Abstract
Purpose
Deficiency of adenosine deaminase type 2 (ADA2) (DADA2) is a rare inborn error of immunity caused by deleterious biallelic mutations in ADA2. Clinical manifestations are diverse, ranging from severe vasculopathy with lacunar strokes to immunodeficiency with viral infections, hypogammaglobulinemia and bone marrow failure. Limited data are available on the phenotype and function of leukocytes from DADA2 patients. The aim of this study was to perform in-depth immunophenotyping and functional analysis of the impact of DADA2 on human lymphocytes.
Methods
In-depth immunophenotyping and functional analyses were performed on ten patients with confirmed DADA2 and compared to heterozygous carriers of pathogenic ADA2 mutations and normal healthy controls.
Results
The median age of the patients was 10 years (mean 20.7 years, range 1–44 years). Four out of ten patients were on treatment with steroids and/or etanercept or other immunosuppressives. We confirmed a defect in terminal B cell differentiation in DADA2 and reveal a block in B cell development in the bone marrow at the pro-B to pre-B cell stage. We also show impaired differentiation of CD4+ and CD8+ memory T cells, accelerated exhaustion/senescence, and impaired survival and granzyme production by ADA2 deficient CD8+ T cells. Unconventional T cells (i.e. iNKT, MAIT, Vδ2+ γδT) were diminished whereas pro-inflammatory monocytes and CD56bright immature NK cells were increased. Expression of the IFN-induced lectin SIGLEC1 was increased on all monocyte subsets in DADA2 patients compared to healthy donors. Interestingly, the phenotype and function of lymphocytes from healthy heterozygous carriers were often intermediate to that of healthy donors and ADA2-deficient patients.
Conclusion
Extended immunophenotyping in DADA2 patients shows a complex immunophenotype. Our findings provide insight into the cellular mechanisms underlying some of the complex and heterogenous clinical features of DADA2. More research is needed to design targeted therapy to prevent viral infections in these patients with excessive inflammation as the overarching phenotype.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-021-01141-0.
Keywords: ADA2 deficiency, DADA2, T cell exhaustion, Humoral immunodeficiency, SIGLEC-1, Type I IFN signature, Monocytes
Introduction
In 2014, biallelic loss-of-function mutations in ADA2 (encoding adenosine deaminase type 2 [ADA2]) were described as the cause of a novel rare inborn error of immunity [1, 2]. The clinical phenotype of human ADA2 deficiency includes not only recurrent fevers and vasculitis (ranging from livedo racemosa to polyarteritis nodosa and lacunar stroke) but also immunodeficiency and cytopenia, either due to autoimmunity or bone marrow (BM) failure and hematological malignancy [3–5]. The pathophysiology of deficiency of ADA2 (DADA2) is far from resolved. Disrupted interaction between the vascular endothelium and monocytes is believed to be central to at least the vascular phenotype of the disease, with a skewing of macrophages to a pro-inflammatory M1 phenotype [1]. For patients with a predominant vasculitis phenotype, the mainstay of treatment is TNF inhibition [6]. However, cytopenias are mostly refractory to treatment with TNF inhibitors, growth factors and immunosuppressives [7, 8]. Hematopoietic stem cell transplantation results in excellent survival and cures ADA2-deficient patients from all disease manifestations, demonstrating the hematopoietic-intrinsic etiology of this condition [9, 10]. In terms of immunodeficiency, up to 30% of DADA2 patients have panhypogammaglobulinemia (IgG, IgM, IgA) [3] and can present with a common variable immunodeficiency-like phenotype, including recurrent upper and lower respiratory tract infections, bronchiectasis and associated gastrointestinal involvement. However, some patients also exhibit a phenotype mimicking auto-immune lymphoproliferative syndrome (ALPS) [11–13]. Moreover, DADA2 patients can have particular susceptibility to herpes virus infections—cytomegalovirus (CMV), Epstein Barr virus (EBV), herpes simplex virus 1, human herpes virus 6 (HHV6)—as well as HPV and Molluscum contagiosum [12, 14].
While features of vasculitis in DADA2 are well described, mechanisms underlying immunodeficiency have been less well explored. Initial descriptions noted B cell lymphopenia, progressive hypogammaglobulinemia occasionally mimicking agammaglobulinemia, increased numbers of naïve and CD21lo B cells, but reductions in switched memory B cells and plasmablasts [1, 15, 16]. In vitro findings of decreased T-dependent Ig secretion and increased B cell apoptosis inferred that a B cell–intrinsic defect underlies humoral immunodeficiency in DADA2 [1, 16]. However, while circulating T follicular helper (cTfh) cells—the CD4+ T cell subset that guides B cell differentiation [17]—have been reported to be present in normal or increased proportions in DADA2 patients, ADA2-deficient CD4+ T cells apparently exhibit features of impaired help for B cell differentiation such as reduced IL-21 production and CD40L expression in vitro [16]. Thus, functionally impaired cTfh cells may also contribute to the B cell defect in DADA2. Further cellular aberrations in DADA2 include increased proportions of CD4−CD8− αß+ TCR+ cells [1, 13, 18, 19] consistent with an ALPS-like phenotype as well as reductions in frequencies of central and effector memory CD4+ and CD8+ T cell subsets [1, 13, 16]. In order to further delineate peripheral immune defects in DADA2, we conducted an in-depth clinical, immunophenotyping and functional study in 10 patients with bi-allelic pathogenic ADA2 variants from 6 unrelated families.
Material and Methods
Patients
Patients with DADA2 were recruited from the University Hospitals Leuven Primary Immunodeficiency Clinic, Leuven, Belgium, and from St Vincent’s, Liverpool and Westmead Hospitals in Sydney, Australia. Healthy controls were recruited from volunteers, or purchased as donor buffy coats from the Australian Red Cross Blood Service. The study was approved by the respective ethics review boards of the participating institutes, including the ethics committees of University Hospitals Leuven (S63077, S63807 and S54866), Sydney Local Health District RPAH Zone Human Research Ethics Committee and Research Governance Office, Royal Prince Alfred Hospital, Camperdown, NSW, Australia (Protocol X16-0210/LNR/16/RPAH/257); the South East Sydney Local Health District Human Research Ethics Committee, Prince of Wales/Sydney Children’s Hospital, Randwick, NSW, Australia (Protocol HREC/11/POWH/152). Written informed consent for genetic investigations and immunological analyses, as well as the publication of data, was obtained from each family. DADA2 was diagnosed based on the demonstration of biallelic deleterious mutations in ADA2, in association with deficient plasma ADA2 enzyme activity.
Detailed Immunophenotyping and Functional Analysis of ADA2-Deficient Lymphocytes
PBMCs from ADA2-deficient patients (n = 10), heterozygous healthy carriers (n = 5) and healthy donors (n = 27) were analyzed as described previously to determine proportions of B cells (CD20+), T cells (CD3+), NK cells (CD3−CD56+) and dendritic cells (DCs) (CD19−CD3−CD56−CD235a−CD14−CD20− HLA-DR+) [20]. Subsets of T cells (CD4+, CD8+, iNKT [TCR Vα24Vβ11+], MAIT [TCR Vα7.2+CD161+], Vγδ [TCR Vγδ+], Vαβ [TCR Vαβ+]) were also determined [20]. Frequencies of naïve (CD45RA+CCR7+), central memory (TCM, CCR7+CD45RA−), effector memory (TEM, CCR7−CD45RA−) and revertant memory TEMRA, CD45RA+CCR7−) cells within the CD4+ and CD8+ T-cell populations and of Treg (CD4+CD25hiCD127−) and Tfh cells (CD4+CD45RA−CXCR5+) were enumerated as percentages of total CD4+ or CD8+ T cells. Th1, Th2, Th17 and Th1/Th17 (Th1*)-like phenotypes within memory CD4+ and Tfh cells were defined by differential expression of CXCR3 and CCR6 [17, 20–22]. Frequencies of transitional (CD27−CD10+), naïve (CD27−CD10−), total memory (CD10−CD27+), class-switched memory (IgD−IgM−, IgG+ or IgA+) and CD21low (CD19+CD21−) CD20+ B cells were also determined. Subsets of total NK cells were defined: immature CD56bright, mature CD56dimCD57- and terminally differentiated CD56dimCD57+ cells. Classical (CD14+CD16−), intermediate (CD14+CD16+) and non-classical (CD14−CD16+) subsets of monocytes were analyzed [23]. Lastly, DCs were assessed by quantifying proportions of plasmacytoid DCs (CD123+CD11c−), myeloid DCs (mDCs, CD11c+CD123) and mDC subsets: DC1 (CD141+ mDCs, CD16+ mDCs, CD1c+ mDCs and CD1clo/−. BM aspirates were incubated with mAbs against CD34, CD19, CD20, CD10, IgM and IgD. Populations of B-lineage cells (CD19+), as well as pro-B (CD19+CD34+CD10hiCD20−IgM−), pre-BI (CD19+CD34−CD10hiCD20−IgM−), pre-BII (CD19+CD34−CD10hiCD20dimIgM−/+), immature (CD19+CD34−CD10intCD20+IgM+) and recirculating mature (CD19+CD34−CD10−CD20+) B cells, were quantified [24–26].
Functional Analysis of ADA2-Deficient Lymphocytes
Naive and memory CD4+ T cells were isolated after excluding Tregs (CD25hiCD127lo) and sorting CD4+CD45RA+CCR7+ and CD4+CD45RA− cells respectively. Total CD8+ T and naive B cells were isolated as CD8+CD4− and CD20+CD10−CD27−IgG− cells, respectively. Sorted lymphocyte populations were labeled with the division-tracing dye CellTrace™ Yellow (CTY). CD4+ T cells were cultured under Th0, or Th1-, or Th17-polarizing conditions and cytokine expression and production were determined [27]. B cells were cultured with CD40L (200 ng/ml) alone or in the absence or presence of IL-21 (50 ng/ml). Cells were harvested, stained with Zombie dye to determine viability and enumerated with calibrate beads. Ig secretion was determined by ELISA as described [28]. CD8+ T cells were cultured with T cell expansion and activation (TAE; anti-CD3, anti-CD28, anti-CD2 mAb) beads (Miltenyi Biotech, Bergisch Gladbach, Germany). After 4 days of culture, supernatants were harvested and assayed for production of interferon (IFN)γ, tumor necrosis factor (TNF)-α, interleukin (IL)-2 and Granzyme A and Granzyme B by cytometric bead array (Becton Dickinson) [29]. For all cultures, cell viability was determined by Zombie Aqua™ live/dead discrimination dye, and proliferation was assessed by the dilution of CTY.
ADA2 Testing
For all patients, plasma ADA2 enzyme activity was measured by HPLC [30] and spectrophotometric [31] assays, as described previously. The ADA2 activity in extracts of dried plasma spots was measured as described [32].
IFN Score
The expression of six interferon-stimulated genes (ISGs) was measured by quantitative PCR, and the median fold change was used to calculate an interferon score (IS) for each subject compared to a previously derived panel of 20 or 13 local controls, respectively, in the Leuven and Sydney sites (where a reading + 2 SDs above the mean of healthy donors, i.e. an IS of > 2.466 or > 2.188 for Leuven and Sydney respectively is considered abnormal) as previously described [33].
