Abstract
This study evaluated the performance of immunoblotting with Leishmania infantum soluble antigens for the diagnosis of visceral leishmaniasis in human immunodeficiency virus (HIV)-infected and immunocompetent patients and assessed the humoral responses of patients coinfected with HIV and Leishmania. In this work, the results of the immunoblot analysis were compared to those of parasitological examination (Giemsa-stained smears and/or parasite isolation in Novy, Nicolle, and MacNeal medium from bone marrow) and indirect immunofluorescence and counterimmunoelectrophoresis techniques. Patients were considered to be infected if one or more of the comparison techniques gave a positive result. Immunoblotting was considered to be positive if at least one band was present. For 198 HIV-positive patients with a clinical suspicion of visceral leishmaniasis, immunoblot analysis had a sensitivity of 70.6%, a specificity of 73.2%, and an accuracy of 72.7%. For a separate group of 40 immunocompetent patients not infected with Leishmania, the immunoblot analysis was negative for all patients (100% specificity), and for a third group of 32 immunocompetent patients with confirmed visceral leishmaniasis, the immunoblot analysis was positive for all patients (100% sensitivity). Sera of coinfected patients recognized few bands and recognized bands at lower intensities compared with sera from immunocompetent patients. The most frequently detected band was 63 to 66 kDa (55.9%), with the difference being statistically significant compared to frequency of detection of the other bands. This study confirms that the humoral response in patients coinfected with HIV and Leishmania is much lower than that in immunocompetent patients and that the immunoblot method is a sensitive, noninvasive, and specific serological technique for the diagnosis of visceral leishmaniasis in immunocompromised patients.
Leishmaniasis is present in 88 countries, and, worldwide, more than 350 million people are exposed to the infection (25). In the Mediterranean basin, the visceral form of leishmaniasis is a significant cause of morbidity. Mediterranean visceral leishmaniasis (VL) is often acquired at an early age as infant kala-azar (2). Since the mid-1980s there has been a dramatic increase in the number of leishmanial infections in human immunodeficiency virus (HIV)-infected patients in the Mediterranean areas where Leishmania infantum is endemic. This increase has led to a marked shift in the age pattern of VL infection, from infants to adults. In southern Europe, 50% of adult VL cases are now related to HIV infection (10).
Definitive diagnosis of VL is mainly based on the demonstration of the parasite in bone marrow or by the detection of antileishmanial antibodies in serum, which has the advantage of being noninvasive. Antileishmanial antibodies have been proven to be of high diagnostic value in immunocompetent patients and can be detected by various methods, such as indirect fluorescent immunoassay (IFI), enzyme-linked immunosorbent assay, counterimmunoelectrophoresis (CIE), or direct agglutination test, performed with the whole parasite or crude promastigote lysates. However, in patients with AIDS the humoral immune response to L. infantum may be weak or negative (6). Since early diagnosis is of great importance for the effective treatment of this potentially fatal infection, it seems necessary to develop a simple, noninvasive, sensitive, and specific tool for the laboratory diagnosis of VL in immunocompromised patients. The immunoblot technique for the detection of antibodies to the 14- and 16-kDa antigens has been successful in the diagnosis of VL in immunocompetent patients, in whom it shows a high sensitivity (100%) and a high specificity (98%) (18).
The aim of the present study was to investigate whether the immunoblot technique can be used to diagnose VL in immunocompromised patients when the results of the immunoblot technique were compared with those of parasitological examination, IFI, and CIE.
MATERIALS AND METHODS
Patients.
Four groups of human sera were studied. These belonged to (i) 198 HIV-positive adult patients with clinical suspicion of VL (patients who showed signs and symptoms of VL, with fever and hepatosplenomegaly found to be the most common); (ii) 32 immunocompetent people (19 adults and 13 children) confirmed to have VL (patients with positive results by parasitological examination or a positive result by at least one serological test); (iii) 11 healthy blood donors with negative results by serological tests (CIE and IFI); and (iv) 29 immunocompetent patients with other confirmed diseases (4 with toxoplasmosis, 3 with malaria, 6 with trypanosomiasis, 2 with helminthiasis, 4 with leptospirosis, 4 with tuberculosis, 4 with hepatitis, and 2 with cutaneous leishmaniasis [1 infected with the L. infantum MON-29 zymodeme and another infected with L. guyanensis]) but with no antileishmanial antibodies as detected by IFI and CIE.
Parasitological and serological methods.
