Abstract
Background
Data on the long-term impact of SARS-CoV-2 infection in children and young people (CYP) are conflicting. We assessed evidence on long-term post-COVID symptoms in CYP examining prevalence, risk factors, type and duration. Methods: Systematic search of published and unpublished literature using 13 online databases between 01/12/2019 and 31/07/2021. Eligible studies reported CYP ≤19 years with confirmed or probable SARS-CoV-2 with any symptoms persisting beyond acute illness. Random effects meta-analyses estimated pooled risk difference in symptom prevalence (controlled studies only) and pooled prevalence (uncontrolled studies also included). Meta-regression examined study characteristics hypothesised to be associated with symptom prevalence. Prospectively registered: CRD42021233153.
Findings
Twenty two of 3357 unique studies were eligible, including 23,141 CYP. Median duration of follow-up was 125 days (IQR 99–231). Pooled risk difference in post-COVID cases compared to controls (5 studies) were significantly higher for cognitive difficulties (3% (95% CI 1, 4)), headache (5% (1, 8)), loss of smell (8%, (2, 15)), sore throat (2% (1, 2)) and sore eyes (2% (1, 3)) but not abdominal pain, cough, fatigue, myalgia, insomnia, diarrhoea, fever, dizziness or dyspnoea. Pooled prevalence of symptoms in post-COVID participants in 17 studies ranged from 15% (diarrhoea) to 47% (fatigue). Age was associated with higher prevalence of all symptoms except cough. Higher study quality was associated with lower prevalence of all symptoms, except loss of smell and cognitive symptoms.
Interpretation
The frequency of the majority of reported persistent symptoms was similar in SARS-CoV-2 positive cases and controls. This systematic review and meta-analysis highlights the critical importance of a control group in studies on CYP post SARS-CoV-2 infection.
Keywords: COVID-19, Long COVID, Post-COVID syndrome, Children and young people, Paediatric, SARS-CoV-2
Research in context.
Evidence before this study
While there has been much recent interest in persistent symptoms in children and young people (CYP) post SARS-CoV-2 infection, the majority of studies to date have been open to significant bias. The lack of a control group in many studies has made it hard to separate symptoms due to infection from those due to the pressures of a pandemic. Prior to our study, a search of Medline, Cochrane, medRxiv and PROSPERO identified one published narrative review and no meta-analyses specifically examining persistent symptoms in children and young people following SARS-CoV-2 infection.
We systematically searched published and unpublished literature using 13 online databases on 31/07/2021 to identify studies reporting symptoms in CYP ≤19 years persisting beyond acute SARS-CoV-2 infection. Although all studies were analysed, our meta-analysis primarily focused on pooled risk difference in symptom prevalence in controlled studies (with SARS-CoV-2 negative CYP).
Added value of this study
We did a systematic review of 22 studies from 12 countries including 23,141 CYP. We found that although the pooled prevalence of symptoms across all studies was high, when we restricted our meta-analysis to only those with a SARS-CoV-2 negative control group, most reported persistent symptoms were equally common in SARS-CoV-2 positive cases and SARS-CoV-2 negative controls. Higher study quality was associated with lower prevalence of all symptoms, except loss of smell and cognitive symptoms.
Small but significant increases in the pooled risk difference were seen for cognitive difficulties (3% (95% CI 1, 4)), headache (5% (1, 8)), loss of smell (8%, (2, 15)), sore throat (2% (1, 2)) and sore eyes (2% (1, 3)) in CYP following confirmed SARS-CoV-2 infection compared to negative controls.
Implications of all the available evidence
To the best of our knowledge, this is the first study to systematically review and meta-analyse persistent symptoms following SARS-CoV-2 infection in CYP. Our study shows that estimates of symptom prevalence are considerably lower in controlled studies, highlighting the importance of scientific quality in investigating emerging phenomena such as post-COVID syndromes.
Alt-text: Unlabelled box
Introduction
Children and young people (CYP) are more likely to be asymptomatic or develop a mild, transient illness following SARS-CoV-2 infection compared to adults, whose risk of severe COVID-19, hospitalisation and death increases with age. Whilst most CYP recover quickly, a small proportion may have on-going symptoms persisting for weeks to months after SARS-CoV-2 infection.
There are a number of terms in use to describe post-COVID symptoms. “Long-COVID” is a term created by patients in May 2020 as a hashtag on social media outlet Twitter.1 , 2 Other descriptions include “long-haul COVID”, “Post COVID-19 syndrome”, “Chronic COVID syndrome (CCS) and “post-acute sequelae of COVID-19 (PASC), the latter a term mostly used in the United States (US).3, 4, 5 Persistent post-COVID symptoms are emerging as a broad spectrum of manifestations in adults and CYP. The syndrome has been described as a complex multisystem disease appearing during the typical convalescent phase of illness, with persistent, heterogenous and recurring symptoms which may wax and wane, lasting beyond four weeks from the date of SARS-CoV-2 infection.6 , 7 There is no universally accepted standardised case definition of the syndrome, but despite this lack of consensus, different categorisations are emerging. In the United Kingdom, the National Institute for Health and Care Excellence (NICE) working guidelines have developed terminology that can be used to describe post COVID-19 syndrome.4 “Ongoing symptomatic COVID-19″ is defined as signs and symptoms that persist between 4 and 12 weeks from onset of the infection and “Post COVID-19 syndrome” is defined as signs and symptoms persisting beyond 12 weeks from the date of onset.4 Alternatively, the US Centres for Disease Control and Prevention (CDC), define “Post COVID-19 Conditions” as an umbrella term for a wide range of health consequences that are present more than four weeks after acute infection.8 Furthermore, the UK National Institute for Health Research (NIHR) has proposed that post COVID-19 syndrome may consist of different clinical syndromes comprising of post-intensive care syndrome, post-viral fatigue syndrome, long-term COVID-19 syndrome and chronic illness which may arise from organ damage due to COVID-19, with patients potentially suffering from more than one syndrome and some experiencing different clusters and patterns of symptoms.9 , 10 An Italian study following hospitalised patients after discharge noted three different syndromes, separating those related to post-viral chronic fatigue to those due to post-critical illness syndrome or post-traumatic stress disorder.11 , 12
Whilst CYP generally experience less severe COVID-19 than adults, there is emerging evidence that CYP may also develop post-acute symptoms of COVID-19. This condition is distinct from “Paediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS)” or “Multisystem Inflammatory Syndrome in Children (MIS-C)”, a novel paediatric hyperinflammatory disease phenotype with features of Kawasaki disease and Toxic Shock Syndrome that typically occurs 2–4 weeks after SARS-CoV-2 infection in CYP.13, 14, 15, 16, 17, 18
Follow-up of adults with COVID-19 has identified multiple persistent and highly variable longer-term symptoms, including fatigue, persistent cough, low-grade fever, headache, chest pain, hair loss, loss of taste and smell amongst many others.7 , 19 , 20 CYP have also been reported to develop similar symptoms after acute SARS-CoV-2 infection, including fatigue, chronic cough, myalgia, headache, cognitive impairments, dyspnoea and chest pain.21, 35, 39 Because of a lack of consensus about case definitions, estimates of post COVID-19 syndrome prevalence range from very low to very high rates across different studies, and the existing literature is dominated by small, uncontrolled and often single-centre studies, although controlled studies are beginning to emerge. The high prevalence of many somatic symptoms in healthy teenage populations, particularly headache and fatigue,22 means that uncontrolled studies may inflate post COVID-19 syndrome prevalence, making comparison with non-infected control groups critical. While narrative reviews are beginning to emerge,23 there is an urgent need for systematic review and meta-analysis of existing literature, particularly focusing on controlled studies.
