INTRODUCTION
The term anaphylaxis was originally coined by Charles Richet and Paul Portier in 1902 based on experiments intended to immunize dogs against toxins from the Mediterranean snakelocks sea anemone (Anemonia sulcata).1 However, in contrast to expectations, subsequent vaccinations caused the dogs to react with wheezing, vomiting, and death. Richet and Portier labeled this lack of protection as anaphylaxis (ana = absence + phylaxis = protection in Greek).
ANAPHYLAXIS DIAGNOSIS
Although anaphylaxis is a potentially life-threatening allergic reaction, reactions can range in severity from mild and self-limited to fatal. Although allergic reactions are typically limited to a single organ system (eg, skin), anaphylaxis typically, although not always, involves multiple organ systems. The diagnosis of anaphylaxis is challenging because of the wide range of potential clinical manifestations and the fact that the line differentiating an allergic reaction and anaphylaxis is not always easily discernible. The difficulty in diagnosing anaphylaxis has resulted in under-recognition and undertreatment in the emergency department.2
The clinical diagnosis of anaphylaxis can be aided by the use of diagnostic criteria. Currently, the most widely accepted clinical diagnostic criteria are the National Institutes of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network (NIAID/FAAN) criteria (Box 1).3 The NIAID/FAAN criteria were proposed by an international multidisciplinary symposium in 2005 and consist of 3 criteria. Only 1 criterion needs to be met for the clinical diagnosis of anaphylaxis to be highly likely. The first criterion requires the acute onset of signs or symptoms associated with mucocutaneous manifestations along with signs or symptoms of respiratory system involvement and/or cardiovascular involvement. For example, a patient who experiences the sudden onset of hives associated with difficulty breathing would fulfill the first criterion even in the absence of a clear inciting allergen. The second criterion requires sudden onset of symptoms after exposure to a likely allergen or other trigger along with signs or symptoms involving 2 organ systems, including mucocutaneous, respiratory, cardiovascular, or gastrointestinal. The third criterion requires sudden onset of hypotension after exposure to a known allergen.
Box 1. National Institutes of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network clinical criteria for diagnosing anaphylaxis.
Anaphylaxis is highly likely when any of the following 3 criteria are fulfilled:
-
Acute onset of an illness (minutes to several hours) with involvement of the skin, mucosal tissue, or both (eg, generalized hives, pruritus or flushing, swollen lips-tongue-uvula)
And at least one of the following- Respiratory compromise (eg, dyspnea, wheeze-bronchospasm, stridor, reduced peak expiratory flow [PEF], hypoxemia)
- Reduced blood pressure (BP) or associated symptoms of end-organ dysfunction (eg, hypotonia [collapse], syncope, incontinence)
- Two or more of the following that occur rapidly after exposure to a likely allergen for that patient (minutes to several hours)
- Involvement of the skin-mucosal tissue (eg, generalized hives, itch-flush, swollen lips-tongue-uvula)
- Respiratory compromise (eg, dyspnea, wheeze-bronchospasm, stridor, reduced PEF, hypoxemia)
- Reduced BP or associated symptoms (eg, hypotonia [collapse], syncope, incontinence)
- Persistent gastrointestinal symptoms (eg, crampy abdominal pain, vomiting)
- Reduced BP after exposure to known allergen for that patient (minutes to several hours)
- Infants and children: low systolic BP (age specific) or greater than 30% decrease in systolic BPa
- Adults: systolic BP of less than 90 mm Hg or greater than 30% decrease from that person’s baseline
aLow systolic blood pressure for children is defined as less than 70 mm Hg from 1 month to 1 year, less than (70 mm Hg + [2 × age]) from 1 to 10 years, and less than 90 mm Hg from 11 to 17 years.
From Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report–second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med 2006;47(4):373 to 80; with permission.
The NIAID/FAAN criteria have been widely adopted4 and both retrospectively5 and prospectively studied. They were found to be 95% sensitive and 71% specific in a prospective validation study among emergency department patients.6 This means that for every 100 patients with anaphylaxis, 95 will meet one of the criteria. But that among 100 patients who meet NIAID/FAAN criteria, only 71 will have anaphylaxis. Thus, it is remains imperative that clinicians use clinical judgment when diagnosing anaphylaxis.
In 2019, the World Allergy Organization proposed a revision to the NIAID/FAAN criteria (Box 2).7 The rationale for the proposed refinement was to simplify the existing criteria and recognize that some cases of anaphylaxis may involve primarily respiratory (eg, wheezing), laryngeal (eg, stridor, vocal changes or odynophagia), or cardiovascular symptoms (eg, hypotension) in the absence of other organ system involvement. Although this is likely to be a small subset of patients, this is a critical subset to recognize. Furthermore, the revision recognizes the potential for delayed presentations that can occur with alpha-Gal mediated reactions or with immunotherapy. Future studies are needed to determine the clinical utility of the revised criteria.
Box 2. Amended criteria for the diagnosis of anaphylaxis, proposed by the WAO Anaphylaxis Committee, 2019.
Anaphylaxis is highly likely when any of the following 2 criteria are fulfilled:
-
Acute onset of an illness (minutes to several hours) with involvement of the skin, mucosal tissue, or both (eg, generalized hives, pruritus or flushing, swollen lips-tongue-uvula)
And at least one of the following:- Respiratory compromise (eg, dyspnea, wheeze-bronchospasm, stridor, reduced PEF, hypoxemia)
- Reduced BP or associated symptoms of end-organ dysfunction (eg, hypotonia [collapse], syncope, incontinence)
- Severe gastrointestinal symptoms (eg, severe crampy abdominal pain, repetitive vomiting), especially after exposure to nonfood allergens
Acute onset of hypotensiona or bronchospasmb or laryngeal involvementc after exposure to a known or highly probable allergend for that patient (minutes to several hourse), even in the absence of typical skin involvement
aHypotension defined as a decrease in systolic BP greater than 30% from that person’s baseline, or. i. Infants and children under 10 y: systolic BP less than (70 mm Hg + [2 × age in years]). ii. Adults: systolic BP less than less than 90 mm Hg.
bExcluding lower respiratory symptoms triggered by common inhalant allergens or food allergens perceived to cause inhalational reactions in the absence of ingestion.
cLaryngeal symptoms include: stridor, vocal changes, odynophagia.
dAn allergen is a substance (usually a protein) capable of triggering an immune response that can result in an allergic reaction. Most allergens act through an IgE-mediated pathway, but some non-allergen triggers can act independent of IgE (for example, via direct activation of mast cells).
eMost allergic reactions occur rapidly, but delayed reactions, with onset up to 10 hours after ingestion, may occur for some food allergens (eg, alpha-Gal) or secondary to immunotherapy.
Adapted from Cardona V, Ansotegui IJ, Ebisawa M, et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J 2020;13(10):100472; with permission.