Parasitological examination was performed by microscopic observation of Giemsa-stained smears and/or parasite isolation in Novy, Nicolle, and MacNeal (NNN) medium (19) from bone marrow for 35 of the 198 immunocompromised patients. For all patients with negative results by direct examination, cultures were also carried out. Cultures were incubated at 24°C, subcultured, and examined weekly for 5 weeks.
Serological methods were carried out for all 198 immunocompromised patients and the groups of healthy blood donors and patients with other diseases.
IFI was carried out by the technique described previously (1). The antigen was prepared from the L. infantum MON-1 zymodeme, which was maintained by weekly passage in NNN medium. A dilution of 1:50 was considered the cutoff point (4).
CIE was performed as described elsewhere (5). The antigen was prepared from L. infantum MON-1 as described by Mansueto et al. (16). Serum samples were used undiluted. All reactions with at least one precipitation arc were considered positive (6).
Immunoblot assay.
Stationary-phase promastigotes from L. infantum MON-1 with no more than five subcultures in NNN medium were used as antigen. Extraction of total parasite antigens was carried out as described by Santos-Gomes and Abranches (22). Briefly, promastigotes were centrifuged at 1,000 × g for 15 min at 22°C. The pellet was resuspended in Locke's solution and was washed three times. The final pellet was resuspended in Tris-buffered saline (TBS; 20 mM Tris-HCl, 150 mM NaCl [pH 7.4]) with 3% (vol/vol) protease inhibitors (N-δ-p-tosyl-l-lysine chloromethyl ketone, 1-chloro-4-phenyl-3-tosylamido-l-butane, and phenylmethylsulfonyl fluoride). The membrane-bound proteins were released by the addition of 10% (wt/vol) sodium dodecyl sulfate (SDS). The lysates were passed repeatedly through a fine-gauge needle to shear the DNA. The samples were incubated at 22°C for 30 min. The insoluble material was removed after centrifugation at 10,000 × g for 10 min.
The antigens for Western blotting (Wb) were separated by SDS-polyacrylamide gel electrophoresis (PAGE) with a 10% (wt/vol) polyacrylamide gel. SDS-PAGE was performed as described by Laemmli (15). The separated antigens were transferred to nitrocellulose membranes (Gelman Sciences, Ann Arbor, Mich.) after being briefly stained with Ponceau S (BDH, Poole, England) and blocked in TBS–3% bovine serum albumin (Boehringer Mannheim, GmbH, Mannheim, Germany), overnight. The nitrocellulose was cut into vertical strips, and each strip was incubated with human serum for 1 h. The sera were used at a fixed dilution (1:100), as described by Marty et al. (17). After being washed and incubated for 1 h with anti-human immunoglobulin G (IgG) (γ chain specific) alkaline phosphatase conjugate (Sigma Chemical Company, St. Louis, Mo.) diluted 1:1,000 in TBS–3% bovine serum albumin, the sera were developed with alkaline phosphatase substrate buffer (0.04 mM 5-bromo-4-chloro-3-indolyl phosphate [Sigma Chemical Company], 0.37 mM nitroblue tetrazolium [Sigma Chemical Company], 10 mM NaCl, 10 mM Tris base, 0.25 mM MgCl2). The color reaction was stopped by washing with distilled water. All assays were performed with 50 μg of total protein per strip.
The criterion for positivity is the presence of at least one band, since the sera from the control groups (healthy blood donors and patients with other diseases) did not recognize any leishmanial antigen.
Statistical methods.
For quality control, we assessed the quality of the tests by calculating their sensitivity (Tp/Tp + Fn), specificity (Tn/Tn + Fp), and accuracy (Tp + Tn/P + N), where Tp is the number of true-positive specimens, Fp is the number of false-positive specimens, Tn is the number of true-negative specimens, Fn is the number of false-negative specimens, P is the total number of positive specimens, and N is the total number of negative specimens. Accuracy indicates the proportion of patients correctly classified by the test (11). The choice of a reference test for positive results was difficult because classical serological reactions have very low sensitivity as tests for the diagnosis of VL in immunocompromised patients (6) and parasitological examination is generally positive for patients with large parasite loads (3). Thus, patients were considered to be positive if one or more of the comparison techniques gave a positive result and were considered to be negative if all the comparison techniques gave a negative result.
The McNemar χ2 test was used when comparing Wb and other diagnostic tests and was considered significant with a 5% significance level (P < 0.05). The statistical test with a normal distribution was applied to assess the significance of the frequency of bands by Wb. Results were considered significant with a 5% significance level (P < 0.05).
