Dear Editor,
Highly infectious Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-caused Coronavirus Disease 2019 (COVID-19) has brought about massive medical and economic burdens on communities worldwide. Accumulating evidence suggests that interleukin-6 (IL-6) are closely associated with the deteriorating health of COVID-19 patients and their deaths.1 Tocilizumab (TCZ), an IL-6 receptor inhibitor, therefore, was proposed to be a promising candidate for COVID-19 therapy. Numerous randomized clinical trials (RCTs) and cohort studies on the efficacy and safety of TCZ in hospitalized COVID-19 patients have been published, and these, as would be expected, bear contradictory findings. Those early meta-analyses had limited value to the broader picture of the pandemic because they mostly assessed retrospective cohort studies, or scrutinized available published or preprinted RCTs alone or along with observational studies.2, 3, 4 Given that more RCTs and cohort studies have been published recently, we conducted a updated meta-analysis, by systematically searching common databases between 2019 and August 11, 2021.
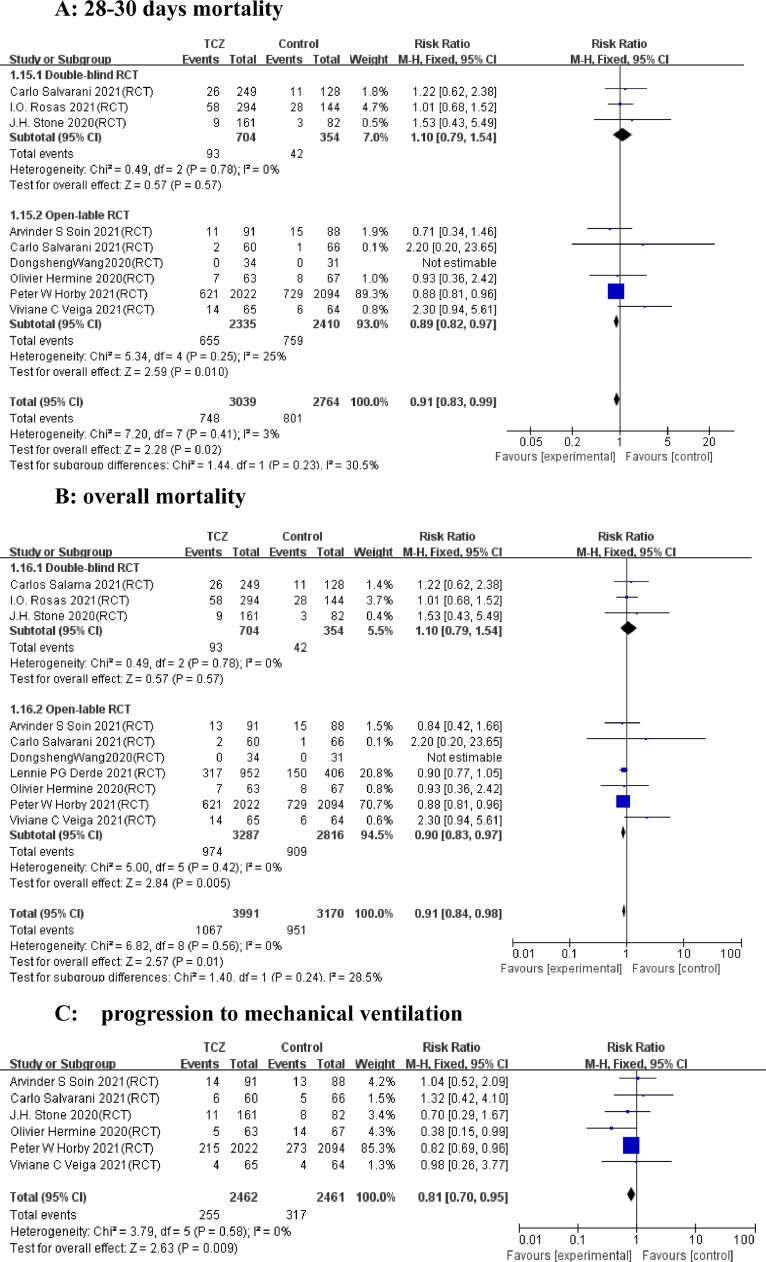
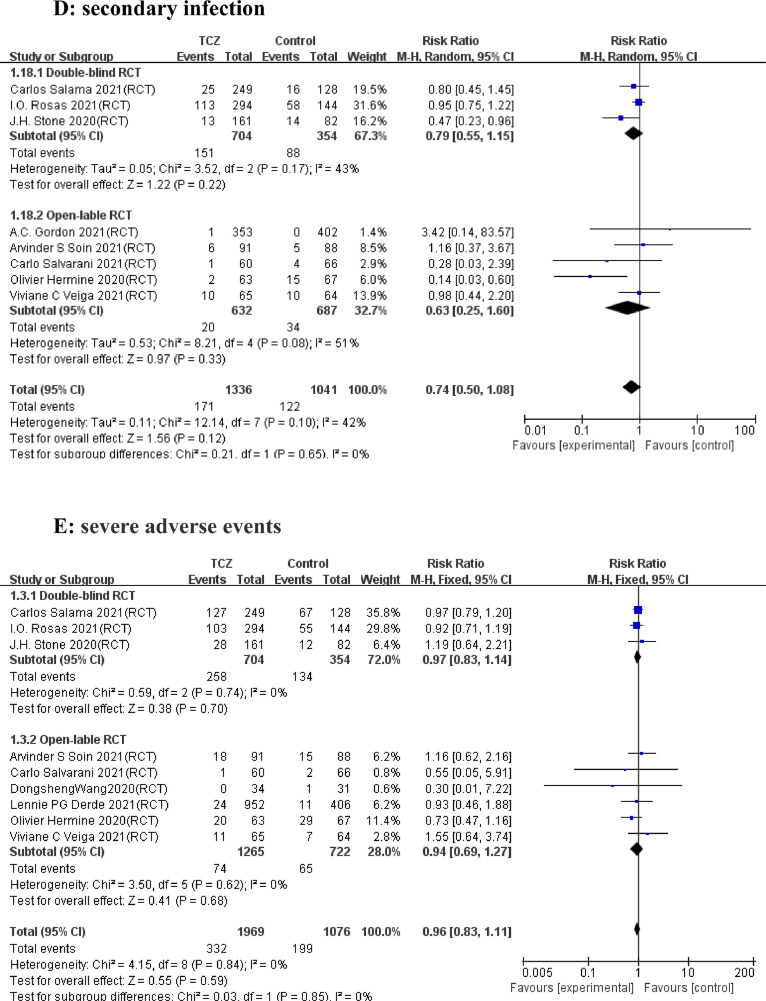
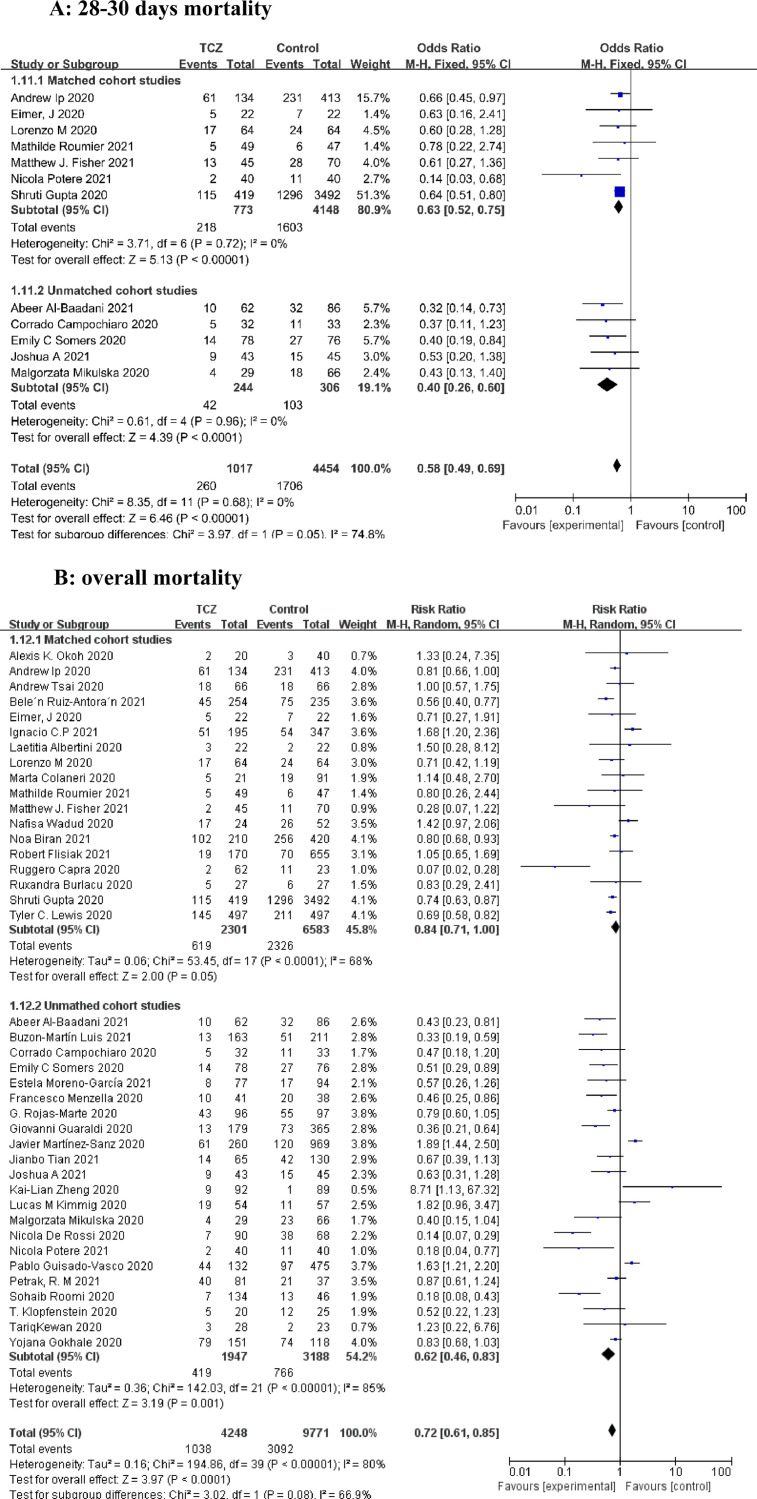
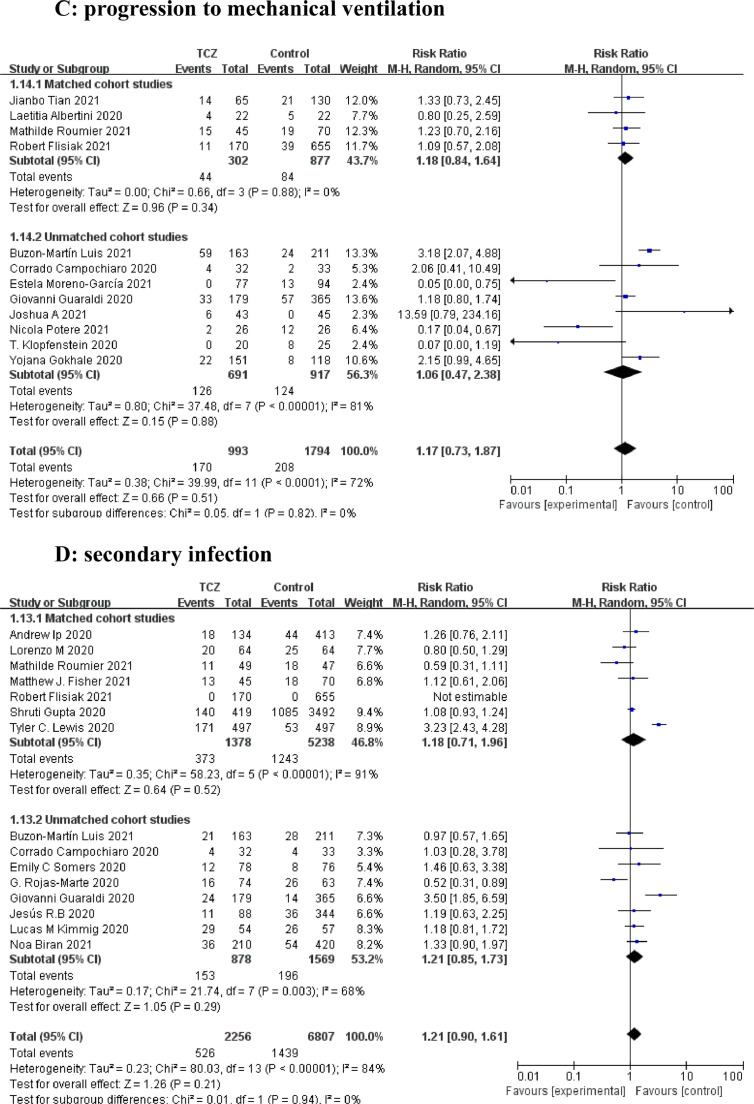
A total of 53 articles with 21,656 patients were identified, including 11 articles on 10 RCTs and 42 cohort studies. Detailed characteristics of the RCTs and cohort studies are described, respectively in Supplementary Table 1 and 2. Results from 9 RCTs showed that TCZ decreased 28–30 days mortality (Relative Risk [RR]=0.91; 95% confidence interval [CI], 0.83–0.99; P = 0.02; I2=3%) (Fig. 1 A). Additionally, TCZ administration in 9 RCTs instigated improved overall mortality (RR=0.91 [0.84–0.98]; P = 0.01; I2=0%) (Fig. 1B). However, the largest RCT (RECOVERY NCT04381936)(5) greatly interfered with the pooled result on short-term and overall mortality, possibly because its sample size was much larger than those of other RCTs. Analyses of the 42 cohorts yielded consistent results that TCZ significantly reduced the short-term and overall mortality (Fig. 2 A and B). Furthermore, TCZ decreased the risk of mechanical ventilation in RCTs (RR=0.81[0.70–0.95]; P = 0.009; I2=0%) (Fig. 1C) but not in cohorts (RR= 1.17 [0.73–1.87]; P = 0.51; I2=72%) (Fig. 2C). RCTs data revealed TCZ had no risk of increased secondary infection (RR=0.74 [ 0.50–1.08]; P = 0.12; I2=42%) (Fig. 1D) and severe adverse events (SAE) (RR=0.96 [0.83–1.11]; P = 0.59; I2=0%) (Fig. 1E), as did the cohorts with a pooled RR for secondary infection of 1.21 [0.90–1.61] (P = 0.21; I2=84%) (Fig. 2D).