Fortunately, most allergic and anaphylactic reactions are self-limited and not life-threatening. However, the inability to predict when a reaction will become life-threatening necessitates early recognition and prompt treatment with epinephrine to prevent progression. Furthermore, some allergic reactions should be treated with epinephrine before anaphylaxis diagnostic clinical criteria are met. For example, a patient who has a history of a peanut allergy with prior severe anaphylactic reactions and develops hives after a peanut exposure should be treated promptly to halt reaction progression. Conversely, a patient whose symptoms have resolved by the time of his or her emergency department evaluation may no longer require epinephrine even if the initial symptoms met anaphylaxis diagnostic criteria. In a study of epinephrine administration for emergency department anaphylaxis patients, allergist-immunologists agreed with the emergency department management for 98% of patients despite the fact that only 70% of patients received epinephrine either before or during their emergency department evaluation.8 These findings demonstrate that some patients who have experienced resolution of their anaphylactic reaction prior to emergency department arrival do not require epinephrine administration. However, even if patients do not require epinephrine administration in the emergency department, they should still receive the diagnosis of anaphylaxis, a prescription for self-injectable epinephrine, education regarding risk of biphasic and future reactions, and referral for follow-up with an allergist-immunologist.2,9
DEFINITIONS OF PERSISTENT, REFRACTORY, AND BIPHASIC ANAPHYLAXIS
Following treatment with intramuscular epinephrine, many patients with anaphylaxis experience symptom resolution. However, some patients have persistent symptoms necessitating treatment with additional epinephrine doses or life-saving resuscitative interventions (eg, positive pressure ventilation for patients with respiratory failure or vasopressors for those in shock).10 On the other hand, some patients may develop recurrent symptoms following an initial asymptomatic period and without repeat exposure to the original trigger, referred to as biphasic or late phase reactions.10,11 Taking into account the possibility that symptoms may return, ED clinicians must determine the need for prolonged observation or whether to hospitalize patients for monitoring.
Until recently, there were inconsistent definitions used to describe these disparate clinical courses, thus making it challenging to conduct comparative studies to identify the prevalence and risk factors for these outcomes, or to standardize ED management guidelines including optimal lengths of observation or hospitalization criteria. To account for the lack of standardized anaphylaxis outcome definitions, a multidisciplinary group of researchers developed consensus definitions for persistent, refractory, and biphasic anaphylaxis (Box 3) to harmonize outcomes in clinical care and research.10 Application of the definitions in clinical care will help standardize communication among providers, patients, and families, and their use in research will help elucidate the true prevalence and risk factors for these outcomes with the ultimate goal of optimizing and standardizing emergency department management guidelines.
Box 3. Clinical criteria for diagnosing persistent, refractory, and biphasic anaphylaxis.
Persistent anaphylaxis is highly likely when there isa presence of symptoms/examination findings that fulfill the 2006 NIAID/FAAN anaphylaxis criteria that persist for at least 4 hours1
Refractory anaphylaxis is highly likely when both of the following 2 criteria are fulfilled:b
Presence of anaphylaxis following appropriate epinephrine dosing and symptom-directed medical management (eg, intravenous fluid bolus for hypotension)
The initial reaction must be treated with 3 or more appropriate doses of epinephrine (or initiation of an intravenous epinephrine infusion)c
Biphasic anaphylaxis is highly likely when all of the following 4 criteria are fulfilledd:
New/recurrent symptoms/examination findings must fulfill the 2006 NIAID/FAAN anaphylaxis criteria1
Initial symptoms/examination findings must completely resolve prior to the onset of new/recurrent symptoms/examination findings
There cannot be allergen repeat exposure prior to the onset of new/recurrent symptoms/examination findings
New/recurrent symptoms/examination findings must occur within 1 to 48 hours from complete resolution of initial symptoms/examination findings.
