Abstract
A feeding trial was conducted to evaluate the effects of Bacillus-based probiotics on growth performance, intestinal histo-morphology, gut microbial population and immune response in broilers. A total of 2000 Hubbard Classic day-old chicks were randomly enrolled in four experimental groups and 4 replicates of 500 birds in each group, and reared for 35 days under a low- level of biosecurity measures. The trial groups were assigned treatment-1 (T1): basal diet(control), treatment-2 (T2): basal diet plus Bacillus licheniformis (DSM17236), treatment-3 (T3): basal diet plus Bacillus subtilis (PB6), and treatment-4 (T4) basal diet plus 4% Flavomycin. All four groups were fed with maize-soybean based prepared feeds (starter, grower and finisher). Dietary inclusion of B. licheniformis significantly improved body weight gain and lessened FCR in T2 compared to other groups (p < 0.05). Probiotics increased the population of Bacillus spp. and decreased the population of Clostrium perfringens, Salmonella spp. and Escherichia coli in the jejunum and ileum in broiler birds on day 21 and 35 (p < 0.05). The highest antibody production was observed in B. licheniformis treated group (T2) compared to other probiotic treated group (T1). Taken together, the study findings suggest that B. licheniformis probiotics could be used as a feasible alternative to antimicrobials in the broiler production considering beneficial impacts at low biosecurity broiler farms.
Keywords: Bacillus licheniformis, Bacillus subtilis, broiler chicken, probiotic, gut histomorphology, intestinal microflora, immune response, low biosecurity
1. Introduction
Antimicrobial agents have been used in commercial poultry production as feed supplements more than 50 years ago because of feed efficiency and growth promotion via modulation of intestinal microflora, and disease prevention as the effect of improved host immunity (Bunyan, Jeffries, Sayers, Gulliver, & Coleman, 1977). However, imprudent antimicrobial use (AMU) in the poultry production have developed antimicrobial residues including the emergence of antimicrobial resistant (AMR) microbes, which could lead to great risk to public health (Shivaramaiah et al., 2011). In addition, intensive poultry rearing causes stress in birds, resulting reduction of immune response. This phenomenon significantly enhances intestinal colonization of pathogens (O'Dea et al., 2006). Therefore, alternative options are needed that act similar to antibiotics targeted for control of disease producing microorganisms, and support to the growth performance of poultry. Thus, replacement of prohibited antibiotic growth promoters (AGP) has grew more attention during the recent past (Deniz et al., 2011). Probiotics as a live microbial feed supplement provide beneficial effects on the host through enhancing intestinal microbial balance (Fuller, 1989). At present, a number of microbial species have been used as probiotics, including species of Bacillus, Bifidobacterium, Enterococcus, Lactobacillus, Lactococcus, Streptococcus, Pediococcus, Aspergillus, Candida, and Saccharomyces etc. (O'Dea et al., 2006), of which species under the Lactobacillus and Bifidobacterium are widely used (Park et al., 2021). Notwithstanding, years of experience for safe commercial use of Bacillus spp. including B. subtilis, B. licheniformis and B. coagulans as potent probiotics in animal production has increased, but their application has been shadowed by the use of these two dominant genera both in human and animal health (AlGburi et al., 2016; Mahoney et al., 2019). In different ways, probiotics can work in the gastrointestinal tract (GIT) of poultry through competitive exclusion and antagonism process (Kabir et al., 2005; Kizerwetter-Swida & Binek, 2009), modification metabolism through intensifying digestive enzyme activity, and reducing ammonia production including bacterial enzyme activity (Yoon et al., 2004), improving feed intake, digestion and absorption capacity (Awad, Böhm, Razzazi-Fazeli, Ghareeb, & Zentek, 2006), and boost body immune response (Apata, 2008; Mathivanan & Kalaiarasi K., 2007). However, major benefits of using probiotics in livestock and poultry production to prevent disease, and growth improvement by enhancing feed efficiency and reducing mortality rate as well have been observed (Deniz et al., 2011).
Enteric diseases are considered to be immense burden to the poultry industry as they are associated with decreased weight gain, higher FCR, increased mortality rate, higher medication costs, and increased likelihood of contamination in poultry products with zoonotic pathogens for human infection (Timbermont, Haesebrouck, Ducatelle, & Van Immerseel, 2011). Intestinal diseases are associated with the overgrowth of Clostridium perfringens, Salmonella spp., and even Escherichia coli in the GIT of poultry. Necrotic enteritis (NE) frequently occurs in poultry, which is caused by Clostridium perfringens and typically occurs in broiler chickens between 2 to 6 weeks of age. Bacillus licheniformis strain of probiotics would prevent NE and to improve growth in broiler poultry (Cheng et al., 2017). Other important bacterial pathogens like Salmonella spp. (Shivaramaiah et al., 2011) and E. coli (Cooper, Songer, & Uzal, 2013), which may cause enormous economic loss to the poultry industry through diminishing overall performance and increasing mortality rate in poultry. All these persuade a very high economic burden to the farmers including public health risks.
The use of Bacillus based probiotics (B. licheniformis and B. subtilis) could be potential AGP replacements in the broiler feed considering the emergence of AMR, and support to the cost-effective poultry production in low resource settings like Bangladesh. Therefore, this study was conducted to evaluate influence of Bacillus- based probiotics in growth performance, intestinal histo-morphology, gut microbial population and immune response status in low biosecurity broiler flocks in comparison with AGP. The findings of this study will promote to scale up this practice in the majority broiler production systems in Bangladesh where lower standard of biosecurity measures is most common.
2. Materials and Methods
2.1. Experimental design, diet and bird management
Two thousand (2000) day-old chicks of commercial Hubbard Classic broiler with an average initial body weight of 45.2 ± 0.2 g were collected from a local chick supplier and randomly assigned into 4 dietary treatment groups which included 500 birds in each group with 4 replicates (125 birds/replicate). The birds were kept under low level of biosecurity measurements of the sector three poultry production systems of FAO classification (Dolberg, 2008). The potential characteristics of low biosecurity parameters were as inadequate provision of perimeter fencing and netting of the poultry farm/shed, quarantine facilities, footbath, separate boot and clothing of the poultry workers for farm use, poultry waste management including cleaning and disinfection practices (FAO, 2008; Akther et al., 2018).
The experimental trial was conducted for a 35 day-period and the birds were fed with a prepared poultry ration (starter, grower and finisher) and divided into four groups based on desired combination: (1) treatment-1/control (T1): birds were fed as a basal diet only; (2) treatment-2 (T2): birds were fed a basal diet plus Bacillus licheniformis DSM17236 (Gallipro Tect®, Chr. Hansen Holding A/S, Denmark, 3 × 109CFU/g), 1.0 Kg/metric ton (MT) feed; (3)treatment-3 (T3): birds fed a basal diet plus Bacillus subtilis PB6 (6.6 × 109 CFU/g), 1.0 Kg/MT feed, and (4) treatment-4 (T4): birds were fed a basal diet plus 4% Flavomycin, 0.3 Kg/MT feed.
