Abstract
Objective:
To evaluate the odds of a behavioral health diagnosis among youth with a difference of sex development (DSD) or congenital adrenal hyperplasia (CAH) compared with matched controls in the PEDSnet database.
Study design:
All youth with a diagnosis of DSD (n=1,216) or CAH (n=1,647) and at least one outpatient encounter were extracted from the PEDSnet database and propensity-score matched on 8 variables (1:4) to controls (n=4,864 and 6,588, respectively) using multivariable logistic regression. The likelihood of having behavioral health diagnoses was examined using generalized estimating equations.
Results:
Youth with a DSD had higher odds of a behavioral health diagnosis (OR: 1.7 [95% CI: 1.4, 2.1], p<0.0001) and neurodevelopmental diagnosis (1.7 [95% CI: 1.4, 2.0], P < .0001 compared with matched controls. Youth with CAH did not have increased odds of a behavioral health diagnosis (1.0 [95% CI: 0.9, 1.1], p=0.9) compared with matched controls but did have higher odds of developmental delay (1.8 [95% CI: 1.4, 2.4], p<0.0001).
Conclusions:
Youth with a DSD diagnosis have higher odds of a behavioral health or neurodevelopmental diagnosis compared with matched controls. Youth with CAH have a higher odds of developmental delay, highlighting the need for screening in both groups.
Keywords: congenital adrenal hyperplasia, disorder of sex development, difference of sex development, psychiatric, neurodevelopmental, depression, anxiety, developmental delay, intellectual disability
Introduction
Individuals with a difference of sex development (DSD) or congenital adrenal hyperplasia (CAH) may be at increased risk of behavioral health comorbidities. We separated these two diagnoses because we included males with a diagnosis of CAH (who would not be considered to have a DSD) and CAH has been shown to be associated with a higher risk of neurodevelopmental conditions.1-3 Furthermore, other studies have shown that families affected by CAH do not identify with the term DSD.4 Individuals with DSD are at increased risk of having central nervous system conditions or learning difficulties.5, 6 adults with a DSD have a high prevalence of psychiatric disorders and suicide attempts.7, 8 Individuals with CAH, particularly those with the salt-wasting form, have been found to have a lower intelligence quotient (IQ) than controls or unaffected siblings.1-3 women and men with CAH have higher odds of psychiatric disorders compared with controls.9, 10 Furthermore, there are known sex differences in the prevalence of behavioral health conditions in the general population,11-13 and sex chromosomes and sex steroids play important roles in brain development and differentiation.14-16
There are limited data on the behavioral health outcomes of youth with DSD or CAH receiving care at large pediatric health centers in the United States (U.S). We aim to fill this gap by utilizing a large pediatric database, to evaluate the odds of having any behavioral health diagnosis (primary outcome) and specific behavioral health diagnoses (secondary outcomes) among youth with a DSD or CAH compared with matched controls.
Methods
Patients
Data for this analysis were obtained from PEDSnet (https://pedsnet.org/), a pediatric Learning Health System and a clinical research network in PCORnet, a national, patient-centered resource, with a Common Data Model. Six pediatric health systems participated in this PEDSnet dataset, collectively including over 6 million children: Children’s Hospital Colorado, Children’s Hospital of Philadelphia, Nemours Children’s Health System (locations in Florida and Delaware), Nationwide Children’s Hospital, St. Louis Children’s Hospital, and Seattle Children’s Hospital. Clinical data are available from the electronic health record (EHR) of these health systems from 2009 onward for patients with an in-person encounter with a provider. All youth (any age) with a diagnosis of a DSD or CAH (by PEDSnet concept ID, Table 4 [available at www.jpeds.com]) includes codes extracted from the EHR problem list or diagnosis code from any encounter) and at least one outpatient visit from 2009-2019 were extracted from the PEDSnet database in November 2019. We chose one outpatient visit as a criterion for cases and controls to not oversample from those who were only seen in the health systems for urgent/emergent care. Although individuals with sex chromosome aneuploidies are classified as having a DSD by the 2006 guidelines,17 we did not include them here and performed separate analyses for those with Turner Syndrome and Klinefelter Syndrome (data not shown). We did include those with a diagnosis of mixed gonadal dysgenesis (45,X/46,XY). We excluded diagnosis codes for late-onset or non-classic CAH but based on the vagueness of some of the diagnosis codes, cannot be certain exactly which types of CAH were included. A random sample of 197,042 patients with at least one outpatient visit who did not have a diagnosis of CAH or other DSD were used as a pool of controls. To ensure these controls were representative of the general PEDSnet population, we evaluated the prevalence of well characterized pediatric diagnoses (asthma, type 1 diabetes, and acute lymphoid leukemia) to ensure the prevalence in the controls was similar to PEDSnet as a whole.
Table 4.
