample mean and SD. Because odorant ratings did not distribute normally (Pleasantness: Shapiro-Wilk W = 0.98, P = 0.001; Intensity: W = 0.96, P = 0.002), we applied a linear mixed model with factors of Sex and Odor and random effects of Participant, which revealed that HEX had no impact on perceived odorant pleasantness (Sex: F1,123 = 1.13, P = 0.29; Odor: F1,123 = 0.12, P = 0.73; Sex × Odor: F1,123 = 0.10, P = 0.7), and no overall impact on perceived intensity but a statistically significant interaction (Sex: F1,123 = 3.66, P = 0.06; Odor: F1,123 = 0.6, P = 0.44; Sex × Odor: F1,123 = 6.07, P = 0.02). Post hoc pairwise contrasts applied to the fitted model (Tukey adjustment for multiple comparisons), however, revealed that this interaction reflected opposing insignificant effects in men and women (men Control: mean rating ± SD = −0.02 ± 0.73; men HEX: mean rating ± SD = −0.22 ± 0.77, t123 = 1.10, P = 0.69; women Control: mean rating ± SD = −0.09 ± 0.60; women HEX: mean rating ± SD = 0.38 ± 0.96, t123 = 2.35, P = 0.09) (Fig. 1, D and E). In summary, the addition of HEX to the carrier had no impact on perceived stimulus pleasantness and minimal impact on perceived stimulus intensity.
Fig. 1. HEX did not significantly shift stimulus perception.
(A) The between-subjects TAP included four groups exposed to either control [100 μl, 10% eugenol in propylene glycol (PG)] or HEX (100 μl, 0.083 M HEX in 10% eugenol in PG). Control n = 34 men and 31 women, HEX n = 33 men and 29 women. Blue and orange refer to the sex of the recipient: blue for women and orange for men. The light and dark shades refer to the odor condition, HEX, and Control, respectively. (B) TAP odorant pleasantness ratings along the VAS. Each dot is a participant, the thick horizontal line is the median, the rectangle reflects the interquartile range (IQR) (25th to the 75th percentiles), and the whiskers are no more than 1.5 * IQR of the upper and lower hinges. Outlying points are plotted individually. (C) TAP odorant Intensity ratings along the VAS. Elements as in (B). (D) Mean Intensity and confidence interval of 95% for 11 consecutive exposures to HEX and Control in men. The dotted line is the point-by-point P value for the HEX-Control two-sample, two-tailed t test. The horizontal dotted line represents the significance threshold P value, corrected for multiple comparisons (Bonferroni correction), set to 0.0045. (E) Same as in (D) in women.
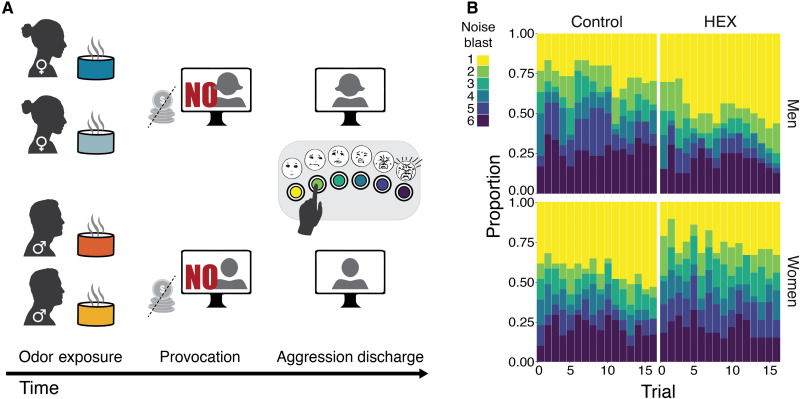
The TAP begins with a provocation phase where the participant plays an on-screen version of the ultimatum game (29) against a purported game partner who is a game algorithm (Fig. 2A). In each round of the ultimatum game, both “players” are allotted a sum of money (~$9) that they can keep if they agree on how to distribute it between them. The game is programmed such that the purported partner agrees only to distributions that substantially discriminate against the participant. Five such rounds served as an effective provocation. At this point, there were no differences between the groups (figs. S1 and S2). Following this is an aggression discharge phase where the participant is again misled to believe that they are playing against the same partner (who previously provoked them) but now in a reaction-time task where they compete to identify a change in shape of a target. The first to react is then allowed to blast his/her opponent with a loud noise blast. The volume applied by the participant is taken as a measure of aggression (Fig. 2B) (26, 30). The game was rigged such that the participant was faster than the fictitious opponent on 16 of 27 trials, and in trials where the fictitious opponent was faster, the participant endured noise blasts randomly ranging in volume.
Fig. 2. Path to provoking and gauging human aggressive behavior.
(A) In a between-subjects design, participants were exposed to an odorant (HEX or control) and then played a game where their online partner was unfair toward them in monetary distribution (provocation) and then another game where they could blast that same (nonexistent) person with noise blasts (aggression discharge). (B) Complete distribution of noise blasts applied in the study by men (n = 67) and women (n = 60) under HEX or control (yellow = mild, purple = harsh). The colors of the buttons on the response box were added here for illustration clarity.
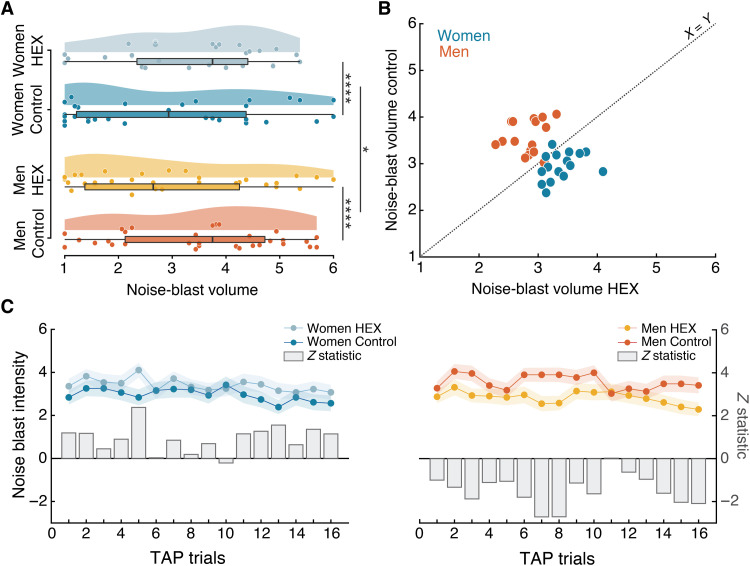
Noise-blast volume was entered into a repeated-measures ordinal logistic regression analysis with factors of odorant (HEX/Control), Sex (men/women), and random effect of Participant. We observed a trend toward an effect of odorant (Z = 1.84, P = 0.07), no effect of Sex (Z = 1.63, P = 0.10), and a significant odorant × Sex interaction (Z = 2.03, P = 0.04, effect size: generalized η2 = 0.04). This significant interaction reflected that HEX significantly lowered aggression in men [men Hex: n = 34, mean blast volume = 2.76 ± 2.03; men Control: n = 33, mean blast volume = 3.60 ± 1.98, Mann-Whitney (trials compacted as repeated measure), Z = 5.855, P < 0.0001, effect size r = 0.7] yet significantly increased aggression in women [women Hex: n = 29, mean blast volume = 3.40 ± 1.91; women Control: n = 31, mean blast volume = 2.96 ± 2.03, Mann-Whitney (trials compacted as repeated measure), Z = 3.655, P = 0.0003, effect size r = 0.47] (Fig. 3A).
Fig. 3. Exposure to HEX modulated aggressive behavior in a sex-dependent manner across participants.
(A) Group-mean noise-blast volume. Men: n = 67 (34 Control). Women: n = 60 (31 Control). Each dot is the mean of a participant, the thick vertical line is the median, the rectangle reflects the IQR (25th to the 75th percentiles), and the whiskers are no more than 1.5 * IQR of the upper and lower hinges. (B) Trial-wise mean noise-blast volume. Each dot is the mean of a trial (1 to 16) of all men (orange) or women (blue) participants. The y axis reflects the trial mean during Control, and the x axis reflects the trial mean during HEX. The dotted line is the unit slope line (X = Y) such that data under the line reflect increased aggression in HEX and data above the line reflect decreased aggression in HEX. (C) Trial-by-trial depiction across participants. The dots reflect mean ratings, the shaded area reflects the SE, and the gray bars reflect a trial-by-trial Z statistic; both plots share the y axis. All tests were two-tailed, and all error bars represent SEM. *P < 0.05, ****P < 0.0005.