RESULTS
Wb was positive for 68 of 198 (34.3%) serum samples from HIV-positive patients. For 24 samples Wb and at least one of the other tests were positive, while for 44 samples Wb was positive and the other tests were negative. For a total of 34 samples for which at least one of the other techniques was positive, Wb was negative for 10 (Table 1). Parasitological examination was positive for 16 patients (45.7%) and was negative for another 19 patients, and the Wb result matched these results for 14 serum samples from the first group and 8 samples from the second group. In one of two patients with a positive result by parasitological examination and a negative result by Wb, L. donovani MON-18 was the zymodeme responsible for infection. For 198 serum samples tested by IFI, only 8 had significant titers (4.0%), with 6 of them being positive by Wb. The Wb result was also positive for 62 serum samples without significant titers by IFI. CIE was tested with serum samples from the same 198 patients and was positive for 27 (13.6%); for 18 of these samples and for 50 serum samples which were negative by CIE, the Wb result was positive (Table 1).
TABLE 1.
Comparison of results of immunoblotting and the reference tests for immunocompromised patients suspected of having VL
| Wb result | No. of specimens with the indicated result by the following test:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Parasitological examination
|
IFI
|
CIE
|
Reference testa
|
|||||
| Positive | Negative | Positiveb | Negative | Positivec | Negative | Positive | Negative | |
| Positive | 14 | 8 | 6 | 62 | 18 | 50 | 24 | 44 |
| Negative | 2 | 11 | 2 | 128 | 9 | 121 | 10 | 120 |
| Total | 16 | 19 | 8 | 190 | 27 | 171 | 34 | 164 |
The reference test was considered positive if the results of any one or more of three methods (IFI, CIE, or parasitological examination) were positive.
Titer, ≥1:50.
At least one precipitation arc.
Wb was positive for 100% of the 32 serum samples from immunocompetent patients with VL and was negative for 100% of the serum samples from 11 healthy blood donors and 29 patients with other confirmed diseases.
For the immunocompromised patient group, the McNemar χ2 test showed significant differences when the Wb result was compared with those of each of the other serological tests (P < 0.05) but did not show significant differences when the Wb result was compared to the result of the parasitological examination (P > 0.05). For these patients, Wb showed a sensitivity of 70.6%, a specificity of 73.2%, and an accuracy of 72.7% compared to the results of the reference test.
For the 40 immunocompetent patients known to not be infected with Leishmania, the specificity of Wb was 100%.
Sera from both the immunocompromised and immunocompetent patients recognized leishmanial antigens with molecular masses ranging from 200 to 21 kDa (Table 2). The antigenic bands most frequently recognized were 63 to 66 and 95 to 97 kDa. The difference between the frequency of the band of 63 to 66 kDa and the frequency of each of the other bands is statistically significant (P < 0.001).
TABLE 2.
Frequency of L. infantum MON-1 antigens recognized by sera from 68 immunocompromised patients suspected of having VL and 32 immunocompetent patients confirmed to have VL
| Polypeptide molecular mass (kDa) | % of sera from:
|
|
|---|---|---|
| Immunocompromised patients suspected of having VL | Immunocompetent patients with VL | |
| 200 | 10.3 | |
| 150 | 4.4 | |
| 116 | 7.4 | 9.4 |
| 97–95 | 30.9 | 71.9 |
| 81 | 5.9 | 28.1 |
| 75–70 | 13.2 | 21.9 |
| 67 | 9.4 | |
| 66–63 | 55.9 | 93.8 |
| 60 | 6.3 | |
| 58–55 | 4.4 | 59.4 |
| 50 | 17.6 | 9.4 |
| 45 | 13.2 | 15.6 |
| 40 | 9.4 | |
| 38–35 | 8.8 | 40.6 |
| 31 | 11.8 | 15.6 |
| 26–21 | 4.4 | 3.1 |
| <21 | 2.9 | |
For the immunocompromised patients, the antigenic binding patterns were very heterogeneous, with 33 of 68 (48.5%) serum samples recognizing one band and only 2 of 68 (2.9%) serum samples recognizing five or more bands. The intensities of the bands was generally lower for immunocompromised patients than for immunocompetent patients (Fig. 1). For the immunocompetent patients, the antigenic binding patterns were as follows: sera from 15 patients (46.9%) recognized five or more bands and serum from only one patient (3.1%) recognized one band.
