Abstract
Due to the vague symptomatology of the disease and a lack of effective screening methods, most patients with epithelial ovarian cancer (EOC) present late in their disease. Despite advances in chemotherapeutic agents, the prognosis of these patients has uniformly been extremely poor. Although cisplatin-based chemotherapy regimens induce responses in most of these patients, the patients invariably experience disease progression or relapses. In an attempt to improve the treatment outcome using a different therapeutic approach, immunotherapy was investigated nearly 20 years ago. Many tumor antigens that are potentially suitable for specific immunotherapy were identified, and many immunotherapeutic approaches were attempted. However, although some responses were observed, the results from clinical studies were generally disappointing. Recent advances in immunoengineering and successes observed among patients treated for refractory/relapsed hematologic malignancies have rekindled the interest to revisit specific cellular immunotherapy in EOC. In this review, we provide the rationale for immunotherapy of EOC, discuss the results of some of the historical studies on the use of cellular immunotherapy in EOC, outline the principles of modern immunoengineering that could be applied to treat the disease, and propose the re-evaluation of the cancer-testis antigen, Sperm protein 17, for targeting by using modern immunoengineering technology.
Keywords: Sperm protein 17, tumor antigens, epithelial ovarian cancer, cell-based immunotherapy, immunoengineering
Graphical abstract

Sperm protein 17, a normal testicular protein aberrantly expressed in epithelial ovarian cancer, is a highly immunogenic tumor antigen discovered more than 20 years ago. The authors provide a review of how this antigen could be revisited for modern immunotherapy of ovarian cancer in the era of immunoengineering.
Introduction
Epithelial ovarian cancer (EOC) remains one of the commonest causes of cancer-related deaths in women in the 21st century. In the United States, there were approximately 235,000 EOC patients in 2018.1 It is estimated that there will be 21,410 new cases, with 13,770 deaths, in the United States in 2021.1 Although EOC is potentially a curable disease in its early stages, due to the generally vague and nonspecific symptoms and lack of effective screening strategies, most patients present late in their disease when metastasis has already occurred. Despite the advances in modern chemotherapeutic agents and personalized medicine, a significant inroad into the treatment of EOC has not been made. Cytoreduction through primary surgery followed by platinum-based chemotherapy has remained the first-line treatment for women with newly diagnosed EOC,2 and this has not changed significantly in the last three decades. While a high proportion of patients will respond to the combination of surgical debulking followed by adjuvant chemotherapy with carboplatin and paclitaxel, disease recurrence/progression invariably occurs.2
New approaches for advanced EOC in the past decade include antiangiogenic agents3, 4, 5 and poly(ADP-ribose) polymerase (PARP) inhibitors.6,7 The most commonly used and well-studied antiangiogenic agent in the treatment of EOC is bevacizumab. However, as reported in ICON-78 and GOG 281,9 bevacizumab added to platinum-based chemotherapy did not increase overall survival (OS) in the study populations as a whole but did show a significant difference in OS in a predefined subgroup of patients with poor-prognosis disease in ICON-7 and stage IV disease in GOG 218. PARP inhibitors, on the other hand, have shown meaningfully prolonged progression-free survival (PFS) for the first time after decades of studying different chemotherapy approaches.10 The well-studied PARP inhibitors for the treatment of high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer include olaparib, rucaparib, niraparib, and veliparib. Most of them are approved by the Food and Drug Administration to be used for first- or second-line maintenance therapy after cytotoxic chemotherapy or as the single agent for third- or fourth-line therapy. Optimal use of these newer agents remains to be determined, but at present the clinical benefits in OS are modest. The lack of successes with the currently available chemotherapeutic agents suggests that other approaches are much needed.
In this review, we discuss briefly the principles and history of immunotherapy in EOC, dissect Sperm protein 17 (Sp17) as a target for immune targeting, and examine how the current advances in immunoengineering might be exploited to utilize Sp17 for cell-based therapy of EOC.
Principles and history of immunotherapy in EOC
Immunotherapy has always been an attractive cancer therapeutic option, since it is not only more specific but also kills tumor cells via molecular mechanisms different from those induced by chemotherapeutic agents. It may, therefore, be used to either circumvent chemoresistance or in conjunction with chemotherapy to augment the cytotoxicity. Various clinical and laboratory data support the applicability of immunotherapy in EOC.
Both extracellular and intracellular tumor antigens have been demonstrated in ovarian cancer. Examples of these antigens are shown in Table 1.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Most of these antigens arise due to either overexpression or aberrant expression of normal cellular proteins. Extracellular antigens include Sp17,18 MUC1,11 and mesothelin.14 Intracellular tumor antigens in ovarian cancer cells have also been demonstrated in various studies, including the MAGE family of antigens,16 NY-ESO1,17 and LACE-1.17 Other antigens including neoantigens24, 25, 26, 27 may have arisen from random mutations associated with neoplastic process, although genomic analysis of EOC has found the tumor cells to harbor low mutational burden.28
Table 1.
Tumor antigens in epithelial ovarian cancer
| Class | Antigens | Comments |
|---|---|---|
| Overexpressed normal cellular antigens | MUC1 | these are normal cellular proteins that are overexpressed on EOC cells. Antigen-reactive T cells within the host immune repertoire are expected to be of low affinity for the tumor cells, since any high-affinity T cells would have been deleted from the immune repertoire during development to avoid autoimmunity |
| Wilms’ tumor 1 (WT1) | ||
| Telomerase | ||
| Mesothelin | ||
| Erb | ||
| Cancer-testis antigens (CTA) | MAGE family | these are normal testicular proteins that are aberrantly expressed by EOC cells. They are generally highly immunogenic because testes are immune sanctuary sites, hence high-affinity antigen-reactive T cells are not deleted by the thymus during development. Furthermore, being whole proteins, immune responses tend to be polyclonal, directed at more than one epitope within the antigens, providing high in vivo effector/target ratios. Even though CTA are testicular-restricted proteins, highly sensitive qPCR has detected leakage of expression of some of these genes, at low levels, in normal tissues. Their expression in tumor cells is believed to be mediated, in most cases, by changes in the methylation status of the gene during carcinogenesis |
| NY-ES O 1 | ||
| LACE-1 | ||
| Sp17 | ||
| AKAP3 | ||
| AKAP4 | ||
| GAGE-1/2 | ||
| PRAME | ||
| TRAG-3 | ||
| Neoantigens | mutated KRas | these antigens arise due to point mutations that cause amino acid substitutions. Being neoantigens, they are highly immunogenic. However, unless there are numerous mutations within the genes, immune responses against the neoantigens will be monoclonal, directed at only one single epitope, restricting the resulting in vivo effector/target ratios |
| mutated p53 | ||
| mutated Erb | ||
| mutated ARID1a |
EOC cells are susceptible to the cytotoxic machinery of T cells. Peripheral blood lymphocytes primed to MUC1,29 MAGE,30,31 Sp17,32 and mesothelin33 have demonstrated the ability to lyse EOC cells. Similarly, susceptibility of EOC cells to killing by T cells has been demonstrated in mouse models of EOC.34,35 Clinically, the correlation between the intensity of tumor-infiltrating lymphocytes (TILs) and clinical outcomes36, 37, 38 further supports this notion. Clinical responses were observed in EOC patients treated with TILs expanded ex vivo.39, 40, 41, 42
Since ovarian tumor cells harbor immunogenic antigens and tumor-reactive T cells are present in the immune repertoire of the autologous hosts, harnessing the host de novo antitumor immune responses through immunotherapy is an attractive option. Unfortunately, the results from cell-based immunotherapy in EOC have thus far been uniformly disappointing (Table 2).39, 40, 41, 42, 43, 44, 45, 46 One of the major reasons for the lack of successes is an immune repertoire that has been contracted severely by chemotherapy, rendering the host response to be unable to mount an efficient effector/target ratio in vivo. This notion is clearly seen in the clinical use of immune checkpoint inhibitors in EOC patients. Due to low tumor mutational burden (17) and thus a low number of neoantigen-reactive T cells, the response rate has been uniformly disappointing. Advances in immunoengineering provide a mean to increase the effector/target ratio. However, this approach is applicable only for known antigens and not for unidentified neoantigens that arise from random genomic mutation in EOC.
