Highlights
-
•
Older age was associated with reductions in inhibition related activity.
-
•
Older drinkers had greater reductions in frontal activity than younger drinkers.
-
•
Men and women had opposite correlations between alcohol use severity and activity.
Keywords: Response inhibition, Alcohol use disorders, Accelerated aging, Error monitoring, Sex differences, Neuroimaging
Abstract
Background
Long-term, heavy alcohol consumption has been associated with impairments in control over alcohol use, but whether this extends to other areas of cognitive and behavioral control such as response inhibition remains unclear. Understanding individual differences in the neural correlates of response inhibition will provide further insight into the neurobiology of heavy drinking. The current study investigated response inhibition in a large sample of moderate to heavy drinkers
Methods
One hundred fifty-three individuals completed a stop signal task while undergoing functional magnetic resonance imaging. Multiple regression analyses focused on blood oxygen level-dependent (BOLD) response contrasts of correct inhibition and failed inhibition as dependent variables and included age, sex, and hazardous drinking (as measured by the Alcohol Use Disorders Identification Test (AUDIT)), and their interactions, as independent variables
Results
Age was negatively associated with BOLD response in lateral inferior and middle frontal gyri, anterior cingulate cortex, and inferior parietal lobe for both successful inhibition and failed inhibition contrasts. In addition, there was a significant age × AUDIT interaction in the successful inhibition contrast in the left middle frontal gyrus, with significant negative correlations between AUDIT and BOLD response in older participants, and a significant positive correlation between AUDIT and BOLD response in younger participants
Conclusions
Age appears to be a particularly important factor in predicting BOLD response and may be a critical variable to include in future studies of heavy drinking and alcohol use disorder, particularly those that assess cognitive function. Finally, the age × AUDIT interaction observed in the current study may represent evidence for accelerated aging effects of alcohol on cognitive function.
1. Introduction
Alcohol use disorder (AUD) is a global health crisis affecting 283 million people and causing roughly 3 million alcohol related deaths every year (World Health Organization, 2018). Long-term, heavy alcohol use is associated with myriad symptoms, including executive dysfunction and reduced behavioral control (Crews and Boettiger, 2009). Impaired ability to control ones’ consumption of alcohol, such as drinking a greater quantity or for a longer duration than intended (Riley et al., 2018, Haeny et al., 2020, Fairlie et al., 2019, Pearson and Henson, 2013) and failed attempts to quit or cut down on one’s drinking (Reyes-Huerta et al., 2018) are symptomatic of AUD and can result in alcohol-related problems. Identifying those mechanisms that contribute to these core symptoms of AUD will be critical for identifying potential treatment targets.
In addition to impairments in drinking-related control, considerable evidence has found impulsivity to be associated with alcohol-related problems, and may be a key contributor to increased AUD severity (Dick et al., 2010, Congdon and Canli, 2005, Verdejo-García et al., 2008, Sher and Trull, 1994). Impulse control disorders are associated with reduced capacity and motivation to regulate behavior, which may lead to increased risk for developing and maintaining substance use disorders (Crews and Boettiger, 2009, Wiers et al., 2007). For example, poor behavioral control/impulsivity during adolescence is predictive of future substance use disorders (Wiers et al., 2007, Nigg et al., 2006), and prolonged heavy alcohol use may lead to further impairment in neural substrates that support behavioral control (Crews and Boettiger, 2009). Individuals who engage in binge drinking show diminished capacity to actively inhibit responses compared to those who do not engage in binge drinking (Poulton et al., 2016) and individuals with AUD, as compared to individuals without AUD, have higher rates of commission errors when performing common tasks of response inhibition (Bjork et al., 2004, Noël et al., 2007). While behavioral indices of response inhibition are not related to alcohol use severity (Liu et al., 2019), there is evidence that neural responses during response inhibition may be related to AUD severity (Claus et al., 2013) and differ between individuals with an AUD and those without AUD (Li et al., 2009). For example, BOLD response in lateral and medial frontal networks during response inhibition in a Go/No-go task was negatively correlated with AUD severity in our previous study (Claus et al., 2013). Identifying those mechanisms of behavioral control that are affected as a function of AUD severity may be key for understanding the negative impacts of alcohol on executive functioning and subsequent attempts to reduce drinking.
When considering individual differences in behavioral control, it is important to consider other potential factors such as sex and age. These factors may be especially critical given potential sex differences in the effects of alcohol on brain structure and function (Thayer et al., 2016), and accelerated aging that may be present in individuals who report heavy drinking (Guggenmos et al., 2017, Bachi et al., 2017, Zhao et al., 2020). In one example of sex differences, men showed greater responses than women within anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC), middle and medial frontal cortices (Li et al., 2006) when comparing inhibition trials to control trials, and greater response in men compared to women in lateral orbital and superior frontal gyrus, perigenual ACC, and pre-supplementary motor area (SMA) when examining correct versus incorrect inhibition trials (Li et al., 2009). In contrast to these findings, no differences were observed between men and women during correct inhibition trials, but when examining trials that signaled a need for inhibition, women showed greater response in right lateralized SMA/pre-SMA, precentral gyrus, and inferior frontal gyrus (IFG) than men (Gaillard et al., 2020). In addition to potential sex differences, age appears to correlate with response inhibition performance on tasks such as the stop signal and Go/No-go (Rey-Mermet and Gade, 2018) and engagement of neural networks implicated in inhibition (Coxon et al., 2016). Previous studies report slowed engagement of response inhibition processes in older individuals (Rey-Mermet and Gade, 2018), and a negative relationship between age and inhibition related BOLD response, such that older individuals had reduced engagement of regions such as IFG compared to younger participants (Sebastian et al., 2013, Kleerekooper et al., 2016). To date, only one study has investigated the effects of alcohol use severity and sex while controlling for age in the context of a response inhibition task (Ide et al., 2018), although this study focused on error trials rather than successful inhibition trials. Nonetheless, these authors reported greater response in the error contrast (incorrect inhibition trials vs. correct go trials) in middle/superior temporal gyrus, dorsal ACC, and the thalamus in women compared to men. Regarding AUD severity, there were no significant correlations between error related responses and AUDIT score in the overall group, but a significant interaction between sex and AUDIT emerged, such that women showed a significantly greater negative correlation between AUDIT scores and error related responses in the thalamus compared to men (Ide et al., 2018).