aThe diagnosis of persistent anaphylaxis is independent of the management of the initial reaction.
bRefractory anaphylaxis is not dependent on the duration of symptoms/examination findings.
cAppropriate epinephrine dosing: 0.01 mg/kg intramuscular epinephrine, maximum single dose 0.5 mg. Also includes manufacturer recommended dosing for epinephrine autoinjectors.
dThe diagnosis of biphasic anaphylaxis is independent of the management of the initial reaction.
From Dribin TE, Sampson HA, Camargo Jr CA, et al. Persistent, refractory, and biphasic anaphylaxis: A multidisciplinary Delphi study. J Allergy Clin Immunol 2020;146(5):1089–96; with permission.
SEVERITY GRADING SYSTEM FOR ACUTE ALLERGIC REACTIONS
As noted previously, anaphylaxis occurs on a severity continuum from mild (requiring minimal interventions) to potentially life-threatening or fatal reactions. Unfortunately, clinical care and research are hampered by the lack of a uniformly accepted grading system to measure reaction severity during the course of reactions, including for initial, persistent, and recurrent/new symptoms. This makes it difficult to evaluate the true prevalence of severe reactions, and to tailor management and therapeutic strategies accordingly. To account for this gap, researchers recently developed a consensus severity grading system for acute allergic reactions to standardize research outcomes and communication among providers, patients, and families (Fig. 1).12 The grading system is optimal, because it can be used to measure reaction severity for anaphylactic and nonanaphylactic reactions, for all patient ages (children and adults), and it accounts for subjective symptoms (eg, throat tightness) in addition to symptoms specific to infants and young children. Before the grading system can be applied in clinical care, it must be validated prospectively; therefore, it is not intended to be used to inform management decisions including whether to administer epinephrine.
Fig. 1.
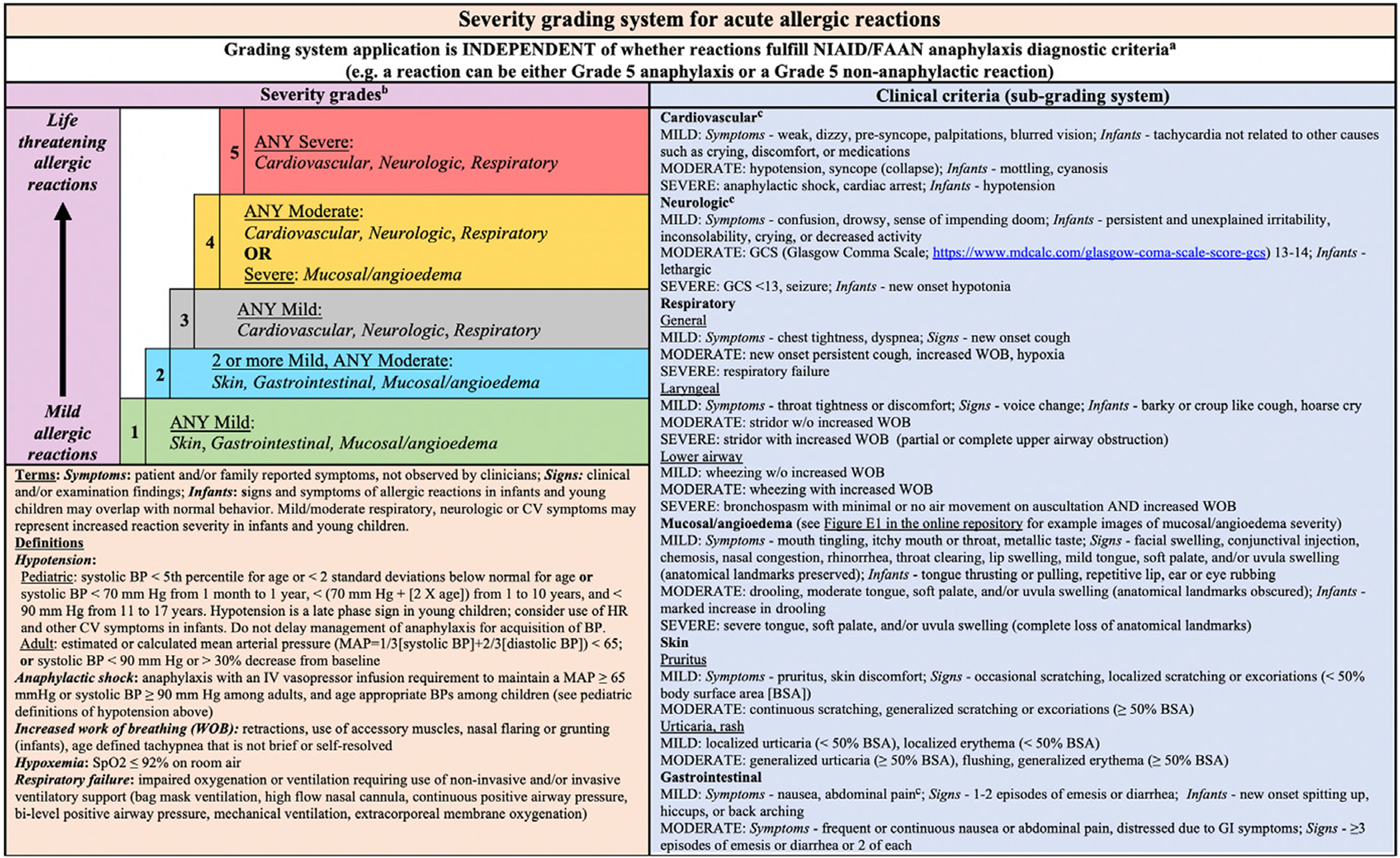
Severity grading system for acute allergic reactions. aFor patients with multiple symptoms, reaction severity is based on the most severe symptom; symptoms that constitute more severe grades always supersede symptoms from less severe grades. The grading system can be used to assign reaction severity at any time during the course of reactions; reactions may progress rapidly (within minutes) from one severity grade to another. The grading system does not dictate management decisions; reactions of any severity grade may require treatment with epinephrine. bPatients with severe cardiovascular and/or neurologic involvement may have urinary or stool incontinence. However, the significance of incontinence as an isolated symptom is unclear, and it is therefore not included as a symptom in the subgrading system. cAbdominal pain may also result from uterine cramping. (From Dribin TE, Schnadower D, Spergel JM, et al. Severity grading system for acute allergic reactions: a multidisciplinary Delphi study J Allergy Clin Immunol 2021;148(1):173–181: with permission.)
ANAPHYLAXIS PATHOPHYSIOLOGY
Anaphylaxis is typically a multiorgan phenomenon involving a broad range of effector cells including mast cells, basophils, neutrophils, macrophages, and platelets. From a mechanistic standpoint, anaphylaxis can be categorized as immunologic, nonimmunologic, or idiopathic, with the latter category caused by an unidentified allergen or underlying mastocytosis (clonal mast cell disorder) (Fig. 2).13 Immunologic anaphylaxis can be further subcategorized to immunoglobulin E (IgE)-mediated (eg, food, drugs, and insect stings) and IgE-independent forms, which include immunoglobulin G (IgG)-dependent anaphylaxis (eg, high molecular weight iron dextran, infusion of human monoclonal antibodies such as infliximab) and complement-mediated (eg, oversulfated chondroitin sulfate-contaminated heparin and polyethylene glycols). Mixed reactions involving both IgE and non-IgE mediated pathways can also occur with chemotherapy. Nonimmunologic anaphylaxis may be caused by direct mediator release from mast cells and basophils (eg, opioids), physical factors (eg, exercise, heat, and sunlight/UV radiation), contact system activation (eg, dialysis membranes), and arachidonic acid metabolism disruptions (eg, nonsteroidal anti-inflammatory drugs [NSAIDs]).
Fig. 2.
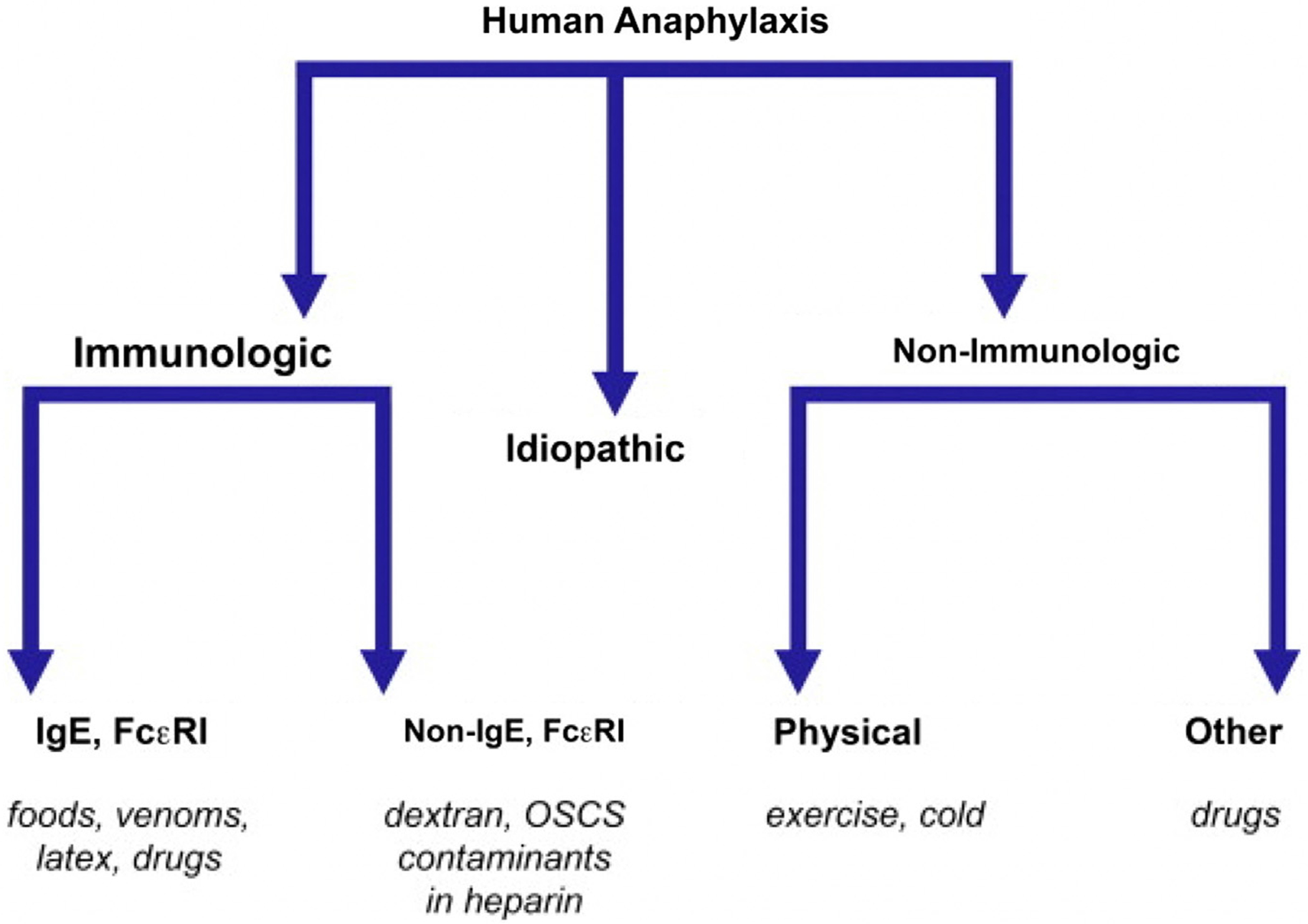
Mechanisms underlying human anaphylaxis. (From Simons FE. Anaphylaxis. J Allergy Clin Immunol 2010;125(2 Suppl 2):S161–8; with permission.)
