Abstract
Purpose:
In pre-planned observational analysis of the POWER-remote trial, we examined the impact of weight loss on patient-reported outcomes (PROs). We hypothesized a priori that survivors with ≥5% weight loss would have improved physical function (PF) at 6 months v. those who did not.
Methods:
Patients with stage 0-III breast cancer who completed local therapy and chemotherapy with BMI ≥25 kg/m2 were randomized to POWER-remote (telephone coaching; diet/activity tracking) or self-directed weight-loss (booklet). Participants completed PROs at baseline, 6 and 12 months: PROMIS PF, pain, fatigue, anxiety, depression, sleep; FACT-endocrine symptoms; MOS-sexual function. Changes in PROs among those with ≥5% weight loss v. those with <5% were tested with multivariable mixed effects models, across randomized groups.
Results:
Of 94 women who completed PROs, 84 and 69 participants were evaluable at 6 and 12 months, respectively. Regardless of intervention, PF improved in those with ≥5% weight loss v. those with <5% at 6-months (4.4 v. 0.3 points; p=0.02) and 12-months (3.6 v. 0 points; p=0.04). While endocrine symptoms, fatigue, and anxiety improved at 6-months in those who lost ≥5%, differences were not significant v. those who lost <5%. There was no significant change within or between groups in sexual function, depression, or sleep. Findings at 12 months were similar, except pain improved in those losing ≥5%.
Conclusions:
These results support the benefits of weight loss in overweight/obese breast cancer survivors.
Implications for Cancer Survivors:
Weight management in breast cancer survivors may improve PF.
Keywords: breast cancer, obesity, physical function, patient reported outcomes
Introduction
Accumulating evidence suggests that obesity influences the course of cancer, as well as overall well-being and survival.1 At breast cancer diagnosis, excess weight (BMI≥25) is noted in 31-69% of women younger than age 65 and in 69-82% of women age 65 and older.2 Obesity at and following breast cancer diagnosis is associated with poor quality of life and increased risk of adverse treatment effects. Loss of sexual interest3,4, neuropathy5-7, lymphedema8,9 and chronic fatigue10,11 are more common in breast cancer patients who are obese compared to normal-weight. As a consequence of side effects of treatment and physical inactivity secondary to treatment, many patients also live with compromised physical function (PF).12 Physical function is the ability to perform both the basic and instrumental activities of daily living, which are considered essential for maintaining independence. For example, the NIH Patient-Reported Outcome Measurement Information System (PROMIS) PF measure assesses coordination, functional mobility, strength, and upper extremity function. While the majority of patients recover the year after treatment is completed, individuals who experience a persistent decline in PF are at risk of functional decline and early mortality, especially women 60 years of age or older.13,14 Weight gain after diagnosis and treatment may also increase risk of recurrence by 40-50% and breast cancer-related mortality by 53-60%15-17. The NCCN survivorship guidelines recommend that “weight loss should be a priority for overweight/obese survivors.”18
The original Practice-based Opportunities for Weight Reduction (POWER) study in obese individuals with a risk for cardiovascular disease demonstrated equivalent weight loss outcomes between in-person coaching and a remote intervention.19 The POWER study measured various patient-reported outcomes (PROs) and found improvement in multiple-aspects of well-being.20 Physical domains were most affected, especially in participants with at least 5% weight loss. Other studies have similarly found improvements in PF associated with weight loss.21-24 In another study which involved postmenopausal women undergoing a diet and exercise intervention, average weight loss of 10.8% predicted improvement in PF, vitality, and mental health at 12-months.22,25 These results support the importance of weight loss in PRO improvement, particularly physical domains.
Our group already compared the remote-based POWER intervention (telephone calls by a coach, access to online learning materials, online self-directed dietary/activity monitoring) to self-directed weight loss in overweight or obese survivors of early-stage breast cancer.19 At 6-months 51% of women randomized to POWER-remote lost ≥5% of their baseline body weight, compared to 12% in the self-directed arm (OR=7.9, 95% CI 2.6-23.9, p=0.0003).26 This analysis investigates the impact of weight loss on PROs, which were a secondary endpoint of the POWER-remote study in breast cancer survivors. We hypothesized a priori that weight loss would be associated with improved PF at 6 months, regardless of study arm.
Methods
Study design
The study titled “POWER-remote weight loss program in early stage breast cancer” enrolled participants at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center and Greater Baltimore Medical Center (POWER-remote trial; clinicaltrials.gov identifier #NCT01871116). The study was approved by the local Institutional Review Board, and all participants provided written informed consent.
Previous publications include details regarding the study’s design and enrollment.26 Briefly, eligible participants included patients diagnosed with stage 0-III breast cancer survivors within 5 years who had completed recommended primary breast surgery, radiation, and/or chemotherapy at least 3 months prior to enrollment. Additional criteria included body mass index (BMI) ≥25 kg/m2, weight ≤400 pounds, and willingness to lose at least 5% of their body weight. Participants were randomized 1:1 to either POWER-remote or self-directed weight loss. Participants randomized to POWER-remote received the same 12-month behavioral weight loss intervention as individuals in the original POWER study, which included telephone-based behavioral weight loss coaching and use of a web-based self-monitoring and learning platform19. Participants could record dietary intake, exercise, and weight on the web-based platform. The coaching sessions were weekly for the first three months, and then once a month for nine subsequent months. Coaches reviewed completion of educational modules, reinforced key learning points, promoted individualized goal-setting, assessed and identified barriers to adherence, and encouraged regular recording of weight, food intake, and activity. Participants randomized to the self-directed weight loss control group received the National Heart, Lung, and Blood Institute (NHLBI) publication “Aim for A Healthy Weight” and met with a weight-loss coach one time during the baseline visit.