In the current study, we aimed to examine the independent contributions of AUD severity, sex, and age on behavioral and neural measures of response inhibition and errors of commission. Similar to prior studies of response inhibition (Claus et al., 2013, Li et al., 2009), we hypothesized reduced response in lateral IFG and SFG during response inhibition in individuals with greater AUD severity. We also hypothesized that men would show greater activity in dorsolateral prefrontal cortex (DLPFC) and perigenual ACC compared to women during response inhibition, consistent with Li et al (Li et al., 2006, Li et al., 2009), and that women would demonstrate greater response in thalamus compared to men (Ide et al., 2018) during error trials. Finally, we expected to find a negative relationship between age and activity during response inhibition in several regions including inferior parietal lobe, insula, and IFG.
2. Methods
2.1. Participants
Participants were recruited from the Albuquerque metropolitan area via flyers, newspaper, online, and radio advertisements, and direct recruitment occurred at local beer and wine festivals. Recruitment targeted individuals who self-identified as a “moderate to heavy drinker”, “binge drinker”, or “weekly drinker”. The sample was recruited for a larger longitudinal study of non-treatment seeking hazardous drinkers. Institutional Review Board-approved written informed consent was obtained for all participants.
To be eligible for the study, participants had to be between the ages of 22 and 55, report “harmful and hazardous drinking” as determined by the Alcohol Use Disorder Identification Test (AUDIT) (SAUNDERS et al., 1993) with scores > 7 for men and > 6 for women (Dearing et al., 2013); have a breath alcohol concentration of 0.000 g% at the screening appointment, and be right-handed as assessed by the Edinburgh handedness questionnaire (Oldfield, 1971). Potential participants were excluded if they met criteria for current psychiatric illness (not including mood or anxiety disorders), prior head injury, or any contraindications for MRI (e.g., pregnancy, non– removable metallic implants), past year substance use disorder (other than alcohol, cannabis, and nicotine), estimated IQ < 80, or were seeking treatment for AUD. Initially, 235 participants were enrolled in the study, but 45 participants were excluded for not meeting eligibility criteria, 19 were lost to follow-up prior to the baseline scanning session, 3 did not complete the stop signal task, 7 did not meet imaging quality control standards, and 8 had task performance issues (n = 6 with go correct < 85%, n = 2 with stop signal reaction time > mean go response time (Verbruggen et al., 2019).
2.2. Stop signal task and behavioral outcome measures
The stop signal task was based on prior studies utilizing this task (Aron and Poldrack, 2006). On each trial, an empty circle was presented for 500 ms, followed by a circle with a left- or right-pointing arrow for up to 1000 msec. On each trial, participants were instructed to press a button with the index finger if the arrow pointed to the left and the middle finger if the arrow pointed to the right (i.e., Go trials). On 25% of the trials, the space within the circle turned red after a variable delay (the stop signal delay (SSD)), indicating to participants to withhold their response (i.e., Stop trials). The SSD was determined using an adaptive algorithm that increased or decreased the delay by 50 ms to ensure that participants were able to successfully withhold their response on approximately 50% of signal trials; the SSD was increased after correctly inhibited responses and decreased after trials in which a response was recorded. Participants were instructed to respond as quickly and accurately as possible, without slowing down their responses in anticipation of a stop signal. Each run of the stop signal task included 96 Go trials and 32 Stop trials; participants completed two runs of the task. The task was administered using E-Prime software (Psychology Software Tools, PA, USA).
Three primary behavior outcome variables were computed from the response time (RT) and accuracy data from the stop signal task. As recommended by the consensus manuscript on the stop signal task (Verbruggen et al., 2019), we used the integration method to compute stop signal reaction times (SSRT). Briefly, this measure is computed by determining the proportion of incorrect Stop trials, which is used to determine the quantile of the go RTs that corresponded to the end of the stop process. SSRT is computed by subtracting the mean stop signal delay time from the nth Go RT. Post error slowing was computed by calculating the difference between the RT for Go trials that followed incorrect Stop trials and Go trials that immediately preceded incorrect Stop trials on a pairwise basis, and the mean of these differences was computed across trials for each participant (Dutilh et al., 2012).
2.3. MRI acquisition
Scans were acquired on a Siemens 3 T Trio TIM scanner equipped with a 32-channel head coil. Structural scans were acquired using a high-resolution five-echo T1-weighted magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequence (TR/TE/TI = 2530/5.36/1200 ms; flip angle = 7 degrees; matrix size = 256 × 256; 92 sagittal slices; voxel size = 1 mm isotropic). Functional scans were acquired with a gradient echo simultaneous multi-slice (SMS) EPI (https://www.cmrr.umn.edu/multiband) sequence (TR/TE = 460/29 ms; flip angle = 44 degrees; multi-band acceleration factor = 8, matrix size = 82 × 82; 56 axial slices, voxel size = 3 mm isotropic, phase encoding direction = AP, bandwidth = 2772 Hz/Px, 740 volumes, scan duration = 5.67 min).
2.4. fMRI analysis
All raw fMRI data were checked for quality using MRIQC v. 0.5.2 and preprocessing of MRI/fMRI data was performed using fMRIPrep v. 1.5.4 (see Supplement). Briefly, functional images were corrected for distortion, slice-time corrected, motion corrected, and normalized to the MNI 152 template. Functional data were resampled to 2 mm isotropic voxels, smoothed with a 6 mm Gaussian kernel, and ICA-AROMA (Independent Component Analysis Automated Removal of Motion Artifacts) (Pruim et al., 2015) was used to identify and remove motion artifacts.
