Abstract
Background & Aims:
Hepatitis B virus (HBV) consists of 9 major genotypes (A to I), one minor strain (designated J) and multiple subtypes, which may have different natural history, disease progression and treatment response. As only cell lines expressing genotype D3 have been established, here we aim to establish stable cell lines producing high-titer cell culture-generated HBV (HBVcc) of different genotypes and to explore their infectivity, virological features and treatment response.
Methods:
Stable cell lines producing high titer of HBV with genotypes A2, B2, C1, E, F1b and H were generated by transfecting replication-competent 1.3×length HBV genome in a plasmid containing an antibiotic marker in HepG2 cells that can support HBV replication. Clones with highest levels of HBV DNA and/or HBeAg were selected and expanded for large-scale purification of HBVcc. HBVcc of different genotypes were tested in cells and humanized chimeric mouse model.
Results:
All HBVcc infected mouse-passaged primary human hepatocytes (PXB cells) and genotypes exhibit different responses to human IFN-α with variable kinetics of reduction in HBV DNA, HBeAg and HBsAg. HBVcc of all genotypes were infectious in humanized chimeric mice but with variable kinetics of viremia and viral antigen production. Treatment of infected mice with human IFN-α resulted in modest and variable reductions of viremia and viral antigenemia. HBVcc passaged in humanized chimeric mice (HBVmp) infected PXB cells much more efficiently than that of the original HBVcc viral stock.
Conclusions:
Here we generate stable cell lines producing HBV of various genotypes that are infectious in vitro and in vivo. We observe genotype-associated variations in viral antigen production, infection kinetics and responses to human IFN-α treatment in these models.
Keywords: Virus genotypes, stable cell lines, liver disease, treatment, infection model
Graphical Abstract

Lay summary:
Stable cell lines producing high-titer cell culture-generated HBV of various genotypes were established. HBV genotypes showed stable infectivity in both in vitro and in vivo models which are valuable tools for antiviral development.
Introduction
HBV is a leading cause of liver disease worldwide: an estimated 250–260 million individuals are chronically infected with over 800,000 deaths each year, and approximately one third of the world’s population have serologic evidence of exposure1–3. In endemic regions like Southeast Asia and Africa, prevalence rates are at least 8% in many populations4–6. Many people, despite having serologic evidence of resolved HBV infection, harbor viral cccDNA reservoir in their liver and are at risk of reactivation2,3. Highly effective vaccines to prevent HBV infection has been available for nearly 40 years, yet thousands of people worldwide continue to acquire HBV infection because of the large reservoir of HBV infected people and less than optimal implementation of prevention and vaccine strategies in the world2,6–8. Many infected people develop chronic infection that can be suppressed by current therapies but effective curative regimen is still lacking9. Chronic HBV infection leads to liver inflammation, with long-term risks of cirrhosis and hepatocellular carcinoma (HCC)10,11.
Since HBV-associated mortality remains high worldwide, the United Nations Sustainable Development Goals set the challenge of eliminating HBV infection as a public health threat by 20301. The current goal for therapeutic development is the achievement of a “functional cure”12. Curing HBV infection requires a detailed and robust understanding of the diversity of HBV, which consists of 9 major genotypes (A to I), one minor strain (designated J) and multiple subtypes based on divergence of the entire genomic sequence of >7.5% and 4–7.5%, respectively13,14. Different genotypes or subtypes may affect HBV natural history, disease progression and treatment response. The mechanisms by which HBV genotypes display diverse disease manifestation and treatment responses remain largely undefined.
Tools and models to investigate HBV genotypes have been limited2,15. Studies using transiently transfected DNA do not replicate the natural pathway for HBV transcription and replication. HBV protein expression is affected by transfection efficiency of the encoding plasmid16. While the covalently closed circular DNA (cccDNA) can be generated in artificial cell culture systems17, its natural biogenesis cannot be fully studied. Studies utilizing in vitro models of HBV infection have been limited to a single viral inoculum of genotype D3 derived from stably transformed cell lines15,18. Thus stable cell lines producing HBV of different genotypes to study their behaviors in vitro and in vivo are sorely needed.
Here we report the generation of stable cell lines that produce high-titer HBV of different genotypes that can be passaged in vitro and in vivo and used for functional and biological studies. We also describe variable infection courses, virological features and treatment responses among different genotypes, underscoring the importance of these model systems in studying HBV infection and developing more effective therapies.
Materials and methods
Described in Supplementary Data.
Results
Establishment of cell lines producing HBVcc of different genotypes
After transfection of HBV genomes of various genotypes into HepG2 and selection with hygromycin B, we obtained a total of 180 clones of genotype A2 with 28 positive for HBeAg production in the culture supernatant; 192 clones of genotype B2 with 31 positive for HBeAg; 189 clones of genotype C1 with 28 positive for HBeAg; 96 clones of genotype E with 18 positive for HBeAg; 180 clones of genotype F1b with 31 positive for HBeAg; 96 clones for genotype H with 16 clones positive for HBeAg. It was apparent from the selection protocol that variable expressions of HBeAg were noted among the various clones of each genotypes. For those clones with positive HBeAg expression, we analyzed HBV DNA levels in the supernatant and selected one clone per genotype with the highest level of HBV DNA as our candidate stable cell line for each genotype (Table 1). We then expanded these clones and collected and concentrated the supernatant for subsequent infection experiment. We seeded the same number of the selected stable cell clone of each genotype into 48-well plate and collected the supernatant on day 3 for HBV DNA, HBeAg and HBsAg. All these clones produced and secreted high levels of HBV DNA (>106 copies/mL) in the supernatant with the exception of genotype H clone being much lower. They produced variable levels of HBsAg and HBeAg (Fig. S2).
Table 1.
Stable cell clones producing infectious HBV genotype viruses.
| Genotype | Genome size (nt) | # Clones screened1 | # Clones selected2 | HBV DNA (copies/mL) of the highest producing clone | Reference/Accession Number |
|---|---|---|---|---|---|
| A2 | 3221 | 180 | 28 | 5.6×106 | (Boscs’ et al. 2000)35/AF305422 |
| B2 | 3215 | 192 | 31 | 1.7×106 | (Vitina Sozzi, et al. 2016)16/ MT111595 |
| C1 | 3215 | 189 | 28 | 2.4×106 | (Vitina Sozzi, et al. 2016)16/ MT111596 |
| E | 3212 | 96 | 18 | 1.7×107 | (Bannister, E.G, et al. Unpublished)/KU736895 |
| F1b | 3215 | 180 | 31 | 9.6×105 | (Torres, C., et al. 2011)36/DQ823095 |
| H | 3215 | 96 | 16 | 9.3×105 | (Arauz-Ruiz, P., et al. 2002)37/AY090460 |
The genomic size of genotype D3 is 3182 nt.