Patient Reported Outcomes (PROs)
We collected PROs using PatientViewpoint, a website for PRO data collection which was already in use through several clinical investigations at Johns Hopkins.27 Participants received e-mail reminders to go to the website to complete the PRO questionnaire at baseline, 6 and 12 months. Pain (6 items), fatigue (7 items), anxiety (7 items), depression (8 items), sleep (6 items), and PF (10 items) were assessed using the NIH Patient-Reported Outcome Measurement Information System (PROMIS) v1 short form questionnaires; endocrine symptoms with the FACT-endocrine symptoms, and sexual function with the MOS-Sexual Function.28-34
PROMIS domains are scored by summing the responses and transforming the raw scores to standardized T-scores using a conversion table with a general population mean of 50 and a standard deviation (SD) of 10. A higher T-score represents more of the concept being measured (i.e., higher T-scores are worse for symptoms such as fatigue, but better for functional domains such as PF). Reliability and validity have been previously reported.28-30 Endocrine symptoms were assessed with the Functional Assessment of Cancer Therapy (FACT)-Endocrine subscale, which was designed for use with the FACT-B (Functional Assessment of Cancer Therapy for breast cancer patients), but has been tested on its own.32 It comprises 18 items in which patients indicate how true a statement has been for them over the prior 7 days using a 5-point scale. A higher score corresponds to improved symptoms. Changes in sexual function were evaluated using the MOS Sexual Functioning Scale, which consists of four items assessing interest in sex, ability to relax and enjoy sex, arousal, and ability to have an orgasm. Items are rated on a 5-point scale (1=not a problem, 2=little of a problem, 3=somewhat of a problem, 4=very much of a problem, and 5=not applicable). A higher score corresponds with worse sexual symptoms. It has demonstrated good reliability and construct validity in patients with chronic diseases including cancer33, and has been used in breast cancer patients in particular.35-37
Our primary PRO endpoint was change in PF, and we hypothesized a priori that PF would show greater improvement from baseline to 6-months in participants who lost ≥5% of their baseline weight v. those who did not lose 5%, regardless of study arm. As a secondary analysis of change in PF, we compared the proportion of patients who demonstrated a minimally important change of 5 points in PF scores by weight loss (<5% v. ≥5% of baseline body weight). Studies in cancer patients suggest a 4-6 point change in PF score using the PROMIS Cancer-PF questionnaire to be clinically meaningful.38,39 Since one approach to estimating the minimally important difference (MID) is to take half the standard deviation,40 and in conjunction with the aforementioned studies, we selected 5 points as the MID. Exploratory analyses included changes in the other PRO domains by weight loss (<5% v. ≥5%) at 6 and 12 months, and changes in all PRO domains by randomized arm.
Statistical approach
The trial was powered to detect differences in the proportion of women who lost ≥5% weight after 6 months. All patients (regardless of degree of weight loss) who completed PROs were included in the secondary analysis. Characteristics of study participants according to whether they achieved ≥5% weight loss were compared using descriptive statistics, t-tests, and Fisher’s exact test. The primary PRO analysis evaluated the effect of weight loss (<5% v ≥5%) on continuous changes in PF at 6 months. For these analyses, we used a multi-level mixed effects regression modeling approach with the PF score as the outcome, a random intercept for each patient and fixed effect terms for group (weight loss or randomized study arm), time point (indicator of 6 and 12 months), and their interaction. For comparisons according to weight loss, models also included study arm, age, and baseline weight as covariates. As this was a pilot study, the primary analysis of PF would conclude a significant benefit for the group with weight loss over the group without weight loss if the interaction p value < 0.10 for the difference at 6 months.
A responder analysis of PF categorized patients into those whose PF score improved by the MID of at least 5 points, was stable (change of less than 5 points), or worsened by at least 5 points. The proportion of patients in each category was compared between the groups defined by weight loss with Fisher’s exact test.
Exploratory PRO analyses examined continuous changes in the other PROs by weight loss and all PROs by study arm. The sexual function, pain, and depression scores were log-transformed prior to analysis. Similar to the primary PRO analyses, we used a multi-level mixed effects regression modeling approach with the PRO score as the outcome, a random intercept for each patient and fixed effect terms for group (weight loss or randomized study arm), time point (indicator of 6 and 12 months), and their interaction. For comparisons according to weight loss, models also included study arm, age, and baseline weight as covariates. Analyses were completed with R version 3.6.0.41
Results
From 2013-2015, 96 women were enrolled in the POWER-remote trial, and 87 were evaluable for the primary analysis at 6 months. Of those, 85 women completed PROs at baseline, 83 at 6 months, and 68 at 12 months and were evaluable for the PROs analyses. Missing domain scores were rare: one participant had incomplete data for sexual function at 6 and 12 months, and two participants had incomplete data for depression at 12 months.
Patient characteristics comparing weight loss groups are described in Table 1. Among the group that lost <5% of baseline weight (N=57) and the group that achieved ≥5% weight loss (N=28) at 6 months, baseline BMI (median: 31.6 v 29.9), use of endocrine therapy (79%v 82%), and postmenopausal status (74%v 71%) were similar. Those who lost at least 5% weight at 6 months had a lower median baseline weight (173 v 189 pounds, p=0.05), had lower alcohol consumption of >3 drinks/month (p=0.04), and were less likely to have received chemotherapy (p=0.006) compared to the group who lost <5% of their body weight.