The degranulation of mast cells and basophils leads to the release of mediators that orchestrate the various systemic manifestations that define anaphylaxis. Such mediators include histamine, platelet-activating factor (PAF), cysteinyl leukotrienes (CysLTs), and anaphylatoxins. Histamine targets multiple organ systems and triggers various signs and symptoms involving the upper respiratory (sneezing and angioedema), lower respiratory (cough and wheezing), digestive (vomiting and diarrhea), cardiovascular (tachycardia and hypotension), and skin systems (flushing and urticaria) (Fig. 3).14 PAF is a lipid-derived mediator of anaphylaxis produced by platelets, neutrophils, mast cells, and macrophages. PAF has effects on the skin and cardiovascular systems, and its effects are thought to be independent of mast cell degranulation.15 Increased levels of PAF have been shown to correlate with increasing anaphylaxis grade severity.16 CysLTs represent a third mediator category and include LTB4, LTC4, and LTD4.17 CysLTs are produced from arachidonic acid by mast cells, basophils, and macrophages. CysLTs and their metabolites are increased during anaphylaxis and have been shown to induce wheal and flare reactions along with bronchoconstriction.18 Anaphylatoxins (C3a, C4a, and C5a) represent the fourth category of mediators. These are small polypeptides that are potent inflammatory mediators that activate mast cells and basophils. Elevated levels of anaphylatoxins have also been correlated with anaphylaxis severity.14 Based on mouse models, anaphylatoxins may mediate similar effects as other mediators and work in a redundant fashion.14 Anaphylaxis may also induce changes in other mediators including prostaglandins, chemokines/cytokines, and tryptase. With regards to the latter, tryptase is a serine protease released by mast cells and basophils that possesses pro-inflammatory effects triggering tissue edema, chemokine secretion, and subsequent neutrophil recruitment. Tryptase is a biomarker that can be measured 15 minutes to 180 minutes after symptom onset, and, although timely results may not be available during the emergency department evaluation, it may aid in the final diagnosis of anaphylaxis. Elevated tryptase of more than 12.4 ng/mL in the emergency department has a positive predictive value of up to 93% and negative predictive value as low as 17%.19 An elevated tryptase may support the clinical diagnosis of anaphylaxis, although it may not be elevated in all cases, particularly in cases of food-induced anaphylaxis.20 Of note, recent research has linked a similar group of mediators (histamine, tryptase, interleukin (IL)-16, IL-10, and tumor necrosis factor [TNF]-receptor 1), with both reaction severity and protracted reactions suggesting that protracted reactions may be closely linked to initial reaction severity.21
Fig. 3.
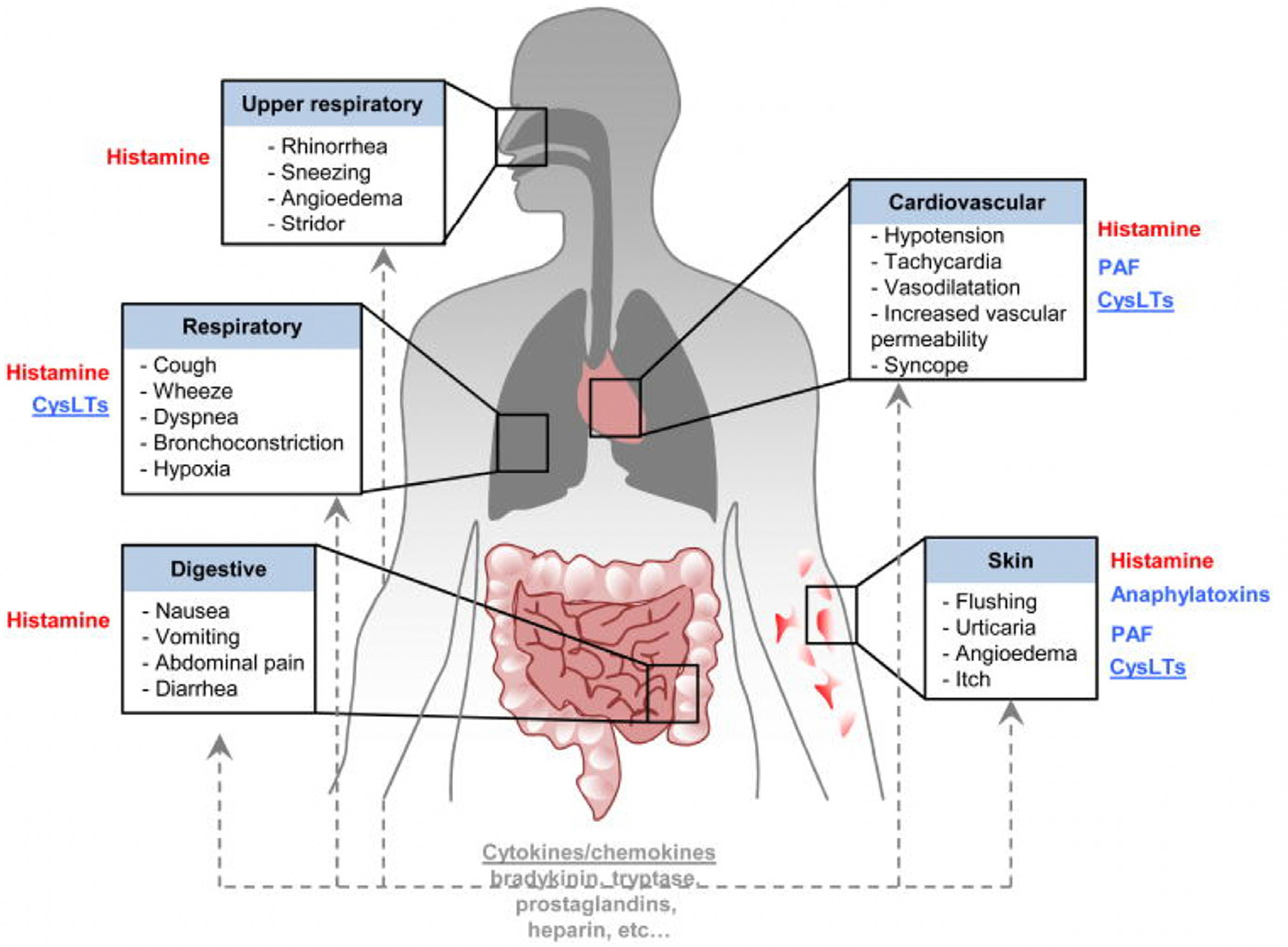
Pathophysiological changes in anaphylaxis and mediators that have been implicated in these processes. (From Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol 2017;140(2):335–48; with permission.)