The total number clones screened with HBeAg ELISA kit;
The total number clones with high level of HBeAg expression selected.
Infection of PXB and HepG2-NTCP cells by HBVcc genotypes
To examine the infectivity of virus produced by these stable cell clones, we first concentrated and purified the HBVcc with heparin column and then infected PXB (human hepatocytes passaged and expanded in Alb-uPA/Scid mice) cells at 50 MOI. We collected the supernatant at different time points to monitor the expression of HBV DNA, HBeAg, and HBsAg (from day 3 to 14 post-infection). As shown in Fig. 1A, the levels of these viral markers varied among the different genotypes, although for the most part, the general trend showed a gradual increase. The production of viral markers was blocked by Myrcludex B at the time of infection, as reflected by a much lower level of viral markers with time (Fig. 1A, dotted lines). The values of the Myrcludex B-treated samples probably represent input viral makers. The kinetics of viral markers production were somewhat variable among the genotypes. To directly compare the levels of viral markers, we plotted the day 14 data from all genotype-infected cells on one graph (Fig. 1B). Cells infected with different genotypes produced variable HBV DNA and viral markers. Genotype B2 appeared to exhibit the highest levels of viral markers while genotype C1 the lowest (Table S2).
Fig. 1. HBVcc genotypes infect PXB cells with different infectivity.
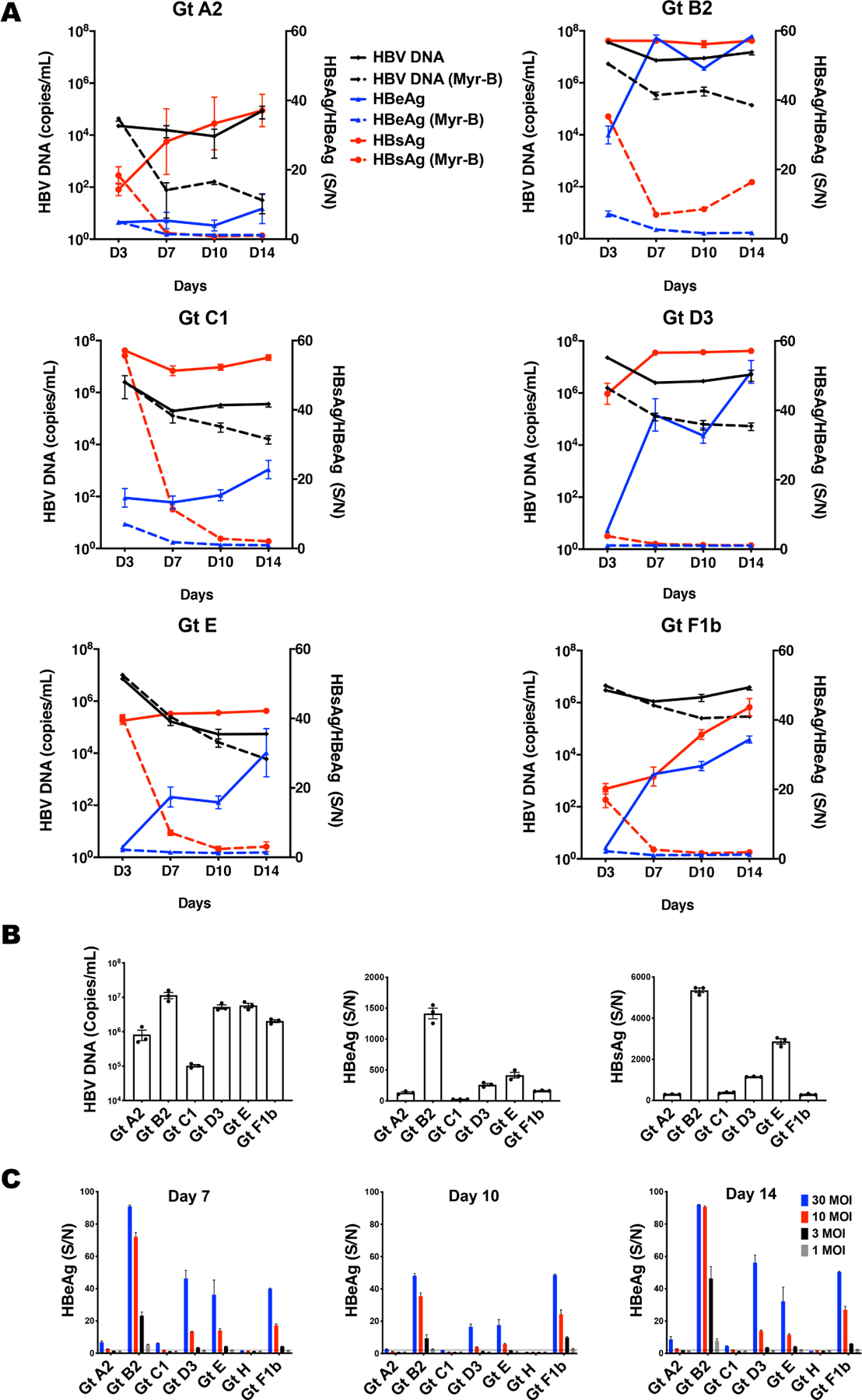
PXB cells were infected with HBVcc of different genotypes at an MOI of 50. (A) HBV DNA was performed with real-time qPCR and HBeAg /HBsAg were tested with ELISA at day 3, 7, 10 and 14 post-infection. The data from the ELISA is calculated as signal to noise ratio (S/N), with N defined as the average OD reading of multiple un-infected culture medium control. The ELISA is generally linear up to a S/N of 50. (B) Comparison of HBV DNA, HBeAg and HBsAg levels among different genotypes at day 14 post-infection. For samples with S/N greater than 50, they were diluted for further analysis. (C) PXB cells were infected with HBVcc genotypes at MOI of 30, 10, 3, and 1. HBeAg was tested with ELISA at day 7, 10 and 14 post-HBVcc infection. Dotted line shows the negative cutoff. Data are means ± SEM of triplicates. The results are representative of three independent experiments.
To further characterize the genotypic differences here, we infected PXB cells with various HBVcc genotypes at MOI of 1, 3, 10 and 30. As showed in Fig. 1C, HBVcc of genotype B2 successfully infected PXB cells at 1 MOI, genotype A2 at 10 MOI, genotype C1 at 30 MOI, genotype D3, E and F at 3 MOI, whereas no infection was detected for genotype H, even at 30 MOI. Thus HBVcc of different genotypes produced by their respective cell clones showed different infection efficiencies in PXB cells.