Table 1:
Patient Characteristics Based on Weight Loss at 6 Months
| Variables | < 5% WL 6-month | ≥ 5% WL 6-month | P-Value* | Total |
|---|---|---|---|---|
| Sample Size | N = 57 | N = 28 | N = 85 | |
| Age - Median (Q1, Q3) | 54 (30, 73) | 59.5 (37, 71) | 0.09 | 56 (30, 73) |
| Baseline BMI - Median (Q1, Q3) | 31.6 (26.2, 45.3) | 29.9 (26.2, 49.2) | 0.12 | 31.45 (26.2, 49.2) |
| Baseline Weight (lb) - Median (Q1, Q3) | 189.4 (151.9, 253.1) | 172.8 (138.7, 268.7) | 0.05 | 186.29 (138.7, 268.7) |
| Race - n (%) | ||||
| White | 40 (70.2) | 24 (85.7) | 0.18 | 64 (75.3) |
| Non-White | 17 (29.8) | 4 (14.3) | 21 (24.7) | |
| Menopause Status - n (%) | ||||
| Pre-Menopause | 15 (26.3) | 8 (28.6) | >0.99 | 23 (27.1) |
| Post-Menopause | 42 (73.7) | 20 (71.4) | 62 (72.9) | |
| Drinking - n (%) | ||||
| Less than 1 per month | 19 (36.5) | 7 (25) | 0.04 | 26 (32.5) |
| 1-3 per month | 7 (13.5) | 11 (39.3) | 18 (22.5) | |
| More than 3 per month | 26 (50) | 10 (35.7) | 36 (45) | |
| Unknown | 5 | 0 | 5 | |
| Smoking - n (%) | ||||
| Yes | 13 (25) | 11 (39.3) | 0.21 | 24 (30) |
| No | 39 (75) | 17 (60.7) | 56 (70) | |
| Unknown | 5 | 0 | 5 | |
| Prior Chemotherapy - n (%) | ||||
| No | 20 (35.1) | 19 (67.9) | 0.006 | 39 (45.9) |
| Yes | 37 (64.9) | 9 (32.1) | 46 (54.1) | |
| Hormone Therapy - n (%) | ||||
| None | 12 (21.1) | 5 (17.9) | 0.95 | 17 (20) |
| Tamoxifen | 22 (38.6) | 12 (42.9) | 34 (40) | |
| Aromatase inhibitor | 23 (40.4) | 11 (39.3) | 34 (40) | |
P value for Fisher’s exact test for differences in categorical measures and for the Wilcoxon rank sum test for differences in continuous measures
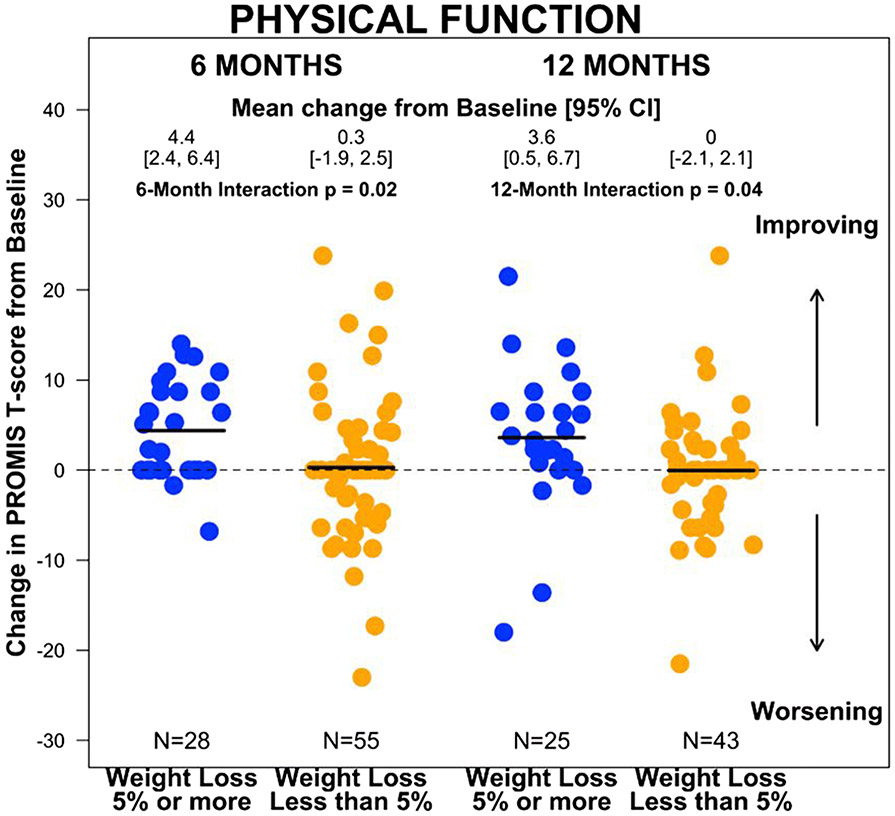
Changes from baseline to 6 and 12 months in PROs by whether patients lost ≥5% baseline weight at 6 months and 12 months, adjusted for age, study arm, and baseline weight, are reported in Table 2. At 6 months, the group that achieved ≥5% weight loss had improved PF scores (4.4; 95%CI: 2.4 to 6.4), whereas the group that did not lose weight had no change (0.3; 95% CI: −1.8 to 2.4; interaction p = 0.02) (Supplement A). At 12 months, the improvement in PF persisted for the group who lost weight (3.6; 95%CI: 0.6 to 6.6), and remained unchanged for those who did not (0.0; 95%CI: −1.9 to 1.8; interaction p=0.04). PF was the only domain that differed statistically significantly between groups. Within the group with ≥5% weight loss at 6 months, there were improvements in endocrine symptoms (2.9; 95%CI: 0.9 to 4.9), fatigue (−3; 95%CI −4.9 to −1), and anxiety (−2.3; 95%CI −4.7 to 0.1) at 6 months. Improvements in endocrine symptoms (2.7; 95%CI 0.7 to 4.7), fatigue (−3.5; 95%CI −6.1 to −0.9), and anxiety (−3.3; 95%CI −5.8 to −0.9) were maintained at 12 months; pain also improved at 12 months (−2.6; 95%CI −0.51 to −0.2). However, there were no significant differences between groups for these PROs. There were no significant changes in sexual function, depression, or sleep disturbance at any time point for the group with ≥5% weight loss. We found no significant changes in PROs over time within the group that had <5% weight loss.