To gain further insight into the dynamics of aggressive behavior in the TAP, we looked at the 16 repeated trials separately. When depicting the trial-by-trial mean, there was an apparent small yet highly consistent difference between the conditions in both sexes (Fig. 3, B and C). To again quantify this, we compared the trial-by-trial means using a Mann-Whitney test, which provided a trial-wise Z value for each comparison (Fig. 3C). A one-way independent two-tailed t test on the distribution of the trial-by-trial z values again revealed a significant effect of condition within sex: significantly decreasing aggression in men exposed to HEX (trial-wise blast volume: control = 3.57 ± 0.34, HEX = 2.91 ± 0.39, t15 = −6.37, P < 10−5) yet significantly increasing it in women (trial-wise blast volume: control = 2.84 ± 0.55, HEX = 3.34 ± 0.33, t15 = 4.70, P < 0.0003) (these numbers differ from the previous analysis because they reflect trial-wise blast volume, whereas the previous analysis reflected participant-wise blast volume), again indicating a significant dissociation between the two sexes (t15 = −11.18, P < 10−7) [these differences again reemerged in a validation permutation test (fig. S3)]. Behavioral differences were not associated with perceptual valence differences between the two conditions (Fig. 1), were not related to vigilance (fig. S4), and were not reflected in group levels of cortisol or testosterone (fig. S5). In summary, three separate analyses of the TAP implied that exposure to HEX reduced aggression in men but increased aggression in women.
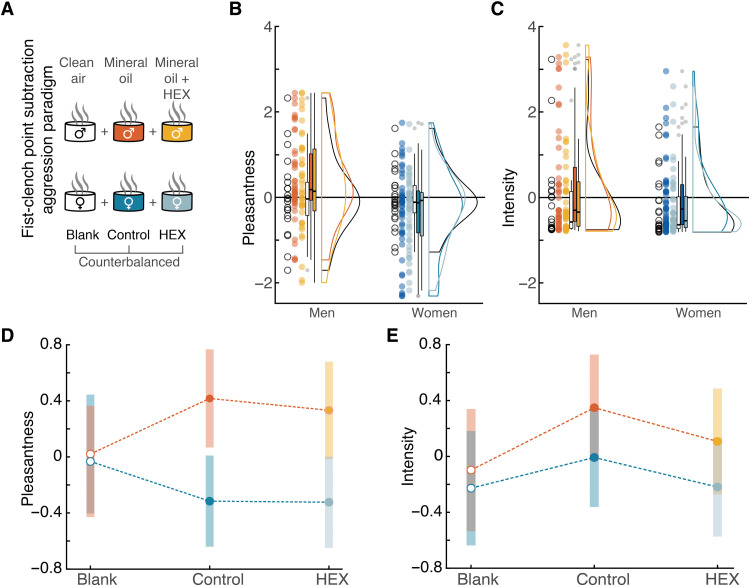
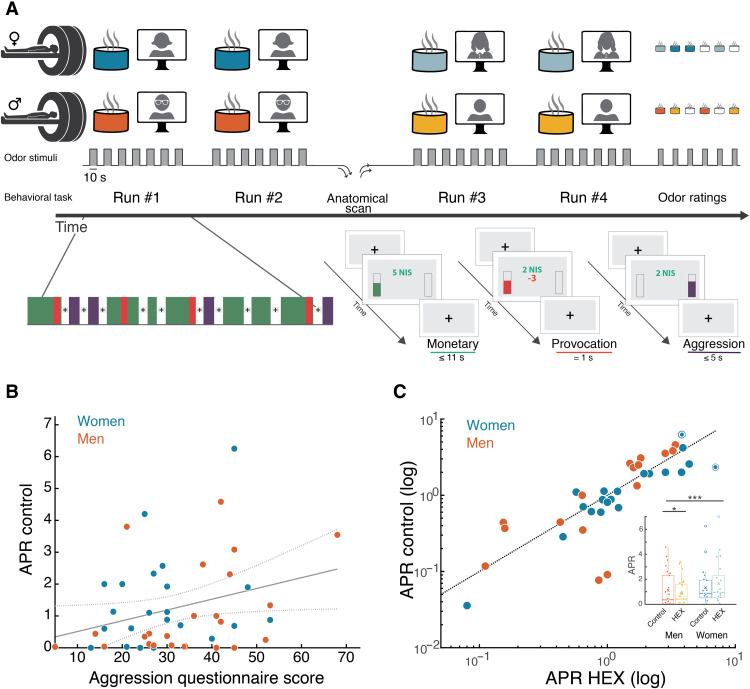
We next used functional magnetic resonance imaging (fMRI) to ask where and how in the brain does HEX affect aggression. We used the point subtraction aggression paradigm (PSAP), another well-established paradigm in aggression research that has been used in fMRI (31, 32). It allows imaging of brain activity during provocation (having your money taken away from you) and aggression (subtracting money from others, yet not for monetary profit). To provide for a more naturalistic aggressive output, we substituted the response buttons with fist-clench (FC) pressure sensors. Participants were misled to think that the response device provides binary output alone, but we measured FC effort as a naturalistic implicit continuous measure of aggression extent. In support of this method, we observed a modest but significant correlation between measured aggression at baseline and basal aggressive tendencies as estimated by a standard aggression questionnaire (AGQ) (Pearson r = 0.30, P = 0.04) (fig. S6) (33). These correlations typically emerge only in very large samples, so their emergence here underlies the power of the FC-PSAP. In a within-subjects, double-blind design (Fig. 4A), 49 participants (24 women; mean age, 26.98 ± 3.92; range, 19 to 36) completed an average of 134.39 (±15.33) trials (of all types), about half concurrent with HEX (HEX dissolved in mineral oil, which served as the HEX condition) and about half with carrier (mineral oil, which served as the Control condition) alone, counterbalanced for order (for full table of events, see table S1). Clean air was used between odor stimuli and during the anatomical scan and is referred to as blank odor.
Fig. 4. HEX did not shift stimulus perception.
(A) The within-subjects FC-PSAP included two groups: men (n = 25) and women (n = 24). Both were exposed to Blank (clean air), Control (mineral oil), and HEX (0.083 M HEX dissolved in mineral oil). Blue and orange refer to the sex of the recipient: blue for women and orange for men. The light and dark shades refer to the odor condition, HEX and Control, respectively. White color refers to the additional Blank air that participants were exposed to during the experiment. (B) FC-PSAP odorant pleasantness ratings along the VAS. Each dot is a participant, the thick horizontal line is the median, the rectangle reflects the IQR (25th to the 75th percentiles), and the whiskers are no more than 1.5 * IQR of the upper and lower hinges. Outlying points are plotted individually. (C) FC-PSAP odorant intensity ratings along the VAS. Elements as in (B). (D) FC-PSAP odorant pleasantness ratings mean and confidence interval of 95%. (E) FC-PSAP odorant intensity ratings mean and confidence interval of 95%.
HEX again blocked aggression in men but triggered aggression in women
To again ask whether the addition of HEX was associated with an explicit percept, participants used a VAS to rate the stimuli along the primary dimensions of human olfactory perception, namely, intensity and pleasantness (Fig. 4, B and C) (28). Odor ratings were standardized with respect to the entire sample mean and SD. Because odorant ratings again did not distribute normally (Pleasantness: W = 0.98, P = 0.001; intensity: W = 0.75, P = 2.2 × 10−16), we applied a linear mixed model with factors of Sex and Odor and random effects of Participant. Analysis of the pleasantness ratings revealed a significant difference between the sexes in overall pleasantness in the FC-PSAP, no effect of Odor, and a just significant interaction (Sex: F1,43 = 8.07, P = 0.007; Odor: F2,175 = 0.007, P = 0.94; Sex × Odor: F2,175 = 3.08, P = 0.05). Post hoc pairwise contrasts applied to the fitted model (Tukey adjustment for multiple comparisons) revealed that this difference arose from men’s ratings of the odorless control. In other words, this reflected a basic sex difference in reported valence (men Control–women Control: men mean ratings ± SD = 0.3 ± 1.05; women mean ratings ± SD = −0.26 ± 0.87, t43 = 3.09, P = 0.04; men Control–women HEX: men mean VAS = 0.3 ± 1.05; women mean ratings ± SD = −0.32 ± 0.83, t43 = 3.12, P = 0.04). All other comparisons are nonsignificant (all t < 2.78, all P > 0.09) (Fig. 4D). To conclude, there were no differences within sex and a difference across sexes arising from men’s ratings of the odorless control only. The same model applied to intensity ratings revealed no main effect of Sex and no interaction but an effect of Odor (Sex: F1,43 = 1.69, P = 0.2; Odor: F2,175 = 3.88, P = 0.02; Sex × Odor: F2,175 = 0.41, P = 0.66). Post hoc contrasts applied to the fitted model (Tukey adjustment for multiple comparisons) revealed that the intensity differences in the FC-PSAP arose from Control-Blank comparisons (blank mean ratings ± SD = −0.17 ± 0.88; control mean ratings ± SD = 0.15 ± 1.08, t175 = 2.57, P = 0.03), but not Control-HEX comparisons (control mean ratings ± SD = 0.15 ± 1.08; HEX mean ratings ± SD = −0.07 ± −0.96, t175 = 2.14, P = 0.08) (Fig. 4E). In other words, mineral oil is detectable versus blank, but HEX had no impact on perception in this experiment.