We also tested the infectivity of HBVcc of various genotypes in HepG2-NTCP cells, which are commonly used for in vitro HBV infection studies. As shown in Fig. S3, only genotype D3 and E infected HepG2-NTCP cells at an MOI of 100. With our current HBV production protocol, it is difficult to generate any higher viral stock for a much higher MOI without causing untoward effects on the cells. These data indicate that HepG2-NTCP cells are less susceptible to infection by HBVcc than the PXB cells.
HBVcc genotypes respond differently to antiviral effect of hIFN-α2a in PXB cells
Previous clinical studies suggested that patients infected with different HBV genotypes may have different responses to hIFN-α treatment19. We thus evaluated the response of HBVcc genotypes to hIFN-α2a in this model. We treated PXB cells infected with HBVcc genotypes (MOI of 50) with 500 IU/mL hIFN-α2a or nucleoside analog entecavir on day 1 post-infection and continued for 2 weeks. As shown in Fig. 2A, hIFN-α2a suppressed HBV DNA levels in all the genotypes, though the extents of suppression were somewhat different among the genotypes. In particular, genotype C1 seemed to respond least well (<1 log reduction on day 14) and genotype B2 had a much higher reduction (>2 log) of HBV DNA compared to no treatment samples. The relative lack of suppression of HBV DNA by hIFN-α2a in genotype C1 infected cells could be attributed to a much lower HBV infection and replication of genotype C1. hIFN-α2a suppressed production of HBeAg and HBsAg to a similar extent in all genotypes (Fig. 2B and 2C). Entecavir showed little effect on HBeAg and HBsAg levels, but effectively suppressed HBV DNA levels in all genotype-infected cells.
Fig. 2. Antiviral activities of hIFN-α2a and entecavir in HBVcc genotypes-infected PXB cells.
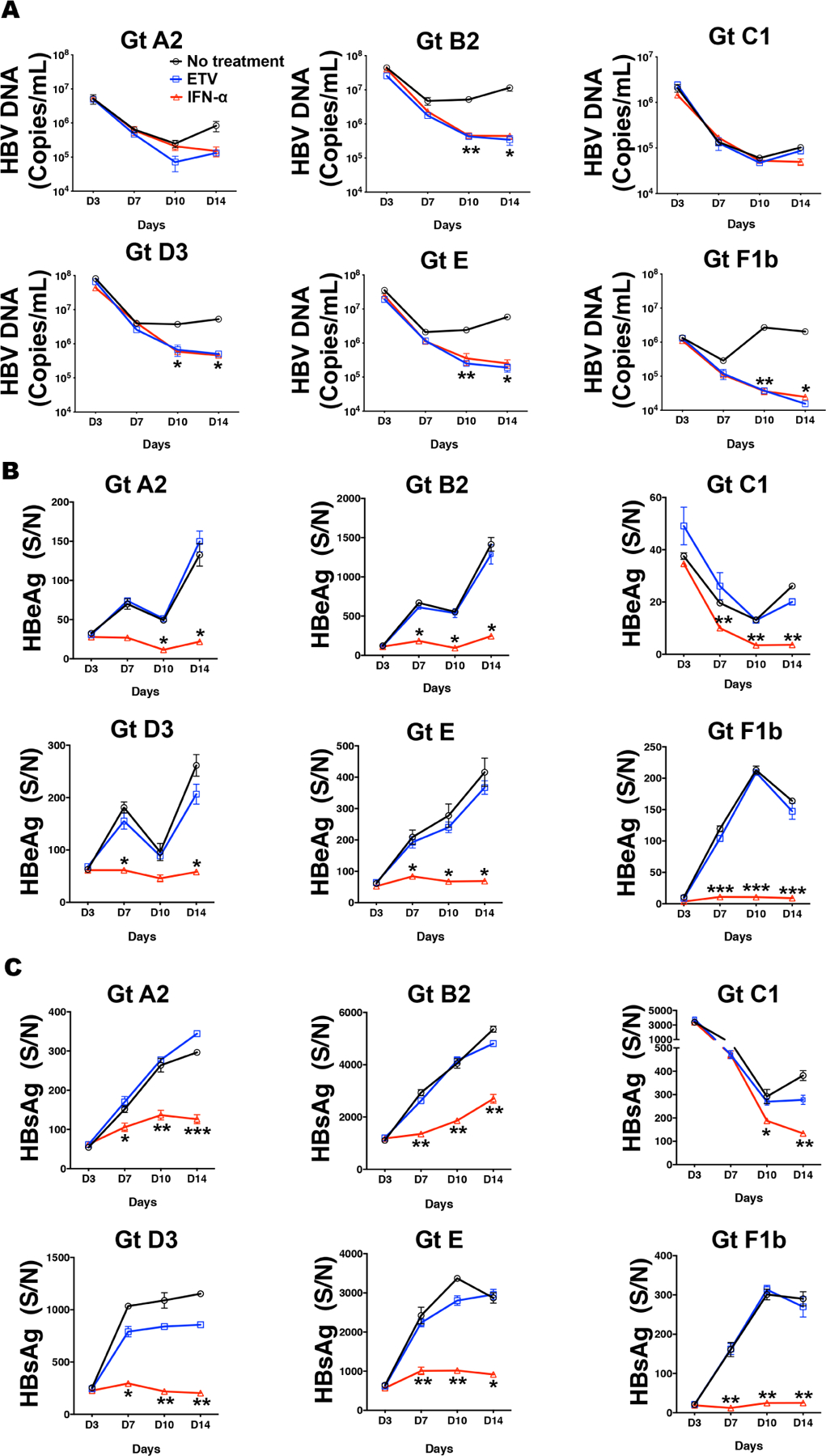
PXB cells were infected with HBV genotypes A2, B2, C1, D3, E and F1b at 50 MOI and further treated with hIFN-α2a (500 U/mL) for 14 days from day 1 post-viral infection. (A, B and C) The anti-HBV effect of hIFN-α2a and entecavir on production of HBV DNA, HBeAg and HBsAg at day 3, 7, 10 and 14 were evaluated. Data are shown as means ± SEM of triplicates. *** P<0.001; ** P<0.01; * P<0.05. The results are representative of three independent experiments.
