Abstract
Background & Aims
Through FXR and TGR5 signaling, bile acids (BAs) modulate lipid and glucose metabolism, inflammation and fibrosis. Hence, BAs returning to the liver after enteric secretion, modification and reabsorption may contribute to the pathogenesis of non-alcoholic steatohepatitis (NASH). Herein, we characterized the enterohepatic profile and signaling of BAs in preclinical models of NASH, and explored the consequences of experimental manipulation of BA composition.
Methods
We used high-fat diet (HFD)-fed foz/foz and high-fructose western diet-fed C57BL/6J mice, and compared them to their respective controls. Mice received a diet supplemented with deoxycholic acid (DCA) to modulate BA composition.
Results
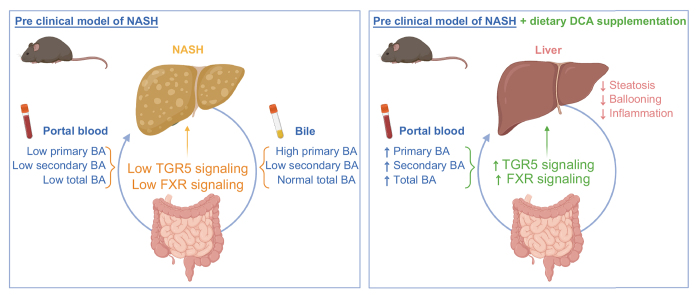
Compared to controls, mice with NASH had lower concentrations of BAs in their portal blood and bile, while systemic BA concentrations were not significantly altered. Notably, the concentrations of secondary BAs, and especially of DCA, and the ratio of secondary to primary BAs were strikingly lower in bile and portal blood of mice with NASH. Hence, portal blood was poor in FXR and TGR5 ligands, and conferred poor anti-inflammatory protection in mice with NASH. Enhanced primary BAs synthesis and conversion of secondary to primary BAs in NASH livers contributed to the depletion in secondary BAs. Dietary DCA supplementation in HFD-fed foz/foz mice restored the BA concentrations in portal blood, increased TGR5 and FXR signaling, improved the dysmetabolic status, protected from steatosis and hepatocellular ballooning, and reduced macrophage infiltration.
Conclusions
BA composition in the enterohepatic cycle, but not in systemic circulation, is profoundly altered in preclinical models of NASH, with specific depletion in secondary BAs. Dietary correction of the BA profile protected from NASH, supporting a role for enterohepatic BAs in the pathogenesis of NASH.
Lay summary
This study clearly demonstrates that the alterations of enterohepatic bile acids significantly contribute to the development of non-alcoholic steatohepatitis in relevant preclinical models. Indeed, experimental modulation of bile acid composition restored perturbed FXR and TGR5 signaling and prevented non-alcoholic steatohepatitis and associated metabolic disorders.
Keywords: NAFLD, NASH, TGR5, FXR, metabolic syndrome
Abbreviations: ASBT, apical sodium-dependent BA transporter; BA, bile acid; CA, cholic acid; CDCA, chenodeoxycholic acid; CYP2A12, bile acid 7α-hydroxylase; CYP27A1, sterol 27-hydroxylase; CYP7A1, cholesterol 7α-hydroxylase; CYP7B1, oxysterol 7α-hydroxylase; CYP8B1, sterol 12α-hydroxylase; DCA, deoxycholic acid; FABP6, fatty acid binding protein 6; FGF15, fibroblast growth factor 15; FGFR4, fibroblast growth factor receptor 4; FXR, Farnesoid X receptor; GLP-1, glucagon-like peptide-1; HFD, high-fat diet; LCA, lithocholic acid; LPS, lipopolysaccharide; αMCA, α-muricholic acid; βMCA, β-muricholic acid; ωMCA, ω-muricholic acid; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; ND, normal diet; OGTT, oral glucose tolerance test; OST, organic solute transporter; SHP, small heterodimer protein; TGR5, Takeda G-protein coupled receptor 5; TLCA, tauro-lithocholic acid; TNFα, tumor necrosis factor α; WDF, western and high-fructose diet; WT, wild-type
Graphical abstract
Highlights
-
•
The enterohepatic bile acid profile is profoundly altered in mice with NASH.
-
•
Bile acid signaling through FXR and TGR5 is dampened in mice with NASH.
-
•
Modulation of bile acid composition prevents obesity, insulin resistance and NASH.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a progressive disease ranging from simple steatosis to non-alcoholic steatohepatitis (NASH). Steatosis is described as the presence of lipid droplets in more than 5% of hepatocytes, while superimposed lobular inflammation and hepatocyte ballooning with variable fibrosis define NASH.1 NAFLD is considered as the hepatic manifestation of the metabolic syndrome and strongly associates with obesity, insulin resistance and type 2 diabetes.1 In line with the rising obesity rates, NAFLD is becoming increasingly common worldwide, with a global prevalence of 25% in the adult population.1 Despite this, the pathogenesis of NAFLD is still not fully understood and efficient pharmacological treatments are lacking.
Primary bile acids (BAs) are synthesized from cholesterol in hepatocytes. After conjugation with taurine or glycine, they are secreted through the bile in the intestine where they enhance the digestion and the absorption of lipids and liposoluble vitamins. In the gut, bacterial enzymes can deconjugate and dehydroxylate primary BAs to form secondary BAs.2 Most of the BAs are reabsorbed and brought back to the liver by the portal flow. A small proportion escapes this enterohepatic cycle and is excreted in feces or enters the systemic circulation.3
Primary and secondary BAs interact with receptors such as the nuclear farnesoid X receptor (FXR) and the membrane Takeda G-protein coupled receptor 5 (TGR5) with specific affinities. Primary chenodeoxycholic acid (CDCA) activates, while tauro α- and tauro β-muricholic acids (Tα- and Tβ-MCA) inhibit FXR.4 Secondary lithocholic and deoxycholic acids (LCA and DCA) have high affinity for and activate TGR5.4 The two receptors are expressed on cells along, but also outside, the enterohepatic cycle. Notably, BA-induced FXR activation in enterocytes stimulates the production of FGF19 (or FGF15 the mouse ortholog), a key component of enterohepatic FXR signaling.5 FXR orchestrates BA metabolism,5,6 while both FXR and TGR5 regulate lipid and glucose homeostasis,[7], [8], [9], [10], [11] energy expenditure,[12], [13], [14] inflammation[14], [15], [16], [17] and fibrosis.18,19 Thereby, any modification of BA pool size and composition would modulate TGR5 and FXR signaling and potentially impact on NAFLD pathogenesis, making BAs attractive candidates for therapeutic development.
Literature reports that explore the association between BA composition and NAFLD are discordant.[20], [21], [22], [23], [24], [25], [26], [27], [28] Proper interpretation of available data is difficult and speculative because the sampling of BAs in humans is often restricted to systemic blood and feces, as sampling of enterohepatic BAs is challenging. BAs in feces and systemic blood have escaped the enterohepatic cycle and thereby do not reflect the BA cycling between the liver and the gut. Hence, the question about alterations of enterohepatic BA pool and their impact on FXR and TGR5 signaling remains elusive in patients with NASH.
With this study, we aimed to evaluate the composition, metabolism and signaling of BAs in the enterohepatic compartment of two validated mouse models of NASH, that recapitulate the metabolic context and the histological hepatic hallmarks of human NASH. We found low BA concentrations and important changes in BA composition of bile and portal blood that resulted in a reduction of BA signaling through FXR and TGR5 in mice with NASH. Dietary DCA supplementation to restore FXR and TGR5 signaling significantly protected from the development of key histological NASH features. Taken together, our data support the contribution of a shift in BA composition to the pathogenesis of NASH in relevant preclinical models, highlighting the therapeutic potential of BA manipulation.
Materials and methods
Animals and diets
Six-week-old male foz/foz (Alsm1-/-) and wild-type (WT, Alms1+/+) mice on a NOD.B10 background,29,30 co-housed in the same cage were fed a high-fat diet (HFD, ResearchDiets D12492) for 12 weeks. As described,31 HFD-fed foz/foz mice (n = 6) were used as a model of obesity, insulin resistance and NASH (NAFLD activity score [NAS] ≥7) while HFD-fed WT mice (NAS ≤2, Fig. S1) served as healthy controls (n = 5-6/group).