FIG. 1.
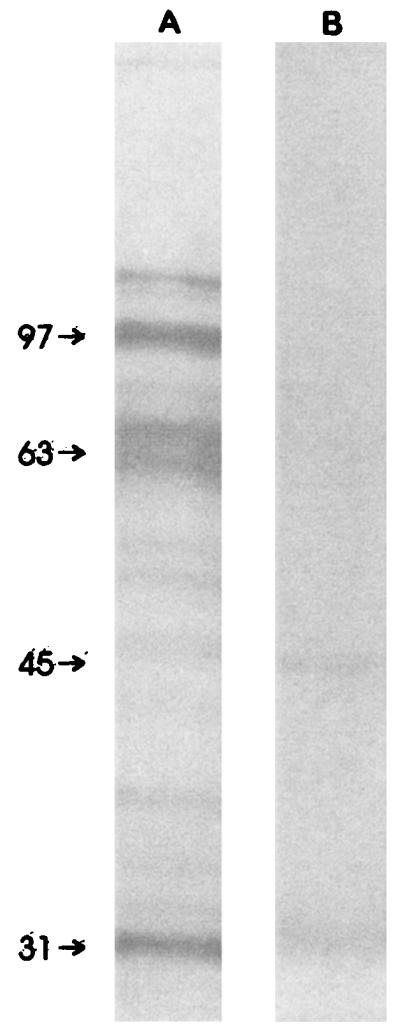
Immunoblot of sera from immunocompetent (A) and immunocompromised (B) patients with VL, showing the differences in both the numbers and the intensities of the bands. The molecular mass markers (indicated on the left) are in kilodaltons.
DISCUSSION
This study confirms that the specificity of Wb can be extremely high (100%) when it is used to test for VL in immunocompetent people, even in patients infected with diseases which often produce cross-reactions when classical serological methods are used, such as African trypanosomiasis, tuberculosis, and malaria (13, 14). In two cases of cutaneous leishmaniasis in immunocompetent patients (one caused by L. infantum MON-29 and the other caused by L. guyanensis [7]), the immunoblotting result was negative. However, this is not necessarily related to the use of heterologous antigen because the production of antibodies is not usual in patients with noncomplicated cutaneous leishmaniasis. The same conclusion can be applied to a patient with AIDS and VL from whom L. donovani MON-18 was isolated, since in a significant number of coinfected patients antibodies are not found (6). However, we did not observe the high degree of specificity found for sera from immunocompetent individuals when we tested sera from immunocompromised patients (specificity of Wb, 73.2%), a fact that could be related to the lack of sensitivity of the reference tests.
Techniques like IFI and CIE have been widely used as tools for the diagnosis of VL in immunocompetent patients, showing high values of sensitivity and specificity (12, 16); nevertheless, in a previous study of patients who were coinfected with HIV and Leishmania and who had a positive classical parasitological examination, the sensitivities of IFI and CIE were 36.8 and 47.0%, respectively (6). Evidence from the present study comparing the results of Wb, IFI, and CIE to those of parasitological examination suggests that Wb may have a higher sensitivity than IFI and CIE. Furthermore, the test was positive for 44 serum samples from patients for whom all the other tests were negative. It is possible that some of the patients who were positive by Wb and negative by classical serological tests could be infected with L. infantum, and as a consequence, the real sensitivity of Wb with sera from coinfected patients could be higher than 70.6% and the true specificity could also be increased.
In this study the antigenic binding patterns obtained by immunoblotting for immunocompromised patients were different from those found by ourselves and other investigators for immunocompetent people (18). Among the sera from the group of 198 immunocompromised patients studied, only 2.9% recognized five or more antigenic fractions. In contrast, 46.9% of 32 VL immunocompetent patients' sera recognized five or more bands. The existence of a great number of antigenic bands in immunocompetent patients has also been reported by Rolland et al. (21).
In this work we found that in immunocompromised people it was not possible to recognize a characteristic band or range of bands associated with VL. The most frequently recognized band was 63 to 66 kDa, and it was detected in 55.9% of the sera. This band could be related to the major superficial antigen gp63 of the parasite. This antigen has been proposed as a potential diagnostic antigen by several investigators (8, 20, 23) but was found by others not to induce a significant antibody response (24). The use of cultured virulent promastigotes from the stationary phase could be important as a means of obtaining an immunodominant antigen. Santos-Gomes and Abranches (22) showed that gp63 from virulent L. infantum strains is much more reactive against an anti-gp63 serum than gp63 obtained from attenuated strains.