Statistical Analysis
Significant differences were determined by multiple t-tests with P < 0.05 indicating statistical significance or by Mann–Whitney U tests (GraphPad Prism v. 8.4.2, La Jolla, CA, USA). Differences in mean values were considered significant at P < 0.05.
Results
Clinical Characteristics, ADA2 Mutations and Plasma Enzyme Activity of ADA2-Deficient Patients
Ten DADA2 patients (age range: 1 year and 10 months to 44 years) from 6 unrelated families, 5 carriers (from 3 families) (age range: 43–73 years) and 27 healthy controls (age range: 18–65 years) were included. Details on the clinical phenotype, ADA2 genotype and ADA2 enzymatic activity of patients, heterozygous carriers and healthy donors are listed in Table 1. Assessment of ADA2 function confirmed that all patients with bi-allelic ADA2 variants had dramatically reduced to undetectable levels of serum ADA2 activity, while all heterozygous carriers exhibited detectable ADA2 levels, albeit reduced compared to healthy donors.
Table 1.
Genetic diagnosis, ADA2 enzyme activity and clinical data of ADA2-deficient patients
| Kindred | Genetic diagnosis | ADA2 level mU/g protein | Age at sample | Viral phenotype | Treatment at sampling | Hypo-gamma | Type I IFN score | Major clinical phenotype at sampling | Other findings | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A | c.973-2 T > C / c. del1240-1442 | 1.1 | 8y6m | Warts, mollusca | (-) SCIG, amoxicilline | y | 19 | V (livedo), W, N, T, AIHA, | Severe AE to CPV |
| 2 | A | c.973-2 T > C / c. del1240-1442 | 2.7 | 10y8m | Warts, mollusca | (-) | y | 7 | V (livedo), R, | |
| 3 | B | c.140G > T (p.Gly47Val)/ c.506G > A(p.Arg169Gln) | 3.3 | 1y10m | (-) | n | 4 | None; now V (livedo), CNS, N, | ||
| 4 | B | c.140G > T (p.Gly47Val)/ c.506G > A(p.Arg169Gln) | 2.1 | 4y8m | Etanercept | y | 22,5 | V (livedo, cutaneous papules), now N | ||
| 5 | C | c.973-2 T > C / c.973-2 T > C | 1.6 | 5y8m | Etanerecept | n | 5 | V (livedo | ||
| 6 | D | p.Gly47Arg / p.Gly47Arg | 1.9 | 11y11m | Tocilizumab | n | 3 | Castleman disease V (livedo), | Later: CNS microbleeds, testicular thrombosis | |
| 7 | E | c.506G > A, c.1358A > G | 2.6 (2.6 HPLC) | 33y5m | Recurrent VZV, warts |
Prednisone 7.5 mg daily, IVIG (previously received mtx, cpm, mmf) |
y | 4.3* | DVT at 10y, V intestinal, S, CNS at 16y, HT | AE to UPV, Candida infections esophagus, skin, nails |
| 8 | E | c.506G > A, c.1358A > G | 0 (2.9 HPLC) | 40y | Viral encephalitis, Shingles | (-) | y | 6.7* | CNS at 3,5y, V, HT | AE to UPV |
| 9 | E | c.506G > A, c.1358A > G | 2.1 HPLC | 44y | Shingles, EBV | (-) | y (IgM) | 6.95* | V | AE to UPV |
| 10 | F | c.1397_1403del;c.1397_1403del p.Lys466Thrfs*2/p.Lys466Thrfs*2 | 0 (2.9 HPLC) | 42y | (-) recent EBV and HSV1, not at sampling | SCIG | y | 15.5* | P, HSM, A | Hepatitis (not at sampling) |
A Alopecia; AE adverse event; AIHA auto-immune hemolytic anemia; CNS central nervous system vasculitis manifestation, lacunar infarction, cranial nerve palsy, stroke, hemorrhage; cpm cyclophosphamide; CPV conjugated pneumococcal vaccine; DVT deep venous thrombosis; HG hypogammaglobulinemia; HSM hepatosplenomegaly; HT hypertension; IVIG intravenous immunoglobulins; mtx methotrexate; mmf mofetil mycophenolate; N neutropenia; P pancytopenia; R Raynaud phenomenon; S splenomegaly; SCIG subcutaneous immunoglobulins; T thrombocytopenia; UPV unconjugated pneumococcal vaccine; V vasculitis
*IFN score analyses performed in Sydney site
All patients suffered from vasculitis, ranging from livedo racemosa to CNS vasculopathy (transient ischemic attack to lacunar infarction). Severe viral infections were present in five out of ten patients ranging from warts to viral encephalitis. Six of the 10 patients had hypogammaglobulinemia. Interestingly, two of ten patients experienced severe local reactions as well as fever and general malaise upon vaccination with unconjugated pneumococcal vaccine. Another patient experienced fever malaise upon vaccination with conjugated pneumococcal vaccine and live attenuated MMR vaccine after which vasculitis occurred for the first time. Overall, there was significant phenotypic heterogeneity in the severity of vasculitis and spectrum of organ involvement within individual kindreds.
Human B Cell Development and Differentiation Is Compromised by ADA2 Deficiency
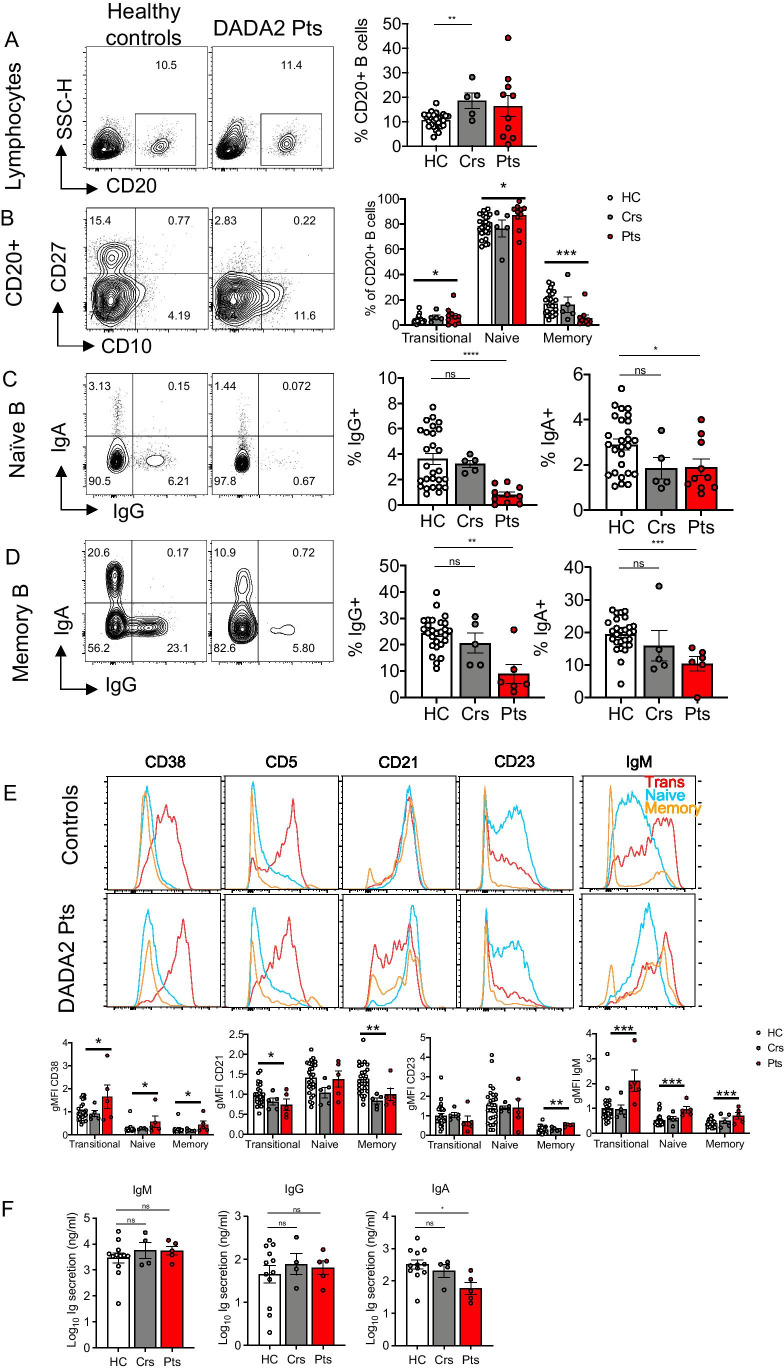
Flow cytometric analysis revealed comparable frequencies of CD20+ B cells in peripheral blood of DADA2 patients (mean = 16.4%) and healthy controls (10.6%; Fig. 1A). Delineation of B cell subsets revealed significant increases in percentages of transitional and naïve B cells in DADA2 patients compared to healthy controls. Consistent with previous studies [1, 12, 13, 16], memory B cells were significantly reduced in DADA2 patients compared to healthy donors (Fig. 1B).
Fig. 1.
Impairment in peripheral B cell development and differentiation in DADA2 patients. Immunophenotyping was determined by flow cytometry on PBMCs from healthy controls (HC), heterozygous carriers (Crs) and DADA2 patients (Pts). Flow cytometric plots and graphs showing the frequencies of A CD20+ B cells within the lymphocyte population; B transitional (CD10+CD27−), naïve (CD10−CD27−) and memory (CD10−CD27+) subsets within the CD20+ B cell population; C, D IgA+ and IgG+ cells within the (C) CD27− and (D) CD27+ memory B cell populations; E Representative histogram plots (top panels) show expression of CD38, CD21, CD23, and IgM on transitional (red), naïve (blue) and memory (orange) B cells of healthy donors and DADA2 patients, and summarized in graphs (bottom panels) depicting geometric mean fluorescent intensity (gMFI) normalized to the transitional B cell population of healthy controls. F Sort-purified naïve B cells from healthy controls (HC), heterozygous carriers (Crs) and DADA2 patients (Pts) were stimulated in vitro with CD40L/IL-21 for 7 days. IgM, IgG and IgA secretion was determined by ELISA. Graphs represent the mean ± S.E.M.; each symbol represents an individual. Significant differences were determined by multiple t-tests with P < 0.05 indicating statistical significance or by Mann–Whitney t-tests with *P < 0.05; **P < 0.01; ***P < 0.001
We next assessed Ig class switching in vivo by quantifying the frequencies of B cells that expressed IgG or IgA. In healthy donors, the vast majority of class-switched B cells are contained within the CD27+ memory B cell subset; however, a small proportion of IgG+ and IgA+ B cells can also be detected within the CD27− B cell subset [34]. Analysis of these B cell subsets demonstrated significantly decreased proportions (~ fivefold) of IgG+ and IgA+ B cells in both the CD27− (naïve) (Fig. 1C) and CD27+ (memory) (Fig. 1D) B cell compartments. While frequencies of total B cells were increased in healthy heterozygous carriers (Fig. 1A), proportions of transitional, naïve, memory B cells (Fig. 1B) and class-switched B cells (Fig. 1C, D) in heterozygous carriers were similar to healthy donors. Together, these findings indicate that ADA2-deficiency compromises not only the generation and/or maintenance of the memory B cell pool, but also the ability of B cells to undergo Ig class switching.