Eight-week-old male C57BL/6J mice were fed a normal diet (ND, SAFE diets A03) or a western diet containing 0.5% cholesterol (WD, ResearchDiets D05011404) and 30% fructose in drinking water for 20 weeks (WDF). After 20 weeks, C57BL/6J mice fed a WDF have NASH (NAS ≥7) while C57BL/6J mice fed a ND have no liver disease (NAS ≤1, n = 10/group, Fig. S2).
For modulation of the BA composition, foz/foz mice were randomized into 3 groups based on their body weight and glycemia and received a HFD or a HFD supplemented with 0.03% or 0.1% (w/w) DCA for 12 weeks, and were compared to WT mice fed a HFD for 12 weeks (n = 6-7/group).
Body weight, glycemia and food intake were measured weekly. For sacrifice, mice were fasted for 12 hours then refed for 4 hours (to ensure synchronization for intestinal bile secretion) and anesthetized with ketamine-xylazine. Portal and systemic blood were collected in heparin-coated tubes and plasma stored at -80°C. Bile, liver and distal ileum were harvested, weighed, snap frozen in liquid nitrogen and stored at -80°C or fixed in 4% formalin.
Animal care was provided in accordance with the guidelines for humane care for laboratory animals as per the European regulations and data reported in conformity with ARRIVE guidelines. The study protocol was approved by the university ethics committee for the use of experimental animals under the reference 2016/UCL/MED/016 and 2020/UCL/MD/018.
BA analyses
BAs were extracted from bile, portal and systemic plasma by precipitation with iced methanol.32 The BA species were quantified by high-performance liquid chromatography (UFLC-XR device, Shimadzu) coupled to tandem mass spectrometry (QTRAP5500 hybrid system, equipped with a Turbo VTM ion source, Sciex) using 5 deuterated BAs (d4-cholic acid [CA], d4-glycocholid acid, d4-taurocholic acid, d4-CDCA, d4-glycochenodeoxycholic acid) as internal standards. CA, CDCA, ursodeoxycholic acid, αMCA and βMCA are mouse primary BAs. DCA, LCA, ωMCA and hyodeoxycholic acid are mouse secondary BAs.
TGR5 ligand activity
HEK293T (ATCC CRL-3216) were cultured in DMEM containing 10% FBS and 1% Penicillin/Streptomycin. At 80% confluence in a 96 well plate, cells were transfected with 20 ng of pCMV-SPORT6 human TGR5 (Harvard Medical School MGC:40597), 40 ng of pGL4.29 (CRE-luciferase, Promega) and 5 ng of pGL4.73 (SV40-Renilla, Promega) using Lipofectamine 2000. Twenty-four hours later, cells were incubated with FBS-free medium or FBS-free medium containing tauro-lithocholic acid (TLCA) 10 μM, or portal plasma (20% or 40%) for 3 hours. Then, cells were lysed and assayed according to the Dual-Luciferase Reporter Assay System (Promega E1910). Firefly and renilla luminescences were quantified using a GloMax 20/20 Luminometer. The firefly luciferase signal was normalized to the renilla luciferase signal as an internal control of the transfection rate. The signal is TGR5-dependent as no signal was detected in cells transfected with pGL4.29 and pGL4.73 only, without pCMV-SPORT6 human TGR5 (Fig. S3).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8. Data are presented as mean ± standard deviation and graphed as individual dots as per the ARRIVE guidelines. Outliers were removed based on Grubbs' test. Normality was assessed using the Shapiro-Wilk test. When comparing 2 groups, an unpaired 2-tailed t test or Mann-Whitney test were used to calculate significance. When comparing more than 2 groups, one-way or two-way ANOVA followed by post hoc Bonferroni correction or Kruskal-Wallis followed by Dunn’s multiple comparisons test was used to calculate significance.
For further details regarding the materials and methods used, please refer to the CTAT table and supplementary information.
Results
In HFD-fed foz/foz mice with NASH, enterohepatic BA composition is altered
As previously described,31,33 foz/foz mice fed a HFD for 12 weeks are obese, severely insulin resistant and have developed the histological hepatic features of human NASH (NAS ≥7). Their co-housed WT littermates fed the same HFD for the same duration do not have metabolic or liver disease (NAS ≤2, Fig. S1).
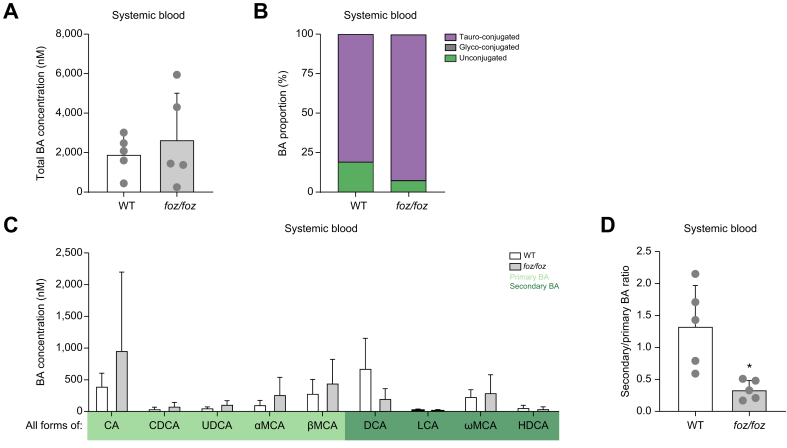
We first quantified and profiled BAs in systemic blood, as commonly sampled in humans. Total BA concentration (Fig. 1A) and proportions of unconjugated and conjugated BAs (Fig. 1B) were similar between foz/foz and WT mice. The concentrations of individual BA species in systemic blood were not significantly different between the 2 groups (Fig. 1C), although the concentrations of CA and DCA tended to be respectively higher and lower in foz/foz mice. The only significant difference was the higher proportion of primary BAs in foz/foz compared to WT mice (Fig. S4A); hence there was a lower ratio of secondary to primary BAs in the systemic blood in foz/foz mice (Fig. 1D).
Fig. 1.
HFD-fed foz/foz mice with NASH do not have an altered systemic BA profile.
(A) Total BA concentrations, (B) proportions of conjugated and unconjugated BAs and (C) individual BA concentrations (sum of conjugated and unconjugated BA) and (D) ratio of secondary to primary BAs in systemic plasma of HFD-fed foz/foz and WT mice (n = 5/group). Mean ± standard deviation. Unpaired 2-tailed t test: ∗p <0.05. BA, bile acid; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; HDCA, hyodeoxycholic acid; HFD, high-fat diet; LCA, lithocholic acid; αMCA, α-muricholic acid; βMCA, β-muricholic acid; ωMCA, ω-muricholic acid; NASH, non-alcoholic steatohepatitis; UDCA, ursodeoxycholic acid; WT, wild-type.
