Abstract
Objectives:
Little is known about the behavioral and psychosocial correlates of pediatric nonalcoholic fatty liver disease (NAFLD). Given diet contributes to the development and persistence of NAFLD, we examined (1) the prevalence of unhealthy eating behaviors (UEB), (2) whether these varied by NAFLD or non-alcoholic steatohepatitis (NASH) presence, and explored (3) the association of psychopathology with NAFLD.
Methods:
Prior to metabolic and bariatric surgery (MBS), adolescents (N=159; Mage=16.4; MBMI=53.7 kg/m2, 73% Female, 62.3% White) self-reported presence/absence of 10 UEB (Questionnaire on Eating and Weight Patterns-Revised, Night Eating Questionnaire, Look AHEAD). NAFLD and NASH presence was assessed by intraoperative liver biopsy. Height/weight, blood pressure and blood specimens were obtained. A medical comorbidity index was created (pre-diabetes/diabetes, dyslipidemia, elevated blood pressure). Psychopathology was assessed in a subgroup completeding the Youth Self-Report (N=98).
Results:
Binge eating disorder symptomatology was associated with higher odds of NAFLD while frequent eating out was associated with lower odds of NAFLD. Among those with NAFLD frequent eating out was associated with higher odds of NASH while nocturnal eating was associated with lower odds of NASH. Separate models identified internalizing psychopathology as associated with higher odds of NAFLD after controlling for demographics, number of UEB, and medical comorbidities.
Conclusions:
Results suggest potential phenotypical differences between adolescents presenting for MBS with/without NAFLD, with implications for behavioral/psychosocial targets for screening and intervention. Replication should occur in a sample with greater gender and ethnic diversity to improve generalizability. Understanding differences in the context of surgical weight loss and comorbidity resolution is indicated.
Keywords: Severe Obesity, Metabolic and Bariatric Surgery, Eating Behaviors, Adolescent
Introduction
Little is known about the behavioral and psychosocial correlates of pediatric nonalcoholic fatty liver disease (NAFLD), an obesity-related comorbidity reported in 26% of youth with obesity and 59% of adolescents with severe obesity undergoing metabolic and bariatric surgery (MBS) 1,2. Diet is critical to the development and persistence of NAFLD and is targeted, together with physical inactivity, as first line treatment3–5. While mixed, there is evidence associating unhealthy eating behavior (UEB) with poor dietary quality, decreased physical fitness, and weight gain in children and adults 6–9. UEB in adults, specifically binge eating symptoms10 and rapid eating11, have been associated with NAFLD, independent of obesity. Studies in youth with NAFLD are more limited, with one reporting higher, though non-significant, restrained eating rates and higher external and emotional eating compared to children with obesity without NAFLD 12. UEB13,14 and NAFLD15,16 have independently been associated with psychopathology symptoms; however the relationships among these variables have yet to be explored in a pediatric population with severe obesity presenting for MBS.
This study examined the prevalence of UEB in a multicenter cohort of adolescents with severe obesity undergoing MBS with and without biopsy-confirmed NAFLD. Participants with NAFLD were hypothesized to report significantly higher UEB. Whether UEB prevalence varied based on NAFLD severity (i.e., NAFLD-not Nonalcoholic Steatohepatitis [NASH]) relative to more severe NAFLD (borderline/definite NASH) was explored. Finally, we explored the association of psychopathology with NAFLD, controlling for the number of UEB and medical comorbidities, with the goal of informing psychosocial targets of assessment and intervention for this unique patient population.
Methods
Study design overview
Building on previous work,1 this study used baseline data from 159 participants with/without NAFLD, confirmed by intraoperative liver biopsy at time of surgery, who participated in the Teen Longitudinal Assessment of Bariatric Surgery Consortium (Teen-LABS; 2007–2012) cohort, a prospective, observational study evaluating the safety and efficacy of MBS in 242 adolescents (ages 13–19) across five academic medical centers in the United States. The majority (99%) of biopsies occurred at three sites where intraoperative biopsies were standard of care. A subgroup of eligible (i.e., age≤18) participants also participated in a Teen-LABS ancillary (TeenView) focused on the psychosocial effects of MBS. Eligibility criteria for both studies have been previously described.17,18 Institutional Review Boards at each institution approved protocols.
