Abstract
Background:
Feeling alive and invigorated, or vitality, is examined within the framework of a stress paradigm. The current study investigated whether endocrine and cardiovascular responses to acute psychological stress predict declines in vitality.
Methods:
A sample of 90 undergraduate students completed an in-lab stressor. We measured anxiety, state vitality, cortisol levels, heart rate, and blood pressure before the task, and measured changes in state vitality, cortisol, heart rate, and blood pressure in response to the stressor. We investigated whether pre-task anxiety predicted changes in state vitality, and whether such changes were explained by physiological responses.
Results:
Results indicate that cognitive and somatic anxiety preceding a stressor predict changes in vitality, which is mediated by the magnitude of diastolic (95% CI [0.017, 0.517]; [0.006, 0.454] and systolic (95% CI [0.038, 0.705]) blood pressure responses to the task. Cortisol reactivity was associated with somatic anxiety (F(6, 83) = 3.34, p < .01, β = 0.401) but was not related to changes in vitality.
Conclusions:
Together, these results contribute to the understanding of how physiological reactivity to a stressor can deplete vitality.
Keywords: subjective vitality, cardiovascular reactivity, stress, blood pressure, heart rate, cortisol, well-being
1. Background
Everyone experiences times where they feel dead and drained, and other times where they feel alive and invigorated. This subjective feeling of both physical and mental aliveness is often referred to as vitality (Ryan & Frederick, 1997; Nix et al., 1999). Theory considers vitality to be an integrated index of somatic and psychological functioning, serving both a regenerative and restorative function which lends flexibility in the face of stressors (Gunnell, et al., 2017; Peterson & Seligman, 2004; Rozanski et al., 2005). In other words, vitality is deemed an essential part of well-being necessary for regulation of emotional and physiological responses (Gunnell et al., 2017).
The presence of vitality is thought to provide functional energy for coping and adaptation, as well as investment in and motivation to pursue daily challenges (Nix et al., 1999; Rozanski & Cohen, 2016; Rozanski et al., 2005), similar to Hans Selye’s (1936; 1959) classic concept of “adaptation energy.” Once this adaptation resource becomes completely depleted, it creates a vulnerable period where one can no longer cope with additional stressors. Over time, this resource is exhausted, over and over, resulting in accumulative wear-and-tear, which is thought to contribute to risk for disease and increased mortality rate (McEwen, 2006; Selye, 1959). Indeed, low levels of vitality inhibit one’s ability to flexibly respond to subsequent stress, contributing to vital exhaustion, which increases risk for coronary heart disease (Rozanski & Cohen, 2016).
Conversely, evidence suggests a link between higher levels of emotional vitality and a lower incidence of coronary heart disease, stroke, and hypertension (Kubzansky & Thurston, 2007; Lambiase et al., 2015; Trudel-Fitzgerald et al., 2014). Consequently, vitality has been suggested as an important psychosocial risk factor when assessing biopsychosocial outcomes, especially related to cardiovascular health and reactivity (Rozanski et al., 2005).
Psychophysiological models which aim to understand how physiological reactivity to stress may contribute to or protect from poor cardiovascular outcomes focus on both the magnitude and intensity of physiological responses to stress. The Cardiovascular Reactivity (CVR) hypothesis argues that extreme cardiovascular responses to acute psychological stress may contribute to the development of cardiovascular disease (CVD) (Chida & Steptoe, 2010). Cross-sectional and prospective research on this relationship suggests a positive relationship between the magnitude of cardiovascular responses and cardiovascular risk (e.g., Carroll et al, 2011, 2003; Matthews et al., 1998), such as atherosclerosis (Barnett et al., 1997), hypertension (Carroll et al., 2001; 2003), increased left ventricular mass (Allen et al., 1997), and an increased risk for CVD mortality (Carroll et al., 2012).
Similarly, there is a body of research suggesting that the hypothalamic-pituitary-adrenal (HPA) axis plays an important role in the stress response through the release of cortisol. Irregular function of the HPA axis is suggested to be one of the possible pathways by which psychosocial stress may increase cardiovascular risk (Dekker et al., 2008; Hamer et al., 2010; Whitworth et al., 2005). Exaggerated cortisol responses to stress have, indeed, been linked to poor outcomes such as hypertension (Hamer & Steptoe, 2012) and coronary artery calcification (Hamer et al., 2010).
While blunted or diminished physiological responses to stress were once believed to be adaptive or benign, accumulating evidence indicates an association between blunted responses and adverse behavioral and health outcomes. Diminished cardiovascular responses to stress are associated with heart failure mortality (Sherwood et al., 2017) and subclinical CVD in adults (Ginty et al., 2016), depression, disordered eating and impulsivity (Phillips, Ginty & Hughes, 2013). In a similar manner, blunted cortisol responses to acute stress have been linked to poor health outcomes including alcohol and substance addictions, obesity, and depression (Phillips, Ginty & Hughes, 2013).
Though there is a robust literature on acute psychological stress and physiological responses, it is important to note that much of this literature fails to address the role of stress appraisals. Perception plays a significant role in the relationship between stress and reactivity. In order for an event to be “stressful,” it must be first perceived or appraised as stressful or anxiety-provoking (Ginty & Conklin, 2011; Keller et al., 2012; Richardson et al., 2012). Physiological responses are evidenced to reflect the appraisal of the stressor (e.g., Maier et al., 2003), and differences in appraisals may affect both the intensity and pattern of the response. For example, the biopsychosocial model of challenge and threat (Blascovich & Tomaka, 1996) suggests that when an individual believes their resources exceed situational demands (i.e. challenge) they exhibit a physiological response characterized by increased sympathetic nervous system (SNS) activation paired with low levels of vascular resistance and increased cardiac output (Mendes et al., 2008) In contrast, when an individual perceives threat because they believe the demands outweigh their resources, they exhibit a cardiovascular pattern characterized by increased SNS activation, increased vascular resistance and less cardiac efficiency (Mendes et al., 2008). Previous work indicates that compared to challenge perceptions, threat perceptions are more likely to be associated with high levels of anxiety (Moore et al., 2012; Skinner & Brewer, 2002; Williams et al., 2010).
Both anxiety and stress are suggested to heavily tax vitality (Niemiec et al., 2006; Penninx et al., 2000; Rozanski et al., 2005), but it is not clear as to whether this deficit is due to psychological appraisals, psychological strain, the physiological reaction, or both. By definition, vitality possesses both somatic and psychological components (Ryan & Frederick, 1997), and it is possible that anxiety related to a task may drain vitality by way of physiological responses. Since vitality is a consciously accessible state, one could argue that indicators of these physiological responses necessarily be consciously accessible as well. In other words, anxiety may induce physiological responses, but the rate at which these responses drain vitality may depend on one’s awareness or accessibility of the response. For example, one may be aware of cardiovascular responses, while being unaware of HPA activation and cortisol release. Following this logic, cardiovascular responses may be more draining in terms of vitality compared to cortisol responses.