In the PSAP, aggressive behavior is typically reported as the aggression/provocation ratio (APR), namely, the aggressive responses divided by the number of provocation events participants experienced. Higher APR values imply increased aggression. Using the within-subjects FC-PSAP, we observed the same behavioral pattern we previously observed using the between-subjects TAP (Fig. 5C). More specifically, a Kruskal-Wallis test on the delta APR with a factor of Sex revealed a significant effect of Sex between odor conditions (χ2 = 9.24, df = 1, P = 0.002, effect size η2 = 0.18). This interaction reflected that HEX drove a significant reduction in aggression by men (APR control = 1.12 ± 1.43, APR HEX = 0.88 ± 1.08, Wilcoxon signed rank test: Z = 2.18, P = 0.03, effect size r = 0.45), yet increased aggression by women (we note that two women were 3 SDs away from the APR mean: one of them from the Control APR mean, and the other from the HEX APR mean; removing these two women from the analysis, the effect in women is APR control = 1.07 ± 1.03, APR HEX = 1.32 ± 1.31, Wilcoxon signed rank test: Z = 2.15, P = 0.03, effect size r = 0.43; if we do not remove these two outliers, we get APR control = 1.34 ± 1.46, APR HEX = 1.66 ± 1.76, Wilcoxon signed rank test: Z = 1.90, P = 0.06, effect size r = 0.41) (Fig. 5C). Thus, in two consecutive experiments, one across-participants and one within-participants, HEX decreased aggression in men but increased aggression in women.
Fig. 5. Exposure to HEX modulated aggressive behavior in a sex-dependent manner within participants.
(A) In a within-subjects design, participants were exposed to an odorant (HEX and Control, counterbalanced for order), while they played a game where their online partner stole their money occasionally (provocation). During the game, they could aggress against that same (nonexistent) person by deducting money from them (Aggression). Each run duration was 8 min, and the anatomical scan duration was 5 min. (B) Pearson’s correlation between basal levels of aggression as measured here [aggression/provocation ratio (APR) in the control condition] and a standard AGQ, r = 0.30, P = 0.04. (C) Scatterplot of the APR under HEX and the APR in the control condition. Each dot is a participant, women (blue) and men (orange). The scatterplot is summarized in a box plot graph. Dotted circles represent outliers (>mean ± 3 SDs). In the box plot, empty circles represent values larger than 1.5 * IQR, thick horizontal lines represent the median, the X is the group mean, the rectangle reflects the IQR (25th to the 75th percentiles), and the whiskers are no more than 1.5 * IQR of the upper and lower hinges. All tests were two-tailed, all centers reflect mean, and all error bars reflect SEM. *P < 0.05, ***P < 0.005.
HEX increased activity in a brain area implicated in the perception of social cues
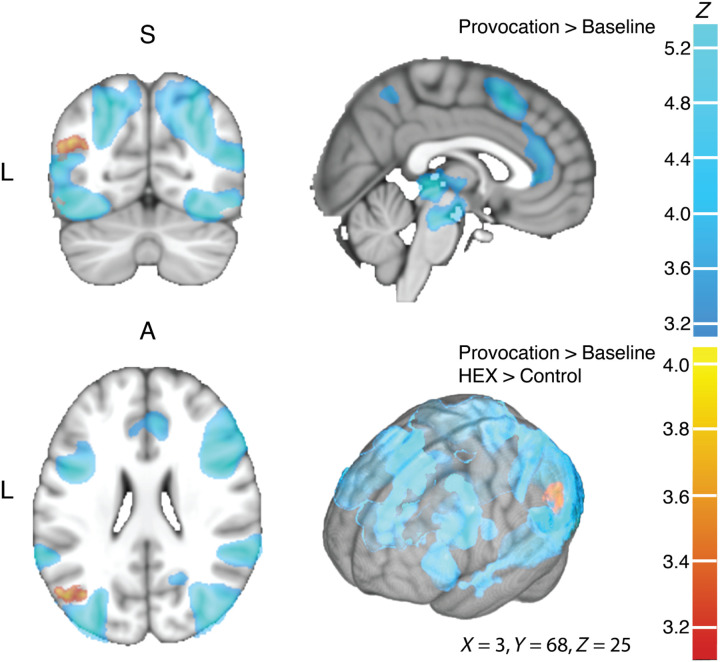
To investigate the impact of HEX on brain response, we conducted a whole-brain voxel-wise statistical parametric map. The analysis of variance (ANOVA) contrast of provocation versus baseline (monetary) revealed a typical salience network pattern of activation (Fig. 6).
Fig. 6. Provocation recruited an extensive brain network.
The group image for both women and men (n = 49), in coronal (top left), sagittal (top right), axial (bottom left), and surface (bottom right) views. In all panels, shades of blue reflect the Provocation > Baseline contrast, and shades of orange reflect the Provocation > Baseline with an additional HEX > Control contrast. In blue, provocation induced activity in the fusiform gyrus, OFC, insula, superior temporal gyrus, anterior cingulate cortex, inferior frontal gyrus, pre-SMA, precuneus, ventral tegmental area, periaqueductal gray area, and thalamus (for full list, coordinates, and peak activation, see table S2). In orange, HEX induced activation in left angular gyrus (AG). Z statistic images were thresholded using clusters determined by Z > 3.1 and a (corrected) cluster significance threshold of P = 0.05 (as described in Materials and Methods).
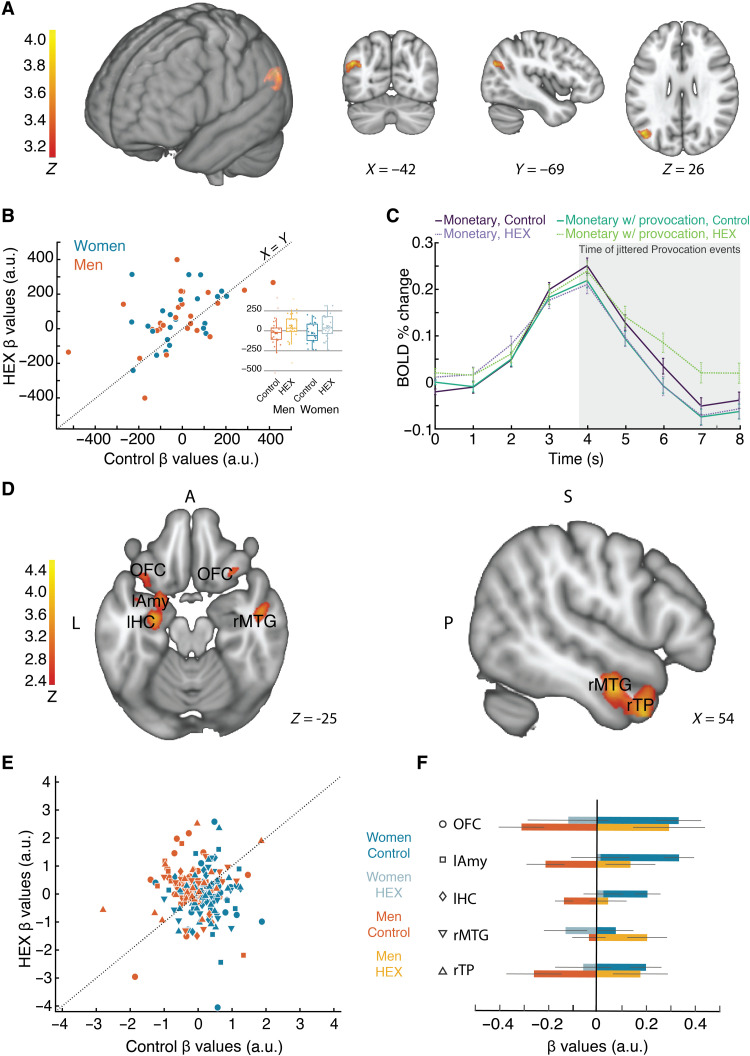
In turn, the ANOVA contrast of provocation versus baseline (monetary; see Materials and Methods) with the added level of HEX versus control in men and women revealed only one but very pronounced significant activation in the left angular gyrus (AG), with no difference between men and women (Figs. 6 and 7A). Although the statistical significance associated with this result is the mapping statistic (P < 0.001, corrected for multiple comparisons), to verify the directional drivers of this effect, we visualized the β values in the resultant left AG region of interest (ROI) (Fig. 7, B and C). A two-tailed ANOVA applied to the β values revealed a main effect of odor (F97 = 6.79 P = 0.01), but no effect of sex (F1,97 = 0, P = 0.95) and no interaction (F1,97 = 0.03, P = 0.87). The main effect of odor reflected increased activation during provocation under HEX [mean β auxiliary units (a.u.) HEX = 57.74 ± 22.76 (SE), mean β (a.u.) control = −26.26 ± 22.37] (we reiterate that these statistics are merely to verify directionality as the statistic associated with this finding remains the mapping statistic alone, namely, P < 0.001, corrected for multiple comparisons). In previous research, the left AG has been implicated in the perception of social cues (34) and was recruited by perception of contextual social cues (35). Moreover, the left AG has been associated with brain mechanisms of aggression (36), and it is hypoactive in aggressive individuals (37). In other words, the AG is part of a social network related to aggression, and here, it was recruited by a body volatile.