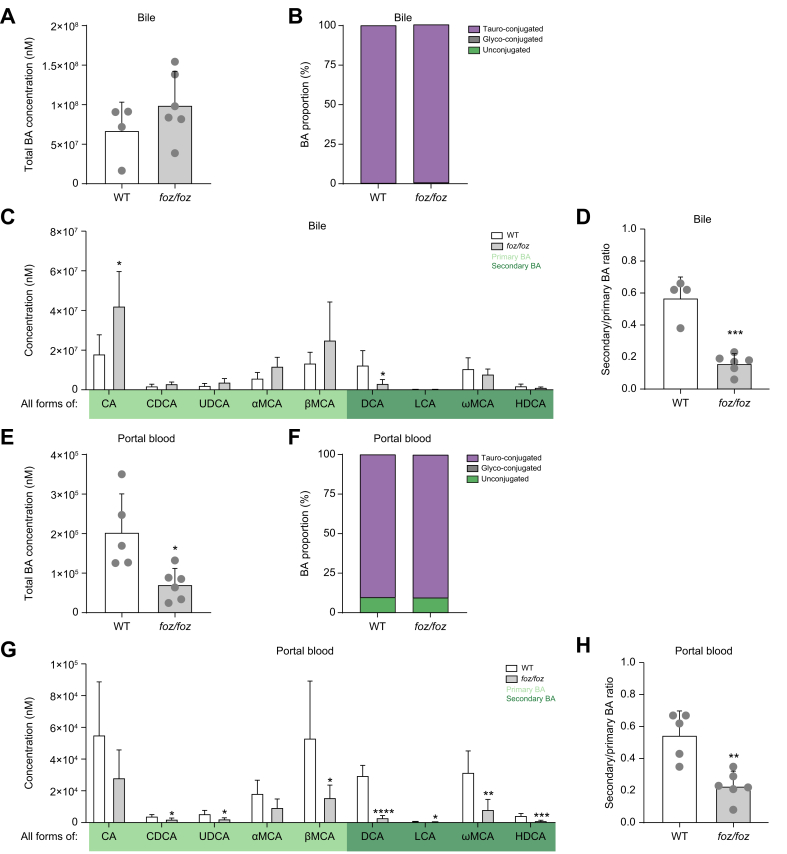
As the systemic BA composition was not significantly altered, we focused on the enterohepatic cycle and profiled BA species in bile and portal blood. In bile, the total BA concentration was similar between foz/foz and WT mice (Fig. 2A) and was composed exclusively of tauro-conjugated BAs (Fig. 2B). The proportion of primary BAs was higher in foz/foz than in WT mice (Fig. S4B), mainly due to a 2-fold higher concentration of CA (Fig. 2C). Accordingly, the proportion of secondary BAs was lower in foz/foz compared to WT mice (Fig. S4B), owed to a lower DCA concentration (Fig. 2C). Hence, the ratio of secondary to primary BAs was 3-fold lower in bile of foz/foz than of WT mice (Fig. 2D).
Fig. 2.
HFD-fed foz/foz mice with NASH have an altered enterohepatic BA composition.
Total BA concentrations, proportions of conjugated and unconjugated BAs, individual BA concentrations (sum of conjugated and unconjugated BA) and ratio of secondary to primary BAs in bile (A-D) and portal plasma (E-H) of HFD-fed foz/foz and WT mice (n = 4-6/group). Mean ± standard deviation. Unpaired 2-tailed t test: ∗p <0.05; ∗∗p <0.01, ∗∗∗p <0.001 and ∗∗∗∗ p <0.0001. BA, bile acid; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; HDCA, hyodeoxycholic acid; HFD, high-fat diet; LCA, lithocholic acid; αMCA, α-muricholic acid; βMCA, β-muricholic acid; ωMCA, ω-muricholic acid; NASH, non-alcoholic steatohepatitis; UDCA, ursodeoxycholic acid; WT, wild-type.
In portal blood, the total BA concentration was significantly lower in foz/foz than in WT mice (Fig. 2E), with similar proportions of conjugated BAs (Fig. 2F) but with a global reduction in the concentration of all primary and secondary BA species (although not significant for CA and αMCA, Fig. 2G). The proportion of secondary BAs was lower in the portal blood of foz/foz than WT mice (Fig. S4C) and, consequently, the ratio of secondary to primary BAs was reduced in the portal blood of foz/foz mice (Fig. 2H).
Altogether, these data suggest that BA composition is significantly altered in the enterohepatic circulation of foz/foz mice with NASH, with a depletion in secondary BAs in bile and portal blood. Interestingly, among all BAs considered, DCA is the only BA whose concentration is significantly different both in the bile and portal blood. By contrast, there is no significant difference in systemic BA profile, indicating that the analysis of BAs in systemic blood is not representative of the enterohepatic BA composition.
In mice with NASH, hepatic FXR signaling is reduced and BA synthesis through the classical pathway increased
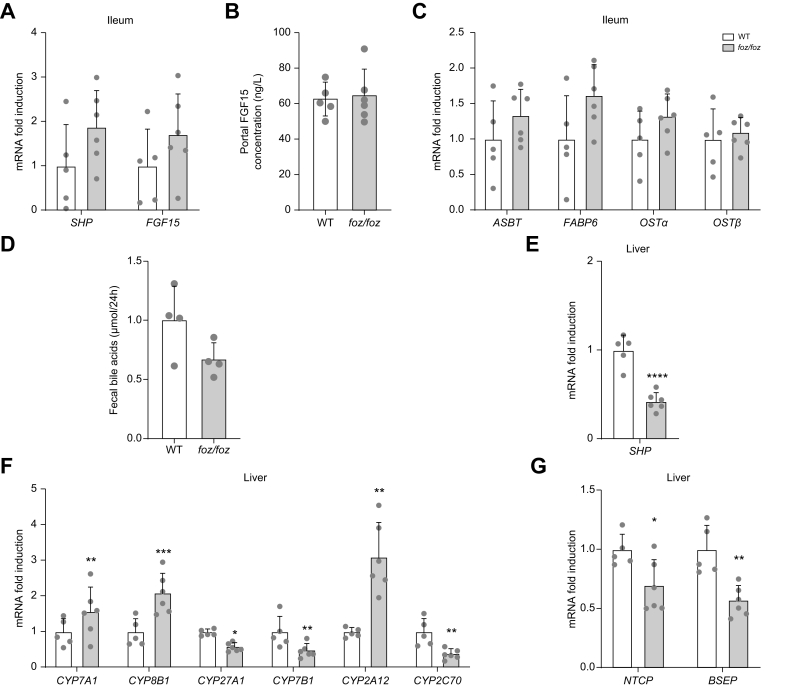
Since FXR regulates BA synthesis and transport, we investigated FXR signaling in the ileum and the liver. When BAs activate FXR in enterocytes, FGF15 is produced, released in portal blood and stimulates the hepatic FGFR4-βKlotho complex, thereby inhibiting hepatic BA synthesis.5 In accordance with similar amounts of biliary BAs secreted by the liver (Fig. S5A-C), the ileal expression of FXR target genes, small heterodimer partner (SHP) and FGF15, as well as the FGF15 portal concentration were similar between foz/foz and WT mice (Fig. 3A-B). The similar ileal gene expression of BA transporters, modulated by FXR activation,3 and similar fecal BA loss in foz/foz and WT mice indicates that intestinal BA reabsorption was not altered (Fig. 3C-D). In hepatocytes, BAs also activate FXR to inhibit BA synthesis in a SHP-dependent manner.6 Compared to WT mice, lower hepatic SHP expression in foz/foz mice supported a reduction in hepatic FXR signaling (Fig. 3E), in accordance with lower total BA concentration in portal blood (Fig. 2E). The expression of CYP7A1 and CYP8B1, key enzymes of the classical pathway for BA synthesis, was accordingly upregulated and that of CYP27A1 and CYP7B1, key enzymes of the alternative pathway, downregulated in foz/foz livers (Fig. 3F). Altogether, this supports a low FXR stimulation and an increased BA synthesis through the classical pathway in foz/foz mice. Interestingly, CYP2A12 and CYP2A22 were induced in the liver of foz/foz mice (Fig. 3F & Fig. S6A). In the mouse liver, CYP2A12 (and likely CYP2A22 which is highly homologous) is the BA 7α-hydroxylase that transforms secondary DCA and LCA back to primary CA and CDCA, respectively.34 In addition, CYP2C70 (that forms α- and β-MCA35,36), the Na+-taurocholate cotransporting polypeptide (NTCP) and the bile salt export pump (BSEP) were downregulated in foz/foz livers (Fig. 3F-G).
Fig. 3.
In HFD-fed foz/foz mice with NASH, hepatic FXR signaling is reduced and BA synthesis through the classical pathway increased.
(A) Ileal expression of genes involved in BA metabolism, (B) concentration of FGF15 in the portal plasma and (C) ileal gene expression of BA transporters in HFD-fed foz/foz and WT mice (n = 5-6/group). (D) Total fecal BA loss per day (n = 4/group). Gene expression of (E) SHP, enzymes involved in BA (F) metabolism and (G) transport in the liver of HFD-fed foz/foz and WT mice (n = 5-6/group). Mean ± standard deviation. Unpaired 2-tailed t test: ∗p <0.05; ∗∗p <0.01, ∗∗∗p <0.001 and ∗∗∗∗ p <0.0001. BA, bile acid; HFD, high-fat diet; NASH, non-alcoholic steatohepatitis; WT, wild-type.