Preprocessed functional images were brain extracted using Brain Extraction Tool (Smith, 2002); grand-mean intensity normalized using a single multiplicative factor, and high-pass temporal filtered (Gaussian-weighted least-squares straight line fitting, with sigma = 45.0 s). Single subject analysis used the general linear model as implemented in FEAT (FMRI Expert Analysis Tool) version 6.0 using FILM with local autocorrelation correction. Custom timing files representing Correct Go, Incorrect Go, Correct Stop, and Failed Stop trials were created using the log files from E-prime; these timing files were used as regressors in the general linear model (GLM) along with their temporal derivatives. In addition, six movement parameters and their first order derivatives were added into the GLM. Voxel-wise beta maps were generated for each regressor of interest and contrast maps representing Correct Stop > Go, Incorrect Stop > Go, and Correct Stop > Incorrect Stop were derived for each task run. Individuals runs within a participant were combined using a fixed effects model in FEAT.
2.5. Group analysis
Analyses of behavioral outcomes (i.e., SSRT, Go RT, and PES) was performed using a multiple regression approach with sex, age, and AUDIT as independent predictors (e.g., SSRT = β0 + β1Age + β2AUDIT + β3Sex). In addition, we tested a model that included all two-way and three-way interactions between predictor variables (e.g., SSRT = β0 + β1Age + β2AUDIT + β3Sex + β4Age*AUDIT + β5Age*Sex + β6Sex*AUDIT + β7Age*AUDIT*Sex). Analyses of fMRI contrasts at the group level were performed using random effects modeling with FLAME 1 (FMRIB’s Local Analysis of Mixed Effects). The main effect analysis across the entire sample used a single group with no regressors. Our second model included effects of biological sex, age, and AUDIT as well as all two-way and three-way interactions between variables. When two-way and three-way interactions showed no significant effects, those terms were removed from the final model. For example, in the analysis of Correct Stop > Go, no significant effects emerged for the main effect of Sex, or the Sex × AUDIT or Sex × AUDIT × Age interaction terms, so these were removed from the final model. In all models, we also included three binary covariates: diagnosis of mood disorder, diagnosis of anxiety disorder, and self-reported use of cannabis in the previous 90 days.
Group level maps were corrected for multiple comparisons using cluster-based correction with an initial voxel-wise threshold of z > 3.1 (p < 0.001) and a cluster threshold of p < 0.05, in accordance with recent recommendations (Eklund et al., 2016). Thresholded statistical maps were projected onto surfaces for display purposes using Nilearn (Abraham et al., 2014).
3. Results
3.1. Descriptives
The individuals included in the current study (n = 153) were predominantly male (n = 89, 58.2%) and 50% (n = 76) reported Hispanic ethnicity. The racial breakdown of the sample included: White (n = 78; 60.8%), Black/African American (n = 8, 5.2%), American Indian/Alaska Native (n = 31, 20.3%), Native Hawaiian (n = 1, 0.7%), Asian (n = 8, 5.2%), more than one race (n = 18, 11.8%), and no race reported (n = 31, 20.3%). The sample had an average age of 34.53 years old with average AUDIT scores suggestive of hazardous drinking (Mean (SD) = 14.6 (6.6)). According to the Structured Clinical Interview for DSM-5 (SCID-5) (First et al., 2015), 132 participants met criteria for AUD, seven participants met diagnostic criteria for a depressive disorder in the past 12 months and 18 participants met diagnostic criteria for an anxiety disorder. All participants provided demographic information and completed several self-report, behavioral, and imaging measures. The measures used in the current study are described below with data presented in Table 1.
Table 1.
Sample characterization.
| Men | Women | |
|---|---|---|
| n | 89 | 64 |
| Age (years) | 35.5 (9.7) | 33.2 (10.3) |
| Alcohol Use Disorder | 83.1% | 90.6% |
| AUDIT | 14.0 (6.0) | 15.5 (7.4) |
| Drinks per drinking day (standard drinks) | 6.1 (4.1) | 5.7 (3.4) |
| Drinks per day (standard drinks) | 3.1 (2.8) | 3.0 (3.1) |
| Percent heavy drinking days | 25.2 (25.6) | 29.8 (25.8) |
| Percent heavy drinking while drinking | 49.3 (34.2) | 56.6 (32.9) |
| Nicotine Use | 36.0% | 37.5% |
| Cannabis Use | 40.4% | 40.6% |
| Depressive Disorder | 2.2% | 7.8% |
| Anxiety Disorder | 7.9% | 17.2% |
Displayed are means and standard deviations (mean(sd)) and percentages of participants meeting criteria for a given diagnosis or drug use category.
3.2. Group analysis of behavior
First order correlations between independent variables of interest (age, sex, and AUDIT) and primary behavior outcomes on the stop signal (i.e. Go RT, SSRT, post-error slowing) are shown in Supplemental Table 1. Multiple regression analyses of median Go RT revealed that men (mean (SD) RT = 472.8 (71.1) msec) responded faster on average than women (496.7 (72.8); t(1 4 6) = 2.18, p = 0.03), and that RT was positively associated with age (t(1 4 6) = 3.69, p < 0.001); AUDIT scores were not significantly related to Go RT (t(1 4 6) = 1.34, p = 0.18). In the model with interaction terms, there were no significant terms that predicted Go RT (all p’s > 0.4). In the multiple regression analysis of SSRT, no effects emerged as significant in the model with no interaction (all p’s > 0.38) or in the model with interactions (all p’s > 0.2). Finally, we tested whether post-error slowing was present in the current sample and found no evidence of post-error slowing (mean (SD) = -1.28 (36.1); t(1 5 2) = 0.44, p = 0.66). In the analysis of post error slowing, no significant effects emerged with age, AUDIT, or sex (all p’s > 0.3). In the multiple regression analyses of post-error slowing that included two-way and three-way interactions, no effects emerged as significant (all p’s > 0.5). Means and standard deviations for performance on the stop signal task are presented in Table 2.