A starter diet (crumble feed) from day 1 to 14, a grower diet (pellet feed) from day 15 to 28 including a finisher diet (pellet feed) from day 29 to 35 were provided depending upon age of the birds. In this trial, each replicate was housed in a clean and disinfected floor pen (15 m2/125 birds) with rice husk litter before the first day. The birds were fed on ad libitum basis with 24 h, 23 h, 20 h, 16 h and 12h artificial lighting conditions were maintained at the age of 1-3 days, 4-7 days, 8-14 days (second week), 15-21 days (third week) and onwards, respectively. Temperature was adjusted on age of the birds, that included 32°C in the first week with 200 watt tungsten light bulb, 30°C in the second week, 28°C in the third week, and then 25°C to the end of the study with artificial LED lights (30 watt) as described earlier (Cao et al., 2013). Since the study was conducted during hotter summer season, no artificial lighting was needed during day-time considering environmental temperature. The birds were immunized against Newcastle disease (ND) and Infectious bursal disease (IBD) at 5 and 9 days of age and booster/second dose at 22 and 17 days of age, respectively.
2.2. Feed formulation and preparation
The basal diet was prepared as per standard method (National Research Council, 1994) and was used as feed during the trial in 3 stages: day 1 – 14, day 15 – 28 and day 21 – 35 (Table 1). The feed ingredients (raw materials) were collected and weighed separately and blended properly with all feed additives (vitamins, minerals etc.) from a commercial feed miller. The probiotics and antibiotics were added in powder form in the basal ration according to the experimental design. Moisture, crude protein, crude fat, crude fiber and ash of the trial feed were analyzed for proximate analysis of feed, and however, amount of calcium and phosphorus and soluble chloride were analyzed as a part of mineral analysis.
Table 1.
Ingredients and composition of the basal feed in broiler starter, grower and finisher ration (as feed basis, %).
| Ingredients (%) | Starter(1 to 14 day) | Grower(15 to 28 day) | Finisher(29 to 35 day) |
|---|---|---|---|
| Maize | 47.40 | 45.00 | 49.00 |
| Rice polish- grade A | 2.50 | 2.28 | 6.60 |
| Wheat Flour Feed grade | 7.00 | 7.00 | 10.00 |
| Broken Rice | - | 5.00 | 5.00 |
| Soybean full fat (extruded) | 7.00 | 8.00 | 3.00 |
| Soybean meal 46% CP | 23.5 | 19.30 | 16.00 |
| Protein Concentration | 4.00 | 4.00 | 3.00 |
| Oil | 2.00 | 3.00 | 3.00 |
| Rape seed Meal 36% | 3.00 | 3.00 | 1.00 |
| Limestone | 1.95 | 1.04 | 1.04 |
| DCP1 | 0.50 | 0.50 | 0.50 |
| Methionine MHA | 0.20 | 0.32 | 0.32 |
| Lysine-L | 0.20 | 0.25 | 0.23 |
| Vitamin and mineral premix 2 | 0.20 | 0.20 | 0.20 |
| Enzyme | 0.05 | 0.05 | 0.05 |
| Salmonella killer3 | 0.10 | 0.15 | 0.15 |
| Toxin (UTPP Biotect) 4 | 0.10 | 0.15 | 0.15 |
| Salt | 0.30 | 0.25 | 0.25 |
| Sodium Bicarbonate | 0.01 | 0.10 | 0.10 |
| Choline5 | - | 0.08 | 0.08 |
| Pro-plus6 | - | 0.03 | 0.03 |
| Betaine7 | - | 0.05 | 0.05 |
| Phyzyme 10000 FTU8 | - | 0.005 | 0.005 |
| Mycocurb9 | - | 0.10 | 0.10 |
| Quixalud10 | - | 0.05 | 0.05 |
| Safe Guard11 | - | 0.05 | 0.05 |
| Lysoforte12 | - | 0.05 | 0.05 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated analysis metabolizable energy(Kcal/kg) |
3039.82 | 3091.65 |
3095.8 |
| Moisture | 10.82 | 10.95 | 10.69 |
| CP13 | 22.16 | 21.01 | 19.61 |
| CF14 | 3.08 | 3.21 | 3.14 |
| Ca | 1.04 | 0.98 | 0.90 |
| P | 0.73 | 0.74 | 0.73 |
Vitamin and mineral premix provided the following nutrients in per kilogram of content: vitamin A, 600,000 IU; vitamin D3, 300,000 IU; vitamin E, 300 mg; vitamin B2, 600 mg; vitamin B6, 60 mg; vitamin B12, 4 mg; vitamin K3, 40 mg; nicotinic acid, 440 mg; folic acid, 100 mg; Zn, 14,400 mg; Cu, 7,200 mg; Mn, 14,400 mg; Se, 108 mg; essential amino acid, 64,800 mg.
DCP = Dicalcium Phosphate (a source of Ca)
Salmonella killer = feed additives which protects birds from Salmonella colonization and invasion
Toxin (UTPP Biotect) = essential components used in broiler ration for prevention of toxin generated from mold/fungus
Choline = vitamin-like essential nutrient in poultry feed for maintaining cell structure and fat metabolism
Pro-plus = protein tonic in poultry feed
Betaine = improves production performance as dietary supplementation in broiler ration
Phyzyme 10000 FTU = concentrated phytase feed enzyme in poultry feed for enhance digestibility to reduce feed cost
Mycocurb = mold inhibitor in poultry feed
Quixalud = effective against bacterial, fungi, and protozoa for improve feed conversion
Safe Guard = used as treatment and control of adult Ascaridia galli in broiler chickens
Lysoforte = a nutritional emulsifier in poultry ration designed for enhance digestion and absorption of energy-rich feed ingredients
CP = crude protein
CF = crude fiber
2.3. Growth performance
The body weight of birds was measured individually at the beginning of the study and every 7 days interval up to 35 day. For measuring average body weight of each replicate for a particular age, birds were selected randomly. A total of 60 birds were weighted and recorded the total weight at day 0, 7, 14 and 21 from each replicate using a measuring scale. However, 30 birds were weighted at the day 28 and 35 from each replicate. Thus, the mean value of live weight gain was measured from each replicate at 0, 7, 14, 21, 28 and 35 day. The feed consumption of each replicate was recorded in hardcopies on daily basis by subtracting the weight of the residual feed from the total quantity of feed offered. Successively, cumulative feed consumption for 7 days was determined for each replicate. Average daily growth and feed intake, feed conversion ratio (FCR), and percentage of mortality were calculated following the procedure described earlier (Khatun et al., 2017) to evaluate the growth performance of broilers included under this study.
2.4. Sample collection and processing
Samples were collected from broiler birds at day 21 and 35 for bacteriological evaluations. Intestinal lesion scoring and gut histopathology were accomplished from collected intestinal samples on day 28. However, serum was obtained from the collected blood samples on the same day (day 28) to perform a hemagglutination inhibition (HI) test for the assessment of antibody titer against Newcastle disease in each group of birds. For bacteriological evaluations, 12 birds (3 birds/replicate) were randomly selected from each group on day 21 and 35. These birds were euthanized after recording live body weight for collection of samples following aseptic measures. About 5 cm length of jejunum and ileum with their contents were collected in sterile zipper bags with sterile scissors and forceps, and transferred to the laboratory maintaining cool chain. Intestinal samples (1 g) were homogenized with 9 mL of 0.1% peptone water using a mortar and pestle, and a 10-fold dilution of sample was obtained. Subsequently, serial dilutions of each of the samples were accomplished using 0.1% peptone water.