Low-molecular-mass antigens like the 32-kDa (9, 24) and the 14- to 16-kDa (18) antigens, usually reported as immunodominant antigens, were rarely found in this study. However, strict comparisons between our results and others reported in the literature are rather difficult because of the variability in techniques and the use of different antigens.
The work performed in the study described here confirms that the humoral response in patients coinfected with HIV and Leishmania is much lower than that in immunocompetent ones, as shown by differences in the numbers and intensities of bands. It has also been demonstrated that the immunoblot method used in the present study is a relatively sensitive, noninvasive serological technique for the diagnosis of VL caused by the L. infantum MON-1 zymodeme in patients coinfected with HIV and Leishmania. For these reasons it seems that immunoblotting is a promising technique, although it needs further evaluation for application in routine diagnostic laboratories.
ACKNOWLEDGMENTS
We are grateful to M. Luck and F. Exposto for critical review of the manuscript; B. A. Fernandes, K. Mansinho (Hospital Egas Moniz), and F. Antunes (Hospital Santa Maria) for providing us with some of the sera; J. Ramada, J. M. Cristovão, and M. Horta for technical help; and P. Aguiar and L. Gonçalves for performing the statistical analyses. We thank Jean-Claude Dujardin for gifts of sera from patients with trypanosomiasis.
This study was supported by Comissão Nacional de Luta Contra a SIDA of the Ministério da Saúde of Portugal (Proc. 8A-1.10.6/94).
REFERENCES
- 1.Abranches P. Ph.D. thesis. Lisbon, Portugal: Faculdade de Ciências Médicas da Universidade Nova de Lisboa; 1984. O kala-azar da área metropolitana de Lisboa e da região de Alcácer do Sal. Estudos sobre os reservatórios doméstico e silvático e sobre a população humana em risco de infecção. [Google Scholar]
- 2.Abranches P, Santos-Gomes G, Campino L. Epidemiology of leishmaniasis in Portugal. Arch Inst Pasteur Tunis. 1993;70:349–355. [PubMed] [Google Scholar]
- 3.Alvar J, Cañavete C, Gutiérrez-Solar B, Jiménez M, Laguna F, López-Vélez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campino L, Abranches P. Indirect fluorescent immunoassay in the diagnosis of infantile and adult kala-azar. Trans R Soc Trop Med Hyg. 1991;85:476. doi: 10.1016/0035-9203(91)90225-n. [DOI] [PubMed] [Google Scholar]
- 5.Campino L, Riça Capela M J, Maurício I, Osensoy S, Abranches P. O kala-azar em Portugal. IX. A região do Algarve: inquérito epidemiológico sobre o reservatório canino no concelho de Loulé. Rev Portug Doenç Infec. 1995;18:189–194. [Google Scholar]
- 6.Campino L, Santos-Gomes G, Pratlong F, Antunes F, Maurício I, Dedet J P, Abranches P. HIV/Leishmania co-infections in Portugal: diagnosis and isoenzyme characterization of Leishmania. Ann Trop Med Parasitol. 1997;91:433–436. doi: 10.1080/00034989761067. [DOI] [PubMed] [Google Scholar]
- 7.Campino L, Santos-Gomes G, Pratlong F, Gomes-Pereira S, Riça Capela M J, Pires R C, Nina J, Carmona H, Mansinho K, Antunes F, Abranches P. Género Leishmania em Portugal. Zimodemes isolados a partir do reservatório doméstico e silvático, do vector e de casos humanos autóctones e importados. Acta Parasitol Portug. 1997;4:65. [Google Scholar]
- 8.Chiller T M, Samudio M A, Zoulek G. IgG antibody reactivity with Tripanossoma cruzi and Leishmania antigens in sera of patients with Chagas disease and leishmaniasis. Am J Trop Med Hyg. 1990;43:650–656. doi: 10.4269/ajtmh.1990.43.650. [DOI] [PubMed] [Google Scholar]
- 9.Evans T G, Krug E C, Wilson M E, Vasconcelos A W, Alencar J E, Pearson R D. Evaluation of antibody responses in American visceral leishmaniasis by ELISA and immunoblot. Mem Inst Oswaldo Cruz. 1989;84:157–166. doi: 10.1590/s0074-02761989000200003. [DOI] [PubMed] [Google Scholar]