Table 2.
Stop signal task performance.
| Men | Women | |
|---|---|---|
| Go median RT (msec) | 482.6 (93.6) | 512.7 (111.1) |
| Go quantile (msec) | 475.8 (73.0) | 498.8 (73.6) |
| Go proportion correct | 97.0% (3.2%) | 97.7% (2.5%) |
| Stop proportion correct | 49.6% (9.0%) | 51.3% (10.7%) |
| SSD mean (msec) | 248.8 (92.7) | 270.5 (103.0) |
| SSRT quantile (msec) | 225.5 (49.3) | 228.3 (49.0) |
| Post-error slowing (msec) | −4.0 (29.9) | 2.5 (31.1) |
Means and standard deviations are presented for each task variable (mean(sd)).
3.3. fMRI analysis
Main effects of the task across all participants are included in the supplement.
3.3.1. Correct Stop > Correct Go
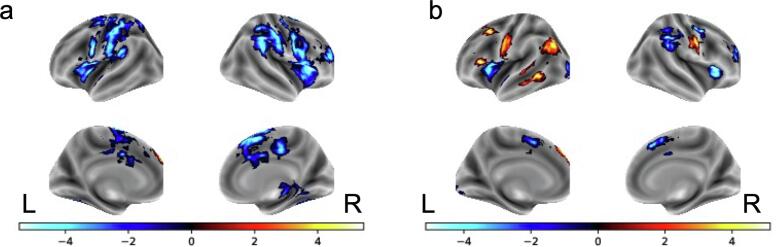
There were significant negative relationships with age and the Correct Stop > Correct Go contrast in several regions including right middle frontal gyrus (MFG), bilateral IFG and anterior insula (aIns), dorsal ACC, right putamen, SMA, right thalamus, and bilateral inferior/superior parietal lobe (IPL/SPL; see Fig. 1 and Table S1). A significant positive relationship with AUDIT and Correct Stop > Correct Go contrast emerged in the cerebellum in left Crus I. Men and women did not differ in their neural response during correct inhibition trials.
Fig. 1.
Effects of age on BOLD response Negative relationships between age and the Stop correct > Go correct contrast. b. Positive (orange) and negative (blue) relationships between age and the Stop incorrect > Go Correct contrast.
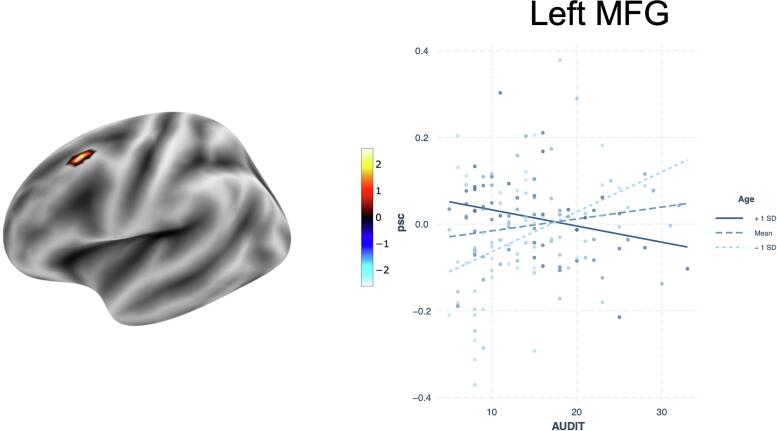
In the interaction model, the interaction of AUDIT and age was significant in predicting Correct Stop > Correct Go in left MFG. Specifically, younger participants had a strong positive relationship
between AUDIT scores and signal change, whereas older individuals demonstrated a strong negative relationship (Fig. 2). No other interactions were significant for this contrast.
Fig. 2.
Interactive effect of age and AUDIT on percent signal change (psc) in the Correct Inhibition vs. Correct Go contrast.
3.3.2. Incorrect Stop > Correct Go
We found significant positive relationships with age and the Incorrect Stop > Correct Go contrast in left superior frontal gyrus (SFG), left MFG, left lateral occipital cortex, and the left temporooccipital portion of the middle temporal gyrus. Significant negative relationships with age emerged in several regions including right MFG, bilateral IFG/aIns SMA, and bilateral IPL/SPL (see Fig. 1 and Table S2).
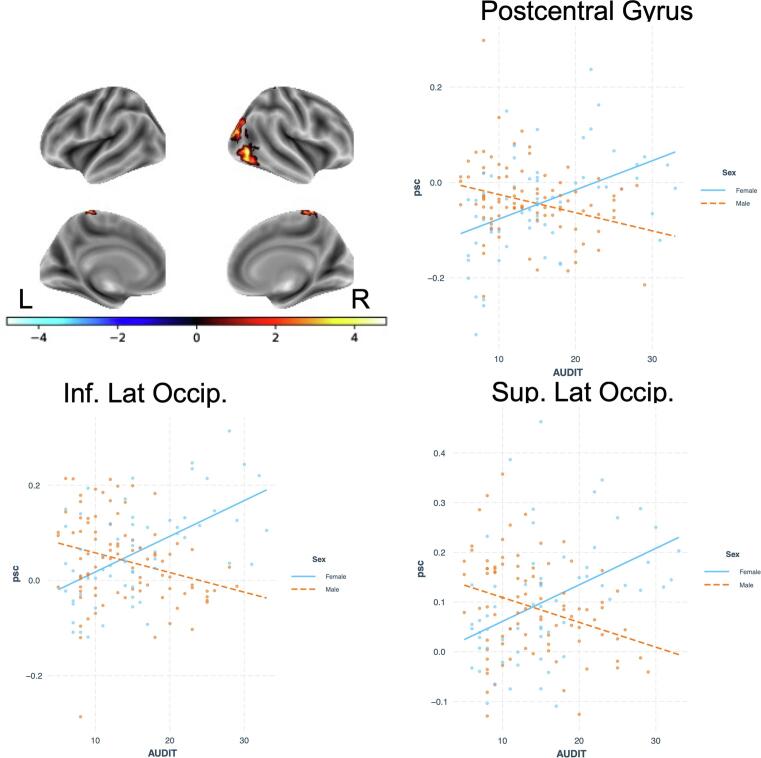
There were no significant relationships between BOLD response during incorrect inhibition trials and AUDIT scores or sex in the first multiple regression model. However, when including interactions in the model, sex differences emerged in a cluster that spanned right pre/post-central gyrus, SPL, and precuneus, as well as right lateral occipital cortex in the inferior and superior divisions. In both cases, men had greater BOLD response than women.