Fig. 7. HEX drives sex-specific functional connectivity in the brain substrates of aggression.
(A) Contrast ANOVA statistical parametric map depicting activity greater during provocation versus baseline in HEX versus Control (P < 0.001, corrected). (B) β values extracted from the left AG. Each dot is a woman (blue) or man (orange) participant. The y axis reflects participant’s mean β during HEX, and the x axis reflects participant’s mean β during Control (all during a Provocation). The dotted line is the unit slope line (x = y) such that data under the line reflect increased activation in Control and data above the line reflect increased activation in HEX. (C) Percent signal change in blood oxygen level–dependent (BOLD) activity in the left AG ROI, depicted by condition. (D) Contrast statistical parametric map depicting functional connectivity with the left AG greater during provocation versus baseline in HEX versus Control in Men > Women (P < 0.01, corrected). OFC, orbitofrontal cortex; lAmy, left amygdala; lHC, left hippocampus; rMTG, right medial temporal gyrus; rTP, right temporal pole. (E) β values for functional connectivity with the angular gyrus. Each dot is a woman (blue) or man (orange) participant; shape of dot is as depicted in (F). (F) Bar graph of the β values of functional connectivity of Provocation > Baseline, HEX > Control, and Women > Men for whole-brain connectivity analysis with the AG as a seed region.
HEX modulated functional connectivity in brain networks of aggression
To investigate how the left AG may be modulating aggression under HEX, we investigated its functional connectivity with the entire brain. We applied whole-brain psychophysiological interaction (PPI) analysis (38) using the left functional AG ROI as a seed. We observed a pronounced dissociation by sex, which mirrored behavior. More specifically, HEX significantly modified left AG functional connectivity with three brain regions: the right temporal pole (extended to the middle temporal gyrus), the left amygdala (extended to the left hippocampus), and bilateral lateral orbitofrontal cortex (OFC) (Fig. 7D). Although the statistic associated with these HEX-related changes in functional connectivity is the PPI mapping statistic [P < 0.01 corrected for multiple comparisons (we note that the temporal pole result is stronger, surviving P < 0.001 corrected)], to further explore directionality, we applied ANOVAs to the β values of connectivity. We observed that under provocation, HEX systematically increased connectivity to these regions in men but decreased it in women. This effect recurred in the right temporal pole (F1,97 = 5.60, P = 0.02, men: mean β values HEX = 0.18 ± 0.16; mean β values Control = −0.25 ± 0.16; women: mean β values HEX = −0.05 ± 0.16; mean β values Control = 0.20 ± 0.09), in the left amygdala (F1,97 = 6.49, P = 0.01, men: mean β values HEX = 0.14 ± 0.14, mean β values Control = −0.21 ± 0.11; women: mean β values HEX = 0.01 ± 0.17; mean β values Control = 0.33 ± 0.09), and in the lateral OFC (F1,97 = 8.40, P = 0.005, men: mean β values HEX = 0.29 ± 0.21; mean β values Control = −0.31 ± 0.13; women: mean β values HEX = −0.12 ± 0.24, mean β values Control = 0.33 ± 0.13) (Fig. 7, E and F] (we reiterate that these statistics are merely to verify directionality, as the statistic associated with this finding remains the mapping statistic alone, namely, P < 0.01 corrected for multiple comparisons).
We observe that the temporal pole, amygdala, and OFC are all parts of two brain networks involved in aggressive behavior: social and emotional evaluation and decision-making (39). Thus, our results imply that these regions may act in concert under the modulation of the AG, whereby increased functional connectivity with these regions is associated with reduced aggression (as in men), but decreased connectivity is associated with increased aggression (as in women). Last, when looking at the shared change in FC for both women and men, we see an increase in FC for left motor areas: premotor cortex (PMC) and supplementary motor area (SMA) (all participants were right-handed and used mostly the right hand for aggressive response). This is consistent with previous reports of increased activity in motor and premotor areas in response to provocations (see fig. S7) (11).
DISCUSSION
Impulsive aggression is a major factor in the human condition, yet how exactly aggression is triggered or blocked in the human brain remains unclear (11, 12). Moreover, real-world human impulsive aggression is one of the most sexually dimorphic behaviors (40), yet what brain mechanisms underlie this dimorphism also remains unclear (12). In animals ranging from insects to rodents, aggression is sexually dimorphic at levels ranging from genes to cells, and this dimorphism in aggression has been linked to dimorphism in the olfactory system (41). Here, we find the same in humans. We observed that sniffing a body volatile, namely, HEX, significantly decreased aggression in men yet significantly increased aggression in women. In both men and women, HEX increased brain activity in the AG, a cross-modal integrating hub involved in social cognition (42). In other words, in humans, like in rodents, a “social odor” activates the “social brain.” Moreover, HEX modulated functional connectivity between these substrates of social appraisal (AG) and a network previously associated with aggression. This included modulation of functional connectivity with the temporal pole (TP), an area similarly implicated in social appraisal (43) and aggression (44), and modulation of functional connectivity with the amygdala and OFC, namely, substrates implicated in aggressive execution (10, 12). All this modulation occurred in a sex-dependent manner consistent with behavior: HEX increased connectivity in men but decreased it in women. Thus, HEX may lead to increased or decreased aggression through increased or decreased control by the AG over the amygdala through a circuit involving the TP and OFC. This modulation of social behavior through modulation of functional brain connectivity was similarly observed following intranasal administration of oxytocin, which reduced OFC connectivity with the amygdala, and in this may have reduced negative emotional arousal (45). This further points to what may be considered a physiological counterpart of this brain mechanism: As stress increases, men become more aggressive and women become less aggressive (46). As stress decreases, men become less aggressive and women become more aggressive (47). In this manner, a non–sex-specific effect of HEX on the stress response (always reducing stress) may evolve into a sex-specific effect of HEX on aggression (increased aggression in women yet decreased aggression in men).
The above detailed neuroanatomy and mechanism may underlie a direct circuit from reception to action without the mediation of conscious perception. This echoes rodent circuitry, where OR37B projections bypass the olfactory cortex and connect directly to the paraventricular nucleus of the hypothalamus, where HEX reduces activity in corticotrophin-releasing cells, thus reducing activation of the hypothalamus-pituitary-adrenal axis (16). Although we cannot trace connectivity of a single olfactory receptor subtype in humans, it is tempting to liken the downstream activation in the absence of olfactory cortex activation we observed in response to HEX to the circuit detailed in rodents.
The sex dimorphism in our behavioral and brain results dovetails with previous findings obtained using functional brain imaging (48) and EEG (15) to depict a level of functional brain sex dimorphism in response to social odors that is not matched by any other sensory stimulus that we are aware of. Human functional brain responses to basic auditory and visual cues are generally nondissociable by sex (49), yet here, we could use them alone to discriminate men from women at 79.6% accuracy. This begs the question: what behavioral setting could underlie selection for a body volatile that increases aggression in women but decreases it in men? Or in other words, what could be the ecological relevance of these results? In this respect, we call attention to the setting of infant rearing. Parents across cultures are encouraged to sniff their babies (50), an action that activates brain reward circuits in women (51). Our results imply that sniffing babies may increase aggression in mothers but decrease aggression in fathers. Whereas maternal aggression has a direct positive impact on offspring survival in the animal world (52), paternal aggression has a negative impact on offspring survival (53). This is because maternal aggression (also termed maternal defense behavior) is typically directed at intruders, yet paternal aggression, and more so nonpaternal male aggression, is often directed at the offspring themselves (54, 55). If babies had a mechanism at their disposal that increased aggression in women but decreased it in men, this would likely increase their survival. With the hypothesis in mind that HEX provides babies with exactly such a mechanism, we first note that infant rearing is the one social setting where humans have extensive exposure to conspecific feces, a rich source of HEX (22). We also turned to a recently published analysis of baby-head volatiles (56), yet in contrast to our hypothesis, this report did not mention HEX. We turned to the authors of that report, who explained that the published analysis was not tuned to the near semivolatile range of HEX. With our question in mind, they (now coauthors T.U. and M.O.) sampled an additional 19 babies (fig. S10A), using gas chromatography (GC) × GC–mass spectrometry, and observed that HEX is one of the most abundant baby-head volatiles, evident in 17 of the 19 babies (fig. S10, B and C). Moreover, they also searched for the two additional known ligands of OR37, namely, pentadecanal and heptadecanal, and found both, albeit at levels much lower than HEX. Pentadecanal was evident in 15 babies but only at an average peak area of 56% that of HEX, and heptadecanal was evident in 16 babies but only at average peak area of 45.5% that of HEX (HEX greater than pentadecanal and peptadecanal: Kruskal-Wallis χ2 = 7.65, df = 2, P = 0.02) (fig. S10C). This outcome renders our overall ecological hypothesis plausible and retrospectively supports our selection of HEX as a testing target. In summary, babies emit HEX from their head. This is expected to trigger aggression in women but block aggression in men, and both of these impacts are expected to increase baby survival.