Table 2:
Changes in patient reported outcomes based on weight loss
| Weight loss at 6 months | Weight loss at 12 months | |||
|---|---|---|---|---|
| < 5% | ≥ 5% | < 5% | ≥ 5% | |
| Physical Function | ||||
| No. in analysis | 55 | 28 | 43 | 25 |
| BL, mean (SD)* | 49.8 (8.1) | 49.3 (6.6) | 49.6 (8.3) | 49.7 (6.8) |
| Follow-up, mean (SD) | 50.1 (8.2) | 53.7 (8.6) | 49.6 (7.9) | 53.3 (8.3) |
| Δ from BL, mean [95% CI] | 0.3 [−1.8, 2.4] | 4.4 [2.4, 6.4] | 0 [−1.9, 1.8] | 3.6 [0.6, 6.6] |
| P, within group** | >0.99 | 0.009 | 0.72 | 0.04 |
| P, between groups*** | 0.02 | 0.04 | ||
| Sexual Function | ||||
| No. in analysis | 55 | 27 | 43 | 24 |
| BL, mean (SD)* | 40.2 (42.4) | 28.2 (35.3) | 36.4 (40.4) | 27.2 (35.2) |
| Follow-up, mean (SD) | 34.2 (38.9) | 18.5 (27.5) | 36.6 (39.2) | 24.8 (31.2) |
| Δ from BL, mean [95% CI] | −5.9 [−11.2, −0.7] | −9 [−16.7, −1.3] | 0.2 [−7.2, 7.6] | −1.3 [−9.1, 6.4] |
| P, within group** | 0.31 | 0.29 | 0.85 | 0.84 |
| P, between groups*** | 0.76 | 0.95 | ||
| Endocrine Symptoms | ||||
| No. in analysis | 55 | 28 | 43 | 25 |
| BL, mean (SD)* | 39.4 (11.2) | 42.4 (8.9) | 40.7 (10.8) | 43.4 (8.7) |
| Follow-up, mean (SD) | 40 (10.3) | 45.4 (9.1) | 41.4 (10.8) | 46.1 (8.5) |
| Δ from BL, mean [95% CI] | 0.7 [−1.8, 3.1] | 2.9 [0.9, 4.9] | 0.7 [−1.8, 3.1] | 2.7 [0.7, 4.7] |
| P, within group** | 0.47 | 0.02 | 0.53 | 0.006 |
| P, between groups*** | 0.43 | 0.3 | ||
| Pain Interference | ||||
| No. in analysis | 55 | 28 | 43 | 25 |
| BL, mean (SD)* | 49.3 (8) | 48.8 (8.8) | 48.6 (8.2) | 47.7 (8.6) |
| Follow-up, mean (SD) | 49.9 (7.6) | 48.4 (8.5) | 49.1 (8.8) | 45 (6) |
| Δ from BL, mean [95% CI] | 0.6 [−1.4, 2.7] | −0.4 [−3.3, 2.6] | 0.5 [−1.6, 2.5] | −2.6 [−5.1, −0.2] |
| P, within group** | 0.56 | 0.83 | 0.8 | 0.07 |
| P, between groups*** | 0.61 | 0.19 | ||
| Fatigue | ||||
| No. in analysis | 55 | 28 | 43 | 25 |
| BL, mean (SD)* | 51.4 (7.8) | 48.7 (7.7) | 50.4 (8) | 47.7 (7.3) |
| Follow-up, mean (SD) | 51 (8.5) | 45.7 (7.6) | 49.4 (8.7) | 44.2 (6.9) |
| Δ from BL, mean [95% CI] | −0.4 [−2.5, 1.6] | −3 [−4.9, −1] | −1 [−3.3, 1.2] | −3.5 [−6.1, −0.9] |
| P, within group** | 0.91 | 0.09 | 0.54 | 0.02 |
| P, between groups*** | 0.28 | 0.23 | ||
| Depression | ||||
| No. in analysis | 55 | 28 | 41 | 25 |
| BL, mean (SD)* | 45.7 (8.9) | 45.3 (7.2) | 45.6 (9.3) | 45.4 (7.3) |
| Follow-up, mean (SD) | 46.3 (9.5) | 44.6 (8) | 44.6 (8.4) | 43.7 (8.2) |
| Δ from BL, mean [95% CI] | 0.6 [−1.3, 2.5] | −0.6 [−2.4, 1.1] | −1 [−3.3, 1.3] | −1.7 [−4.3, 0.8] |
| P, within group** | 0.75 | 0.42 | 0.33 | 0.21 |
| P, between groups*** | 0.45 | 0.84 | ||
| Anxiety | ||||
| No. in analysis | 55 | 28 | 43 | 25 |
| BL, mean (SD)* | 49.2 (8.6) | 48.9 (7.9) | 48.9 (8.8) | 48.7 (8.2) |
| Follow-up, mean (SD) | 49 (8.7) | 46.6 (8.4) | 48.2 (8.3) | 45.3 (8.1) |
| Δ from BL, mean [95% CI] | −0.2 [−2.3, 1.9] | −2.3 [−4.7, 0.1] | −0.7 [−3.4, 2] | −3.3 [−5.8, −0.9] |
| P, within group** | 0.73 | 0.05 | 0.67 | 0.008 |
| P, between groups*** | 0.16 | 0.18 | ||
| Sleep Disturbance | ||||
| No. in analysis | 55 | 28 | 43 | 25 |
| BL, mean (SD)* | 52 (9.2) | 49 (7.9) | 51.8 (9.2) | 48.9 (8.4) |
| Follow-up, mean (SD) | 51.7 (9.6) | 48.1 (8.3) | 51.8 (6.8) | 49.2 (4.7) |
| Δ from BL, mean [95% CI] | −0.3 [−2.4, 1.8] | −0.9 [−3.6, 1.7] | −0.1 [−2.1, 2] | 0.3 [−2.2, 2.7] |
| P, within group** | 0.71 | 0.25 | 0.85 | 0.86 |
| P, between groups*** | 0.27 | 0.99 | ||
Values are the mean (SD) of baseline measures among those with data at the follow-up time point