Circulating B Cells in ADA2-Deficient Patients Exhibit a Block in Maturation
The increase in transitional and decrease in total and class-switched memory B cells led us to further examine the maturation status of B cell subsets in DADA2 patients. This was achieved by assessing the expression of a range of surface molecules that are differentially expressed as human B cells mature from transitional to naïve, and naïve to memory B cells [24, 35, 36]. In healthy donors, CD38 and IgM are highly expressed on transitional B cells and then downregulated on naïve and memory B cells [24, 35, 36] (Fig. 1E). While this pattern was observed for ADA2-deficient B cell subsets (Fig. 1E), the absolute levels of CD38 and IgM expressed were significantly (~ twofold) higher on transitional, naïve and memory B cells from DADA2 patients than those from healthy donors (Fig. 1E).
CD21 and CD23 increase as transitional B cells from healthy donors develop into naïve B cells, and then plateau/decrease as naïve B cells differentiate into memory B cells [24, 36] (Fig. 1E). Compared to healthy donors, CD21 was consistently and significantly reduced on ADA2-deficient transitional B cells and failed to be upregulated on ADA2-deficient memory B cells (Fig. 1E). CD23 was also lower on ADA2-deficient transitional B cells (Fig. 1E). While CD23 was upregulated normally on ADA2-deficient naive B cells, it continued to be more highly expressed on ADA2-deficient memory B cells compared to memory B cells from healthy donors (Fig. 1E). No differences were observed for CD19 or BAFF-R expression on transitional, naïve or memory B cells from healthy donors and DADA2 patients (data not shown). The phenotype of transitional, naïve and memory B cells from healthy heterozygous carriers was comparable to that of healthy donors (Fig. 1E). Collectively, our detailed analysis of peripheral B cell subsets indicates that ADA2 deficiency compromises B cell differentiation, resulting in B cell populations that are developmentally less mature than those in healthy donors.
To further explore B cell–intrinsic defects due to ADA2-deficiency, we assessed the ability of naïve B cells to proliferate and differentiate in vitro in response to CD40L with or without IL-21, which mimics T-dependent B-cell activation [17, 37]. There were no significant differences in survival (Fig. S1A) or proliferation (Fig. S1B) of naïve B cells from DADA2 patients, heterozygous carriers or healthy donors 5 days post-stimulation. Similarly, induction of IgM and IgG secretion by CD40L/IL-21 stimulation was unaffected by heterozygous or bi-allelic mutations in ADA2 (Fig. 1F). However, IgA secretion by naïve ADA2-deficient B cells was significantly reduced compared to healthy donor naïve B cells (Fig. 1F). Thus, naïve B cells in DADA2 patients have an intrinsically impaired ability to differentiate into IgA-secreting plasmablasts. Overall, ADA2 deficiency impacts differentiation of peripheral naive B cells to memory B cells, as well as class switching in vivo and generating of IgA-secreting cells in vitro.
Arrested B Cell Development in ADA2-Deficient Bone Marrow
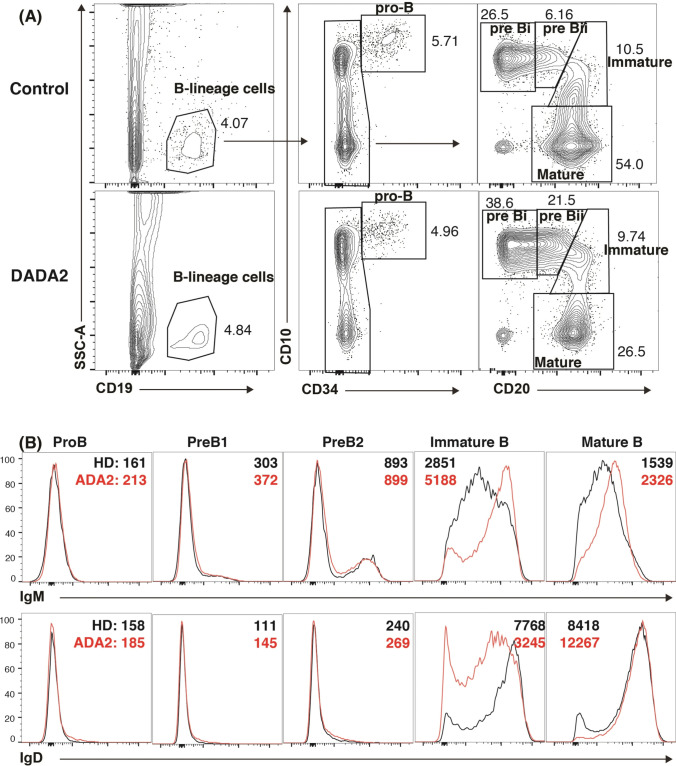
To extend our observations into the defect in B cell development observed in peripheral blood, we quantified proportions of B-lineage cells (CD19+), as well as pro-B (CD19+CD34+CD10hiCD20−IgM−), pre-BI (CD19+CD34−CD10hiCD20−IgM−), pre-BII (CD19+CD34−CD10hiCD20dimIgM−/+), immature (CD19+CD34−CD10intCD20+IgM+) and mature (CD19+CD34−CD10−CD20+) B cells in the BM of a DADA2 patient who did not receive any immunosuppressive treatment or anti-TNF agent. Frequencies of total B-lineage cells and pro-B cells were comparable in the patient and healthy donors (Fig. 2A). However, pre-BI and pre-BII cells were markedly increased, and mature recirculating B cells reduced, in DADA2 BM compared to those in control BM (Fig. 2A). Although proportions of immature B cells were similar in the patient and controls, ADA2-deficient immature B cells exhibited features of altered maturation, evidenced by delayed down-regulation of CD10 (Fig. 2A), elevated expression of IgM (Fig. 2B), and reduced expression of IgD (Fig. 2B). Consistent with fewer class-switched memory B cells in the blood of DADA2 patients (Fig. 1C), > 98% of mature ADA2-deficient BM B cells were IgMhiIgD+, compared to ~ 80–85% in healthy controls (Fig. 2B). Thus, the aberrant distribution and phenotype of peripheral B cell subsets in ADA2-deficient patients likely result from a block in B cell development in the BM, evidenced by an accumulation of pre-B cells and underdeveloped immature B cells.
Fig. 2.
ADA2 mutations impede B cell development in the bone marrow at the preB to immature B cell stage. A, B BM aspirates from healthy donors, or a DADA2 patient were labeled with mAbs against CD34, CD19, CD20, CD10, IgM and IgD. A Proportions of B-lineage cells (CD19+), pro-B (CD19+CD34+CD10hi), pre-BI (CD19+CD34−CD10hi), pre-BII (CD19+CD34−CD10hi), immature (CD19+CD34−CD10int) and recirculating mature (CD19+CD34−CD10−CD20+) B cells. B Each BM B cell subset in healthy donors (black histogram) and DADA2 patients (red histogram) was assessed for expression of IgM (upper) and IgD (lower panel). Values in B correspond to the gMFI of IgM and IgD expression on BM B cell subsets from in healthy donors (black) and the DADA2 patient (red)
CD4+ and CD8+ Memory T cells and Regulatory T Cells Are Reduced In Vivo in DADA2 Patients
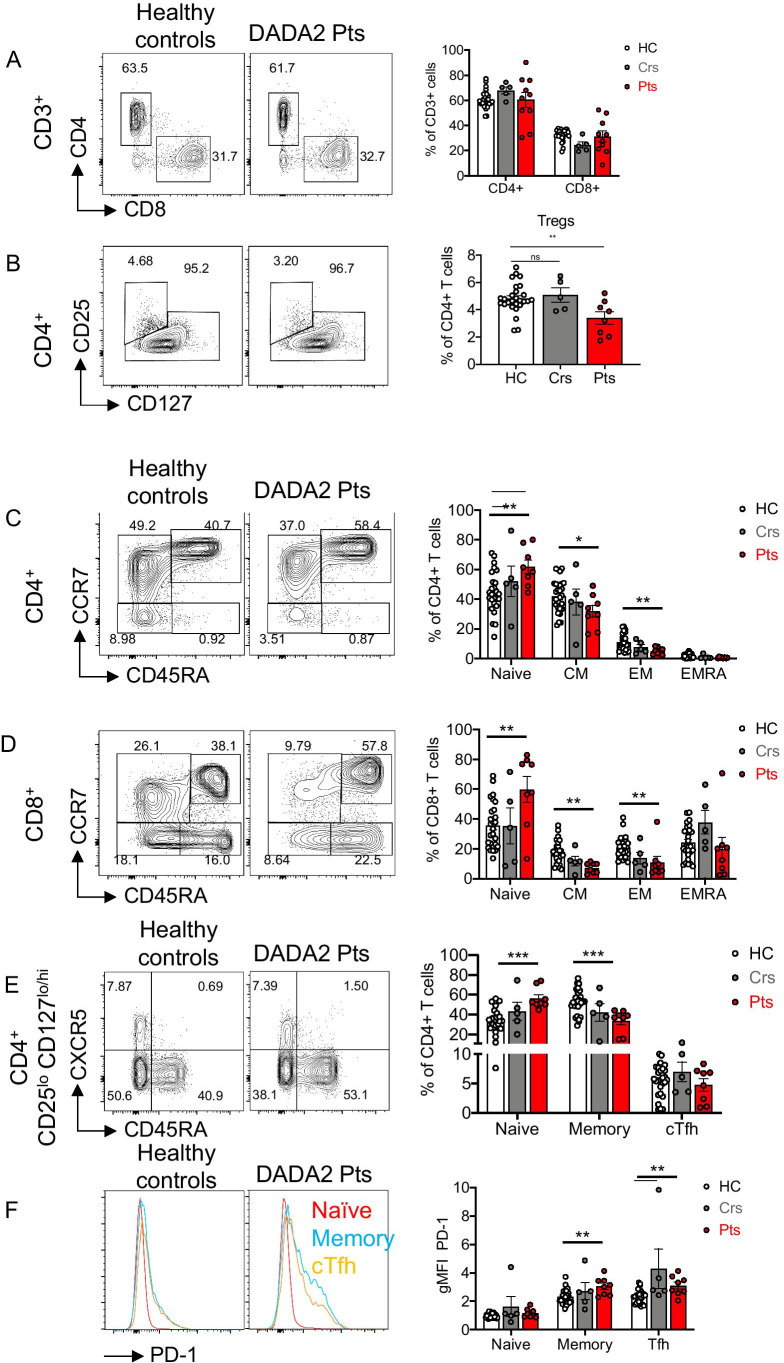
We next examined the distribution and maturation status of T cells in DADA2 patients. Proportions of total (CD3+) T cells (Fig. S2A), as well as CD4+ and CD8+ T cells (Fig. 3A), in peripheral blood of DADA2 patients and healthy donors were comparable. Heterozygous carriers had modest but significantly increased CD4+ T cells and reduced CD8+ T cells (Fig. 3A). Within the population of CD4+ T cells, Treg cells (CD25hiCD127lo) were significantly reduced in DADA2 patients compared to those in healthy donors and heterozygous carriers (Fig. 3B). Frequencies of naïve (CD45RA+CCR7+) T cells were significantly increased, whereas central memory (TCM, CD45RA−CCR7+) and effector memory (TEM, CD45RA−CCR7−) T cells were significantly reduced within both the CD4+ and CD8+ T cell subsets in DADA2 (Fig. 3C, D). There were no differences in proportions of CD4+ or CD8+ TEMRA (CD45RA+CCR7−) cell subsets. In general, the distribution of these CD4+ and CD8+ T cell subsets in healthy heterozygous carriers was comparable to healthy donors (Fig. 3C, D).