2. The Current Research
To date, research on vitality is limited, especially in relation to stress and physiology. This is, to the best of our knowledge, the first empirical study to investigate vitality levels throughout the course of a frequently used laboratory stress paradigm. The purpose of the current research was to a) examine whether anxiety depletes vitality by way of physiological responses, and b) whether this pattern is dependent on accessibility of these responses. Accordingly, it was predicted that the relationship between anxiety about the impending stress task and declines in vitality would be mediated in part by the magnitude of the physiological response to the stress task. However, it was hypothesized that this would only be true of cardiovascular responses (as compared to cortisol responses), as one may be more aware of increases in CVR.
3. Method
3.1. Participants and Procedure
Ninety undergraduates (Mage = 20.07, SDage = 2.12; 30 males, 59 females, 1 non-binary) at a large state university participated for partial course credit. While we did not conduct an a-priori power analysis, we arrived at this sample size based on past research which investigated similar constructs using similar models (Chauntry et al., 2019; John-Henderson et al., 2020; Lustyk et al., 2010; Wright et al., 2014). For research purposes not related to the current research question, approximately half (47.1%) of the participants recruited were American Indian. As part of a pre-screening process, participants were asked to report any chronic health conditions. Participants who reported any chronic health conditions were not eligible for participation. Eligible participants signed up for via SONA systems for a 2-hour study examining stress and health. All participants were greeted by 2 research assistants upon entering the laboratory. After providing informed consent, participants completed a set of intake questionnaires on the computer using Qualtrics software. A Research assistant measured participant height, weight, and hip and waist circumference. Participants were set up with a standard blood pressure cuff positioned over the brachial artery on the non-dominant arm (GE Dinamap v100, Milwaukee, WI) and were asked to sit comfortably still for the next ten minutes for a 10-minute adaptation period during which participants adjusted to sitting in the chair and became accustomed with the equipment. After the adaptation period was finished, participants were asked to remain sitting quietly for a 10-minute baseline period. During this baseline period, blood pressure and heart rate were measured and record discontinuously every 2 minutes. At minute seven of the baseline period, participants were instructed to provide a salivary cortisol sample. At the end of the baseline period, participants were told that they would complete a commonly used stress task, the Paced Auditory Serial Addition Task (PASAT; Gronwall, 1977), and were given the instructions by the experimenter. Before completing the task, participants completed the Immediate Anxiety Measurement Scale (IAMS; Thomas et al., 2002) to assess cognitive and somatic anxiety prior to the task. Upon completion of the IAMS, participants began the PASAT. Blood pressure and heart rate were measured and recorded discontinuously every 2 minutes during the task. After the task, there was a 10-minute recovery period during which blood pressure and heart rate were again measured discontinuously every 2 minutes. In the final minute of the recovery period (i.e. 10 minutes after completion of task), a cortisol sample was collected once again. Participants were fully debriefed and dismissed.
3.2. Measures and Materials
Subjective Vitality Scale
We used the seven-item Subjective Vitality Scale to measure state vitality, or one’s current perception of their vitality levels (Ryan & Frederick, 1997). State vitality was measured both before (M = 28.43, SD = 7.18; α = .81) and after (M = 23.07, SD = 8.89, α = .79) the stress task, and the difference between the two was recorded as change in vitality (M = 5.36, SD = 3.92). In addition to state vitality, trait vitality was also assessed amidst the initial measures (M = 30.14, SD = 9.00, α = .85).
Immediate Anxiety Measurement Scale.
The Immediate Anxiety Measurement Scale (IAMS) is a validated measurement of cognitive and somatic anxiety (Thomas, Hanton, & Jones, 2002). Immediately prior to the administration of the stressor, participants received definitions of each dimension of anxiety as defined by Thomas and colleagues (2002). Cognitive anxiety was described as the mental aspect of anxiety (i.e., worries or concerns about the task), and somatic anxiety was described as the perception of the physical aspect of anxiety (i.e., increased heart rate or butterflies in stomach related to the task). After reading these definitions, participants completed the IAMS by responding to the following statements, “I am cognitively anxious about the upcoming task,” and “I am somatically anxious about the upcoming task.” Participants responded to these statements using a 7-point Likert scales from 1(not at all) to 7(extremely) to indicate the level of cognitive and somatic anxiety they felt before the task. (Cognitive Anxiety: M = 3.66 SD = 1.63; Somatic Anxiety; M=2.96 SD= 1.38).
Blood Pressure and Heart Rate
Blood pressure and heart rate were measured discontinuously every two minutes during the adaptation period and the baseline period, every two minutes during the stress task, and during the post-stress recovery period. Blood pressure measurements were collected using a standard blood pressure cuff positioned over the brachial artery on the non-dominant arm, and a semi-automatic sphygmomanometer (GE Dinamap v100, Milwaukee, WI). Blood pressure and heart rate measurements were averaged across each period (adaptation, baseline, stress task, post-stress recovery period).
Salivary cortisol
Saliva samples were obtained using stimulated salivettes (Salimetrics, USA) 7 min into the baseline period, and 10 minutes after the completion of the acute psychological stress task. For each sample, participants placed the salivette in their mouth and gently chewed for approximately one minute. Participants returned the swab to the salivette tube and all samples were frozen at −80 C within 4 hours of collection. Samples were thawed on the day of analyses and centrifuged at 1500 x g for 15 min. All salivary cortisol samples were processed using a High Sensitivity ELISA (Salimetrics). The inter-assay and intra-assay coefficients were below 8%.
Acute Psychological Stress Task
Participants completed the Paced Auditory Serial Addition Task (PASAT; Gronwall, 1977) to induce acute psychological stress. The PASAT has been shown to consistently inflate cardiac activity in previous studies (e.g., Ginty et al., 2015). Following a practice round, participants completed the ten-minute stress phase, where they are presented with a series of single-digit numbers and asked to add consecutive numbers while remembering the most recent number so it could be added to the following number presented. Participants begin with 1000 points and are deducted for each error, which would be signified by the experimenter hitting a buzzer. Participants were also told that they had to watch themselves in the mirror, and that they were going to be videotaped so that the videos could be reviewed by a panel of research assistants. These evaluative components of the task were included to heighten levels of psychological stress. Blood pressure recordings were taken during the task, every two minutes.
Hospital Anxiety and Depression Scale
We used the 14-item Hospital and Anxiety Scale to examine trait depressive symptoms (M = 5.38, SD = 2.55, α = .82) and trait anxiety (M = 4.62, SD = 2.04, α = .86). Seven items from this scale relate to anxiety (e.g., “I feel tense or wound up”) and seven items relate to depression (e.g., “I feel as if I am slowed down”), rated on a four-point conditional scale. This scale is commonly utilized for detection of anxiety and depressive symptoms independent of somatic symptoms associated with illness. Items from the anxiety and depression subscales are summed; higher scores indicate higher levels of reported symptomology. Trait anxiety and trait depression were measured as covariates.
4. Results
The authors have a data file which includes all measures which is available upon request. Statistical analyses were conducted using SPSS version 26 (IBM Corp, USA). Paired-samples t-tests, using baseline and post-stress-task values of vitality, average blood pressure, average heart rate, and average cortisol were carried out to determine whether the PASAT affected these variables. Then, the associations between anxiety prior to the PASAT, changes in subjective vitality, and reactivity were analyzed using mediation analyses, adjusted for age, sex, depressive symptomology, and trait anxiety.
4.1. Correlational analyses
See Table 1 for correlation coefficients and descriptive statistics.
Table 1.
