Abstract
Tocilizumab decreases inflammatory response in the cytokine storm which is one of the mechanisms behind the development of ARDS in COVID-19 patients. The objective of our study was to determine response of tocilizumab in patients suffering from COVID-19 by analyzing clinical parameters and inflammatory markers. A single-arm observational retrospective study was conducted from March 15, 2020 to March 15, 2021. Clinical outcomes in terms of mortality, weaning from mechanical ventilator, improvement in laboratory parameters including inflammatory cytokines, and length of hospital stay were documented. Reduction in values of inflammatory markers, and patients discharged home in stable condition were defined as an improvement after tocilizumab administration. A total of 514 patients received tocilizumab, majority of whom were critically sick 333 (64.8%). Out of the total sample 363 (70.6%) patients were discharged home in stable condition. Overall mean length of stay was 11.50 ± 8.4 days. There was significant difference in length of stay of patients who required invasive mechanical ventilation as compared to those who were kept only on supplemental oxygen (p < 0.05). Patients who were discharged home showed significant improvement in inflammatory markers and neutrophil to lymphocyte ratio as compared to those who expired (p < 0.05). A total of 21 (4.1%) patients had positive blood culture while 57 (11.1%) had positive culture of tracheal aspirate. Hence, tocilizumab is found to be a reasonable therapeutic option for worsening COVID-19 pneumonia by decreasing the need for mechanical ventilation. However, it is associated with adverse events including bacterial and fungal infections.
Keywords: COVID-19, SARS-CoV-2, Tocilizumab, Cytokine release syndrome
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has rapidly spread globally across continents [1], [2]. The clinical presentation of COVID-19 varies from being an asymptomatic disease to potentially life-threatening pneumonia which may eventually lead to acute respiratory distress syndrome (ARDS) [3]. Though most of the COVID-19 cases can be categorized as mild to moderate, nonetheless, around 15% develop severe disease requiring oxygen support and about 5% develop critical disease requiring mechanical ventilation [4].
Cytokine release syndrome (CRS) is thought to be the underlying mechanism of development of ARDS through hyperactive immune responses [3]. CRS is a systemic inflammatory response mediated by inflammatory cytokines. Excessive systemic inflammation and raised interleukin-6 (IL-6) levels, due to unregulated immune response of the host, are associated with poor outcomes in patients suffering from COVID-19 [5]. Along with raised IL-6 levels, data also shows an association between increased mortality and high levels of several inflammatory markers including C-reactive protein (CRP), ferritin, D-dimer, and lactate dehydrogenase (LDH) [4].
Several randomized controlled trials (RCTs) and cohort studies have been conducted in search of effective therapies for COVID-19 with conflicting results [1], [6], [7], [8], [9], [10], [11], [12]. Systemic corticosteroids were the first of these treatments which showed mortality benefits in patients who received oxygen and ventilatory support in the RECOVERY trial [10]. Tocilizumab, being an IL-6 antagonist, has been hypothesized to decrease the inflammatory response in the cytokine storm, hence, it minimizes the incidence of catastrophic complications of COVID-19, like ARDS, and thus decreases fatality rate and improves outcomes [2]. Literature review shows few RCTs and observational studies on the efficacy of tocilizumab with contradictory findings. The REMAP-CAP trial showed improved outcomes with IL-6 antagonists [13], however, the COVACTA trial demonstrated no significant difference in clinical outcomes or mortality with tocilizumab [14].
The objective of our study was to determine the response of tocilizumab in patients suffering from COVID-19 by analyzing clinical parameters and inflammatory markers. This study further elaborates the findings of our previous study, that included a sample size of 40 patients, which showed tocilizumab as a possible treatment option of COVID-19 [15].
2. Materials and Methods:
A single-arm retrospective observational study was conducted at The Aga Khan University Hospital Karachi, Pakistan after approval was obtained from the institutional ethical review committee (ERC 2020-4752-10750). The hospital designated a separate facility (COVID-19 Diagnosis and Treatment Zone-CDTZ) for the management of COVID-19 patients. The facility contains around 100 beds and is equipped with low, intermediate and high acuity level beds. The study included all adult patients aged > 18 years admitted with COVID-19 in CDTZ from March 15, 2020 to March 15, 2021. Patients were labeled as having COVID-19 if they had a positive nasopharyngeal swab for SARS-CoV-2 through RT-PCR. Furthermore, the severity of the disease was classified according to the national guidelines into non-severe, severe and critical disease [16]. Any patient who had confusion, was in shock or had respiratory failure requiring invasive mechanical ventilation or non-invasive ventilation (NIV) was labeled as having a critical disease. All patients who required supplemental oxygen support were labeled as having a severe disease while the rest were considered as having a non-severe disease.