Fig. 3.
Aberrant CD4+ and CD8+ T cell differentiation in DADA2 patients. Immunophenotyping by flow cytometry on PBMCs from healthy controls (HC), heterozygous carriers (Crs) and DADA2 patients (Pts) was performed. Flow cytometric plots (left) and graphs (right) show the frequencies of A CD4+ and CD8+ T cells within the CD3+ lymphocyte population; B CD25hiCD127loTreg within CD4+ T cells; C, D naïve (CD45RA+CCR7+), central memory (CM, CD45RA−CCR7+), effector memory (EM, CD45RA−CCR7−) and terminally differentiated effector memory (EMRA, CD45RA+CCR7−) subsets within C CD4+ T cells and D CD8+ T cells; E naïve (CD45RA−CXCR5−CCR7+), memory (CD45RA−CXCR5−) and cTfh (CD45RA−CXCR5+) within CD4+CD25loCD127lo/hI T cells. F Representative histogram plots (left) showing PD-1 expression on naïve (red), memory (blue) and cTfh (orange) CD4+ T cells from healthy controls and DADA2 patients, and summarized in graphs (right) as gMFI normalized to naïve CD4+ T cells of healthy controls. A–F Graphs represent the mean ± S.E.M.; each symbol represents an individual. Significant differences were determined by multiple t-tests with P < 0.05 indicating statistical significance or by Mann–Whitney U-tests with *P < 0.05; **P < 0.01; ***P < 0.001
We also analyzed CD4+ T cells for cTfh cells based on differential expression of CD45RA, CXCR5 and PD-1 (CD45RA-CXCR5+). The cTfh cells were present in comparable frequencies in DADA2 patients, heterozygous healthy carriers and healthy donors (Fig. 3E). This was even more apparent when expressed as a proportion of memory CD4+ T cells (HC: 9.7 ± 1.1%; carriers: 16 ± 3.4%; ADA2: 15.5 ± 3.3%). The Tfh cells can also be characterized by assessing PD-1 expression, as well as delineation into distinct subsets defined by differential expression of the chemokine receptors CCR6 and CXCR3 [21, 38]. Consistent with our previous findings [21], PD-1 was expressed at low levels on naïve CD4+ T cells, and upregulated on memory CD4+ and cTfh cells from healthy donors (Fig. 3F). While similar expression patterns of PD-1 were observed for memory and cTfh cells from healthy heterozygous carriers, PD-1 was significantly increased on memory CD4+ T and cTfh cells from DADA2 patients (Fig. 3F). Proportions of Th1 (CXCR3+CCR6−), Th17 (CXCR3−CCR6+), Th2 (CXCR3−CCR6−) and Th1/Th17 (CXCR3+CCR6+)-type cells within both memory CD4+ T cells and cTfh cell populations were also comparable between DADA2 patients and healthy donors (Fig. S2B, C). Thus, although the generation of memory T cells is compromised of ADA2 mutations, differentiation of memory CD4+ T cells to distinct effector fates remains intact. Consequently, there is a quantitative rather than qualitative effect of ADA2-deficiency on memory CD4+ T cells.
CD4+ and CD8+ T Cell Differentiation Is Compromised by ADA2 Deficiency
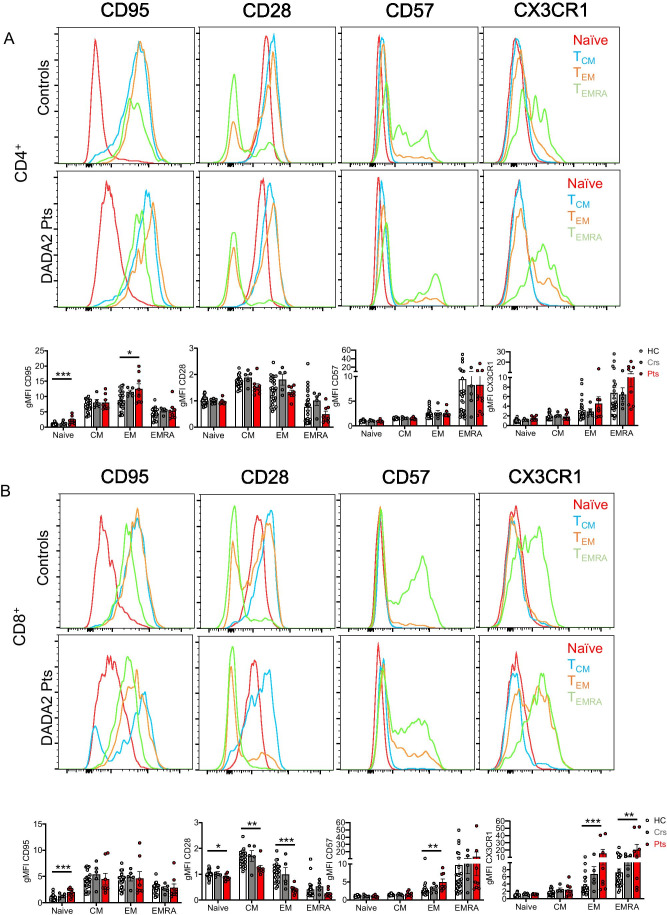
The expression of the surface receptors CD28, CD95, CD57 and CX3CR1, which change during differentiation of naïve CD4+ and CD8+ T cells to TEMRA subsets, was examined to determine whether ADA2-deficient T cells exhibited features of premature senescence or exhaustion [39, 40]. CD28 is constitutively expressed by naïve CD4+ and CD8+ T cells, slightly increased on TCM cells and then downregulated on TEM and TEMRA cells from healthy donors (Fig. 4A, B). In contrast, CD95, CD57 and CX3CR1 are absent from naïve T cells, and then incrementally increased as CD4+ and CD8+ T cells differentiate (Fig. 4A, B) [40, 41]. Compared to healthy donors, DADA2 patients exhibited significantly increased expression of CD95 on naïve CD4+ and CD8+ T cells (Fig. 4A, B). CD28 expression was lower on naïve CD8+ T cells in DADA2 patients and then further and significantly diminished on ADA2-deficient CD8+ TCM, TEM and TEMRA cells compared to healthy donors (Fig. 4B). Within the memory T cell subsets, we observed significantly elevated levels of CD95 on CD4+ TEM cells, CD57 on CD8+ TEM cells and CX3CR1 on CD8+ TEM and TEMRA cells (Fig. 4A, B). Thus, ADA2-deficiency exacerbates T cell differentiation, resulting in greater proportions of exhausted/senescent CD4+ and CD8+ T cells.
Fig. 4.
CD4+ and CD8+ T cells in DADA2 patients display an exacerbated senescent/exhausted phenotype. Expression of CD95, CD28, CD57 and CX3CR1 on naïve (red), CM (blue), EM (orange), and EMRA (green) subsets of A CD4+ and B CD8+ T cells from healthy donors, heterozygous carriers and DADA2 patients. Data are depicted as histogram plots for healthy controls and DADA2 patients (top panels) and summarized in graphs (bottom panels) which as gMFI normalized to the naive CD4+ (A) or CD8+ (B) T cells from healthy controls. Graphs represent the mean ± S.E.M.; each symbol represents an individual. Significant differences were determined by multiple t-tests with *P < 0.05; **P < 0.01; ***P < 0.001, indicating statistical significance
ADA2 Deficiency Impairs Granzyme Production by CD8+ TEM and TEMRA Cells
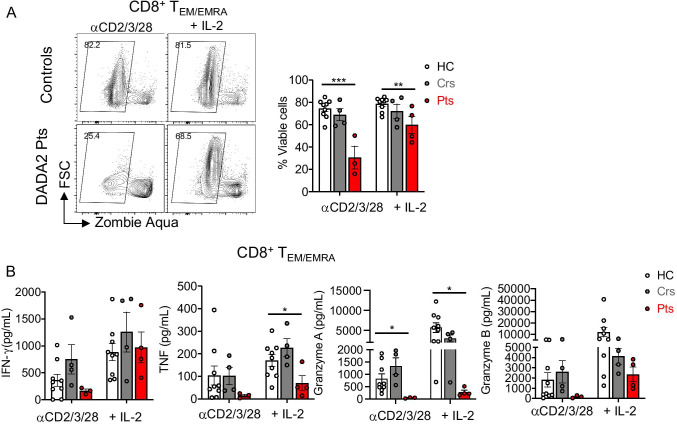
To correlate alterations in surface phenotype with cell function, we sorted CD8+ TEM/TEMRA cells from healthy donors, heterozygous carriers and DADA2 patients and examined survival and secretion of different cytokines after in vitro stimulation with anti-CD2/CD3/CD28 mAbs alone or together with IL-2 for 4 days. The viability of cultured ADA2-deficient CD8+ TEM/TEMRA cells was significantly reduced compared to healthy donors (Fig. 5A). While exogenous IL-2 improved recovery of CD8+ T cells from DADA2 patients, the overall survival of these cells continued to be significantly less than that of CD8+ T cells from healthy donors (Fig. 5A).
Fig. 5.
Reduced viability and impaired cytokine secretion by ADA2-deficient CD8+ TEM/EMRA cells. Sort-purified combined TEM/EMRA CD8+ T cells from PBMCs of healthy controls, heterozygous carriers and DADA2 patients were stimulated in vitro with anti-CD2/CD3/CD28 mAbs beads ± IL-2 for 4 days and were analyzed for viability and secretion of cytokines and cytotoxic granules. A Representative flow cytometric plot (left) showing viable cells. The values on the contour plots represent the percentage of ZombieAqua− cells, and thus viable cells, and are summarized in graphs (right). B Secretion of IFN-γ, TNF, granzyme A and granzyme B by TEM/EMRA CD8+ T cells. Graphs represent the mean ± S.E.M.; each symbol represents an individual. Significant differences were determined by multiple t-tests with *P < 0.05; **P < 0.01; ***P < 0.001, indicating statistical significance
The production of IFN-γ and TNF-α by ADA2-deficient CD8+ TEM and TEMRA cells was intact following in vitro stimulation. However, ADA2-deficient CD8+ TEM/TEMRA cells secreted significantly lower levels of granzyme A compared to CD8+ T cells from healthy donors (Fig. 5B). Granzyme B secretion by ADA2-deficient CD8+ T cells tended to be reduced but did not reach statistical significance (Fig. 5B). CD8+ TEM and TEMRA cells from heterozygous carriers produced similar levels of cytokines and granzymes as CD8+ T cells from healthy donors (Fig. 5B). Reduced granzyme A secretion by activated TEM and TEMRA CD8+ T cells from DADA2 patients, combined with poor viability of and increased CD57 expression by these cells, is consistent with ADA2-deficient CD8+ T cells undergoing premature exhaustion and/or senescence. Unlike CD8+ T cells, survival, cytokine secretion and effector function of CD4+ T cells were unaffected by ADA2 deficiency (Fig. S3).