Thus, FXR signaling is reduced in the liver of mice with NASH, but not in the ileum, and as expected associates with increased hepatic BA synthesis.
TGR5 activation is reduced in mice with NASH
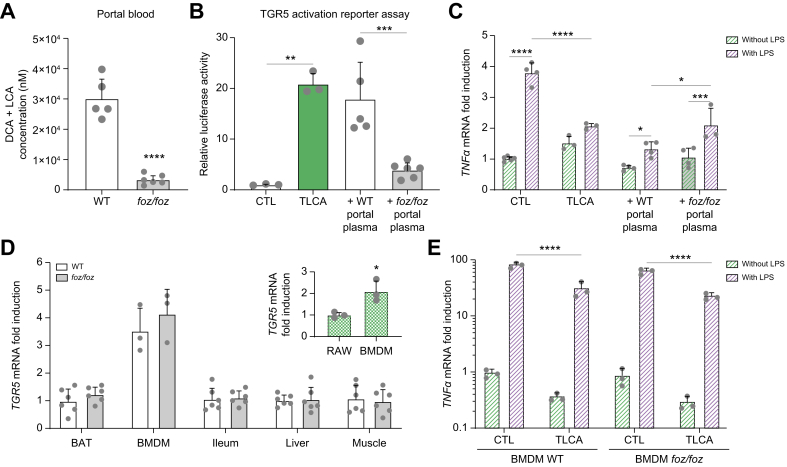
Because DCA and LCA – considered natural TGR5 agonists – were 8-fold less concentrated in the portal blood of foz/foz compared to WT mice (Fig. 4A), we questioned the amount of TGR5 ligands in portal blood by using a TGR5 activation reporter assay. The foz/foz portal plasma was indeed less effective in activating TGR5 than the WT portal plasma (Fig. 4B). TLCA was used as a positive control and the TGR5 dependency was proven, as no signal was detected in cells not transfected with the TGR5 plasmid (Fig. S3).
Fig. 4.
TGR5 activation is reduced in HFD-fed foz/foz mice with NASH.
(A) Total concentration of LCA and DCA in portal plasma of HFD-fed foz/foz and WT mice (n = 5-6/group). (B) TGR5 activation by medium containing TLCA (used as a positive control), WT or foz/foz portal plasma in the cell reporter assay. (C) TNFα gene expression in RAW264.7 macrophages stimulated or not with LPS and treated with medium (CTL) containing TLCA, WT or foz/foz portal plasma (n = 3-4/group). (D) TGR5 gene expression in brown adipose tissue (BAT), BMDMs, distal ileum, liver and quadriceps of WT and foz/foz mice (n = 3-6/group), normalized to TGR5 gene expression in BAT of WT mice. (E) TNFα gene expression in BMDMs of WT and foz/foz mice stimulated or not with LPS and treated with medium (CTL) containing TLCA, normalized to TNFα gene expression in untreated BMDMs WT. For each condition, TNFα was significantly upregulated by LPS. Mean ± standard deviation. Unpaired 2-tailed t test for (A) and for comparison between RAW264.7 and BMDM in (D), One-way ANOVA followed by post hoc Bonferroni correction for (B) and Two-way ANOVA followed by post hoc Bonferroni correction for (C), (D) and (E). ∗p <0.05; ∗∗p <0.01, ∗∗∗p <0.001 and ∗∗∗∗p <0.0001. BMDM, bone marrow-derived macrophage; DCA, deoxycholic acid; HFD, high-fat diet; LCA, lithocholic acid; LPS, lipopolysaccharide; NASH, non-alcoholic steatohepatitis; TLCA, tauro-lithocholic acid; WT, wild-type.
As TGR5 activation is known to reduce the pro-inflammatory cytokine response in macrophages,15,17 we investigated whether a deficit in TGR5 agonists in portal blood promotes inflammation in foz/foz mice. In RAW264.7 macrophages, the lipopolysaccharide (LPS)-induced upregulation of tumor necrosis factor α (TNFα) was significantly blunted when cells where exposed to TLCA or to portal plasma of foz/foz or WT mice (Fig. 4C). However, this reduction was of a lesser magnitude when macrophages were exposed to portal plasma of foz/foz mice (Fig. 4C). Thus, compared to WT, the foz/foz portal plasma is less effective in reducing LPS-induced expression of pro-inflammatory cytokines, in line with higher macrophage activation in the liver of foz/foz mice (Fig. S1F).
Of note, TGR5 was similarly expressed in brown adipose tissue (BAT), bone-marrow derived macrophages (BMDMs), ileum, liver and muscle of foz/foz and WT mice (Fig. 4D). Also, the foz/foz genotype did not alter TGR5 functionality as LPS similarly enhanced TNFα gene expression and TLCA blunted it to the same extent in BMDMs from both genotypes (Fig. 4E). As foz/foz mice carry a mutation in the Alms1 gene involved in primary cilium function30 and as TGR5 might be found on primary cilia of cholangiocytes,37 we verified the hepatic bile flow, the absence of ductular reaction and the gene expression of the apical sodium-dependent BA transporter (ASBT) in the liver of these mice: all the parameters were similar to WT mice (Fig. S5A-F), ruling out that foz/foz mice are ill equipped for TGR5 signaling.
In conclusion, a lower concentration of circulating TGR5 agonists in the portal blood of mice with NASH dampens TGR5 activation.
Alterations in BA signaling are not model specific
To determine whether BA alterations are model specific, we compared C57BL/6J mice fed a western and high-fructose diet (WDF) for 20 weeks to mice fed a ND. WDF-fed mice became obese and insulin resistant (Fig. S2A-C). Glucose tolerance was globally maintained by hyperinsulinemia (Fig. S2B-C). Similarly to foz/foz mice,31 WDF-fed mice had a higher liver weight and lower liver density (a surrogate for liver steatosis) and recapitulated all histological features of NASH, resulting in a NAS >7, whereas ND-fed mice had normal livers (Fig. S2D-G).
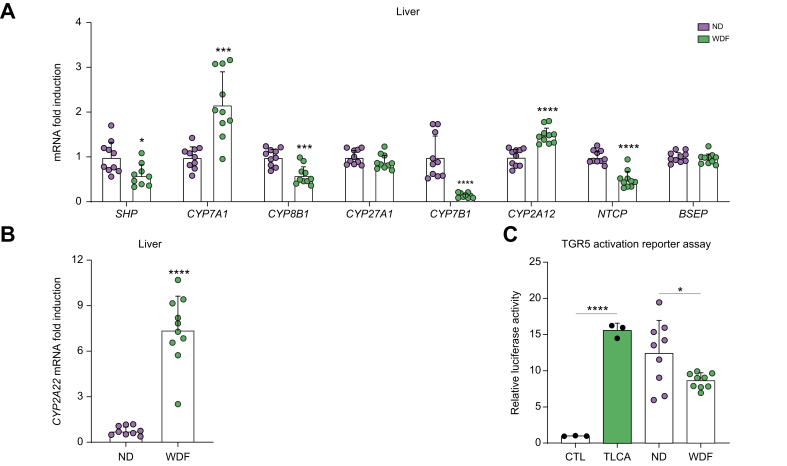
Reminiscent of the changes in foz/foz livers, FXR was less activated in the liver of WDF-fed mice, as shown by a downregulation of SHP expression (Fig. 5A). Accordingly, the gene expression of CYP7A1 was upregulated, and that of CYP8B1 and CYP7B1 downregulated in the liver of WDF- compared to ND-fed mice (Fig. 5A). Notably, CYP2A12 and CYP2A22, converting secondary into primary BAs, were also upregulated in the liver of WDF-fed mice (Fig. 5A-B). Strikingly, TGR5 was also less activated by the portal plasma of WDF- than ND-fed mice (Fig. 5C).
Fig. 5.
Alterations in FXR and TGR5 signaling are not model specific.