Table 2.
Samples of clinical studies of cell-based immunotherapy in epithelial ovarian cancer
| Authors | No. of patients (n) | Intervention | Outcome | Comments |
|---|---|---|---|---|
| Kershaw et al.43 | 8 in cohort 1; 6 in cohort 2 | T cells with reactivity against the ovarian cancer–associated antigen α-folate receptor (FR) were generated from autologous T cells with a chimeric gene incorporating an anti-FR single-chain antibody linked to the signaling domain of the Fc receptor γ chain. Eight patients in cohort 1 received a dose escalation of T cells in combination with high-dose interleukin-2, and six patients in cohort 2 received dual-specific T cells (reactive with both FR and allogeneic cells) followed by immunization with allogeneic peripheral blood mononuclear cells | no response was observed. 111In-labeled adoptively transferred T cells in cohort 1 did not show any tumor localization except in one patient where some signals were detected in the peritoneal deposit | phase 1 study in which all 14 patients had metastatic disease and had undergone surgical debulking and combination chemotherapy |
| Haas et al.44 | 15 patients in total: 6 in cohort 1; 3 each in cohorts 2, 3, and 4 | CAR was directed at mesothelin and contained CD3z and 4-1BB in the lentiviral construct. Cohorts 1 and 2 patients received 1–3 × 107 m2 and cohorts 3 and 4 patients received 1–3 × 108/m2 CAR-T cells. Patients in cohorts 2 and 4 also received lymphodepletion with cyclophosphamide, 1.5 g/m2 3 ± 1 day prior to CAR-T infusion | stable disease was observed in 11/15 patients on day 28 | phase 1 study that included 5 patients each, with ovarian cancer, malignant pleural mesothelioma, and pancreatic cancer |
| Aoki et al.39 | 17 patients with advanced/recurrent EOC | 7 patients received TILs following one dose of cyclophosphamide (group A) and 10 received alternating cisplatin-containing regimen and TILs (group B) | 1 CR and 4 PR in group A, and 7 CR and 2 PR in group B | responses in both primary and metastatic lesions lasted 3–5 months in group A |
| Fujita et al.40 | 13 | all patients were treated in the adjuvant setting after surgical debulking and cisplatin regimen. Each received >1 × 109 TILs | 3-year DFS was 81.2% and OS 100% | compared with a control group of 11 patients, DFS and OS were significantly prolonged |
| Freedman et al.41 | 8 patients with advanced EOC refractory to platinum-based chemotherapy | all patients received 1010–1011 TILs intraperitoneally and recombinant interleukin-2. One patient received two doses of TILs | no measurable response was observed in any of the patients | pilot study to evaluate feasibility and clinical response of intraperitoneal TILs |
| Liu et al.45 | 92 | all patients were treated in the adjuvant setting following surgical debulking and 6–8 cycles of carboplatin/paclitaxel; 46 patients were treated with monthly CIK cells and 46 in the control group with observation only | PFS was 37.7 months in the treatment group compared with 22.2 months in the control group (p = 0.004). OS was not improved | paired study of monthly maintenance CIK cells |
| Pedersen et al.42 | 6 | all patients had platinum-resistant progressive metastatic EOC. They all received TILs followed by rIL-2 | 4 patients showed stable disease for 3 months and 2 for 5 months | high number of infused TILs expressed LAG-3 and PD-1 |
| Wright et al.46 | 7 | all patients had recurrent EOC confined to the peritoneal cavity. They received peripheral blood lymphocytes stimulated with MUC1 intraperitoneally | 1 patient attained CR | no significant reduction in serum CA125 |
CR, complete response; PR, partial response; DFS, disease-free survival; OS, overall survival; PFS, progression-free survival; TILs, tumor-infiltrating lymphocytes; CIK, cytokine-induced killer; rIL-2, recombinant interleukin-2; MUC1, Mucin 1; LAG-3, lymphocyte activation gene 3; PD-1, programmed cell death protein 1; CA125, carbohydrate antigen 125.
Sperm protein 17 as a target for immunotherapy
Sp17 gene and protein
Human Sp17 gene, located on chromosome 11q24.2, encodes a highly conserved antigenic protein of 17.4 kDa molecular weight expressed in spermatozoa and is involved in acrosome reactions during fertilization.47 Immunohistochemistry on tissue arrays and RT-PCR of a panel of tissue RNA48 showed Sp17 to be restricted in its normal tissue distributions, being expressed primarily in normal testes. Immunotherapy targeting Sp17 will, therefore, be more specific and less toxic.
Sp17 is a highly immunogenic protein. Many vasectomized normal males develop autoantibodies against Sp17.49 It was, therefore, the target of investigation as an immunocontraceptive.50 Sp17 is primarily an extracellular protein and promotes heparan sulfate-mediated cell-cell adhesion.51,52 Although it is an extracellular protein, Sp17 undergoes modulation and is shed into the serum of cancer patients.53
Sp17 as a tumor antigen
Sp17 was first identified in our laboratory as an aberrantly expressed tumor antigen in multiple myeloma.54 It has since been found to be expressed in non-Hodgkin's lymphoma55 and non-small cell lung cancer.56 Interestingly, despite being a normal testicular protein, Sp17 expression on tumor cells does not appear to be gender biased. In fact, Sp17 is expressed in high proportions of patients with gynecologic cancers such as ovarian cancer, endometrial cancer, and cervical cancer (Table 3).18,54, 55, 56, 57, 58, 59, 60, 61, 62 In these conditions, Sp17 was not only expressed at the mRNA level but also at the protein level, arguing for a possible function of the protein in tumor cells. Sp17 expression is correlated with chemoresistance in clear-cell adenocarcinoma.63 It also has roles in metastasis and drug resistance; for example, EOC with high Sp17 expression shows resistance to paclitaxel,64 the first line of EOC treatment. EOC cells with enhanced Sp17 expression demonstrate increased migration.65 Progressive tumor growth by ID8 cells in mice is dependent on Sp17 expression.66 Immunotherapy targeting Sp17 is, therefore, attractive and may be used to eradicate the chemoresistance clones to augment the efficacy of chemotherapy.