Patients with high HBV DNA levels have been reported to respond less well to IFN-α treatment20. To assess the antiviral effect of hIFN-α2a in cells with sustained and high level of HBV replication, we tested the effect of hIFN-α2a on day 10 post-infection when HBV DNA level was high and continued the treatment for 7 days. As shown in Fig. S4, hIFN-α2a remained effective in suppressing HBV DNA, HBeAg and HBsAg among all genotype infected cells. Again genotype C1 showed the least response to hIFN-α2a in HBV DNA.
Infection of humanized chimeric mice by HBVcc genotypes
To demonstrate the infectivity of various HBVcc genotypes produced in vivo, humanized chimeric mice (n=3 per genotype) were inoculated with a titer of 1×106 copies (all diluted in 100 μL PBS for injection) of each genotype. Weekly samples were obtained and measured for hAlb, HBsAg, HBeAg and HBV DNA. As HBVcc of genotype C1 did not show evidence of infection with a titer of 1×106 copies, we re-inoculated the mice with a higher titer of 5×106 copies, which resulted in infection. As shown in Fig. 3A, all inoculated mice demonstrated gradual increases of serum HBV DNA, HBeAg, and HBsAg. The hAlb levels were stable in all mice during the experimental time period, indicating the maintenance of the engrafted human liver cells. HBVcc of genotype H, which did not demonstrate any robust infectivity in cell culture models, was equally infectious in mice. The HBeAg and HBsAg levels and kinetics were different among the genotypes. Genotype F1b showed the highest levels of both HBeAg and HBsAg levels compared to the other genotypes. Genotype B2 and C1 showed somewhat lower HBeAg, HBsAg and HBV DNA levels compared to other genotypes after 10 weeks of infection (Fig. 3B and Table S3).
Fig. 3. HBVcc genotypes infect humanized chimeric mice and produce variable levels of HBV DNA, HBeAg and HBsAg.
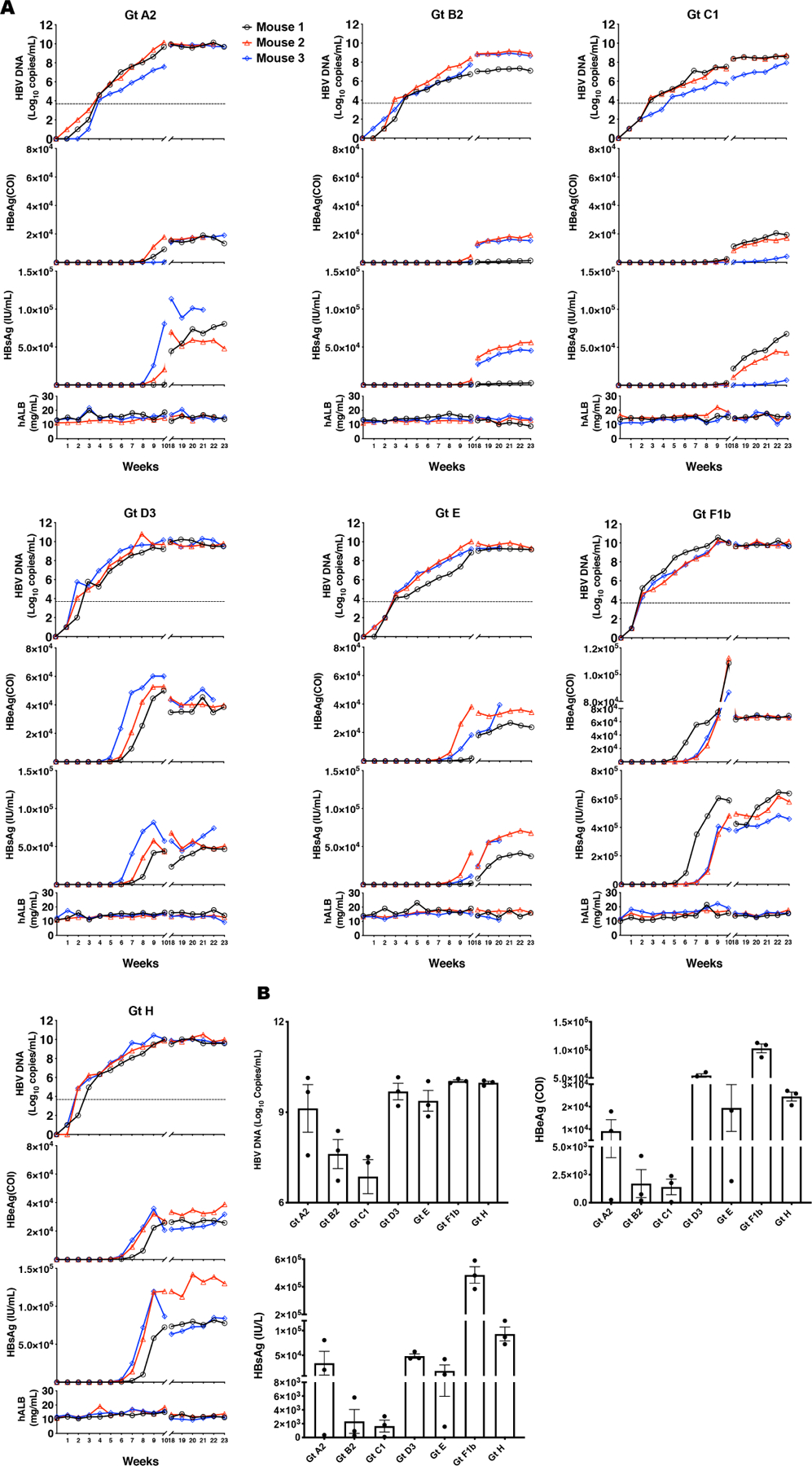
Humanized chimeric mice were infected intravenously through tail vein injection with 1×106 copies of HBVcc genotypes A2, B2, D3, E, F1b or H and 5×106 copies of HBVcc genotype C1 (3 mice per group). Serum was harvested weekly post-infection up to 23 weeks post-infection. (A) Serum was analyzed for HBV DNA, HBeAg, HBsAg and hAlb levels through real time qPCR and ELISA kits. The data of each mouse are denoted by a different color of symbols & lines. (B) Comparison of HBV DNA, HBeAg and HBsAg levels at week 10 post-infection. Data are shown as means ± SEM of triplicates.