Participants
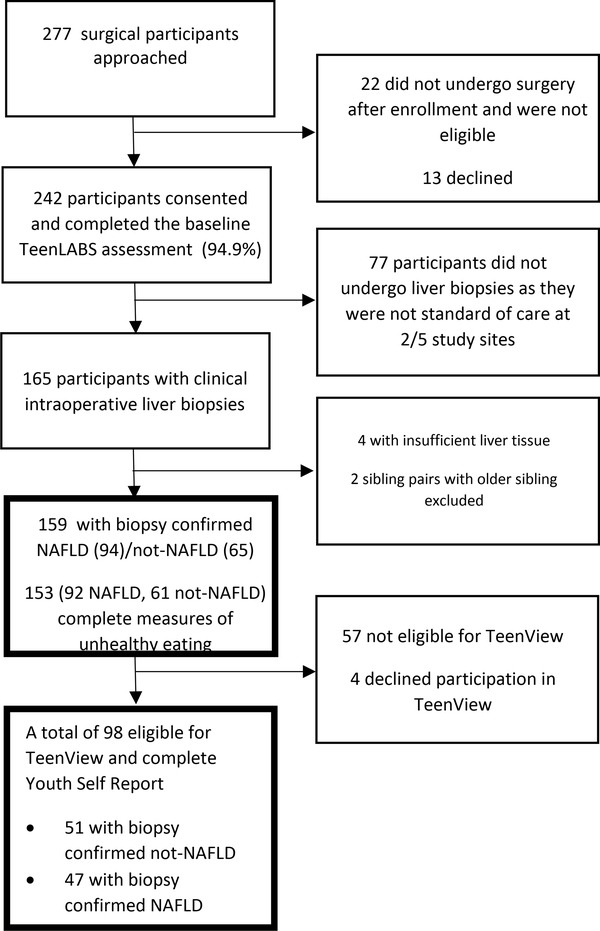
Teen-LABS participants with/without biopsy-confirmed NAFLD (biopsy-confirmed; total n=159; NAFLD n =94; not-NAFLD n = 65) were included, with sub-group analyses completed for those who completed the Youth Self-Report (YSR) in TeenView at baseline (total n = 98; NAFLD = 47; not-NAFLD n = 51). Recruitment and inclusion were previously reported1 and are detailed in Figure 1.
Figure 1.
Teen-LABS participant flow from approached to analysis sample
Procedures
After obtaining written consent/assent, adolescents completed baseline (within 30 days of surgery) self-report measures using standardized protocols administered by trained study personnel at each care center. Trained personnel also measured height, weight, and blood pressure, and obtained blood specimens with standardized procedures17 which were used along with presurgical data from medical records, physical exam, and participant interview, to determine the presence or absence of weight-related comorbidities (i.e., dyslipidemia, hypertension, prediabetes/diabetes). Intraoperative liver biopsies were obtained immediately prior to the MBS procedure.
Liver Histology
The characterization of liver histology in this population has been described previously.1 In short, liver biopsies were categorized as definite NASH, borderline NASH, or NAFLD-not NASH (NAFL) based on the aggregate presence and pattern of the individual histologic features of fatty liver disease. Borderline zone 3 NASH had some features of NASH but not enough to meet full criteria for definite NASH (steatosis, lobular inflammation and ballooning). Borderline zone 1 NASH has portal predominant lesions (portal and periportal inflammation and periportal fibrosis) and is predominantly seen in children. If there was no evidence of abnormal steatosis (>5% of hepatocytes with macrovesicular steatosis), or injury consistent with NAFLD or NASH, the biopsy was designated as “Not-NAFLD”.
Measures
Unhealthy eating behaviors.
Self-report items adapted from the psychometrically sound Questionnaire on Eating and Weight Patterns-Revised (QEWP-R)19 to assess unplanned eating, unplanned eating with loss of control, binge eating, binge eating with loss of control, and binge eating disorder (BED) symptomatology. Frequency of skipping meals, eating out, eating when not hungry and eating when full were assessed with items originally adapted from the Look AHEAD (Action for Health in Diabetes) study20,21 for LABS-2, the development of which has been previously described.22 Nocturnal eating was based on adapted items from the validated Night Eating Questionnaire (NEQ)23. See Table, Supplemental Digital Content 1 for descriptions and definitions for dichotomizing each UEB, with a UEB index created by summing UEBs endorsed as present, ranging from 0 to 10.
Psychopathology.
The Youth Self-Report (YSR)24 is a validated measure assessing adolescent symptomatology over the past 6 months. The internalizing scale includes anxious-depressed, withdrawn-depressed, and somatic symptomatology. Externalizing symptoms include aggressive, rule-breaking, and intrusive behavior. Higher scores indicate greater symptomatology, with “borderline” (T>60) cut-points indicating elevated symptomatology based on age-/sex-instrument normative values.
Medical comorbidities.
Sex/age specific standard definitions were used to determine the presence or absence of weight-related comorbidities (i.e., dyslipidemia, hypertension, prediabetes/diabetes; see Table, Supplemental Digital Content 1)17. A medical comorbidity index ranging from 0–3 comorbidities was created to characterize the comorbidity burden carried by each participant.
Medications potentially impacting liver health.