Table 3.
Expression of Sperm protein 17 in tumor specimens
| Authors | Tumor type | Comments |
|---|---|---|
| Lim et al.54 | multiple myeloma | detected by RT-PCR and western blot analysis in 12/47 (26%) of specimens |
| Straughn et al.18 | ovarian cancer | detected by RT-PCR, northern blot analysis, western blot analysis, and immunohistochemistry in 7/11 (64%) of papillary serous or mixed, 4/8 (50%) endometrioid, 1/1 primary peritoneal carcinoma, 2/2 clear cell, 3/3 ovarian unspecified. Overall, 17/25 (68%) specimens |
| Liggins et al.55 | diffuse large B cell lymphoma | detected by RT-PCR, western blot analysis, and immunohistochemistry in 6/11 (55%) specimens |
| Zhou et al.57 | invasive breast cancer | detected by RT-PCR and immunohistochemistry in 27/100 (27%) specimens. Sp17 expression correlated with lymph node metastasis |
| Mirandola et al.58 | invasive breast cancer | detected by immunohistochemistry in 10/22 (45%) invasive breast cancers and 17/36 (47%) triple-negative breast cancers |
| Li et al.59 | endometrial and cervical cancer | detected by immunohistochemistry in 33/50 (66%) endometrial cancers and 19/31 (61%) cervical cancers |
| Grizzi et al.60 | neuroectodermal tumors | detected by immunohistochemistry in 6/28 (21%) tumors |
| Gupta et al.61 | esophageal squamous cell cancer | detected by RT-PCR in 26/30 (86%) and by immunohistochemistry in 60/80 (75%) specimens |
| Xia et al.62 | hepatocellular cancer | detected by immunohistochemistry in 36/45 (80%) specimens |
| Mirandola et al.56 | non-small cell lung cancer | detected by RT-PCR and immunocytochemistry in 16/40 (40%) specimens |
We and others have previously demonstrated the ability to generate Sp17-specific human leukocyte antigen (HLA) class I-restricted cytotoxic T cells from patients with various cancer types,32,67,68 including ovarian cancer. These cytotoxic T cells were able to kill fresh autologous ovarian tumor cells.32 T cell epitopes restricted by HLA-A1 and HLA-A2 have been identified.68,69 These works suggest that, despite being a surface antigen, Sp17 protein is also processed and presented in conjunction with the major histocompatibility complex (MHC). Furthermore, they also clearly demonstrate that despite the presence of active cancer and previous chemotherapy, Sp17-reactive T cells were still present in the immune repertoire of these patients. However, while molecules of MHC I expression are retained, Sp17-positive tumor cells have lower expression of MHC II molecules along with an increase in STAT3.66 STAT3 overexpression is associated with invasive and metastatic properties of EOC cells.70
Regulation of Sp17 expression
Unless the tumor antigen is a driver protein needed for tumor cell survival, progressive downregulation of a tumor antigen gene often occurs. This is a form of immune tumor escape mechanism and poses one of the biggest obstacles to successful immunotherapy. Although there is currently no evidence supporting progressive downregulation of Sp17 expression, Sp17 is heterogeneously expressed within individual tumor specimens,18 suggesting the risk of selecting for Sp17-negative tumor clones. Sp17 gene expression is regulated through promoter methylation71 and its expression may be upregulated by DNA-hypomethylating agents such as 5-azacytidine,71 providing a means to circumvent the obstacle due to antigen downregulation and heterogeneity of the antigen.
Sp17-targeted immunotherapy in the era of modern immunoengineering
Sp17 appears to be an ideal tumor target for modern immunotherapy of EOC for the following reasons: (1) it is expressed on the surface of a high proportion of EOC cells; (2) it is processed and presented by the MHC molecules on the surface of EOC cells; (3) it shows restricted normal tissue distribution; and (4) its expression may be regulated pharmacologically. Being a surface antigen, Sp17 may be amenable to specific antibody targeting. However, Sp17 monoclonal antibodies have not been tried in the clinic and the feasibility of this approach remains to be determined. Anti-Sp17 monoclonal antibody was found to have some antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity in vitro against Sp17-expressing ovarian cancer cells.72 Potential obstacles to its successful use include the presence of shed Sp17 protein in the serum of these patients.53 However, it remains to be determined whether the functions of ADCC remain intact and efficient in patients with advanced EOC.
On the other hand, data showing the susceptibility of ovarian tumor cells to T cells would support the use of T cell immunotherapy for EOC. In mice injected with ID8 ovarian cancer cells, coadministration of recombinant Sp17 and CpG oligonucleotides every 30 days for a total of ten doses prevented ovarian tumor formation for up to 300 days with a survival rate of 77% and 80% in prophylactic and therapeutic groups, respectively.73 It generated strong immune response evident by an increase in T-helper 17 cells, increases in tumor necrosis factor α, interferon-γ, and granulocyte-macrophage colony-stimulating factor in serum, and a decrease in regulatory T cells. Oral coadministration of M cell-targeted microparticles loaded with T and B cell immune-dominant region of recombinant Sp17 and CpG oligonucleotide triggered humoral and cellular immune responses in mice injected with ID8 ovarian cancer cells and retarded ovarian tumor growth.74 Human anti-Sp17 monoclonal cytotoxic T lymphocytes, obtained either from advanced ovarian cancer patients or from healthy donors, directly migrated to tumor sites and eradicated the tumor in mice injected with human ovarian cancer cells.75 Clinically, dendritic cells pulsed with the Sp17 recombinant protein were administered to one patient with EOC.76 Although no obvious clinical benefits were observed in this patient, there were transient drops in serum CA125. The transient drop in the tumor marker most likely reflects the low in vivo effector/target ratio generated by the dendritic cells within a very restricted immune repertoire. A modern immunoengineering approach would be an ideal platform for a cell-based immunotherapy for EOC targeting Sp17. Two approaches are currently available, chimeric antigen receptor T (CAR-T) cells or T cell receptor T (TCR-T) cells. Both approaches involve the procurement of autologous T cells by apheresis, followed by ex vivo expansion and genetic modification of the T cells in the laboratory to become either CAR-T or TCR-T, before the T cells are reinfused back into the patients (Figure 1). The ex vivo expansion will provide a means to increase the effector/target ratio following infusion of the genetically modified T cells.