2.5. Intestinal morphology
Intestinal lesion scoring and histopathological examination were conducted on day 28 for the evaluation of intestinal morphology. The samples were collected from euthanized birds of randomly selected two birds of a replication on day 28 (32 birds in total) for intestinal lesion scoring as per standard methods (Dahiya, Hoehler, Wilkie, Van Kessel, & Drew, 2005). In this scoring, a scale from 0 to 4 was used, where 0= apparently normal with no lesion; 0.5 = severely congested serosa and blood engorgement in mesentery; 1 = thin and friable intestine with small red petechiae; 2 = focal necrotic lesions; 3 = 1 – 2 cm long patches of necrosis; and 4 = diffused necrosis.
In histopathological evaluation, samples were collected from one of the two same euthanized bird of each replication (16 birds in total). A portion of 2 cm longitudinal section of the jejunum from each bird was collected in 10 percent phosphate buffered formalin. Further, four percent (4%) freshly prepared paraformaldehyde was used for fixation of jejunum tissues followed by embedding in paraffin blocks and sliced into 5 µm, deparaffinized in xylene, rehydrated, and finally, stained with hematoxylin and eosin stain. The tissue sections were examined by light microscope (100X). The grading of histopathological changes was performed as per standard protocol (Gholamiandehkordi et al., 2007) with moderate modification. In brief, histological scores are mainly based on the degree of villi fusion, erosion of the villi tips with loss of villi cellular content, abnormalities in epithelial tissues including volume of proteinaceous substances in the lumen of broiler intestine.
2.6. Bacteriological examination
Serial diluted (10−1 – 10−8 dilution) 0.1 mL each sample was inoculated into different selective agar media for bacterial culture. In this assessment, total Bacillus, Bacillus licheniformis, Bacillus subtilis, Escherichia coli, Salmonella spp., and Clostridium perfringens were enumerated using different culture media, viz., Hichrome Bacillus agar, MacConkey agar, Xylose lysine deoxycholate (XLD) agar, and Tryptose sulphite cycloserine (TSC) agar (Hi-Media, Mumbai, India). Plates of Hichrome Bacillus agar were incubated at 37°C for 24 h for total Bacillus, B. licheniformis and B. subtilis enumeration. Yellowish green to green colonies and irregular or creeping yellow colonies surroundings with yellow hue were considered for total B. subtilis and B. licheniformis counts respectively (Němečková, Solichová, Roubal, Uhrová, & Šviráková, 2011). In contrast, MacConkey agar, XLD agar culture media were used for E. coli and Salmonella respectively, and incubated at 37 °C for 24 h. However, TSC agar was used for C. perfringens, and incubated anaerobically using at 37°C for 24 h. Concentration of C. perfringens was tested on Tryptose-Sulfite-Cycloserine (TSC) agar, combined with perfringens (TSC) selective supplement (HiMedia, Mumbai, India) and 30% egg yolk emulsion following pour plate technique (Olnood, Beski, Choct, & Iji, 2015). After incubation of the agar plate, colonies were counted. Cultured plate containing 30 to 300 distinct colonies were counted and the number of colonies was then multiplied by the dilution factor to get respective bacterial counts. Thus, the result obtained was presented in log10 colony-forming units per gram of intestinal contents.
2.7. Immune responses
Twelve birds from each group (3 birds/replicate) were selected randomly and 0.1 mL of 0.5% sheep red blood cell (SRBC) was injected in brachial vein for immune response to SRBC on day 30. The immune response was measured after 5 days of inoculation by the micro titer haemagglutination method (Khaksefidi & Ghoorchi, 2006). Similar number of birds were included for the detection of serum antibody titers against NDV by hemagglutination inhibition (HI) test on day 28. The HI titer was expressed in log2 reciprocal of the highest serum dilution resulting in complete inhibition of hemagglutination activity.
2.8. Statistical analyses
Data for all parameters were analyzed for statistical significance using the Statistical Package for the Social Sciences (SPSS) version 23.0, IBMⓇ Co., USA. Variance among the treatment groups was measured using the one-way ANOVA. The significant differences among the treatment groups were further determined by the Tukey honestly significant difference (HSD) test under significant result of ANOVA.
3. Results
3.1. Body growth performance
Live weight gain (BWG), average daily weight gain (ADWG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were monitored weekly basis during the entire trial period (5 weeks). The live weight of trial birds at different ages are presented in Table 2. The BWG of T2 and T3 at the age of day 21 and 35 was found significantly higher (p < 0.05). However, lower BWG was observed in T1 and T4 at the age of day 21 and 35 (p < 0.05). The highest BWG was observed in T2 at the age of day 21 – 35 (Table 2).
Table 2.
The effects of dietary treatments on growth performance of broilers at day 0, 7, 21, 28, 35 in four trial groups.1
| Parameters2 | Treatment | SEM3 | p-value | |||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |||
| BWG (g/chick) | ||||||
| 0 d | 45.29 ± 0.11a | 45.33 ± 0.08a | 45.46 ± 0.12a | 45.20 ± 0.08a | 0.05 | 0.37 |
| 7 d | 204.92± 0.50a | 206.41 ± 0.46a | 205.75 ± 0.78a | 204.00 ± 0.59a | 0.35 | 0.068 |
| 14 d | 535.94 ± 3.93a | 549.89 ± 2.67a | 545.57 ± 3.15a | 546.86 ± 3.36a | 2.00 | 0.057 |
| 21 d | 974.00 ± 3.34a | 993.24 ± 2.97b | 985.75 ± 3.68ab | 984.89 ± 5.60ab | 2.52 | 0.038* |