There was also a significant interaction of sex × AUDIT in postcentral gyrus and two clusters in lateral occipital cortex; in all cases, women had strong positive relationships between signal change and AUDIT whereas men showed the opposite effect (Fig. 3). All other interactions failed to show any significant effects.
Fig. 3.
Interactive effect of sex and AUDIT on percent signal change (psc) in the Incorrect Inhibition vs. Correct Go contrast.
3.3.3. Correct stop vs. Incorrect stop
There were no significant effects in either model in predicting BOLD responses in the contrast of correct and incorrect inhibition trials.
4. Discussion
The present study investigated the effects of sex, hazardous drinking, and age on neural responses to successful and unsuccessful response inhibition. Contrary to our hypotheses and prior study (Claus et al., 2013, Hu et al., 2016), we failed to find negative correlations between AUDIT scores and BOLD response in right IFG or ACC; instead, we found a positive relationship with AUDIT scores in the left cerebellum Crus I. We observed a few sex differences, which are inconsistent with previous studies of the stop signal task (Li et al., 2006, Li et al., 2009, Gaillard et al., 2020). The effects of age were particularly robust and consistent with previous research, with significant negative relationships in several regions including IFG, MFG, and dorsal ACC/SMA during both successful and unsuccessful response inhibition. Finally, we found two notable interactions: an age * AUDIT interaction in the correct inhibition contrast in MFG and a sex * AUDIT interaction in the contrast of incorrect inhibition trials. Although we did not replicate previous work, the results nonetheless point to the need to carefully consider task differences as well as potential confounding variables such as age and sex.
While our initial hypotheses regarding AUDIT scores were not supported, the interactions between AUDIT and age during successful inhibition and between AUDIT and sex during failed inhibitions do suggest a moderating role of hazardous drinking on BOLD response. First, the interaction between age and AUDIT in the left MFG provides additional evidence of accelerated aging in AUD that has been reported in studies of grey and white matter integrity (Bachi et al., 2017, Zhao et al., 2020). Specifically, older individuals demonstrated significantly reduced engagement of MFG as AUDIT scores increased, whereas younger participants showed the opposite effect. The MFG is implicated in motor planning and its involvement in response inhibition may be the result of conflict between going and stopping (Stock et al., 2016), and reduced engagement in older adults may be suggestive of decreased ability of this region to engage when response conflict is high, whereas younger individuals with more severe AUD may increase resources in order to successfully inhibit responses. This possibility should be explored in future studies that specifically manipulate cognitive load to determine whether there is a threshold at which older individuals are able to engage this region.
Second, we found a sex * AUDIT interaction during error trials, such that men had negative correlations between AUDIT and BOLD response and women showed positive relationships in lateral occipital cortex and postcentral gyrus. The lateral occipital cortex is implicated in object identification, and may be key for signaling other regions in a distributed behavioral control network (Gonzalez Alam et al., 2018), whereas the postcentral gyrus response appears to correspond to the force applied during button pressing (Thickbroom et al., 1998, Mizuguchi et al., 2014), which may differ during error and go trials in men and women. Of note, we did not replicate previous findings reporting an interaction of sex and AUDIT score on activity in the thalamus during error trials (Ide et al., 2018). Our sample size was nearly identical to the previous study, but the overall drinking levels and AUDIT scores were higher in the current sample, which may have influenced the findings. Given the importance of sex differences and the potential for differential effects of alcohol on men and women, future research is necessary to better understand the mechanisms of these sex differences.
Given that previous studies have reported sex differences in contrasts measuring response inhibition, we were surprised to find no significant differences in the current study. Because the participants in the current study had significant drinking histories, it is possible that neurotoxic effects of alcohol differentially affected men and women, which could lead to a reduced likelihood of finding significant effects. However, careful inspection of the available literature suggests that sex differences in response inhibition may not be highly reliable. For instance, whereas two early studies (Li et al., 2006, Li et al., 2009) reported greater neural response in men compared to women during response inhibition in medial and lateral frontal cortex, a subsequent study reported greater activation in women compared to men when averaging across Correct Stop > Correct Go and Incorrect Stop > Correct Go contrasts (Gaillard et al., 2020). In addition, a fourth study with a large sample of healthy control participants (n = 103) examined effects of sex differences and age, and found no effects of sex (Hu et al., 2012). Future studies that incorporate heavy drinking and control samples across the lifespan will be instrumental in advancing our understanding of sex differences in response inhibition related processes.