Figure 1.
Schematic illustration of immunoengineering of CAR-T or TCR-T cells for treating patients with epithelial ovarian cancer
(1) Autologous CD3+ T cells first are procured by apheresis from the patient. (2) T cells are genetically modified, most commonly using a lentiviral construct, to express chimeric antigen receptors (CARs) or engineered T cell receptors (TCRs). (3) CAR-T or TCR-T cells are expanded ex vivo to obtain required number. (4) Engineered T cells are infused into the same EOC patient to lyse EOC cells. Patients are commonly given chemotherapy via fludarabine and/or cyclophosphamide to lymphodeplete the immune repertoire prior to infusion of the engineered T cells. Lymphodepletion facilitates the homeostatic expansion of the infused engineered T cells.
Chimeric antigen receptor T cells
CAR-T cells are autologous peripheral blood T cells engineered to display antigen-binding fragments of a specific antibody fused to intracellular T cell signaling domains. Human antibody variable fragments directed at Sp17 may be obtained through immunizing transgenic mice carrying the human immunoglobulin genes. CAR-T has produced encouraging clinical results in those with refractory B cell acute lymphoblastic leukemia,77 B cell non-Hodgkin's lymphoma,78 and multiple myeloma.79 CAR-T in hematologic malignancies have thus far relied primarily on targeting differentiation antigens to avoid potential antigen downregulation as a form of tumor escape from immunosurveillance.
In EOC, there no suitable differentiation antigens have been identified so far. Sp17 is, therefore, a suitable alternative. CAR-T cells targeting Sp17 may be engineered to bind either the intact surface Sp17 or the HLA class I/Sp17 peptide complex (Figure 2) on EOC cells. Unlike other tumor antigens, Sp17 expression can be upregulated using DNA-hypomethylating agents.71 Intermittent upregulation of Sp17 expression may be sufficient to prevent tumor immunosurveillance escape and provide comparable efficacy with CAR-T that is directed at a differentiation antigen. The presence of both surface antigens and HLA class I/tumor peptide complex on the surface provides the opportunity for dual CAR-T therapy using a combination of CAR-T directed at the intact surface Sp17 protein and the HLA class I/tumor peptide complex to improve the efficacy of tumor cell killing and heterogeneity of antigen expression.
Figure 2.
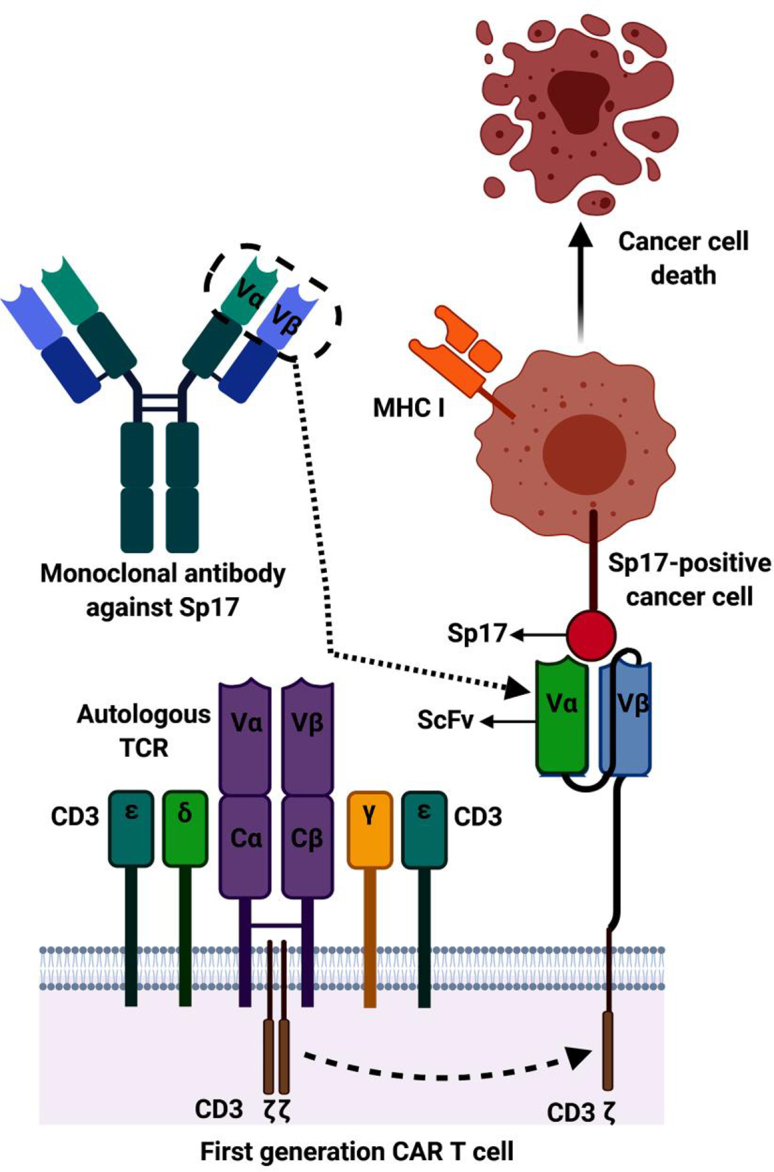
CARs are fusion protein receptors consisting of an extracellular single-chain fragment of variable regions (ScFv) derived from Sp17 monoclonal antibody
This is most commonly accomplished through cloning of the immunoglobulin variable genes, v genes (VH and VL), encoding the Sp17 monoclonal antibodies. These v genes are connected through a linker and are cloned into a viral vector encoding the transmembrane CD3ζ, derived from T cells, to mediate CAR-T activation. Sp17-specific ScFv binds to EOC cells expressing surface Sp17 protein and mediates cell death through T cell cytotoxic mechanisms.
T cell receptor T cells
Cell-based immunotherapy targeting Sp17 may also be accomplished by TCR-T cells, which are autologous peripheral blood T cells engineered to display antigen-binding fragments of a specific TCR fused to intracellular T cell signaling domains (Figure 3). Successes in various laboratories32,67,68 to generate cytotoxic T cells directed against Sp17 suggest that this could be readily accomplished by cloning TCR from the cytotoxic T cell lines generated. TCR-T cells are currently being explored in various tumor types.80, 81, 82, 83 However, since TCR does not undergo hypermutation and affinity maturation like an antibody, the affinity of TCR-T cells for their targets will invariably be considerably lower than those of CAR-T.
Figure 3.
Schematic diagram of TCR T cell structure
(A) The TCR of an autologous T cell is a heterodimer surrounded by CD3 chains. It recognizes intracellular antigens presented by the MHC molecules to initiate cell death. (B) The autologous TCR-T is genetically engineered by cloning of the TCR variable genes (Vα and Vβ) expressed by Sp17-reactive T cells to a viral vector expressing the TCR C genes (Cα and Cβ) linked to the transmembrane CD3ζ and transduced into autologous T cells. (C) Sp17 immunogenic peptides presented on the EOC cell surface in association with the MHC I molecules is recognized by TCR-T cells to initiate cell death.