| 28 d | 1606.55 ± 16.98a | 1658.11 ± 9.77a | 1644.11 ± 11.86a | 1637.62 ± 11.48a | 7.52 | 0.079 |
| 35 d | 2176.16 ± 14.57a | 2258.17 ± 7.02b | 2236.21 ± 10.49b | 2231.39 ± 13.82b | 9.43 | 0.003* |
| ADWG (g/bird/day) | ||||||
| 0-7 d | 22.80 ± 0.08a | 23.01 ± 0.08a | 22.90 ± 0.13a | 22.69 ± 0.09a | 0.05 | 0.162 |
| 8-14 d | 47.29 ± 0.6a | 49.07 ± 0.32a | 48.55 ± 0.52a | 48.98 ± 0.45a | 0.28 | 0.08 |
| 15-21 d | 62.58 ± 0.25a | 63.34 ± 0.59a | 62.88 ± 0.92a | 62.58 ± 0.77a | 0.32 | 0.842 |
| 22-28 d | 90.36 ± 2.58a | 94.98 ± 1.16a | 94.05 ± 1.97a | 93.25 ± 1.83a | 0.98 | 0.408 |
| 29-35 d | 81.38 ± 4.08a | 85.72 ± 1.75a | 84.59 ± 2.42a | 84.83 ± 1.04a | 1.22 | 0.663 |
| 0-35 d | 60.88 ± 0.41a | 63.23 ± 0.20b | 62.59 ± 0.30b | 62.46 ± 0.39b | 0.27 | 0.002* |
| ADFI (g/bird/day) | ||||||
| 0-7 d | 25.40 ± 0.03a | 25.34 ± 0.16a | 25.20 ± 0.11a | 25.20 ± 0.11a | 0.06 | 0.514 |
| 8-14 d | 64.21 ± 0.23a | 64.70 ± 0.25a | 65.09 ± 0.19a | 65.23 ± 0.45a | 0.17 | 0.124 |
| 15-21 d | 93.12 ± 0.55a | 93.50 ± 0.45a | 93.06 ± 0.74a | 93.35 ± 0.6a | 0.27 | 0.946 |
| 22-28 d | 131.58 ± 1.26a | 130.29 ± 2.06a | 134.63 ± 1.49a | 133.37 ± 2.08a | 0.89 | 0.358 |
| 29-35 d | 147.31 ± 3.54a | 142.72 ± 1.87a | 138.91 ± 3.66a | 145.32 ± 2.00a | 1.53 | 0.247 |
| 0-35 d | 95.18 ± 0.83a | 93.05 ± 0.29a | 94.10 ± 0.48a | 94.10 ± 0.39a | 0.31 | 0.098 |
| FCR | ||||||
| 0-7 d | 1.11 ± 0.006a | 1.10 ± 0.005a | 1.10 ± 0.008a | 1.11 ± 0.006a | 0.003 | 0.22 |
| 0-14 d | 1.28 ± 0.009a | 1.25 ± 0.004a | 1.26 ± 0.009a | 1.26 ± 0.005a | 0.004 | 0.069 |
| 0-21 d | 1.38 ± 0.003a | 1.36 ± 0.007b | 1.37 ± 0.002ab | 1.37 ± 0.006ab | 0.003 | 0.02* |
| 0-28 d | 1.42 ± 0.01a | 1.37 ± 0.01a | 1.37 ± 0.007a | 1.40 ± 0.02a | 0.01 | 0.179 |
| 0-35 d | 1.55 ± 0.03a | 1.47 ± 0.01b | 1.49 ± 0.01ab | 1.50 ± 0.01ab | 0.01 | 0.021* |
| Mortality (%) | ||||||
| 0-35 d | 4.60 ± 0.38a | 3.40 ± 0.6a | 4.00 ± 0.33a | 3.60 ± 0.4a | 0.23 | 0.278 |
a,b Within a row, means with different superscripts differ significantly (p < 0.05), one way ANOVA, Tukey's test).
The results are reported as means ± standard error.
Represents significant variation (p < 0.05) within a row.
BWG = Body Weight Gain; ADWG = Average Daily Weight Gain; ADFI = Average Daily Feed Intake; FCR = Feed Conversion Ratio; g = Gram; d = day.
SEM= Standard error of mean
The average daily weight gain (ADWG) during 0 – 35 day varied significantly among the four trial groups (p < 0.05) with a highest ADWG was observed in T2 (Table 2). However, T3 and T4 groups obtained nearly a similar ADWG, though T1 was found to have lowest body weight (Table 2).
Average daily feed intake (ADFI) among the different treatment groups with a 35 days trial period was found non-significant (p>0.05). However, a lower ADFI was observed in T2 and T3 (probiotic-supplemented groups) at 5th week (Table 2). Additionally, during a 35 days trial period, the lowest ADFI was recorded in T2 (supplemented with Bacillus licheniformis DSM17236).
The impact of different dietary treatments on FCR of broiler poultry was found significantly varied (p < 0.05) at day 21 and 35 (Table 2). In this evaluation, a 35 days trial-period, T1 was found to be the highest FCR (1.55) than the other groups [T2 (1.47), T3 (1.49) and T4 (1.50)]. A lower FCR was documented in probiotic treated groups (T2 and T3) in each week of trial period where the birds were fed with Bacillus licheniformis DSM17236 (T2) and the FCR was found to be satisfactory.
A total of 78 broiler birds died in four trial groups during the entire study period where the highest mortality was recorded in T1 (n = 23) and the lowest in T2 (n = 17) trail group. However, second-highest mortality was observed in T3 (n = 20) (supplemented with Bacillus subtilis PB6). However, the mortality rate in broiler birds among different trial groups was found to be non-significant (Table 2).
3.2. Gut histopathology
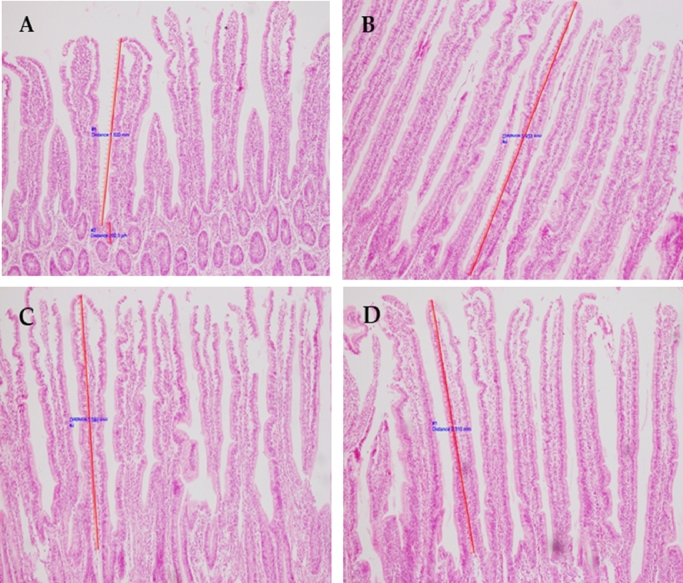
Histopathology findings of small intestine (jejunum) tissue samples of all trial groups are presented in Table 3 and Fig. 1. A moderate erosion in the tips of the villi with loss of villi materials including flattening of the upper portion of the villi were observed in histopathology section of birds in T1, T3 and T4 trial groups. However, histopathology section of T2 group birds was found to be marginally better villi, slight erosion with some separation of the villi tips. The gut histomorphology was found to be relatively better in T2 group than the others. However, the histopathology scoring was found to be non-significant among the trial groups (Table 3).
Table 3.
Comparison of histopathology, intestinal lesion scoring and immune response of broiler birds in four trial groups.1
| Parameters | Treatment | SEM2 | p-value | |||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |||
| Gut histopathology (n = 4) | 2.00 ± 0.00 | 1.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 0.11 | - |
| Intestinal lesion scoring (n = 8) | 2.88 ± 0.13a | 2.25 ± 0.25a | 2.75 ± 0.14a | 2.88± 0.24a | 0.11 | 0.13 |
| Antibody titer (log2) to SRBC antigen (n = 12) | 2.67 ± 0.76a | 3.17 ± 0.56a | 2.75 ± 0.62a | 2.83± 0.79a | 0.33 | 0.959 |
| Antibody titer (log2) against Newcastle virus (n = 12) | 4.31 ± 0.50a | 4.44 ± 0.48a | 4.25 ± 0.25a | 4.31± 0.34a | 0.18 | 0.99 |
Values are mean and standard error. Mean values in the same row with different letters differ significantly (p<0.05). n = Number of birds of a treatment group examined for each test. 2 SEM = Standard error of mean
Fig. 1.