Response of HBVcc genotypes to hIFN-α2a in humanized chimeric mice
After the establishment of infection in humanized chimeric mice (week 10 post-HBVcc infection), pegIFN-α2a (30ug/kg) was administered twice weekly for 4 weeks. The mice were then followed for 4 more weeks off treatment. Serum samples were obtained weekly and hAlb, HBV DNA, HBeAg and HBsAg were tested. Interferon stimulated genes (ISGs) were upregulated in the livers of humanized chimeric mice after pegIFN-α2a treatment (Fig. S5). As shown in Fig. 4A, pegIFN-α2a suppressed serum HBV DNA levels in all genotype infected mice during the treatment period. As expected, HBV DNA rebounded gradually after the termination of pegIFN-α2a treatment. We determined the area under curve (AUC) of changes in HBV DNA of different genotypes during the 4-week treatment (Fig. 4B). Overall, suppression of HBV DNA levels was variable among the genotypes, with genotypes B2 and C1 having the highest suppression (Table S4). HBeAg and HBsAg, on the other hand, were not reduced much by pegIFN-α2a treatment (Fig. S6). In some genotypes, there were surprisingly no reductions or even slight increases in HBeAg or HBsAg levels with IFN treatment. The effects on viral antigens did not seem to track the effect on HBV DNA levels.
Fig. 4. Inhibition of HBV DNA, HBeAg and HBsAg by pegIFN-α2a in infected humanized chimeric mice.
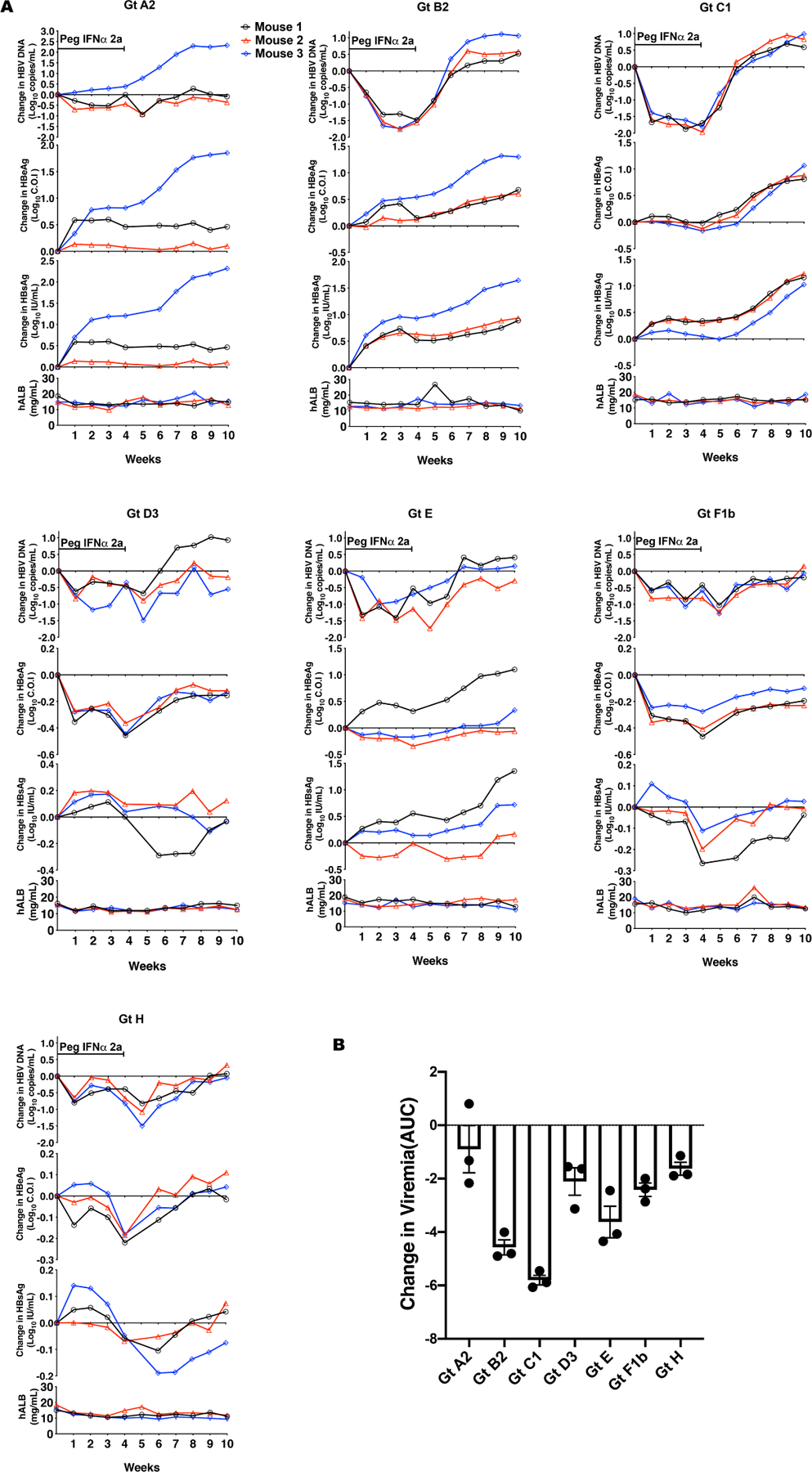
HBVcc-infected humanized chimeric mice were treated with pegIFN-α2a (30 μg/kg) at week 10 post-infection and continued for 4 weeks. Mice serum was harvested weekly at indicated time points during the treatment period and continued for 6 weeks after treatment cessation. HBV DNA and hAlb in mice serum were analyzed using real time PCR and ELISA kit accordingly. (A) The changes of HBV DNA, HBeAg and HBsAg levels from the pre-treatment levels (week 10 of infection) were calculated and shown. The data of each mouse are denoted by a different color of symbols & lines. (B) the cumulative changes of HBV DNA during 4 weeks of pegIFN-α2a treatment were calculated as area under curve (AUC) from week 1–4 of treatment. The data are shown as means ± SEM of triplicates.
Efficient infection of PXB cells by HBVcc genotypes passaged in humanized chimeric mice
As the infectivity of HBVcc of some genotypes appears to be low in cell culture, even in PXB cells, we examined the infectivity of the HBVcc passaged in the humanized chimeric mice (HBVmp) in PXB cells. We tested infection of HBVmp in PXB cells at different MOIs. As shown in Fig. S7, all HBVmp of different genotypes could infect PXB cells even at an MOI of 1 and produced high levels of HBV DNA, HBeAg and HBsAg. Interestingly, when we compared the infectivity of HBVmp and HBVcc in PXB cells (at an MOI of 1), HBVmp showed a much more robust infection with a substantially higher production of HBV DNA and HBeAg than those of HBVcc (Fig. 5A).
Fig. 5. Infectivity and density gradient analysis of HBVmp and HBVcc.