Means, standard deviations, and correlations of model variables.
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|
| 1. Pre-task Cognitive Anxiety | 3.66 | 1.63 | ||||||
| 2.Pre-task Somatic Anxiety | 2.96 | 1.38 | .48** | |||||
| 3. Change in Vitality | 5.36 | 3.92 | .39** | .28* | ||||
| 4. DBP Reactivity | 13.22 | 7.32 | .21 | .17 | .28* | |||
| 5. SBP Reactivity | 13.74 | 10.20 | .19 | .10 | .33** | .56** | ||
| 6. HR Reactivity | 14.20 | 11.37 | .05 | −.01 | .22* | .49** | .54** | |
| 7. Cortisol Reactivity | 0.09 | 0.09 | .10 | .39** | .11 | −.02 | .06 | .05 |
Notes: DBP: Diastolic Blood Pressure, SBP: Systolic Blood Pressure, HR: Heart Rate;
p < .05
p < .01
4.2. Manipulation checks
A paired-samples t-test compared average DBP [t(83) = −14.24, p < .001] before and during the PASAT stress task. Average DBP before the task (M = 67.69) was significantly lower than during the stress task (M = 79.83), t(89) = −14.24, p < .001. Similarly, a paired-samples t-test compared SBP before and after the PASAT stress task. As with DBP, average baseline SBP was significantly lower (M = 118.47) than average SBP during the task (M = 131.82), [t(89) = −12.71, p < .001], illustrating that the manipulation was successful in inducing acute stress. A separate paired-samples t-test comparing HR before and after the PASAT, indicated that baseline HR was significantly lower (M=65.26) than HR during the PASAT (M=78.07), [t(84) = −10.62, p < .001]. Lastly, a paired-samples t-test was used to compare cortisol production before and after the stress task. Cortisol significantly increased from baseline (M = 0.122) to 10-minutes post-stressor (M = 0.202), suggesting that the stress task did, indeed, increase cortisol production [t(89) = −9.329, p < .001].
In addition, a paired-samples t-test was also conducted comparing self-reported subjective vitality before the stress task to self-reported subjective vitality after the task. Vitality levels before the task (M = 28.52) were significantly higher than vitality levels after the stress task (M = 22.83), [t(89) = 11.83, p < .01], providing evidence toward the hypothesis that stressful tasks may contribute to lowered vitality levels.
4.3. Anxiety and Vitality
Separate linear regressions tested whether cognitive and somatic anxiety predicted changes in vitality. As expected, higher levels of cognitive and somatic anxiety predicted larger decreases in vitality [F(6,81) = 3.34, p < 0.01, β = 0.454; F(4,89) = 4.37, p < .01, β = 0.405], even when controlling for related factors (age, sex, race, depressive symptoms, and anxiety symptoms; see Table 2).
Table 2.
Linear regression models for changes in vitality regressed onto anxiety.
| B | SE B | β | t | p | R2 | ||
|---|---|---|---|---|---|---|---|
| Cognitive Anxiety | |||||||
| Model 1 | 1.118 | 0.249 | 0.433 | 4.485 | <.001 | .213 | |
| Model 2 | 1.265 | 0.258 | 0.490 | 4.913 | <.001 | .264 | |
| Somatic Anxiety | |||||||
| Model 1 | 1.170 | 0.284 | 0.398 | 4.125 | <.001 | .192 | |
| Model 2 | 1.205 | 0.286 | 0.409 | 4.207 | <.001 | .219 |
Notes: Model 1 includes baseline vitality; Model 2 additionally includes age, sex, depressive symptoms, and trait anxiety symptoms.
4.4. Pre-task Anxiety and Reactivity
Linear regression analyses examined the relationships between anxiety, blood pressure reactivity, and vitality. First, linear regressions were carried out to test if cognitive anxiety prior to the task significantly predicted DBP and SBP reactivity, while controlling for other factors (age, sex, race, depressive symptoms, anxiety symptoms, and average baseline blood pressure). Cognitive anxiety significantly predicted both DBP reactivity (F(7, 86) = 5.06, p < .001, β = 0.315) and SBP reactivity (F(7, 86) = 8.33, p < .05, β = .301), such that higher cognitive anxiety associated with higher blood pressure levels, when accounting for other related variables. Similarly, somatic anxiety significantly predicted both DBP reactivity (F(5, 88) = 5.65, p < .001, β = 0.221) and SBP reactivity (F(5, 88) = 2.86, p < .05, β = .220), such that higher somatic anxiety associated with higher blood pressure levels, when accounting for other related variables. Neither cognitive anxiety nor somatic anxiety predicted HR reactivity (F(1, 82) = 1.49, p = .203, β = 0.050) and (F(1, 82) = 1.49, p = .929 β = 0.050) respectively.
Additionally, separate linear regressions were carried out to test if cognitive and somatic anxiety prior to the task significantly predicted cortisol reactivity, while controlling for related factors (age, sex, race, depressive symptoms, anxiety symptoms, and baseline cortisol). Cognitive anxiety failed to predict cortisol reactivity, F(6, 81) = 0.88, p = .51, β = 0.285); however, somatic anxiety successfully predicted cortisol reactivity, such that greater levels of somatic anxiety led to larger spikes in cortisol, F(6,83) = 3.34, p < .01, β = 0.401 (see Table 3).
Table 3.
Linear regression models for reactivity regressed onto cognitive anxiety.
| B | SE B | β | t | p | R2 | ||
|---|---|---|---|---|---|---|---|
| DBP reactivity | |||||||
| Model 1 | 0.810 | 0.481 | 0.166 | 1.686 | .096 | .240 | |
| Model 2 | 1.094 | 0.512 | 0.224 | 2.138 | .038 | .288 | |
| SBP reactivity | |||||||
| Model 1 | 1.261 | 0.675 | 0.195 | 1.870 | .065 | .142 | |
| Model 2 | 1.456 | 0.720 | 0.225 | 2.021 | .047 | .166 | |
| HR reactivity | |||||||
| Model 1 | 0.322 | 0.763 | 0.047 | 0.422 | .674 | .002 | |
| Model 2 | 0.346 | 0.790 | 0.050 | 0.437 | .663 | .088 | |
| Cortisol reactivity | |||||||
| Model 1 | 0.005 | 0.006 | 0.089 | 0.805 | .423 | .021 | |
| Model 2 | 0.007 | 0.007 | 0.115 | 0.988 | .326 | .080 |
Notes: Model 1 includes baseline blood pressure, pulse rate, or cortisol; Model 2 additionally includes age, sex, depressive symptoms, and anxiety symptoms.