Cross sectional view of small intestine (jejunal villus) of broiler birds at 28 day of age (H & E X 100). (A): Moderate erosion of the villi tips with loss of villi material and flatten of the upper portion of the villi (T1); (B) Mild erosion with some separation of the tips and separation of the cells from the basement membrane (T2); (C) Moderate erosion of the villi tips with loss of villi material and flatten of the upper portion of the villi (T3), and (D) Moderate erosion of the villi tips with loss of villi material and flatten of the upper portion of the villi (T4).
3.3. Intestinal lesion scoring
The birds from T1 and T4 had a greater average lesion score (2.88) than T2 and T3. However, average intestinal lesion score was found to be deviated marginally among two probiotic-supplemented groups (T2 and T3). The lowest intestinal lesion score was recorded in birds of T2 (Bacillus licheniformis probiotic supplemented group) with an average lesion score of 2.25. However, no significant difference (p > 0.05) of intestinal lesion scores among different treatment groups was found at the age of 28 day (Table 3).
3.4. Enumeration of intestinal bacteria
The intestinal (jejunal and ileal) microflora composition of broilers was examined at day 21 and 35 of age (Table 4). Unrelatedly to the trial groups, significant differences (p<0.05) were observed on the microbial populations at day 21 of age in jejunum samples for total Bacillus count (TBC) and day 35 of age for total Bacillus count (TBC), total E. coli count (TEC), total Bacillus licheniformis count (TBlC) and total Bacillus subtilis count (TBsC). The concentration of Clostridium perfringens, Escherichia coli, Salmonella was found to be decreased with age, at the same time, the colonization of both Bacillus licheniformis and Bacillus subtilis was elevated in both jejunum and ileum samples. In most cases, microbial count was presented higher in the ileum than jejunum samples at day 21 and 35.
Table 4.
Comparison of bacterial enumeration (Log10 CFU/g) in intestinal (jejunum and ileum) content of birds with different treatments on day 21 and 35.1
| Sample source | Differentbacterial count | Treatment | SEM 2 | p-value | |||
|---|---|---|---|---|---|---|---|
| T1(n = 12) | T2(n = 12) | T3(n = 12) | T4(n = 12) | ||||
| Jejunum | 21 day | ||||||
| TCpC | 2.63 ± 0.52a | 1.53 ± 0.63a | 2.47 ± 0.12a | 2.43 ± 0.67a | 0.26 | 0.482 | |
| TSC | 2.46 ± 0.67a | 2.05 ± 0.58a | 1.91 ± 0.84a | 2.32 ± 0.99a | 0.36 | 0.958 | |
| TEC | 7.36 ± 0.46a | 5.35 ± 0.81a | 5.53 ± 0.49a | 7.07 ± 0.71a | 0.37 | 0.096 | |
| TBC | 7.40 ± 0.13a | 9.05 ± 0.07b | 8.42 ± 0.19cb | 8.27 ± 0.23cd | 0.17 | <0.001* | |
| TBlC | 7.41 ± 0.71a | 8.03 ± 0.20a | 7.93 ± 0.71a | 7.99 ± 0.21a | 0.24 | 0.815 | |
| TBsC | 7.03 ± 0.29a | 7.24 ± 0.90a | 7.84 ± 0.15a | 7.39 ± 0.28a | 0.24 | 0.706 | |
| 35 day | |||||||
| TCpC | 2.28 ± 0.70a | 1.13 ± 0.05a | 1.61 ± 0.35a | 2.02 ± 0.91a | 0.29 | 0.569 | |
| TSC | 1.92 ± 0.82a | 2.18 ± 0.06a | 1.76 ± 0.36a | 2.60 ± 0.47a | 0.24 | 0.676 | |
| TEC | 6.95 ± 0.25a | 3.17 ± 0.70b | 4.59 ± 0.38ab | 5.23 ± 1.49ab | 0.52 | 0.05* | |
| TBC | 8.83 ± 0.15a | 10.06 ± 0.20b | 9.91 ± 0.19cb | 9.20 ± 0.14ac | 0.15 | 0.001* | |
| TBlC | 8.79 ± 0.22a | 9.41 ± 0.33a | 8.89 ± 0.19a | 8.82 ± 0.13a | 0.12 | 0.232 | |
| TBsC | 8.80 ± 0.14a | 9.27 ± 0.23ab | 9.82 ± 0.20b | 8.76 ± 0.20a | 0.14 | 0.007* | |
| Ileum | 21 day | ||||||
| TCpC | 2.72 ± 0.73a | 1.92 ± 0.82a | 2.59 ± 0.11a | 2.64 ± 0.48a | 0.28 | 0.77 | |
| TSC | 3.26 ± 0.35a | 2.75 ± 0.30a | 2.18 ± 0.80a | 2.81 ± 0.23a | 0.24 | 0.489 | |
| TEC | 7.10 ± 0.85a | 5.69 ± 0.62a | 5.44 ± 0.93a | 6.19 ± 0.43a | 0.37 | 0.422 | |
| TBC | 8.50 ± 0.05a | 8.97 ± 0.07a | 8.88 ± 0.15a | 8.58 ± 0.17a | 0.08 | 0.051 | |
| TBlC | 7.49 ± 0.73a | 8.40 ± 0.21a | 7.75 ± 0.68a | 8.19 ± 0.11a | 0.25 | 0.603 | |
| TBsC | 7.88 ± 0.13a | 8.10 ± 0.16a | 7.89 ± 0.80a | 7.96 ± 0.18a | 0.19 | 0.981 | |
| 35 day | |||||||
| TCpC | 2.95 ± 0.74a | 1.63 ± 0.98a | 2.25 ± 0.44a | 2.62 ± 1.17a | 0.41 | 0.744 | |
| TSC | 2.95 ± 0.63a | 1.04 ± 0.40a | 2.03 ± 0.29a | 2.77 ± 0.93a | 0.34 | 0.167 | |
| TEC | 7.66 ± 0.07a | 4.31 ± 0.67b | 5.00 ± 0.48b | 5.75 ± 0.56ab | 0.39 | 0.003* | |
| TBC | 8.67 ± 0.17a | 10.32 ± 0.12b | 10.21 ± 0.18bc | 9.02 ± 0.12a | 0.20 | <0.001* | |
| TBlC | 8.17 ± 0.17a | 10.09 ± 0.10b | 9.57 ± 0.20bc | 8.96 ± 0.20cd | 0.20 | <0.001* | |
| TBsC | 8.34 ± 0.02a | 9.65 ± 0.15b | 9.96 ± 0.18bc | 8.86 ± 0.26a | 0.18 | <0.001* | |
Values of each row are mean and standard error of 12 birds of a trial group (n = Number of birds of a trial group examined for each test). Mean values in the same row with different letters differ significantly (p < 0.05). *Indicates significant variation (p < 0.05) within a row. TCpC = Total Clostridium perfringens count, TSC = Total Salmonella count, TEC = Total E. coli count, TBC = Total Bacillus count, TBlC = Total Bacillus licheniformis count, TBsC = Total Bacillus subtilis count. 2 SEM= Standard error of mean
3.5. Immune responses
Antibody response in different trial groups at day 35 has been presented in Table 3. Regarding antibody production against SRBC, no significant difference (p > 0.05) was found among different trial groups. However, antibody titer was found to be higher after 5 days of inoculation of SRBC in T2, T4 and T3 trial group respectively than the control group (T1). A similar pattern of immune response against NDV was observed in HI test. Result of HI antibody titer at day 28 among the different groups is shown in Table 3. Moderately higher antibody titer was documented in T2. However, no significant difference (p > 0.05) was observed in antibody titer to NDV among the groups.