P value for differential change in the PRO within weight loss groups at 6 and 12 months, estimated from a linear mixed effects regression model with the PRO as the outcome and terms for weight loss group (1 indicator variable)
P value for differential change in the PRO between weight loss groups at 6 and 12 months, estimated from a linear mixed effects regression model with the PRO as the outcome and terms for weight loss group (1 indicator variable), time point (2 indicator variables), and their interaction (2 terms)
In the responder analysis, we compared the proportion of patients whose PF scores improved by ≥5 points, were stable, or worsened by ≥5 points from baseline to 6 and 12 months by weight loss group (≥5% v. not) (Table 3). At 6 months, of the group that achieved ≥5% weight loss, 50% had improved PF, 46% were stable, and 4% had worsened PF v. 18%, 58%, and 24%, respectively, in the group who lost <5% (p = 0.004). The median weight loss (kg) from baseline to 6 months was 4.5 (2.1, 9.3) in those with improved PF, 1.9 (−0.2, 4.6) in those with stable PF, and 0.1 (−1, 2.4) in those with worsened PF. Median change in PF from baseline to 6 months was 9.3 (6.5, 12.7) for those with improved PF, 0 (0, 0) for those with stable PF, and −7.7 (−8.7, −6.4) in those with worsened PF. At 12 months, the difference in changes in PF persisted between groups (40% improved, 52% stable, and 8% worsened in the group that achieved ≥5% weight loss v. 16%, 63%, and 21%, respectively, in the group who lost <5%, p=0.05). The median weight loss (kg) from baseline to 12 months was 4.4 (1.9, 9.2) in those with improved PF, 1.5 (−1.6, 5.4) in those with stable PF, and 1.2 (−2.6, 2.9) in those with worsened PF. Median change in PF from baseline to 12 months was 8.7 (6.4, 12.7) for those with improved PF, 0 (0, 2.3) for those with stable PF, and −8.4 (−11.3, −6.4) in those with worsened PF.
Table 3:
Physical Function Responder Analysis by Weight Loss at 6 and 12 months
| < 5% Weight loss n (%) |
≥ 5% Weight loss n (%) |
P value** | Total | |
|---|---|---|---|---|
| Physical Function Status at 6 months* |
N = 55 | N = 28 | N = 83 | |
| Improved | 10 (18.2) | 14 (50.0) | 0.004 | 24 (28.9) |
| Stable | 32 (58.2) | 13 (46.4) | 45 (54.2) | |
| Worsened | 13 (23.6) | 1 (3.6) | 14 (16.9) | |
| Physical Function Status at 12 months* |
N = 43 | N = 25 | N = 68 | |
| Improved | 7 (16.3) | 10 (40.0) | 0.05 | 17 (25.0) |
| Stable | 27 (62.8) | 13 (52.0) | 40 (58.8) | |
| Worsened | 9 (20.9) | 2 (8.0) | 11 (16.2) |
Participants were grouped into 3 categories based on changes in NIH PROMIS Version 1.0 Short Form physical function: Improved (score increase of 5 or more), Stable (score change of less than 5) and Worsened (score decrease of 5 or more)
P value for Fisher’s exact test for differences in the distribution of change in physical function at 6 months and 12 months between groups defined according to weight loss
In analyses of change in PROs from baseline to 6 and 12 months by randomized arm, the POWER-remote group had improved PF scores from baseline to 6 months (2.2; 95%CI 0.3 to 4.1) v. control (0.9; 95%CI −1.5- to 3.3) (interaction p=0.08) (Table 4). At 12 months, the POWER-remote group also had improvements in endocrine symptoms (1.5; 95%CI −0.3 to 3.3), pain (−1.7; 95%CI −3.7 to 0.3), and fatigue (−1.8; 95%CI −3.8 to 0.2). However, there were no significant differences between groups for these PROs. Within the POWER-remote arm, there were no changes in sexual function, depression, anxiety, or sleep disturbance at any time point. We found no changes within the self-directed arm at either time point.