(A-B) Hepatic expression of genes involved in BA metabolism and transport. (C) TGR5 activation by medium (CTL) containing TLCA, portal plasma of C57BL/6J fed a ND or a WDF for 20 weeks (n = 9-10/group) in the cell reporter assay. Mean ± standard deviation. Unpaired 2-tailed t test for (A) and (B) and One-way ANOVA followed by post hoc Bonferroni correction for (C): ∗p <0.05; ∗∗∗p <0.001 and ∗∗∗∗p <0.0001. BA, bile acid; ND, normal diet; TLCA, tauro-lithocholic acid; WDF, western high-fructose diet.
Altogether, these results confirm that FXR and TGR5 signaling are reduced in another model of NASH and that the alterations are independent of the genetic background and of the Alms1 mutation.
Modulation of BA composition and recovery of FXR and TGR5 signaling prevent obesity, insulin resistance and NASH
Then, we hypothesized that the altered portal BA composition that compromises FXR and TGR5 signaling contributes to NASH pathogenesis. To test this, we modulated BAs in foz/foz mice to increase FXR and TGR5 activation and study the effect on glucose metabolism and liver pathology. Since DCA concentration was consistently low in foz/foz bile and portal blood (Fig. 2C&G), we supplemented the HFD with 0.03% and 0.1% of DCA for 12 weeks and compared them with foz/foz mice fed a HFD without supplementation.
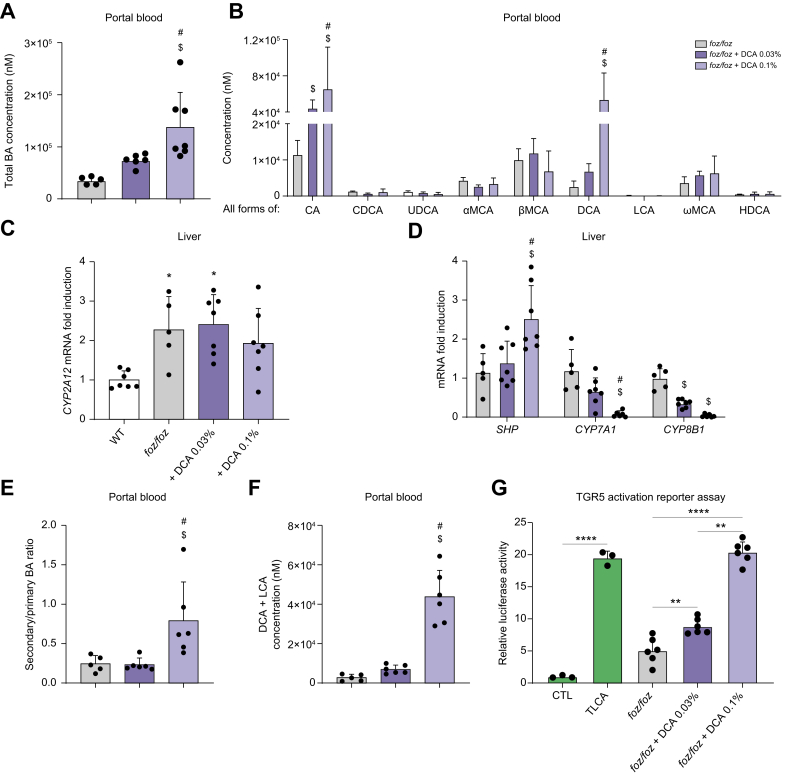
The supplementation with 0.1%, but not 0.03% DCA, increased total BA concentrations in the portal blood of foz/foz mice (Fig. 6A). Feeding foz/foz mice 0.03% DCA failed to significantly increase portal DCA but increased CA (Fig. 6B). The high expression of CYP2A12 and CYP2A22, which convert DCA to CA, in the liver of foz/foz mice (Fig. 6C and Fig. S7A) could explain the increased CA concentration (Fig. 6B). By contrast, 0.1% DCA increased DCA and CA portal concentrations in foz/foz mice (Fig. 6B). In accordance, FXR was activated, as shown by the induction of SHP and the downregulation of CYP7A1 and CYP8B1 in the liver of foz/foz mice treated with 0.1% DCA (Fig. 6D). As expected, because of the higher gut BA concentration, FXR was also activated in enterocytes, as shown by the upregulation of SHP and FGF15 in foz/foz mice treated with 0.1% DCA (Fig. S7B). In addition, while slightly (or not) modified by 0.03% DCA supplementation, the ratio of secondary to primary BAs, the concentration of DCA and LCA considered as natural TGR5 agonists, and the TGR5 ligand activity were strikingly increased in the portal blood of foz/foz mice supplemented with 0.1% DCA (Fig. 6E-G). Thus, supplementation with the secondary BA DCA modulated BA profile and in consequence, restored FXR and TGR5 activation in foz/foz mice.
Fig. 6.
Dietary 0.1% DCA modulates BA composition and restores FXR and TGR5 signaling in HFD-fed foz/foz mice.
(A) Total BA and (B) individual BA concentrations (sum of conjugated and unconjugated BA) in portal plasma of HFD-fed foz/foz, foz/foz + DCA 0.03% or foz/foz + DCA 0.1% mice (n = 5/group). Hepatic gene expression of (C) CYP2A12, (D) SHP and enzymes involved in BA synthesis. (E) Ratio of secondary to primary BAs and (F) total concentration of LCA and DCA in portal plasma (n = 5-6/group) and (G) TGR5 activation by medium (CTL) containing TLCA, portal plasma of foz/foz, foz/foz + DCA 0.03% or foz/foz + DCA 0.1% mice (n = 6/group) in the cell reporter assay. Mean ± standard deviation. One-way ANOVA followed by post hoc Bonferroni correction. Statistical significance (p <0.05) is represented by ∗ when compared to WT; $ when compared to foz/foz and # when compared to foz/foz + DCA 0.03% for (A) to (F) and by ∗∗p <0.01 and ∗∗∗∗p <0.0001 for (G). BA, bile acid; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; HDCA, hyodeoxycholic acid; HFD, high-fat diet; LCA, lithocholic acid; αMCA, α-muricholic acid; βMCA, β-muricholic acid; ωMCA, ω-muricholic acid; TLCA, tauro-lithocholic acid; UDCA, ursodeoxycholic acid; WT, wild-type.
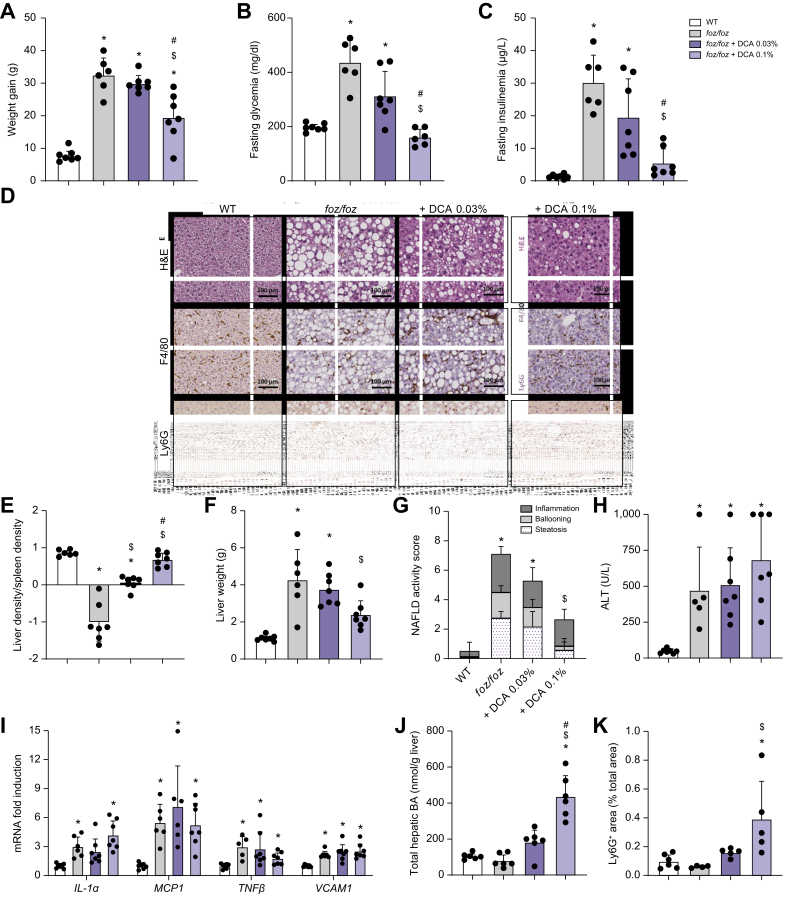
We then investigated the impact of the BA modulation on NASH and associated metabolic features. Feeding the foz/foz mice a HFD supplemented with 0.03% DCA did not alter body weight gain, fat mass, glucose intolerance, fasting hyperglycemia and hyperinsulinemia (Fig. 7A-C & Fig. S7C-G). By contrast, DCA 0.1% supplementation significantly reduced body weight gain and fat mass in HFD-fed foz/foz mice, in spite of slightly increased food intake compared to untreated foz/foz mice (Fig. 7A & Fig. S7C-E). Glucose intolerance, fasting glycemia and insulinemia were also reduced by DCA 0.1% (Fig. 7B-C & Fig. S7F-G).