4.5. Physiological Reactivity and Vitality
4.5 Reactivity and Vitality
Linear regression analyses examined the relationships between reactivity and vitality. First, a linear regression was carried out to test if DBP reactivity significantly predicted changes in vitality, while controlling for other factors (age, sex, race, depressive symptoms, anxiety symptoms, and average baseline DBP). The results indicated that these variables accounted for 17.9% of the variance in vitality change, and that the model was significant, F(8, 88) = 2.17, p < .03. DBP reactivity significantly predicted changes in vitality within the model (β = 0.351, p < .05). Similarly, a linear regression tested whether average SBP during the task predicted changes in vitality, while controlling for related variables (age, sex, race, depressive symptoms, anxiety symptoms, and average baseline SBP). The results indicated that these variables accounted for 22.8% of the variance and that the model was significant, F(8, 88) = 2.96, p < .05. SBP reactivity significantly predicted changes in vitality, controlling for the other variables (β = 0.498, p < .05). Separately, a linear regression was carried out to test if HR reactivity significantly predicted changes in vitality, while controlling for other factors (age, sex, race, depressive symptoms, anxiety symptoms, and average baseline HR). The variables in this model accounted for about 19% of the variance in vitality, and heart rate reactivity significantly predicted changes in vitality controlling for the other variables (β = 0.475, p < .001). Thus, the results of these regression analyses provide support for the hypothesis that CVR may contribute to changes in subjective vitality. See Table 4 for regression statistics.
Table 4.
Linear regression models for changes in vitality regressed onto reactivity.
| B | SE B | β | t | p | R2 | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| DBP reactivity | |||||||
| Model 1 | 0.194 | 0.061 | 0.353 | 3.157 | .002 | .120 | |
| Model 2 | 0.194 | 0.064 | 0.353 | 3.032 | .003 | .130 | |
| SBP reactivity | |||||||
| Model 1 | 0.175 | 0.042 | 0.425 | 4.178 | <.001 | .189 | |
| Model 2 | 0.179 | 0.043 | 0.435 | 4.207 | <.001 | .210 | |
| HR reactivity | |||||||
| Model 1 | 0.193 | 0.045 | 0.444 | 4.34 | <.001 | .187 | |
| Model 2 | 0.207 | 0.046 | 0.475 | 4.52 | <.001 | .199 | |
| Cortisol reactivity | |||||||
| Model 1 | 4.572 | 4.550 | 0.111 | 1.005 | .318 | .015 | |
| Model 2 | 4.668 | 4.754 | 0.113 | 0.982 | .329 | .031 | |
Notes: Model 1 includes baseline blood pressure, heart rate or cortisol; Model 2 additionally includes age, sex, depressive symptoms, and anxiety symptoms.
Lastly, a simple linear regression tested whether the magnitude of cortisol reactivity predicted changes in vitality. As predicted, cortisol reactivity in response to the stressor was unrelated to changes in vitality over the course of the stressor, F(6, 83), 0.536, p = .78, β = 0.135. Thus, further analyses were unnecessary (see Table 4).
4.6. Blood Pressure Mediation Analyses
In order to further test the primary hypothesis, analyses examined whether average blood pressure during the task would mediate the relationship between cognitive anxiety and changes in subjective vitality. Using Hayes’ (2013) PROCESS macro (Model 4) with 5000 bootstrapped samples, we found evidence of a significant indirect effect of cognitive anxiety on changes in vitality through changes in SBP and DBP, even when adjusting for self-reported depressive and anxiety symptoms, age, sex, race, pre-task state vitality, and baseline blood pressure, (95% CI [0.017, 0.517]) and (95% CI [0.038, 0.705]) respectively. These results support the main hypothesis, suggesting that cognitive anxiety contributes to changes in vitality by way of blood pressure responses (See Figures 1 and 2).
Figure 1.
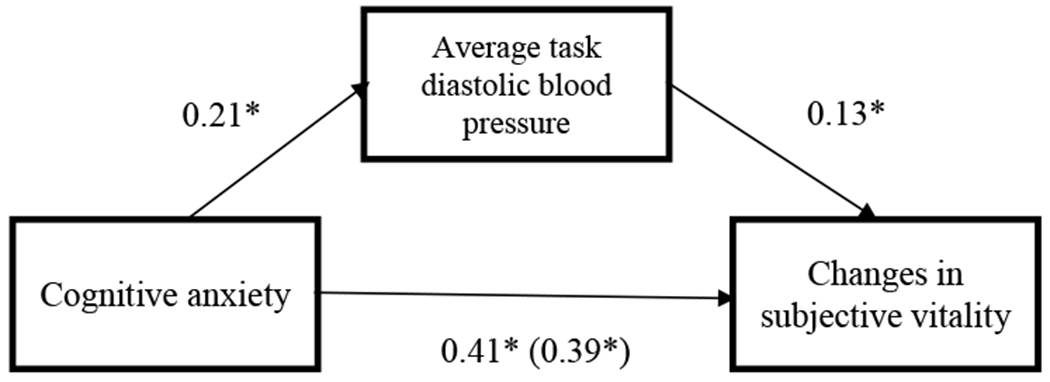
Standardized beta-weights for the relationship between cognitive anxiety at the beginning of the task and changes in subjective vitality before and after the stress task, as mediated by DBP reactivity.
*p < .05
Figure 2.
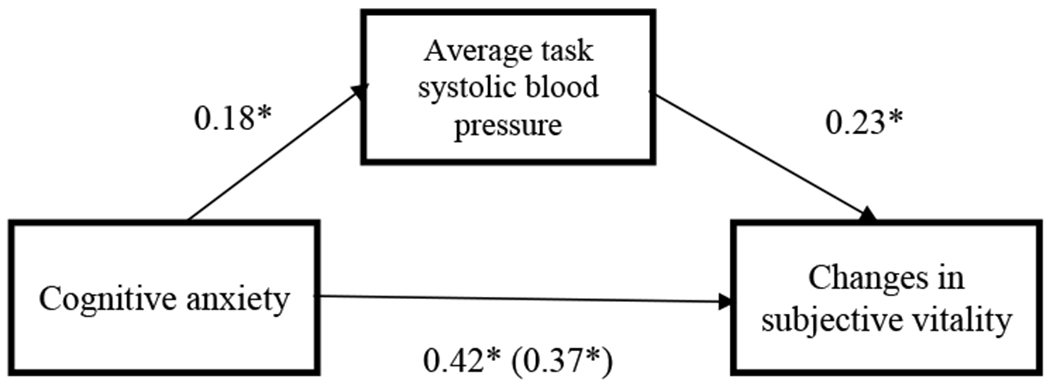
Standardized beta-weights for the relationship between cognitive anxiety at the beginning of the task and changes in subjective vitality before and after the stress task, as mediated by SBP reactivity.
*p < .05
Similarly, the PROCESS macro (Model 4) was used to test whether average blood pressure during the task would mediate the relationship between somatic anxiety and changes in subjective vitality, when controlling for self-reported depression, age, sex, race, pre-task state vitality, and baseline blood pressure. There was evidence of a significant indirect effect for diastolic blood pressure reactivity (95% CI [0.006, 0.454]), but not for systolic blood pressure reactivity (95% CI [−0.015, 0.639]). These results do not directly support the hypothesis (See Figures 3 and 4).
Figure 3.