ANAPHYLAXIS EPIDEMIOLOGY
The lifetime prevalence of anaphylaxis in the general population (from all triggers) has been estimated to be between 0.05% and 2%.22 Numerous studies have demonstrated increasing rates of anaphylaxis in Western countries, including the United States, Canada, Australia, Finland, Sweden, the United Kingdom, as well as in Asia, including Korea and Hong Kong.23 Among children under 10 years of age, boys have higher incidence rates of anaphylaxis than girls.23 However, after age 10, girls have comparable or higher rates of anaphylaxis. Among adults, anaphylaxis rates are higher among women than men.23,24
Time Trends
In the United States, anaphylaxis-related ED visits are increasing.25 Based on a large national administrative claims database study, the overall rate of anaphylaxis per 100,000 enrollees increased by 101% from 2005 to 2014. Similar results were published in a recent national cross-sectional study that revealed a 3.2-fold increase in anaphylaxis-related emergency department visits from 2008 to 2016.26
Triggers
Food represents the leading cause of pediatric anaphylaxis and is the leading cause of anaphylaxis presenting to emergency departments in the United States, with about 30,000 cases per year. The most common specific food trigger varies by age group, with cow’s milk more common in infants, peanuts in children, and shellfish and tree nuts in young adults and adults.27 In an observational study examining national time trends of pediatric food-induced anaphylaxis-related emergency department visits from 2005 to 2014, anaphylaxis caused by a food trigger increased by 214%, with infants and toddlers (0–2 years of age) comprising most of those visits.28 A retrospective cohort study examining 37 pediatric hospitals from 2007 to 2012 reported a similar trend of increasing rates of food-induced anaphylaxis emergency department visits.29 However, there was no increase in the proportion of patients admitted to the hospital or intensive care unit (ICU) for food-related anaphylaxis.
A novel food allergy syndrome was recently defined over the past decade. Referred to as alpha-Gal syndrome, it is associated with 2 distinct presentations: (1) delayed allergic reaction, typically 2 to 6 hours after ingestion of mammalian meat; and (2) anaphylaxis immediately upon receiving the chemotherapy agent cetuximab commonly used to treat colorectal and head and neck cancers.30 Patients are typically adults, many of whom tolerated meats previously and who present with symptoms ranging from localized urticaria and angioedema to severe anaphylaxis requiring emergency department management and hospital admission. The reactions are caused by the development of a novel IgE antibody response to galactose-alpha-1,3-galactose (alpha-Gal). Alpha-Gal is an oligosaccharide epitope present in the saliva of some ticks, mammalian meat, and on the antigen-binding fragment (Fab) portion of the cetuximab heavy chain. It is hypothesized that the introduction of alpha-Gal that occurs with a tick bite results in the production of IgE, which later causes anaphylaxis to mammalian meat or cetuximab (Fig. 4).30 The distinctive delay between ingestion of mammalian meat and symptom onset is because of the time required for digestion and then presentation of the antigen to mast cells in peripheral tissues. In the United States, the Lone Star tick (Amblyomma americanum) is the primary cause of alpha-Gal syndrome, whereas different species of ticks are responsible for this disease in other countries. Management of alpha-Gal syndrome involves avoidance of red meat and mammalian organs, with up to 20% of patients also needing to avoid gelatin and dairy products. Patients are also advised to avoid tick bites, as further tick bites may maintain or increase the titer of IgE specific to alpha-Gal.
Fig. 4.
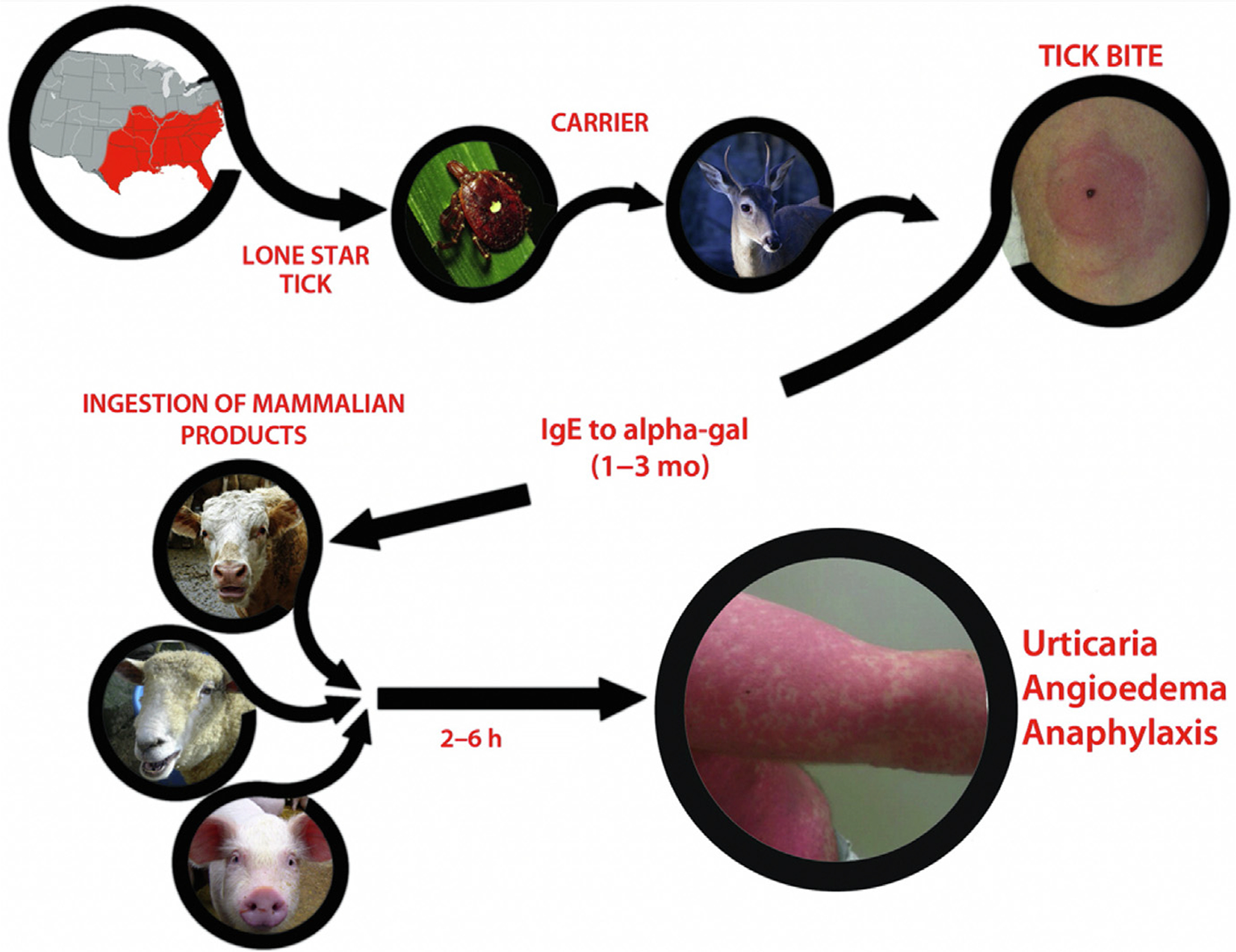
Summary of alpha-Gal sensitization leading to clinical symptoms of red meat allergy. The southeastern section of the United States is where most of the reactions to red meat have been reported. This region overlaps with the distribution of the lone star tick. The current hypothesis is that persons are bitten by lone star ticks carried by deer into rural and urban areas. After a period of time, IgE to alpha-Gal develops. Once IgE to alpha-Gal reaches sufficient levels, ingestion of red meat can trigger reactions. Several of the images used in this figure are licensed under a Creative Commons CC BY-NC 2.0 (Attribution-NonCommercial 2.0 Generic) license (Cow: https://flic.kr/p/adgjhp by user Plashing Vole; Deer: https://flic.kr/p/jeZwq7 by user Cherry Bream; Sheep: https://flic.kr/p/4WirD by user Lauren; Tick: https://flic.kr/p/cdnNaY by user Katja Schulz; Pig: https://flic.kr/p/N7gpc by user Anne). (From Steinke JW, Platts-Mills TA, Commins SP. The alpha-Gal story: lessons learned from connecting the dots. J Allergy Clin Immunol 2015;135(3):589–96; with permission.)