Given all the above, should we label HEX as a human pheromone? Sniffing human bodily secretions such as sweat and tears drives assorted behavioral and physiological effects (7, 24), and body odors may reflect assorted emotional states (57), including aggression (13, 14), but the identity of specific molecular components involved in human social chemosignaling has remained elusive (58). Moreover, the current view on human social chemosignals is that, to the extent that they exist, they likely entail alterations in the ratios of components in complex body odor bouquets and not single molecular species (59). Yet, here, we identify one component, namely, HEX, whose effects can be seen as consistent with those of a mammalian pheromone (60). Previously, the steroidal molecules estratetraenol (EST) and androstadienone (AND) had been proposed as human pheromones, yet this labeling was often rejected, primarily because EST and AND do not clearly trigger or block behavior, nor do they have obvious ecological relevance (59, 61). Here, HEX had a pronounced effect on behavior and, moreover, on the behavior of aggression, a domain dominated by pheromonal communication in most mammals (3). The notion of pheromonal communication was once considered dependent on a functional vomeronasal system, a system that humans may not have (62). More recent views, however, blur this distinction and highlight pheromonal communication through the main olfactory system as well (63–65). Given all this, we think that had we presented an equivalent set of results obtained from mice, very few would argue the pheromone label. In turn, if HEX is a human pheromone, is it a cuing pheromone that is emitted consistently by the sender or a signaling pheromone that is emitted only during appropriate behavioral context (61)? Here, we reach at the primary limitation of this study: Although we think of HEX as a signal, we did not measure its emission as a function of behavior. Had we measured HEX emission under different conditions and found that its emission increased under the endurance of aggression, this would have closed the loop of a signaling pheromone. Such an effort, however, was far beyond the scope of the current study and remains the key missing component for labeling HEX a human signaling pheromone.
Beyond this, we would like to acknowledge several additional limitations in this study: First, although we used various control conditions across experiments (eugenol, mineral oil, and blank air), we did not test any other potential OR37 ligands. The rationale for selecting HEX was detailed in Introduction, but future testing of additional potential ligands remains an important question. Second, we do not know whether the concentrations of HEX that we used were physiological. This is because we do not know the concentrations that humans emit (the existing reports are relative), and we do not know the concentration that actually reached our participants using the current paradigms (e.g., experiment 1 sniff-jar versus experiment 2 olfactometer). Third, regarding our imaging results, we reiterate that correlation is not causation. We identify a brain pattern associated with HEX, and it is tempting to suggest that this pattern is responsible for the observed effects, yet this can only be proven by experiments where the brain mechanism is perturbed, experiments that are very difficult to conduct in human participants. Last, we also acknowledge that our suggested ecological relevance in infant rearing was not directly tested in this study. One may note that there are various forms of aggression, and whereas our tasks measured interpersonal aggression, our infant-rearing hypothesis alludes to paternal/maternal aggression. Thus, although we think it is a plausible hypothesis, it remains to be experimentally verified, and here serves only as an example of possible ecological relevance for our results.
Despite the above limitations, we conclude in stating that sniffing the body odor constituent HEX blocks aggression in men but triggers aggression in women. HEX may exert its effects by modulating functional connectivity between the brain substrates of social appraisal and the brain substrates of aggressive execution. This places chemosignaling at the mechanistic heart of human aggression and poses but one added example to the rapidly growing body of evidence implicating social chemosignaling as a major, albeit mostly subconscious, power in human behavior.
MATERIALS AND METHODS
Taylor aggression paradigm
Participants
To estimate the number of needed participants, we conducted a power analysis. We used the effect size obtained with aggression-related chemosignals (13) and using G*Power software (66) estimated at α = 0.05 and 80% power, a necessary sample of at least 105 participants. On this basis, a total of 127 participants (67 men; mean age, 25.48 ± 3.46; range, 21 to 34) were recruited to a modified TAP (25, 26, 67) after providing written informed consent to procedures that were approved by the Weizmann Institute Institutional Review Board (IRB). Participants were recruited using advertisements and had no history of psychiatric drug use or any neurological or nasal conditions.
Odorant delivery
We used methods we have applied extensively in the past (24). In an across-subjects double-blind design, half of the participants were exposed to carrier alone (10% eugenol, 100 μl, diluted in propylene glycol) (control) and half to HEX masked in 10% eugenol (100 μl, 0.083 M). In brief, before commencement of the TAP, participants were first exposed to the odorant. Exposure started with 11 rating trials (intertrial interval = 25 s, mean ± SD duration of the odorant delivery phase = 363.99 ± 116.13 s). On each trial, the participant sniffed from an unmarked odorant jar for 3 s and then rated pleasantness, intensity, and familiarity of the odorant along a VAS. After the ratings session, an adhesive pad containing 30 μl of the odorant was pasted onto the upper lip of the participant for continued exposure throughout the experiment. Participants and experimenters were of the same sex (three alternating men experimenters and four alternating women experimenters), and it was also noted to participants that their unseen game partner is of the same sex.
Provocation phase
Provocation is a necessary catalyst for aggression in laboratory studies (68). To this end, participants were first antagonized toward a purported game partner (Fig. 1) in a form of the ultimatum game (29). In five rounds, participants were asked to distribute an amount of money (~$9) between themselves and their fictitious game partner. If they were to reach agreement, the distributed amount would be added to their earnings in the experiment. However, the interaction was rigged such that the fictitious game partner refused any fair distribution and agreed only to distributions where he/she received almost the entire amount (>$8). To maintain reliability, the time it took the purported game partner to respond to offers was U-shaped, shorter for low and high amounts. The apparent egregious behavior of the fictitious game partner served as the aggression-provoking mechanism. The mean ± SD duration of the provocation phase was 178.79 ± 55.14 s.
Aggression discharge phase
After the provoking phase, participants engaged in a modified TAP. Participants were again misled to believe that they are playing against the same partner (from the provocation phase), this time in a reaction-time task where they compete to identify a change in shape of an on-screen target. If they were faster to identify, they could blast their opponent with an unpleasant noise (noise blast) at a volume of their choosing. If their game partner was faster, they would endure a noise blast induced by their game partner. Determining which of the participants was faster was done using a responsive calculation. This assured that participants will induce noise blasts in 16 of 27 trials, yet with respect to their actual reaction times. In addition, this served the purpose of, again, maintaining reliability of the experimental setup. Noise blasts were induced using a button box, with six buttons labeled with informative faces (modified from a pain scale for children) (Fig. 2A) (69). The faces portrayed the intensity of the noise blast from the mildest (1) to the most severe (6). Noise blasts that participants heard were randomized in length (~4 s) and volume (mean = 90 dB, coming from a speaker in front of them). The mean ± SD duration of the aggression discharge phase was 254.94 ± 69.48 s.
Questionnaires
Before any experimental manipulation, self-reported mood was measured using a commonly applied 17-item mood questionnaire (fig. S1) (70). At the end of the experiment, we asked participants to rate, using a VAS, general questions probing their social evaluation and willingness to interact with their game partner (figs. S8 and S9). In addition, participants completed the autism quotient questionnaire (AQ), state anxiety questionnaire (STAI), and the Buss and Perry AGQ (fig. S6); details on these questionnaires are in (24). The mean ± SD duration of the exit questionnaire was 160.16 ± 154.28 s. The mean ± SD duration of the mood questionnaire was 37.30 ± 10.11 s.
Statistical analysis
Odor ratings were standardized with respect to the entire sample mean and SD. Odor ratings did not distribute normally (Pleasantness TAP: Shapiro-Wilk W = 0.98, P = 0.001; intensity TAP: W = 0.96, P = 0.002; Pleasantness FC-PSAP: W = 0.98, P = 0.001; intensity FC-PSAP: W = 0.75, P = 2.2 × 10−6). Therefore, we applied a linear mixed model with factors of Sex, Odor, and random effect of participant. Noise-blast volume was entered into a repeated-measures ordinal logistic regression analysis (cumulative link mixed-model fitted with the Laplace approximation) with factors of odorant (HEX/Control), Sex (men/women), and random effects of Participant. We further explored the data using the trial-by-trial mean for each group (16 separate noise-blast trials for each participant). To quantify this, we conducted first a Mann-Whitney test to compare between groups within trial and then an independent two-tailed t test on the trial-by-trial z values. For further validation, we used a random permutation test. We shuffled the odor conditions of the real data and conducted a one-sample two-tailed t test on the shuffled z distributions. We repeated the procedure 10,000 times and created a distribution of the t statistics, resulting from statistical tests on the shuffled z distributions. For reaction times (RT), we first z-scored with respect to the entire group’s mean and SD. RTs did not distribute normally (Shapiro-Wilk: W = 0.49, P < 2.2−16). Therefore, we applied a linear mixed model with factors of Odor, Sex, round, and participant as a random effect. For the exit questionnaire, responses were z-scored to each participant’s range. Self-reported mood was analyzed using a linear mixed model with factors of Sex, Odor, question, and Participant as a random effect.