Fig. 7.
Restoration of the FXR and TGR5 signaling prevents obesity, insulin resistance and NASH in HFD-fed foz/foz mice.
(A) Body weight gain, (B) fasting glycemia and (C) insulinemia of WT, foz/foz, foz/foz + DCA 0.03% and foz/foz + DCA 0.1% mice (n = 6-7/group). (D) Representative H&E, F4/80 and Ly6G staining of liver sections (bar size: 100 μm). (E) Liver density, (F) weight and (G) NAS, (H) plasmatic ALT levels, (I) hepatic expression of pro-inflammatory genes, (J) hepatic concentration of total BA and (K) quantification of neutrophils in the liver of WT, foz/foz, foz/foz + DCA 0.03% and foz/foz + DCA 0.1% mice (n = 4-7/group). Mean ± standard deviation. One-way ANOVA followed by post hoc Bonferroni correction for (A-F) and (H-K), and Kruskal-Wallis followed by Dunn’s multiple comparisons test for (G). Statistical significance (p <0.05) is represented by ∗ when compared to WT; $ when compared to foz/foz and # when compared to foz/foz + DCA 0.03%. ALT, alanine aminotransferase; BA, bile acid; DCA, deoxycholic acid; HFD, high-fat diet; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; WT, wild-type.
Low 0.03% DCA supplementation partly reduced steatosis and corrected liver density but did not significantly change the severity of liver disease, as shown by a marginal reduction in the histological NAS (Fig. 7D-G). Accordingly, serum transaminase levels were unchanged compared to HFD-fed foz/foz mice (Fig. 7H & Fig. S7H). By contrast, 0.1% DCA reduced the liver weight of HFD-fed foz/foz mice and protected them from hepatic steatosis and hepatocellular ballooning (Fig. 7D-G). Despite a reduction in the number of macrophage aggregates (Fig. 7D, F4/80 staining), the histological inflammation score, hepatic Il1β (interleukin-1β), Mcp1 (monocyte chemoattractant protein-1), Tnfα and Vcam1 (vascular cell adhesion molecule 1) mRNA and transaminase levels were unchanged by 0.1% DCA (Fig. 7G-I & Fig. S7H). In the light of high hepatic BA concentration, neutrophilic infiltration and increased number of apoptotic hepatocytes and mitotic figures (Fig. 7D&J-K, Ly6G staining), we suspected a BA-induced hepatotoxicity.38 Owing to the pronounced effect on steatosis and ballooning, the treatment significantly decreased NAS, such that only 1 out of the 7 foz/foz mice treated with 0.1% DCA presented NASH vs. 6 out of the 6 untreated foz/foz mice (Fig. 7G).
Discussion
In this study, we characterized the enterohepatic BA profile, metabolism and signaling in different mouse models of NASH. Our data showed that BA composition was profoundly altered in bile and portal blood of mice with NASH. Indeed, concentrations of total BAs as well as of secondary BAs were lower in mice with NASH compared to controls that do not have liver disease. Hence, BA perturbations resulted in a reduction of BA signaling through FXR and TGR5, with consequences on BA, glucose and lipid metabolism, as well as on the inflammatory response. In addition, we demonstrated the contribution of BAs to NASH pathogenesis, as correcting BA composition restored FXR and TGR5 signaling and prevented the development of NASH and associated metabolic disorders.
Herein, we used validated models of NASH in which, contrary to numerous other mouse models,[39], [40], [41] mice exhibit the histological hepatic features of human NASH in a dysmetabolic context.29 By comparing hyperphagic foz/foz mice and their WT littermates fed the same HFD, we isolated the effects due to dysmetabolism and liver disease from those directly influenced by food composition. By comparing C57BL/6J mice fed a WDF and C57BL/6J fed a ND, we validated that the alterations were independent of the genetic background and the Alms1 mutation. Strikingly, all the perturbations observed in HFD-fed foz/foz mice were validated in WDF-fed C57BL/6J mice.
Mice with NASH have less total BAs in their portal blood, but not in their bile. The concentrations of BAs arriving to the liver are lower in mice with NASH and, consequently, FXR is less activated, SHP downregulated and CYP7A1 upregulated. Thus, increased primary BA production through the classical synthesis compensates for low hepatic exposure to BAs, but completely changes the balance between primary and secondary BAs secreted by the liver. Our data in preclinical models are similar to reports of upregulated CYP7A1 expression and increased BA synthesis in livers of NASH vs. no-NASH patients,23,25,27 which indicate reduced FXR signaling.
The low concentration of total BAs in the portal blood of mice with NASH could be caused by reduced intestinal BA reabsorption. Conjugated BAs are mostly actively reabsorbed by the ASBT into the enterocytes, while unconjugated secondary BAs are mostly passively reabsorbed through diffusion across the intestinal epithelium.3 Logically, loss of ASBT-driven absorption drastically increases BA fecal loss and hepatic synthesis and, consequently, changes the BA pool composition.42 However, BA reabsorption was similar in HFD-fed foz/foz and WT mice, invalidating this hypothesis.
The most striking and constant modification observed in mice with NASH is the low concentration of secondary BAs in all compartments. A defective production of secondary BAs by the gut microbiota might be an explanation. Conjugated BAs can be deconjugated by the bile salt hydrolase activity widely distributed in the commensal bacteria of the small intestine and colon.43 Then, unconjugated primary BAs, under the operation of enzymes produced by anaerobic bacteria, may undergo transformation into 7α-dehydroxylated secondary BAs.2 Thereby, a reduced 7α-dehydroxylase activity could explain the lower concentration of secondary BAs. Gut microbiota tightly control BA metabolism, as in germ-free and antibiotic-treated mice, the BA pool is depleted in secondary BAs.44 However, in our experiment, HFD-fed WT and foz/foz littermates were co-housed, an experimental setting that levels out microbial differences.45 In addition, their ratios of conjugated vs. unconjugated BAs in portal blood, as well as their levels of intestinal FXR activation (directly linked to active BA reabsorption) were similar and invalidate massive perturbation in BA gut deconjugation. By contrast, the BA 7α-hydroxylase CYP2A12 and CYP2A22, expressed in murine hepatocytes, are substantially upregulated in the liver of HFD-fed foz/foz and WDF-fed C57BL/6J mice compared to their respective controls. Back transformation of secondary into primary BAs likely explains the shift in BA proportions. In support of this, in mice receiving a 0.03% DCA-supplemented diet, the constant DCA dietary supply does not increase the DCA concentration but significantly increases CA, even though de novo synthesis through CYP7A1 is repressed. With supraphysiological supply (DCA 0.1%), there is a rise in DCA concentration, but accompanied by a major increase in primary CA concentration despite the silencing of the enzymatic machinery for de novo synthesis. The hypothesis that upregulated CYP2A12 contributes to decreased secondary BA concentrations in mice with NASH is reinforced by a recent study reporting that Cyp2a12 knockout mice had higher proportion of secondary BAs and lower proportion of primary BAs in their BA pool than WT controls.34 In addition, Honda et al. suggested that CYP2A22, highly homologous to CYP2A12, could also hydroxylate secondary BAs at the 7α position.34
Insulin resistance, a risk factor and key player in NAFLD pathogenesis, also influences the metabolism of BAs. Haeusler et al. showed that proper insulin signaling inhibits FoxO1, an activator of the sterol 12α-hydroxylase CYP8B1, and reduces the synthesis of 12α-hydroxylated CA.46 In addition, Legry et al. reported that BA alterations were correlated with insulin resistance rather than NASH.27 Herein, we showed that CYP8B1 expression and the biliary concentration of CA were higher in insulin-resistant HFD-fed foz/foz mice than in their controls. On the contrary, CYP8B1 was downregulated in the liver of WDF-fed C57BL/6J mice. This could be explained by the less pronounced insulin resistance in WDF-fed C57BL/6J than in HFD-fed foz/foz mice. Although treatment with DCA 0.1% improved insulin resistance in HFD-fed foz/foz mice and downregulated CYP8B1 expression, the concentration of CA was increased (most likely by a 7α-hydroxylation of DCA in the liver of mice with NASH).