Participants self-reported whether they were currently taking medications that may influence their NAFLD phenotype, including use of psychotropic medications (i.e., antidepressants, major tranquilizers, minor tranquilizers, mood stabilizers), glucocorticoids, methotrexate, tetracycline antibiotics, vitamin E, purified omega 3 polyunsaturated fatty acids, sitagliptin and rosiglitazone. A dichotomized variable was created to capture the presence or absence of taking a medication that may impact liver health.
Other measures.
Participants and caregivers completed a demographic questionnaire self-reporting sex, race, ethnicity, age, and caregiver education level. Adolescent height and weight data were used to calculate BMI (kg/m2).
Statistical Analyses
Missing data (3.5–6.2%) were handled via maximum likelihood estimation. Nesting of participants within sites was controlled via specialized in Mplus v7.3 to avoid Type-1 errors. T-tests and chi-square tests were used to examine using medication that may impact liver health, demographic factors and BMI for NAFLD versus not-NAFLD groups, with significant differences controlled in subsequent analyses.
For Aims 1 and 2, frequencies for UEB were calculated in participants with/without biopsy-confirmed NAFLD as well as in those with less severe (NAFLD-not NASH) and more severe NAFLD (borderline/definite NASH). A series of logistic regressions were completed with each UEB as the independent variable and group (NAFLD versus non-NAFLD; NAFLD-not NASH versus borderline/definite NASH) as the dependent variable. For Aim 3, separate logistic regressions were used to examine the associations of concurrent psychopathology (elevated internalizing or externalizing symptomatology) and NAFLD. Sex, race, age, caregiver education, and indices of UEB and medical comorbidities were controlled.
Results
The majority of participants self-identified as female, white, and living with a caregiver who had more than a high school education (Table 1). Over half (n=94 of 159; 59.1%) were determined by biopsy to have NAFLD. There were no significant demographic or BMI differences between adolescents with and without NAFLD. Additionally, no significant group differences were identified between participants with NAFLD (n=26 of 94; 27.7%) and those without NAFLD (n=15 of 65, 23.1%; X2=0.42; p=0.52) in use of medications that may influence NAFLD phenotype.
Table 1.
Demographic characteristics of adolescents undergoing bariatric surgery and their families.
| Demographic characteristics | NAFLD Mean ± SD n (%) | Not-NAFLDMean ± SD n (%) | p a |
|---|---|---|---|
| Baseline | n=94 | n=65 | |
| Gender (% Female) | 64 (68.1%) | 52 (80.0%) | 0.10 |
| Race (% White) | 62 (66.0%) | 37 (56.9%) | 0.25 |
| Age (in years) | 16.6±1.5 | 16.1±1.6 | 0.06 |
| BMI (kg/m2) | 54.9±9.7 | 52.0±9.6 | 0.06 |
| Caregiver Education (% ≤ High School Graduation)b | 41 (43.6%) | 19 (29.2%) | 0.08 |
| Surgical Procedure | |||
| Gastric Bypass | 72 (76.6%) | 40 (61.5%) | |
| Sleeve Gastrectomy | 22 (23.4%) | 25(38.5%) | |
| Taking Medication that may Impact Liver Healthc | 26 (27.7%) | 15 (23.1%) | 0.52 |
| NASH | 35 (37.2%) | - |
Abbreviations: BMI=body mass index
p-values are based on two-tailed independent t-tests when examining mean values and on Chi-Square tests when examining percentages.
Missing for n=1 in not-NAFLD
Endorsing taking at least one of the following medications: psychotropic medications (i.e., antidepressants, major tranquilizers, minor tranquilizers, mood stabilizers), glucocorticoids, methotrexate, tetracycline antibiotics, vitamin E, purified omega 3 polyunsaturated fatty acids, sitagliptin and rosiglitazone
UEB occurred frequently in participants with and without NAFLD (Table 2). Almost half or more participants engaged in eating when not hungry, eating when full, and skipping meals. In logistic regressions controlling for sex, BMI, race, and age, endorsing BED symptomatology was associated with significantly higher odds of NAFLD. Frequent eating out (i.e., eating out for at least one meal three or more times a week) was associated with lower odds of NAFLD. No other significant associations were identified.
Table 2.