Stinging insect venom are a major cause of adult and pediatric anaphylaxis including children and adolescents. Although insect allergy is more common in young adults, fatal anaphylaxis caused by insect stings are more likely to occur in older adults, a likely consequence of underlying comorbidities and impaired compensatory physiologic responses.31 The most common venom triggers include hymenopterans (yellow jacket, hornet, wasp, and honeybee) and the imported fire ant (Solenopsis invicta and Solenopsis richteri). Among the stinging insects, yellow jackets cause the most frequent insect sting reactions in the United States. More than 10% of all anaphylaxis-related emergency department visits are caused by insect allergy. Systemic reactions to insect stings may affect up to 0.8% of children and 3% of adults, with at least 40 fatal stings per year nationally.32 For patients, the first sting related reaction may be fatal. Venom content varies among the different stinging insect families and demonstrates seasonal and geographic variation, which may explain the variability of allergic reactions with individual stings.
Medications represent the third most common anaphylaxis trigger. Generally, adverse drug reactions occur in up to 10% of the general population, and of those, 10% are drug hypersensitivity reactions.33 The incidence of anaphylaxis caused by medication triggers is increasing.25,34 In the United States, the most commonly identified drug culprits include antibiotics (penicillin, cephalosporins, and sulfonamides), along with aspirin and other NSAIDs. In the United States as well as globally in Australia, the United Kingdom, and New Zealand, medications are a common cause of fatal anaphylaxis.35
In the emergency department, the exact trigger can often be difficult to identify, and thus referral to an allergist-immunologist specialist is recommended. In a prior retrospective study, more than one-third of patients with suspected anaphylaxis in the emergency department had a change in the diagnosis or suspected trigger after allergy consultation.36 Moreover, for those with suspected venom-induced anaphylaxis, allergy specialists can offer venom immunotherapy, which may not only help to reduce a patient’s risk of subsequent anaphylaxis from 30% to 60% to less than 5%, but also improve quality of life by reducing anxiety related to risk of future reactions.37–39
RISK FACTORS FOR SEVERE, BIPHASIC, AND FATAL ANAPHYLAXIS
Severe Anaphylaxis
It is challenging to evaluate risk factors for severe anaphylaxis given the preponderance of research related to this topic uses inconsistent outcome definitions (eg, need for ICU admission, hospitalization, or repeat epinephrine administration) and study designs. Despite this, recent research has identified several potential risk factors for severe anaphylaxis (Box 4): older patient age (>65 years), history of mastocytosis, medication trigger, and comorbidities including pulmonary (eg, asthma) and cardiac disease (coronary disease, heart failure).40,41 Although history of asthma may be a risk factor for severe reactions, it is unclear whether patients with a history of asthma should be managed more conservatively (eg, extended observation periods, hospital admission) than patients without asthma to monitor for biphasic reactions.40,42
Box 4. Potential risk factors for severe, biphasic, and fatal anaphylaxis.
Patient factors: age ≥65 years, male sex
Comorbidities: cardiac or lung disease (eg, chronic obstructive pulmonary disease [COPD], asthma), prior emergency department visit or hospitalization for anaphylaxis, mastocytosis
Triggers: medication, insect venom, iatrogenic
P factors: use of beta blockers or angiotensin-converting enzyme (ACE) inhibitors in proximity to allergen exposure, vigorous physical activity
Comorbidities: prior anaphylaxis
Triggers: unknown trigger
Examination findings: wide pulse pressure, hypotension, wheezing, diarrhea
Reaction features: delayed epinephrine administration, greater than 1 dose of epinephrine Fatal anaphylaxis7,35,53–55
Patient factors: elderly patients, male sex
Comorbidities: asthma, cardiovascular disease, mastocytosis
Reaction features: delayed epinephrine administration
Biphasic Anaphylaxis
Although there is wide variability in the reported prevalence (1% to 20%) and risk factors for biphasic reactions, it is important for emergency department clinicians to be aware of potential risk factors when making management decisions including determining the length of emergency department observation or need for hospitalization (see Box 4).11 Recently published anaphylaxis guidelines recommend (albeit a weak recommendation based on low evidence) extended observation periods to monitor for biphasic reactions for patients with resolved severe anaphylaxis (eg, hypotension) and those who receive greater than 1 dose of epinephrine.42 Although antihistamines and systemic steroids are commonly used to treat anaphylaxis and have a theoretic role in preventing biphasic reactions, the same guidelines recommend against their routine use to prevent biphasic reactions given insufficient supporting data.42
Fatal Anaphylaxis
Despite an apparent increase in the prevalence of anaphylaxis globally (reflected in rising emergency department visits and hospitalizations),25,26 there does not appear to be a parallel increase in anaphylaxis fatalities, which fortunately remain rare events. Recent studies report population fatality rates between 0.47 and 0.69 per million persons, and emergency department and inpatient fatality rates between 0.25% and 0.33%.35,42 Establishing the true prevalence and risk factors for fatal anaphylaxis is challenging given the preponderance of data are from retrospective registries and case series from which it is difficult to develop reliable predictive models. Still, clinicians must be knowledgeable of potential risk factors for this rare outcome, and optimize management strategies to mitigate patient risk. This is challenging given risk factors for fatal anaphylaxis vary by allergen.35,43 Potential risk factors for drug-induced fatalities include underlying cardiovascular disease and older age (age >65), whereas delayed epinephrine administration may be a risk factor for food-induced fatalities and cardiovascular disease and mastocytosis for inset sting fatalities.35 Given the unpredictable nature of fatal anaphylaxis, it is essential that patients adhere to strict allergen avoidance. Likewise, clinicians should seek to optimize the management of predisposing comorbidities (eg, asthma, immunotherapy for sting allergy), and ensure patients have access to epinephrine autoinjectors and are educated on their use.42
DIFFERENTIAL DIAGNOSIS
Clinicians must maintain a broad differential when treating patients with suspected anaphylaxis (Box 5), especially for patients who do not respond to standard anaphylaxis management. Additionally, because anaphylaxis is under-recognized and under-treated (specifically around treatment with epinephrine),2 it is critical for emergency department providers to consider anaphylaxis in the differential diagnosis for patients whose symptoms overlap with those of anaphylaxis (eg, upper airway obstruction, wheezing, angioedema, flushing, syncope, hypotension) given delayed treatment with epinephrine may be a risk factor for adverse outcomes including biphasic and fatal anaphylaxis. Likewise, recognition of anaphylaxis may be especially challenging for noncommunicative patients including infants and young children who may present with nonspecific symptoms that overlap with normal infant behavior (eg, fussiness, drooling, spitting up).44
Box 5. Differential diagnosis of anaphylaxis.