4. Discussion
The poultry sector in Bangladesh has expanded rapidly since 1990s and this sector has an enormous contribution to the national economy regarding woman empowerment, income generation and supply of cheapest source animal protein for the country people (Joardar & Rahman, 2018). The commonly reported broiler diseases in Bangladesh are IBD, ND, Coccidiosis (Rahman et al., 2019), however, low prevalence of NE was reported in Bangladesh. Such disease distribution has confirmed based on the history, clinical findings including postmortem lesions (Hassan et al., 2016). This disease may be underreported or misdiagnosed with other broiler diseases in Bangladesh as the robust diagnostic tools are not being used. NE has a serious impact globally on poultry production causing severe economic losses due to reduced growth performance, increased mortality, huge treatment costs, and poor flock uniformity (Skinner, Bauer, Young, Pauling, & Wilson, 2010; Timbermont, Haesebrouck, Ducatelle, & Van Immerseel, 2011). The causal agent of NE is C. perfringens, a normal inhabitant in chicken intestinal microflora, usually found in low numbers in the posterior part of the gut. This pathogen cause disease through increase in number and toxins production in favorable condition (Jayaraman, Das, Saini, Roy, & Chatterjee, 2017; Timbermont, Haesebrouck, Ducatelle, & Van Immerseel, 2011). Therefore, appropriate management strategies should be included in the control approaches to curb the infection as an alternative to AGPs. Competitive exclusion by prebiotics, probiotics, enzymes and organic acids would be potential to support better gut health and to lessen incidence and severity of diseases, confirmed by several reports (Kaldhusdal, Schneitz, Hofshagen, & Skjerve, 2001; Zhou, Deng, Tao, Hu, & Hou, 2009).
In poultry production, this is very crucial to select safe and appropriate antibiotic alternatives that will give economic returns. Growth performances including BWG, ADWG, ADFI, and FCR are the major determinants used to assess the economic returns in broiler production (Zhang et al., 2021). In this study, BWG in T2 and T3 trial groups was found significantly higher at the age of day 21 and 35. Similarly, significant progress in ADWG of the birds during the entire production cycle (0-35 d) was observed as feed efficiency enhanced in probiotic supplemented trial groups (T2 and T3). Moreover, the feed conversion ratio (FCR) was found to be improved significantly (p<0.001) in probiotic treated groups. These findings are in agreement with several published reports where increased BWG and ADWG in probiotic supplemented feed (Deniz et al., 2011; Jayaraman et al., 2017; Kabir et al., 2005; Khatun et al., 2017; Lin et al., 2017; Zhang et al., 2021).
In this study, BWG was found to be significantly increased in the later weeks of a production cycle. This finding is corroborated by a study conducted in India as an improvement in growth performance was observed when probiotic microbial agent was supplemented to the finisher diet of broilers (Mohan, Kadirvel, Natarajan, & Bhaskaran, 1996). The beneficial effect of B. licheniformis and B. subtilis on growth performance including higher bone strength in broiler poultry (Mutuş et al., 2006)) due to the improved feed digestibility and rise of serum Ca and P by the action of beneficial bacteria in the gut (Rizzoli & Biver, 2020). The proper probiotic supplementation would render a suitable environment to assist microflora colonization in intestine and support to the better growth performance of birds (Jayaraman et al., 2017).
Moderate to mild erosion of the villi tips with loss of villi material and flatten of the upper portion of the villi were found in all trial groups, however, degree changes was relatively lesser in T2 trial group. This finding is sparsely supported by other research (Al-Baadani, Abudabos, Al-Mufarrej, & Alzawqari, 2016). However, in our study, there had no significant variation regarding gut histopathology and intestinal lesion scoring among the different groups. Moreover, T3 (B. subtilis PB6 supplemented group) demonstrated significant FCR along with an improved villi morphology. Nevertheless, a second highest mortality rate (4.00 ± 0.33%) with a poor immune response (against SRBC antigen and NDV) recorded in this trial group. This may be due to closely similar intestinal lesion score and gut histopathology score in T1, T3 and T4 groups. This signifies an imbalance condition of gut microflora including higher number of pathogenic bacteria especially Clostridium perfringens in the gut of broiler poultry. Moreover, birds were reared under low biosecurity farm this might enhance a higher colonization of pathogenic bacteria in the poultry gut causing nearly similar level of pathogenic lesions.
Higher antibody response in the probiotic groups (T2 and T3) than the control group was observed in this study could attribute to the Bacillus probiotics that enabling to assimilation of essential nutritional components for stimulating the immune production cells (Khaksefidi & Ghoorchi, 2006). A higher antibody titer was observed in the probiotic supplemented groups at 5 days of post-immunization is consistent with the finding of the present study (Khaksefidi & Ghoorchi, 2006). Further, the ability of probiotics to facilitate for production of serum and intestinal natural antibodies against several foreign antigens in chickens has been validated (Haghighi et al., 2005). The direct immunomodulatory impact of probiotics could stimulate the lymphatic tissue and the indirect impact through conservation of a balanced microflora in the intestine (Khaksefidi & Ghoorchi, 2006).
The results of present study relating to the intestinal microflora populations demonstrated that the dynamics of gut microorganism are affected by the feed supplementation of probiotics in broiler ration, and is responsible for significantly less growth of pathogenic bacteria, like Clostridium perfringens, Escherichia coli (Markowiak & Śliżewska, 2017). The concentration of Clostridium perfringens, Escherichia coli, Salmonella was found to be decreased with the age, at the same time, the colonization of both Bacillus licheniformis and Bacillus subtilis were found to be elevated in jejunum and ileum samples in probiotic treatment groups (T2 and T3). In most cases, microbial count was found higher in the ileum than jejunum samples at day 21 and 35. Nevertheless, a significant increase of other beneficial microorganisms like Lactobacillus spp. in the digestive tract of chickens was confirmed in several studies after inclusion of Bacillus probiotics in chicken feed (Whelan et al., 2019).
The main limitation of this study was that samples were taken from a fewer number of birds of trial groups that was not representative for histopathology, intestinal lesion scoring and immune response evaluations. Therefore, this makes insignificant result. Additionally, this could not be possible to establish a substantial association in all studied parameters of probiotic trial groups as the efficiency of a probiotic application depends on numerous factors, like type of strains, doses of probiotics, route of application, feed, and hygienic condition of the farm (Patterson & Burkholder, 2003). However, the study was conducted in broiler flocks with lower level of biosecurity measurements in hotter summer season. These might be the reasons for insignificant results in the stressful environments.