This systematic review and meta-analysis was undertaken to estimate the prevalence of persistent symptoms following SARS-CoV-2 infection compared with uninfected controls and to identify potential risk factors associated with development of post-COVID symptoms in CYP.
Methods
This systematic review was performed according to PRISMA guidelines,24, 25, 26 the protocol was registered with PROSPERO on 01 Mar 2021 (Reference: CRD42021233153).
Eligibility
Studies meeting the following criteria were included:
-
1
Population: CYP aged ≤19 years with confirmed evidence of SARS-CoV-2 infection (Reverse transcription polymerase chain reaction (RT-PCR), lateral flow antigen test (LFT) or serology) or probable COVID-19 (clinician defined or suspected COVID-19) who have persistent symptoms as defined by the study authors. We included studies reporting participants from any source but excluded studies where all participants were admitted to intensive care to increase generalisability. Studies including participants of all ages but reporting CYP outcomes separately were eligible.
-
2
Study type: any study design excluding systematic reviews or other reviews. We included published, preprint and grey literature.
-
3
Outcomes: the type, prevalence and duration of persistent symptoms in the study population or risk factors for development of persistent symptoms in CYP. We included all symptoms described in each eligible study and included all studies of persistent symptoms regardless of time after infection.
There were no restrictions or limitations on language, date of acceptance or of publications of studies. Google translate was used to translate any non-English publications.
Searches
A systematic search was conducted by the primary reviewer (SAB) from 1st December 2019 to 31st July 2021 in 7 electronic databases (MEDLINE (via OVID), EMBASE (via OVID), CINAHL (via EBSCO), ProQuest Coronavirus Research Database, COVID-19 Living Overview of the Evidence (L-OVE) subset of Episteminokos, Cochrane Covid-19 Study Registry and the World Health Organization (WHO) Covid-19: Global literature on coronavirus disease) and 5 preprint databases (ZBMed's preview database of COVID-related preprints from medRxiv, bioRxiv, ChemRxiv, ResearchSquare and preprints.org). We supplemented searches by a) manual searching of various COVID-19 specialised sources to identify published, unpublished and grey literature (NICE evidence reviews, Up to Date, COVID-END, CADTH COVID-19 pandemic database, Centre for Evidence-based Medicine-Oxford COVID-19 Evidence Service, Cochrane COVID Review Bank, National COVID-19 Clinical Evidence Task Force, John Hopkins centre for humanitarian help, Don't Forget the Bubbles, and BMJ Best Practice COVID-19); cross-examined reference lists in published reviews for relevant studies and forward search of citations through Google Scholar; searching of reference lists of all included studies; and identifying studies through our professional networks. Each database was searched by using medical subject heading (MeSH) terms and free words including synonyms (in the title and abstract) for the concepts “COVID-19″, “children”, “adolescents”, “long-COVID”, “sequelae” and “persistent symptom” (combined with the Boolean logic operation “OR”/ “AND”, (Table A2)).
Study selection and data extraction
Titles and abstracts of all studies were screened independently by SAB and independently verified by a second reviewer (SF), with disagreements resolved by consensus or a third reviewer (OS). Data including methods of diagnosis of infection, recruitment source, study characteristics, symptom prevalence and population demographics, were extracted independently by SAB and SB with disagreements resolved by consensus.
Risk of bias
The methodological quality of included studies was assessed independently by SAB and a second assessor (AZ) using the Newcastle-Ottawa Scale (NOS) for observational studies.27 , 28 The Joanna Briggs Institute (JBI) critical appraisal checklist was used for the cross-sectional and case-series studies.29 , 30
Analyses
The primary analysis was restricted to controlled studies: participants with confirmed SARS-CoV-2 infection (cases) were compared with subjects who tested negative for SARS-CoV-2 (controls). We used random effects meta-analyses to examine the pooled risk difference in prevalence of each symptom or symptom combination in cases with confirmed SARS-coV-2 infection compared with controls. Analyses were undertaken in R using the metafor package. I 2 estimates the proportion of the variance across study estimates that is due to heterogeneity and was considered as small if I 2 < 50%, and large if statistical heterogeneity between the results of the studies was I2 ≥ 50%. Given that different patterns and numbers of symptoms were reported by different studies, meta-analysis was only undertaken for symptoms with ≥3 studies providing data. The small number of controlled trials meant that we were unable to undertake meta-regression of study-level moderators nor examine publication bias.
Our secondary analyses examined the pooled prevalence of persistent symptoms only in CYP post-COVID, including uncontrolled studies and positive cases from controlled trials, and used meta-regression to examine study-level factors hypothesised to be associated with prevalence of symptoms. Study-level factors included compositional factors related to study population (mean age and proportion of females, both of which were hypothesised to be associated with higher prevalence), duration of follow-up (hypothesised to be associated with lower prevalence) and study quality factors (study size, risk of bias, recruitment source and degree to which participants had objectively confirmed infection). Because there were a wide range of reported persistent symptoms (many in only a small number of studies) we conducted meta-analysis and meta-regression only for symptoms where 8 or more studies provided data. Because multiple analyses were undertaken, only associations with p<0.01 were considered significant. We did not investigate publication bias given the recency of this literature and due to poor performance of standard tests in prevalence studies.31 Data for symptoms with <8 studies were described but not pooled. Where individual studies identified predictors of symptom prevalence, we reported these descriptively, but data did not allow for pooling of these results.
Results
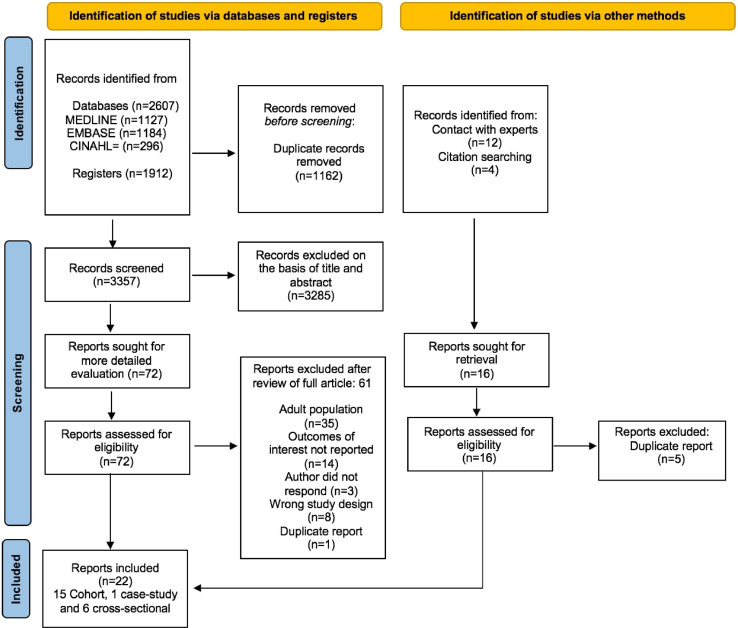
The search flow is shown in Fig. 1 . We identified 3357 articles after removal of duplicates 72 were reviewed in full-text and 22 were included in the review32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53: Half of the studies (n = 11) were identified through databases and registers and the other half through other methods. Included studies are described in Table 1 . Fifteen (68%) were cohort studies32, 36, 37, 38, 40, 41, 43, 44, 45, 46, 48, 50, 51, 52, 53, six (27%) cross-sectional studies33, 34, 35, 42, 47, 49 and one was a case report39. Eight of the 22 studies included population-based control groups32, 36, 42, 43, 46, 49, 52, 53. Nine (41%) recruited from a mix of previously hospitalised and non-hospitalised CYP34 , 35 , 41, 42, 43 , 45 , 48, 49, 50 nine (41%) recruited from non-hospitalised CYP,32 , 33 , 36 , 38 , 39 , 46 , 40, 52, 53 and four (18%) recruited hospitalised CYP post-discharge.37 , 44 , 47 , 51 One study of non-hospitalised CYP34 included CYP from an on-line post COVID-19 syndrome support group of participants who considered their CYP to have post COVID-19 syndrome.