(A) PXB cells were infected with HBVcc and the corresponding HBVmp harvested at week 10 of each infected mouse; an MOI of 1 based on HBV DNA copy number was used. Culture media on day 10 were harvested and assayed for HBV DNA and HBeAg. The data for HBVmp are shown as means ± SEM of individual mouse data, and HBVcc shown as means ± SEM in triplicates. The results are representative of three independent experiments. (B) Concentrated HBVcc genotype A2 (500 μL of 4.2×109 HBV DNA copies/mL) and D3 (500 μL of 6×109 HBV DNA copies/mL) stocks, HBVmp Gt D3 (100 μL of 5×109 HBV DNA copies/mL) and HBVpt (500 μL of 9.4×108 HBV DNA copies/mL) were layered on top of a preformed iodixanol density gradient and subjected the centrifugation density gradient analysis as described in the Methods. After centrifugation, 20 fractions (500 μL per fraction) were collected from each gradient. HBsAg, HBcAg and HBV DNA were measured in each fraction with ELISA kits or qPCR. The boxed number above fraction 10 where Dane particles are represents the relative infectivity, which was calculated by dividing HBV DNA level of PXB cells at day 10 post-infection over the infecting HBV DNA titer of fraction 10.
We next compared the infectivity of HBVmp among different genotypes in PXB cells with an MOI of 1, 0.3 and 0.1 (Fig. S8). In contrast to the HBVcc data (Fig. 1C), we found that all genotypes of HBVmp exhibited similar infectivity. HBVmp could establish infection in PXB cells even at an MOI of 0.1.
We also tested the infectivity of HBVmp in the HepG2-NTCP cells, which, based on our earlier data, could only be infected by HBVcc genotypes D3 and E. In this experiment, HBVmp of various genotypes at an MOI of 100 were more infectious than HBVcc and able to establish infection in HepG2-NTCP cells, with genotype D3 and E being the most infectious (Fig. S9).
Sequence analyses of HBVmp
HBVcc and HBVmp appeared to behave quite differently in infection of PXB cells. We sequenced the circulating viruses of humanized chimeric mice infected with various HBVcc genotypes to assess whether adaptive mutations might have occurred in vivo. Serum samples of week 10 after infection from all infected mice (n=3 per genotype) were PCR-amplified with 4 sets of primers covering overlapping regions of the entire HBV genome (Table S1). The PCR fragments were then subjected to Sanger sequencing. Sequence heterogeneity (>10%) can be detected by analyzing the sequencing chromatograms. Comparing with sequences of the original HBV genotype clones, no mutations, even as a minor population, were observed.
HBVcc and HBVmp have distinct features by density gradient analysis
To understand the striking difference in infectivity between HBVcc and HBVmp, we performed density gradient analysis of both viral preparations (Fig. 5B). We selected HBVcc genotype A2 and D3, HBVmp genotype D3 and patient’s serum (HBVpt) with HBV genotype D infection, for detailed analysis. The distinct distributions of HBV DNA, HBcAg and HBsAg in the gradient provided a possible explanation for the difference in infectivity. First both HBVmp and HBVpt displayed similar profiles with peaks of HBV DNA, HBsAg and HBcAg in fractions where infectious Dane particles are known to reside (fractions at a density of 1.15 g/mL). The HBcAg level detected by ELISA was much lower in the HBVpt comparing to the HBVmp sample. The presence of pre-existing anti-HBc antibodies in patient sample could have masked the detection of HBcAg, which is done by an antibody-based detection immunoassay.
In contrast, HBcAg peak was detected in HBVcc samples at fractions with higher density (1.2 g/mL) containing the unenveloped naked-capsids. Interestingly, HBVmp and HBVpt did not show any peak for the naked capsid, supporting the presence of more mature HBV virions and thus higher infectivity in samples from both patients and animals. We also analyzed HBVcc of other genotypes by density gradient analysis and showed a similar profile with substantial amounts of naked capsids (Fig. S10). Therefore, the MOI for HBVcc may be overestimated as compared with that of HBVmp. For HBVcc of genotype A2, HBV DNA peaked at the naked capsid fraction, whereas HBV DNA of HBVcc genotype D3 peaked at the Dane particle fraction (Fig. 5B), potentially explaining a higher infectivity of HBVcc genotype D3 than that of HBVcc genotype A2 (Fig. 1).
To assess the infectivity of each fraction, we infected PXB cells with aliquot (20 μL) of each fraction from the density gradient. As shown in Fig. S11A, fraction 10 which contained Dane particles exhibited the highest production of HBV DNA in the supernatant. We also stained for HBV core antigen in PXB cells infected by fraction 10 and confirmed the data of HBV DNA levels (Fig. S11B).
Discussion
Based on mounting clinical evidence, HBV genotypes exhibit diverse disease manifestations and treatment responses. HBV genotypes C and F are associated with a higher risk of HCC than other genotypes21,22. Comparing to genotype C and D, patients with genotype A and B have higher rates of HBeAg seroconversion under treatment of IFN-α20,23. Genotype D appears to be associated more with acute liver failure than other genotypes24. Although racial/ethnic background, environment or other confounding factors could explain the differences, HBV genotypes likely play an important but yet-to-be defined role in the pathogenesis of liver disease. Few studies have utilized infection models to address the functional importance and virological mechanisms of genotypic differences, mostly because of the lack of convenient tools and model systems to study various HBV genotypes. The only HBV genotype that has been studied most extensively in cell culture is genotype D3 isolated from Europe, as it is the best characterized molecular clone of HBV17,25,26. While this molecular clone and its derived cell lines producing infectious virus have been an indispensable tool in studying the biology of HBV, it may not fully represent the biology of other diverse genotypes infecting the rest of the world. Transient transfection studies of other genotypes have identified differences in HBV replication phenotype16,27. Thus, convenient model systems of various genotypes are necessary to study the biological mechanisms of genotypic differences.
In this study, we established cell culture models for production of major HBV genotypes with well defined molecular clones and showed that HBVcc are infectious in human hepatoma-derived cells (HepG2-NTCP) and human adult hepatocytes (PXB cells). In addition, these viruses can be successfully passaged in humanized chimeric mouse models (human hepatocyte-engrafted Alb-uPA/Scid mice) and the passaged viruses (HBVmp) are highly infectious in the cell culture systems.