Rates of unhealthy eating behaviors in Teen-LABS participants with and without NAFLD and in participants with less severe (NAFLD-not NASH) and more severe NAFLD (borderline/definite NASH)
| NAFLDa n=92 | Not-NAFLDa n=61 | β | OR | 95% CI OR | p | NASH n=35 | Not-NASHb n=57 | β | OR | 95% CI OR | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modelc | Modeld | |||||||||||
| 1. Unplanned Eating | 45.7% | 42.6% | 0.16 | 1.17 | 1.00–1.37 | 0.06 | 42.9% | 47.4% | −0.05 | 0.95 | 0.24–3.74 | 0.94 |
| 2. Unplanned Eating with Loss of Control | 25.0% | 26.2% | −0.05 | 0.96 | 0.34–2.71 | 0.93 | 22.9% | 26.3% | −0.12 | 0.89 | 0.37–2.14 | 0.80 |
| 3. Binge Eating | 42.4% | 42.6% | −0.13 | 0.88 | 0.62–1.25 | 0.47 | 40.0% | 43.9% | −0.21 | 0.81 | 0.30–2.20 | 0.69 |
| 4. Binge Eating With Loss of Control | 31.5% | 29.5% | −0.09 | 0.92 | 0.52–1.63 | 0.77 | 28.6% | 33.3% | −0.40 | 0.67 | 0.18–2.53 | 0.56 |
| 5. Binge Eating Disorder Symptomatology | 13.0% | 4.9% | 1.03 | 2.79 | 2.33–3.36 | <0.001 | 14.3% | 12.3% | 0.21 | 1.23 | 0.49–3.10 | 0.66 |
| 6. Skipping Meals | 50.0% | 54.1% | −0.20 | 0.82 | 0.50–1.34 | 0.43 | 54.3% | 47.4% | 0.24 | 1.27 | 0.33–4.87 | 0.73 |
| 7. Eating Oute | 38.0% | 43.3% | −0.27 | 0.77 | 0.61–0.96 | 0.02 | 48.6% | 31.6% | 0.63 | 1.87 | 1.04–3.38 | 0.04 |
| 8. Nocturnal Eatingf, g | 23.3% | 11.7% | 0.71 | 2.03 | 0.73–5.61 | 0.17 | 14.3% | 29.1% | −1.09 | 0.34 | 0.13–0.88 | 0.03 |
| 9. Eating When Not Hungry | 68.5% | 60.7% | 0.47 | 1.59 | 0.81–3.14 | 0.18 | 65.7% | 70.2% | −0.20 | 0.82 | 0.29–2.35 | 0.71 |
| 10. Eating When Full | 58.7% | 50.8% | 0.52 | 1.68 | 0.78–3.60 | 0.19 | 65.7% | 54.4% | 0.45 | 1.56 | 0.91–2.67 | 0.11 |
All measures of unhealthy eating were missing for n=2 of 94 NAFLD and n=4 of 65 not-NAFLD.
All measures of unhealthy eating were missing for n=2 of 59 not-NASH.
Each model is based on a logistic regression with NAFLD group (0=not-NAFLD, 1=NAFLD) as the dependent variable and eating behavior (0=absence, 1=presence) as the independent variable. Sex, race, age, and BMI were controlled in each model. Unstandardized betas (β), odds ratios (OR) with 95% confidence intervals (CI), and p-values are provided.
Each model is based on a logistic regression with NASH group (0=not-NASH, 1=NASH) as the dependent variable and eating behavior (0=absence, 1=presence) as the independent variable. Sex, race, age, and BMI were controlled in each model. Unstandardized betas (β), odds ratios (OR) with 95% confidence intervals (CI), and p-values are provided.
Missing for n=1 not-NAFLD
Missing for n=2 NAFLD and n=1 not-NAFLD
For nocturnal eating, an additional n=2 missing for not-NASH
Among participants with NAFLD, approximately 37% had borderline or definite NASH. Groups did not differ on any demographic variables or BMI. Groups were also not significantly different on use of medications that may affect liver health (NASH: n=10 of 35, 28.6%; not NASH: n=16 of 59, 27.1%; X2=0.02, p=0.88). Logistic regression analyses controlling for sex, BMI, race, and age revealed that frequent eating out was associated with higher odds of having borderline/definite NASH. Nocturnal eating was associated with lower odds of having borderline/definite NASH. (Table 2).
For the subsample who participated in the TeenView study (NAFLD: n=51; Not-NAFLD: n=47), separate logistic regressions were used to test associations between the presence of elevated internalizing symptoms and externalizing symptoms with the presence of NAFLD (Table 3). Demographics, BMI, number of UEB, and number of medical comorbidities were controlled. Notably, a greater number of medical comorbidities (NAFLD: M=1.55+0.82; Not-NAFLD: M=1.18+0.75) was significantly associated with higher odds of NAFLD in both models, while the index of UEB was not significant (NAFLD: M=2.92+1.68; Not-NAFLD: M=2.78+1.81). When examining psychopathology, internalizing symptoms (NAFLD: 41.2%; Not-NAFLD: 19.1%), but not externalizing symptoms (NAFLD:11.8%; Not-NAFLD:19.1%), were associated with higher odds of NAFLD.
Table 3.