Fig. 1.
PRISMA 2020 flow diagram for included studies.
Table 1.
Characteristics of Included Studies
| Study ID (author) | Country | Sample size (n) | Study Design | Age (years) mean±SD median (IQR) or [Range] | Sex (% Female) | Baseline severity of COVID-19 | Diagnostic Criteria | Duration of Follow-up: mean±SD, median (IQR) or [Range] | Pre-existing Comorbidities | Inclusion Criteria |
|---|---|---|---|---|---|---|---|---|---|---|
| Blankenburg32 | Germany | 188 Seropositive | Cohort (Preprint) | Seropositive: 15 (14-17) | 55% Seropositive | NR | Serology (100%) | NR | NR | 14-17 year-old students in 14 secondary schools with seroprevalence assessment |
| 1365 Seronegative | Seronegative: 15 (14-16) | 56% Seronegative | ||||||||
| Brackel33 | The Netherlands | 89 | Cross-sectional | 13 (9-15) | NR | 18% hospitalised | RT-PCR - 53%, Serology - 35%,CD - 38%,Suspected -9% | ≥12 weeks after diagnosis of COVID-19 | NR | CYP referred to pediatricians across hospitals in The Netherlands for long-COVID assessment |
| Buonsenso (a)34 | UK | 510 | Cross-Sectional (Preprint) | 10.3±3.8 | 56% | 12% asymptomatic, 74% managed at home, 4% hospitalised, 9% attended hospital (not admitted) | RT-PCR-28%,LFT-1%,CD-31%, Suspected 41% | >4 weeks after symptom onset | 56% had comorbidities | CYP with symptoms persisting for more than 4 weeks included. Self-selected from online patient group |
| Buonsenso (b)35 | Italy | 129 | Cross-Sectional | 11±4.4 | 48% | 26% asymptomatic, 74% symptomatic, 5% hospitalised, 2% PICU | RT-PCR (100%) | 163 ±114 days after microbiological diagnosis | 10% neurological, 5% skin problems, 4% asthma, 3% allergic rhinitis | All CYP ≤18 years diagnosed with microbiologically confirmed COVID-19 presenting to single hospital |
| Chevinsky36 | USA | 305 inpatients2,368 outpatients | Matched cohort | Range [≤1-17] | 44% inpatient51% outpatient | NR | CD (100%) | [Range: 31-120 days] after diagnosis of COVID-19 | NR | CYP aged <18 years identified from all payer databases including inpatient and outpatient data from April-June 2020 |
| Denina37 | Italy | 25 | Cohort | 7.8 [Range: 0.4-15] | 52% | 28% mild, 56% moderate, 16% severe | Serology or RT-PCR | 130 days from discharge (IQR 106–148) | 1 cystic fibrosis 1 congenital heart disease | CYP admitted with COVID-19 from March 1 to June 1, 2020 |
| Dobkin41 | USA | 29 | Cohort | 13.1±3.9 [Range: 4-19] | 59% | 93% symptomatic, 14% hospitalised, 3% MIS-C | RT-PCR or confirmed close household contacts with positive SARS-CoV-2 testing | 3.2 ± 1.5 months[Range: 1.3-6.7 months] after SARS-CoV-2 PCR testing or confirmed close household contact | 62% overweight / obese,38% asthma | CYP referred to pulmonary clinic at single hospital with history of SARS-CoV-2 positivity or confirmed close household contact |
| Knoke42 | Germany | 73 SARS-CoV-2 +45 SARS-CoV-2 - | Cross-sectional(Preprint) | SARS-CoV-2 +: 10.8±-3.3SARS-CoV-2 - : 10±3.5 | 52%62% | 36% symptomatic,64% asymptomatic | Serology or RT-PCR | 2.6 months [Range 0.4–6.0] “following COVID-19” | SARS-CoV-2 +: 23% pulmonary diseaseSARS-CoV-2 -10% pulmonary disease | SARS-CoV-2 positive CYP 5-18 years, both inpatients and outpatients or seropositive from community study. Seronegative children served as controls |
| Ludvigsson39 | Sweden | 5 | Case report | 12 [Range: 9-15] | 80% | 100% mild disease | CD (100%) | 6-8 months after clinical diagnosis of COVID-19 | 1 comorbidity (asthma, allergies and mild autism spectrum disorder) | Inclusion of CYP whose parents contacted the study author after experiencing symptoms more than 2 months after clinical diagnosis of COVID-19 |
| Miller38 | England and Wales | 4678 (175 with evidence of past or present SARS-Cov-2 infection) | Cohort (Preprint) | Age <2: 7% Age 2-11 years: 54% Age 12-17 years: 39% | 41% | NR | 63% RT-PCR, 27% serology, 10% RT-PCR and serology | ≥28 days after symptom onset | 8% had at least 1 comorbidity | Household cohort study. CYP ≤17 years who “a) had answered the questions about persistent symptoms in the 3rd monthly survey or b) whose household had participated in at least 3 weekly surveys in a 5-week period before 20th of January 2021” |
| Molteni43 | UK | 1734 cases 1734 controls | Cohort | Cases: 13 (10-15)Controls: 13 (10-15) | Cases 50%,Controls 50% | 2% of cases visited hospital2% of controls visited hospital | RT-PCR or lateral flow test | ≥28 days after diagnosis of COVID-19 | 13% cases had asthma13% controls had asthma | Data from a mobile smartphone application. Cases: CYP 5-17 years with positive SARS-CoV-2 test Controls: CYP 5-17 years with negative SARS-CoV-2 test |
| Nogueira López40 | Spain | 8 | Cohort | 11.8 (9.8-13.9) | 50% | None hospitalised | 25% RT-PCR, Otherwise CD or confirmed COVID-19 contact | 52.5 (25–60.5) days after diagnosis with COVID-19 | 13% had comorbidities | CYP ≤18 years old with confirmed or probable diagnosis of COVID-19 followed up after discharge from hospital between March and June 2020 |
| Osmanov44 | Russia | 518 | Cohort | 10.4 (3–15.2) | 52% | None hospitalised, 3% required ventilation | RT-PCR (100%) | 256 days (223-271) after hospital admission | 27% had 1 comorbidity, 17% had ≥2 comorbidities | CYP ≤18 years old with RT-PCR confirmed SARS-CoV-2 infection admitted to single hospital between April and August 2020 |
| Petersen45 | Faroe Islands | 21 | Cohort | [Range: 0-17] | NR | None hospitalised | RT-PCR (100%) | 125± 17 days [Range: 45-153] after symptom onset | NR | All consecutive RT-PCR positive patients in the Faroe Islands from March to April 2020 |