Enhancing the efficacy of cell-based immunotherapy targeting Sp17
The efficacy of CAR-T cells and TCR-T cells may also be enhanced if the antigen-binding fragment is linked to the T cell costimulatory domains such as CD2784 or CD2885 (Figure 4). It may also be possible to improve the efficacy of the infused CAR-T or TCR-T cells by using them in combination with immune checkpoint inhibitors or sequential administration of chemotherapy. Other approaches to enhance the efficacy of cell-based immunotherapy for solid tumors include enhancing tumor cell apoptosis via iCaspase-9 release86 and strategies to overcome the adverse immunologic conditions induced by cytokines, such as transforming growth factors that impair T cell functions in the tumor microenvironment.87
Figure 4.
To enhance the efficacy of the TCR-T cell and CAR-T cell mediated immune response, the costimulatory domains of CD27 or CD28 are incorporated into the viral construct for either TCR-T cells or second-generation CAR-T cells
Conclusions
Recent advances in immunoengineering have rekindled interest in cell-based immunotherapy in EOC. It is expected that increasing efforts will be made to revisit the tumor antigens previously identified in solid tumors, especially cancer-testis antigens. However, although cell-based immunotherapy has transformed the treatment paradigm in a number of hematologic malignancies, it has so far not made significant inroads into solid tumors. Tumor microenvironments associated with solid tumors, such as malignant ascites and various immunosuppressive cytokines associated with EOC,87 will likely be the main obstacles impairing the efficacy of cell-based immunotherapy. Although there are many tumor antigens that may be suitable for targeting, in addition to enhancing T cell activation and upregulating antigen expression pharmacologically, successful cell-based immunotherapy for EOC may need to incorporate strategies that include sequential chemotherapy and methods to overcome the deleterious tumor microenvironmental cytokines.
Acknowledgments
Figures were created with BioRender.com.
Author contributions
M.P.: initial data collection and writing of the first draft of the manuscript. D.D.: initial data collection, editing of the drafts, and drawing of the figures. Y.L.: editing of the drafts and initial conceptualization of the review. S.H.L.: overall responsibilities, conceptualization of the review, supervision, and writing of final draft.
Declaration of interests
The authors declare no competing interests.
References
- 1.Howlader N., Noone A.M., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., et al. National Cancer Institute; 2021. SEER Cancer Statistics Review, 1975-2018.https://seer.cancer.gov/csr/1975_2018/ based on November 2020 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Matulonis U.A., Sood A.K., Fallowfield L., Howitt B.E., Sehouli J., Karlan B.Y. Ovarian cancer. Nat. Rev. Dis. Primers. 2016;2:16061. doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gadducci A., Lanfredini N., Sergiampietri C. Antiangiogenic agents in gynecological cancer: state of art and perspectives of clinical research. Crit. Rev. Oncol. 2015;96:113–128. doi: 10.1016/j.critrevonc.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Jackson A.L., Eisenhauer E.L., Herzog T.J. Emerging therapies: angiogenesis inhibitors for ovarian cancer. Expert Opin. Emerg. Drugs. 2015;20:331–346. doi: 10.1517/14728214.2015.1036739. [DOI] [PubMed] [Google Scholar]
- 5.Matulonis U.A., Berlin S., Ivy P., Tyburski K., Krasner C., Zarwan C., Berkenblit A., Campos S., Horowitz N., Cannistra S.A., et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J. Clin. Oncol. 2009;27:5601. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman R.L., Sill M.W., Bell-McGuinn K., Aghajanian C., Gray H.J., Tewari K.S., Rubin S.C., Rutherford T.J., Chan J.K., Chen A., et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation—an NRG oncology/gynecologic oncology group study. Gynecol. Oncol. 2015;137:386–391. doi: 10.1016/j.ygyno.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandhu S.K., Schelman W.R., Wilding G., Moreno V., Baird R.D., Miranda S., Hylands L., Riisnaes R., Forster M., Omlin A., et al. The poly (ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 8.Perren T.J., Swart A.M., Pfisterer J., Ledermann J.A., Pujade-Lauraine E., Kristensen G., Carey M.S., Beale P., Cervantes A., Kurzeder C., et al. ICON7 Investigators A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 9.Tewari K.S., Burger R.A., Enserro D., Norquist B.M., Swisher E.M., Brady M.F., Bookman M.A., Fleming G.F., Huang H., Homesley H.D., et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J. Clin. Oncol. 2019;37:2317–2328. doi: 10.1200/JCO.19.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirza M.R., Coleman R.L., González-Martín A., Moore K.N., Colombo N., Ray-Coquard I., Pignata S. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann. Oncol. 2020;31:1148–1159. doi: 10.1016/j.annonc.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Engelstaedter V., Heublein S., Schumacher A.L., Lenhard M., Engelstaedter H., Andergassen U., Guenthner-Biller M., Kuhn C., Rack B., Kupka M., et al. Mucin-1 and its relation to grade, stage and survival in ovarian carcinoma patients. BMC Cancer. 2012;12:600. doi: 10.1186/1471-2407-12-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W., Lu X., Cui P., Piao C., Xiao M., Liu X., Wang Y., Wu X., Liu J., Yang L. Phase I/II clinical trial of a Wilms' tumor 1-targeted dendritic cell vaccination-based immunotherapy in patients with advanced cancer. Cancer Immunol. Immunother. 2019;68:121–130. doi: 10.1007/s00262-018-2257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng P.S., Iwasaka T., Yamasaki F., Ouchida M., Yokoyama M., Nakao Y., Fukuda K., Matsuyama T., Sugimori H. Telomerase activity in gynecologic tumors. Gynecol. Oncol. 1997;64:171–175. doi: 10.1006/gyno.1996.4523. [DOI] [PubMed] [Google Scholar]
- 14.Hassan R., Ho M. Mesothelin targeted cancer immunotherapy. Eur. J. Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baxevanis C.N., Sotiropoulou P.A., Sotiriadou N.N., Papamichail M. Immunobiology of HER-2/neu oncoprotein and its potential application in cancer immunotherapy. Cancer Immunol. Immunother. 2004;53:166–175. doi: 10.1007/s00262-003-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada A., Kataoka A., Shichijo S., Kamura T., Imai Y., Nishida T., Itoh K. Expression of MAGE-1, MAGE-2, MAGE-3/-6 and MAGE-4a/-4b genes in ovarian tumors. Int. J. Cancer. 1995;64:388–393. doi: 10.1002/ijc.2910640607. [DOI] [PubMed] [Google Scholar]