Unconventional T Cells and NK Cell Subsets Are Severely Reduced in DADA2 Patients
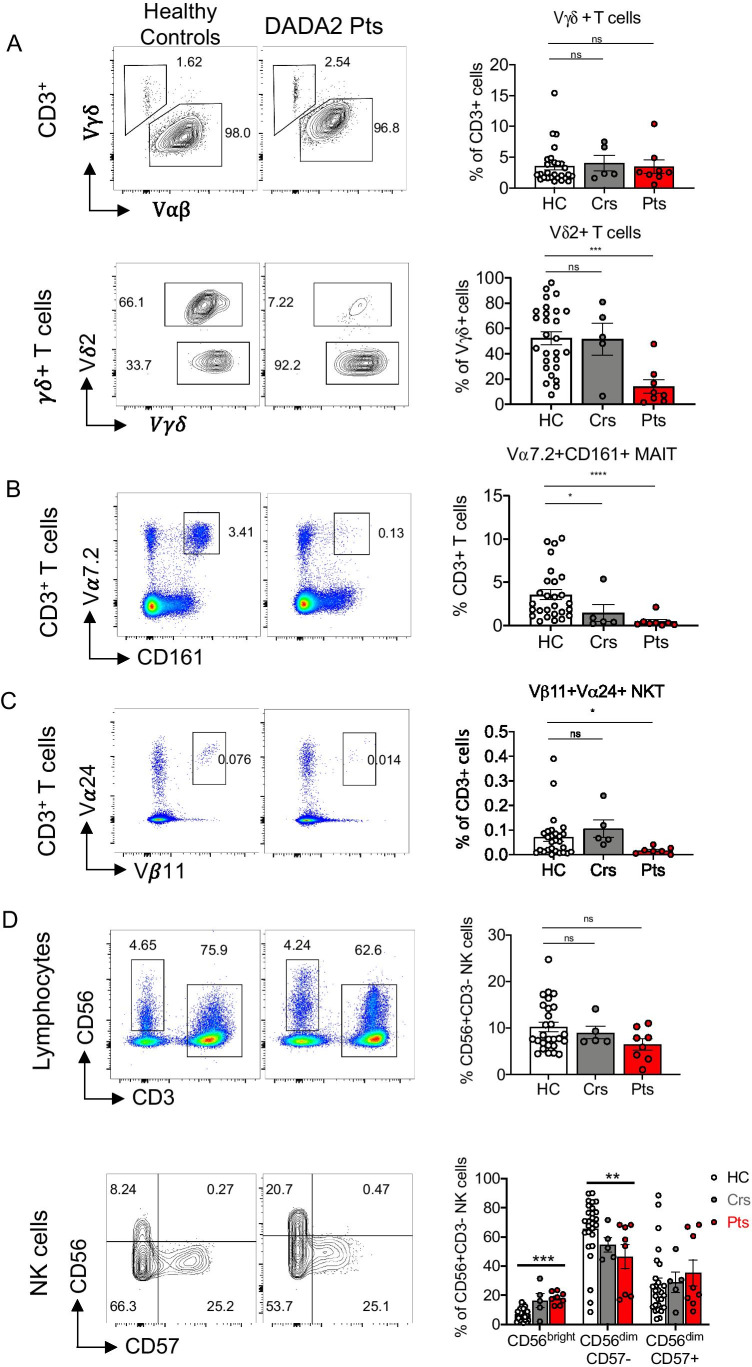
The consequences of ADA2 deficiency on unconventional T cells have not been investigated. Thus, we quantified proportions of γδ+ T cells, Vα7.2+CD161+ mucosal-associated invariant T (MAIT) cells and Vβ11+Vα24+ invariant natural killer T (iNKT) cells within CD3+ T cells. The proportions of γδ+ T cells were similar in healthy donors, heterozygous carriers and DADA2 patients (Fig. 6A top). However, within the γδ+ T cell subset, DADA2 patients had significantly fewer Vδ2+ cells than healthy donors (Fig. 6A bottom). MAIT cells (Fig. 6B) and iNKT cells (Fig. 6C) were also drastically (5–10-fold) and significantly reduced in DADA2 patients compared to healthy donors.
Fig. 6.
Diminished unconventional T cells and NK cell subsets in DADA2 patients. Proportions of A γδ+ T cells (top) and Vδ2+ within γδ+ T cells (bottom), B Vα7.2+CD161+ MAIT cells, and C Vβ11+Vα24+ NKT were analyzed in healthy donors, heterozygous carriers and DADA2 patients, presented as flow cytometry plots (A-C left) and summarized in graphs (A–C right). D representative flow cytometry plots showing total CD56+CD3− NK cells as a proportion of lymphocytes (top), and proportions of immature CD56bright, mature CD56dimCD57− and terminally differentiated CD57dimCD57+ NK cell subsets within the total NK cell population (bottom). Graphs represent the mean ± S.E.M.; each symbol represents an individual. Significant differences were determined by Mann–Whitney t-tests with *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. A–D or multiple t-tests with P < 0.05 indicating statistical significance (D bottom)
Within the lymphocyte population, NK cells were present in comparable proportions in healthy controls, heterozygous carriers and DADA2 patients (Fig. 6D, top). However, when we quantified distinct NK cell subsets [42, 43], immature CD56bright NK cells were markedly increased and mature CD56dimCD57− NK cells were significantly reduced in DADA2 patients (Fig. 6D, bottom). Thus, pathogenic ADA2 mutations compromise the generation and/or maintenance of MAIT cell, NKT cells and Vδ2+ γδ+ T cells, as well as NK cell differentiation.
Effect of ADA2 Deficiency on Dendritic Cells and Monocytes
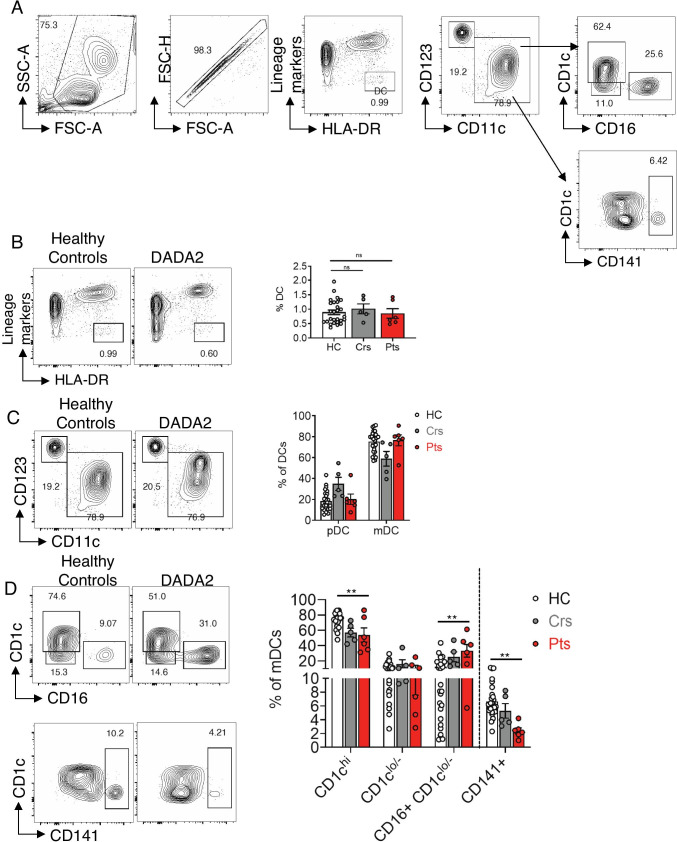
ADA2 is highly expressed in dendritic cells (DCs) and monocytes [44]. DCs were identified in PBMCs as HLA-DR+ and lineage marker (CD19, CD20, CD3, CD56, CD14, CD235a)− cells (Fig. 7A). Proportions of DCs in healthy donors, heterozygous carriers and DADA2 patients were similar (Fig. 7B). Two major DC subsets can be resolved: CD123+CD11c− plasmacytoid DCs (pDCs) and CD123−CD11c+ myeloid DCs (mDCs or conventional DCs) [45]. These subsets were detected at comparable frequencies in DADA2 patients and healthy controls (Fig. 7C). When mDC subsets were further analyzed, consistent with previous studies, we found that CD1chi mDCs were the major mDC subset in healthy donors, while CD141+ mDCs were least frequent (Fig. 7D) [46]. Compared to healthy donors, DADA2 patients had significantly reduced proportions of CD1chi and CD141+ mDCs subsets, comparable proportions of CD1clo/− mDCs and significantly increased proportions of CD16+CD1clo/− mDC (Fig. 7D). Altogether, these data showed that while ADA2-deficiency does not impact overall numbers and proportions of dendritic cells, it does alter the distribution of mDC subsets.
Fig. 7.
Dendritic cell subsets in ADA2 deficiency. A Gating strategy for DC subsets. A broad gate containing lymphocyte and monocyte populations was first gated against SSC-A vs FSC-A, followed by single cells gated as FSC-H vs FSC-A. DCs were defined as negative for lineage markers (CD3, CD19, CD20, CD56, CD14, CD235a), and HLA-DR+. DCs were gated as CD123 and CD11c to distinguish CD123+CD11c− plasmacytoid DCs (pDC) and CD123−CD11c+ myeloid (conventional) DCs. mDCs were gated as CD1c and CD16 to distinguish CD1chi, CD1clo/− and CD16+CD1clo/− mDCs and for CD1c and CD141 to identify CD141+ mDCs. B–D Representative flow cytometry plots and summary graphs showing proportions of B Lin−HLA-DR+ DCs, C pDCs and mDCs within the DC population and D CD1chi, CD1clo/−, CD16 + CD1clo/− and CD141+ mDC subsets. Graphs represent mean ± S.E.M.; each symbol represents an individual. Significant differences were determined by Mann–Whitney (B) or multiple (C, D) t-tests (**P < 0.01)
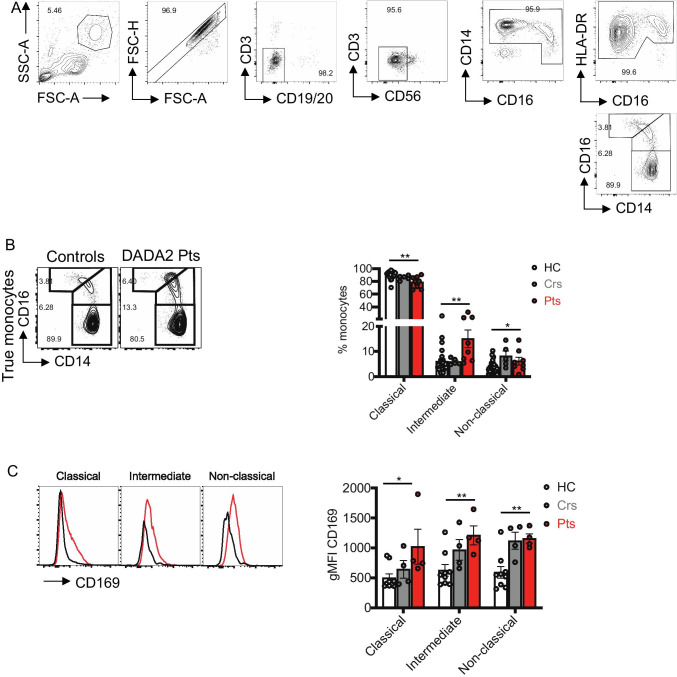
The predominant monocyte subset in healthy donors, heterozygous controls and DADA2 patients is the classical (CD14+CD16−) subset, followed by intermediate (CD14+CD16+) and then non-classical (CD14−CD16+) subsets (Fig. 8A, B). Compared to healthy donors, proportions of classical monocytes were significantly reduced in DADA2 patients, with a concomitant and significant increase in intermediate and non-classical monocytes (Fig. 8B). The increase in non-classical monocytes was also significant in the carrier group (Fig. 8B).