SNOMED codes and linked PEDSnet concept IDs
| SNOMED Name | PEDSnet Concept ID |
|---|---|
| Difference of Sex Development (DSD) | |
| 46,XX disorder of sex development with anorectal anomalies syndrome | 37116741 |
| 46,XX disorder of sex development with skeletal anomalies syndrome | 37116740 |
| 46, XX true hermaphrodite | 4004776 |
| Ambiguous genitalia | 4062097 |
| Androgen receptor absent | 4035130 |
| Androgen resistance - infertile male | 4127074 |
| Androgen resistance syndrome | 440359 |
| Chimera 46, XX; 46, XY | 4007565 |
| Chondrodysplasia with disorder of sex development syndrome | 36715303 |
| Complete androgen insensitivity syndrome | 45770921 |
| Complete testicular feminization syndrome | 4176428 |
| Disorder of androgen receptor | 4322687 |
| Disorder of sex development with intellectual disability syndrome | 36714286 |
| Dysmorphism, short stature, deafness, disorder of sex development syndrome | 37118645 |
| Female pseudohermaphroditism | 4338827 |
| Gynandromorphism syndrome | 4048536 |
| Hermaphroditism | 4028941 |
| History of intersex surgery | 4323227 |
| Incomplete testicular feminization syndrome | 442636 |
| Indeterminate sex | 46270485 |
| Indeterminate sex and pseudohermaphroditism | 73584 |
| Intersex | 46273637 |
| Male pseudohermaphroditism | 4009631 |
| Male pseudohermaphroditism due to 5-alpha-reductase deficiency | 42538057 |
| Mixed gonadal dysgenesis | 4308443 |
| Meacham syndrome | 36716451 |
| Mosaic XO/XY | 4005274 |
| Ovotestis | 4077758 |
| Partial androgen insensitivity syndrome | 45757367 |
| Pseudohermaphroditism | 4326589 |
| Pure gonadal dysgenesis | 4316871 |
| Pure gonadal dysgenesis 46,XX | 4317840 |
| Pure gonadal dysgenesis 46,XY | 4317951 |
| Receptor-positive androgen resistance syndrome | 4289899 |
| Reifenstein syndrome | 4242435 |
| Testicular feminization | 436379 |
| Testosterone 17-beta-dehydrogenase deficiency | 4174657 |
| XX males | 4251774 |
| XY females | 4219609 |
| XY, female phenotype | 4004649 |
| XY type gonadal dysgenesis with associated anomalies syndrome | 37116728 |
| Congenital Adrenal Hyperplasia (CAH) | |
| 3 beta-Hydroxysteroid dehydrogenase deficiency | 4182535 |
| 17 alpha-Hydroxyprogesterone aldolase deficiency | 4169253 |
| Adrenogenital disorder | 196369 |
| Adrenal virilism | 4156662 |
| CAH - desmolase deficiency | 4028928 |
| Classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency | 37397203 |
| Cholesterol monooxygenase (side-chain cleaving) deficiency | 4195771 |
| Congenital adrenal hyperplasia | 4029573 |
| Congenital adrenal hyperplasia due to cytochrome P450 oxidoreductase deficiency | 37396060 |
| Congenital lipoid adrenal hyperplasia due to STAR deficiency | 37397202 |
| Deficiency of steroid 11-beta-monooxygenase | 4051374 |
| Deficiency of steroid 17-alpha-monooxygenase | 4050764 |
| Feminization-adrenogenital syndrome | 4028925 |
| Feminizing syndrome of adrenal origin | 4150530 |
| Pseudohermaphrodite, female with adrenocortical disorder | 4035119 |
| Pseudohermaphrodite, male with adrenocortical disorder | 4035118 |
| Salt-losing congenital adrenal hyperplasia | 4029574 |
| Salt-losing congenital adrenal hyperplasia with virilism | 45757615 |
| Steroid 21-monooxygenase deficiency, salt wasting type | 4324258 |
| Virilization-adrenogenital syndrome | 4028926 |
| Virilizing syndrome of adrenal origin | 4066281 |
Outcomes
Composite diagnoses were created for behavioral health diagnoses based on SNOMED clinical terms concept terminology (medical term codes). SNOMED codes are used in U.S. Federal Government systems for exchange of electronic clinical health information (www.snomed.org). PEDSnet concept IDs were mapped to Athena Ancestor Tables (developed by Observational Health Data Sciences and Informatics, Columbia University). Athena is the application that contains standardized vocabularies used in PEDSnet. In addition to an overall neurodevelopmental composite, we created additional composites for developmental delay, feeding delay, learning disorders, motor delay, and speech and language disorders (Table 5; available at www.jpeds.com). Similarly, a self-harm composite comprised of all codes reflective of self-injurious behavior and suicidality was made. If n was <10 in any cell, that cell was omitted. Controls did not have a diagnosis of gender dysphoria as this group was also used as a control group for a related study evaluating mental health in individuals with gender dysphoria. Therefore, the prevalence of gender dysphoria is presented in the results, but no odds ratios were generated, as the controls did not have gender dysphoria.
Table 5.
SNOMED codes for outcomes
| SNOMED code | |
|---|---|
| Behavioral Health Diagnoses | 74732009, 700364009, 229729009, 231543005, 268734000, 29164008, 27172100, 248290002, (exclude 123526007, 129104009 & 66936004) |
| Adjustment disorder | 17226007 |
| Anxiety disorder | 197480006 |
| Obsessive compulsive disorder | 191736004 |
| Panic disorder | 371631005 |
| Post-traumatic stress disorder | 47505003 |
| Conduct disorder* | 430909002 |
| Eating disorder | 72366004 (exclude 192016008, 426881004) |
| Impulse control disorder | 66347000 |
| Mood disorder | 46206005 |
| Bipolar disorder | 13746004 |
| Depressive disorder | 35489007 |
| Neurosis | 111475002 |
| Personality disorder | 33449004 |
| Psychotic disorder | 69322001 |
| Tic disorder | 568005 |
| Self-Harm Composite (not a subcategory of psychiatric disorders) | 248062006, 6471006, 82313006, 77434001, 276853009 |
| Neurodevelopmental Diagnoses | 700364009, 229729009, 231543005, 268734000, 29164008, 27172100, 248290002, (exclude 123526007) |
| Attention deficit hyperactivity disorder | 406506008 |
| Autism spectrum disorder | 408856003 |
| Developmental delay composite | 442059001, 716710007, 609225004, 248290002 (exclude 307653008, 123526007, 703477003, 430099007) |
| Feeding delay composite | 78164000, 426881004 |
| Intellectual disability | 110359009 |
| Learning disorder composite | 1855002, 59770006, 192138007 |
| Motor delay composite | 268674003, 4949009, 302289002 |
| Speech/language disorder composite | 229729009, 231543005, 268734000, 29164008, 271721006 |
in SNOMED, conduct disorder is classified as both a mental disorder and a neurodevelopmental disorder. Psychiatric diagnoses are termed “mental disorder” in SNOMED.