Table 4:
Changes in patient reported outcomes based on randomized group
| 6 months | 12 months | |||
|---|---|---|---|---|
| POWER-remote | Self-Directed | POWER-remote | Self-Directed | |
| Physical Function | ||||
| No. in analysis | 43 | 41 | 38 | 31 |
| BL, mean (SD)* | 49.2 (7.3) | 50.2 (8) | 49.6 (7.5) | 49.7 (8) |
| Follow-up, mean (SD) | 51.4 (8.9) | 51.1 (8) | 50.8 (8.6) | 51 (7.7) |
| Δ from BL, mean [95% CI] | 2.2 [0.3, 4.1] | 0.9 [−1.5, 3.3] | 1.2 [−0.8, 3.3] | 1.3 [−1, 3.5] |
| P, within group** | 0.08 | 0.49 | 0.31 | 0.59 |
| P, between groups*** | 0.485 | 0.787 | ||
| Sexual Function | ||||
| No. in analysis | 42 | 41 | 37 | 31 |
| BL, mean (SD)* | 29.3 (37.4) | 42.5 (42.5) | 27.6 (36.3) | 41.8 (41.5) |
| Follow-up, mean (SD) | 25.9 (34.3) | 31.4 (37.9) | 30.6 (35) | 36.5 (40.3) |
| Δ from BL, mean [95% CI] | −2.8 [−7.5, 1.9] | −11 [−17.6, −4.4] | 3.9 [−1.9, 9.6] | −5.3 [−14.2, 3.5] |
| P, within group** | 0.83 | 0.13 | 0.2 | 0.48 |
| P, between groups*** | 0.352 | 0.161 | ||
| Endocrine Symptoms | ||||
| No. in analysis | 43 | 41 | 38 | 31 |
| BL, mean (SD)* | 41 (10) | 39.9 (11) | 42.8 (8.4) | 40.1 (11.7) |
| Follow-up, mean (SD) | 42.2 (10.6) | 41.7 (9.8) | 44.3 (9.2) | 41.1 (11.5) |
| Δ from BL, mean [95% CI] | 1.2 [−0.7, 3] | 1.8 [−0.9, 4.6] | 1.5 [−0.3, 3.3] | 1 [−2, 4] |
| P, within group** | 0.27 | 0.27 | 0.07 | 0.5 |
| P, between groups*** | 0.716 | 0.688 | ||
| Pain Interference | ||||
| No. in analysis | 43 | 41 | 38 | 31 |
| BL, mean (SD)* | 49.8 (8.9) | 48.2 (7.4) | 48.6 (8.6) | 48 (7.9) |
| Follow-up, mean (SD) | 49.8 (7.8) | 49.3 (8.1) | 46.9 (7.7) | 48.2 (8.6) |
| Δ from BL, mean [95% CI] | 0 [−2.3, 2.2] | 1.1 [−1.3, 3.5] | −1.7 [−3.7, 0.3] | 0.2 [−2.2, 2.6] |
| P, within group** | 0.91 | 0.29 | 0.07 | 0.87 |
| P, between groups*** | 0.491 | 0.176 | ||
| Fatigue | ||||
| No. in analysis | 43 | 41 | 38 | 31 |
| BL, mean (SD)* | 49.8 (8) | 51.2 (7.5) | 48.7 (7.7) | 50.6 (8.1) |
| Follow-up, mean (SD) | 48.7 (9.1) | 49.7 (7.8) | 46.9 (8.5) | 48.6 (8.4) |
| Δ from BL, mean [95% CI] | −1.1 [−3, 0.9] | −1.5 [−3.6, 0.7] | −1.8 [−3.8, 0.2] | −2 [−4.7, 0.6] |
| P, within group** | 0.29 | 0.28 | 0.04 | 0.17 |
| P, between groups*** | 0.914 | 0.725 | ||
| Depression | ||||
| No. in analysis | 43 | 41 | 38 | 29 |
| BL, mean (SD)* | 46.2 (8.7) | 44.8 (7.9) | 46.1 (9) | 45.1 (8.1) |
| Follow-up, mean (SD) | 46.8 (9.9) | 44.4 (7.9) | 45.5 (9.4) | 42.9 (6.3) |
| Δ from BL, mean [95% CI] | 0.7 [−1.2, 2.5] | −0.4 [−2.2, 1.4] | −0.6 [−2.6, 1.3] | −2.2 [−4.9, 0.5] |
| P, within group** | 0.73 | 0.83 | 0.54 | 0.18 |
| P, between groups*** | 0.685 | 0.462 | ||
| Anxiety | ||||
| No. in analysis | 43 | 41 | 38 | 31 |
| BL, mean (SD)* | 48.9 (7.9) | 49 (8.9) | 48.7 (8.3) | 49.3 (8.9) |
| Follow-up, mean (SD) | 47.6 (8.8) | 48.6 (8.7) | 47 (9.1) | 47.5 (7.1) |
| Δ from BL, mean [95% CI] | −1.4 [−3.5, 0.8] | −0.4 [−2.6, 1.8] | −1.7 [−4.1, 0.7] | −1.8 [−4.7, 1.1] |
| P, within group** | 0.17 | 0.94 | 0.12 | 0.33 |
| P, between groups*** | 0.391 | 0.815 | ||
| Sleep Disturbance | ||||
| No. in analysis | 43 | 41 | 38 | 31 |
| BL, mean (SD)* | 51.6 (9.6) | 50.4 (8) | 50.7 (9.3) | 50.5 (8.5) |
| Follow-up, mean (SD) | 50.9 (10.5) | 50.1 (7.8) | 50.8 (6.2) | 50.5 (6.4) |
| Δ from BL, mean [95% CI] | −0.7 [−3.2, 1.8] | −0.3 [−2.3, 1.7] | 0.1 [−1.9, 2.1] | −0.1 [−2.4, 2.2] |
| P, within group** | 0.65 | 0.86 | 0.95 | 0.91 |
| P, between groups*** | 0.834 | 0.973 | ||
Values are the mean (SD) of baseline measures among those with data at the follow-up time point
P value for differential change in the PRO within treatment groups at 6 and 12 months, estimated from a linear mixed effects regression model with the PRO as the outcome and terms for treatment group (1 indicator variable)
P value for differential change in the PRO between treatment groups at 6 and 12 months, estimated from a linear mixed effects regression model with the PRO as the outcome and terms for treatment group (1 indicator variable), time point (2 indicator variables), and their interaction (2 terms)
Discussion
Our results demonstrate a significant improvement in PF in overweight or obese patients with early breast cancer with weight loss of ≥5% compared to those with <5% weight loss at 6-months, and this improvement persisted at 12-months. With an increase in the diagnosis of breast cancer and improvements in treatment, the prevalence of breast cancer survivors is likely to increase over the next decade, including survivors with a decline in PF due to the disease, its treatment, and aging .42 Our finding that weight loss of ≥5% improves PF is consistent with studies in a non-cancer population of postmenopausal women undergoing a lifestyle intervention,43 and documents the potential value of weight loss, aside from its impact on other aspects of health. While the group who lost weight did not achieve a clinically meaningful difference of 5 points, this may be attributable to the conservative selection of 5 points as the MID. The literature suggests that a difference of 4-6 points seems to be clinically meaningful.38,39 In the responder analysis, among the group with ≥5% weight loss, only 4-8% had worsened PF compared to 21-24% in the group who lost <5%.