| Radtke46 | Switzerland | Seropositive 109 | Cohort | [Range: 6-16] | 53% seropositive, | None hospitalised | Serology (100%) | >4 weeks,>12 weeks and6-month follow-up after serological testing | 16% had 1 comorbidity in seropositive group | Children from 55 randomly selected primary and secondary schools in Zurich in October/November 2020. Seropositive (cases) and seronegative (controls) |
| Seronegative 1246 | 54% seronegative | 20% had 1 comorbidity in seronegative group | ||||||||
| Rusetsky47 | Russia | 79 | Cross-sectional | 12.9±3.4 | 53% | All hospitalised | RT-PCR (100%) | 60 days after hospital discharge | NR | CYP ≥5 years admitted with SARS-CoV-2 at single hospital |
| Sante49 | Italy | 12 Long-COVID | Cross-sectional | Long-COVID: 10.3±4.5 | 33% Long-COVID | Long-COVID: 8% asymptomatic 92% mild, 0% hospitalised | RT-PCR (100%) | 98.5 ± 41.5 “days after acute SARS-CoV-2 infection” | Long-COVID: 25% had comorbidities | CYP “fully recovered or with PASC assessed in a dedicated post-COVID outpatient service” |
| 17 Recovered | Recovered: 7.7±5.5 | 36% Recovered | Recovered: 12% asymptomatic, 59% mild, 18% moderate, 12% severe, 29% hospitalised | Recovered: 18% had comorbidities | ||||||
| Say48 | Australia | 12 | Cohort | 3.7±3.5 | 42% | 92% mild, 8% severe50% admitted to hospital | “Children who tested positive for SARS-CoV-2” | [Range 3-6 months] after diagnosis | 17% chronic respiratory condition, 8% congenital cardiac disease | CYP aged ≤18 years referred to a dedicated COVID-follow up clinic |
| Smane50 | Latvia | 30 | Cohort | 9.2±5.2Range [3 months-17 years] | 43% | 17% asymptomatic80% mild, 3% moderate,17% hospitalised | RT-PCR (100%) | 101 ± 7 days after infection | 23% had comorbidities | SARS-CoV-2 positive CYP 0-17 years enrolled at a post-acute outpatient centre |
| Stephenson53 | England | 3065 RT-PCR +3739 RT-PCR - | Cohort (Preprint) | Age: 11-15PCR + (56%)Age: 16-17PCR + (44%)64% PCR +63% PCR - | 65% of PCR + asymptomatic35% of PCR + symptomatic | RT-PCR (100%) | 14.9 weeks (13.1-18.9) after testing | NR | SARS-CoV-2 PCR-positive CYP aged 11-17 years selected from a national database of test results held by Public Health England from January-March 2021 | |
| Age: 11-15PCR - (57%)Age: 16-17PCR - (43%) | 92% of PCR - asymptomatic8% of PCR- symptomatic | |||||||||
| Sterky51 | Sweden | 55 | Cohort | [Range: <1-18] | 42% | 9 children had MIS-C, 2 of which required ICU | RT-PCR (100%) | 219 days (123-324) after hospital admission | 35% had comorbidities | CYP aged 0-18 years who were admitted to one of the two paediatric hospitals in the Stockholm Region and RT-PCR positive for SARS-CoV-2 |
| Other reasons for admission: 38% dehydration, 35% “infection observation”, 23% for “inhalations” | ||||||||||
| Zavala52 | UK | Case: 472 | Cohort (Preprint) | 10 (6-13) | 50% cases, | Cases: 68% symptomatic, 32% asymptomatic | RT-PCR (100%) | >1 month after testing | 7% had one or more co-morbidities | CYP aged 2-16 years tested for SARS-CoV-2 by RT-PCR identified from the national testing data in England during the first week of January 2021 |
| Control: 387 | 47% controls | Controls: 40% symptomatic 60% asymptomatic | ||||||||
NOTE: Data are means ± standard deviations, medians with interquartile ranges (IQR) or [ranges]. Abbreviations: RT-PCR: Positive Reverse transcription Polymerase chain reaction; NR: not reported; CD: Clinical Diagnosis, LFT: Lateral Flow Test; MIS-C: Multisystem Inflammatory Syndrome in Children; ICU: Intensive Care Unit; PICU: Paediatric Intensive Care Unit; PASC: Post-Acute Sequelae of SARS-CoV-2
Ten studies were assessed to have high risk of bias34, 37, 38, 40, 41, 44, 45, 48, 50, 51, six moderate32, 33, 35, 42, 47, 49 and six low risk of bias36, 39, 43, 46, 52, 53 (Table A4). All studies were published during 2020–21 and included participants from high and upper middle income countries; Australia, Faroe Islands, Germany, Italy, Latvia, the Netherlands, Russia, Spain, Sweden, Switzerland, United Kingdom, and the United States. Eight were in pre-print.32 , 34 , 38 , 41 , 42 , 49 , 53 , 52 Sample size ranged from 5 to 6804 CYP with a total of 23,141 participants (median 109). Eleven studies included less than 100 participants. All studies assessed outcomes at >4 weeks after infection (range 28- 324 days), with 15 (68%) assessing outcomes at >12 weeks. Across all studies, 101 symptoms were reported, with 46 symptoms reported in at least 2 studies and 32 symptoms reported in at least 3 studies (Table A5).
Controlled studies
Five controlled studies provided sufficient data for meta-analyses32, 43, 46, 52, 53. Four were community studies32, 46, 52, 53 and one included a mix of hospitalised and non-hospitalised CYP and hospital recruitment43. All were rated as good (four studies) or fair (one study) quality. One study used self-reported evidence of SARS-CoV-2 infection43 with the other four studies reporting evidence where results were independently verified32, 46, 52, 53.
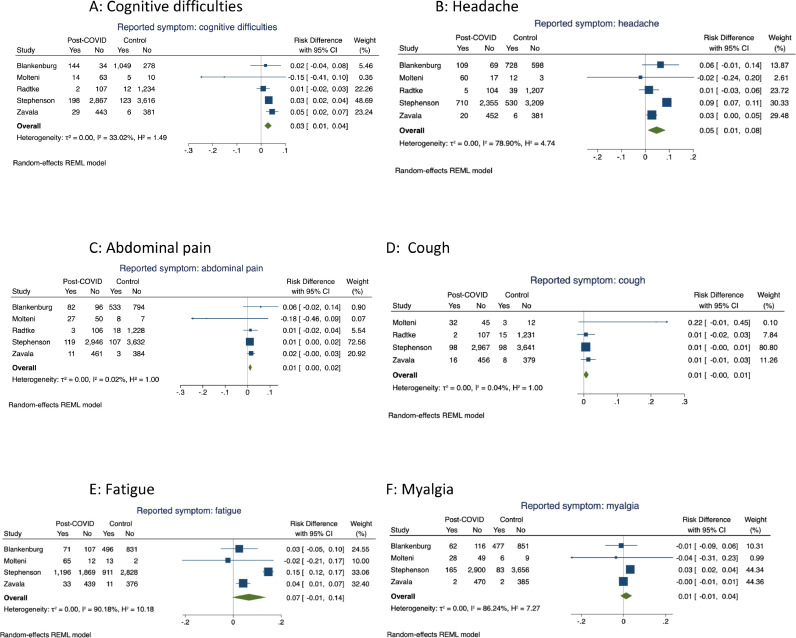
Meta-analyses were undertaken for 14 symptoms within the controlled studies. Four or more controlled studies provided data on cognitive difficulties, headache, abdominal pain, cough, myalgia and fatigue, with forest plots for these meta-analyses shown in Fig. 2 . There were significantly higher pooled estimates of proportions of symptoms in the cases with confirmed SARS-CoV-2 infection for cognitive difficulties (pooled risk difference 3% (95% CI 1, 4)) and headache (5% (1, 8)) but not for abdominal pain, cough, fatigue or myalgia. Heterogeneity was low for cognitive difficulties, abdominal pain and cough but high for headache, fatigue and myalgia.
Fig. 2.
Meta-analyses of risk difference in symptom prevalence between cases and control participants in controlled studies: analyses including symptoms reported in 4 or more studies.