Statistical analysis
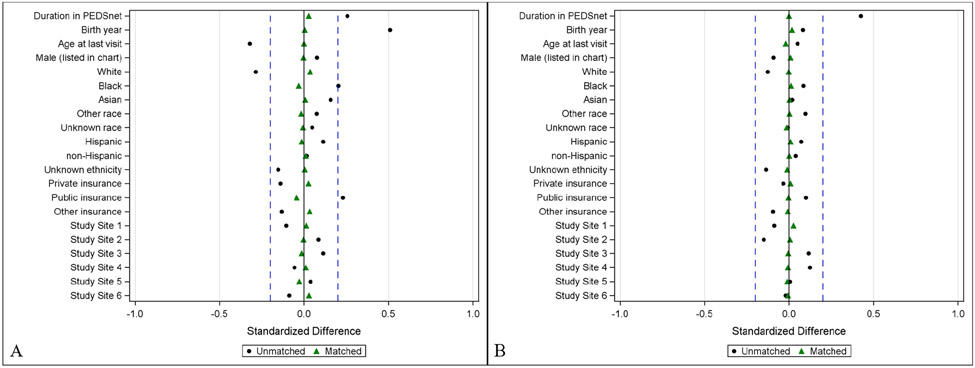
Propensity scores were generated for cases (individuals with a DSD or CAH code) via multivariable logistic regression and used to match every 1 case to 4 controls.18 The propensity score was defined as the probability of having DSD or CAH given the following youth characteristics: sex listed in chart, year of birth, age at last medical visit, PEDSnet site, race, ethnicity, payer status (public/private/none), and duration in the PEDSnet database (time between first and last encounter). Cases and controls were matched on the predicted probability of having the diagnosis (DSD or CAH) using a greedy match algorithm and a caliper of width 0.10.19 The balance of covariates between the cases and control groups (ie, the similarity of the covariate distributions) was evaluated as a reduction in standardized mean difference, using a decision criterion of <0.20 to indicate that a covariate was balanced (Figure 1; available at www.jpeds.com).20 Variable missingness was handled by the widely accepted approach of including missing as another category for the variable.21
Figure 1: Standardized differences in population baseline characteristics in youth with a difference of sex development (A) or congenital adrenal hyperplasia (B) vs. controls before (dots) and after (triangles) matching.
Dotted lines are at 0.2.
Differences in diagnoses of interest between cases and controls were examined using generalized estimating equations (GEE), which accounted for potential correlation between the cases and matched controls.22 Sex listed in the chart was included as interaction term in the regression model to evaluate whether the associations between diagnoses and case/control status differed by sex (a p-value cutoff of <0.05 was used for interaction terms). Descriptive statistics including the prevalence of outcomes in the cases versus control groups, odds ratios and 95% confidence intervals, were computed. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). A conservative p-value of <0.0025 was considered significant given the multiple comparisons.
Results
Demographics are in Table I for those with a DSD (n=1,216) or CAH (n=1,647) and their matched controls (n=4,864 and n=6,588, respectively).
Table 1.
Demographics
| DSD n=1,216 |
Controls n=4,864 |
CAH n=1,647 |
Controls n=6,588 |
|
|---|---|---|---|---|
| Sex listed in chart | ||||
| Female | 655 (53.9) | 2,613 (53.7) | 1,025 (62.2) | 4,124 (62.6) |
| Male | 561 (46.1) | 2,251 (46.3) | 622 (37.8) | 2,464 (37.4) |
| Race | ||||
| White | 656 (53.9) | 2,539 (52.2) | 1,017 (61.7) | 4,074 (61.8) |
| Black | 186 (15.3) | 798 (16.4) | 186 (11.3) | 720 (10.9) |
| Asian | 77 (6.3) | 300 (6.2) | 55 (3.3) | 218 (3.3) |
| Other | 175 (14.4) | 729 (15.0) | 250 (15.2) | 995 (15.1) |
| Unknown | 122 (10.0) | 498 (10.2) | 139 (8.4) | 581 (8.8) |
| Ethnicity | ||||
| Non-Hispanic | 939 (77.2) | 3,736 (76.8) | 1,288 (78.2) | 5,151 (78.2) |
| Hispanic | 188 (15.5) | 777 (16.0) | 231 (14.0) | 907 (13.8) |
| Unknown | 89 (7.3) | 351 (7.2) | 128 (7.8) | 530 (8.0) |
| Insurance type | ||||
| Private | 564 (46.4) | 2,196 (45.1) | 850 (51.6) | 3,374 (51.2) |
| Public | 539 (44.3) | 2,261 (46.5) | 626 (38.0) | 2,513 (38.1) |
| Other | 92 (7.6) | 282 (5.8) | 142 (8.6) | 461 (7.0) |
| Unknown | 21 (1.7) | 125 (2.6) | 29 (1.8) | 240 (3.6) |
| Age at first visit (years) | 0.2 (0.0, 4.3) | 1.0 (0.1, 5.0) | 2.9 (0.1, 8.0) | 3.0 (0.4, 8.2) |
| Age at last visit (years) | 8.8 (3.9, 15.4) | 10.1 (4.2, 15.3) | 12.9 (7.3, 17.6) | 13.6 (8.3, 16.7) |
| Duration in PEDSnet (years) | 5.3 (2.2, 10.2) | 5.4 (1.5, 10.7) | 7.1 (2.7, 11.4) | 7.2 (2.0, 12.2) |
| Saw a provider who could make a behavioral health diagnosis | 452 (37) | 1,073 (22) | 429 (26) | 1,508 (23) |
| Age at first diagnosis (years) | 1.0 (0.0, 9.0) | --- | 6.0 (0.0, 11.0) | --- |
| Age at last diagnosis (years) | 6.0 (1.0, 14.0) | --- | 10.0 (5.0, 15.0) | --- |
Data are shown as n (%), mean ± standard deviation or median (25-75th %ile). Age at first visit refers to the age at the first visit in PEDSnet (not necessarily a visit related to DSD/CAH). Age at first diagnosis refers to the first diagnosis of DSD or CAH in the chart.