Fig. 8.
Classical monocyte are reduced and IFN-signature detected in ADA2 deficiency. A Gating strategy for monocyte subsets. A broad monocyte gate was set according to SSC-A vs FSC-A, then single cells defined by FSC-H by FSC-A. Lineage markers CD3, CD19/CD20 and CD56 were used to exclude T, B and NK cells. CD14−CD16− cells were also excluded. HLA-DRint/+ cells that were CD16− or CD16+ were defined as “true” monocytes. Finally, three subsets were defined: CD14+CD16− classical, CD14+CD16+ intermediate and CD14−CD16+ non-classical monocytes (23). B Flow cytometry plots (left) showing the frequencies of classical (CD14+CD16−), intermediate (CD14+CD16+) and non-classical (CD14−CD16+) monocytes and summarized in graphs (right). C Histograms showing CD169 expression on classical, intermediate and non-classical monocytes, and gMFI of CD169 summarized in graphs (right). Significant differences were determined by multiple t-tests (*P < 0.05; **P < 0.01)
Several studies have reported a predominant type I IFN signature, in some papers in combination with a neutrophil signature, or type II IFN signature, in DADA2 patients [47–50]. We here also report increased type I IFN signature in all patients, but not in carriers, using a 6-gene signature as previously described [33] (Table 1). For this reason, we analyzed the expression of CD169/SIGLEC1, a cell adhesion protein induced by type I IFN-signaling and a potential myeloid cell-specific biomarker for an overt type I IFN response [51] on monocytes. While CD169/SIGLEC1 is expressed at similar levels on classical, intermediate and non-classical monocyte subsets present in healthy donors, its level of expression was found to be twofold higher on all subsets of ADA2-deficient monocytes (Fig. 8C). Thus, ADA2-deficiency not only alters the distribution of myeloid cell subsets but also modulates the phenotype of monocytes in terms of type I IFN dysregulation.
Discussion
In this report, we provide extensive immunophenotyping and in vitro functional analyses of leukocytes from 10 patients with DADA2. We confirm the defect in terminal B cell differentiation in ADA2 deficiency [1, 2, 16, 52], evidenced by lower proportions of the total as well as IgG- and IgA-expressing memory B cells in these patients. Furthermore, the phenotype of ADA2-deficient B cell subsets suggested these cells were less mature than corresponding B cells in healthy donors. Moreover, the peripheral B cell defect correlated with a block in B cell development in the BM at the pre–B cell stage, as well as an intrinsic failure of DADA2 B cells to differentiate into IgA-producing B cells. As the proportions of CD27+ memory B cells increase in the peripheral blood of healthy individuals with age, it could be argued that the memory B cell deficiency in DADA2 reflects the younger age of some of the patients in our cohort (< 10 years old, Table 1). However, this is unlikely because frequencies and numbers of memory B cell in healthy individuals reach adult levels by 2–4 years of age [24, 53, 54]. Indeed, all but 1 patient (P7) had a paucity of memory B cells, including P8, P9 and P10 who were > 40 years old, indicating age is not a determinant of impaired B cell differentiation in DADA2. Collectively, these data demonstrating an intrinsic maturation B cell defect due to ADA2-deficiency may explain the hypogammaglobulinemia and B cell lymphopenia characteristic of DADA2 patients. Moreover, there was no improvement in hypogammaglobulinemia despite treatment with etanercept (Table 1, data not shown) [12]. This may reflect the requirement for TNF in establishing and maintaining the anatomical structure of B-cell follicles in mice [55], and/or the impact of TNF blockade on memory B cell maintenance in secondary lymphoid tissues [56].
Extended lymphocyte immunophenotyping and functional analysis also revealed several anomalies in DADA2 patients. First, we found a significantly reduced proportion of Treg cells. This has also been noted previously in some [13], but not all [16] studies. This is interesting as patients with DADA2 experience multiple auto-immune features including cytopenia and auto-antibody production [3]. Moreover, the frequencies of naïve CD4+ and CD8+ T cells were increased while TCM and TEM cells were reduced, pointing to a T cell differentiation defect. Second, ADA2-deficient CD4+ and CD8+ memory T cells exhibited a dysfunctional phenotype—elevated expression of PD-1, CD95, CX3CR1 and CD57; diminished expression of CD28—that has been associated with poor proliferation, terminal differentiation and exhaustion/senescence [57]. Third, ADA2-deficient memory CD8+ T cells exhibited diminished survival and granzyme production in vitro. Fourth, DADA2 patients were also found to have significantly lower proportions of MAIT, NKT, Vγδ2+ T cells and mature CD56dimCD57− NK cells, but increased proportions of immature CD56bright NK cells. Interestingly, Vγδ2 T cells are the most abundant γδT cells in peripheral blood of healthy individuals and display increased cytotoxicity against multiple viral and tumoral antigens [58, 59]. Indeed, an inverse correlation between proportions of Vδ2+ T cells and EBV reactivation has been observed following HSCT [60, 61].
Taken together, it is likely that the senescent/exhausted phenotype and impaired production of cytolytic molecules by ADA2-deficient CD8+ T cells, coupled with decreased frequencies of Vγδ2+ T cells and an accumulation of less mature NK cells, contribute to refractory/recurrent viral infections in ADA2-deficient patients [62–64]. From a mechanistic perspective, ADA2 deficiency may lead to increased activation of adenosine 2A receptors, reducing T cell activation, in addition to reduced TCR signaling and lytic activity of cytototoxic lymphocytes [65]. These findings are reminiscent of our previous analysis of the CD8 + T cell compartment in individuals with pathogenic bi-allelic inactivating variants in DOCK8 [40, 66, 67] or gain-of-function variants in PIK3CD [39] who are also susceptible to recurrent infections with herpes viruses and have an abundance of dysfunctional exhausted-type CD8+ T cells. The defect in ADA2-deficient cytotoxic lymphocytes may also explain why some DADA2 patients present with hemophagocytic lymphohistiocytosis (unpublished observation) or CD3+CD8+ large granular lymphocytes [68, 69].
Interestingly, DC phenotyping showed significantly increased proportions of CD16+CD1clo/− DCs. These DCs or non-classical like monocytes have potent T cell stimulatory capacity and produce proinflammatory cytokines upon TLR stimulation by TNF-α, IL6, IL12. Thus, these cells may play a role in maintaining the inflammatory state observed in DADA2. In addition, reduced proportions of mDCs may be associated with the overall defect in T and B cell immune responses observed in vivo.
DADA2 patients exhibited significantly reduced proportions of classical monocytes and increased proportions of intermediated and non-classical monocytes. Moreover, CD169/SIGLEC1 expression on each monocyte subset in DADA2 patients was significantly elevated compared to healthy controls. The increased non-classical monocytes in DADA2 may account in part for the observed inflammation in these patients. Indeed, nonclassical monocytes are considered proinflammatory and a prominent source of TNF-α compared to classical monocytes [70, 71]. Increased CD169/SIGLEC1 expression is in line with reported evidence of upregulated type I IFN signaling in DADA2. The concomitant increase in proportions of intermediate monocytes is interesting, as increases in both intermediate and nonclassical monocyte subsets have been observed in patients with lupus and sepsis, which are also characterized by type 1 IFN signatures [72]. Intriguingly, several patients with DADA2 were initially diagnosed with lupus based on clinical features and serum anti-dsDNA auto-antibodies [1, 11]. Interestingly, increased expression of SIGLEC-1 on myeloid cells has been implicated in viral dissemination in models of HSV1 and CMV infections, among other viruses [73]. Further study of the effect of increased SIGLEC-1 expression on the observed viral infections in DADA2 may shed light on the intriguing coalescence of the type I IFN signature and susceptibility to viral infection. Finally, increased SIGLEC-1 expression on monocytes from heterozygous carriers is consistent with the clinical observation that some carriers of pathogenic ADA2 mutations exhibit signs and symptoms compatible with DADA2, although the carriers tested here were reportedly asymptomatic. This biological parallel of clinical phenotype in carriers is also evident from the investigation of ADA2 deficient neutrophils [74].
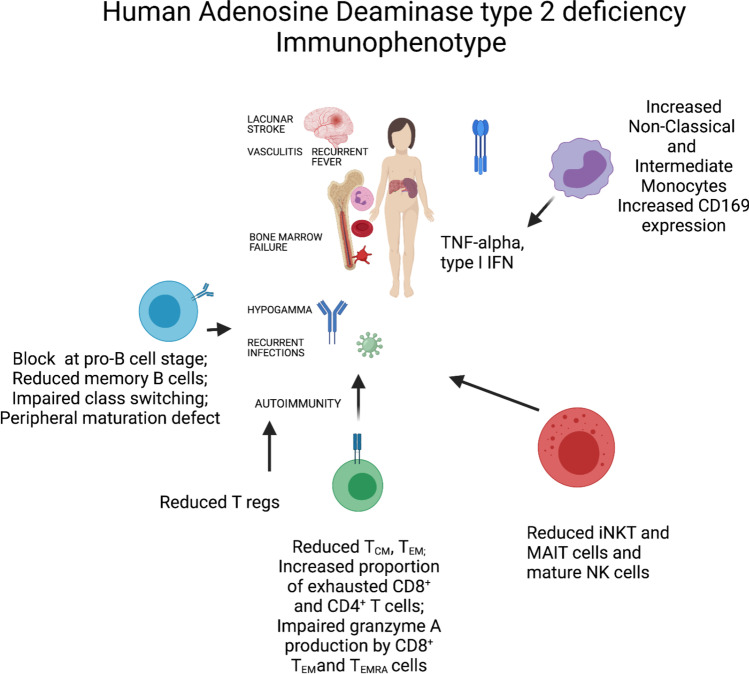
In summary, we have identified a complex immunophenotype in DADA2 with impaired differentiation of CD4+ and CD8+ T cells and B cells, in addition to an exhausted/senescent CD8+ T cell phenotype. Unconventional T cells are diminished whereas pro-inflammatory monocytes and CD56bright immature NK cells are increased (Fig. 9). These findings suggest a delicate balance between type I and type II IFN as well as TNF-α-driven hyperinflammation next to a predisposition to severe viral infection and diminished antibody responses. Our findings confirm and extend a recently published report [75, 76]. Although these are associations, they invite a large prospective survey on the immunophenotype of ADA2-deficient patients to further our understanding of the variable immunodeficiency manifestations. Moreover, as DADA2 may underlie humoral immunodeficiency, patients with an immunophenotype in line with these finding could be selected to have ADA2 enzyme activity tested [12]. Our study is unique as several patients were not on any treatment. Also, the median age of the patients was young, which may lead us to believe that there is less environmental influence on the findings, in terms of longer ongoing inflammation and multiple treatments. Ultimately, better knowledge may aid in designing targeted therapy to prevent viral infections in these patients with excessive inflammation as the overarching phenotype.