5. Conclusion
The study has demonstrated the impact of probiotics and AGP on the growth performance, gut morphology, intestinal microbiota and immune response in broiler chicken. Data of the present study confirmed that Bacillus based probiotics especially Bacillus licheniformis in broiler feed can support to enhance body weight gain, efficient feed conversion ratio including immune response in broiler chicken. Therefore, this probiotic could be used in broiler feed to facilitate cost effective broiler meat production and to minimize antimicrobial resistant associated with broiler rearing considering public health and food safety grounds.
CRediT authorship contribution statement
Mohammad Arif: Formal analysis, Writing – original draft. Md. Akteruzzaman: Methodology. : Formal analysis. Sk Shaheenur Islam: Formal analysis, Writing – review & editing. Bidhan Chandra Das: Conceptualization, Methodology. Mahbubul Pratik Siddique: Writing – review & editing. S. M. Lutful Kabir: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
Funding
This study was funded by Planet Feeds Ltd., Delta Dahlia (7th Floor), 36 Kemal Ataturk Avenue, Banani, Dhaka-1213, Bangladesh (Project ID: 2019/1/PFL).
Ethical statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to this research. The experimental trial was approved by Animal Welfare and Experimentation Ethical Committee (AWEEC) of Bangladesh Agricultural University (AWEEC/BAU/2019/15). The birds were handled humanely throughout the study period.
Data availability
The data that support the findings of this study are included in the manuscript.
Acknowledgments
The authors thanks to Planet Hatchery Project, Sirajgonj, Bangladesh, and Planet Feeds Ltd. Vhaluka, Mymensingh, Bangladesh for providing day-old-chicks and trial sheds to conduct the present study. The authors would like also extend their deep appreciation to the Bangladesh Livestock Research Institute (BLRI), Savar, Bangladesh for support to conducting histopathology and HI test.
References
- Akhter A.H.M.T., Islam S.S., Sufian M.A., Hossain M., Rahman S.M.M., Hossain M.M. Implementation of code of practices (CoP) in selected poultry farms of Bangladesh. Asian-Australasian Journal of Food Safety and Security. 2018;2:45–55. [Google Scholar]
- Al-Baadani H.H., Abudabos A.M., Al-Mufarrej S.I., Alzawqari M. Effects of dietary inclusion of probiotics, prebiotics and synbiotics on intestinal histological changes in challenged broiler chickens. South African Journal of Animal Science. 2016;46:157–165. doi: 10.4314/sajas.v46i2.6. https://doi.org/ [DOI] [Google Scholar]
- AlGburi A., Volski A., Cugini C., Walsh E.M., Chistyakov V.A., Mazanko M.S., Bren A.B., Dicks M.L.T., Chikindas M.L. Safety properties and probiotic potential of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895. Advances in Microbiology. 2016;6:432–452. doi: 10.4236/AIM.2016.66043. https://doi.org/ [DOI] [Google Scholar]
- Apata D.F. Growth performance, nutrient digestibility and immune response of broiler chicks fed diets supplemented with a culture of Lactobacillus bulgaricus. Journal of the Science of Food and Agriculture. 2008;88:1253–1258. doi: 10.1002/JSFA.3214. https://doi.org/ [DOI] [Google Scholar]
- Awad W.A., Böhm J., Razzazi-Fazeli E., Ghareeb K., Zentek J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poultry Science. 2006;85:974–979. doi: 10.1093/PS/85.6.974. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Bunyan J., Jeffries L., Sayers J.R., Gulliver A.L., Coleman K. Antimicrobial substances and chick growth promotion: The growth-promoting activities of antimicrobial substances, including fifty-two used either in therapy or as dietary additives. British Poultry Science. 1977;18:283–294. doi: 10.1080/00071667708416364. https://doi.org/ [DOI] [Google Scholar]
- Cao G.T., Zeng X.F., Chen A.G., Zhou L., Zhang L., Xiao Y.P., Yang C.M. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poultry Science. 2013;92:2949–2955. doi: 10.3382/ps.2013-03366. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chen Y., Li X., Yang W., Wen C., Kang Y., Wang A., Zhou Y. Effects of synbiotic supplementation on growth performance, carcass characteristics, meat quality and muscular antioxidant capacity and mineral contents in broilers. Journal of the Science of Food and Agriculture. 2017;97:3699–3705. doi: 10.1002/JSFA.8230. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Cooper K.K., Songer J.G., Uzal F.A. Diagnosing clostridial enteric disease in poultry. Journal of Veterinary Diagnostic Investigation. 2013;25:314–327. doi: 10.1177/1040638713483468. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Dahiya J.P., Hoehler D., Wilkie D.C., Van Kessel A.G., Drew M.D. Dietary glycine concentration affects intestinal Clostridium perfringens and lactobacilli populations in broiler chickens. Poultry Science. 2005;84:1875–1885. doi: 10.1093/ps/84.12.1875. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Deniz G., Orman A., Cetinkaya F., Gencoglu H., Meral Y., Turkmen I.I. Effects of probiotic (Bacillus subtilis DSM 17299) supplementation on the caecal microflora and performance in broiler chickens. Revue de Medecine Veterinaire. 2011;162:538–545. [Google Scholar]
- Dolberg, F. (2008). Poultry sector country review, Bangladesh. Retrieved August 26, 2021, from https://agris.fao.org/agris-search/search.do?recordID=XF2016009026.
- FAO. (2008). Biosecurity for highly pathogenic avian influenza : issues and options. Retrieved August 26, 2021, from http://www.fao.org/3/i0359e/i0359e00.htm.