Presurgical correlates of NAFLD in Teen-LABS/TeenView participants
| Modelsa | β | OR | 95% CI OR | p |
|---|---|---|---|---|
| Model 1 Internalizing | ||||
|
| ||||
| Sex (0=Female; 1=Male) | −0.14 | 0.87 | 0.20–3.82 | 0.86 |
| Race (0=White; 1=Other) | −0.32 | 0.73 | 0.45–1.17 | 0.19 |
| Age | 0.31 | 1.36 | 1.11–1.66 | 0.003 |
| Caregiver Education (0= more than High School education; 1= High School graduate or less) | 0.63 | 1.87 | 0.94–3.72 | 0.08 |
| BMI | 0.01 | 1.01 | 0.96–1.06 | 0.75 |
| Index of Medical Comorbidities | 0.68 | 1.98 | 1.57–2.50 | <0.001 |
| Index of Unhealthy Eating Behaviors | −0.11 | 0.90 | 0.75–1.07 | 0.23 |
| Internalizing Symptomatologyb | 1.42 | 4.12 | 1.92–8.85 | <0.001 |
|
| ||||
| Model 2 Externalizing | ||||
|
| ||||
| Sex (0=Female; 1=Male) | 0.06 | 1.06 | 0.23–4.82 | 0.94 |
| Race (0=White; 1=Other) | −0.32 | 0.73 | 0.46–1.16 | 0.18 |
| Age | 0.24 | 1.27 | 1.15–1.42 | <0.001 |
| Caregiver Education (0= more than High School education; 1= High School graduate or less) | 0.45 | 1.57 | 1.04–2.38 | 0.03 |
| BMI | 0.01 | 1.01 | 0.95–1.07 | 0.75 |
| Index of Medical Comorbidities | 0.59 | 1.81 | 1.50–2.19 | <0.001 |
| Index of Unhealthy Eating Behaviors | 0.01 | 1.02 | 0.87–1.18 | 0.85 |
| Externalizing Symptomatologyb | −0.29 | 0.75 | 0.55–1.02 | 0.06 |
Abbreviations: BMI: Body mass index; CI: Confidence Interval; OR: Odds ratio
Each model is a logistic regression with NAFLD group as the dependent variable.
Internalizing and Externalizing symptomatology on the Youth Self Report were dichotomized, with elevated symptomatology defined as exceeding the T score (>60) for borderline/clinical levels.
Discussion
Previous studies have demonstrated that NAFLD,1 UEB13 and psychopathology25 are common in adolescents with severe obesity who undergo MBS. The present study is the first to examine these factors together comparing adolescents with severe obesity who present with versus without biopsy-confirmed NAFLD in that clinical setting. Results identified commonalities and differences between groups. Prevalence estimates of UEB were high in both groups, suggesting that many adolescents with severe obesity who progress to MBS engage in dysregulated eating. However, significant differences in UEB were found among those with and without NAFLD (i.e., frequent eating out and BED symptomology) as well as those with and without borderline/definite NASH (i.e., frequent eating out and nocturnal eating). Additionally, differences in internalizing symptomology for those with NAFLD relative to those without were demonstrated. Taken together these findings suggest potential phenotypical differences between groups.
In general, these data suggest significant dysregulated eating occurs in adolescents with severe obesity and NAFLD. Approximately half of participants with NAFLD endorsed eating when not hungry, eating when full, and skipping meals, behaviors that may act as barriers to weight loss, and by association, the resolution of NAFLD. Endorsing BED symptomatology occurred significantly more often in individuals with NAFLD, supporting preliminary findings in the adult NAFLD literature, suggesting patients with NAFLD may have a higher prevalence of BED10. Indeed, BED symptomatology in participants with NAFLD (13%) was more than twice as high as participants without NAFLFD (4.9%) whose rates were similar to those found in a non-MBS sample with pediatric obesity (5.3%).26
Although individuals with NAFLD endorsed eating out less frequently than those without NAFLD, those individuals with borderline/definite NASH endorsed more frequent eating out than individuals with NAFL-not NASH. Diets that are high in saturated or trans fats and sugar have been linked to the development of NASH in animal models27 and in human cohorts,28 both of which are often found in fast food meals29. Eating out three or more times a week has been associated with elevated serum aminotransferase levels, markers of liver disease, particularly inflammation30 and our data suggests that it also may be indicative of more severe histological liver disease (i.e., borderline/definite NASH).
Finally, nocturnal eating occurred more often in individuals with NAFLD (29.1%) as opposed to those with NASH (14.3%), indicating that this behavior may be associated more with the accumulation of fat in the liver versus inflammation. One adult study found that meal frequency for individuals with NAFLD was shifted towards nighttime as compared to a control group32; however, no significant group differences were found. Taken together, these studies show a potential signal for the significance of nighttime eating within this population, which warrants further study.