Fig. 9.
Graphical overview of major immunophenotypic findings in ADA2 deficiency. Arrows depict a hypothetical link of the immunophenotype with a clinical presentation (drafted in BioRender)
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients and their families for their trust, enthusiasm and participation in this study. We would also like to thank the DADA2 foundation for continuous support, and the Clinical Trials and Biorepository Team, St Vincent’s Centre for Applied Medical Research (St Vincent’s Hospital, Darlinghurst, NSW, Australia) for sample collection, processing and biobanking.
Author Contribution
Conceptualization: SGT, IM; methodology: JYY, SGT, LM, CSM, IM; formal analysis and investigation: JYY, LM, GH, SGT, IM; writing—original draft preparation: JYY, SGT, IM; writing—review and editing: all authors; funding acquisition: SGT, IM; resources: all authors; supervision: SGT, IM.
Funding
This study was supported by the Office of Health and Medical Research of the NSW Government, the Jeffrey Modell Foundation, SPHERE Triple I Clinical Academic Group and UNSW Medicine Infection, Immunology and Inflammation Theme, the Ross Trust, the John Brown Cook Foundation and the Sydney Children’s Hospital Network. S.G.T was a Principal Research Fellow (1042925) of the NHMRC and is currently a recipient of an NHMRC Leadership 3 Investigator Grant (1176665) and NHMRC program grant (1113904). C.S.M is supported by an Early-Mid Career Research Fellowship from the Ministry of Health of the New South Wales Government. IM is a Senior Clinical Investigator at the FWO – Flanders, and is supported by the CSL Behring Chair of Primary Immunodeficiencies, KU Leuven C1 Grant C16/18/007, a VIB GC PID Grant, FWO Grants G0C8517N, G0B5120N and G0E8420N and the Jeffrey Modell Foundation. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 948959).
Availability of Data and Materials
Available upon request to the corresponding authors.
Declarations
Ethics Approval
This study was approved by the Sydney Local Health District RPAH Zone Human Research Ethics Committee and Research Governance Office, Royal Prince Alfred Hospital, Camperdown, NSW, Australia (Protocol X16-0210/LNR/16/RPAH/257); the South East Sydney Local Health District Human Research Ethics Committee, Prince of Wales/Sydney Children’s Hospital, Randwick, NSW, Australia (Protocol HREC/11/POWH/152) and by the Ethical Committee of the University Hospitals Leuven (Protocol numbers S63077, S63807, S58466).
Consent to Participate
Written informed consent was obtained from participants or their guardians.
Consent for Publication
Patients signed informed consent regarding publishing their data.
Conflict of Interest
IM holds a CSL Behring Chair, Paid to Institution. All of the other authors have no conflicts of interest to declare.
Footnotes
Carine Wouters, Lien De Somer, Giorgia Bucciol, Selket Delafontaine, and Isabelle Meyts are members of the European Reference Network for Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases -Project ID No 739543.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stuart G. Tangye and Isabelle Meyts contributed equally to this study as senior authors.
Contributor Information
Stuart G. Tangye, Email: s.tangye@garvan.org.au
Isabelle Meyts, Email: isabelle.meyts@uzleuven.be.
References
- 1.Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370(10):911–920. doi: 10.1056/NEJMoa1307361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navon Elkan P, Pierce SB, Segel R, Walsh T, Barash J, Padeh S, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 2014;370(10):921–931. doi: 10.1056/NEJMoa1307362. [DOI] [PubMed] [Google Scholar]
- 3.Meyts I, Aksentijevich I. Deficiency of adenosine deaminase 2 (DADA2): updates on the phenotype, genetics, pathogenesis, and treatment. J Clin Immunol. 2018;38(5):569–578. doi: 10.1007/s10875-018-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashem H, Egler R, Dalal J. Refractory pure red cell aplasia manifesting as deficiency of adenosine deaminase 2. J Pediatr Hematol Oncol. 2017;39(5):e293–e296. doi: 10.1097/MPH.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 5.Alabbas F, Elyamany G, Alsharif O, Hershfield M, Meyts I. Childhood Hodgkin Lymphoma: Think DADA2. J Clin Immunol. 2019;39(1):26–29. doi: 10.1007/s10875-019-0590-7. [DOI] [PubMed] [Google Scholar]
- 6.Ombrello AK, Qin J, Hoffmann PM, Kumar P, Stone D, Jones A, et al. Treatment strategies for deficiency of adenosine deaminase 2. N Engl J Med. 2019;380(16):1582–1584. doi: 10.1056/NEJMc1801927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooray S, Omyinmi E, Hong Y, Papadopoulou C, Harper L, Al-Abadi E, et al. Anti-tumour necrosis factor treatment for the prevention of ischaemic events in patients with deficiency of adenosine deaminase 2 (DADA2). Rheumatology (Oxford). 2021. [DOI] [PubMed]
- 8.Lee PY, Kellner ES, Huang Y, Furutani E, Huang Z, Bainter W, et al. Genotype and functional correlates of disease phenotype in deficiency of adenosine deaminase 2 (DADA2) J Allergy Clin Immunol. 2020;145(6):1664–72 e10. doi: 10.1016/j.jaci.2019.12.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashem H, Kumar AR, Muller I, Babor F, Bredius R, Dalal J, et al. Hematopoietic stem cell transplantation rescues the hematological, immunological, and vascular phenotype in DADA2. Blood. 2017;130(24):2682–2688. doi: 10.1182/blood-2017-07-798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashem H, Bucciol G, Ozen S, Unal S, Bozkaya IO, Akarsu N, et al. Hematopoietic cell transplantation cures adenosine deaminase 2 deficiency: report on 30 patients. J Clin Immunol. 2021;41(7):1633–47. 10.1007/s10875-021-01098-0. [DOI] [PMC free article] [PubMed]
- 11.Schepp J, Bulashevska A, Mannhardt-Laakmann W, Cao H, Yang F, Seidl M, et al. Deficiency of adenosine deaminase 2 causes antibody deficiency. J Clin Immunol. 2016;36(3):179–186. doi: 10.1007/s10875-016-0245-x. [DOI] [PubMed] [Google Scholar]
- 12.Schepp J, Proietti M, Frede N, Buchta M, Hubscher K, Rojas Restrepo J, et al. Screening of 181 patients with antibody deficiency for deficiency of adenosine deaminase 2 sheds new light on the disease in adulthood. Arthritis Rheumatol. 2017;69(8):1689–1700. doi: 10.1002/art.40147. [DOI] [PubMed] [Google Scholar]
- 13.Van Eyck Jr L, Hershfield MS, Pombal D, Kelly SJ, Ganson NJ, Moens L, et al. Hematopoietic stem cell transplantation rescues the immunologic phenotype and prevents vasculopathy in patients with adenosine deaminase 2 deficiency. J Allergy Clin Immunol. 2015;135(1):283–7 e5. doi: 10.1016/j.jaci.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arts K, Bergerson JRE, Ombrello AK, Similuk M, Oler AJ, Agharahimi A, et al. Warts and DADA2: a Mere Coincidence? J Clin Immunol. 2018;38(8):836–843. doi: 10.1007/s10875-018-0565-0. [DOI] [PubMed] [Google Scholar]
- 15.Betrains A, Staels F, Schrijvers R, Meyts I, Humblet-Baron S, De Langhe E, et al. Systemic autoinflammatory disease in adults. Autoimmun Rev. 2021;102774. [DOI] [PubMed]
- 16.Schena F, Penco F, Volpi S, Pastorino C, Caorsi R, Kalli F, et al. Dysregulation in B-cell responses and T follicular helper cell function in ADA2 deficiency patients. Eur J Immunol. 2020;51(1):206–219. doi: 10.1002/eji.202048549. [DOI] [PubMed] [Google Scholar]
- 17.Tangye SG, Ma CS. Regulation of the germinal center and humoral immunity by interleukin-21. J Exp Med. 2020;217(1). [DOI] [PMC free article] [PubMed]
- 18.Alsultan A, Basher E, Alqanatish J, Mohammed R, Alfadhel M. Deficiency of ADA2 mimicking autoimmune lymphoproliferative syndrome in the absence of livedo reticularis and vasculitis. Pediatr Blood Cancer. 2017;65(4). [DOI] [PubMed]
- 19.Barzaghi F, Minniti F, Mauro M, Bortoli M, Balter R, Bonetti E, et al. ALPS-like phenotype caused by ADA2 deficiency rescued by allogeneic hematopoietic stem cell transplantation. Front Immunol. 2018;9:2767. doi: 10.3389/fimmu.2018.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne K, Li W, Salomon R, Ma CS. OMIP-063: 28-color flow cytometry panel for broad human immunophenotyping. Cytometry A. 2020;97(8):777–781. doi: 10.1002/cyto.a.24018. [DOI] [PubMed] [Google Scholar]
- 21.Ma CS, Wong N, Rao G, Avery DT, Torpy J, Hambridge T, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol. 2015;136(4):993–1006 e1. doi: 10.1016/j.jaci.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abeles RD, McPhail MJ, Sowter D, Antoniades CG, Vergis N, Vijay GK, et al. CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14(hi) /CD16(neg) monocytes: Expansion of CD14(hi) /CD16(pos) and contraction of CD14(lo) /CD16(pos) monocytes in acute liver failure. Cytometry A. 2012;81(10):823–834. doi: 10.1002/cyto.a.22104. [DOI] [PubMed] [Google Scholar]
- 24.Avery DT, Kane A, Nguyen T, Lau A, Nguyen A, Lenthall H, et al. Germline-activating mutations in PIK3CD compromise B cell development and function. J Exp Med. 2018;215(8):2073–2095. doi: 10.1084/jem.20180010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Zelm MC, van der Burg M, de Ridder D, Barendregt BH, de Haas EF, Reinders MJ, et al. Ig gene rearrangement steps are initiated in early human precursor B cell subsets and correlate with specific transcription factor expression. J Immunol. 2005;175(9):5912–5922. doi: 10.4049/jimmunol.175.9.5912. [DOI] [PubMed] [Google Scholar]
- 26.Uckun FM. Regulation of human B-cell ontogeny. Blood. 1990;76(10):1908–1923. [PubMed] [Google Scholar]
- 27.Ma CS, Wong N, Rao G, Nguyen A, Avery DT, Payne K, et al. Unique and shared signaling pathways cooperate to regulate the differentiation of human CD4+ T cells into distinct effector subsets. J Exp Med. 2016;213(8):1589–1608. doi: 10.1084/jem.20151467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207(1):155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bier J, Rao G, Payne K, Brigden H, French E, Pelham SJ, et al. Activating mutations in PIK3CD disrupt the differentiation and function of human and murine CD4(+) T cells. J Allergy Clin Immunol. 2019;144(1):236–253. doi: 10.1016/j.jaci.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnappauf O, Sampaio Moura N, Aksentijevich I, Stoffels M, Ombrello AK, Hoffmann P, et al. Sequence-based screening of patients with idiopathic polyarteritis nodosa, granulomatosis with polyangiitis, and microscopic polyangiitis for deleterious genetic variants in ADA2. Arthritis Rheumatol. 2021;73(3):512–519. doi: 10.1002/art.41549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muraoka T, Katsuramaki T, Shiraishi H, Yokoyama MM. Automated enzymatic measurement of adenosine deaminase isoenzyme activities in serum. Anal Biochem. 1990;187(2):268–272. doi: 10.1016/0003-2697(90)90455-i. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Ami T, Revel-Vilk S, Brooks R, Shaag A, Hershfield MS, Kelly SJ, et al. Extending the clinical phenotype of adenosine deaminase 2 deficiency. J Pediatr. 2016;177:316–320. doi: 10.1016/j.jpeds.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 33.Rice GI, Melki I, Fremond ML, Briggs TA, Rodero MP, Kitabayashi N, et al. Assessment of type I interferon signaling in pediatric inflammatory disease. J Clin Immunol. 2017;37(2):123–132. doi: 10.1007/s10875-016-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–45 e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Prat M, Alvarez-Sierra D, Aguilo-Cucurull A, Salgado-Perandres S, Briongos-Sebastian S, Franco-Jarava C, et al. Extended immunophenotyping reference values in a healthy pediatric population. Cytometry B Clin Cytom. 2019;96(3):223–233. doi: 10.1002/cyto.b.21728. [DOI] [PubMed] [Google Scholar]