- 17.Odunsi K., Jungbluth A.A., Stockert E., Qian F., Gnjatic S., Tammela J., Intengan M., Beck A., Keitz B., Santiago D., et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. [PubMed] [Google Scholar]
- 18.Straughn J.M., Jr., Shaw D.R., Guerrero A., Bhoola S.M., Racelis A., Wang Z., Chiriva-Internati M., Grizzle W.E., Alvarez R.D., Lim S.H., et al. Expression of sperm protein 17 (Sp17) in ovarian cancer. Int. J. Cancer. 2004;108:805–811. doi: 10.1002/ijc.11617. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S., Qian F., Keitz B., Driscoll D., Scanlan M.J., Skipper J., Rodabaugh K., Lele S., Old L.J., Odunsi K. A-kinase anchoring protein 3 messenger RNA expression correlates with poor prognosis in epithelial ovarian cancer. Gynecol. Oncol. 2005;99:183–188. doi: 10.1016/j.ygyno.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal S., Saini S., Parashar D., Verma A., Sinha A., Jagadish N., Batra A., Suri S., Gupta A., Ansari A.S., et al. The novel cancer-testis antigen A-kinase anchor protein 4 (AKAP4) is a potential target for immunotherapy of ovarian serous carcinoma. Oncoimmunology. 2013;2:e24270. doi: 10.4161/onci.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann M., Ruschenburg I. mRNA detection of tumor-rejection genes BAGE, GAGE, and MAGE in peritoneal fluid from patients with ovarian carcinoma as a potential diagnostic tool. Cancer. 2002;96:187–193. doi: 10.1002/cncr.10622. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W., Barger C.J., Eng K.H., Klinkebiel D., Link P.A., Omilian A., Bshara W., Odunsi K., Karpf A.R. PRAME expression and promoter hypomethylation in epithelial ovarian cancer. Oncotarget. 2016;7:45352–45369. doi: 10.18632/oncotarget.9977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan Z., Feller A.J., Toh H.C., Makastorsis T., Seiden M.V. TRAG-3, a novel gene, isolated from a taxol-resistant ovarian carcinoma cell line. Gene. 1999;229:75–81. doi: 10.1016/s0378-1119(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 24.Lindsay C.R., Shaw E.C., Blackhall F., Blyth K.G., Brenton J.D., Chaturvedi A., Clarke N., Dick C., Evans T.R.J., Hall G., et al. Somatic cancer genetics in the UK: real-world data from phase I of the Cancer Research UK Stratified Medicine Programme. ESMO Open. 2018;3:e000408. doi: 10.1136/esmoopen-2018-000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermeij R., Leffers N., Melief C.J., Daemen T., Nijman H.W. Antigen-specific immunotherapy in ovarian cancer and p53 as tumor antigen. Curr. Pharm. Des. 2012;18:3804–3811. doi: 10.2174/138161212802002805. [DOI] [PubMed] [Google Scholar]
- 26.Lin W.L., Kuo W.H., Chen F.L., Lee M.-Y., Ruan A., Tyan Y.-S., Hsu J.-D., Chiang H., Han C.P. Identification of the coexisting HER2 gene amplification and novel mutations in the HER2 protein-overexpressed mucinous epithelial ovarian cancer. Ann. Surg. Oncol. 2011;18:2388–2394. doi: 10.1245/s10434-011-1572-z. [DOI] [PubMed] [Google Scholar]
- 27.Takeda T., Banno K., Okawa R., Yanokura M., Iijima M., Irie-Kunitomi H., Nakamura K., Iida M., Adachi M., Umene K., et al. ARID1A gene mutation in ovarian and endometrial cancers (Review) Oncol. Rep. 2016;35:607–613. doi: 10.3892/or.2015.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin S.D., Brown S.D., Wick D.A., Nielsen J.S., Kroeger D.R., Twumasi-Boateng K., Holt R.A., Nelson B.H. Low mutation burden in ovarian cancer may limit the utility of neoantigen-targeted vaccines. PLoS One. 2016;11:e0155189. doi: 10.1371/journal.pone.0155189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannides C.G., Fisk B., Jerome K.R., Irimura T., Wharton J.T., Finn O.J. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J. Immunol. 1993;151:3693–3703. [PubMed] [Google Scholar]
- 30.Batchu R.B., Gruzdyn O.V., Moreno-Bost A.M., Szmania S., Jayandharan G., Srivastava A., Kolli B.K., Weaver D.W., van Rhee F., Gruber S.A. Efficient lysis of epithelial ovarian cancer cells by MAGE-A3-induced cytotoxic T lymphocytes using rAAV-6 capsid mutant vector. Vaccine. 2014;32:938–943. doi: 10.1016/j.vaccine.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 31.Yamada A., Kawano K., Harashima N., Niiya F., Nagai K., Kobayashi T., Mine T., Ushijima K., Nishida T., Itoh K. Study of HLA class I restriction and the directed antigens of cytotoxic T lymphocytes at the tumor sites of ovarian cancer. Cancer Immunol. Immunother. 1999;48:147–152. doi: 10.1007/s002620050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiriva-Internati M., Wang Z., Salati E., Timmins P., Lim S.H. Tumor vaccine for ovarian carcinoma targeting sperm protein 17. Cancer. 2002;94:2447–2453. doi: 10.1002/cncr.10506. [DOI] [PubMed] [Google Scholar]
- 33.Yokokawa J., Palena C., Arlen P., Hassan R., Ho M., Pastan I., Schlom J., Tsang K.Y. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin. Cancer Res. 2005;11:6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung C.-F., Tsai Y.-C., He L., Wu T.-C. Control of mesothelin-expressing ovarian cancer using adoptive transfer of mesothelin peptide-specific CD8+ T cells. Gene Ther. 2007;14:921–929. doi: 10.1038/sj.gt.3302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gritzapis A.D., Sotiriadou N.N., Papamichail M., Baxevanis C.N. Generation of human tumor-specific CTLs in HLA-A2.1-transgenic mice using unfractionated peptides from eluates of human primary breast and ovarian tumors. Cancer Immunol. Immunother. 2004;53:1027–1040. doi: 10.1007/s00262-004-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang W.-T., Adams S.F., Tahirovic E., Hagemann I.S., Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol. Oncol. 2012;124:192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen J.S., Sahota R.A., Milne K., Kost S.E., Nesslinger N.J., Watson P.H., Nelson B.H. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 38.Sato E., Olson S.H., Ahn J., Bundy B., Nishikawa H., Qian F., Jungbluth A.A., Frosina D., Gnjatic S., Ambrosone C., et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aoki Y., Takakuwa K., Kodama S., Tanaka K., Takahashi M., Tokunaga A., Takahashi T. Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian cancer. Cancer Res. 1991;51:1934–1939. [PubMed] [Google Scholar]