Exclusions: Participants
Three participants were excluded from the TAP analysis because of technical faults of the acquisition system. Five participants did not answer the exit questionnaire but were included in all other analyses. Trials: In the reaction time analysis, we removed outliers of >mean ± 3 SDs. This resulted in removal of 99 of 3456 trials. This constitutes 2.86% of the total trials and 18.5% of trials in the one participant with the most exclusions.
FC point subtraction aggression paradigm
Participants
To estimate the number of needed participants, we conducted a power analysis. We used the effect size obtained in the TAP to estimate group size using G*Power software. This implied that at α = 0.05 and 80% power, we need to test a sample of 50 participants. On this basis, a total of 58 participants, of which 49 were included in the final analyses (24 women; mean age, 26.98 ± 3.92; range, 19 to 36), participated in a modified PSAP after providing written informed consent to procedures that were approved by the Wolfson Hospital Helsinki Committee and the Weizmann Institute IRB. Participants were recruited using advertisements and had no history of psychiatric drug use or any neurological or nasal conditions. All participants were right-handed. None of the participants participated in both the TAP and the FC-PSAP.
Fist-clench PSAP
Participants were told that their goal is to earn as much money as possible, and that we are interested in the dynamics of the interaction between them and the other participants. Participants actuated the PSAP using FC-activated pressure sensors (MR-compatible). The FC devices were built in-house. They constituted a rubber ball in the hands of the participant, which was linked via 1/4-inch Tygon tubing to a pressure sensor (All Sensors, 1-INCH-Dx-4V-MINI). The sensors were powered at 5 V (DAQ NI USB-6008), and the resultant signal was read using custom software written in MATLAB (code available at GitLab, https://gitlab.com/worg_wis/eva-aggression) and LabChart7 software (ADInstruments, New South Wales, Australia). The software includes electrical noise removal and calculation of a threshold. In the FC-PSAP, participants pressed both sensors simultaneously to earn money (monetary event), using one sensor, no matter which they deducted money from the other participant (aggression). Provocation onsets were prerandomized and held constant across participants. The experimental timeline was such that the first MRI head scout was performed while participants calibrated the pressure sensors using alternating strong and weak presses, repeated three times. This served both the purpose of adapting the system to individual differences in pressure applied and also having the participants accustomed to the use of the FC pressure sensors. Ensuing run duration was 8 min. After completing two runs with one odor condition, an anatomical scan was obtained. During the duration of the anatomical scan (5 min), air in the bore was high throughput vacuumed. Before starting the third run, participants were told that their (fictitious) game partner was replaced, and now, they are interacting with a new participant. This was done to minimize transferring of emotions, strategies, etc., between odor conditions.
Odorant delivery in the MRI
Odorants were delivered using a computer-controlled air-dilution olfactometer that was optimized for naturalistic delivery in the MRI (71). The olfactometer emitted a constant flow of olfactometer air (1.5 l/min) that carried embedded 10-s pulses of odorant (HEX or control). Nasal airflow was constantly precisely monitored using a nasal cannula linked to spirometer (ML141, ADInstruments) and instrumentation amplifier (Power-Lab 16SP, ADInstruments). Because HEX at this concentration and mineral oil have no perceivable odor, we validated precise olfactometer timing with a photoionization detector (RAE Systems, model ppbRAE 3000). In each experiment, there were a total of four runs: two with HEX (CAS #629-80-1, 0.083 M, supplied by TCI and Cayman) and two with control (mineral oil alone, CAS #8042-47-5, Sigma-Aldrich). The order of conditions was counterbalanced, and the given condition was unknown to participants or experimenters. Only 2 of 58 participants reported that they smelled any odor during the experiment. Odor ratings were completed inside the scanner after completion of the FC-PSAP. At the end of the experiment, participants completed an exit questionnaire probing their thoughts, strategies, and attitudes toward their game partners, a general demographics questionnaire, AGQ, AQ, and STAI.
MRI data acquisition
MRI scanning was performed on a 3-T Siemens MAGNETOM Prisma scanner using a 32-channel head coil. Functional data were collected using a T2*-weighted gradient-echo planar imaging sequence. In each run, there were 240 repetitions comprising 56 slices, axial slices tilted 15° toward the anterior commissure-posterior commissure (AC-PC) plane [repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 75°, field of view = 240 × 240 mm, matrix size = 96 × 96, slice thickness of 2.5 mm with no gap, and 2.5 × 2.5 mm2 in-plane resolution], covering the whole brain. Anatomical images were acquired at three-dimensional (3D) T1-weighted magnetization prepared rapid gradient-echo sequence at high resolution (1 × 1 × 1 mm3 voxel, TR = 2300 ms, TE = 2.98 ms, inversion time = 900 ms, and flip angle = 9°).
fMRI data analysis
Neuroimaging data were analyzed using FMRIB’s Software Library (FSL 5.0.9), FMRI Expert Analysis Tool (FEAT v6.00), SPM12 (for PPI analysis), and MATLAB (R2017b, R2018b, and R2020b). FMRI data processing was carried out using FEAT version 6.00, part of FSL (www.fmrib.ox.ac.uk/fsl). Registration of the functional data to the high-resolution structural image was carried out using the boundary-based registration algorithm (72). Registration of the high-resolution structural to standard space images was carried out using FMRIB’s linear image registration tool (FLIRT) (73) and was then further refined using FMRIB’s nonlinear image registration tool (FNIRT) nonlinear registration (74). The following pre-statistics processing was applied: motion correction using MCFLIRT (73), nonbrain removal using brain extraction tool (BET) (73), spatial smoothing using a Gaussian kernel of full width at half maximum 6 mm, grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, and high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with σ = 62.5 s). For each participant, a first-level general linear model included three regressors of monetary (<11 s), provocation (=1 s), and aggressive events (<5 s); onset and offset were determined according to the visual stimuli presented to participants. Only offset times of monetary events are dependent on participants’ behavior. In addition, we regressed out failed events (“none” regressor) and temporal derivatives and modeled out single TRs with excessive motion according to frame-wise displacement >0.9. The signal was convolved with double-gamma hemodynamic response function. First, we modeled each run separately for each participant (four in total) and then combined the runs, adding the odor present for each run (two runs exposed to HEX, two to control, counterbalanced). Then, we grouped the data while adding a parametric modulation of participants’ sex. Time-series statistical analysis was carried out using FMRIB’s improved linear model (FILM) with local autocorrelation correction (75). The second-level analysis, which separated different odor condition runs and combined same odor runs, was carried out using a fixed-effects model by forcing the random effects variance to zero in FLAME (FMRIB’s Local Analysis of Mixed Effects) (76). The group-level analysis, which added participants’ sex and averaged across all group, was carried out using FLAME stage 1 (76). Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P = 0.05. Analysis focused on provocation events for several reasons. First, because the response in question is impulsive aggression, the moment of the provocation is the moment when the reactive spontaneous response happens; this is, by definition, the provocation event. In addition, provocation events were well defined temporally and most reproducible across participants. Last, some participants did not have enough aggressive responses to allow analysis. Moreover, some participants had aggressive responses only in one odor condition and not the other, which severely skews the analysis. In the manuscript, we report a contrast of Provocation > Baseline. Notably, because of the particular design in which provocation events occurred only during monetary events, although technically the contrast is defined as provocation versus baseline, it is de facto a Provocation > Monetary contrast that we are estimating. We refer to it throughout the text as Provocation > Baseline. Because of this design, we also refrain from calculating the percent change and refer to β values as a measure of activation.
PPI analysis
To explore functional connectivity with the AG during provocation, we conducted a whole-brain PPI analysis (38) using the AG ROI as a seed region. The AG ROI was functionally defined from the general linear model (GLM) analysis of the group level. We conducted a whole-brain PPI analysis using FSL, with the first regressor being the provocation events regressor (psychological regressor), the second was the time course of the AG ROI (physiological regressor), and the third was the PPI regressor of the convoluted response (interaction regressor) generated using SPM12. Other regressors were the task regressors as in the GLM model (monetary, aggression, none, and motion according to frame-wise displacement >0.9). The following pre-statistics processing was applied: grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor. Time-series statistical analysis was carried out using FILM (75). Second-level analysis, in which we contrasted the odor conditions and combined same-odor runs, was carried out using a fixed-effects model by forcing the random effects variance to zero in FLAME (76). Group-level analysis, in which we added the sex parameter and averaged across participants, was carried out using FLAME stage 1 (76). Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z > 2.58 and a (corrected) cluster significance threshold of P = 0.05.
Exclusions
One participant reported that she did not believe that the purported participant was real, and was therefore excluded from further analyses. Additional participants were excluded from analyses because of technical problems (n = 5), AQ score above 32 (n = 1 women) [in accordance with (77)], or excessive head movements during the scan (n = 1), retaining 24 women and 25 men in all reported analyses.