- 10.Gorgolas M, Miles M A. Visceral leishmaniasis and AIDS. Nature. 1994;372:734. doi: 10.1038/372734b0. [DOI] [PubMed] [Google Scholar]
- 11.Greenhalgh T. Papers that report diagnostic or screening tests. Br Med J. 1997;315:540–543. doi: 10.1136/bmj.315.7107.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harith A E, Kolk A A, Kager P A, Leeuwenburg J, Faber F J, Muigai R, Kiugu S, Laarman J J. Evaluation of a newly developed direct agglutination test (DAT) for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis: comparison with IFAT and ELISA. Trans R Soc Trop Med Hyg. 1987;81:603–606. doi: 10.1016/0035-9203(87)90423-8. [DOI] [PubMed] [Google Scholar]
- 13.Harith A E, Kolk A A, Kager P A, Leeuwenburg J, Muigai R, Kingu S, Kingu S, Laarman J J. A simple and economical direct agglutination test for serodiagnosis and sero-ecoepidemiological studies of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1986;80:583–587. doi: 10.1016/0035-9203(86)90149-5. [DOI] [PubMed] [Google Scholar]
- 14.Jaffe C L, Zakis M. Use of purified parasite proteins from Leishmania donovani for the rapid serodiagnosis of visceral leishmaniasis. J Infect Dis. 1988;157:1212–1220. doi: 10.1093/infdis/157.6.1212. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Mansueto S, Picone D, Di Rosa S, La Cascia C. La contraimmunoelecttroforesi (CIEP) nella diagnosti della leishmaniose viscerale. Boll Ist Sieroter Milan. 1978;57:623–630. [PubMed] [Google Scholar]
- 17.Marty P, Levievre A, Quaranta J-F, Rahal A, Gari-Toussaint M, Le Fichoux Y. Use of the leishmanin skin test and Western blot analysis for epidemiological studies in visceral leishmaniasis areas: experience in a highly endemic focus in Alpes-Maritimes (France) Trans R Soc Trop Med Hyg. 1994;88:658–659. doi: 10.1016/0035-9203(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 18.Mary C, Lamouroux D, Dunan S, Quilici M. Western blot analysis of antibodies to Leishmania infantum antigens: potential of the 14-kD and 16-kD antigens for diagnosis and epidemiological purposes. Am J Trop Med Hyg. 1992;47:764–771. doi: 10.4269/ajtmh.1992.47.764. [DOI] [PubMed] [Google Scholar]
- 19.Nicolle C. Isolement et culture des corps de Leishman. Arch Inst Pasteur Tunis. 1908;2:55–56. [Google Scholar]
- 20.Reed S G, Shreffler W G, Burns J M, Jr, Scott J M, Orge M G, Ghalib H W, Siddig M, Badaró R. An improved serodiagnostic procedure for visceral leishmaniasis. Am J Trop Med Hyg. 1990;43:632–639. doi: 10.4269/ajtmh.1990.43.632. [DOI] [PubMed] [Google Scholar]
- 21.Rolland L, Zilberfarb V, Furtado A, Gentilini M. Identification of a 94-kilodalton antigen on Leishmania promastigote forms and its specific recognition in human and canine visceral leishmaniasis. Parasite Immunol. 1994;16:599–608. doi: 10.1111/j.1365-3024.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 22.Santos-Gomes G, Abranches P. Comparative study of infectivity caused by promastigotes of Leishmania infantum MON-1, L. infantum MON-24 and L. donovani MON-18. Folia Parasitol. 1996;43:7–12. [PubMed] [Google Scholar]
- 23.Schreffler W G, Burns J M, Jr, Badaró R, Ghalib H W, Button L L, McMaster R W, Reed S G. Antibody responses of visceral leishmaniasis patients to gp63, a major surface glycoprotein of Leishmania species. J Infect Dis. 1993;167:426–430. doi: 10.1093/infdis/167.2.426. [DOI] [PubMed] [Google Scholar]
- 24.Tebourski F, El Gaied A, Louzir H, Ben-Ismail R, Kammoun R, Dellagi K. Identification of an immunodominant 32-kilodalton membrane protein of Leishmania donovani infantum promastigotes suitable for specific diagnosis of Mediterranean visceral leishmaniasis. J Clin Microbiol. 1994;32:2474–2480. doi: 10.1128/jcm.32.10.2474-2480.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Rapport sur la Santé dans le Monde. Combattre la Maladie, Promouvoir le développement. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]