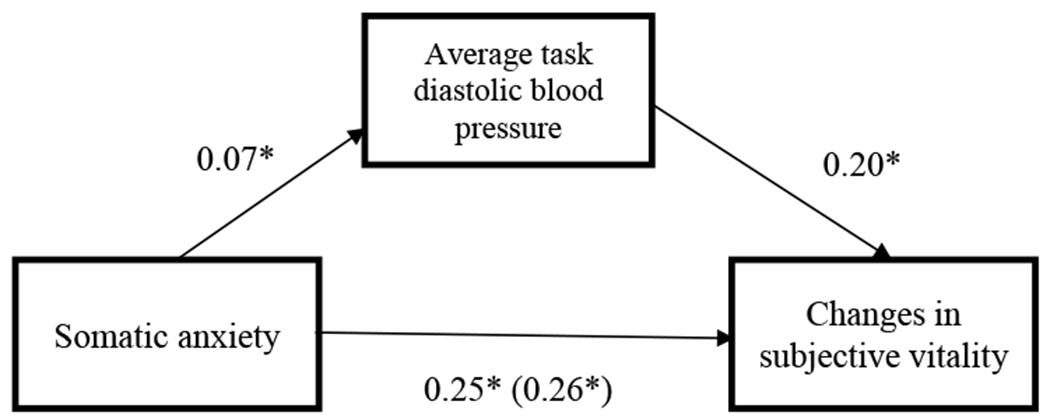
Standardized beta-weights for the relationship between cognitive anxiety at the beginning of the task and changes in subjective vitality before and after the stress task, as mediated by DBP reactivity.
*p <.05
Figure 4.
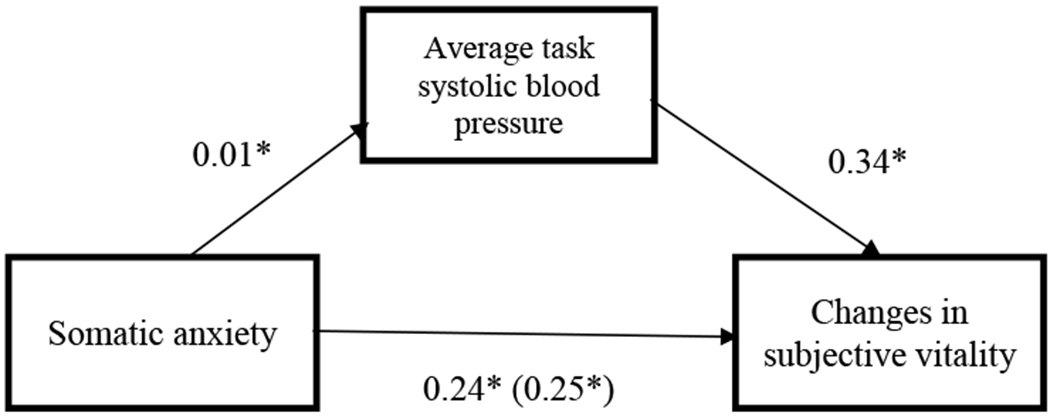
Standardized beta-weights for the relationship between cognitive anxiety at the beginning of the task and changes in subjective vitality before and after the stress task, as mediated by SBP reactivity.
*p <.05
5. Discussion
To our knowledge, the current study provides the first data on the relationships between physiological responses (i.e. cardiovascular and endocrine) to acute psychological stress and vitality levels in college students. Using an in-laboratory study, we examined the relationships between anxiety (cognitive and somatic) preceding an acute stressor, subsequent changes in vitality, and considered whether physiological reactivity would mediate observed relationships.
Because both physical and psychological factors contribute to one’s state vitality level (Ryan & Frederick, 1997; Penninx et al., 2000), we hypothesized that both anxiety and physiological reactivity responses to acute psychological stress would deplete subjective vitality. We further predicted that this relationship would be dependent on one’s awareness of reactivity responses; CVR, posited to be more accessible than cortisol reactivity, was hypothesized to drain vitality more than cortisol reactivity. The results partially supported these hypotheses: individuals who reported higher levels of anxiety preceding the task mounted larger blood pressure responses to an acute mathematical stressor, which was associated with larger decreases in vitality over the course of the study session, even when controlling for theoretically relevant constructs such as age, race, and reported symptoms of depression and anxiety. However, neither cognitive nor somatic anxiety predicted HR reactivity, although HR reactivity was a significant predictor of changes in vitality, with higher heart rate during the task predicting greater declines in state vitality following the task. The different relationships observed between anxiety and blood pressure and anxiety and HR are in line with previous work which found that state anxiety predicted blood pressure reactivity, but not HR reactivity (Pointer et al., 2012). More research is needed to understand why anxiety may be more closely linked to blood pressure responses compared to HR. It is important to note that the ability to perceive blood pressure dynamics is imperfect. Compared to HR dynamics which can be readily perceived, inferences about blood pressure are more likely linked to psychological states (James et al., 1986; Pointer et al., 2012; Steffen & Larson, 2015). Unlike blood pressure and HR, cortisol reactivity was not related to changes in vitality. These results provide some support for the notion that while pre-task anxiety may positively relate to some physiological responses to stressors, individuals may only experience loss of vitality when they can access reactivity cues. However, in the current study, our post-task cortisol sample did not correspond with the timing of our post-task vitality measurement. In future work, it will be important to synchronize assessment of these outcomes to more accurately understand the correspondence between cortisol reactivity and changes in vitality.
Unfortunately, the current work did not include a measure of participants’ perception of the task as either challenging or threatening. Based on previous work indicating that challenge perceptions predict lower task-related anxiety compared to threat perceptions (Moore et al., 2012; Skinner & Brewer, 2002; Williams et al., 2010), and based on the current findings, it is possible that challenge perceptions of a task would reduce the degree to which stressful tasks deplete vitality. Future work should include measures of challenge and threat appraisals, in addition to expanded cardiovascular measures (i.e. cardiac output, vascular resistance), in order to better understand the role of appraisals in the current findings, and to understand the pattern of cardiovascular responses which may relate to greater declines in vitality. If data indicate that challenge appraisals of a stressor predict smaller declines in vitality, then brief interventions used in prior work which encourage individuals to view tasks as a challenge as opposed to a threat (Jamieson et al., 2018) could be helpful in reducing the impact of stressors on vitality levels.
The current data suggest that stressors, even when acute, consume vital energy. These findings provide support for existing literature which claims that vitality can be lessened through exposure to stress and psychological tension (Penninx et al., 2000). It is important to note, however, that this is only a small piece of the larger picture of stress and reactivity; stressors are not limited to a single, isolated event, they can occur randomly and repeatedly in daily life. Stress is accumulated over time, so it is equally likely that vitality also fluctuates over time. A separate, supporting body of literature suggests that repeated exposure to stress may lead to chronically low levels of vitality, termed “vital exhaustion” (Appels et al., 1987; Frestad & Prescott, 2017; Nicolson & van Diest, 2000; Rozanski & Cohen, 2017). Vital exhaustion (VE), or a chronic lack of vitality, is often accompanied by fatigue, increased irritability, feelings of demoralization, and the inability to cope with daily stressors (Appels, 1990; Nicolson & van Diest, 2000). Day-to-day stressors accumulate, slowly diminishing vitality, and when depleted, may limit flexible responding and regulation to subsequent stressors. Through this pathway, it is suggested that stress-induced lack of vitality may contribute to future risk for disease (Rozanski et al., 2005). Prospective evidence indicates that VE leads individuals presently free of cardiac disease to be twice as likely to experience myocardial infarction, even when controlling for lifestyle and demographic factors (Appels & Mulder, 1988; Appels & Otten, 1992). Similarly, other clinical data links vital exhaustion to a number of other adverse health outcomes, such as stroke (Kornerup et al., 2010), heart failure (Rod et al., 2011), and all-cause mortality (Frestad & Prescott, 2017). Together, these findings suggest that vitality and vital exhaustion may be important psychosocial risk factors to consider when attempting to understand the pathways that lead to the development of adverse cardiac outcomes.