Our results support research suggesting that weight loss may be key to enhancing quality of life, particularly PF, in breast cancer survivors who are overweight or obese, regardless of the type of intervention utilized to achieve this.44-46 Compared to other PROs, PF may be more directly influenced by weight loss due to changes in body composition, such as preservation of lean body mass and reduction in sarcopenia. Improvements in endocrine symptoms, fatigue, and anxiety at both 6 and 12 months in the group who lost ≥5% were observed. Pain at 12 months also improved compared to baseline within this group. However, these changes were not significantly different compared to the group who lost <5%. While another study has demonstrated improvements in vitality and mental health after a dietary and exercise intervention at 12-months,22,25 a greater amount of weight loss (e.g., ≥10%) may be necessary to observe changes in these particular PROs.
Our study has limitations. First, the sample size is relatively small. A study with a larger sample size would have greater statistical power to detect more modest, but clinically relevant effect sizes. Second, in this behavioral weight loss trial, participants could not be blinded to randomized group or to the extent of changes in weight and exercise. The possibility that patients allocated to the behavioral intervention subjectively felt better and therefore reported better PF cannot be ruled out, nor can the possibility that patients attaining significant weight loss would report better PF. However, if this were the case, one might expect similar changes in other PROs, but such changes did not occur. The study also has several strengths. First, the study population was diverse, with ~25% black participants. Second, rates of follow-up and data collection were high.
Healthcare providers need to address the topic of excess weight and identify the best ways to support breast cancer survivors in making and maintaining healthy lifestyle changes for weight loss.47,48 Weight loss may provide a reliable means of improving PF and lead to other benefits (e.g. endocrine symptoms, fatigue, anxiety, pain). Furthermore, retrospective studies find that weight gain may also increase risk of recurrence by 40-50% and breast cancer-related mortality by 53-60%.15-17 The POWER-remote intervention has demonstrated efficacy in weight loss26 and may be a practical weight loss strategy for busy clinics due to its remote-supported and scalable nature. Further research is needed to define impacts of weight loss on breast cancer specific outcomes (such as recurrence and overall survival), as well as develop effective strategies that can be incorporated into clinical practice.
In conclusion, while addressing overweight and obesity continues to be a challenge for patients and providers, but advances in treatment, such as the POWER-Remote intervention, provide a means to improve the quality of life and potentially survival outcomes in women with early stage breast cancer.
Figure 1.
Per our hypothesis, physical function scores improved at 6 months in those who lost 5% of their weight compared to those who didn’t (p=0.02), and the difference between groups was sustained at 12 months (p=0.04). PF improved 4.4 points at 6 months and 3.6 points at 12 months in the group who lost 5% of their weight, vs 0.3 and 0 points, respectively, in the group who did not. These differences approach, but do not reach, the pre-established minimally important difference of 5 points.
Funding:
Breast Cancer Research Foundation, Cigarette Restitution Fund, and National Institutes of Health (P30 CA006973).
Footnotes
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animal performed by any of the authors.
Consent to participate: Informed consent was obtained on all individual participants included in the study.
Consent for publication: Not applicable
Availability of data and material: Not applicable
Code availability: Not applicable
Conflicts of Interest: No conflicts for JS, ALB, DL, and AC. CS: grants from Genentech and Pfizer to institution. CAS: research funding from Pfizer, Astrazeneca, BMS, Novartis, GSK; and advisory board for BMS, Seattle Genetics, Genomic Health, Athenex, Polyphor, Halozyme. VS: grants to the institution from Abbvie, Biocept, Pfizer, Novartis and Puma Biotechnology; member of data safety monitoring board for Immunomedics, Inc. KLS: research funding from Pfizer, family member with stock in ABT Labs, Abbvie.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2012;21(8):1244–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maliniak ML, Patel AV, McCullough ML, et al. Obesity, physical activity, and breast cancer survival among older breast cancer survivors in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res Treat 2018;167(1):133–45. [DOI] [PubMed] [Google Scholar]
- 3.Rojas KE, Matthews N, Raker C, et al. Body mass index (BMI), postoperative appearance satisfaction, and sexual function in breast cancer survivorship. J Cancer Surviv Res Pract 2017; [DOI] [PubMed] [Google Scholar]
- 4.Imayama I, Alfano CM, Neuhouser ML, et al. Weight, inflammation, cancer-related symptoms and health related quality of life among breast cancer survivors. Breast Cancer Res Treat 2013;140(1):159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mustafa Ali M, Moeller M, Rybicki L, Moore HCF. Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res Treat 2017;166(2):519–26. [DOI] [PubMed] [Google Scholar]
- 6.Greenlee H, Hershman DL, Shi Z, et al. BMI, Lifestyle Factors and Taxane-Induced Neuropathy in Breast Cancer Patients: The Pathways Study. J Natl Cancer Inst 2017;109(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res Treat 2016;159(2):327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meeske KA, Sullivan-Halley J, Smith AW, et al. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Res Treat 2009;113(2):383–91. [DOI] [PubMed] [Google Scholar]
- 9.Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer 2011;19(6):853–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt ME, Wiskemann J, Schneeweiss A, Potthoff K, Ulrich CM, Steindorf K. Determinants of physical, affective, and cognitive fatigue during breast cancer therapy and 12 months follow-up. Int J Cancer 2018;142(6):1148–57. [DOI] [PubMed] [Google Scholar]
- 11.Herath K, Peswani N, Chitambar CR. Impact of obesity and exercise on chemotherapy-related fatigue. Support Care Cancer 2016;24(10):4257–62. [DOI] [PubMed] [Google Scholar]
- 12.Evangelista AL, Santos EMM, Maciel M do S, et al. Associations of Quality of Life, Physical Activity and Mood States in Women with Breast Cancer Treated with Curative Intent. Appl Res Qual Life 2016;11(2):445–59. [Google Scholar]