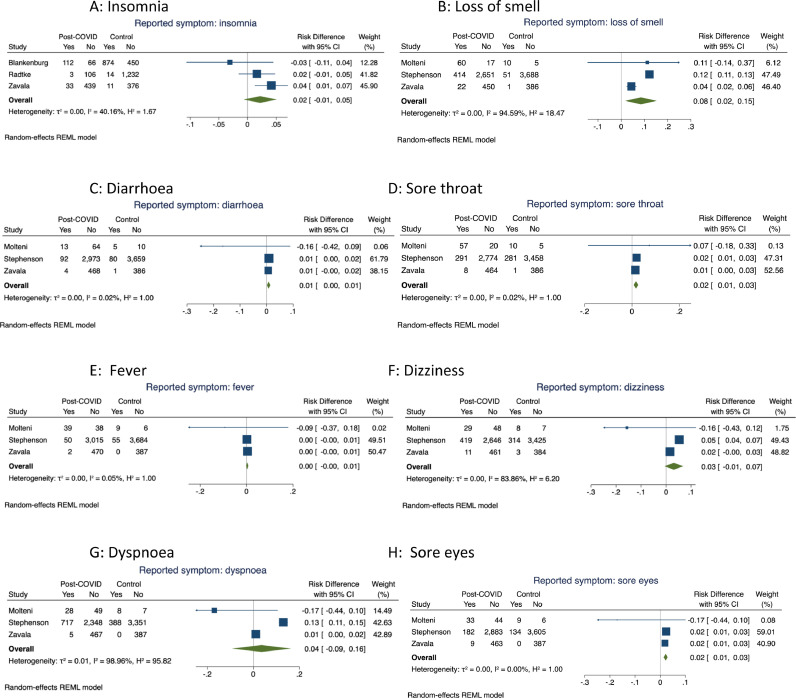
Pooled estimates for symptoms where only three studies provided data are shown in Fig. 3 (insomnia, loss of smell, diarrhoea, sore throat, fever, dizziness, dyspnoea and sore eyes). Pooled risk differences were significant for loss of smell (8%, (2, 15)), sore throat (2% (1, 2)) and sore eyes (2% (1, 3)) but not for insomnia, diarrhoea, fever, dizziness or dyspnoea. Heterogeneity was low for insomnia, diarrhoea, sore throat and eyes and fever but high for loss of smell, dizziness and dyspnoea.
Fig. 3.
Meta-analyses of risk difference in symptom prevalence between cases and control participants in controlled studies: analyses including symptoms reported in 3 or more studies.
Only two studies provided data on multiple persistent symptoms and were, therefore, not eligible for meta-analysis. Both studies46 , 53 found no difference in the proportions of cases and controls with 1 or 2 persistent symptoms. One study53 which involved teenagers completing questionnaires about their own health status, found a significantly higher proportion of cases than controls had three or more persistent symptoms (risk difference 14% (12, 16)), whilst another study,46 which used proxy reporting of symptoms by parents, did not find a significant difference (5% (0, 10)).
Other persistent symptoms were reported by <3 studies and therefore not included in the meta-analyses. These included loss of appetite, nausea, vomiting, constipation, swallowing difficulties, joint pain, chest pain/tightness, nasal congestion, tiredness/weakness, chills, palpitations, otalgia, tinnitus, paraesthesia, seizures, altered taste, hypersomnia, listlessness, low mood, mood swings, anxiety, rash, urticaria, blisters/skin peeling, hoarse voice, communication difficulties, blurred vision and hair loss.
Prevalence and predictors of symptoms in post-COVID CYP
Across all study types, 10 symptoms had data from ≥8 studies allowing meta-analysis and meta-regression: cognitive difficulties, headache, fatigue, fever, myalgia, cough, dyspnoea, abdominal pain, diarrhoea and anosmia / altered sense of smell.
Seventeen studies provided data for these analyses: Five studies included SARS-CoV-2 positive cases from controlled studies32, 43, 46, 52, 53 and 12 were uncontrolled studies33, 34, 35, 38, 40, 41, 42, 44, 48, 49, 50, 51. Seven were community studies32, 33, 38, 40, 46, 52, 53, two had hospital recruitment of cases44, 51 and eight had a mix of hospitalised and non-hospitalised CYP recruitment34, 35, 41, 42, 43, 48, 49, 50.
Table 2 shows pooled prevalence (95% CI) of symptoms in SARS-CoV-2 positive CYP, alongside findings from meta-regressions for hypothesised moderators for each meta-analysis. Pooled prevalence of symptoms ranged from 15% (diarrhoea) to 47% (fatigue), with high heterogeneity across all symptom analyses. Meta-regression of study participant characteristics showed that higher study age was associated with higher prevalence of all symptoms with the exception of lower prevalence of cough, and that a higher proportion of female participants was associated with higher prevalence of fatigue, headache, myalgia, diarrhoea, loss of smell and dyspnoea and lower prevalence of cough and abdominal pain.
Table 2.
Pooled estimates and univariable meta-regression coefficients for all studies reporting prevalence estimates (%) of symptoms.
| Pooled estimates |
Meta-regression |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence | N | n | Age | Female proportion | Study size(/100) | Follow-up (months) | Community versus mixed recruitment | Risk of bias: Reference=Low | % confirmed diagnosis | ||
| Moderate risk | High risk of bias | ||||||||||
| Cough | 17(7, 27) | 13 | 4656 | 0.99(0.98,0.99)* | 0.99(0.997,0.99)* | 0.999(0.998,0.999)* | 0.99(0.99,1.00) | 0.85(0.83,0.87)* | 0.99(0.97,1.01) | 1.14(1.11,1.17)* | 0.995(0.994,0.996)* |
| Fever | 18(5, 32) | 8 | 4241 | 1.02(1.01,1.03)* | 1.001(1.00,1.001) | 1.000(0.999,1.000) | 1.00(1.00,1.001) | 0.74(0.71,0.77)* | 1.02(0.98,1.05) | 1.33(1.28,1.38)* | 0.994(0.993,0.995)* |
| Fatigue | 47(32, 62) | 15 | 4817 | 1.09(1.07,1.10)* | 1.014(1.012,1.016)* | 1.002(1.001,1.003)* | 1.02(1.01,1.03)* | 0.74(0.72,0.76)* | 1.12(1.08,1.17)* | 1.45(1.40,1.49)* | 0.988(0.987,0.989)* |
| Headache | 35(19, 51) | 13 | 4795 | 1.12(1.11,1.14)* | 1.009(1.008,1.011)* | 1.001(1.001,1.002)Ψ | 0.99(0.98,0.99)Ψ | 0.66(0.64,0.68)* | 1.16(1.11,1.20)* | 1.56(1.51,1.61)* | 0.986(0.985,0.986)* |
| Cognitive difficulties | 26(8, 44) | 10 | 4264 | 1.15(1.14,1.16)* | 1.000(0.999,1.001) | 0.999(0.998,1.000) | 0.99(0.98,0.99)* | 0.95(0.91, 1.000) | 1.44(1.39,1.49)* | 0.96(0.94,0.98)* | 0.99(0.986,0.993)* |
| Myalgia | 25(11, 40) | 10 | 4665 | 1.10(1.08,1.11)* | 1.004(1.003,1.005)* | 1.001(1.001,1.002)* | 1.01(1.01,1.02)* | 0.65(0.63,0.67)* | 1.20(1.16,1.25)* | 1.28(1.25,1.31)* | 0.985(0.984,0.986)* |
| Abdominal pain | 25(9, 42) | 10 | 4762 | 1.08(1.06,1.09)* | 0.998(0.997,0.999)* | 0.998(0.997,0.998)* | 0.98(0.98,0.99)* | 0.80(0.78,0.81)* | 1.05(1.03,1.08)* | 1.59(1.54,1.64)* | 0.983(0.982,0.984)* |
| Diarrhoea | 15(4, 26) | 8 | 4475 | 1.05(1.03,1.07)* | 1.001(1.00,1.002) | 1.000(0.999,1.001) | 1.00(1.00,1.007) | 0.93(0.91,0.95)* | 1.01(0.98,1.03) | 1.28(1.24,1.32)* | 0.991(0.99,0.992)* |
| Loss of smell | 18(2, 34) | 9 | 3986 | 1.00(0.99,1.01) | 1.004(1.003,1.006)* | 1.003(1.002,1.004)* | 1.01(1.01,1.02)* | 0.95(0.92,0.98)Ψ | 0.91(0.89,0.93)* | 1.05(0.99,1.12) | 1.007(1.005,1.009)* |
| Dyspnoea | 43(18, 68) | 8 | 3882 | 1.28(1.26,1.30)* | 1.021(1.019,1.022)* | 1.007(1.006,1.008)* | 1.05(1.05,1.06)* | 0.50(0.47,0.53)* | 1.67(1.53,1.82)* | 1.25(1.21,1.30)* | 0.99(0.988,0.992)* |
N= number of studies, n=pooled total sample size, Ψp<0.01, *p<0.001
Meta-regression analyses of study characteristics found that some study quality markers (higher proportion of objectively confirmed cases; low risk of bias; community compared with a mix of hospitalised and non-hospitalised CYP recruitment) were consistently associated with lower prevalence of all symptoms, except loss of smell and cognitive symptoms. However, study size was inconsistently associated with symptom prevalence.