5.1. Limitations and Future Directions
Though the current study provides insight into the relationship between stress and vitality, there are important limitations to note. First, generalizability of the findings is limited in that the sample was comprised of healthy college aged students, and these relationships may differ in clinical populations. It is possible that vitality may change across the lifespan. Further, approximately half of the participants were American Indian (AI), which could potentially alter the generalizability of the findings. As compared to non-Hispanic White college students, AI students have been shown to illustrate blunted SBP, DBP, HR and cortisol responses to acute psychological stress (John-Henderson et al., 2020). Though inclusion of race as a covariate did not significantly alter the results, future studies could explore reactivity differences amongst racial groups, specifically examining the relationship between blunted reactivity and vitality levels.
Future work should consider whether in-laboratory stressors affect vitality in a similar manner to stress in daily life in the natural environment. Daily life stress has been illustrated to activate similar reactivity responses to laboratory tasks, and although responses to daily life stress may lead to more accurately reflect pathways that lead to disease, laboratory investigations of stress and the associated responses are still reliable (Zanstra & Johnston, 2011). Another significant limitation is that the current study is cross-sectional. In real life, stress is not limited to episodes of acute stress, instead the effects can accumulate over time (e.g., Slopen et al., 2018). It is realistic that subjective vitality, too, ebbs and flows over time. Stressors, physical and psychological, could affect vitality levels, leaving an individual with less flexibility when dealing with subsequent stressors (Ryan & Frederick, 1997). Future studies should examine the accumulation of stress over time in relation to vitality and examine how vitality fluctuates over time. The current study only captures one sliver of the stress/vitality cycle, and a longitudinal approach would be more suitable for capturing cumulative effects.
The current research was limited by discontinuous measurement of blood pressure and HR. Changes in methodology to increase frequency of data collection could also allow for more accurate, momentary estimates of blood-pressure reactivity in real-time. Additionally, operationalizations of subjective vitality were informed purely through self-report. Manipulations of vitality (e.g., exercise, exposure to nature; Peterson & Seligman, 2004) prior to exposure to acute lab stressors would add support for the relationship between stress and vitality. Another measurement issue is related to our sampling of cortisol. In the current research, we only obtained one salivary sample for measurement of post-stressor levels of cortisol. Our post-stressor sample was taken 10 minutes after the cessation of the task. In a previous review, peak cortisol responses were found to occur most often 10 minutes after a stressor, however it is possible that they might occur 5 or 20 minutes after stressor (Allen et al., 2014). Increased sampling for measurement of cortisol levels in future work would allow us to better understand the relationships between pre-task anxiety, cortisol reactivity, and changes in subsequent levels of vitality.
Though the current work provides important findings that inform the way physiology may affect vitality, we only touch on one aspect of the relationship between stress and vitality. Theoretical research alludes to the notion of subjective vitality as being protective against the threat of both psychological and physical stressors (Polk et al., 2005). This would suggest that those high in subjective vitality would be less affected by stressors, compared to those low in subjective vitality. However, the current research presents data that suggests subjective vitality may act as a resource that is drawn from in times of stress, rather than a shield against the effects of stress. Future research could further examine the protective competencies of subjective vitality and the findings should be replicated using a larger sample.
The current research focuses on ways in which subjective vitality is diminished. Though this research is important, further research should also examine ways in which vitality can be replenished. This knowledge could then be used to inform interventions that could promote overall well-being. Activities that increase vitality may maximize the amount of energy one has available for dealing with daily stressors, which would, ideally, reduce time spent in the exhaustion phase of the stress response and, potentially, reduce the amount of wear-and-tear on the system. With further research on how to increase subjective vitality, this may provide insight into its protective function, utilizing subjective vitality as an adaptive coping mechanism against the stress and the development of disease (Rozanski et al., 2005).
5.2. Conclusion
To our knowledge, this is the first empirical paper to examine subjective vitality within the framework of stress research. These results provide novel findings, illustrating that cardiovascular responses, but not cortisol reactivity responses, to stress are implicated in changes in the phenomenological experience of subjective vitality. While this research is a first step, subjective vitality remains an under-researched construct, predominantly supported by theoretical research. Because stress is suggested to play a significant role in risk for disease, more research is needed to understand the role of vitality in this relationship.
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103474 and U54GM115371. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of Interest: The authors have no conflicts of interest to disclose.
Data availability statement:
Authors have a data set which can be shared upon reasonable request.
References
- Allen MT, Matthews KA, & Sherman FS (1997). Cardiovascular reactivity to stress and left ventricular mass in youth. Hypertension (Dallas, Tex: 1979), 30(4), 782–787. 10.1161/01.hyp.30.4.782 [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, & Clarke G (2014). Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neuroscience and Biobehavioral Reviews, 38, 94–124. 10.1016/j.neubiorev.2013.11.005 [DOI] [PubMed] [Google Scholar]
- American College Health Association. American College Health Association-National College Health Assessment II: Reference Group Executive Summary Spring 2018. Silver Spring, MD: American College Health Association; 2018. [Google Scholar]
- Appels A (1990). Mental precursors of myocardial infarction. The British Journal of Psychiatry : the Journal of Mental Science, 156, 465–471. 10.1192/bjp.156.4.465 [DOI] [PubMed] [Google Scholar]
- Appels A, & Mulder P (1988). Excess fatigue as a precursor of myocardial infarction. European Heart Journal, 9(7), 758–764. 10.1093/eurheartj/9.7.758-764. [DOI] [PubMed] [Google Scholar]
- Appels A, & Otten F (1992). Exhaustion as precursor of cardiac death. British Journal of Clinical Psychology, 31(3), 351–356. 10.1111/j.2044-8260.1992.tb01004.x [DOI] [PubMed] [Google Scholar]
- Appels A, Höppener P, & Mulder P (1987). A questionnaire to assess premonitory symptoms of myocardial infarction. International Journal of Cardiology, 17(1), 15–24. 10.1016/0167-5273(87)90029-5 [DOI] [PubMed] [Google Scholar]