Data analysis tools
All analyses and statistical analyses were done using MATLAB R2018a, R2018b, and R2020b (The MathWorks Inc.); FSL 5.0.9 (78); FEAT 6.00 (75); SPM12 (79); and R 3.6.1 (80) (packages: readxl version 1.3.1, tidyverse 1.2.1, Hmisc version 4.3-0, plyr version 1.8.4, RColorBrewer version 1.1-2, reshape2, lme4 version 1.1-21, emmeans version 1.3.5, nlme version 3.1-140, pwr version 1.3-0, and DescTools version 0.99.35).
Acknowledgments
Funding: This work was funded by an ERC AdG grant (SocioSmell 670798) awarded to N.S. We thank M. Buades-Rotger, U. Kramer, K. Bertsch, J. Mumford, Y. Yeshurun, and A. Arzi for valuable guidance. T.U. and M.O. thank T. Hariyama, N. Kanayama, H. Suzuki, and C. Senoh at Hamamatsu Medical University, Japan, for help in collecting baby odor samples. T.U. and M.O. also thank GL Science Inc. (Tokyo, Japan) for the technical support on odor collection and application and LECO JAPAN CORPORATION (Tokyo, Japan) for the technical support on chemical analyses by GC × GC-MS.
Author contributions: N.S., H.B., J.S., and E.M. conceptualized the idea. E.M. planned and created the experiments, acquired, analyzed, interpreted, and visualized the data. D.A. acquired men data for the TAP. E.M., T.W., and K.S. analyzed imaging data. D.H., A.W., and E.M. created the pressure sensor apparatus. E.L. and L.G. handled the olfactometer. S.K. and E.M. performed GCMS analyses. E.M., D.K., and R.Z. performed the enzyme-linked immunosorbent assays. A.R. wrote code for the TAP. R.W., T.S., Y.E.-S., S.A., L.R., N.R., A.R., L.G., E.L., and E.F.-H. helped run participants. T.U. and M.O. contributed baby-head GC-MS results and analysis. E.M. and N.S. wrote the manuscript.
Competing interests: The Weizmann Institute of Science has filed for a patent on the use of HEX to modify human behavior and mood. Other than this, all authors declare that they have no other competing interests.
Data and materials availability: The physical body odors collected for fig. S10 cannot be shared as they dissipate in the gas chromatograph. All other data and materials are available as follows: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. In addition, all the behavioral data are available on Mendeley at https://data.mendeley.com/datasets/6nkh8j8733/1 (dataset DOI: doi:10.17632/6nkh8j8733.1). All brain imaging data are available on OpenNeuro at https://openneuro.org/datasets/ds003791/versions/1.0.0. (dataset DOI: 10.18112/openneuro.ds003791.v1.0.0). All experiment and analyses codes are available on Zenodo at https://zenodo.org/badge/latestdoi/408786167 (DOI: 10.5281/zenodo.5519358).
Supplementary Materials
This PDF file includes:
Figs. S1 to S10
Tables S1 and S2
REFERENCES AND NOTES
- 1.Mykytowycz R., Goodrich B. S., Skin glands as organs of communication in mammals. J. Invest. Dermatol. 62, 124–131 (1974). [DOI] [PubMed] [Google Scholar]
- 2.Novotny M., Harvey S., Jemiolo B., Alberts J., Synthetic pheromones that promote inter-male aggression in mice. Proc. Natl. Acad. Sci. U.S.A. 82, 2059–2061 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamero P., Marton T. F., Logan D. W., Flanagan K., Cruz J. R., Saghatelian A., Cravatt B. F., Stowers L., Identification of protein pheromones that promote aggressive behaviour. Nature 450, 899–902 (2007). [DOI] [PubMed] [Google Scholar]
- 4.McGlone J. J., Curtis S. E., Banks E. M., Evidence for aggression-modulating pheromones in prepuberal pigs. Behav. Neural Biol. 47, 27–39 (1987). [DOI] [PubMed] [Google Scholar]
- 5.Ferrero D. M., Moeller L. M., Osakada T., Horio N., Li Q., Roy D. S., Cichy A., Spehr M., Touhara K., Liberles S. D., A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nature 502, 368–371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanas U., Terkel J., Mole-rat harderian gland secretions inhibit aggression. Anim. Behav. 54, 1255–1263 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Gelstein S., Yeshurun Y., Rozenkrantz L., Shushan S., Frumin I., Roth Y., Sobel N., Human tears contain a chemosignal. Science 331, 226–230 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Oh T. J., Kim M. Y., Park K. S., Cho Y. M., Effects of chemosignals from sad tears and postprandial plasma on appetite and food intake in humans. PLOS ONE 7, e42352 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chernyak N., Leimgruber K. L., Dunham Y. C., Hu J., Blake P. R., Paying back people who harmed us but not people who helped us: Direct negative reciprocity precedes direct positive reciprocity in early development. Psychol. Sci. 30, 1273–1286 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Nelson R. J., Trainor B. C., Neural mechanisms of aggression. Nat. Rev. Neurosci. 8, 536–546 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Fanning J. R., Keedy S., Berman M. E., Lee R., Coccaro E. F., Neural correlates of aggressive behavior in real time: A review of fMRI studies of laboratory reactive aggression. Curr. Behav. Neurosci. Rep. 4, 138–150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanigan M. E., Russo S. J., Recent advances in the study of aggression. Neuropsychopharmacology 44, 241–244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutic S., Parma V., Brünner Y. F., Freiherr J., You smell dangerous: Communicating fight responses through human chemosignals of aggression. Chem. Senses 41, 35–43 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Mutic S., Brünner Y. F., Rodriguez-Raecke R., Wiesmann M., Freiherr J., Chemosensory danger detection in the human brain: Body odor communicating aggression modulates limbic system activation. Neuropsychologia 99, 187–198 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Pause B. M., Storch D., Lübke K. T., Chemosensory communication of aggression: Women’s fine-tuned neural processing of male aggression signals. Philos. Trans. R. Soc. B 375, 20190270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein B., Bautze V., Maier A. M., Deussing J., Breer H., Strotmann J., Activation of the mouse odorant receptor 37 subsystem coincides with a reduction of novel environment-induced activity within the paraventricular nucleus of the hypothalamus. Eur. J. Neurosci. 41, 793–801 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Tirindelli R., Dibattista M., Pifferi S., Menini A., From pheromones to behavior. Physiol. Rev. 89, 921–956 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Nieberding C. M., Fischer K., Saastamoinen M., Allen C. E., Wallin E. A., Hedenström E., Brakefield P. M., Cracking the olfactory code of a butterfly: The scent of ageing. Ecol. Lett. 15, 415–424 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Tsunoda M., Miyamichi K., Eguchi R., Sakuma Y., Yoshihara Y., Kikusui T., Kuwahara M., Touhara K., Identification of an intra-and inter-specific tear protein signal in rodents. Curr. Biol. 28, 1213–1223.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Hoppe R., Lambert T. D., Samollow P. B., Breer H., Strotmann J., Evolution of the “OR37” subfamily of olfactory receptors: A cross-species comparison. J. Mol. Evol. 62, 460–472 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Mainland J. D., Keller A., Li Y. R., Zhou T., Trimmer C., Snyder L. L., Moberly A. H., Adipietro K. A., Liu W. L. L., Zhuang H., Zhan S., Lee S. S., Lin A., Matsunami H., The missense of smell: Functional variability in the human odorant receptor repertoire. Nat. Neurosci. 17, 114–120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lacy Costello B., Amann A., al-Kateb H., Flynn C., Filipiak W., Khalid T., Osborne D., Ratcliffe N. M., A review of the volatiles from the healthy human body. J. Breath Res. 8, 014001 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Jha S. K., Marina N., Liu C., Hayashi K., Human body odor discrimination by GC-MS spectra data mining. Anal. Methods 7, 9549–9561 (2015). [Google Scholar]
- 24.Endevelt-Shapira Y., Perl O., Ravia A., Amir D., Eisen A., Bezalel V., Rozenkrantz L., Mishor E., Pinchover L., Soroka T., Honigstein D., Sobel N., Altered responses to social chemosignals in autism spectrum disorder. Nat. Neurosci. 21, 111–119 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Taylor S. P., Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression 1. J. Pers. 35, 297–310 (1967). [DOI] [PubMed] [Google Scholar]
- 26.M. Elson, FlexibleMeasures. com: Competitive reaction time task (2016); 10.17605/OSF.IO/4G7FV. [DOI]
- 27.Jacob S., McClintock M. K., Psychological state and mood effects of steroidal chemosignals in women and men. Horm. Behav. 37, 57–78 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Khan R. M., Luk C. H., Flinker A., Aggarwal A., Lapid H., Haddad R., Sobel N., Predicting odor pleasantness from odorant structure: Pleasantness as a reflection of the physical world. J. Neurosci. 27, 10015–10023 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Güth W., On ultimatum bargaining experiments—A personal review. J. Econ. Behav. Organ. 27, 329–344 (1995). [Google Scholar]
- 30.Chester D. S., Lasko E. N., Validating a standardized approach to the Taylor Aggression Paradigm. Soc. Psychol. Personal. Sci. 10, 620–631 (2019). [Google Scholar]