Data regarding patients’ demographics, clinical presentation, comorbidities, laboratory parameters including details of infections, and outcomes were retrieved from medical records using International Classification of Diseases-10 (ICD-10) coding for COVID-19. Tocilizumab was administered only to those patients who had severe or critical disease. As per the national guidelines, tocilizumab was administered if there was clinical deterioration after 24 to 48 hours of standard care including systemic corticosteroids [16]. Patients who had a confirmed or suspected bacterial infection indicated by a raised procalcitonin (PCT) or positive cultures (blood, urine, sputum, or tracheal) were not considered for treatment with tocilizumab. Written informed consent was taken from patients or their next of kin before administration of tocilizumab and risks and benefits were explained to them in detail as per hospital policy. Serum levels of inflammatory markers like neutrophil–lymphocyte ratio (NLR), CRP, ferritin, D-dimer, and LDH were recorded before and after tocilizumab administration. The highest value of these markers was noted before administration while the lowest value was noted after administration of tocilizumab. Clinical outcomes in terms of mortality, weaning from mechanical ventilator, improvement in laboratory parameters including inflammatory cytokines, and length of hospital stay were also recorded. Reduction in values of inflammatory markers including NLR, CRP, ferritin, D-dimer, and LDH, and patients discharged home in stable condition was defined as an improvement after tocilizumab administration.
Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 22.0. Quantitative variables like age, length of stay, and values of inflammatory markers were presented as mean ± SD. Qualitative variables like gender, comorbidities, clinical presentation, disease severity, use of mechanical ventilator, details of infections and outcomes were presented as frequency and percentages. Wilcoxon sign ranked test was used to determine the significance of difference in values of inflammatory markers before and after tocilizumab administration. P value of <0.05 was taken as significant.
3. Results:
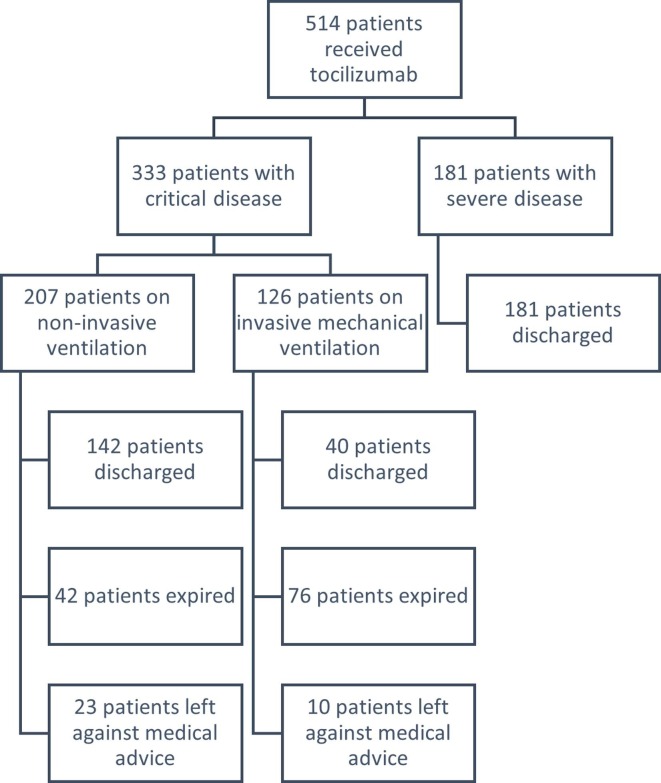
A total of 514 patients received tocilizumab during the study period. Mean age was 59.87 ± 12.96 years and 384 (74.7%) patients were males. The most prevalent presenting symptom was fever which was present in 436 (84.8%) patients. Hypertension was the most common comorbidity which was present in 264 (51.4%) patients while 143 (28%) had no prior comorbidity. Multi-morbidities were found in 229 (44.6%) patients. Most of our patients 438 (85.2%) received a single dose of tocilizumab. The dose of tocilizumab administered was 400 mg. Fig. 1 shows the number of patients in each category. Critically sick patients 333 (64.8%) constituted majority of the study population while there were no cases of non-severe COVID-19. Out of these 333 patients, 207 (40.3%) patients were on NIV support.
Fig. 1.
Graphical representation of the outcome of patients having severe and critical COVID-19.
A total of 363 (70.6%) patients were safely discharged home. All of those who had severe disease were discharged home in stable condition. Among patients who had critical disease, 182 (54.7%) were discharged home in stable condition. There were 33 (6.4%) patients who left the hospital against medical advice due to financial constraints or personal reasons. The overall mean length of stay was found to be 11.50 ± 8.4 days. There was a significant difference in the length of stay of patients who required an invasive mechanical ventilator as compared to those who were kept only on supplemental oxygen (p < 0.05). Baseline characteristics of all patients are summarized in Table 1 .
Table 1.