The duration of persistent symptoms was reported in 13 studies34, 34, 34 , 38, 39, 40, 41 , 43 , 44 , 48 , 50 , 51 , 53 with a median of 125 days (IQR 99–231) after acute SARS-CoV-2 infection. In meta-regression, longer follow-up duration was associated with lower prevalence of cough, headache, cognitive difficulties, abdominal pain but higher prevalence of fatigue, myalgia, loss of smell and dyspnoea. Not all these associations were significance, hence should be taken as indicative.
Small/limited number of available studies at present meant that we were unable to undertake meta-analysis of number of persistent symptoms nor of a range of other symptoms. These symptoms are reported in Table A6.
Risk factors
Few studies examined risk factors associated with persistent post-COVID symptoms in CYP. Osmanov et al. reported that persistent symptoms were more common amongst CYP aged 6–11 (odds ratio 2.74, 95% CI, 1.37 to 5.75) and those 12–18 years (OR 2.68, 95% CI, 1.41 to 5.4) compared to those aged <2 years, as well as amongst CYP with a history of allergic diseases (OR 1.67, 95% CI, 1.04 to 2.67).44 Molteni et al. reported that older CYP (12–17 years) were more likely to manifest symptoms ≥28 days in comparison with younger CYP (5–11 years) (5.1% vs. 3.1%).43 Miller et al. reported that persistent symptom prevalence was higher in females (OR 1.79 [95% CI, 1.07 to 2.99]), teenagers (OR 2.67 [95% CI, 1.56 to 4.57]) and CYP with long-term health conditions (OR 2.95 [95% CI, 1.59 to 5.45]).38 Females also reported a consistently higher prevalence of neurocognitive and pain symptoms compared to males in Blankenburg et al., with age being positively correlated with nearly all neurocognitive and pain symptoms.32 Stephenson et al. reported that for both SARS-CoV-2-positive and SARS-CoV-2-negative CYP, in those assigned to the latent class with “multiple symptoms” at three months, being female, older and having poorer physical and mental health before COVID-19 were important risk factors.53
Discussion
In this comprehensive systematic review and meta-analysis of 22 studies, we identified 101 symptoms reported to be persistent after SARS-CoV-2 infection in CYP, across cardiovascular, respiratory, gastrointestinal, musculoskeletal, skin and nervous systems as well as general somatic symptoms. Our analyses focused on persistence of individual symptoms and combination of symptoms where these were reported by multiple studies. Data were sufficient for us to examine 14 of the most common symptoms in controlled studies and 10 symptoms in uncontrolled analyses. The lack of an agreed case definition means that we were unable to comment on the prevalence of post COVID-19 syndrome(s) in CYP.
The majority of the included studies were of poor quality, predominantly uncontrolled and retrospective, and open to selection bias. There are a number of reasons why symptoms reported in many of these studies may not be specific to SARS-CoV-2, including the high prevalence of somatic symptoms such as fatigue and headache in healthy CYP, the overlap of symptoms such as fatigue, poor concentration and headache, with mental health symptoms (which rose during the pandemic), and potential attribution bias. Our primary analysis therefore focused on controlled studies and found that the frequency of the majority of reported persistent symptoms was similar in SARS-CoV-2 positive cases and controls. Risk differences for abdominal pain, cough, myalgia, insomnia, diarrhoea, fever, and dizziness were each very close to zero and not significant. However, loss of smell occurred in 8% more cases than controls, as did headaches (5%), cognitive difficulties (3%) and sore throat and eyes (2% each). Fatigue occurred in 7% more cases than controls although confidence intervals included zero. Combinations of persistent symptoms could not be included in meta-analyses but the two studies that considered this found no difference between cases and controls in the proportions with 1 or 2 persistent symptoms. Estimates of the excess proportion of cases with 3 or more symptoms were 5 and 14% in these studies.
The excess in the proportion of cases with specific symptoms compared to controls was much lower than the pooled estimates of symptom prevalence in the secondary analyses of cases alone. This was true across all symptoms studied. Pooled estimates were particularly high for fatigue (47%) and headache (35%), approximately 7-fold higher than in controlled studies, highlighting the importance of including a control group.
Our meta-regressions, whilst performed at study level rather than at the level of individual participants, suggested that older age and female sex were associated with increased risk of persistent symptoms. Higher study quality, community recruitment and test-confirmed diagnosis of infection were each strongly and consistently associated with lower prevalence, highlighting the importance of scientific quality in investigating emerging phenomena such as post-COVID syndromes.
Comparison with the literature
One previous narrative review noted the high prevalence of multiple symptoms in the majority of studies of persistent post-COVID symptoms, however this study did not undertake meta-analysis of symptom prevalence.23 We found that somatic or constitutional symptoms such as fatigue (47%) and headache (35%) were amongst the most commonly reported symptoms in CYP post-COVID. This is consistent with other systematic reviews in adults and CYP,20 , 23 , 54 , 55 yet in controlled studies that accounted for high background prevalence in non-infected CYP, we found that the excess in cases over controls was much lower at 5% (headache) and 7% (fatigue). It is important to note that post-infection fatigue appears to be common in CYP with post COVID-19 syndrome and have also been reported after other human coronaviruses such as Middle East Respiratory Syndrome (MERS) and severe acute respiratory syndrome (SARS) as well as Epstein-Barr, Dengue, Zika, Ebola and Chikungunya viruses.56 , 57 Headache is a commonly reported neurological symptom in acute SARS-CoV-2 infection and can persist after acute infection.58
We found evidence that female sex, underlying comorbidities, and increasing age were associated with increased risk of persistent symptoms after SARS-CoV-2 infection in CYP. For sex this is consistent with a higher risk observed with other post-viral syndromes61 and in adults with post COVID-19 syndrome.23 , 55 , 59, 60, 62
Limitations
Our findings are subject to a number of limitations. Low study quality is discussed above. The majority of the meta-analyses had high heterogeneity, almost certainly due to both measurement issues across studies and to differing samples, recruitment strategies and follow-up times. Because of this we used a random effects meta-analysis to take account of unmeasured between-study factors. Our findings were limited by lack of data for many symptoms, particularly combinations of symptoms. Very few studies provided data on the impact of symptoms on daily functioning amongst CYP. We were unable to assess publication bias; however, this is likely to play less of a role in a highly topical new area.