- 40.Fujita K., Ikarashi H., Takakuwa K., Kodama S., Tokunaga A., Takahashi T., Tanaka K. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin. Cancer Res. 1995;1:501–507. [PubMed] [Google Scholar]
- 41.Freedman R.S., Edwards C.L., Kavanagh J.J., Kudelka A.P., Katz R.L., Carrasco C.H., Atkinson E.N., Scott W., Tomasovic B., Templin S., et al. Intraperitoneal adoptive immunotherapy of ovarian carcinoma with tumor-infiltrating lymphocytes and low-dose recombinant interleukin-2: a pilot trial. J. Immunother. 1994;16:198–210. doi: 10.1097/00002371-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen M., Westergaard M.C.W., Milne K., Nielsen M., Borch T.H., Poulsen L.G., Hendel H.W., Kennedy M., Briggs G., Ledoux S., et al. Adoptive cell therapy with tumor-infiltrating lymphocytes in patients with metastatic ovarian cancer: a pilot study. Oncoimmunology. 2018;7:e1502905. doi: 10.1080/2162402X.2018.1502905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kershaw M.H., Westwood J.A., Parker L.L., Wang G., Eshhar Z., Mavroukakis S.A., White D.E., Wunderlich J.R., Canevari S., Rogers-Freezer L., et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haas A.R., Tanyi J.L., O'Hara M.H., Gladney W.L., Lacey S.F., Torigian D.A., Soulen M.C., Tian L., McGarvey M., Nelson A.M., et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol. Ther. 2019;27:1919–1929. doi: 10.1016/j.ymthe.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J., Li H., Cao S., Zhang X., Yu J., Qi J., An X., Yu W., Ren X., Hao X. Maintenance therapy with autologous cytokine-induced killer cells in patients with advanced epithelial ovarian cancer after first-line treatment. J. Immunother. 2014;37:115–122. doi: 10.1097/CJI.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 46.Wright S.E., Rewers-Felkins K.A., Quinlin I.S., Phillips C.A., Townsend M., Philip R., Dobrzanski M.J., Lockwood-Cooke P.R., Robinson W. Cytotoxic T-lymphocyte immunotherapy for ovarian cancer: a pilot study. J. Immunother. 2012;35:196–204. doi: 10.1097/CJI.0b013e318243f213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frayne J., Hall L. A re-evaluation of sperm protein 17 (Sp17) indicates a regulatory role in an A-kinase anchoring protein complex, rather than a unique role in sperm-zona pellucida binding. Reproduction. 2002;124:767–774. doi: 10.1530/rep.0.1240767. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y., Wang Z., Robinson W.R., Lim S.H. Combined real time PCR and immunohistochemical evaluation of sperm protein 17 as a cancer-testis antigen. Eur. J. Haematol. 2004;73:280–284. doi: 10.1111/j.1600-0609.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 49.Lea I.A., Adoyo P., Michael G.O. Autoimmunogenicity of the human sperm protein Sp17 in vasectomized men and identification of linear B cell epitopes. Fertil. Steril. 1997;67:355–361. doi: 10.1016/S0015-0282(97)81923-1. [DOI] [PubMed] [Google Scholar]
- 50.Diekman A.B., Herr J.C. Sperm antigens and their use in the development of an immunocontraceptive. Am. J. Reprod. Immunol. 1997;37:111–117. doi: 10.1111/j.1600-0897.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 51.Lacy H.M., Sanderson R.D. Sperm protein 17 is expressed on normal and malignant lymphocytes and promotes heparan sulfate–mediated cell-cell adhesion. Blood. 2001;98:2160–2165. doi: 10.1182/blood.v98.7.2160. [DOI] [PubMed] [Google Scholar]
- 52.Wen Y., Richardson R.T., Widgren E.E., O'Rand M.G. Characterization of Sp17: a ubiquitous three domain protein that binds heparin. Biochem. J. 2001;357:25–31. doi: 10.1042/0264-6021:3570025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunette L.L., Mhawech-Fauceglia P.Y., Ji L., Skeate J.G., Brand H.E., Lawrenson K., Walia S., Chiriva-Internati M., Groshen S., Roman L.D., et al. Validity and prognostic significance of sperm protein 17 as a tumor biomarker for epithelial ovarian cancer: a retrospective study. BMC Cancer. 2018;18:970. doi: 10.1186/s12885-018-4880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim S.H., Wang Z., Chiriva-Internati M., Xue Y. Sperm protein 17 is a novel cancer-testis antigen in multiple myeloma. Blood. 2001;97:1508–1510. doi: 10.1182/blood.v97.5.1508. [DOI] [PubMed] [Google Scholar]
- 55.Liggins A.P., Lim S.H., Soilleux E.J., Pulford K., Banham A.H. A panel of cancer-testis genes exhibiting broad-spectrum expression in haematological malignancies. Cancer Immun. 2010;10:8. [PMC free article] [PubMed] [Google Scholar]
- 56.Mirandola L., Figueroa J.A., Phan T.T., Grizzi F., Kim M., Rahman R.L., Jenkins M.R., Cobos E., Jumper C., Alalawi R., et al. Novel antigens in non-small cell lung cancer: SP17, AKAP4, and PTTG1 are potential immunotherapeutic targets. Oncotarget. 2015;6:2812. doi: 10.18632/oncotarget.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y., Qiu J., Wang Y., Liu P., Lv Q., Du Z. Sperm protein antigen 17 expression correlates with lymph node metastasis and worse overall survival in patients with breast cancer. Front Oncol. 2019;9:710. doi: 10.3389/fonc.2019.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mirandola L., Pedretti E., Figueroa J.A., Chiaramonte R., Colombo M., Chapman C., Grizzi F., Patrinicola F., Kast W.M., Nguyen D.D., et al. Cancer testis antigen sperm protein 17 as a new target for triple negative breast cancer immunotherapy. Oncotarget. 2017;8:74378. doi: 10.18632/oncotarget.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li F., Liu Q., Han Y., Wu B., Yin H. Sperm protein 17 is highly expressed in endometrial and cervical cancers. BMC Cancer. 2010;10:429. doi: 10.1186/1471-2407-10-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grizzi F., Gaetani P., Franceschini B., Di Ieva A., Colombo P., Ceva-Grimaldi G., Bollati A., Frezza E.E., Cobos E., Rodriguez y Baena R., et al. Sperm protein 17 is expressed in human nervous system tumours. BMC Cancer. 2006;6:23. doi: 10.1186/1471-2407-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta G., Sharma R., Chattopadhyay T.K., Gupta S.D., Ralhan R. Clinical significance of sperm protein 17 expression and immunogenicity in esophageal cancer. Int. J. Cancer. 2007;120:1739–1747. doi: 10.1002/ijc.22463. [DOI] [PubMed] [Google Scholar]
- 62.Xia Q., Liu S., Li F., Huang W., Shi L., Zhou X. Sperm protein 17, MAGE-C1 and NY-ESO-1 in hepatocellular carcinoma: expression frequency and their correlation with clinical parameters. Int. J. Clin. Exp. Pathol. 2013;6:1610. [PMC free article] [PubMed] [Google Scholar]