Baseline characteristics of all patients (N = 514).
| Characteristics | N (%) |
|---|---|
| Age (years) | 59.87 ± 12.96 |
| Gender | |
| Male | 384 (74.7%) |
| Female | 130 (25.3%) |
| Comorbidities | |
| Hypertension | 264 (51.4%) |
| Diabetes mellitus | 248 (48.2%) |
| Ischemic heart disease | 78 (15.2%) |
| Chronic obstructive pulmonary disease/Asthma | 49 (9.53%) |
| Chronic kidney disease | 22 (4.28%) |
| Others | 57 (11.1%) |
| Symptoms | |
| Fever | 436 (84.8%) |
| Shortness of breath | 401 (78.0%) |
| Cough | 333 (64.8%) |
| Others | 63 (12.3%) |
| Clinical severity | |
| Critical | 333 (64.8%) |
| Severe | 181 (35.2%) |
| Number of doses | |
| Single | 438 (85.2%) |
| Double | 76 (14.8%) |
| Mean length of stay (days) | 11.50 ± 8.473 |
| Outcome | |
| Discharged | 363 (70.6%) |
| Expired | 118 (23.0%) |
| Left against medical advice | 33 (6.4%) |
Table 2 shows median values of various inflammatory markers (CRP, LDH, ferritin, D-dimer, and PCT) and NLR. Patients who were discharged home showed significant improvement in all inflammatory markers and NLR as compared to those who died (p < 0.05). No significant difference was observed in serum ferritin and NLR levels after tocilizumab administration in patients who expired. Interestingly, D-dimer levels remained significantly elevated even after tocilizumab administration in these patients. A significant increase in PCT levels was also seen after tocilizumab administration in the overall study population as well as in both discharged home and expired groups.
Table 2.
Median and interquartile ranges of inflammatory markers and neutrophil to lymphocyte ratio (NLR) before and after tocilizumab administration.
| Overall (N = 514) |
Patients who were discharged (N = 363) |
Patients who expired (N = 118) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Before TCZ | After TCZ | P value* | Before TCZ | After TCZ | P value* | Before TCZ | After TCZ | P value* | |
| NLR | 9.6 [7–12.8] | 4.3 [1.5–10.6] | 0.015 | 9.5 [6.8–12.6] | 3.5 [2.2–7.8] | <0.001 | 12.9 [9.9–17.5] | 10.3 [8.5–15.3] | 0.231 |
| CRP | 139 [78.2–181] | 8.3 [2.11–19.5] | <0.001 | 140.8 [79.2–182.5] | 5.99 [1.75–15.3] | <0.001 | 124 [71.9–180] | 16.9 [5.64–62.0] | <0.001 |
| LDH | 548 [438.3–666] | 461 [365–605] | <0.001 | 523 [421–636] | 413 [335–513] | <0.001 | 619 [460.5–736] | 673 [520–977.5] | <0.001 |
| Ferritin | 1092 [577–1541] | 977 [620–1398] | <0.001 | 1094 [582.3–1525.3] | 929 [619–1324] | <0.001 | 1104 [535–1532.8] | 1200 [659–1627.3] | 0.1333 |
| D-dimer | 1.0 [0.6–2.2] | 1.2 [0.6–3.23] | 0.723 | 0.9 [0.5–1.75] | 0.9 [0.5–1.8] | 0.041 | 1.55 [0.8–3.3] | 3.3 [1.6–6.95] | <0.001 |
| PCT | 0.23 [0.12–0.44] | 0.65 [0.13–5.2] | <0.001 | 0.22 [0.11–0.40] | 1.57 [0.11–5.68] | <0.001 | 0.25 [0.15–0.51] | 0.59 [0.22–3.58] | <0.001 |
NLR: Neutrophil to lymphocyte ratio; CRP: C-reactive protein; LDH: Lactate dehydrogenase; PCT: Procalcitonin.
Based on Wilcoxon signed rank test.
Out of 333 critically sick patients, 207 (40.3%) patients required NIV while 126 (24.5%) required invasive ventilatory support. Out of 207 patients who required NIV, 142 (68.5%) patients were discharged home safely while out of 126 patients who required invasive mechanical ventilation, 40 (31.7%) patients were safely extubated and discharged home in stable condition (p < 0.05). Patients who required invasive ventilatory support were found to have a significantly longer length of stay in the hospital as compared to patients who required supplemental oxygen and NIV support.
Almost all our patients 504 (98.05%) received corticosteroids along with tocilizumab. A total of 32 (6.2%) patients underwent hemodialysis due to acute kidney injury, out of which only nine (1.8%) were known cases of chronic kidney disease.
Regarding development of infections after tocilizumab administration, 21 (4.1%) patients had a positive blood culture while 57 (11.1%) had a positive culture of tracheal aspirate. Out of these patients, 41 (61.04%) expired during hospital stay with the majority being those who required invasive mechanical ventilation 30 (73.1%). In our study population, 21 (4.1%) patients developed COVID-19 associated pulmonary aspergillosis (CAPA) and were treated with voriconazole. Hospital-acquired pneumonia (HAP) due to Acinetobacter (6.5%) and Pseudomonas (4.5%) was another major complication noted in our patients after receiving tocilizumab. Candidemia occurred in 15 (2.9%) patients. Table 3 shows the detail of various microorganisms in blood and tracheal cultures.