Elevated levels of internalizing symptoms, but not externalizing symptoms, were also associated with NAFLD, even when controlling for the number of UEB and medical comorbidities. This is consistent with previous studies with small sample sizes, which showed a significant relationship between NAFLD and internalizing symptoms (i.e., group differences between average scores in the non-clinical range) in youth.15,16 Future studies with a larger sample size should explore mechanistic explanations for the link between internalizing symptoms and NAFLD, particularly in youth with severe obesity. While no significant group differences were found between amount of individuals with and without NAFLD who were using medications that impacted their liver health (including psychotropic medications), psychotropic medication usage has been associated with increased steatosis in at least one study.34 This is concerning as one in five to ten adolescents with internalizing disorders is estimated to use psychotropic medications. 35 In addition, internalizing symptomatology may be a barrier to needed lifestyle behavior change (e.g., by increasing UEB, decreasing physical activity), further perpetuating their disease process. 36–38
Taken together, it would be beneficial to screen adolescents with severe obesity and NAFLD for frequent eating out, nocturnal eating39, internalizing disorders40,41, and BED42 and refer for adjunctive care when indicated. Having a dietician integrated within the medical clinic would allow for real time guidance around eating behaviors for those who eat out frequently. Additionally, it is important to note that the mainstay of NAFLD treatment includes weight loss, often through encouraging adolescents to change the way they eat (i.e., dieting)43, a risk factor for BED. Likewise internalizing disorders have been associated with lower energy expenditure44 and increased sedentary behavior38 and may influence patient’s ability to engage in physical activity outside of the home. Given that clinic follow-up among youth with NAFLD who report internalizing symptoms can be poor15, when possible, having a mental health provider integrated into the clinic would allow for a warm hand off and timely intervention of evidence-based treatment45,46. Of note, each of these adolescents with severe obesity and NAFLD who completed measures about their UEB and psychopathology preoperatively eventually underwent MBS. Adolescents who undergo MBS often experience a change in UEB (i.e., loss of control eating)47 as well as NAFLD resolution48, yet maintain their psychopathology status25,48. Future studies should determine what role, if any, UEB and internalizing symptoms play in the rate of NAFLD resolution, as this will determine psychosocial targets for intervention post-operatively.
This study has several significant strengths, including the use of a large multicenter cohort with liver biopsies used to determine presence or absence of liver disease. However, results should also be viewed in the light of significant limitations. The Teen-LABS cohort, while large and well characterized, illustrates what are complex and historical health disparities in adolescent bariatric surgery care, with participants self-identifying as primarily White, non-Hispanic, and female.49 Thus, generalizability to clinical practice in demographic groups at very high risk of NAFLD, including Hispanic, indigenous or Native American, or Asian children is unknown. Replication should therefore occur in samples with more males as well as individuals of Asian, Indigenous and/or Hispanic origin50. Additionally, it would be important to determine if these findings also hold true or differ in individuals with NAFLD who do not have severe obesity to determine if they generalize to the broader population of adolescents with NAFLD.
In conclusion, patients with NAFLD present within a context of emotional and behavioral concerns that may be associated with their disease process, thus concomitant assessment and treatment of dietary and mental health concerns is warranted.
Supplementary Material
Table, Supplemental Digital Content 1: Operationalization of unhealthy eating behaviors and weight-related comorbidities
What is known on the subject:
Although diet is critical to the development of nonalcoholic fatty liver disease (NAFLD), an obesity-related comorbidity, limited evidence exists associating unhealthy eating behavior (UEB) with NAFLD in youth.
UEB and NAFLD have independently been associated with psychopathology symptoms, but the relationships among these variables have not been explored in youth presenting for bariatric surgery.
What this study adds:
Significant differences in were found among those with/without NAFLD (frequent eating out, binge eating disorder symptomology, internalizing symptomatology) as well as those with/without Nonalcoholic Steatohepatitis (frequent eating out and nocturnal eating).
These findings suggest potential phenotypical differences between groups.
Conflicts of Interest and Source of Funding:
The Teen-LABS consortium is funded by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), through grants UM1DK072493 (PI, Dr Thomas Inge, University of Colorado, Denver, CO), and UM1DK095710 (PI, Dr Changchun Xie, University of Cincinnati, OH). The TeenView ancillary was supported by grant R01DK080020 (PI: Zeller). Dr Xanthakos’s effort was supported by the NIDDK K23 grant DK080888 (PI S.Xanthakos). Dr. Ley’s effort, in part, was supported by an NIH post-doctoral training grant (T32 DK063929). The aforementioned funding sources played no role in study design, data collection, analysis and interpretation of data, report writing, or in the decision to submit the article for publication. For the remaining authors, no conflicts of interest or funding sources are declared. This manuscript was presented as a poster at the Society of Pediatric Psychology Annual Conference (March 2020; Dallas, TX).