- 13.Winters-Stone KM, Medysky ME, Savin MA. Patient-reported and objectively measured physical function in older breast cancer survivors and cancer-free controls. J Geriatr Oncol 2019;10(2):311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehl M, Lu X, Silliman R, Ganz PA. Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J Cancer Surviv Res Pract 2013;7(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biganzoli E, Desmedt C, Fornili M, et al. Recurrence dynamics of breast cancer according to baseline body mass index. Eur J Cancer 2017;87:10–20. [DOI] [PubMed] [Google Scholar]
- 16.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer 2012;118(23):5937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trentham-Dietz A, Newcomb PA, Nichols HB, Hampton JM. Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res Treat 2007;105(2):195–207. [DOI] [PubMed] [Google Scholar]
- 18.Sanft T, Denlinger CS, Armenian S, et al. NCCN Guidelines Insights: Survivorship, Version 2.2019. J Natl Compr Cancer Netw JNCCN 2019;17(7):784–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appel LJ, Clark JM, Yeh H-C, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011;365(21):1959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin RR, Peyrot M, Wang N-Y, et al. Patient-reported outcomes in the practice-based opportunities for weight reduction (POWER) trial. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 2013;22(9):2389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villareal DT, Chode S, Parimi N, et al. Weight Loss, Exercise, or Both and Physical Function in Obese Older Adults. N Engl J Med 2011;364(13):1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imayama I, Alfano CM, Kong A, et al. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. Int J Behav Nutr Phys Act 2011;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young DR, Coughlin J, Jerome GJ, Myers V, Chae SE, Brantley PJ. Effects of the PREMIER interventions on health-related quality of life. Ann Behav Med Publ Soc Behav Med 2010;40(3):302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson DA, Rejeski J, Lang W, et al. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med 2009;169(2):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obes Silver Spring Md 2012;20(8):1628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santa-Maria CA, Coughlin JW, Sharma D, et al. The Effects of a Remote-based Weight Loss Program on Adipocytokines, Metabolic Markers, and Telomere Length in Breast Cancer Survivors: the POWER-Remote Trial. Clin Cancer Res [Internet] 2020. [cited 2020 Apr 13];Available from: https://clincancerres.aacrjournals.org/content/early/2020/04/10/1078-0432.CCR-19-2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder CF, Jensen R, Courtin SO, Wu AW, Website for Outpatient QOL Assessment Research Network. Patient Viewpoint: a website for patient-reported outcomes assessment. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 2009;18(7):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain 2010;150(1):173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia SF, Cella D, Clauser SB, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol 2007;25(32):5106–12. [DOI] [PubMed] [Google Scholar]
- 30.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 2010;33(6):781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose M, Bjorner JB, Becker J, Fries JF, Ware JE. Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS). J Clin Epidemiol 2008;61(1):17–33. [DOI] [PubMed] [Google Scholar]
- 32.Fallowfield LJ, Bliss JM, Porter LS, et al. Quality of life in the intergroup exemestane study: a randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol 2006;24(6):910–7. [DOI] [PubMed] [Google Scholar]
- 33.Arrington R, Cofrancesco J, Wu AW. Questionnaires to measure sexual quality of life. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 2004;13(10):1643–58. [DOI] [PubMed] [Google Scholar]
- 34.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol Off J Am Soc Clin Oncol 1999;17(9):2659–69. [DOI] [PubMed] [Google Scholar]
- 35.Dahir M, Travers- Gustafson D. Breast Cancer, Aromatase Inhibitor Therapy, and Sexual Functioning: A Pilot Study of the Effects of Vaginal Testosterone Therapy. Sex Med 2014;2(1):8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganz PA, Kwan L, Stanton AL, et al. Quality of Life at the End of Primary Treatment of Breast Cancer: First Results From the Moving Beyond Cancer Randomized Trial. JNCI J Natl Cancer Inst 2004;96(5):376–87. [DOI] [PubMed] [Google Scholar]
- 37.Acquati C, Zebrack BJ, Faul AC, et al. Sexual functioning among young adult cancer patients: A 2-year longitudinal study. Cancer 2018;124(2):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hays RD, Spritzer KL, Fries JF, Krishnan E. Responsiveness and minimally important difference for the patient-reported outcomes measurement information system (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Ann Rheum Dis 2015;74(1):104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol 2011;64(5):507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003:41(5);582–92. [DOI] [PubMed] [Google Scholar]
- 41.R Core Team. R: A language and environment for statistical computing [Internet]. 2019. [cited 2018 May 22];Available from: https://www.R-project.org/
- 42.Rowland JH, Bellizzi KM. Cancer survivorship issues: life after treatment and implications for an aging population. J Clin Oncol 2014;32(24):2662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imayama I, Ulrich CM, Alfano CM, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res 2012;72(9):2314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stout NL, Baima J, Swisher A, Winters-Stone KM, Welsh J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature. (2005 – 2017). PM R 2017;9(9 Suppl 2):S347–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolotkin RL, Norquist JM, Crosby RD, et al. One-year health-related quality of life outcomes in weight loss trial participants: comparison of three measures. Health Qual Life Outcomes 2009;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweegers MG, Altenburg TM, Chinapaw MJ, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 2018;52(8):505–13. [DOI] [PubMed] [Google Scholar]
- 47.Brown JC, Mao JJ, Stricker C, Hwang W-T, Tan K-S, Schmitz KH. Aromatase inhibitor associated musculoskeletal symptoms are associated with reduced physical activity among breast cancer survivors. Breast J 2014;20(1):22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeNysschen CA, Burton H, Ademuyiwa F, Levine E, Tetewsky S, O’Connor T. Exercise intervention in breast cancer patients with aromatase inhibitor-associated arthralgia: a pilot study. Eur J Cancer Care (Engl) 2014;23(4):493–501. [DOI] [PubMed] [Google Scholar]