While two previous studies demonstrated negative correlations between AUD severity and BOLD response during response inhibition (Claus et al., 2013, Hu et al., 2016) and failed inhibition (Claus et al., 2013), the current study found no such relationships. One important difference between the previous study that showed significant relationships between AUDIT scores and response inhibition related activity in the stop signal task (Hu et al., 2016) is the sample of drinkers collected for each study. While Hu et al (Hu et al., 2016) included drinkers and non-drinkers, none of the drinkers met criteria for an AUD whereas 86% of participants in the current study met AUD criteria. This severity difference may account for the difference in findings across the two studies. While Hu et al recruited a very different sample, our previous study (Claus et al., 2013) included participants that were similar in AUD severity and drinking patterns. However, significant task differences exist between the stop signal task and the Go/No-go task that was used in our prior study (Claus et al., 2013). In the Go/No-go task, participants viewed a rapidly presented stream of alternating X's and Y's, and inhibited responses on trials in which the current stimulus matched the stimulus shown immediately prior (Kaufman et al., 2003). In the Go/No-go, inhibition decisions rely on comparisons of a stimulus with representations in working memory, which is more arguably more cognitively demanding than the stop signal task. A meta-analysis of simple and complex response inhibition tasks revealed key differences in regions depending on the nature of the task, with simple tasks engaging fewer regions within frontal cortex (Simmonds et al., 2008). An additional meta-analysis also demonstrated greater BOLD response in Go/No-go tasks compared to stop signal tasks in regions such as inferior frontal gyrus and inferior parietal lobe (Swick et al., 2011). In addition, the adaptive nature of the stop signal task used in the present study reduces potential differences in behavioral performance whereas the Go/No-go task used previously did not include adaptive methods. The degree to which response inhibition is a group-defining difference or an important individual difference factor in predicting severity of AUD or treatment outcomes is still unclear (Kwako et al., 2016, Voon et al., 2020), and future studies may need to address the different types of response inhibition tasks or use a latent variable approach with multiple tasks (Friedman and Miyake, 2004).
Next, our findings demonstrated significant correlations with age. Specifically, we observed significant negative relationships with age across all three contrasts, and some positive correlations within the incorrect inhibition contrast. Our findings are highly consistent with typical aging effects for both the contrast of response inhibition (Sebastian et al., 2013, Hu et al., 2018) and failed inhibition (Hu et al., 2012). Several regions in the response inhibition network such as right lateralized IFG and MFG, dorsal ACC, and IPL were negatively correlated with age. The reduced response observed in this network potentially stems from meeting a “resource ceiling” that limits the degree to which regions can respond in the face of increasing cognitive demand (Sebastian et al., 2013, Hu et al., 2018). The degree to which reductions in gray matter volume influences activation or behavioral performance (Hu et al., 2018) in the current sample is not clear, but future investigations will explore these links to gain a more comprehensive understanding of how heavy alcohol use affects structural and functional integrity. Given the robustness of the effects of age on BOLD response, it will be critical to control for this potentially confounding variable in future examinations, particularly those examining cognitive functioning.
While we used rigorous methods and analysis approaches for the current study, several limitations must be considered. First, the current study does not include any non-drinking or social drinking control participants, which limits interpretability. The parent study was designed as a within subject longitudinal examination of the neural mechanisms of self-change, a design that precluded the inclusion of controls. Regardless, it is still critical to understand how individual variation in response inhibition related BOLD response relates to varying levels of hazardous drinking. Inclusion of non-drinkers or social drinkers across a wide age range would also allow us to examine sex differences in the absence of any neurotoxicity that occurs over years of heavy drinking (Thayer et al., 2016). In addition to the lack of controls, approximately 40% of participants in the current study reported use of cannabis. While we controlled for cannabis use in our analyses, future studies would benefit from including a group of drinkers who do not use cannabis. In addition to the lack of controls, there was an error in the programming of question number two of the AUDIT, which omitted the “10 or more” option from the potential answer options. This may have led to lower AUDIT scores for up to fifteen participants that reported average drinking quantities of 10 or more standard drinks, which could have affected results. However, we identified these participants and conducted a separate set of analyses using AUDIT scores that were one point higher, and all results remained unchanged.
4.1. Conclusions
Overall, the current findings extend the current literature on response inhibition in AUD, by examining the effects of age, sex, and drinking severity on neural responses during a stop signal task. While our findings did not replicate previous reports of AUD severity correlating with BOLD response, two notable findings emerged with respect to age. First, age was a significant predictor of BOLD response in the contrasts of successful inhibition and failed inhibition, suggesting that future studies should include age as a covariate. Second, when examining the age by AUDIT interaction, we found that the direction of the correlation between AUDIT score and BOLD response in the middle frontal gyrus were moderated by age, which may be evidence of accelerated aging that results from prolonged heavy alcohol use. Finally, we observed some sex differences during error trials in postcentral gyrus and lateral occipital cortex, but this needs further exploration in future studies in order to identify sex effects across other tasks and cognitive functions to more fully understand the effects of sex differences resulting from heavy alcohol use.