References
- 1.Xanthakos SA, Jenkins TM, Kleiner DE, et al. High prevalence of nonalcoholic fatty liver disease in adolescents undergoing bariatric surgery. Gastroenterology. 2015;149(3):623–634.e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu EL, Golshan S, Harlow KE, et al. Prevalence of nonalcoholic fatty liver disease in children with obesity. J Pediatr 2019;207:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nobili V, Socha P. Pediatric nonalcoholic fatty liver disease: current thinking. J Pediatr Gastroenterol Nutr 2018;66(2):188–192.. [DOI] [PubMed] [Google Scholar]
- 4.Mencin AA, Loomba R, Lavine JE. Caring for children with NAFLD and navigating their care into adulthood. Nat Rev Gastroentrol Helatol 2015;12(11):617–628. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. [DOI] [PubMed] [Google Scholar]
- 6.Newby PK. Are dietary intakes and eating behaviors related to childhood obesity? A comprehensive review of the evidence. J Law Med Ethics. 2007;35(1):35–60. [DOI] [PubMed] [Google Scholar]
- 7.Leon-Munoz LM, Garcia-Esquinas E, Soler-Vila H et al. Unhealthy eating behaviors and weight gain: A prospective study in young and middle-age adults. Obesity. 2016;24(5):1178–1184. [DOI] [PubMed] [Google Scholar]
- 8.Thivel D, Aucouturier J, Isacco L, et al. Are eating habits associated with physical fitness in primary school children? Eat Behav 2013;14(1):83–86. [DOI] [PubMed] [Google Scholar]
- 9.Mesas AE, Guallar-Castillon P, Leon-Munoz LM, et al. Obesity-related eating behaviors are associated with low physical activity and poor diet quality in Spain. J Nutr 2012;142(7):1321–1328. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Abbasi O, Malevanchik L, et al. Pilot study of the prevalence of binge eating disorder in non-alcoholic fatty liver disease patients. Ann Gastroenterol 2017;30(6):664–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Ko BJ, Gong Y, et al. Self-reported eating speed in relation to non-alcoholic fatty liver disease in adults. Eur J Nutr 2016;55(1):327–333. [DOI] [PubMed] [Google Scholar]
- 12.Gibson PS, Lang S, Gilbert M, et al. Assessment of diet and physical activity in paediatric non-alcoholic fatty liver disease patients: a United Kingdom case control study. Nutrients. 2015;7(12):9721–9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utzinger LM, Gowey MA, Zeller M, et al. Loss of control eating and eating disorders in adolescents before bariatric surgery. Int J Eat Disorder 2016;49(10):947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou F, Xu S, Zhao Y, et al. Effects of emotional symptoms and life stress on eating behaviors among adolescents. Appetite. 2013;68:63–68. [DOI] [PubMed] [Google Scholar]
- 15.Kerkar N, D’Urso C, Van Nostrand K, et al. Psychosocial outcomes for children with nonalcoholic fatty liver disease over time and compared with obese controls. J Pediatr Gastroenterol Nutr 2013;56(1):77–82. [DOI] [PubMed] [Google Scholar]
- 16.Mazzone L, Postorino V, De Peppo L, et al. Paediatric non-alcoholic fatty liver disease: impact on patients and mothers’ quality of life. Hepat Mon 2013;13(3):e7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med 2016;374(2):113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becnel JN, Zeller MH, Noll JG, et al. Romantic, sexual, and sexual risk behaviours of adolescent females with severe obesity. Pediatr Obes 2016; 12 (5) 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nangle DW, Johnson WG, Carr-Nangle RE, Engler LB. Binge eating disorder and the proposed DSM-IV criteria: psychometric analysis of the Questionnaire of Eating and Weight Patterns. Int J Eat Disorder. 1994;16(2):147–157. [DOI] [PubMed] [Google Scholar]
- 20.Jeffery RW, French SA. Preventing weight gain in adults: the pound of prevention study. Am J Public Health. 1999;89(5):747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French SA, Jeffery RW, Murray D. Is dieting good for you?: Prevalence, duration and associated weight and behaviour changes for specific weight loss strategies over four years in US adults. Int J Obes 1999;23(3):320–327. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell JE, King WC, Courcoulas A, et al. Eating behavior and eating disorders in adults before bariatric surgery. Int J Eat Disord 2015;48(2):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allison KC, Lundgren JD, O’Reardon JP, et al. The Night Eating Questionnaire (NEQ): psychometric properties of a measure of severity of the Night Eating Syndrome. Eat Behav 2008;9(1):62–72. [DOI] [PubMed] [Google Scholar]
- 24.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 25.Hunsaker SL, Garland BH, Rofey D, et al. A multisite 2-year follow up of psychopathology prevalence, predictors, and correlates among adolescents who did or did not undergo weight loss surgery. J Adolesc Health. 2018;63(2):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan CM, Yanovski SZ, Nguyen TT, et al. Loss of control over eating, adiposity, and psychopathology in overweight children. Int J Eat Disorder. 2002;31(4):430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohli R, Kirby M, Xanthakos SA, et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52(3):934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mager DR, Patterson C, So S, et al. Dietary and physical activity patterns in children with fatty liver. Eur J Clin Nutr 2010;64(6):628–635. [DOI] [PubMed] [Google Scholar]