Tissue swelling
Idiopathic urticaria
Isolated angioedemaa
Idiopathic
ACE inhibitor-induced
Acquired or hereditary C1 esterase inhibitor deficiency
Conditions mimicking upper airway edema
Dystonic reactions mimicking symptoms of a swollen tongue after taking metoclopramide, prochlorperazine, or antihistamines
Acute esophageal reflux (sudden onset of painful throat swelling)
Endocrine/flushing syndromes
Peptide-secreting tumors (eg, carcinoid syndrome, VIPomasb)
Alcohol-related
Medullary carcinoma of thyroid
Vancomycin Infusion Syndromec
Menopause (flushing, hot flashes)
Hypoglycemia
Neurologic syndromes
Seizure
Stroke
Other causes of syncope
Vasovagal episodes
Sepsis
Shock (septic, cardiogenic, hypovolemic, hemorrhagic, neurologic)
Acute respiratory distress
Asthma
Panic disorders
Globus hystericus
Laryngospasm
Vocal cord dysfunction
Medications
Vancomycin (vancomycin infusion syndrome)
Niacin (flushing)
General anesthetics (hypotension)
Psychosomatic/functional disorders
Panic disorders
Factitious anaphylaxis
Undifferentiated somatoform anaphylaxis
Vocal cord dysfunction
Miscellaneous
Scombroid fish poisoning
Serum sickness
Pheochromocytoma
Systemic mastocytosis
Urticaria pigmentosa
Basophil leukemia
Acute promyelocytic leukemia with tretinoin treatment
aIsolated angioedema lacks any other organ or systemic features and thus by definition is not anaphylaxis
bNeuroendocrine tumors that secrete vasoactive intestinal polypeptide
cVancomycin infusion syndrome is flushing and erythema associated with infusion of vancomycin (or occasionally other antibiotics); it is thought to be caused by histamine release, and may be related to dose or infusion rate
Adapted from Brown SG, Mullins RJ, Gold MS. Anaphylaxis: diagnosis and management. Med J Aust 2006;185(5):283–9; LoVerde D, Iweala OI, Eginli A, et al. Anaphylaxis. Chest 2018;153(2):528–43; with permission.
KEY POINTS.
Allergic reactions and anaphylaxis occur on a severity continuum from mild and self-limited to potentially life-threatening or fatal reactions.
The clinical diagnosis of anaphylaxis can be aided by the use of diagnostic criteria.
Prompt treatment with epinephrine is necessary to prevent progression to a potentially life-threatening reaction.
Risk factors for increased anaphylaxis severity include older age, cardiopulmonary comorbidities, and delayed epinephrine administration.
CLINICS CARE POINTS.
Epinephrine is indicated for patients with potentially life-threatening allergic manifestations even if multiple organ systems are not involved.
Consider alpha-Gal syndrome in patients without a clear inciting allergic trigger.
Inform patients of their risk of a biphasic reaction and ensure that they are adequately prepared to manage it.
Refer patients with anaphylaxis to an allergist for confirmation of the diagnosis and trigger and for possible immunotherapy.
Acknowledgments
Dr. Dribin has received research funding from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 2UL1TR001425-05A1 and Award Number 2KL2TR001426-05A1.
Footnotes
DISCLOSURE
R.L. Campbell is an author for UpToDate and a consultant for Bryn Pharma. All other authors declare no conflicts of interest.
REFERENCES
- 1.Bergmann K-C, Ring J. History of allergy. Chemical immunology and allergy, vol. 100. Basel: Karger; 2014. [Google Scholar]
- 2.Fineman SM, Bowman SH, Campbell RL, et al. Addressing barriers to emergency anaphylaxis care: from emergency medical services to emergency department to outpatient follow-up. Ann Allergy Asthma Immunol 2015;115(4):301–5. [DOI] [PubMed] [Google Scholar]
- 3.Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report–second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med 2006;47(4):373–80. [DOI] [PubMed] [Google Scholar]
- 4.Simons FE, Ebisawa M, Sanchez-Borges M, et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J 2015;8(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of national institute of allergy and infectious diseases/food allergy and anaphylaxis network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol 2012;129(3):748–52. [DOI] [PubMed] [Google Scholar]
- 6.Loprinzi Brauer CE, Motosue MS, Li JT, et al. Prospective validation of the NIAID/FAAN criteria for emergency department diagnosis of anaphylaxis. J Allergy Clin Immunol Pract 2016;4(6):1220–6. [DOI] [PubMed] [Google Scholar]
- 7.Cardona V, Ansotegui IJ, Ebisawa M, et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J 2020;13(10):100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baalmann DV, Hagan JB, Li JT, et al. Appropriateness of epinephrine use in ED patients with anaphylaxis. Am J Emerg Med 2016;34(2):174–9. [DOI] [PubMed] [Google Scholar]
- 9.Campbell RL, Li JT, Nicklas RA, et al. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol 2014;113(6):599–608. [DOI] [PubMed] [Google Scholar]
- 10.Dribin TE, Sampson HA, Camargo CA Jr, et al. Persistent, refractory, and biphasic anaphylaxis: a multidisciplinary Delphi study. J Allergy Clin Immunol 2020;146(5):1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Sadosty AT, Campbell RL. Update on biphasic anaphylaxis. Curr Opin Allergy Clin Immunol 2016;16(4):346–51. [DOI] [PubMed] [Google Scholar]
- 12.Dribin TE, Schnadower D, Spergel JM, et al. Severity grading system for acute allergic reactions: a multidisciplinary Delphi study. J Allergy Clin Immunol 2021; 148(1):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons FE. Anaphylaxis. J Allergy Clin Immunol 2010;125(2 Suppl 2):S161–81. [DOI] [PubMed] [Google Scholar]
- 14.Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol 2017;140(2):335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vadas P, Perelman B, Liss G. Platelet-activating factor, histamine, and tryptase levels in human anaphylaxis. J Allergy Clin Immunol 2013;131(1):144–9. [DOI] [PubMed] [Google Scholar]
- 16.Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med 2008;358(1):28–35. [DOI] [PubMed] [Google Scholar]
- 17.Austen KF. The cysteinyl leukotrienes: where do they come from? What are they? Where are they going? Nat Immunol 2008;9(2):113–5. [DOI] [PubMed] [Google Scholar]
- 18.Weiss JW, Drazen JM, Coles N, et al. Bronchoconstrictor effects of leukotriene C in humans. Science 1982;216(4542):196–8. [DOI] [PubMed] [Google Scholar]
- 19.Buka RJ, Knibb RC, Crossman RJ, et al. Anaphylaxis and clinical utility of real-world measurement of acute serum tryptase in UK Emergency Departments. J Allergy Clin Immunol Pract 2017;5(5):1280–7. e2. [DOI] [PubMed] [Google Scholar]