Some studies were open to misclassification bias, including suspected cases without laboratory confirmation of diagnosis. Definitions and reporting of symptoms differed across studies, and whilst we categorized similar symptoms, together this may have introduced bias. Studies used a mix of child or parent reporting, and some studies had permissive inclusion of symptoms, which may be persistent following acute infection, new-onset of symptoms days to weeks after acute infection, worsening of pre-existing symptoms prior to SARS-CoV-2 infection, as well as waxing and waning of symptoms during follow-up after acute infection. As all participants were aware of their infection status, attribution bias is also likely to have influenced symptom reporting, as seen in other infections.63
Almost all studies (95%) were from high income countries, limiting generalisability for low and middle-income countries. The median duration of follow-up after COVID-19 symptom onset was 125 days (IQR 99–231). This led to substantial disparity in the timelines for symptom onset and assessment in our systematic review and likely influenced the combinability of our estimates of prevalence and symptom duration.
Implications
Persistent symptoms of loss of smell, headaches, cognitive difficulties and sore throat and eyes were estimated to occur in 2 to 8% more CYP after SARS-CoV-2 infection than in those without infection. Two large controlled studies suggest that 5–14% may have multiple persistent symptoms 4 weeks or more after acute infection. However, the majority of the 14 most commonly symptoms reported in CYP post-COVID were no more common in those with documented SARS-CoV-2 infection compared with those without infection. These findings suggest that persistent symptoms occur both singly and in clusters in CYP after SARS-CoV-2 infection, but prevalence is much lower than suggested by many low-quality uncontrolled studies.
Our findings confirm the urgent need to provide health and education services for those with significant post-COVID symptoms and our data provide estimates for planning these. Our review also shows the paucity of data on many aspects of post-COVID symptoms in CYP, particularly on the pathophysiology of symptoms and the functional limitations linked with reported symptoms. Further work is needed to understand frequency of particular clusters of symptoms and severity and functional limitation related to these, in order to inform both preventive and treatment strategies. There is also a need to understand the relationship of mental health problems during the pandemic to symptom clusters in order to prioritise healthcare services and resources to support and minimise the consequences of the pandemic in the CYP population.
Our findings highlight the critical importance of a control group in this area of study. Additional research priorities in developing treatment programs will need to be targeted to symptoms associated with SARS-CoV-2 infection, rather than symptoms which may be attributable to pandemic societal pressures. We hope that this work will act as a stimulus for the design of more high quality prospective controlled studies in this area. Only with these can we really inform the global policy conversation around the health of CYP during the pandemic.
CRediT authorship contribution statement
S.A. Behnood: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. R. Shafran: Conceptualization, Investigation, Methodology, Writing – review & editing. S.D. Bennett: Data curation, Validation, Writing – review & editing. A.X.D. Zhang: Formal analysis, Methodology, Validation, Writing – review & editing. L.L. O'Mahoney: Resources, Writing – review & editing. T.J. Stephenson: Conceptualization, Investigation, Writing – review & editing. S.N. Ladhani: Writing – review & editing. B.L. De Stavola: Formal analysis, Methodology, Visualization, Writing – review & editing. R.M. Viner: Formal analysis, Methodology, Visualization, Validation, Writing – review & editing, Writing – original draft. O.V. Swann: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Supervision.
Declaration of Competing Interest
SAB, RS, SDB, AXDZ, LLO, SNL, BLDS, RMV and OVS have no conflicts of interest. TJS is the Chair of the Health Research Authority for England who reimburse his university for his time. He is not paid personally. He has recused himself from research studies in which he is personally involved and which require ethical approval from the HRA.
Acknowledgments
Acknowledgements
We are grateful to Sherif Fakhry for his valuable contributions as a second reviewer during the screening process.
Funding
None.
Data sharing
No individual patient level data was used during this analysis. Data extracted for this study, including study protocol, individual assessments of study quality and risk of bias in addition to analytical code will be made available following publication. Requests for data and code can be made to the corresponding author, outlining specific data needs, analysis and dissemination plans.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.11.011.
Appendix. Supplementary materials
References
- 1.Perego E., Callard F., Stras L., Melville-Jóhannesson B., Pope R., Alwan N.A. Why the patient-made term 'long COVID' is needed [version 1; peer review: awaiting peer review] Wellcome Open Res. 2020:224. [Google Scholar]
- 2.Callard F., P E. How and why patients made long COVID. Soc Sci Med. 2021;268 doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baig A.M. Chronic COVID syndrome: need for an appropriate medical terminology for long-COVID and COVID long-haulers. J Med Virol. 2021;93(5):2555–2556. doi: 10.1002/jmv.26624. [DOI] [PubMed] [Google Scholar]
- 4.Overview | COVID-19 rapid guideline: managing the long-term effects of COVID-19 | Guidance | NICE [Internet]. Nice.org.uk. 2021 [cited 07 June 2021]. Available from: https://www.nice.org.uk/guidance/NG188.
- 5.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson H. Long-term effects children with long COVID. New Sci. 2021;245(3323):10–11. doi: 10.1016/S0262-4079(21)00303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenhalgh T., Knight M., A'Court C., Buxton M., Husain L. Management of post-acute COVID-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 8.Healthcare Workers [Internet]. Centers for Disease Control and Prevention. 2021 [cited 11 June 2021]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html.
- 9.Yong S.J. Long-haul COVID-19: putative pathophysiology, risk factors, and treatments. Preprintsorg. 2020;53(10):737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Living with Covid19 Second review [Internet]. NIHR Evidence. 2021 [cited 02 October 2021]. Available from: https://evidence.nihr.ac.uk/themedreview/living-with-covid19-second-review/ (accessed 02 October 2021).