A behavioral health diagnosis was listed in the EHR for 382 individuals with a DSD (31.4%), which includes all mental health and neurodevelopmental conditions. Individuals in PEDSnet with a DSD had higher odds of having any behavioral health diagnosis (OR 1.7 [95% CI: 1.4, 2.1], p<0.0001) and higher odds of any neurodevelopmental diagnosis compared with matched controls (1.7 [95% CI: 1.4, 2.0], p<0.0001, Table 2 and Figure 2, A). Individuals with a DSD had higher odds of developmental delay, feeding delay, intellectual disability, motor delay, and a speech language disorder than matched controls. Thirteen (1.1%) individuals with a DSD had a diagnosis of gender dysphoria. There were significant interactions by sex listed in the chart aside for some of the secondary outcomes including depressive disorder (p=0.04), feeding delay (p=0.03) and intellectual disability (p=0.03). Neither males nor females with DSD had a significantly higher odds of depressive disorder than their respective controls. Males with DSD had higher odds of intellectual disability compared with males without DSD (3.9 [95% CI: 2.2, 7.1], p<0.0001); but females with DSD did not (1.4 [95% CI: 0.7, 2.9], p=0.4). Males with DSD had higher odds of feeding delay compared with males without DSD (4.4 [95% CI: 3.2, 6.1], p<0.0001); females also had higher odds of feeding delay than females without DSD (2.6 [95% CI: 1.7, 3.8], p<0.0001).
Table 2.
Prevalence and odd ratios of behavioral health diagnoses in those with DSD vs. matched controls
| DSD n=1,216 (%) |
Control n=4,864 (%) |
OR (95% CI) | p-value | |
|---|---|---|---|---|
| Behavioral Health Diagnoses * | 382 (31.4) | 1,130 (23.2) | 1.7 (1.4, 2.1) | <0.0001 |
| Adjustment disorder | 20 (1.6) | 76 (1.6) | 1.1 (0.6, 1.7) | 0.8 |
| Anxiety disorder | 68 (5.6) | 263 (5.4) | 1.0 (0.8, 1.4) | 0.8 |
| Conduct disorder | 28 (2.3) | 89 (1.8) | 1.3 (0.8, 2.0) | 0.3 |
| Eating disorder | 15 (1.2) | 50 (1.0) | 1.2 (0.7, 2.2) | 0.5 |
| Mood disorder | 50 (4.1) | 220 (4.5) | 0.9 (0.7, 1.3) | 0.5 |
| Depressive disorder | 40 (3.3) | 170 (3.5) | 0.9 (0.7, 1.3) | 0.7 |
| Neurodevelopmental Diagnoses | 288 (23.7) | 763 (15.7) | 1.7 (1.4, 2.0) | <0.0001 |
| Attention deficit hyperactivity disorder | 64 (5.3) | 261 (4.9) | 1.0 (0.7, 1.3) | 0.9 |
| Autism spectrum disorder | 27 (2.2) | 92 (1.9) | 1.2 (0.8, 1.8) | 0.5 |
| Developmental delay composite | 132 (10.9) | 183 (3.8) | 3.1 (2.5, 3.9) | <0.0001 |
| Feeding delay composite | 130 (10.7) | 160 (3.3) | 3.5 (2.8, 4.5) | <0.0001 |
| Intellectual disability | 32 (2.6) | 52 (1.1) | 2.5 (1.6, 3.9) | <0.0001 |
| Learning disorder composite | 22 (1.8) | 68 (1.4) | 1.3 (0.8, 2.1) | 0.3 |
| Motor delay composite | 116 (9.5) | 333 (6.8) | 1.4 (1.1, 1.8) | 0.002 |
| Speech/language disorder composite | 151 (12.4) | 375 (7.7) | 1.7 (1.4, 2.1) | <0.0001 |
Data are shown as n (%), mean ± standard deviation or median (25-75th %ile).
Behavioral health diagnoses include a composite of all outcomes including neurodevelopmental outcomes.
Figure 2: Odds of behavioral health diagnoses among youth with a difference of sex development (DSD) or congenital adrenal hyperplasia (CAH) compared to matched controls.
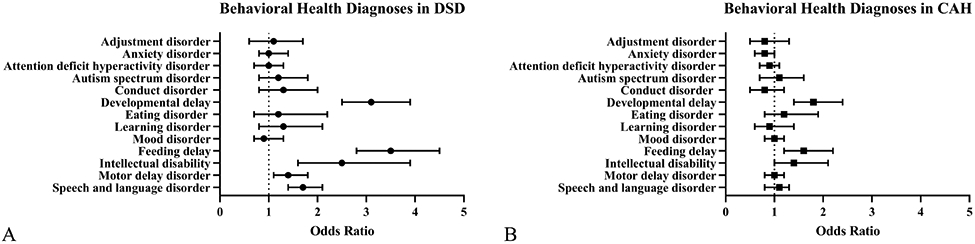
The forest plots show the odds ratios and 95% confidence intervals of behavioral health diagnoses in DSD (A) and CAH (B) compared to controls. Higher odds ratios (>1) indicate that youth with DSD or CAH are more likely to have the listed diagnosis. Lower odds ratios (<1) indicate that youth with DSD or CAH are less likely to have the listed diagnosis. Note that developmental delay, feeding delay, learning disorder, motor delay disorder and speech and language disorder are composite scores.
A behavioral health diagnosis was listed in the EHR for 388 individuals with CAH (23.6%). Individuals in PEDSnet with CAH did not have higher odds of having a behavioral health diagnosis (1.0 [95% CI: 0.9, 1.1], p=0.9) compared with controls. However, individuals with a CAH had higher odds of developmental delay (1.8 [95% CI: 1.4, 2.4], p<0.0001) and feeding delay (1.6 [95% CI: 1.2, 2.2], p=0.002, Table 3 and Figure 2, B). Eleven (0.7%) individuals with CAH had a diagnosis of gender dysphoria. There was a significant interaction by sex for anxiety (p=0.03). Females with CAH had lower odds of anxiety disorder compared with females without CAH (0.7 [95% CI: 0.5, 0.9], p=0.007); but males did not (1.2 [95% CI: 0.8, 1.7], p=0.5).