CRediT authorship contribution statement
Megan Swartz: Writing – original draft, Writing – review & editing. Finnigan Burton: Writing – original draft, Data curation, Visualization. Kishore Vakamudi: Writing – review & editing, Data curation. Kareem Al-Khalil: Writing – review & editing, Data curation. Katie Witkiewitz: Conceptualization, Writing – review & editing, Investigation, Data curation, Funding acquisition. Eric D. Claus: Conceptualization, Writing – original draft, Writing – review & editing, Investigation, Data curation, Funding acquisition, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was support by a grant from NIAAA (R01AA023665 to EDC and KW). NIAAA did not have any input into the design or conduct of the study, and the opinions stated in this article do not necessarily represent those of NIAAA. All authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102875.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abraham A., Pedregosa F., Eickenberg M., Gervais P., Mueller A., Kossaifi J., Gramfort A., Thirion B., Varoquaux G. Machine learning for neuroimaging with scikit-learn. Front Neuroinform. 2014;8 doi: 10.3389/fninf.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci. 2006;26(9):2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachi K., Sierra S., Volkow N.D., Goldstein R.Z., Alia-Klein N. Is biological aging accelerated in drug addiction? Curr Opin Behav Sci. 2017;13:34–39. doi: 10.1016/j.cobeha.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Hommer D.W., Grant S.J., Danube C. Impulsivity in abstinent alcohol-dependent patients: Relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34(2-3):133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Claus E.D., Feldstein Ewing S.W., Filbey F.M., Hutchison K.E. Behavioral control in alcohol use disorders: Relationships with severity. J Stud Alcohol Drugs. 2013;74(1):141–151. doi: 10.15288/jsad.2013.74.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E., Canli T. The endophenotype of impulsivity: Reaching consilience through behavioral, genetic, and neuroimaging approaches. Behav Cogn Neurosci Rev. 2005;4(4):262–281. doi: 10.1177/1534582305285980. [DOI] [PubMed] [Google Scholar]
- Coxon J.P., Goble D.J., Leunissen I., Van Impe A., Wenderoth N., Swinnen S.P. Functional Brain Activation Associated with Inhibitory Control Deficits in Older Adults. Cereb Cortex. 2016;26(1):12–22. doi: 10.1093/cercor/bhu165. [DOI] [PubMed] [Google Scholar]
- Crews F.T., Boettiger C.A. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93(3):237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearing R.L., Witkiewitz K., Connors G.J., Walitzer K.S. Prospective changes in alcohol use among hazardous drinkers in the absence of treatment. Psychol Addict Behav. 2013;27(1):52–61. doi: 10.1037/a0028170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D.M., Smith G., Olausson P., Mitchell S.H., Leeman R.F., O’Malley S.S., Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15(2):217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilh G., van Ravenzwaaij D., Nieuwenhuis S., van der Maas H.L.J., Forstmann B.U., Wagenmakers E.-J. How to measure post-error slowing: A confound and a simple solution. J Math Psychol. 2012;56(3):208–216. [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairlie A.M., Cadigan J.M., Patrick M.E., Larimer M.E., Lee C.M. Unplanned heavy episodic and high-intensity drinking: Daily-level associations with mood, context, and negative consequences. J Stud Alcohol Drugs. 2019;80(3):331–339. doi: 10.15288/jsad.2019.80.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Williams J., Karg R., Spitzer R. American Psychiatric Association; Arlington, VA: 2015. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV) [Google Scholar]
- Friedman N.P., Miyake A. The Relations Among Inhibition and Interference Control Functions: A Latent-Variable Analysis. J Exp Psychol Gen. 2004;133(1):101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Gaillard A., Rossell S.L., Carruthers S.P., Sumner P.J., Michie P.T., Woods W., Neill E., Phillipou A., Toh W.L., Hughes M.E. Greater activation of the response inhibition network in females compared to males during stop signal task performance. Behav Brain Res. 2020;386:112586. doi: 10.1016/j.bbr.2020.112586. [DOI] [PubMed] [Google Scholar]
- Gonzalez Alam T., Murphy C., Smallwood J., Jefferies E. Meaningful inhibition: Exploring the role of meaning and modality in response inhibition. Neuroimage. 2018;181:108–119. doi: 10.1016/j.neuroimage.2018.06.074. [DOI] [PubMed] [Google Scholar]
- Guggenmos M., Schmack K., Sekutowicz M., Garbusow M., Sebold M., Sommer C., Smolka M.N., Wittchen H.-U., Zimmermann U.S., Heinz A., Sterzer P. Quantitative neurobiological evidence for accelerated brain aging in alcohol dependence. Transl Psychiatry. 2017;7(12) doi: 10.1038/s41398-017-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeny A.M., Gueorguieva R., Morean M.E., Krishnan‐Sarin S., DeMartini K.S., Pearlson G.D., Anticevic A., Krystal J.H., O'Malley S.S. The Association of Impulsivity and Family History of Alcohol Use Disorder on Alcohol Use and Consequences. Alcohol Clin Exp Res. 2020;44(1):159–167. doi: 10.1111/acer.14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Chao H.H.A., Winkler A.D., Li C.S.R. The effects of age on cerebral activations: Internally versus externally driven processes. Front Aging Neurosci. 2012;4:1–9. doi: 10.3389/fnagi.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Zhang S., Chao H.H., Krystal J.H., Li C.-S. Association of Drinking Problems and Duration of Alcohol Use to Inhibitory Control in Nondependent Young Adult Social Drinkers. Alcohol Clin Exp Res. 2016;40(2):319–328. doi: 10.1111/acer.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ide J.S., Chao H.H., Castagna B., Fischer K.A., Zhang S., Li C., shan R Structural and functional cerebral bases of diminished inhibitory control during healthy aging. Hum Brain Mapp. 2018;39:5085–5096. doi: 10.1002/hbm.24347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S., Zhornitsky S., Chao H.H., Zhang S., Hu S., Wang W., Krystal J.H., Li C.-S. Thalamic Cortical Error-Related Responses in Adult Social Drinkers: Sex Differences and Problem Alcohol Use. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(10):868–877. doi: 10.1016/j.bpsc.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J.N., Ross T.J., Stein E.A., Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23(21):7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerekooper I., van Rooij S.J.H., van den Wildenberg W.P.M., de Leeuw M., Kahn R.S., Vink M. The effect of aging on fronto-striatal reactive and proactive inhibitory control. Neuroimage. 2016;132:51–58. doi: 10.1016/j.neuroimage.2016.02.031. [DOI] [PubMed] [Google Scholar]