Conversely, BAs fine tune insulin responsiveness. Indeed, FXR and TGR5 regulate glucose and lipid metabolism as well as inflammation, so that pharmacological agonists are proposed for the treatment of obesity, type 2 diabetes and NASH.4,47 Here, it is conceivable that low FXR and TGR5 signaling, as a consequence of the altered BA composition in mice with NASH could worsen insulin resistance. Supporting this, we showed that the restoration of BA composition increases FXR and TGR5 signaling, prevents hyperglycemia, intrahepatic lipid accumulation and insulin resistance in HFD-fed foz/foz mice.
As DCA concentrations were notably lower in mice with NASH, we supplemented their HFD with DCA 0.03% or 0.1%. When administered for 1 week to C57BL/6J mice, these concentrations have been shown to significantly increase secondary BAs without raising total BA concentrations or inducing side effects or toxicity.48,49 In HFD-fed foz/foz mice, DCA 0.03% was not sufficient to correct low secondary BA concentrations and to reset TGR5 and FXR signaling, likely because of the induction of hepatic 7α-rehydroxylation of DCA to CA. Accordingly, DCA 0.03% did not change liver pathology and associated metabolic parameters in HFD-fed foz/foz mice. By contrast, DCA 0.1%, supposedly because the level of DCA overwhelms the enzymatic activities of CYP2A12 and CYP2A22, augmented secondary BA concentrations and, as a result, TGR5 and FXR activation in HFD-fed foz/foz mice. Ensuing from these changes, glucose tolerance, insulin resistance and obesity improved and at liver histology, steatosis and ballooned hepatocytes vanished. Also, macrophage aggregates were far less numerous, supportive of decreased lipotoxicity. By contrast, these livers remain inflamed, although with a switch from focal monocyte-macrophage aggregates in HFD-fed foz/foz mice to a more diffuse polymorphonuclear neutrophil infiltration in those supplemented with DCA 0.1%. In line with a significant (5-fold) increase in liver total BA concentration in mice that received 0.1% DCA and with persistent elevation in transaminases, we suspect BA-induced toxicity concomitant to NASH improvement. Given the positive anti-metabolic syndrome and anti-NASH effects of DCA, it will be of great interest to test, as a proof of principle, the therapeutic effects of strategies that restore the BA profile without increasing the BA pool size in NASH.
One observation of importance regarding translational studies is that the enterohepatic cycle sees the main changes in BAs. Hence, as shown here and by others,50 the alterations are not recapitulated in systemic blood usually sampled in human studies. First, BAs are at least 100-fold less concentrated in the systemic than in the portal blood and, second, the systemic and the portal BA profiles are not similar. Indeed, while the concentrations of nearly all BA species were changed in portal blood of mice with NASH, no corresponding alterations were observed in systemic blood. In addition, the fecal BA excretion is similar in mice with or without NASH. Hence, in the clinic, sampling of systemic blood or feces could provide misleading information with regard to the BA pool and its influence on metabolism and inflammation in the enterohepatic hub. This likely also explains the discordant reports among BA studies in patients with NASH.28
Targeting BA receptors through FXR, TGR5 or dual agonists led to promising results regarding NASH treatment, but this was at the cost of secondary effects, such as decreased HDLc, increased cholesterol, LDLc, pruritus and gallbladder volume.47 This study clearly demonstrates that enterohepatic BA alterations significantly contribute to the development of NASH in relevant preclinical models. In the light of our data, understanding how the enterohepatic BA pool, metabolism and signaling are changed in patients could be the key to developing an effective treatment without side effects and therefore should be fully explored.
Financial support
JG is the recipient of a PhD fellowship from the F.R.S.-FNRS (Belgium, FC33785). The work was supported by grants from the F.R.S.-FNRS (Belgium) to IAL (EQPU.N047.21; T.0141.19) and by an unrestricted research grant from Gilead (Belgium). BS is a recipient of an Advanced ERC grant (694717) and is supported by grants from the French ‘Agence Nationale de la Recherche’ (ANR-10-LBEX-46, ANR-16-RHUS-0006-PreciNASH).
Authors’ contributions
JG and IAL conceived and designed the study. JG performed the in vivo experiments, prepared the material, performed biochemical, histological and gene expression analyses. AT managed the LC-MS/MS analyses. JG and LAC optimized and performed the in vitro experiments. MN acquired and analyzed micro-CT data. CS provided help with histological analyses. JG, BS, LBB, AT and IAL critically reviewed and analyzed the data. JG and IAL wrote the manuscript. All authors read and revised the manuscript.
Data availability statement
All data that support the findings in this study can be found within this article. Nevertheless, raw data on bile acid analysis are available from the corresponding author upon a reasonable request.