Table 3.
Prevalence and odd ratios of behavioral health diagnoses in those with CAH vs. matched controls
| CAH n=1,647 (%) |
Control n=6,588 (%) |
OR (95% CI) | p-value | |
|---|---|---|---|---|
| Behavioral Health Diagnoses * | 388 (23.6) | 1,561 (23.7) | 1.0 (0.9, 1.1) | 0.9 |
| Adjustment disorder | 24 (1.5) | 119 (1.8) | 0.8 (0.5, 1.3) | 0.3 |
| Anxiety disorder | 84 (5.1) | 414 (6.3) | 0.8 (0.6, 1.0) | 0.07 |
| Conduct disorder | 28 (1.7) | 137 (2.1) | 0.8 (0.5, 1.2) | 0.3 |
| Eating disorder | 24 (1.5) | 80 (1.2) | 1.2 (0.8, 1.9) | 0.4 |
| Mood disorder | 93 (5.6) | 383 (5.8) | 1.0 (0.8, 1.2) | 0.8 |
| Depressive disorder | 73 (4.4) | 325 (4.9) | 0.9 (0.7, 1.2) | 0.4 |
| Bipolar disorder | 13 (0.8) | 46 (0.7) | 1.1 (0.6 2.1) | 0.7 |
| Tic disorder | 15 (0.9) | 44 (0.9) | 1.4 (0.8, 2.5) | 0.3 |
| Self-Harm composite | 16 (1.0) | 137 (2.1) | 0.5 (0.3, 0.8) | 0.004 |
| Neurodevelopmental Diagnoses | 240 (14.6) | 970 (14.7) | 1.0 (0.9, 1.2) | 0.9 |
| Attention deficit hyperactivity disorder |
98 (6.0) | 429 (6.5) | 0.9 (0.7, 1.1) | 0.4 |
| Autism spectrum disorder | 31 (1.9) | 117 (1.8) | 1.1 (0.7, 1.6) | 0.8 |
| Developmental delay composite | 91 (5.5) | 203 (3.1) | 1.8 (1.4, 2.4) | <0.0001 |
| Feeding delay composite | 55 (3.3) | 139 (2.1) | 1.6 (1.2, 2.2) | 0.002 |
| Intellectual disability | 34 (2.1) | 97 (1.5) | 1.4 (1.0, 2.1) | 0.09 |
| Learning disorder composite | 26 (1.6) | 111 (1.7) | 0.9 (0.6, 1.4) | 0.8 |
| Motor delay composite | 121 (7.3) | 493 (7.5) | 1.0 (0.8, 1.2) | 0.9 |
| Speech/language disorder composite |
108 (6.6) | 414 (6.3) | 1.1 (0.8, 1.3) | 0.7 |
Data are shown as n (%), mean ± standard deviation or median (25-75th %ile).
Behavioral health diagnoses include a composite of all outcomes including neurodevelopmental outcomes.
Discussion
In this large EHR-based study, youth with a DSD in the PEDSnet database had higher odds of having a behavioral health diagnosis, particularly neurodevelopmental diagnoses compared with matched controls. Those with CAH had higher odds of developmental delay but did not have higher odds of an overall behavioral health diagnosis.
Although much attention is given to the initial gender assignment23, 24 and controversies about surgical intervention in patients with DSD and CAH,25-29 there is less attention given to neurodevelopment. Individuals with DSD may be at increased risk for neurodevelopmental conditions, likely primarily related to the underlying genetic cause of the DSD. Some DSD conditions are isolated to development of the genital or reproductive organs, but others may be a result of a large gene deletion or duplication, sex chromosome aneuploidies (as in 45,X/45,XY mosaicism causing mixed gonadal dysgenesis), or from mutations in a gene that affects multiple developmental pathways.30 In the European I-DSD Registry, about a quarter of cases had an additional associated condition; over 10 times the prevalence of congenital anomalies at birth in the general population.5, 31 The Deciphering Developmental Disorders study in the UK found that DSD phenotypes (including cryptorchidism and hypospadias) occur in 5% of patients with learning difficulties,6 and among the 603 children with a DSD, 61% had at least one neurodevelopmental delay diagnosis, a prevalence much higher than what we report here.6 However, their cohort was enriched for individuals with intellectual disability or developmental delay. An improved understanding of the risk of neurodevelopmental conditions, along with a specific molecular diagnosis, when possible, will improve counseling and screening for patients and families. Specific considerations are warranted for medical and surgical management, and future decision-making capacity should be evaluated in children with a DSD and a neurodevelopmental condition. These children, in particular, would be best served in a multidisciplinary clinic or care center where psychosocial support, genetic counseling and testing, and neurodevelopmental screening and assessment are available on an ongoing basis.
Patients in PEDSnet with CAH had higher odds of developmental delay and feeding delay, but not intellectual disability or a neurodevelopmental diagnosis overall. Others have shown that individuals with CAH, particularly those with the salt-wasting form, have a lower IQ than controls or unaffected siblings.1-3 Risk factors for low IQ in CAH include higher glucocorticoid dose, higher 17-hydroxyprogesterone concentrations, and higher number of hyponatremic episodes,2 which may indicate more severe disease. Those who have poorly controlled disease have lower IQ and higher risk of cognitive deficits compared with well-controlled patients.2 Others have shown that those with the more severe salt-wasting form have lower IQs than those with simple virilizing form,1-3 and those with adrenal crises or abnormal electrolytes in neonatal life may be particularly at risk for future learning problems.32-34 There have been several studies evaluating the effect of prenatal hormonal imbalances in CAH (androgen excess, hypocortisolemia) on brain development.35-38 Finally, although considered experimental, the use of prenatal dexamethasone administered to pregnant mothers of children at risk of having CAH may negatively impact their visual working memory and visual perception.39, 40 Finally, as some of the codes for CAH were non-specific that were included here, the cohort here reflects a range of phenotypes, and results may be different if only those with the salt wasting form are included.