- Kwako L.E., Momenan R., Litten R.Z., Koob G.F., Goldman D. Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biol Psychiatry. 2016;80(3):179–189. doi: 10.1016/j.biopsych.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.-S., Huang C., Constable R.T., Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage. 2006;32(4):1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Li C-S.R., Luo X., Yan P., Bergquist K., Sinha R. Altered impulse control in alcohol dependence: Neural measures of stop signal performance. Alcohol Clin Exp Res. 2009;33(4):740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.-S., Zhang S., Duann J.-R., Yan P., Sinha R., Mazure C.M. Gender differences in cognitive control: An extended investigation of the stop signal task. Brain Imaging Behav. 2009;3(3):262–276. doi: 10.1007/s11682-009-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., van den Wildenberg W.P.M., de Graaf Y., Ames S.L., Baldacchino A., Bø R., Cadaveira F., Campanella S., Christiansen P., Claus E.D., Colzato L.S., Filbey F.M., Foxe J.J., Garavan H., Hendershot C.S., Hester R., Jester J.M., Karoly H.C., Kräplin A., Kreusch F., Landrø N.I., Littel M., Loeber S., London E.D., López-Caneda E., Lubman D.I., Luijten M., Marczinski C.A., Metrik J., Montgomery C., Papachristou H., Mi Park S., Paz A.L., Petit Géraldine, Prisciandaro J.J., Quednow B.B., Ray L.A., Roberts C.A., Roberts G.M.P., de Ruiter M.B., Rupp C.I., Steele V.R., Sun D., Takagi M., Tapert S.F., van Holst R.J., Verdejo-Garcia A., Vonmoos M., Wojnar M., Yao Y., Yücel M., Zack M., Zucker R.A., Huizenga H.M., Wiers R.W. Is (poly-) substance use associated with impaired inhibitory control? A mega-analysis controlling for confounders. Neurosci Biobehav Rev. 2019;105:288–304. doi: 10.1016/j.neubiorev.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Mizuguchi N., Nakata H., Kanosue K. Activity of right premotor-parietal regions dependent upon imagined force level: An fMRI study. Front Hum Neurosci. 2014;8:1–8. doi: 10.3389/fnhum.2014.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J.T., Wong M.M., Martel M.M., Jester J.M., Puttler L.I., Glass J.M., Adams K.M., Fitzgerald H.E., Zucker R.A. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Noël X., Van der Linden M., d’Acremont M., Bechara A., Dan B., Hanak C., Verbanck P. Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology. 2007;192(2):291–298. doi: 10.1007/s00213-006-0695-6. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pearson M.R., Henson J.M. Unplanned drinking and alcohol-related problems: A preliminary test of the model of unplanned drinking behavior. Psychol Addict Behav. 2013;27(3):584–595. doi: 10.1037/a0030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton A., Mackenzie C., Harrington K., Borg S., Hester R. Cognitive Control Over Immediate Reward in Binge Alcohol Drinkers. Alcohol Clin Exp Res. 2016;40(2):429–437. doi: 10.1111/acer.12968. [DOI] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Reyes-Huerta H.E., dos Santos C., Martínez K. Impulsive mechanisms influencing relapse in alcohol drinking. Med Hypotheses. 2018;112:27–29. doi: 10.1016/j.mehy.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Rey-Mermet A., Gade M. Inhibition in aging: What is preserved? What declines? A meta-analysis. Psychon Bull Rev. 2018;25(5):1695–1716. doi: 10.3758/s13423-017-1384-7. [DOI] [PubMed] [Google Scholar]
- Riley E.N., Davis H.A., Milich R., Smith G.T. Heavy, problematic college drinking predicts increases in impulsivity. J. Stud. Alcohol Drugs. 2018;79(5):790–798. doi: 10.15288/jsad.2018.79.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J.B., Aasland O.G., Babor T.F., De La Fuente J.R., Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption‐II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sebastian A., Baldermann C., Feige B., Katzev M., Scheller E., Hellwig B., Lieb K., Weiller C., Tüscher O., Klöppel S. Differential effects of age on subcomponents of response inhibition. Neurobiol Aging. 2013;34(9):2183–2193. doi: 10.1016/j.neurobiolaging.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Sher K.J., Trull T.J. Personality and Disinhibitory Psychopathology: Alcoholism and Antisocial Personality Disorder. J Abnorm Psychol. 1994;103(1):92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Simmonds D.J., Pekar J.J., Mostofsky S.H. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A.-K., Popescu F., Neuhaus A.H., Beste C. Single-subject prediction of response inhibition behavior by event-related potentials. J Neurophysiol. 2016;115(3):1252–1262. doi: 10.1152/jn.00969.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56(3):1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Thayer R.E., Hagerty S.L., Sabbineni A., Claus E.D., Hutchison K.E., Weiland B.J. Negative and interactive effects of sex, aging, and alcohol abuse on gray matter morphometry. Hum Brain Mapp. 2016;37(6):2276–2292. doi: 10.1002/hbm.23172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom G.W., Phillips B.A., Morris I., Byrnes M.L., Mastaglia F.L. Isometric force-related activity in sensorimotor cortex measured with functional MRI. Exp Brain Res. 1998;121(1):59–64. doi: 10.1007/s002210050437. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Band GPH, Beste C, Bissett PG, Brockett AT, et al. (2019): A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. Elife 8: 1–26. [DOI] [PMC free article] [PubMed]
- Verdejo-García A., Lawrence A.J., Clark L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Voon V., Grodin E., Mandali A., Morris L., Doñamayor N., Weidacker K., Kwako L., Goldman D., Koob G.F., Momenan R. Addictions NeuroImaging Assessment (ANIA): Towards an integrative framework for alcohol use disorder. Neurosci Biobehav Rev. 2020;113:492–506. doi: 10.1016/j.neubiorev.2020.04.004. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2018. Global Status Report on Alcohol and Health, Geneva: World Health Organization. Retrieved from http://www.who.int/substance_abuse/publications/global_alcohol_report/msbgsruprofiles.pdf.
- Wiers R.W., Bartholow B.D., van den Wildenberg E., Thush C., Engels R.C.M.E., Sher K.J., Grenard J., Ames S.L., Stacy A.W. Automatic and controlled processes and the development of addictive behaviors in adolescents: A review and a model. Pharmacol Biochem Behav. 2007;86(2):263–283. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Pfefferbaum A., Podhajsky S., Pohl K.M., Sullivan E.V. Accelerated aging and motor control deficits are related to regional deformation of central cerebellar white matter in alcohol use disorder. Addict Biol. 2020;25:1–12. doi: 10.1111/adb.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.