- Bryla CM (1996). The relationship between stress and the development of breast cancer: a literature review. Oncology Nursing Forum, 23(3), 441–448. [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Painter RC, Roseboom TJ, Phillips AC, & de Rooij SR (2012). Systolic blood pressure reactions to acute stress are associated with future hypertension status in the Dutch Famine Birth Cohort Study. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 85(2), 270–273. 10.1016/j.ijpsycho.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Der G, Hunt K, & Benzeval M (2011). Blood pressure reactions to acute mental stress and future blood pressure status: data from the 12-year follow-up of the West of Scotland Study. Psychosomatic Medicine, 73(9), 737–742. 10.1097/PSY.0b013e3182359808 [DOI] [PubMed] [Google Scholar]
- Carroll D, Ring C, Hunt K, Ford G, & Macintyre S (2003). Blood pressure reactions to stress and the prediction of future blood pressure: effects of sex, age, and socioeconomic position. Psychosomatic Medicine, 65(6), 1058–1064. 10.1097/01.psy.0000097330.58739.26 [DOI] [PubMed] [Google Scholar]
- Chauntry AJ, Williams SE, & Whittaker AC (2019). Blunted cardiovascular responses to acute psychological stress predict low behavioral but not self-reported perseverance. Psychophysiology, 56(11), e13449. 10.1111/psyp.13449 [DOI] [PubMed] [Google Scholar]
- Chida Y, & Steptoe A (2010). Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension (Dallas, Tex. : 1979), 55(4), 1026–1032. 10.1161/HYPERTENSIONAHA.109.146621 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. [PubMed] [Google Scholar]
- Dekker MJ, Koper JW, van Aken MO, Pols HA, Hofman A, de Jong FH, Kirschbaum C, Witteman JC, Lamberts SW, & Tiemeier H (2008). Salivary cortisol is related to atherosclerosis of carotid arteries. The Journal of Clinical Endocrinology and Metabolism, 93(10), 3741–3747. 10.1210/jc.2008-0496 [DOI] [PubMed] [Google Scholar]
- Frestad D, & Prescott E (2017). Vital Exhaustion and Coronary Heart Disease Risk: A Systematic Review and Meta-Analysis. Psychosomatic Medicine, 79(3), 260–272. 10.1097/PSY.0000000000000423 [DOI] [PubMed] [Google Scholar]
- Ginty AT, & Conklin SM (2011). High perceived stress in relation to life events is associated with blunted cardiac reactivity. Biological Psychology, 86(3), 383–385. 10.1016/j.biopsycho.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Ginty AT, Brindle RC, & Carroll D (2015). Cardiac stress reactions and perseverance: Diminished reactivity is associated with study non-completion. Biological psychology, 109, 200–205. 10.1016/j.biopsycho.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Greer S, & Watson M (1985). Towards a psychobiological model of cancer: psychological considerations. Social Science & Medicine (1982), 20(8), 773–777. 10.1016/0277-9536(85)90330-2 [DOI] [PubMed] [Google Scholar]
- Gronwall DM (1977). Paced auditory serial-addition task: a measure of recovery from concussion. Perceptual and Motor Skills, 44(2), 367–373. 10.2466/pms.1977.44.2.367 [DOI] [PubMed] [Google Scholar]
- Gunnell K, Mosewich A, McEwen C, Eklund RC & Crocker P (2017). Don’t be so hard on yourself! Changes in self-compassion during the first year of university are associated with changes in well-being. Personality and Individual Differences,107, 43–48. 10.1016/j.paid.2016.11.032. [DOI] [Google Scholar]
- Hamer M, O’Donnell K, Lahiri A, & Steptoe A. (2010). Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. European Heart Journal, 31(4), 424–429. DOI: 10.1093/eurheartj/ehp386. [DOI] [PubMed] [Google Scholar]
- Hamer M, & Steptoe A (2012). Cortisol responses to mental stress and incident hypertension in healthy men and women. The Journal of Clinical Endocrinology and Metabolism, 97(1), E29–E34. 10.1210/jc.2011-2132 [DOI] [PubMed] [Google Scholar]
- Harris A, Ursin H, Murison R, & Eriksen HR (2007). Coffee, stress and cortisol in nursing staff. Psychoneuroendocrinology, 32(4), 322–330. 10.1016/j.psyneuen.2007.01.003 [DOI] [PubMed] [Google Scholar]
- James GD, Yee LS, Harshfield GA, Blank SG, & Pickering TG (1986). The influence of happiness, anger, and anxiety on the blood pressure of borderline hypertensives. Psychosomatic Medicine, 48(7), 502–508. 10.1097/00006842-198609000-00005 [DOI] [PubMed] [Google Scholar]
- Jamieson JP, Crum AJ, Goyer JP, Marotta ME, & Akinola M (2018). Optimizing stress responses with reappraisal and mindset interventions: an integrated model. Anxiety, Stress, and Coping, 31(3), 245–261. 10.1080/10615806.2018.1442615 [DOI] [PubMed] [Google Scholar]
- John-Henderson NA, Gruman HE, Counts CJ, & Ginty AT (2020). American Indian young adults display diminished cardiovascular and cortisol responses to acute psychological stress. Psychoneuroendocrinology, 114, 104583. 10.1016/j.psyneuen.2020.104583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Bell C, Jones M, Farquharson B, Allan J, Schofield P, Ricketts I, & Johnston M (2016). Stressors, Appraisal of Stressors, Experienced Stress and Cardiac Response: A Real-Time, Real-Life Investigation of Work Stress in Nurses. Annals of Behavioral Medicine: a publication of the Society of Behavioral Medicine, 50(2), 187–197. 10.1007/s12160-015-9746-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Meijen C, McCarthy PJ, & Sheffield D (2009). A theory of challenge and threat states in athletes. International Review of Sport and Exercise Psychology, 2(2), 161–180. 10.1080/17509840902829331 [DOI] [Google Scholar]
- Keller A, Litzelman K, Wisk LE, Maddox T, Cheng ER, Creswell PD, & Witt WP (2012). Does the perception that stress affects health matter? The association with health and mortality. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 31(5), 677–684. 10.1037/a0026743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup H, Marott JL, Schnohr P, Boysen G, Barefoot J, & Prescott E (2010). Vital exhaustion increases the risk of ischemic stroke in women but not in men: results from the Copenhagen City Heart Study. Journal of Psychosomatic Research, 68(2), 131–137. 10.1016/j.jpsychores.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, & Thurston RC (2007). Emotional vitality and incident coronary heart disease: benefits of healthy psychological functioning. Archives of General Psychiatry, 64(12), 1393–1401. 10.1001/archpsyc.64.12.1393 [DOI] [PubMed] [Google Scholar]
- Lambiase MJ, Kubzansky LD, & Thurston RC (2015). Positive psychological health and stroke risk: The benefits of emotional vitality. Health Psychology : official journal of the Division of Health Psychology, American Psychological Association, 34(10), 1043–1046. 10.1037/hea0000228 [DOI] [PubMed] [Google Scholar]
- Lazarus RS, & Folkman S (1984). Stress, appraisal, and coping. Springer publishing company. [Google Scholar]
- Lustyk MK, Olson KC, Gerrish WG, Holder A, & Widman L (2010). Psychophysiological and neuroendocrine responses to laboratory stressors in women: implications of menstrual cycle phase and stressor type. Biological Psychology, 83(2), 84–92. 10.1016/j.biopsycho.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Maier KJ, Waldstein SR, & Synowski SJ (2003). Relation of cognitive appraisal to cardiovascular reactivity, affect, and task engagement. Annals of Behavioral Medicine : a publication of the Society of Behavioral Medicine, 26(1), 32–41. 10.1207/S15324796ABM2601_05 [DOI] [PubMed] [Google Scholar]
- Mallorquí-Bagué N, Bulbena A, Pailhez G, Garfinkel SN, & Critchley HD (2016). Mind-Body Interactions in Anxiety and Somatic Symptoms. Harvard Review of Psychiatry, 24(1), 53–60. 10.1097/HRP.0000000000000085 [DOI] [PubMed] [Google Scholar]