Unlike other studies, we did not show an increased risk of mental health diagnoses in those with DSD or CAH. In fact, in this cohort, those with CAH had lower odds of self-harm than matched controls. A large study in Sweden found that women and girls with CAH had higher odds of any psychiatric disorder compared with male and female controls.9 And women and girls with CAH had higher odds of mood disorder, anxiety disorder, and eating disorders compared with male, but not female, controls.9 Studies are more limited in men with CAH, but one study found that men with CAH had higher odds of psychiatric disorders and suicidality compared with controls.10 The dsd-LIFE consortium (6 European countries, n=1,040) reported psychiatric disorders in 45% of the study cohort and a suicide attempt in 6.8% of individuals.7 However, Turner Syndrome and Klinefelter syndrome, which are not always classified as a DSD, represented 50% of the cohort.7 In the dsd-LIFE study, 19.5% of adults met clinical cutoff symptom scores for anxiety, 7.1% for depression, 4.1% for ADHD and 9.1% for autism. Other smaller studies (n<100) of adults with a DSD that excluded sex chromosome aneuploidies have also shown a prevalence of severe psychological symptoms of 26% (vs 14% in controls) and suicidal thoughts and attempts at 38% and 12%, respectively (about 3x that in controls);8 or a prevalence of suicidal tendencies and self-harm comparable with women without a DSD but a history of abuse.41 A recent survey of adults in the U.S. with DSD demonstrated that half rated their mental health as fair or poor.42 Data in the pediatric population are limited. The prevalence of these behavioral health diagnoses is lower in this pediatric cohort than what has been reported in adults. It is unknown if that is secondary to ascertainment bias in our sample, or if the risk of psychiatric diagnoses in young kids (average age ~11 years at most recent follow up) receiving care at large pediatric health centers is actually lower than what is reported in adults. The Pediatric Psychosocial Preventative Health Model can be utilized in DSD/CAH to prevent and accurately diagnosed and treat mental health concerns as they arise.43 Finally, many psychiatric conditions emerge in adolescence and we do not yet know how many children in this cohort will go on to be diagnosed with a psychiatric condition later in life. It is important to note that there may be a subset of individuals who are at higher psychiatric risk,7-10, 42 and more work is needed to understand specific risk factors.
Small studies suggest a prevalence of gender dysphoria of 5% among 46,XX individuals with congenital adrenal hyperplasia, the most common 46,XX DSD44 and as high as >50% for some rare DSDs.45-47 The prevalence of a diagnosis of gender dysphoria in the medical chart was 1.1% for DSD (n=13) and 0.7% of CAH (n=11). Recent studies in the U.S. suggest that 0.6% of adults48 and up to 1.8% of youth49 identify as transgender in the general population, although it is uncertain what percent of people who self-identify as transgender would have a diagnosis of gender dysphoria in their medical record.
There are several limitations to our study. As this study utilized EHR data, cases and outcomes were only captured if a diagnosis was listed in either the problem list or as a billing diagnosis at any point in the EHR. Past medical history is not available in PEDSnet; therefore, individuals with an outcome diagnosis of interest may have been missed if it was not also documented in the problem list or as a billing code. If codes of interest were entered erroneously or omitted, this could alter the prevalence/risk of our outcomes, although we would expect this would be similar for both cases and controls. If an individual only received a diagnosis of interest at a non-PEDSnet site, this would not be captured in the outcomes. This may result in an underreporting of outcomes for individuals with a DSD or CAH. However, the converse may also be true. All six PEDSnet sites presented here (minus the Nemours Delaware location) have a DSD multidisciplinary clinic that includes either a psychologist or psychiatrist (and a social worker in some cases). If children with a DSD or CAH are receiving their care in a multidisciplinary clinic that includes regular behavioral health screening and support, they may be more likely to receive a psychiatric or neurodevelopmental diagnosis. Although we do not know what percentage of patients here received care in a multidisciplinary setting. The age of the patients, particularly those in the DSD cohort, is relatively young. As these participants age, some may go on to be diagnosed with one of the behavioral health conditions here or express gender dysphoria. The risk of these outcomes may change over time or as participants age. Prospective, multi-site data, such as those collected in the DSD Translational Research Network (DSD-TRN, which includes two PEDSnet sites: Children’s Hospital Colorado and Seattle Children’s),50 will help address some of the limitations of the PEDSnet database. Future work will also focus on clarifying how sex is recorded in the chart at each site. Several sites use an EHR smart form that captures gender identity, sex assigned at birth and pronouns. When it becomes possible to capture SNOMED codes that reflect these fields, we will also evaluate differences based on stated gender identity. Finally, although our sample size of youth with DSD or CAH is large and diverse, it may not be representative of patients with these diagnoses in the U.S. as the PEDSnet sites represent large pediatric health systems.
In conclusion, this large PEDSnet sample shows higher odds of behavioral health diagnoses, in particular those affecting neurodevelopment, among youth with DSD and a similar odds of behavioral health diagnoses among youth with CAH compared with rigorously matched controls. However, both groups had higher odds of developmental delay than controls, highlighting the need for appropriate screening and referrals for these groups.
Acknowledgements:
We thank the PEDSnet Data Coordinating Center for their support in the data acquisition and all PEDSnet site contributors. We also thank the team at the Adult and Child Consortium for Health Outcomes Research and Delivery Science (ACCORDS) including prior analysts Jacob Thomas, Bridget Mosley, and Angela Moss, who worked on data cleaning and the early analyses and Elizabeth Juarez-Colunga who helped develop the initial analysis plan.