Table 3.
Details of positive cultures in patients receiving tocilizumab.
| Specimen | Organism | N (%) |
|---|---|---|
| Tracheal aspirate | Aspergillus flavus | 21 (4.1%) |
| Pseudomonas aeruginosa | 23 (4.5%) | |
| Acinetobacter | 33 (6.5%) | |
| Klebsiella pneumoniae | 16 (3.1%) | |
| Others | 24 (4.7%) | |
| Blood | Candida spp. | 15 (2.9%) |
| Staphylococcus aureus | 2 (0.39%) | |
| Klebsiella pneumoniae | 2 (0.39%) | |
| Acinetobacter | 2 (0.39%) |
4. Discussion:
This study presents a comprehensive review of more than 500 sick patients with COVID-19 who received tocilizumab over a period of one year at a tertiary care hospital in Pakistan. Our study showed that in patients with COVID-19 induced hyperinflammatory syndrome (CRS) administration of tocilizumab improved outcomes in patients with severe and critical diseases. More than two-thirds of patients showed recovery after tocilizumab administration which included all those patients with severe disease and around half of those who had critical disease. They also showed improvement in inflammatory markers including CRP, LDH, D-dimer, and PCT and were discharged home in stable condition. However, some patients developed bacterial and fungal infections in blood along with HAP and CAPA.
The overactive immune response in the form of CRS plays an important role in the pathogenesis of COVID-19, characterized by the release of inflammatory cytokines, and implicated in catastrophic multi-organ dysfunction [17], [18]. Raised IL-6 levels have also been implicated in a hypercoagulable state [19]. Hypercoagulability associated with COVID-19 can lead to thrombosis including pulmonary embolism and deep venous thrombosis [20], [21]. An observational study conducted by Galvan Roman et al. in Spain showed that raised IL-6 levels associated with severe disease and predicted favorable response to tocilizumab. This study also demonstrated that tocilizumab administered to patients with raised IL-6 levels showed respiratory improvement in terms of increased PaO2/FiO2 ratio and reduced overall mortality [22]. Since IL-6 levels were not available at our center for the initial major part of our study duration, the decision to administer tocilizumab in our patients was made based on rising levels of CRP, ferritin, LDH, D-dimer, worsening infiltrates on chest X-ray, increasing oxygen requirement, need for non-invasive and invasive ventilation, absence of secondary bacterial infection and a normal PCT level.
CORIMUNO-RCT which was conducted in France showed a reduction in requirement of NIV, high flow oxygen, and mechanical ventilation by day 14 in patients with moderate to severe disease who received tocilizumab as compared to those who did not receive it. The study showed no difference in mortality between the two groups on day 28 [23]. Similarly, a multicenter phase 3 trial also indicated that there was no statistical difference in mortality at day 28 in patients who received tocilizumab as compared to the placebo group [14]. Salvarani et al. from Italy showed no difference in admission to ICU, requirement of mechanical ventilation and frequency of death in patients who received tocilizumab as compared to those who did not receive it [24]. However, another study done in Italy showed decreased requirement of mechanical ventilation and frequency of death in patients who received tocilizumab as compared to those who did not receive it [25].
Food and drug administration (FDA) has now issued emergency use authorization (EUA) for the use of tocilizumab for the treatment of hospitalized adult and pediatric patients who have received corticosteroids and supplemental oxygen, NIV, invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) [2]. Moderate quality of evidence suggests that tocilizumab decreases the risk of intubation in critically ill COVID-19 patients while RCTs showed that use of tocilizumab was not associated with reduction in short-term mortality [9]. Lan et al. in a systematic review reported that all-cause mortality was lower in the tocilizumab group as compared to the control group, but this was not statistically significant and there was no difference in the requirement of mechanical ventilation between the two groups [8].
It is noteworthy that all our patients received usual standard of care which included systemic corticosteroids, antiviral (remdesivir), broad-spectrum antibiotics (if indicated), and prophylactic anticoagulation. Hermine et al. randomized patients to receive tocilizumab plus usual care or usual care alone that included corticosteroids, antiviral agents, antibiotics, vasopressors, and anticoagulants [23]. Therefore, the improvement observed in our patients cannot be solely attributed to tocilizumab.
It is worth mentioning that after receiving tocilizumab all inflammatory cytokines like CRP, ferritin, LDH, D-dimer, and NLR decreased in our patients particularly in patients who improved and were subsequently discharged home. Even though our institution did not have the facility of IL-6 levels, based on the values of other inflammatory markers, it can be postulated that IL-6 levels in critically ill patients would have been higher as compared to those who were severely ill. However, the outcome of critically ill patients in our study was worse as compared to severely ill patients. This may be because the outcome in COVID-19 patients is dependent upon multiple factors in addition to cytokine release syndrome.