Fig. 1.
Forest plot for the efficacy and safety of tocilizumab in randomized controlled trials. (A) 28–30 days mortality. (B) overall mortality. (C) Progression to mechanical ventilation. (D) Secondary infection. (E) Severe adverse events.
Fig. 2.
Forest plot for the efficacy and safety of tocilizumab in cohort studies. (A) 28–30 days mortality. (B) overall mortality. (C) progression to mechanical ventilation. (D) secondary infection.
Our findings that TCZ was associated with a decreased risk of death in both RCTs and cohort studies were partly inconsistent with the conclusions of several other recent meta-analyses, possibly because our meta-analysis enrolled more RCTs and cohorts with larger sample sizes than those previous meta-analyses.4, 5, 6 A recent prospective meta-analysis with more unpublished data from ongoing RCTs, was consistent with our meta-analysis on the beneficial effect of TCZ on 28–30 days mortality.7 The beneficial outcome of TCZ in critical COVID-19 patients can perhaps be attributed to its efficacy in interfering with the cytokine release syndrome. All the recent meta-analyses, including ours, have found no TCZ-induced increase in the risk of secondary infections,4 , 6 , 7 for that TCZ possibly only inhibits IL-6-impacted immune responses and does not interfere with the functioning of immune processes that might help the body fight COVID-19.8 In conclusion, TCZ improves COVID-19 patient outcomes without increasing SAE compared to usual care or placebo.
This study was supported by grant from the National Key R&D Program of China (No.2020YFC0845700).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.11.013.
Appendix. Supplementary materials
References
- 1.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. NovPubMed PMID: 32845568. Pubmed Central PMCID: PMC7460877. Epub 2020/08/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lan S.H., Lai C.C., Huang H.T., Chang S.P., Lu L.C., Hsueh P.R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56(3) doi: 10.1016/j.ijantimicag.2020.106103. SepPubMed PMID: 32712333. Pubmed Central PMCID: PMC7377685. Epub 2020/07/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tharmarajah E., Buazon A., Patel V., Hannah J., Adas M., Allen V., et al. IL-6 inhibition in the treatment of COVID-19: a meta-analysis and meta-regression. J Infect. 2021 doi: 10.1016/j.jinf.2021.03.008. PubMed PMID: 33745918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C.X., Hu F., Wei J., Yuan L.T., Wen T.M., Gale R.P., et al. Systematic review and meta-analysis of tocilizumab in persons with coronavirus disease-2019 (COVID-19) Leukemia. 2021;35(6):1661–1670. doi: 10.1038/s41375-021-01264-8. JunPubMed PMID: 34002026. Pubmed Central PMCID: PMC8127467 Mingsight Parmaceuticals Inc. and CStone Pharmaceuticals. Advisor: Antegene Biotech LLC, Medical Director: FFF Enterprises Inc. Partner: AZACA Inc. Board of Directors: RakFond Foundation for Cancer Research Support. Scientific Advisory Board, StemRad Ltd. All other authors declare no competing interests. Epub 2021/05/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group R.C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. PubMed PMID: 33933206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tleyjeh I.M., Kashour Z., Riaz M., Hassett L., Veiga V.C., Kashour T. Efficacy and safety of tocilizumab in COVID-19 patients: A living systematic review and meta-analysis: first update. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.04.019. Apr 26. PubMed PMID: 33915284. Pubmed Central PMCID: PMC8076756. Epub 2021/04/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar-Hari M., Vale C., Godolphin P., Fisher D., Higgins J., Spiga F., et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A meta-analysis. JAMA. 2021 doi: 10.1001/jama.2021.11330. PubMed PMID: 34228774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo C., Li B., Ma H., Wang X., Cai P., Yu Q., et al. Single-cell analysis of two severe COVID-19 patients reveals a monocyte-associated and tocilizumab-responding cytokine storm. Nat Commun. 2020;11(1):3924. doi: 10.1038/s41467-020-17834-w. PubMed PMID: 32764665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.