Supported in part by NIH/NICHD K23HD092588 and R03HD102773 (to S.D.), NIH/NICHD K12HD057022 (to N.N.), NIH/NCI K08CA248704 (to L.P.), Doris Duke Foundation (to S.D. and N.N.). Contents are the authors’ sole responsibility and do not necessarily represent views of the funders. The funders had no role in the design and conduct of the study. N.N. previously served as a consultant for Antares Pharma, Inc and Neurocrine Biosciences. M.V. has served as a consultant for Antares Pharma, Neurocrine Biosciences, Adrenas Therapeutics, and Eton Pharmaceuticals and has conducted research supported by Neurocrine Biosciences and Spruce Biosciences. The other authors declare no conflicts of interest.
Abbreviations:
- DSD
difference of sex development
- CAH
congenital adrenal hyperplasia
- EHR
electronic health record
- IQ
intelligence quotient
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Johannsen TH, Ripa CP, Reinisch JM, Schwartz M, Mortensen EL, Main KM. Impaired cognitive function in women with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2006;91:1376–81. [DOI] [PubMed] [Google Scholar]
- [2].Hamed SA, Metwalley KA, Farghaly HS. Cognitive function in children with classic congenital adrenal hyperplasia. Eur J Pediatr. 2018;177:1633–40. [DOI] [PubMed] [Google Scholar]
- [3].Nass R, Baker S. Learning disabilities in children with congenital adrenal hyperplasia. Journal of Child Neurology. 1991;6:306–12. [DOI] [PubMed] [Google Scholar]
- [4].Lin-Su K, Lekarev O, Poppas DP, Vogiatzi MG. Congenital adrenal hyperplasia patient perception of 'disorders of sex development' nomenclature. Int J Pediatr Endocrinol. 2015;2015:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cox K, Bryce J, Jiang J, Rodie M, Sinnott R, Alkhawari M, et al. Novel associations in disorders of sex development: findings from the I-DSD Registry. J Clin Endocrinol Metab. 2014;99:E348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gazdagh G, Tobias ES, Ahmed SF, McGowan R. Novel Genetic Associations and Range of Phenotypes in Children with Disorders of Sex Development and Neurodevelopment: Insights from the Deciphering Developmental Disorders Study. Sex Dev. 2016;10:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Falhammar H, Claahsen-van der Grinten H, Reisch N, Slowikowska-Hilczer J, Nordenstrom A, Roehle R, et al. Health status in 1040 adults with disorders of sex development (DSD): a European multicenter study. Endocr Connect. 2018;7:466–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Johannsen TH, Ripa CP, Mortensen EL, Main KM. Quality of life in 70 women with disorders of sex development. European Journal of Endocrinology. 2006;155:877–85. [DOI] [PubMed] [Google Scholar]
- [9].Engberg H, Butwicka A, Nordenström A, Hirschberg AL, Falhammar H, Lichtenstein P, et al. Congenital adrenal hyperplasia and risk for psychiatric disorders in girls and women born between 1915 and 2010: a total population study. Psychoneuroendocrinology. 2015;60:195–205. [DOI] [PubMed] [Google Scholar]
- [10].Falhammar H, Butwicka A, Landén M, Lichtenstein P, Nordenskjöld A, Nordenström A, et al. Increased psychiatric morbidity in men with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. The Journal of Clinical Endocrinology & Metabolism. 2014;99:E554–E60. [DOI] [PubMed] [Google Scholar]
- [11].Li SH, Graham BM. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. The Lancet Psychiatry. 2017;4:73–82. [DOI] [PubMed] [Google Scholar]
- [12].Kuehner C. Why is depression more common among women than among men? The Lancet Psychiatry. 2017;4:146–58. [DOI] [PubMed] [Google Scholar]
- [13].May T, Adesina I, McGillivray J, Rinehart NJ. Sex differences in neurodevelopmental disorders. Current opinion in neurology. 2019;32:622–6. [DOI] [PubMed] [Google Scholar]
- [14].Bakker J. The Sexual Differentiation of the Human Brain: Role of Sex Hormones Versus Sex Chromosomes. Curr Top Behav Neurosci. 2019;43:45–67. [DOI] [PubMed] [Google Scholar]
- [15].Arnold AP. Sexual differentiation of brain and other tissues: Five questions for the next 50 years. Horm Behav. 2020;120:104691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Green T, Flash S, Reiss AL. Sex differences in psychiatric disorders: what we can learn from sex chromosome aneuploidies. Neuropsychopharmacology. 2019;44:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee PA, Houk CP, Ahmed SF, Hughes IA. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006;118:e488–500. [DOI] [PubMed] [Google Scholar]
- [18].Imbens GW, Rubin DB. Causal inference in statistics, social, and biomedical sciences: Cambridge University Press; 2015. [Google Scholar]
- [19].Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. The American Statistician. 1985;39:33–8. [Google Scholar]
- [20].Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine. 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. Journal of the American statistical Association. 1984;79:516–24. [Google Scholar]
- [22].Austin PC. The performance of different propensity score methods for estimating marginal odds ratios. Statistics in medicine. 2007;26:3078–94. [DOI] [PubMed] [Google Scholar]
- [23].Timmermans S, Yang A, Gardner M, Keegan CE, Yashar BM, Fechner PY, et al. Gender destinies: assigning gender in Disorders of Sex Development-Intersex clinics. Sociol Health Illn. 2019;41:1520–34. [DOI] [PubMed] [Google Scholar]
- [24].Fisher A, Ristori J, Fanni E, Castellini G, Forti G, Maggi M. Gender identity, gender assignment and reassignment in individuals with disorders of sex development: a major of dilemma. Journal of Endocrinological Investigation. 2016;39:1207–24. [DOI] [PubMed] [Google Scholar]
- [25].Watch HR. I want to be like nature made me: Medically unnecessary surgeries on intersex children in the US. 2017. [Google Scholar]