Keske et al., in a study done in Turkey on 43 COVID-19 patients, demonstrated that the percentage of lymphocytes was the foremost laboratory investigation that showed marked improvement after tocilizumab administration followed by CRP and IL-6, while ferritin and D-dimer showed only a slight decline [26]. CRP levels are elevated in patients with severe disease in contrast to the patients with non-severe disease [27]. Moreover, it has been described, that higher values of CRP on admission are an independent risk factor for development of critical disease and poor outcomes [28]. A study which was done at our center including 40 COVID-19 patients, in the early phase of the pandemic, also showed a significant reduction in inflammatory cytokines after tocilizumab administration [15]. It is notable that PCT levels increased after receiving tocilizumab in all our patients. This shows that tocilizumab has the potential to develop secondary bacterial infections.
A meta-analysis showed that tocilizumab treated patients had a significant reduction in odds of mortality as compared to the control group [6]. Another systematic review and meta-analysis of 10 observational studies showed a risk reduction in mortality of 12% with the use of tocilizumab [7]. Majority of our patients who received tocilizumab were on NIV (40%). Some of these patients could have been candidates for ICU but could not be assigned to ICU due to limited number of ICU beds assigned for COVID-19 patients at our center as compared to intermediate care unit beds. Intermediate acuity level of care served as a reasonable substitute to high acuity levels of care at our center during the pandemic especially for those patients who did not require invasive ventilation or invasive hemodynamic monitoring [29].
Although, the RECOVERY trial described relatively lower infection rates after corticosteroid administration [10], various studies after the RECOVERY trial have mentioned increased risk of bacterial and fungal infection after the use of systemic corticosteroids [30], [31], [32]. Similarly, tocilizumab, being an immune suppressant drug, can also lead to secondary bacterial and fungal infections. CAPA is an emerging complication associated with critically ill patients on mechanical ventilation [33], [34]. Various studies from Europe have reported a frequency of 5–30% of super-imposed infections with severe COVID-19 [35], [36]. Bloodstream infections (BSI) were reported in 31 (39.7%) patients admitted to ICU in a study conducted in Italy with staphylococcus being the most common isolate [37]. Kumar et al. demonstrated an increase in mortality of COVID-19 patients who developed secondary bacterial or fungal infections as compared to those who did not develop these infections (41% vs 12%) [38]. Campochiaro et al. demonstrated bacteremia in four (13%) patients while CAPA in one (3%) patient [39]. However, Tleyjeh et al. in a meta-analysis did not show an increased risk of infections or adverse events with the use of tocilizumab in RCTs [9].
Twenty-one (4.1%) patients in our study population developed CAPA and were treated with voriconazole. Hospital-acquired pneumonia (HAP) due to Acinetobacter (6.5%) and Pseudomonas (4.5%) was another major complication noted in our patients after receiving tocilizumab. In our cohort, candidemia occurred in 15 (2.9%) patients. The outcome of patients with secondary infections was poor in our study which is comparable with other studies that show higher rates of mortality in the South Asian population [40].
It is important to mention that 118 (23 %) patients died despite receiving tocilizumab. Moreover, 33 (6.4%) patients left against medical advice, hence, their outcomes could not be analyzed completely. Mean length of stay in our cohort was 11.5 ± 8.4 days. Wadud et al. documented that those patients who received tocilizumab had a higher length of hospital stay as compared to those who did not receive tocilizumab [41].
As per our knowledge, this is one of the largest observational studies demonstrating the outcomes of COVID-19 patients treated with tocilizumab. However, our study has several limitations as well. This is a single-center study, hence, results extracted from this study are not easily generalizable. IL-6 levels were not measured in most of our patients before administration of tocilizumab as this test was not available at our center for a major part of the study duration. No control arm was present in our study for comparison of treatment regimens with and without tocilizumab. Our patients also received systemic corticosteroids in addition to tocilizumab. Moreover, treatment was individualized as per the patient’s condition, laboratory and radiological parameters including but not limited to broad-spectrum antibiotics and antifungal medications.
5. Conclusion:
Tocilizumab is a reasonable therapeutic option for worsening COVID-19 pneumonia by reducing the need for mechanical ventilation and improving survival. However, its use is associated with some adverse events including secondary bacterial and fungal infections. Large RCTs with extended follow up would further establish the role of tocilizumab in these patients.