- Fuller R. Probiotics in man and animals. Journal of Applied Bacteriology. 1989;66:365–378. doi: 10.1111/J.1365-2672.1989.TB05105.X. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Gholamiandehkordi A.R., Timbermont L., Lanckriet A., Van Den Broeck W., Pedersen K., Dewulf J., Pasmans F., Haesebrouck F., Ducatelle R., Van Immerseel F. Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathology. 2007;36:375–382. doi: 10.1080/03079450701589118. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Haghighi H.R., Gong J., Gyles C.L., Hayes M.A., Sanei B., Parvizi P., Gisavi H., Chambers J.R., Sharif S. Modulation of antibody-mediated immune response by probiotics in chickens. Clinical and Diagnostic Laboratory Immunology. 2005;12:1387–1392. doi: 10.1128/CDLI.12.12.1387-1392.2005. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M.K., Kabir M.H., Hasan M.A.A., Sultana S., Khokon M.S.I., Kabir S.M.L. Prevalence of poultry diseases in Gazipur district of Bangladesh. Asian Journal of Medical and Biological Research. 2016;2:107–112. doi: 10.3329/AJMBR.V2I1.27575. https://doi.org/ [DOI] [Google Scholar]
- Jayaraman S., Das P.P., Saini P.C., Roy B., Chatterjee P.N. Use of Bacillus Subtilis PB6 as a potential antibiotic growth promoter replacement in improving performance of broiler birds. Poultry Science. 2017;96:2614–2622. doi: 10.3382/ps/pex079. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Joardar J.C., Rahman M.M. Poultry feather waste management and effects on plant growth. International Journal of Recycling of Organic Waste in Agriculture. 2018;7:183–188. doi: 10.1007/S40093-018-0204-Z. https://doi.org/ [DOI] [Google Scholar]
- Kabir S.M.L., Rahman M.M., Rahman M.B., Hosain M.Z., Akand M.S.I., Das S.K. Viability of probiotics in balancing intestinal flora and effecting histological changes of crop and caecal tissues of broilers. Biotechnology. 2005;4:325–330. doi: 10.3923/BIOTECH.2005.325.330. https://doi.org/ [DOI] [Google Scholar]
- Kaldhusdal M., Schneitz C., Hofshagen M., Skjerve E. Reduced incidence of Clostridium perfringens - associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Diseases. 2001;45(1):149–156. doi: 10.2307/1593022. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Khaksefidi A., Ghoorchi T. Effect of probiotic on performance and immunocompetence in broker chicks. Journal of Poultry Science. 2006;43:296–300. doi: 10.2141/jpsa.43.296. https://doi.org/ [DOI] [Google Scholar]
- Khatun M.T., Kabir S.M.L., Islam M.S., Ferdous T.A., Islam M.M., Rahman M.M., Mustafa M.M.H., Latif M.A., Thitisak P., Poonsuk K. Effects of dietary inclusion of a commercially available probiotic on growth performance, cecal microbiota and small intestinal morphology in broiler chickens. International Journal of Livestock Production. 2017;8:33–39. doi: 10.5897/IJLP2017.0365. https://doi.org/ [DOI] [Google Scholar]
- Kizerwetter-Swida M., Binek M. Protective effect of potentially probiotic Lactobacillus strain on infection with pathogenic bacteria in chickens. Polish Journal of Veterinary Sciences. 2009;12:15–20. https://www.academia.edu/download/44991373/Protective_effect_of_potentially_probiot20160422-1034-xysngx.pdf Retrieved from. [PubMed] [Google Scholar]
- Lin Y., Xu S., Zeng D., Ni X., Zhou M., Zeng Y., Wang H., Zhou Y., Shu H., Pan K., Li G. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS One. 2017;12:1–18. doi: 10.1371/journal.pone.0182426. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney R., Weeks R., Zheng T., Huang Q., Dai W., Cao Y., Guo Y., Chistyakov V., Chikindas M.L. Evaluation of an industrial soybean byproduct for the potential development of a probiotic animal feed additive with Bacillus species. Probiotics and Antimicrobial Proteins. 2019;12:1173–1178. doi: 10.1007/S12602-019-09619-5. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Markowiak P., Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi: 10.3390/NU9091021. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan R., Kalaiarasi K. Panchagavya and Andrographis paniculata as alternatives to antibiotic growth promoters on haematological, serum biochemical parameters and immune status of. The Journal of Poultry Science. 2007;44:198–204. doi: 10.2141/jpsa.44.198. Retrieved from https://doi.org/ [DOI] [Google Scholar]
- Mohan B., Kadirvel R., Natarajan A., Bhaskaran M. Effect of probiotic supplementation on growth, nitrogen utilisation and serum cholesterol in broilers. British Poultry Science. 1996;37:395–401. doi: 10.1080/00071669608417870. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Mutuş R., Kocabaǧli N., Alp M., Acar N., Eren M., Gezen Ş.Ş. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poultry Science. 2006;85:1621–1625. doi: 10.1093/PS/85.9.1621. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- National Research Council. (1994). Nutrient requirements of poultry: 1994. Retrieved from https://books.google.com/books?hl=en&lr=&id=bbV1FUqRcM0C&oi=fnd&pg=PT13&dq=32.%09NRC.+Nutrient+Requirements+of+Poultry%3B+Natl.+Acad.+Press,+Washington,+DC.:+1994.&ots=IkhKYFfzSz&sig=TIHKuKWeQmNwKDNJoK6N885Ygog.
- Němečková I., Solichová K., Roubal P., Uhrová B., Šviráková E. Methods for detection of Bacillus sp., B. cereus, and B. licheniformis in raw milk. Czech Journal of Food Sciences. 2011;29:55–60. https://www.agriculturejournals.cz/publicFiles/313_2011-CJFS.pdf Retrieved from. [Google Scholar]
- O'Dea E.E., Fasenko G.M., Allison G.E., Korver D.R., Tannock G.W., Guan L.L. Investigating the effects of commercial probiotics on broiler chick quality and production efficiency. Poultry Science. 2006;85:1855–1863. doi: 10.1093/PS/85.10.1855. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Olnood C.G., Beski S.S.M., Choct M., Iji P.A. Novel probiotics: Their effects on growth performance, gut development, microbial community and activity of broiler chickens. Animal Nutrition. 2015;1:184–191. doi: 10.1016/j.aninu.2015.07.003. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Lee M., Jeong D., Park S., Ji Y., Todorov S.D., Holzapfel W.H. Safety evaluation and in vivo strain-specific functionality of Bacillus strains isolated from korean traditional fermented foods. Probiotics and Antimicrobial Proteins. 2021;13:60–71. doi: 10.1007/S12602-020-09672-5. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poultry Science. 2003;82:627–631. doi: 10.1093/PS/82.4.627. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Rahman M.A., Rahman M.M., Abdullah M.S., Sayeed M.A., Rashid M.H., Mahmud R., Belgrad J.P., Hoque M.A. Epidemiological assessment of clinical poultry cases through the government veterinary hospital-based passive surveillance system in Bangladesh: a case study. Tropical Animal Health and Production. 2019;51:967–975. doi: 10.1007/S11250-018-1782-5. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Rizzoli R., Biver E. Are probiotics the new calcium and vitamin D for bone health? Current Osteoporosis Reports. 2020;18:273–284. doi: 10.1007/S11914-020-00591-6. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Shivaramaiah S., Pumford N.R., Morgan M.J., Wolfenden R.E., Wolfenden A.D., Torres-Rodriguez A., Hargis B.M., Tellez G. Evaluation of Bacillus species as potential candidates for direct-fed microbials in commercial poultry. Poultry Science. 2011;90:1574–1580. doi: 10.3382/ps.2010-00745. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Skinner J.T., Bauer S., Young V., Pauling G., Wilson J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Diseases. 2010;54:1237–1240. doi: 10.1637/9399-052110-REG.1. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathology. 2011;40(4):341–347. doi: 10.1080/03079457.2011.590967. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Whelan R.A., Doranalli K., Rinttilä T., Vienola K., Jurgens G., Apajalahti J. The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poultry Science. 2019;98:3450–3463. doi: 10.3382/ps/pey500. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C., Na C.S., Park J.H., Han S.K., Nam Y.M., Kwon J.T. Effect of feeding multiple probiotics on performance and fecal noxious gas emission in broiler chicks. Korean Journal of Poultry Science. 2004;31(4):229–235. https://www.koreascience.or.kr/article/JAKO200430710410176.page Retrieved from. [Google Scholar]
- Zhang S., Zhong G., Shao D., Wang Q., Hu Y., Wu T., Ji C., Shi S. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poultry Science. 2021;100 doi: 10.1016/J.PSJ.2020.12.032. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.L., Deng Y.F., Tao Q.S., Hu Y.F., Hou J.F. Effects of Gushukang, a Chinese herbal medicine, on bone characteristics and osteoporosis in laying hens. Poultry Science. 2009;88(11):2342–2345. doi: 10.3382/PS.2009-00285. https://doi.org/ [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are included in the manuscript.