HBVcc of different genotypes displayed different profiles of production of HBsAg, HBeAg and HBV DNA both in vitro and in vivo. All HBVcc genotypes were infectious in PXB cells but only genotypes D3 and E could establish infection in HepG2-NTCP cells. In infected PXB cells, genotype B2, D3, E and F1b had a more robust replication with higher production of HBV DNA in the culture supernatant than the other genotypes. Genotype B2 displayed the highest infectivity and replication efficiency compared to all other genotypes. Genotype D3 and E showed similar infectivity, probably because they are more genetically related in the unrooted phylogenetic tree13. Viral antigen productions, such as HBeAg and HBsAg, were also variable among the genotypes. Again genotype B2 produced the highest levels of HBV antigens. HepG2-NTCP cells were less susceptible to HBVcc than the PXB cells, possibly because cellular pathways for efficient infection in HepG2-NTCP cells are less robust because of the transformed nature of the cells.
HBVcc of various genotypes were able to successfully establish productive infection in the humanized chimeric mouse model. It is interesting to note that most HBVcc genotypes were equally infectious using a standard inoculum titer of 106 genome-equivalent of HBV. Genotype C1, however, needed a 5× higher inoculum titer to establish infection, likely reflecting the lower infection capacity of the genotype as described above. The infection courses and kinetics of viremia (HBV DNA) were quite comparable among the genotypes. Peak viremia occurred 8–10 weeks post-infection and remained steady for an extended period of time (up to 23 weeks post-infection). The wide variations of infection and replication observed in the cell culture models were not evident in vivo. HBV antigenemia showed some genotype-associated difference. Genotypes B2 and C1 seemed to have a much lower HBeAg and HBsAg levels during the course of infection. Genotype B has been associated with a lower prevalence of HBeAg positivity at presentation and a higher rate of HBeAg seroconversion than other genotypes28. It is tempting to speculate that a lower HBe antigenemia observed here may explain such a clinical association.
HBVmp genotypes efficiently infected PXB cells with a much lower MOI than the HBVcc, suggesting acquisition of more infectious properties by HBVcc passaged in humanized chimeric mice. With extensive sequence analyses of HBVmp, we did not observe any in vivo acquired mutations in the viral genomes of all genotypes, eliminating the possibility of in vivo adaptative mutations. By density gradient analyses, we showed notable differences between HBVcc and HBVmp. In general, HBVmp had a much higher level of Dane particles in the appropriate density fraction. Interestingly, HBVmp and HBVpt show similar HBsAg, HBcAg and HBV DNA peaks without a clearly defined naked capsid fraction like what was observed in the HBVcc gradient, suggesting much more mature HBV particles formation in vivo.
In general, the Dane particles from different HBVcc genotypes, at least comparing A2 (relative infectivity of 0.150) and D3 (0.166), are similarly infectious. It is interesting that the relative infectivity of HBVmp (0.566) is higher than that of HBVcc of genotype D3, suggesting more mature Dane particles produced in vivo. The difference in infectivity between HBVmp and HBVpt (0.097) of the same genotype is not clear at this point. It should be noted that HBVmp is derived from a molecular clone whereas HBVpt may contain a heterogenous population of viruses with some sequence divergence. Other viral particles, such HBsAg spheres/filaments, empty virions, may also affect infection efficiency.
HBV genotype may play a role in IFN-α treatment of chronic hepatitis B20,23,29. However, conflicting data exist regarding the specific role of HBV genotypes in IFN-α response30. Such results, to some extent, are not surprising as responses to IFN-α therapy represent a complex phenotype that is affected by multiple factors of both virus and host. The model systems described here allow the investigation of viral factors for this phenotype. In cell culture model, we noted somewhat variable responses but no consistent differences. In human chimeric mice, we found that genotypes B2 and C1 responded somewhat better to peg-IFN-α2a than other genotypes. However further studies are required using larger numbers of treated vs untreated mice as well as using additional clones of each HBV genotype to determine there are indeed any genotypic differences in interferon response.
In PXB cells infected with HBVcc, we did not see any substantial genotypic differences in response to IFN-α2a treatment. The only notable observation is that genotype C1 appears to respond less well to IFN-α, probably explained by a much lower level of replication of this genotype. Thus far, the clinical observation of a lower response of certain genotypes to IFN-α therapy was not born out by our in vitro and in vivo models, likely because the response is determined more by host rather than viral factors31–33. Similarly, we did not see much difference in response to entecavir, a nucleoside analog, among the genotypes in our models. This observation is consistent with a comparable response of patients infected with different genotypes to various nucleoside analogs13,34.
Conclusions
We have successfully established stable in vitro and in vivo models of HBV infection with different genotypes and explored some interesting differences among genotypes in their infectivity, antigen expression, replication and treatment response. Further studies of the biology of HBV genotypes using these models are necessary. These models also pave the way for developing curative therapies that would be efficacious against all HBV genotypes – a goal that is critically important for the elimination of HBV worldwide.
Supplementary Material
Highlights:
Stable cell lines producing high-titer cell culture-generated HBV of various genotypes were established.
HBV genotypes showed stable infectivity in both in vitro and in vivo models.
HBV genotypes exhibit different infectivity, antigen expression, replication and treatment response.
These model systems are valuable tools for antiviral development.
Acknowledgements
We thank Dr. Yuchen Xia, Dr. Xiaoming Cheng, Seung Bum Park, and Tadashi Inuzuka for technical support.
Financial support statement:
This work was supported by the Intramural Research Program, NIDDK, NIH.
Abbreviations
- HBV
hepatitis B virus
- HBVcc
cell culture generated HBV
- HBVmp
mice serum generated HBV
- HBVpt
patient serum generated HBV
- HBsAg
hepatitis B surface antigen
- HBeAg
hepatitis B envelope antigen
- cccDNA
covalently closed circular DNA
- CHB
chronic hepatitis B
- HCC
hepatocellular carcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
The authors declare no conflicts of interest that pertain to this work.
Data availability:
The authors will make all the raw data available upon request.