- 11.Venturelli S., Benatti S.V., Casati M., et al. Surviving COVID-19 in bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021;149:e32. doi: 10.1017/S0950268821000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann P., Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2021;106(5):429–439. doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]
- 13.Carter M.J., Shankar-Hari M., Tibby S.M. Paediatric inflammatory multisystem syndrome temporally-associated with SARS-CoV-2 infection: an overview. Intensive Care Med. 2021;47(1):90–93. doi: 10.1007/s00134-020-06273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies P., Evans C., Kanthimathinathan H.K., et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4(9):669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swann O.V., Holden K.A., Turtle L., et al. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waseem M., Shariff M.A., Tay E.T., et al. Multisystem inflammatory syndrome in children. J Emerg Med. 2021;S0736-4679(21) doi: 10.1016/j.jemermed.2021.07.070. 00652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395(10239):1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoste L., Van Paemel R., Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. 2021;180(7):2019–2034. doi: 10.1007/s00431-021-03993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carfì A., Bernabei R., Landi F., for the Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv. 2021;11(1) doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peny V., Valind A. Re: case reports and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021;110(4):1372. doi: 10.1111/apa.15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viner R., Clark C., Taylor S., et al. Longitudinal risk factors for persistent fatigue in adolescents. Arch Pediatr Adolesc Med. 2008;162(5):469–475. doi: 10.1001/archpedi.162.5.469. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann P., Pittet L.F., Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. 2021;40(12):e482–e487. doi: 10.1097/INF.0000000000003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 28.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 29.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., Currie M., Lisy K., Qureshi R., Mattis P., Mu P. In: JBI Manual for Evidence Synthesis. JBI. Aromataris E., Munn Z., editors. 2020. Chapter 7: Systematic reviews of etiology and risk. Appendix 7.4 Critical appraisal checklist for case reports. [Google Scholar]
- 30.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., Currie M., Lisy K., Qureshi R., Mattis P., Mu P. In: JBI Manual for Evidence Synthesis. JBI. Aromataris E., Munn Z., editors. 2020. Chapter 7: Systematic reviews of etiology and risk. Appendix 7.5 Critical appraisal checklist for analytical cross-sectional studies. [Google Scholar]
- 31.Hunter J.P., Saratzis A., Sutton A.J., Boucher R.H., Sayers R.D., Bown M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67(8):897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Blankenburg J., Wekenborg M.K., Reichert J., et al. Mental health of adolescents in the pandemic: long-COVID19 or long-pandemic syndrome? medRxiv. 2021 2021.05.11.21257037. [Google Scholar]
- 33.Brackel C.L.H., Lap C.R., Buddingh E.P., et al. Pediatric long-COVID: an overlooked phenomenon? Pediatr Pulmonol. 2021;56(8):2495–2502. doi: 10.1002/ppul.25521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buonsenso D., Pujol F.E., Munblit D., Mcfarland S., Simpson F. Clinical characteristics, activity levels and mental health problems in children with long COVID: a survey of 510 children. Preprints Org. 2021 doi: 10.2217/fmb-2021-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buonsenso D., Munblit D., De Rose C., et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110(7):2208–2211. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chevinsky J.R., Tao G., Lavery A.M., et al. Late conditions diagnosed 1-4 months following an initial COVID-19 encounter: a matched cohort study using inpatient and outpatient administrative data - United States, March 1-June 30, 2020. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021;73(Supplement_1):S5–S16. doi: 10.1093/cid/ciab338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denina M., Pruccoli G., Scolfaro C., et al. Sequelae of COVID-19 in hospitalized children: a 4-months follow-up. Pediatr Infect Dis J. 2020;39(12):e458–e4e9. doi: 10.1097/INF.0000000000002937. [DOI] [PubMed] [Google Scholar]
- 38.Miller F., Nguyen V., Navaratnam A.M., et al. Prevalence of persistent symptoms in children during the COVID-19 pandemic: evidence from a household cohort study in England and Wales. medrxiv. 2021 doi: 10.1097/INF.0000000000003715. 2021.05.28.21257602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ludvigsson J.F. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2020;110(3):914–921. doi: 10.1111/apa.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nogueira Lopez J., Grasa C., Calvo C., Garcia Lopez-Hortelano M. Long-term symptoms of COVID-19 in children. Acta Paediatr. 2021;110(7):2282–2283. doi: 10.1111/apa.15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leftin Dobkin S.C. Proceedings of the American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference. Vol. 203. 2021. Respiratory findings in children post-COVID-19 infection. ATS. [Google Scholar]
- 42.Knoke L., Schlegtendal A., Maier C., Eitner L., Lücke T., Brinkmann F. More complaints than findings - long-term pulmonary function in children and adolescents after COVID-19. medRxiv. 2021 doi: 10.3389/fped.2022.851008. 2021.06.22.21259273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molteni E., Sudre C.H., Canas L.S., et al. Lancet Child Adolesc Health. 2021. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Epub ahead of print 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osmanov I.M., Spiridonova E., Bobkova P., et al. Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur Respir J. 2021;01 doi: 10.1183/13993003.01341-2021. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen M.S., Kristiansen M.F., Hanusson K.D., et al. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1792. ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radtke T., Ulyte A., Puhan M.A., Kriemler S. Long-term symptoms after SARS-CoV-2 infection in school children: population-based cohort with 6-months follow-up. medrxiv. 2021;326(9):869. doi: 10.1001/jama.2021.11880. Short Report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rusetsky Y., Meytel I., Mokoyan Z., Fisenko A., Babayan A., Malyavina U. Smell status in children infected with SARS-CoV-2. Laryngoscope. 2021;131(8):E2475–E2E80. doi: 10.1002/lary.29403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Say D., Crawford N., McNab S., Wurzel D., Steer A., Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021;5(6):e22–ee3. doi: 10.1016/S2352-4642(21)00124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sante GD, Buensenso D, De Rose C, et al. Immune profile of children with post-acute sequelae of SARS-CoV-2 infection (long COVID) medRxiv. 2021;05.07.21256539 [Google Scholar]
- 50.Smane L., Pucuka Z., Roge I., Pavare J., Stars I. Persistent clinical features in paediatric patients after SARS-CoV-2 virological recovery: a retrospective population-based cohort study from a single centre in Latvia. BMJ Paediatr Open. 2020;4(1) doi: 10.1136/bmjpo-2020-000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sterky E., Olsson-Åkefeldt S., Hertting O., et al. Persistent symptoms in Swedish children after hospitalisation due to COVID-19. Acta Paediatr. 2021;110(9):2578–2580. doi: 10.1111/apa.15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zavala M., Ireland G., Amin-Chowdhury Z., Ramsay M.E., Ladhani S.N. Acute and persistent symptoms in children with PCR-confirmed SARS-CoV-2 infection compared to test-negative children in England: active, prospective, national surveillance. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021:ciab991. doi: 10.1093/cid/ciab991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephenson T., Shafran R., De Stavola B., et al. Long COVID - the physical and mental health of children and non-hospitalised young people 3 months after SARS-CoV-2 infection; a national matched cohort study (The CLoCk) Study. Res Sq. 2021 [Internet]. 2021 [cited 2021 Nov 29]. Available from: https://www.researchsquare.com/article/rs-798316/v1. [Google Scholar]
- 54.Michelen M., Manoharan L., Elkheir N., et al. Characterising long-term covid-19: a rapid living systematic review. medrxiv. 2020 [Google Scholar]
- 55.Salamanna F., Veronesi F., Martini L., Landini M.P., Fini M. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. a systematic review of the current data. Front Med. 2021;8:392. doi: 10.3389/fmed.2021.653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amenta E.M., Spallone A., Rodriguez-Barradas M.C., El Sahly H.M., Atmar R.L., Kulkarni P.A. Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis. 2020;7(12):ofaa509. doi: 10.1093/ofid/ofaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Islam M.F., Cotler J., Jason L.A. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue Biomed Health Behav. 2020;8(2):61–69. [Google Scholar]
- 58.Carlos C.R., Gerardo M.M., Jaime O.G., Isauro G.H.L., Dios A.P.J. Prevalence of neurological manifestations in COVID-19 and their association with mortality. Neurol Perspect. 2021;1(1):11–16. doi: 10.1016/j.neurop.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng Fat L., Scholes S., Boniface S., Mindell J., Stewart-Brown S. Evaluating and establishing national norms for mental wellbeing using the short warwick-edinburgh mental well-being scale (SWEMWBS): findings from the health survey for England. Qual Life Res. 2017;26(5):1129–1144. doi: 10.1007/s11136-016-1454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cella M., Chalder T. Measuring fatigue in clinical and community settings. J Psychosom Res. 2010;69(1):17–22. doi: 10.1016/j.jpsychores.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Straus S.E., Tosato G., Armstrong G., et al. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann Intern Med. 1985;102(1):7–16. doi: 10.7326/0003-4819-102-1-7. [DOI] [PubMed] [Google Scholar]
- 62.Aiyegbusi O.L., Hughes S.E., Turner G., et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021 doi: 10.1177/01410768211032850. 01410768211032850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thein H.H., Butler T., Krahn M., et al. The effect of hepatitis C virus infection on health-related quality of life in prisoners. J Urban Health. 2006;83(2):275–288. doi: 10.1007/s11524-005-9015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.