- 31.Gan G., Preston-Campbell R. N., Moeller S. J., Steinberg J. L., Lane S. D., Maloney T., Parvaz M. A., Goldstein R. Z., Alia-Klein N., Reward versus retaliation—The role of the mesocorticolimbic salience network in human reactive aggression. Front. Behav. Neurosci. 10, 179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Cunha-Bang S., Fisher P. M., Hjordt L. V., Perfalk E., Persson Skibsted A., Bock C., Ohlhues Baandrup A., Deen M., Thomsen C., Sestoft D. M., Knudsen G. M., Violent offenders respond to provocations with high amygdala and striatal reactivity. Soc. Cogn. Affect. Neurosci. 12, 802–810 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buss A. H., Perry M., The aggression questionnaire. J. Pers. Soc. Psychol. 63, 452–459 (1992). [DOI] [PubMed] [Google Scholar]
- 34.Allison T., Puce A., McCarthy G., Social perception from visual cues: Role of the STS region. Trends Cogn. Sci. 4, 267–278 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Zaki J., Hennigan K., Weber J., Ochsner K. N., Social cognitive conflict resolution: Contributions of domain-general and domain-specific neural systems. J. Neurosci. 30, 8481–8488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miczek K. A., de Almeida R. M., Kravitz E. A., Rissman E. F., de Boer S. F., Raine A., Neurobiology of escalated aggression and violence. J. Neurosci. 27, 11803–11806 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raine A., Buchsbaum M., LaCasse L., Brain abnormalities in murderers indicated by positron emission tomography. Biol. Psychiatry 42, 495–508 (1997). [DOI] [PubMed] [Google Scholar]
- 38.Gitelman D. R., Penny W. D., Ashburner J., Friston K. J., Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. Neuroimage 19, 200–207 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Potegal M., Temporal and frontal lobe initiation and regulation of the top-down escalation of anger and aggression. Behav. Brain Res. 231, 386–395 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Archer J., Sex differences in aggression in real-world settings: A meta-analytic review. Rev. Gen. Psychol. 8, 291–322 (2004). [Google Scholar]
- 41.Keverne E. B., Pheromones, vomeronasal function, and gender-specific behavior. Cell 108, 735–738 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Seghier M. L., The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist 19, 43–61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson I. R., Plotzker A., Ezzyat Y., The enigmatic temporal pole: A review of findings on social and emotional processing. Brain 130, 1718–1731 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Franzen E., Myers R., Neural control of social behavior: Prefrontal and anterior temporal cortex. Neuropsychologia 11, 141–157 (1973). [DOI] [PubMed] [Google Scholar]
- 45.Riem M. M. E., van IJzendoorn M. H., Tops M., Boksem M. A. S., Rombouts S. A. R. B., Bakermans-Kranenburg M. J., No laughing matter: Intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology 37, 1257–1266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verona E., Kilmer A., Stress exposure and affective modulation of aggressive behavior in men and women. J. Abnorm. Psychol. 116, 410–421 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Prasad S., Narayanan J., Lim V. K. G., Koh G. C. H., Koh D. S. Q., Mehta P. H., Preliminary evidence that acute stress moderates basal testosterone’s association with retaliatory behavior. Horm. Behav. 92, 128–140 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Savic I., Berglund H., Gulyas B., Roland P., Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in humans. Neuron 31, 661–668 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Joel D., Berman Z., Tavor I., Wexler N., Gaber O., Stein Y., Shefi N., Pool J., Urchs S., Margulies D. S., Liem F., Hänggi J., Jäncke L., Assaf Y., Sex beyond the genitalia: The human brain mosaic. Proc. Natl. Acad. Sci. U.S.A. 112, 15468–15473 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okamoto M., Shirasu M., Fujita R., Hirasawa Y., Touhara K., Child odors and parenting: A survey examination of the role of odor in child-rearing. PLOS ONE 11, e0154392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lundström J. N., Mathe A., Schaal B., Frasnelli J., Nitzsche K., Gerber J., Hummel T., Maternal status regulates cortical responses to the body odor of newborns. Front. Psychol. 4, 597 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maestripieri D., Functional aspects of maternal aggression in mammals. Can. J. Zool. 70, 1069–1077 (1992). [Google Scholar]
- 53.Ebensperger L. A., Strategies and counterstrategies to infanticide in mammals. Biol. Rev. 73, 321–346 (1998). [Google Scholar]
- 54.Dulac C., O’Connell L. A., Wu Z., Neural control of maternal and paternal behaviors. Science 345, 765–770 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isogai Y., Wu Z., Love M. I., Ahn M. H.-Y., Bambah-Mukku D., Hua V., Farrell K., Dulac C., Multisensory logic of infant-directed aggression by males. Cell 175, 1827–1841.e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uebi T., Hariyama T., Suzuki K., Kanayama N., Nagata Y., Ayabe-Kanamura S., Yanase S., Ohtsubo Y., Ozaki M., Sampling, identification and sensory evaluation of odors of a newborn baby’s head and amniotic fluid. Sci. Rep. 9, 12759 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Groot J. H. B., Smeets M. A. M., Human fear chemosignaling: Evidence from a meta-analysis. Chem. Senses 42, 663–673 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Smeets M. A. M., Rosing E. A. E., Jacobs D. M., van Velzen E., Koek J. H., Blonk C., Gortemaker I., Eidhof M. B., Markovitch B., de Groot J., Semin G. R., Chemical fingerprints of emotional body odor. Metabolites 10, 84 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wysocki C. J., Preti G., Facts, fallacies, fears, and frustrations with human pheromones. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 281, 1201–1211 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Stowers L., Marton T. F., What is a pheromone? Mammalian pheromones reconsidered. Neuron 46, 699–702 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Wyatt T. D., The search for human pheromones: The lost decades and the necessity of returning to first principles. Proc. R. Soc. B Biol. Sci. 282, 20142994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meredith M., Human vomeronasal organ function: A critical review of best and worst cases. Chem. Senses 26, 433–445 (2001). [DOI] [PubMed] [Google Scholar]
- 63.Yoon H., Enquist L. W., Dulac C., Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123, 669–682 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Baum M. J., Cherry J. A., Processing by the main olfactory system of chemosignals that facilitate mammalian reproduction. Horm. Behav. 68, 53–64 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Kang N., Baum M. J., Cherry J. A., A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur. J. Neurosci. 29, 624–634 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faul F., Erdfelder E., Lang A.-G., Buchner A., G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Elson M., Mohseni M. R., Breuer J., Scharkow M., Quandt T., Press CRTT to measure aggressive behavior: The unstandardized use of the competitive reaction time task in aggression research. Psychol. Assess. 26, 419–432 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Bushman B. J., Baumeister R. F., Stack A. D., Catharsis, aggression, and persuasive influence: Self-fulfilling or self-defeating prophecies? J. Pers. Soc. Psychol. 76, 367–376 (1999). [DOI] [PubMed] [Google Scholar]
- 69.McGrath P. A., Seifert C. E., Speechley K. N., Booth J. C., Stitt L., Gibson M. C., A new analogue scale for assessing children’s pain: An initial validation study. Pain 64, 435–443 (1996). [DOI] [PubMed] [Google Scholar]
- 70.Levenson R. W., Ekman P., Friesen W. V., Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology 27, 363–384 (1990). [DOI] [PubMed] [Google Scholar]
- 71.Gorodisky L., Livne E., Weiss T., Weissbrod A., Weissgross R., Mishor E., Furman-Haran E., Sobel N., Odor canopy: A method for comfortable odorant delivery in MRI. Chem. Senses 46, bjaa085 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greve D. N., Fischl B., Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenkinson M., Bannister P., Brady M., Smith S., Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002). [DOI] [PubMed] [Google Scholar]
- 74.J. L. Andersson, M. Jenkinson, S. Smith, Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford 2, e21 (2007).
- 75.Woolrich M. W., Ripley B. D., Brady M., Smith S. M., Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14, 1370–1386 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Woolrich M., Robust group analysis using outlier inference. Neuroimage 41, 286–301 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E., The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 31, 5–17 (2001). [DOI] [PubMed] [Google Scholar]
- 78.Smith S. M., Jenkinson M., Woolrich M. W., Beckmann C. F., Behrens T. E. J., Johansen-Berg H., Bannister P. R., de Luca M., Drobnjak I., Flitney D. E., Niazy R. K., Saunders J., Vickers J., Zhang Y., de Stefano N., Brady J. M., Matthews P. M., Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004). [DOI] [PubMed] [Google Scholar]
- 79.W. D. Penny, K. J. Friston, J. T. Ashburner, S. J. Kiebel, T. E. Nichols, Statistical Parametric Mapping: The Analysis of Functional Brain Images (Elsevier, 2011). [Google Scholar]
- 80.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S10
Tables S1 and S2