- 36.Ma CS, Pittaluga S, Avery DT, Hare NJ, Maric I, Klion AD, et al. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J Clin Investig. 2006;116(2):322–333. doi: 10.1172/JCI25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuss AK, Avery DT, Cannons JL, Yu LJ, Nichols KE, Shaw PJ, et al. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176(3):1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 38.Suryani S, Fulcher DA, Santner-Nanan B, Nanan R, Wong M, Shaw PJ, et al. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood. 2010;115(3):519–529. doi: 10.1182/blood-2009-07-234799. [DOI] [PubMed] [Google Scholar]
- 39.Moens L, Tangye SG. Cytokine-mediated regulation of plasma cell generation: IL-21 takes center stage. Front Immunol. 2014;5:65. doi: 10.3389/fimmu.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoki K, Shimada S, Simantini DS, Tun MM, Buerano CC, Morita K, et al. Type-I interferon response affects an inoculation dose-independent mortality in mice following Japanese encephalitis virus infection. Virol J. 2014;11:105. doi: 10.1186/1743-422X-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards ESJ, Bier J, Cole TS, Wong M, Hsu P, Berglund LJ, et al. Activating PIK3CD mutations impair human cytotoxic lymphocyte differentiation and function and EBV immunity. J Allergy Clin Immunol. 2019;143(1):276–91 e6. doi: 10.1016/j.jaci.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 42.Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208(11):2305–2320. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ives ML, Ma CS, Palendira U, Chan A, Bustamante J, Boisson-Dupuis S, et al. Signal transducer and activator of transcription 3 (STAT3) mutations underlying autosomal dominant hyper-IgE syndrome impair human CD8(+) T-cell memory formation and function. J Allergy Clin Immunol. 2013;132(2):400–11 e9. doi: 10.1016/j.jaci.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116(19):3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 45.Della Chiesa M, Pesce S, Muccio L, Carlomagno S, Sivori S, Moretta A, et al. Features of memory-like and PD-1(+) human NK cell subsets. Front Immunol. 2016;7:351. doi: 10.3389/fimmu.2016.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyts I, Aksentijevich I. Deficiency of adenosine deaminase 2 (DADA2): updates on the phenotype, genetics, pathogenesis, and treatment. J Clin Immunol. 2018;38(5):569–578. doi: 10.1007/s10875-018-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villar J, Segura E. The more, the merrier: DC3s join the human dendritic cell family. Immunity. 2020;53(2):233–235. doi: 10.1016/j.immuni.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Rhodes JW, Tong O, Harman AN, Turville SG. Human dendritic cell subsets, ontogeny, and impact on HIV infection. Front Immunol. 2019;10:1088. doi: 10.3389/fimmu.2019.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skrabl-Baumgartner A, Plecko B, Schmidt WM, Konig N, Hershfield M, Gruber-Sedlmayr U, et al. Autoimmune phenotype with type I interferon signature in two brothers with ADA2 deficiency carrying a novel CECR1 mutation. Pediatr Rheumatol Online J. 2017;15(1):67. doi: 10.1186/s12969-017-0193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belot A, Wassmer E, Twilt M, Lega JC, Zeef LA, Oojageer A, et al. Mutations in CECR1 associated with a neutrophil signature in peripheral blood. Pediatr Rheumatol Online J. 2014;12:44. doi: 10.1186/1546-0096-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uettwiller F, Sarrabay G, Rodero MP, Rice GI, Lagrue E, Marot Y, et al. ADA2 deficiency: case report of a new phenotype and novel mutation in two sisters. RMD Open. 2016;2(1):e000236. doi: 10.1136/rmdopen-2015-000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Insalaco A, Moneta GM, Pardeo M, Caiello I, Messia V, Bracaglia C, et al. Variable clinical phenotypes and relation of interferon signature with disease activity in ADA2 deficiency. J Rheumatol. 2019;46(5):523–526. doi: 10.3899/jrheum.180045. [DOI] [PubMed] [Google Scholar]
- 53.Biesen R, Demir C, Barkhudarova F, Grun JR, Steinbrich-Zollner M, Backhaus M, et al. Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis Rheum. 2008;58(4):1136–1145. doi: 10.1002/art.23404. [DOI] [PubMed] [Google Scholar]
- 54.Van Eyck L, Hershfield MS, Pombal D, Kelly SJ, Ganson NJ, Moens L, Frans G, Schaballie H, De Hertogh G, Dooley J, Bossuyt X, Wouters C, Liston A, Meyts I. Hematopoietic stem cell transplantation rescues the immunologic phenotype and prevents vasculopathy in patients with adenosine deaminase 2 deficiency. J Allergy Clin Immunol. 2015;135(1):283–287. doi: 10.1016/j.jaci.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cook MC, Korner H, Riminton DS, Lemckert FA, Hasbold J, Amesbury M, et al. Generation of splenic follicular structure and B cell movement in tumor necrosis factor-deficient mice. J Exp Med. 1998;188(8):1503–1510. doi: 10.1084/jem.188.8.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anolik JH, Ravikumar R, Barnard J, Owen T, Almudevar A, Milner EC, et al. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol. 2008;180(2):688–692. doi: 10.4049/jimmunol.180.2.688. [DOI] [PubMed] [Google Scholar]
- 57.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 58.Liu C, Emami SN, Pettersson J, Ranford-Cartwright L, Faye I, Parmryd I. Vgamma9Vdelta2 T cells proliferate in response to phosphoantigens released from erythrocytes infected with asexual and gametocyte stage Plasmodium falciparum. Cell Immunol. 2018;334:11–19. doi: 10.1016/j.cellimm.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat Immunol. 2013;14(9):908–916. doi: 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, Bian Z, Wang X, Xu LP, Fu Q, Wang C, et al. Inverse correlation of Vdelta2(+) T-cell recovery with EBV reactivation after haematopoietic stem cell transplantation. BJH. 2018;180(2):276–285. doi: 10.1111/bjh.15037. [DOI] [PubMed] [Google Scholar]
- 61.De Rosa SC, Mitra DK, Watanabe N, Herzenberg LA, Herzenberg LA, Roederer M. Vdelta1 and Vdelta2 gammadelta T cells express distinct surface markers and might be developmentally distinct lineages. J Leukoc Biol. 2001;70(4):518–526. [PubMed] [Google Scholar]
- 62.Le Voyer T, Boutboul D, Ledoux-Pilon A, de Fontbrune FS, Boursier G, Latour S, et al. Late-Onset EBV Susceptibility and Refractory Pure Red Cell Aplasia Revealing DADA2. J Clin Immunol. 2020;40(6):948–953. doi: 10.1007/s10875-020-00812-8. [DOI] [PubMed] [Google Scholar]
- 63.Staples E, Simeoni I, Stephens JC, Allen HL, BioResource N, Wright P, et al. ADA2 deficiency complicated by EBV-driven lymphoproliferative disease. Clin Immunol. 2020;215:108443. doi: 10.1016/j.clim.2020.108443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brooks JP, Rice AJ, Ji W, Lanahan SM, Konstantino M, Dara J, et al. Uncontrolled Epstein-Barr Virus as an atypical presentation of deficiency in ADA2 (DADA2) J Clin Immunol. 2021;41(3):680–683. doi: 10.1007/s10875-020-00940-1. [DOI] [PubMed] [Google Scholar]
- 65.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16(3):177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 66.Pillay BA, Avery DT, Smart JM, Cole T, Choo S, Chan D, et al. Hematopoietic stem cell transplant effectively rescues lymphocyte differentiation and function in DOCK8-deficient patients. JCI Insight. 2019;5(11):e127527. doi: 10.1172/jci.insight.127527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pillay BA, Fusaro M, Gray PE, Statham AL, Burnett L, Bezrodnik L, et al. Somatic reversion of pathogenic DOCK8 variants alters lymphocyte differentiation and function to effectively cure DOCK8 deficiency. JCI. 2021;131(3). [DOI] [PMC free article] [PubMed]
- 68.Trotta L, Martelius T, Siitonen T, Hautala T, Hamalainen S, Juntti H, et al. ADA2 deficiency: Clonal lymphoproliferation in a subset of patients. The J Allergy clin Immunol. 2018;141(4):1534–1537. doi: 10.1016/j.jaci.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 69.Saettini F, Fazio G, Corti P, Quadri M, Bugarin C, Gaipa G, et al. Two siblings presenting with novel ADA2 variants, lymphoproliferation, persistence of large granular lymphocytes, and T-cell perturbations. Clin Immunol. 2020;218:108525. doi: 10.1016/j.clim.2020.108525. [DOI] [PubMed] [Google Scholar]
- 70.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168(7):3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 71.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 72.Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep. 2015;5:13886. doi: 10.1038/srep13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez-Zsolt D, Martinez-Picado J, Izquierdo-Useros N. When dendritic cells go viral: the role of Siglec-1 in host defense and dissemination of enveloped viruses. Viruses. 2019;12(1). [DOI] [PMC free article] [PubMed]
- 74.Carmona-Rivera C, Khaznadar SS, Shwin KW, Irizarry-Caro JA, O'Neil LJ, Liu Y, et al. Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood. 2019;134(4):395–406. doi: 10.1182/blood.2018892752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watanabe N, Gao S, Wu Z, Batchu S, Kajigaya S, Diamond C, et al. Analysis of deficiency of adenosine deaminase 2 pathogenesis based on single-cell RNA sequencing of monocytes. J Leukoc Biol. 2021. [DOI] [PubMed]
- 76.Ehlers L, Meyts I. What a difference ADA2 makes: Insights into the pathophysiology of ADA2 deficiency from single-cell RNA sequencing of monocytes. J Leukoc Biol. 2021. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available upon request to the corresponding authors.