References
- [1].McNaughton AL, D’Arienzo V, Ansari MA, Lumley SF, Littlejohn M, Revill P, et al. Insights From Deep Sequencing of the HBV Genome-Unique, Tiny, and Misunderstood. Gastroenterology 2019;156:384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, et al. A global scientific strategy to cure hepatitis B. The lancet Gastroenterology & hepatology 2019;4:545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology 2017;152:1297–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546–1555. [DOI] [PubMed] [Google Scholar]
- [5].Matthews PC, Geretti AM, Goulder PJ, Klenerman P. Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in Sub-Saharan Africa. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 2014;61:20–33. [DOI] [PubMed] [Google Scholar]
- [6].Spearman CW, Afihene M, Ally R, Apica B, Awuku Y, Cunha L, et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. The lancet Gastroenterology & hepatology 2017;2:900–909. [DOI] [PubMed] [Google Scholar]
- [7].Zhang H, Pan CQ, Pang Q, Tian R, Yan M, Liu X. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology 2014. [DOI] [PubMed] [Google Scholar]
- [8].Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. Journal of viral hepatitis 2012;19:e18–25. [DOI] [PubMed] [Google Scholar]
- [9].Xia Y, Liang TJ. Development of Direct-acting Antiviral and Host-targeting Agents for Treatment of Hepatitis B Virus Infection. Gastroenterology 2019;156:311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Global Burden of Disease Liver Cancer C, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA oncology 2017;3:1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. Journal of hepatology 2017;67:302–309. [DOI] [PubMed] [Google Scholar]
- [12].Cornberg M, Lok AS, Terrault NA, Zoulim F, Faculty E-AHTEC. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- [13].Rajoriya N, Combet C, Zoulim F, Janssen HLA. How viral genetic variants and genotypes influence disease and treatment outcome of chronic hepatitis B. Time for an individualised approach? Journal of hepatology 2017;67:1281–1297. [DOI] [PubMed] [Google Scholar]
- [14].Kramvis A. Genotypes and genetic variability of hepatitis B virus. Intervirology 2014;57:141–150. [DOI] [PubMed] [Google Scholar]
- [15].Thomas E, Liang TJ. Experimental models of hepatitis B and C - new insights and progress. Nature reviews Gastroenterology & hepatology 2016;13:362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sozzi V, Walsh R, Littlejohn M, Colledge D, Jackson K, Warner N, et al. In Vitro Studies Show that Sequence Variability Contributes to Marked Variation in Hepatitis B Virus Replication, Protein Expression, and Function Observed across Genotypes. Journal of virology 2016;90:10054–10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sells MA, Zelent AZ, Shvartsman M, Acs G. Replicative intermediates of hepatitis B virus in HepG2 cells that produce infectious virions. J Virol 1988;62:2836–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shen F, Li Y, Wang Y, Sozzi V, Revill PA, Liu J, et al. Hepatitis B virus sensitivity to interferon-alpha in hepatocytes is more associated with cellular interferon response than with viral genotype. Hepatology 2018;67:1237–1252. [DOI] [PubMed] [Google Scholar]
- [19].Revill PA, Tu T, Netter HJ, Yuen LKW, Locarnini SA, Littlejohn M. The evolution and clinical impact of hepatitis B virus genome diversity. Nat Rev Gastroenterol Hepatol 2020;17:618–634. [DOI] [PubMed] [Google Scholar]
- [20].Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 2009;137:2002–2009. [DOI] [PubMed] [Google Scholar]
- [21].Wong GL, Chan HL, Yiu KK, Lai JW, Chan VK, Cheung KK, et al. Meta-analysis: The association of hepatitis B virus genotypes and hepatocellular carcinoma. Alimentary pharmacology & therapeutics 2013;37:517–526. [DOI] [PubMed] [Google Scholar]
- [22].Ching LK, Gounder PP, Bulkow L, Spradling PR, Bruce MG, Negus S, et al. Incidence of hepatocellular carcinoma according to hepatitis B virus genotype in Alaska Native people. Liver Int 2016;36:1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005;365:123–129. [DOI] [PubMed] [Google Scholar]
- [24].Wai CT, Fontana RJ, Polson J, Hussain M, Shakil AO, Han SH, et al. Clinical outcome and virological characteristics of hepatitis B-related acute liver failure in the United States. Journal of viral hepatitis 2005;12:192–198. [DOI] [PubMed] [Google Scholar]
- [25].Galibert F, Mandart E, Fitoussi F, Tiollais P, Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature 1979;281:646–650. [DOI] [PubMed] [Google Scholar]
- [26].Will H, Cattaneo R, Koch HG, Darai G, Schaller H, Schellekens H, et al. Cloned HBV DNA causes hepatitis in chimpanzees. Nature 1982;299:740–742. [DOI] [PubMed] [Google Scholar]
- [27].Sozzi V, Shen F, Chen J, Colledge D, Jackson K, Locarnini S, et al. In vitro studies identify a low replication phenotype for hepatitis B virus genotype H generally associated with occult HBV and less severe liver disease. Virology 2018;519:190–196. [DOI] [PubMed] [Google Scholar]
- [28].Chu CJ, Hussain M, Lok AS. Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology 2002;122:1756–1762. [DOI] [PubMed] [Google Scholar]
- [29].Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sonneveld MJ, Rijckborst V, Cakaloglu Y, Simon K, Heathcote EJ, Tabak F, et al. Durable hepatitis B surface antigen decline in hepatitis B e antigen-positive chronic hepatitis B patients treated with pegylated interferon-alpha2b: relation to response and HBV genotype. Antiviral therapy 2012;17:9–17. [DOI] [PubMed] [Google Scholar]
- [31].Micco L, Peppa D, Loggi E, Schurich A, Jefferson L, Cursaro C, et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J Hepatol 2013;58:225–233. [DOI] [PubMed] [Google Scholar]
- [32].ter Borg MJ, Hansen BE, Herrmann E, Zeuzem S, Cakaloglu Y, Karayalcin S, et al. Modelling of early viral kinetics and pegylated interferon-alpha2b pharmacokinetics in patients with HBeag-positive chronic hepatitis B. Antivir Ther 2007;12:1285–1294. [PubMed] [Google Scholar]
- [33].Rehermann B, Thimme R. Insights From Antiviral Therapy Into Immune Responses to Hepatitis B and C Virus Infection. Gastroenterology 2019;156:369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wiegand J, Hasenclever D, Tillmann HL. Should treatment of hepatitis B depend on hepatitis B virus genotypes? A hypothesis generated from an explorative analysis of published evidence. Antivir Ther 2008;13:211–220. [PubMed] [Google Scholar]
- [35].Bock CT, Malek NP, Tillmann HL, Manns MP, Trautwein C. The enhancer I core region contributes to the replication level of hepatitis B virus in vivo and in vitro. J Virol 2000;74:2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Torres C, Pineiro y Leone FG, Pezzano SC, Mbayed VA, Campos RH. New perspectives on the evolutionary history of hepatitis B virus genotype F. Mol Phylogenet Evol 2011;59:114–122. [DOI] [PubMed] [Google Scholar]
- [37].Arauz-Ruiz P, Norder H, Robertson BH, Magnius LO. Genotype H: a new Amerindian genotype of hepatitis B virus revealed in Central America. J Gen Virol 2002;83:2059–2073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors will make all the raw data available upon request.