- 20.Lin RY, Schwartz LB, Curry A, et al. Histamine and tryptase levels in patients with acute allergic reactions: an emergency department-based study. J Allergy Clin Immunol 2000;106(1 Pt 1):65–71. [DOI] [PubMed] [Google Scholar]
- 21.Brown SG, Stone SF, Fatovich DM, et al. Anaphylaxis: clinical patterns, mediator release, and severity. J Allergy Clin Immunol 2013;132(5):1141–9. e5. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman P, Camargo CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006;97(5): 596–602. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Allen KJ, Suaini NHA, et al. The global incidence and prevalence of anaphylaxis in children in the general population: a systematic review. Allergy 2019;74(6):1063–80. [DOI] [PubMed] [Google Scholar]
- 24.Jensen-Jarolim E, Untersmayr E. Gender-medicine aspects in allergology. Allergy 2008;63(5):610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motosue MS, Bellolio MF, Van Houten HK, et al. Increasing emergency department visits for anaphylaxis, 2005–2014. J Allergy Clin Immunol Pract 2017;5(1): 171–5. e3. [DOI] [PubMed] [Google Scholar]
- 26.Michelson KA, Dribin TE, Vyles D, et al. Trends in emergency care for anaphylaxis. J Allergy Clin Immunol Pract 2020;8(2):767–8. e2. [DOI] [PubMed] [Google Scholar]
- 27.Poowuttikul P, Seth D. Anaphylaxis in children and adolescents. Pediatr Clin North Am 2019;66(5):995–1005. [DOI] [PubMed] [Google Scholar]
- 28.Motosue MS, Bellolio MF, Van Houten HK, et al. National trends in emergency department visits and hospitalizations for food-induced anaphylaxis in US children. Pediatr Allergy Immunol 2018;29(5):538–44. [DOI] [PubMed] [Google Scholar]
- 29.Parlaman JP, Oron AP, Uspal NG, et al. Emergency and hospital care for food-related anaphylaxis in children. Hosp Pediatr 2016;6(5):269–74. [DOI] [PubMed] [Google Scholar]
- 30.Steinke JW, Platts-Mills TA, Commins SP. The alpha-gal story: lessons learned from connecting the dots. J Allergy Clin Immunol 2015;135(3):589–96 [quiz 597]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract 2015;3(3):315–22 [quiz 323]. [DOI] [PubMed] [Google Scholar]
- 32.Graft DF. Insect sting allergy. Med Clin North Am 2006;90(1):211–32. [DOI] [PubMed] [Google Scholar]
- 33.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998;279(15): 1200–5. [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Hess EP, Lohse C, et al. Trends, characteristics, and incidence of anaphylaxis in 2001–2010: a population-based study. J Allergy Clin Immunol 2017; 139(1):182–8. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner PJ, Jerschow E, Umasunthar T, et al. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract 2017;5(5):1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell RL, Park MA, Kueber MA Jr, et al. Outcomes of allergy/immunology follow-up after an emergency department evaluation for anaphylaxis. J Allergy Clin Immunol Pract 2015;3(1):88–93. [DOI] [PubMed] [Google Scholar]
- 37.Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy 2017;72(3):342–65. [DOI] [PubMed] [Google Scholar]
- 38.Lange J, Cichocka-Jarosz E, Marczak H, et al. Natural history of Hymenoptera venom allergy in children not treated with immunotherapy. Ann Allergy Asthma Immunol 2016;116(3):225–9. [DOI] [PubMed] [Google Scholar]
- 39.Confino-Cohen R, Melamed S, Goldberg A. Debilitating beliefs and emotional distress in patients given immunotherapy for insect sting allergy: a prospective study. Allergy Asthma Proc 2009;30(5):546–51. [DOI] [PubMed] [Google Scholar]
- 40.Motosue MS, Bellolio MF, Van Houten HK, et al. Risk factors for severe anaphylaxis in the United States. Ann Allergy Asthma Immunol 2017;119(4):356–61. e2. [DOI] [PubMed] [Google Scholar]
- 41.Worm M, Francuzik W, Renaudin JM, et al. Factors increasing the risk for a severe reaction in anaphylaxis: an analysis of data from The European Anaphylaxis Registry. Allergy 2018;73(6):1322–30. [DOI] [PubMed] [Google Scholar]
- 42.Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol 2020; 145(4):1082–123. [DOI] [PubMed] [Google Scholar]
- 43.Pumphrey R Anaphylaxis: can we tell who is at risk of a fatal reaction? Curr Opin Allergy Clin Immunol 2004;4(4):285–90. [DOI] [PubMed] [Google Scholar]
- 44.Greenhawt M, Gupta RS, Meadows JA, et al. Guiding principles for the recognition, diagnosis, and management of infants with anaphylaxis: an expert panel consensus. J Allergy Clin Immunol Pract 2019;7(4):1148–56. e5. [DOI] [PubMed] [Google Scholar]
- 45.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 2004;114(2):371–6. [DOI] [PubMed] [Google Scholar]
- 46.Calvani M, Cardinale F, Martelli A, et al. Risk factors for severe pediatric food anaphylaxis in Italy. Pediatr Allergy Immunol 2011;22(8):813–9. [DOI] [PubMed] [Google Scholar]
- 47.Clark S, Wei W, Rudders SA, et al. Risk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitals. J Allergy Clin Immunol 2014;134(5):1125–30. [DOI] [PubMed] [Google Scholar]
- 48.Alqurashi W, Stiell I, Chan K, et al. Epidemiology and clinical predictors of biphasic reactions in children with anaphylaxis. Ann Allergy Asthma Immunol 2015;115(3):217–23. e2. [DOI] [PubMed] [Google Scholar]
- 49.Lee JM, Greenes DS. Biphasic anaphylactic reactions in pediatrics. Pediatrics 2000;106(4):762–6. [DOI] [PubMed] [Google Scholar]
- 50.Lee S, Bellolio MF, Hess EP, et al. Predictors of biphasic reactions in the emergency department for patients with anaphylaxis. J Allergy Clin Immunol Pract 2014;2(3):281–7. [DOI] [PubMed] [Google Scholar]
- 51.Lee S, Bellolio MF, Hess EP, et al. Time of Onset and Predictors of Biphasic Anaphylactic Reactions: A Systematic Review and Meta-analysis. J Allergy Clin Immunol Pract 2015;3(3):408–16, e401–402. [DOI] [PubMed] [Google Scholar]
- 52.Lee S, Peterson A, Lohse CM, et al. Further evaluation of factors that may predict biphasic reactions in emergency department anaphylaxis patients. J Allergy Clin Immunol Pract 2017;5(5):1295–301. [DOI] [PubMed] [Google Scholar]
- 53.Pouessel G, Claverie C, Labreuche J, et al. Fatal anaphylaxis in France: analysis of national anaphylaxis data, 1979–2011. J Allergy Clin Immunol 2017;140(2): 610–2. e2. [DOI] [PubMed] [Google Scholar]
- 54.Pouessel G, Turner PJ, Worm M, et al. Food-induced fatal anaphylaxis: from epidemiological data to general prevention strategies. Clin Exp Allergy 2018; 48(12):1584–93. [DOI] [PubMed] [Google Scholar]
- 55.Turner PJ, Gowland MH, Sharma V, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012. J Allergy Clin Immunol 2015;135(4):956–63. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]