6. Financial Disclosure
No funding or grant was received for this study. The authors have no relevant financial interest in this article. The authors declare that they have no conflict of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Klopfenstein T., Gendrin V., Gerazime A., Conrozier T., Balblanc J.-C., Royer P.-Y., Lohse A., Mezher C., Toko L., Guillochon C., Badie J., Pierron A., Kadiane-Oussou N.’.J., Puyraveau M., Zayet S. Systematic Review and Subgroup Meta-analysis of Randomized Trials to Determine Tocilizumab’s Place in COVID-19 Pneumonia. Infect. Dis. Ther. 2021;10(3):1195–1213. doi: 10.1007/s40121-021-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinzon R.T., Wijaya V.O., Buana R.B. Interleukin-6 (IL-6) inhibitors as therapeutic agents for coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Infect. Public Health. 2019;14(2021):1001–1009. doi: 10.1016/j.jiph.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eljaaly K., Malibary H., Alsulami S., Albanji M., Badawi M., Al-Tawfiq J.A. Description and analysis of cytokine storm in registered covid-19 clinical trials: A systematic review. Pathogens. 2021;10:692. doi: 10.3390/pathogens10060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamed D.M., Belhoul K.M., Al Maazmi N.A., Ghayoor F., Moin M., Al Suwaidi M., Narainen M., Makki M., AbdulRahman M. Intravenous methylprednisolone with or without tocilizumab in patients with severe COVID-19 pneumonia requiring oxygen support: A prospective comparison. J. Infect. Public Health. 2021;14(8):985–989. doi: 10.1016/j.jiph.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M., Vale C.L., Godolphin P.J., Fisher D., Higgins J.P.T., Spiga F., Savovic J., Tierney J., Baron G., Benbenishty J.S., Berry L.R., Broman N., Cavalcanti A.B., Colman R., de Buyser S.L., Derde L.P.G., Domingo P., Omar S.F., Fernandez-Cruz A., Feuth T., Garcia F., Garcia-Vicuna R., Gonzalez-Alvaro I., Gordon A.C., Haynes R., Hermine O., Horby P.W., Horick N.K., Kumar K., Lambrecht B.N., Landray M.J., Leal L., Lederer D.J., Lorenzi E., Mariette X., Merchante N., Misnan N.A., Mohan S.V., Nivens M.C., Oksi J., Perez-Molina J.A., Pizov R., Porcher R., Postma S., Rajasuriar R., Ramanan A.V., Ravaud P., Reid P.D., Rutgers A., Sancho-Lopez A., Seto T.B., Sivapalasingam S., Soin A.S., Staplin N., Stone J.H., Strohbehn G.W., Sunden-Cullberg J., Torre-Cisneros J., Tsai L.W., van Hoogstraten H., van Meerten T., Veiga V.C., Westerweel P.E., Murthy S., Diaz J.V., Marshall J.C., Sterne J.A.C. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19. JAMA. 2021;326 doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berardicurti O., Ruscitti P., Ursini F., D’Andrea S., Ciaffi J., Meliconi R., Iagnocco A., Cipriani P., Giacomelli R. Mortality in tocilizumab-treated patients with COVID-19: a systematic review and meta-analysis. Clin. Exp. Rheumatol. 2020;38:1247–1254. [PubMed] [Google Scholar]
- 7.Malgie J., Schoones J.W., Pijls B.G. Decreased Mortality in Coronavirus Disease 2019 Patients Treated with Tocilizumab: A Rapid Systematic Review and Meta-analysis of Observational Studies. Clin. Infect. Dis. 2019;72(2021):742–749. doi: 10.1093/cid/ciaa1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan S.-H., Lai C.-C., Huang H.-T., Chang S.-P., Lu L.-C., Hsueh P.-R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2020;56(3):106103. doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tleyjeh I.M., Kashour Z., Damlaj M., Riaz M., Tlayjeh H., Altannir M., Altannir Y., Al-Tannir M., Tleyjeh R., Hassett L., Kashour T. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin. Microbiol. Infect. 2021;27(2):215–227. doi: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkofide H., Almohaizeie A., Almuhaini S., Alotaibi B., Alkharfy K.M. Tocilizumab and Systemic Corticosteroids in the Management of Patients with COVID-19: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021;110:320–329. doi: 10.1016/j.ijid.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti V., Corbi G., Sellitto C., Sabbatino F., Maci C., Bertini N., De Bellis E., Iuliano A., Davinelli S., Pagliano P., Filippelli A. Effect of tocilizumab in reducing the mortality rate in covid-19 patients: A systematic review with meta-analysis. J. Personalized Med. 2021;11(7):628. doi: 10.3390/jpm11070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The REMAP-CAP Investigators Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., Savic S., Youngstein T., Del Sorbo L., Cubillo Gracian A., De La Zerda D.J., Ustianowski A., Bao M., Dimonaco S., Graham E., Matharu B., Spotswood H., Tsai L., Malhotra A. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia, New Engl. J. Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zain Mushtaq M., Bin Zafar Mahmood S., Jamil B., Aziz A., Ali S.A. Outcome of COVID-19 patients with use of Tocilizumab: A single center experience. Int. Immunopharmacol. 2020;88:106926. doi: 10.1016/j.intimp.2020.106926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical Management Guidelines for COVID-19 Infections, Government of Pakistan - Ministry of National Health Services, Regulations and Coordination. (2020). https://covid.gov.pk/guidelines/pdf/20200402%20Clinical%20Management%20Guidelines%20for%20COVID-19%20infections_1201.pdf (accessed August 15, 2021).