- [26].Mouriquand PD, Gorduza DB, Gay C-L, Meyer-Bahlburg HF, Baker L, Baskin LS, et al. Surgery in disorders of sex development (DSD) with a gender issue: If (why), when, and how? Journal of pediatric urology. 2016;12:139–49. [DOI] [PubMed] [Google Scholar]
- [27].Karkazis K, Tamar-Mattis A, Kon AA. Genital surgery for disorders of sex development: implementing a shared decision-making approach. Journal of Pediatric Endocrinology and Metabolism. 2010;23:789–805. [DOI] [PubMed] [Google Scholar]
- [28].Mouriquand P, Caldamone A, Malone P, Frank J, Hoebeke P. The ESPU/SPU standpoint on the surgical management of disorders of sex development (DSD). Journal of pediatric urology. 2014;10:8–10. [DOI] [PubMed] [Google Scholar]
- [29].Gardner M, Sandberg DE. Navigating surgical decision making in disorders of sex development (DSD). Frontiers in pediatrics. 2018;6:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ohnesorg T, Vilain E, Sinclair AH. The Genetics of Disorders of Sex Development in Humans. Sexual Development. 2014;8:262–72. [DOI] [PubMed] [Google Scholar]
- [31].Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Research Part A: Clinical and Molecular Teratology. 2010;88:1008–16. [DOI] [PubMed] [Google Scholar]
- [32].Collaer ML, Hindmarsh PC, Pasterski V, Fane BA, Hines M. Reduced short term memory in congenital adrenal hyperplasia (CAH) and its relationship to spatial and quantitative performance. Psychoneuroendocrinology. 2016;64:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Johannsen TH, Ripa CP, Reinisch JM, Schwartz M, Mortensen EL, Main KM. Impaired cognitive function in women with congenital adrenal hyperplasia. The Journal of Clinical Endocrinology & Metabolism. 2006;91:1376–81. [DOI] [PubMed] [Google Scholar]
- [34].Maheu FS, Merke DP, Schroth EA, Keil MF, Hardin J, Poeth K, et al. Steroid abnormalities and the developing brain: declarative memory for emotionally arousing and neutral material in children with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2008;33:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ernst M, Maheu FS, Schroth E, Hardin J, Golan LG, Cameron J, et al. Amygdala function in adolescents with congenital adrenal hyperplasia: a model for the study of early steroid abnormalities. Neuropsychologia. 2007;45:2104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. The Journal of Clinical Endocrinology & Metabolism. 2003;88:1760–5. [DOI] [PubMed] [Google Scholar]
- [37].Nass R, Heier L, Moshang T, Oberfield S, George A, New MI, et al. Magnetic resonance imaging in the congenital adrenal hyperplasia population: increased frequency of white-matter abnormalities and temporal lobe atrophy. Journal of child neurology. 1997;12:181–6. [DOI] [PubMed] [Google Scholar]
- [38].Somajni F, Sovera V, Albizzati A, Russo G, Peroni P, Seragni G, et al. Neuropsychological assessment in prepubertal patients with congenital adrenal hyperplasia: preliminary study. Minerva pediatrica. 2011;63:1–9. [PubMed] [Google Scholar]
- [39].Hirvikoski T, Nordenström A, Lindholm T, Lindblad F, Ritzén EM, Wedell A, et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab. 2007;92:542–8. [DOI] [PubMed] [Google Scholar]
- [40].Maryniak A, Ginalska-Malinowska M, Bielawska A, Ondruch A. Cognitive and social function in girls with congenital adrenal hyperplasia -- influence of prenatally administered dexamethasone. Child Neuropsychol. 2014;20:60–70. [DOI] [PubMed] [Google Scholar]
- [41].Schützmann K, Brinkmann L, Schacht M, Richter-Appelt H. Psychological distress, self-harming behavior, and suicidal tendencies in adults with disorders of sex development. Archives of sexual behavior. 2009;38:16–33. [DOI] [PubMed] [Google Scholar]
- [42].Rosenwohl-Mack A, Tamar-Mattis S, Baratz AB, Dalke KB, Ittelson A, Zieselman K, et al. A national study on the physical and mental health of intersex adults in the U.S. PLoS One. 2020;15:e0240088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kazak AE, Schneider S, Didonato S, Pai AL. Family psychosocial risk screening guided by the Pediatric Psychosocial Preventative Health Model (PPPHM) using the Psychosocial Assessment Tool (PAT). Acta Oncol. 2015;54:574–80. [DOI] [PubMed] [Google Scholar]
- [44].Dessens AB, Slijper FM, Drop SL. Gender dysphoria and gender change in chromosomal females with congenital adrenal hyperplasia. Arch Sex Behav. 2005;34:389–97. [DOI] [PubMed] [Google Scholar]
- [45].Cohen-Kettenis P. Psychological long-term outcome in intersex conditions. Horm Res. 2005;64 Suppl 2:27–30. [DOI] [PubMed] [Google Scholar]
- [46].Cohen-Kettenis P. Gender Change in 46,XY Persons with 5α-Reductase-2 Deficiency and 17β-Hydroxysteroid Dehydrogenase-3 Deficiency. Arch Sex Behav. 2005;34:399–410. [DOI] [PubMed] [Google Scholar]
- [47].Kreukels BPC, Kohler B, Nordenstrom A, Roehle R, Thyen U, Bouvattier C, et al. Gender Dysphoria and Gender Change in Disorders of Sex Development/Intersex Conditions: Results From the dsd-LIFE Study. J Sex Med. 2018;15:777–85. [DOI] [PubMed] [Google Scholar]
- [48].Herman JL, Flores AR, Brown TNT, Wilson BDM, & Conron KJ . Age of individuals who identify as transgender in the United States. . Los Angeles, CA: The Williams Institute. 2017. [Google Scholar]
- [49].Johns MM, Lowry R, Andrzejewski J, Barrios LC, Demissie Z, McManus T, et al. Transgender Identity and Experiences of Violence Victimization, Substance Use, Suicide Risk, and Sexual Risk Behaviors Among High School Students - 19 States and Large Urban School Districts, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sandberg DE, Gardner M, Callens N, Mazur T. Interdisciplinary care in disorders/differences of sex development (DSD): The psychosocial component of the DSD-Translational research network. Am J Med Genet C Semin Med Genet. 2017;175:279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]