- 63.Nakazato T., Kanuma T., Tamura T., Faried L.S., Aoki H., Minegishi T. Sperm protein 17 influences the tissue-specific malignancy of clear cell adenocarcinoma in human epithelial ovarian cancer. Int. J. Gynecol. Cancer. 2007;17:426–432. doi: 10.1111/j.1525-1438.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 64.Li F., Han Y., Liu Q., Wu B., Huang W., Zeng S. Overexpression of human sperm protein 17 increases migration and decreases the chemosensitivity of human epithelial ovarian cancer cells. BMC Cancer. 2009;9:323. doi: 10.1186/1471-2407-9-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Q., Li F., Han Y., Wang L., Hou Y. Aberrant expression of sperm protein 17 enhances migration of ovarian cancer cell line HO-8910. Nat. J. Androl. 2008;14:982–986. [PubMed] [Google Scholar]
- 66.Gao Q., Xiang S.D., Wilson K., Madondo M., Stephens A.N., Plebanski M. Sperm protein 17 expression by murine epithelial ovarian cancer cells and its impact on tumor progression. Cancers. 2018;10:276. doi: 10.3390/cancers10080276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiriva-Internati M., Wang Z., Salati E., Bumm K., Barlogie B., Lim S.H. Sperm protein 17 (Sp17) is a suitable target for immunotherapy of multiple myeloma. Blood. 2002;100:961–965. doi: 10.1182/blood-2002-02-0408. [DOI] [PubMed] [Google Scholar]
- 68.Ait-Tahar K., Anderson A.P., Barnardo M., Collins G.P., Hatton C.S.R., Banham A.H., Pulford K. Histocompatibility Class I and II epitope presentation in diffuse large B cell lymphoma patients. Adv. Hematol. 2017;2017:6527306. doi: 10.1155/2017/6527306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiriva-Internati M., Wang Z., Pochopien S., Salati E., Lim S.H. Identification of a Sperm protein 17 CTL epitope restricted by HLA-A1. Int. J. Cancer. 2003;107:863–865. doi: 10.1002/ijc.11486. [DOI] [PubMed] [Google Scholar]
- 70.Wu C.-J., Sundararajan V., Sheu B.-C., Huang R.Y.-J., Wei L.-H. Activation of STAT3 and STAT5 signaling in epithelial ovarian cancer progression: mechanism and therapeutic opportunity. Cancers (Basel) 2019;12:24. doi: 10.3390/cancers12010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z., Zhang Y., Ramsahoye B., Bowen D., Lim S.H. Sp17 gene expression in myeloma cells is regulated by promoter methylation. Br. J. Cancer. 2004;91:1597–1603. doi: 10.1038/sj.bjc.6602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song J., Cao W., Li F., Shi L., Jia X. Anti-Sp17 monoclonal antibody with antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity activities against human ovarian cancer cells. Med. Oncol. 2012;29:2923–2931. doi: 10.1007/s12032-011-0137-0. [DOI] [PubMed] [Google Scholar]
- 73.Chiriva-Internati M., Yu Y., Mirandola L., Jenkins M.R., Chapman C., Cannon M., Cobos E., Kast W.M. Cancer testis antigen vaccination affords long-term protection in a murine model of ovarian cancer. PLoS One. 2010;5:e10471. doi: 10.1371/journal.pone.0010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mattila J., Mirandola L., Chiriva-Internati M. Development of a M cell-targeted microparticulate platform, BSK02™, for oral immunization against the ovarian cancer antigen, sperm protein 17. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107:29–36. doi: 10.1002/jbm.b.34092. [DOI] [PubMed] [Google Scholar]
- 75.Chiriva-Internati M., Weidanz J.A., Yu Y., Frezza E.E., Jenkins M.R., Kennedy R.C., Cobos E., Kast W.M. Sperm protein 17 is a suitable target for adoptive T-cell–based immunotherapy in human ovarian cancer. J. Immunother. 2008;31:693–703. doi: 10.1097/CJI.0b013e31818283d5. [DOI] [PubMed] [Google Scholar]
- 76.Dadabayev A.R., Wang Z., Zhang Y., Zhang J., Robinson W.R., Lim S.H. Cancer immunotherapy targeting Sp17: when should the laboratory findings be translated to the clinics? Am. J. Hematol. 2005;80:6–11. doi: 10.1002/ajh.20415. [DOI] [PubMed] [Google Scholar]
- 77.Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O., et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nastoupil L.J., Jain M.D., Feng L., Spiegel J.Y., Ghobadi A., Lin Y., Dahiya S., Lunning M., Lekakis L., Reagan P., et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J. Clin. Oncol. 2020;38:3119–3128. doi: 10.1200/JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raje N., Berdeja J., Lin Y., Siegel D., Jagannath S., Madduri D., Liedtke M., Rosenblatt J., Maus M.V., Turka A., et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 2019;380:1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei T., Leisegang M., Xia M., Kiyotani K., Li N., Zeng C., Deng C., Jiang J., Harada M., Agrawal N., et al. Generation of neoantigen-specific T cells for adoptive cell transfer for treating head and neck squamous cell carcinoma. Oncoimmunology. 2012;10:1929726. doi: 10.1080/2162402X.2021.1929726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu F., Ma X.J., Jin W.L., Luo Y., Li X. Neoantigen specific T cells derived from T cell-derived induced pluripotent stem cells for the treatment of hepatocellular carcinoma: potential and challenges. Front Immunol. 2021;12:690565. doi: 10.3389/fimmu.2021.690565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orlando D., Miele E., De Angelis B., Guercio M., Boffa I., Sinibaldi M., Po A., Caruana I., Abballe L., Carai A., et al. Adoptive immunotherapy using PRAME-specific T cells in Medulloblastoma. Cancer Res. 2018;78:3337–3349. doi: 10.1158/0008-5472.CAN-17-3140. [DOI] [PubMed] [Google Scholar]
- 83.Tawara I., Kageyama S., Miyahara Y., Fujiwara H., Nishida T., Akatsuka Y., Ikeda H., Tanimoto K., Terakura S., Murata M., et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood. 2017;130:1985–1994. doi: 10.1182/blood-2017-06-791202. [DOI] [PubMed] [Google Scholar]
- 84.Chen H., Wei F., Yin M., Zhao Q., Liu Z., Yu B., Huang Z. CD27 enhances the killing effect of CAR T cells targeting trophoblast cell surface antigen 2 in the treatment of solid tumors. Cancer Immunol. Immunother. 2021;70:2059–2071. doi: 10.1007/s00262-020-02838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R., et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoyos V., Savoldo B., Quintarelli C., Mahendravada A., Zhang M., Vera J., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson K.G., Stromnes I.M., Greenberg P.D. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell. 2017;31:311–325. doi: 10.1016/j.ccell.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]