- 29.Powell LM, Nguyen BT. Fast-food and full-service restaurant consumption among children and adolescents: effect on energy, beverage, and nutrient intake. JAMA Pediatr 2013;167(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee EY, Choi HY, Cho H, et al. Health behavior associated with liver enzymes among obese Korean adolescents, 2009–2014. PLoS One. 2018;13(1):e0190535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boutelle KN, Fulkerson JA, Neumark-Sztainer D, et al. Fast food for family meals: relationships with parent and adolescent food intake, home food availability and weight status. Public Health Nutr 2007;10(1):16–23. [DOI] [PubMed] [Google Scholar]
- 32.Bernsmeier C, Weisskopf DM, Pflueger MO, et al. Sleep disruption and daytime sleepiness correlating with disease severity and insulin resistance in non-alcoholic fatty liver disease: a comparison with healthy controls. PLoS One. 2015;10(11):e0143293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kass AE, Accurso EC, Goldschmidt AB, et al. Picking and nibbling in children and adolescents with eating disorders. Int J Eat Disorder. 2015;48(8):1102–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouzaki M, Yodoshi T, Arce-Clachar AC, et al. Psychotropic medications are associated with increased liver disease severity in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 2019;69(3):339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merikangas KR, He JP, Rapoport J et al. Medication use in US youth with mental disorders. JAMA Pediatr 2013;167(2):141–148. [DOI] [PubMed] [Google Scholar]
- 36.Gray WN, Janicke DM, Ingerski LM, et al. The impact of peer victimization, parent distress and child depression on barrier formation and physical activity in overweight youth. J Dev Behav Pediatr 2008;29(1):26–33. [DOI] [PubMed] [Google Scholar]
- 37.Mauro M, Taylor V, Wharton S, et al. Barriers to obesity treatment. Eur J Intern Med 2008;19(3):173–180. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Bastian K, Ohinmaa A et al. Influence of physical activity, sedentary behavior, and diet quality in childhood on the incidence of internalizing and externalizing disorders during adolescence: a population-based cohort study. Ann Epidemiol 2018;28(2):86–94. [DOI] [PubMed] [Google Scholar]
- 39.Gallant AR, Lundgren J, Allison K, et al. Validity of the night eating questionnaire in children. Ing J Eat Disord 2012;45(7):861–865. [DOI] [PubMed] [Google Scholar]
- 40.Richardson LP, McCauley E, Grossman DC, et al. Evaluation of the patient health questionnaire-9 Item for detecting major depression among adolescents. Pediatrics. 2010;126(6):1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mossman SA, Luft MJ, Schroeder HK, et al. The Generalized Anxiety Disorder 7-item scale in adolescents with generalized anxiety disorder: Signal detection and validation. Ann Clin Psychiatry. 2017;29(4):227–234a. [PMC free article] [PubMed] [Google Scholar]
- 42.Yanovski SZ, Marcus MD, Wadden TA, et al. The questionnaire on eating and weight patterns-5 (QEWP-5): An updated screening instrument for binge eating disorder. Int J Eat Disord 2015;48(3):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West CE, Goldschmidt AB, Mason SM et al. Differences in risk factors for binge eating by socioeconomic status in a community-based sample of adolescents: Findings from Project EAT. Int J Eat Disord 2019;52(6):659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gosmann NP, Salum GA, Schuch F, et al. Association between internalizing disorders and day-to-day activities of low energetic expenditure. Child Psychiatry Hum Dev 2015;46(1):67–74. [DOI] [PubMed] [Google Scholar]
- 45.Melnyk BM, Small L, Morrison-Beedy D, et al. Mental health correlates of healthy lifestyle attitudes, beliefs, choices, and behaviors in overweight adolescents. J Pediatri Health Care. 2006;20(6):401–406. [DOI] [PubMed] [Google Scholar]
- 46.Agras WS, Apple RF. Overcoming your eating disorder: A cognitive-behavioral therapy approach for bulimia nervosa and binge eating disorder. Oxford University Press; 2008. [Google Scholar]
- 47.Goldschmidt AB, Khoury J, Jenkins TM, et al. Adolescent loss-of-control eating and weight loss maintenance after bariatric surgery Pediatrics. 2018;141(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pratt JSA, Browne A, Browne NT, et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg Obes Relat Dis 2018;14(7):882–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunez Lopez O, Jupiter DC, Bohanon FJ, et al. Health disparities in adolescent bariatric surgery: nationwide outcomes and utilization. J Adolesc Health. 2017; 61(5):649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwimmer JB, Deutsch R, Kahen T et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table, Supplemental Digital Content 1: Operationalization of unhealthy eating behaviors and weight-related comorbidities