- 17.Zumla A., Hui D.S., Azhar E.I., Memish Z.A., Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395(10224):e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia. Clin Drug Investig. 2020;40(6):511–518. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stouthard J.M.L., Levi M., Hack C.E., Veenhof C.H.N., Romijn H.A., Sauerwein H.P., Poll T.van.der. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb. Haemost. 1996;76(05):738–742. doi: 10.1055/s-0038-1650653. [DOI] [PubMed] [Google Scholar]
- 20.Levi M. Tocilizumab for severe COVID-19: A promising intervention affecting inflammation and coagulation. Eur J Intern Med. 2020;76:21–22. doi: 10.1016/j.ejim.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortegiani A., Ippolito M., Greco M., Granone V., Protti A., Gregoretti C., Giarratano A., Einav S., Cecconi M. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. 2021;27(1):52–66. doi: 10.1016/j.pulmoe.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.J.M. Galván-Román, S.C. Rodríguez-García, E. Roy-Vallejo, A. Marcos-Jiménez, S. Sánchez-Alonso, C. Fernández-Díaz, A. Alcaraz-Serna, T. Mateu-Albero, P. Rodríguez-Cortes, I. Sánchez-Cerrillo, L. Esparcia, P. Martínez-Fleta, C. López-Sanz, L. Gabrie, L. del Campo Guerola, C. Suárez-Fernández, J. Ancochea, A. Canabal, P. Albert, D.A. Rodríguez-Serrano, J.M. Aguilar, C. del Arco, I. de los Santos, L. García-Fraile, R. de la Cámara, J.M. Serra, E. Ramírez, T. Alonso, P. Landete, J.B. Soriano, E. Martín-Gayo, A. Fraile Torres, N.D. Zurita Cruz, R. García-Vicuña, L. Cardeñoso, F. Sánchez-Madrid, A. Alfranca, C. Muñoz-Calleja, I. González-Álvaro, T. Alvarado, P. Martínez, F. Javier de la Cuerda Llorente, C. del Arco, N. Villalba, M. Negro, E. Contreras, A. del Rey, C. Santiago, M. Junquera, R. Caminero, F.J. Val, S. González, M. Caño, I. López, A. von Wernitz, B. Retana, I. Guerra, J. Sorando, L. Chao, M.J. Cárdenas, V. Espiga, P. Chicharro, P. Rodríguez, I.H. Alday, M. Sampedro, J. Prada, E.R. Aldama, Y. Real, M. Caldas, S. Casabona, A. Lanas-Gimeno, R. de la Camara, A.F. Alvárez, B. Aguadol, A. Morell, A.I. Zurriaga, M.P. Abanades, S.R. García, T.G. Aranda, M. Ruiz, C.M. Nieto, J. Aspa, L. del C. Guerola, E. Fernández, M.J. Calzada, R. Tejedor, J. Iglesias, F. Suarez, J.A. Sánchez, B. Abad, C. Suarez, I. de los Santos, E. Roy, J. Sanz, E. Sanchez, F. Moldenhauer, P. Casado, J. Curbelo, A. Gutierrez, A. Bautista, N.R. Giménez, A. Fernandez, P. Parra, B. Moyano, A. Barrios, D. Real de Asua, B. Sanchez, C. Saez, M. Ciudad, D. Navas, L.C. Domingo, M. del C.C. Torresano, D.D. García, T.A. Cavero, A.G. Blanco, A.M. Ramírez, M.A. Semiglia Chong, A.G. Cobos, A.M. Fraile Torres, C. Sanchez-Gonzalez, A.F. Perpén, C.D. Pérez, J. Soriano, C. Cisneros, E.G. Castillo, F.J. García Pérez, R.M. Girón, C. Marcos, E. Zamora, P.G. García, S. Castañeda, S. Rodríguez-García, I.L. Cubas, E.G. Tomero, N.G. Castañeda, A.M. Ortiz, C. Valero, M. Uriarte, N. Montes, IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study, J Allergy Clin Immunol. 147 (2021) 72–80. https://doi.org/10.1016/j.jaci.2020.09.018. [DOI] [PMC free article] [PubMed]
- 23.Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P. Effect of Tocilizumab vs Usual Care in Adults Hospitalized with COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., Bruzzi P., Boni F., Braglia L., Turrà C., Ballerini P.F., Sciascia R., Zammarchi L., Para O., Scotton P.G., Inojosa W.O., Ravagnani V., Salerno N.D., Sainaghi P.P., Brignone A., Codeluppi M., Teopompi E., Milesi M., Bertomoro P., Claudio N., Salio M., Falcone M., Cenderello G., Donghi L., Del Bono V., Colombelli P.L., Angheben A., Passaro A., Secondo G., Pascale R., Piazza I., Facciolongo N., Costantini M. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized with COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern Med. 2021;181(1):24. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menzella F., Fontana M., Salvarani C., Massari M., Ruggiero P., Scelfo C., Barbieri C., Castagnetti C., Catellani C., Gibellini G., Falco F., Ghidoni G., Livrieri F., Montanari G., Casalini E., Piro R., Mancuso P., Ghidorsi L., Facciolongo N. Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation. Crit. Care. 2020;24:589. doi: 10.1186/s13054-020-03306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keske Ş., Tekin S., Sait B., İrkören P., Kapmaz M., Çimen C., Uğur S., Çelebi İ., Bakır V.O., Palaoğlu E., Şentürk E., Çağlayan B., Çakar N., Tabak L., Ergönül Ö. Appropriate use of tocilizumab in COVID-19 infection. Int J Infect Dis. 2020;99:338–343. doi: 10.1016/j.ijid.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo X., Zhou W., Yan X., Guo T., Wang B., Xia H., Ye L., Xiong J., Jiang Z., Liu Y., Zhang B., Yang W. Prognostic Value of C-Reactive Protein in Patients with Coronavirus 2019. Clin. Infect. Dis. 2020;71:2174–2179. doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almas A., Mushtaq Z., Moller J. Acuity level of care as a predictor of case fatality and prolonged hospital stay in patients with COVID-19: A hospital-based observational follow-up study from Pakistan. BMJ Open. 2021;11(5):e045414. doi: 10.1136/bmjopen-2020-045414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cataño-Correa J.C., Cardona-Arias J.A., Porras Mancilla J.P., García M.T., Chen R.J. Bacterial superinfection in adults with COVID-19 hospitalized in two clinics in Medellín-Colombia, 2020. PLoS ONE. 2021;16(7):e0254671. doi: 10.1371/journal.pone.0254671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obata R., Maeda T., Rizk D., Kuno T. Increased secondary infection in covid-19 patients treated with steroids in New York city. Jpn J Infect Dis. 2021;74(4):307–315. doi: 10.7883/yoken.JJID.2020.884. [DOI] [PubMed] [Google Scholar]
- 32.van Paassen J., Vos J.S., Hoekstra E.M., Neumann K.M.I., Boot P.C., Arbous S.M. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit. Care. 2020;24:696. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F., Hallek M., Jung N., Klein F., Persigehl T., Rybniker J., Kochanek M., Böll B., Shimabukuro‐Vornhagen A. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528–534. doi: 10.1111/myc.v63.610.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19, Lancet. Respir. Med. 2020;8(6):e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Permpalung N., Chiang T.-P.-Y., Massie A.B., Zhang S.X., Avery R.K., Nematollahi S., Ostrander D., Segev D.L., Marr K.A. Coronavirus Disease 2019–Associated Pulmonary Aspergillosis in Mechanically Ventilated Patients. Clin. Infect. Dis. 2021;223:1–9. doi: 10.1093/cid/ciab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jabeen K., Farooqi J., Irfan M., Ali S.A., Denning D.W. Diagnostic dilemma in COVID-19-associated pulmonary aspergillosis. Lancet Infect. Dis. 2021;21(6):767. doi: 10.1016/S1473-3099(21)00066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giacobbe D.R., Battaglini D., Ball L., Brunetti I., Bruzzone B., Codda G., Crea F., De Maria A., Dentone C., Di Biagio A., Icardi G., Magnasco L., Marchese A., Mikulska M., Orsi A., Patroniti N., Robba C., Signori A., Taramasso L., Vena A., Pelosi P., Bassetti M. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Invest. 2020;50(10) doi: 10.1111/eci.v50.1010.1111/eci.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar G., Adams A., Hererra M., Rojas E.R., Singh V., Sakhuja A., Meersman M., Dalton D., Kethireddy S., Nanchal R., Guddati A.K. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int J Infect Dis. 2021;104:287–292. doi: 10.1016/j.ijid.2020.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campochiaro C., Della-Torre E., Cavalli G., de Luca G., Ripa M., Boffini N., Tomelleri A., Baldissera E., Rovere-Querini P., Ruggeri A., Monti G., de Cobelli F., Zangrillo A., Tresoldi M., Castagna A., Dagna L. TOCI-RAF Study Group, Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., Cockburn J., McDonald H.I., MacKenna B., Tomlinson L., Douglas I.J., Rentsch C.T., Mathur R., Wong A.Y.S., Grieve R., Harrison D., Forbes H., Schultze A., Croker R., Parry J., Hester F., Harper S., Perera R., Evans S.J.W., Smeeth L., Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wadud N., Ahmed N., Shergil M., Khan M., Krishna M., Gilani A., el Zarif S., Galaydick J., Linga K., Kooragayalu S., Galea J., Stuczynski L., Osundele M. Improved survival outcome in SARs-CoV-2 (COVID-19) Acute Respiratory Distress Syndrome patients with Tocilizumab administration. Chest. 2020;158:696–697. doi: 10.1016/j.chest.2020.08.654. [DOI] [Google Scholar]