Conflict of interest
The authors declare that they have no conflict of interest in relation to this work to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
We thank Natacha Feza-Bingi, Sébastien Meurice and Mathilde Beka for animal breeding, genotyping, and care; Boris Pirlot, Simon Ravau and Corinne Picalausa for technical assistance; and Paulina Henry for help with histological analyses. We also thank Amandine Descat and Emmanuelle Vallez for their indispensable contribution to BA extraction and dosage.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100387.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Dawson P.A., Karpen S.J. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56:1085–1099. doi: 10.1194/jlr.R054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chávez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–1694. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C.L., McDonald J.G., et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin B., Jones S.A., Price R.R., Watson M.A., McKee D.D., Moore L.B., et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 7.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., et al. TGR5-Mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potthoff M.J., Boney-Montoya J., Choi M., He T., Sunny N.E., Satapati S., et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatnagar S., Dammron H.A., Hillgartner F.B. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284:10023–10033. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kir S., Beddow S.A., Samuel V.T., Miller P., Previs S.F., Suino-Powell K., et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ploton M., Mazuy C., Gheeraert C., Dubois V., Berthier A., Dubois-Chevalier J., et al. The nuclear bile acid receptor FXR is a PKA- and FOXA2-sensitive activator of fasting hepatic gluconeogenesis. J Hepatol. 2018;69:1099–1109. doi: 10.1016/j.jhep.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe M., Houten S.M., Mataki C., Christoffolete M.A., Kim B.W., Sato H., et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 13.Velazquez-Villegas L.A., Perino A., Lemos V., Zietak M., Nomura M., Pols T.W.H., et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat Commun. 2018;9:245. doi: 10.1038/s41467-017-02068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang S., Suh J.M., Reilly S.M., Yu E., Osborn O., Lackey D., et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y.D., Chen W.D., Yu D., Forman B.M., Huang W. The G-Protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54:1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadaleta R.M., Van Erpecum K.J., Oldenburg B., Willemsen E.C.L., Renooij W., Murzilli S., et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 17.Pols T.W.H., Nomura M., Harach T., Lo Sasso G., Oosterveer M.H., Thomas C., et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747–757. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorucci S., Antonelli E., Rizzo G., Renga B., Mencarelli A., Riccardi L., et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Ferrell J.M., Pathak P., Boehme S., Gilliland T., Chiang J.Y.L. Deficiency of both farnesoid X receptor and Takeda G protein–coupled receptor 5 exacerbated liver fibrosis in mice. Hepatology. 2019;70:955–970. doi: 10.1002/hep.30513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lake A.D., Novak P., Shipkova P., Aranibar N., Robertson D., Reily M.D., et al. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol Appl Pharmacol. 2013;268:132–140. doi: 10.1016/j.taap.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puri P., Daita K., Joyce A., Mirshahi F., Santhekadur P.K., Cazanave S., et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534–548. doi: 10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dasarathy S., Yang Y., McCullough A.J., Marczewski S., Bennett C., Kalhan S.C. Elevated hepatic fatty acid oxidation, high plasma fibroblast growth factor 21, and fasting bile acids in nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2011;23:382–388. doi: 10.1097/MEG.0b013e328345c8c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J., Zheng M., Liu J., Luo Y., Yang W., Yang J., et al. Ratio of conjugated chenodeoxycholic to muricholic acids is associated with severity of nonalcoholic steatohepatitis. Obesity. 2019;27:2055–2066. doi: 10.1002/oby.22627. [DOI] [PubMed] [Google Scholar]
- 24.Ferslew B.C., Xie G., Johnston C.K., Su M., Stewart P.W., Jia W., et al. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60:3318–3328. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiao N., Baker S.S., Chapa-Rodriguez A., Liu W., Nugent C.A., Tsompana M., et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1–11. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 26.Grzych G., Chávez-Talavera O., Descat A., Thuillier D., Verrijken A., Kouach M., et al. NASH-related increases in plasma bile acid levels depend on insulin resistance. JHEP Rep. 2021;3:100222. doi: 10.1016/j.jhepr.2020.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legry V., Francque S., Haas J.T., Verrijken A., Caron S., Chávez-Talavera O., et al. Bile acid alterations are associated with insulin resistance, but not with NASH, in obese subjects. J Clin Endocrinol Metab. 2017;102:3783–3794. doi: 10.1210/jc.2017-01397. [DOI] [PubMed] [Google Scholar]
- 28.Chávez-Talavera O., Haas J., Grzych G., Tailleux A., Staels B. Bile acid alterations in nonalcoholic fatty liver disease, obesity, insulin resistance and type 2 diabetes. Curr Opin Lipidol. 2019;30:244–254. doi: 10.1097/MOL.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 29.Farrell G., Schattenberg J.M., Leclercq I., Yeh M.M., Goldin R., Teoh N., et al. Mouse models of nonalcoholic steatohepatitis: toward optimization of their relevance to human nonalcoholic steatohepatitis. Hepatology. 2019;69:2241–2257. doi: 10.1002/hep.30333. [DOI] [PubMed] [Google Scholar]
- 30.Arsov T., Silva D.G., O’Bryan M.K., Sainsbury A., Lee N.J., Kennedy C., et al. Fat aussie—a new Alstro¨m syndrome mouse showing a critical role for ALMS1 in obesity, diabetes, and spermatogenesis. Mol Endocrinol. 2006;20:1610–1622. doi: 10.1210/me.2005-0494. [DOI] [PubMed] [Google Scholar]
- 31.De Rudder M., Bouzin C., Nachit M., Louvegny H., Vande Velde G., Julé Y., et al. Automated computerized image analysis for the user-independent evaluation of disease severity in preclinical models of NAFLD/NASH. Lab Investig. 2020;100:147–160. doi: 10.1038/s41374-019-0315-9. [DOI] [PubMed] [Google Scholar]
- 32.Spinelli V., Lalloyer F., Baud G., Osto E., Kouach M., Daoudi M., et al. Influence of Roux-en-Y gastric bypass on plasma bile acid profiles: a comparative study between rats, pigs and humans. Int J Obes. 2016;40:1260–1267. doi: 10.1038/ijo.2016.46. [DOI] [PubMed] [Google Scholar]
- 33.Poekes L., Gillard J., Farrell G.C., Horsmans Y., Leclercq I.A. Activation of brown adipose tissue enhances the efficacy of caloric restriction for treatment of nonalcoholic steatohepatitis. Lab Investig. 2019;99:4–16. doi: 10.1038/s41374-018-0120-x. [DOI] [PubMed] [Google Scholar]
- 34.Honda A., Miyazaki T., Iwamoto J., Hirayama T., Morishita Y., Monma T., et al. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J Lipid Res. 2020;61:54–69. doi: 10.1194/jlr.RA119000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi S., Fukami T., Masuo Y., Brocker C.N., Xie C., Krausz K.W., et al. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J Lipid Res. 2016;57:2130–2137. doi: 10.1194/jlr.M071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Boer J.F., Verkade E., Mulder N.L., de Vries H.D., Huijkman N., Koehorst M., et al. A human-like bile acid pool induced by deletion of hepatic Cyp2c70 modulates effects of FXR activation in mice. J Lipid Res. 2020;61:291–305. doi: 10.1194/jlr.RA119000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masyuk A.I., Huang B.Q., Radtke B.N., Gajdos G.B., Splinter P.L., Masyuk T.V., et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Liver Physiol. 2013;304:G1013–G1024. doi: 10.1152/ajpgi.00383.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M., Cai S., Boyer J.L. Mechanisms of bile acid mediated inflammation in the liver. Mol Aspects Med. 2017;56:45–53. doi: 10.1016/j.mam.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carino A., Marchianò S., Biagioli M., Bucci M., Vellecco V., Brancaleone V., et al. Agonism for the bile acid receptor GPBAR1 reverses liver and vascular damage in a mouse model of steatohepatitis. FASEB J. 2019;33:2809–2822. doi: 10.1096/fj.201801373RR. [DOI] [PubMed] [Google Scholar]
- 40.Suga T., Yamaguchi H., Ogura J., Shoji S., Maekawa M., Mano N. Altered bile acid composition and disposition in a mouse model of non-alcoholic steatohepatitis. Toxicol Appl Pharmacol. 2019;379:114664. doi: 10.1016/j.taap.2019.114664. [DOI] [PubMed] [Google Scholar]
- 41.Lee G., You H.J., Bajaj J.S., Joo S.K., Yu J., Park S., et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun. 2020;11:4982. doi: 10.1038/s41467-020-18754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao A., Kosters A., Mells J.E., Zhang W., Setchell K.D.R., Amanso A.M., et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet–fed mice. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aaf4823. 357ra122-357ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones B.V., Begley M., Hill C., Gahan C.G.M., Marchesi J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A. 2008;105:13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayin S.I., Wahlström A., Felin J., Jäntti S., Marschall H.U., Bamberg K., et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Robertson S.J., Lemire P., Maughan H., Goethel A., Turpin W., Bedrani L., et al. Comparison of Co-housing and littermate methods for microbiota standardization in mouse models. Cell Rep. 2019;27:1910–1919.e2. doi: 10.1016/j.celrep.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Haeusler R.A., Pratt-Hyatt M., Welch C.L., Klaassen C.D., Accili D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 2012;15:65–74. doi: 10.1016/j.cmet.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiorucci S., Distrutti E., Carino A., Zampella A., Biagioli M. Bile acids and their receptors in metabolic disorders. Prog Lipid Res. 2021;82:101094. doi: 10.1016/j.plipres.2021.101094. [DOI] [PubMed] [Google Scholar]
- 48.Song P., Zhang Y., Klaassen C.D. Dose-response of five bile acids on serum and liver bile acid concentrations and hepatotoxicty in mice. Toxicol Sci. 2011;123:359–367. doi: 10.1093/toxsci/kfr177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Klaassen C.D. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res. 2010;51:3230–3242. doi: 10.1194/jlr.M007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voronova V., Sokolov V., Al-Khaifi A., Straniero S., Kumar C., Peskov K., et al. A physiology-based model of bile acid distribution and metabolism under healthy and pathologic conditions in human beings. Cmgh. 2020;10:149–170. doi: 10.1016/j.jcmgh.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings in this study can be found within this article. Nevertheless, raw data on bile acid analysis are available from the corresponding author upon a reasonable request.