- Martens R, Vealey RS, & Burton D (1990). Competitive anxiety in sport. Champaign, IL: Human Kinetics. [Google Scholar]
- Matthews KA, Owens JF, Kuller LH, Sutton-Tyrrell K, Lassila HC, & Wolfson SK (1998). Stress-induced pulse pressure change predicts women’s carotid atherosclerosis. Stroke, 29(8), 1525–1530. 10.1161/01.str.29.8.1525 [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gump BB, Block DR, & Allen MT (1997). Does background stress heighten or dampen children’s cardiovascular responses to acute stress? Psychosomatic Medicine, 59(5), 488–496. 10.1097/00006842-199709000-00005 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2006). Protective and damaging effects of stress mediators: central role of the brain. Dialogues in Clinical Neuroscience, 8(4), 367–381. 10.31887/DCNS.2006.8.4/bmcewen [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LJ, Vine SJ, Wilson MR, & Freeman P (2012). The effect of challenge and threat states on performance: an examination of potential mechanisms. Psychophysiology, 49(10), 1417–1425. 10.1111/j.1469-8986.2012.01449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Gagné M, & Rosman H (2008). Helpful Self-Control: Autonomy Support, Vitality, and Depletion. Journal of Experimental Social Psychology, 44(3), 573–585. 10.1016/j.jesp.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musante L, Treiber FA, Kapuku G, Moore D, Davis H, & Strong WB (2000). The effects of life events on cardiovascular reactivity to behavioral stressors as a function of socioeconomic status, ethnicity, and sex. Psychosomatic Medicine, 62(6), 760–767. 10.1097/00006842-200011000-00004 [DOI] [PubMed] [Google Scholar]
- Nicolson NA, & van Diest R (2000). Salivary cortisol patterns in vital exhaustion. Journal of Psychosomatic Research, 49(5), 335–342. 10.1016/s0022-3999(00)00166-5 [DOI] [PubMed] [Google Scholar]
- Niemiec CP, Lynch MF, Vansteenkiste M, Bernstein J, Deci EL, & Ryan RM (2006). The antecedents and consequences of autonomous self-regulation for college: a self-determination theory perspective on socialization. Journal of Adolescence, 29(5), 761–775. 10.1016/j.adolescence.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Bandeen-Roche K, Kasper JD, Simonsick EM, Ferrucci L, & Fried LP (2000). The protective effect of emotional vitality on adverse health outcomes in disabled older women. Journal of the American Geriatrics Society, 48(11), 1359–1366. 10.1111/j.1532-5415.2000.tb02622.x [DOI] [PubMed] [Google Scholar]
- Peterson C, & Seligman MEP (2004). Character strengths and virtues: A handbook and classification. New York: Oxford University Press and Washington, DC: American Psychological Association. [Google Scholar]
- Phillips AC, Ginty AT, & Hughes BM (2013). The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. International Journal of Psychophysiology : official journal of the International Organization of Psychophysiology, 90(1), 1–7. 10.1016/j.ijpsycho.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Pointer MA, Yancey S, Abou-Chacra R, Petrusi P, Waters SJ, & McClelland MK (2012). State anxiety is associated with cardiovascular reactivity in young, healthy african americans. International Journal of Hypertension, 2012, 268013. 10.1155/2012/268013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk DE, Cohen S, Doyle WJ, Skoner DP, & Kirschbaum C (2005). State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology, 30(3), 261–272. 10.1016/j.psyneuen.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Richardson S, Shaffer JA, Falzon L, Krupka D, Davidson KW, & Edmondson D (2012). Meta-analysis of perceived stress and its association with incident coronary heart disease. The American Journal of Cardiology, 110(12), 1711–1716. 10.1016/j.amjcard.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rod NH, Andersen I, & Prescott E (2011). Psychosocial risk factors and heart failure hospitalization: a prospective cohort study. American Journal of Epidemiology, 174(6), 672–680. 10.1093/aje/kwr144 [DOI] [PubMed] [Google Scholar]
- Rozanski A, & Cohen R (2017). From Vitality to Vital Exhaustion and Other States of “Tense Tiredness”: A New Biopsychosocial Risk Domain. Psychosomatic Medicine, 79(3), 256–259. 10.1097/PSY.0000000000000452 [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Davidson KW, Saab PG, & Kubzansky L (2005). The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. Journal of the American College of Cardiology, 45(5), 637–651. 10.1016/j.jacc.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Ryan RM, & Frederick C (1997). On energy, personality, and health: subjective vitality as a dynamic reflection of well-being. Journal of Personality, 65(3), 529–565. 10.1111/j.1467-6494.1997.tb00326.x [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Lerman C, Miller SM, Daly M, & Masny A (1995). Coping disposition, perceived risk, and psychological distress among women at increased risk for ovarian cancer. Health Psychology : official journal of the Division of Health Psychology, American Psychological Association, 14(3), 232–235. 10.1037//0278-6133.14.3.232 [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, & Singer BH (2001). Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America, 98(8), 4770–4775. 10.1073/pnas.081072698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H (1936). A syndrome produced by diverse nocuous agents. Nature, 138, 32. [DOI] [PubMed] [Google Scholar]
- Selye H (1946). The general adaptation syndrome and the diseases of adaptation. The Journal of Clinical Endocrinology and Metabolism, 6, 117–230. [DOI] [PubMed] [Google Scholar]
- Selye H (1956). The stress of life. New York: McGraw-Hill. [Google Scholar]
- Selye H (1959). Perspectives in Stress Research. Perspectives in Biology and Medicine, 2(4), 403–416. Johns Hopkins University Press. [DOI] [PubMed] [Google Scholar]
- Selye H (1975). Confusion and controversy in the stress field. Journal of Human Stress, 1(2), 37–44. [DOI] [PubMed] [Google Scholar]
- Selye H (1976). Stress in health and disease . Reading, MA: Butterworth’s. [Google Scholar]
- Skinner N, & Brewer N (2002). The dynamics of threat and challenge appraisals prior to stressful achievement events. Journal of Personality and Social Psychology, 83(3), 678–692. 10.1037/0022-3514.83.3.678 [DOI] [PubMed] [Google Scholar]
- Slopen N, Meyer C, & Williams DR (2018). Cumulative Stress and Health. In The Oxford Handbook of Integrative Health Science (p. 75). Oxford Library of Psychology Somatic Medicine, 59, 488–496. [Google Scholar]
- Steffen PR, & Larson MJ (2015). A brief mindfulness exercise reduces cardiovascular reactivity during a laboratory stressor paradigm. Mindfulness, 6(4), 803–811. 10.1007/s12671-014-0320-4 [DOI] [Google Scholar]
- Steptoe A, & Kearsley N (1990). Cognitive and somatic anxiety. Behaviour Research and Therapy, 28(1), 75–81. 10.1016/0005-7967(90)90057-p [DOI] [PubMed] [Google Scholar]
- Thomas O, Hanton S, & Jones G (2002). An alternative approach to short-form self-report assessment of competitive anxiety: A research note. International Journal of Sport Psychology, 33(3), 325–336. [Google Scholar]
- Trotman GP, Gianaros PJ, Veldhuijzen van Zanten J, Williams SE, & Ginty AT (2019). Increased stressor-evoked cardiovascular reactivity is associated with reduced amygdala and hippocampus volume. Psychophysiology, 56(1), e13277. 10.1111/psyp.13277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel-Fitzgerald C, Boehm JK, Kivimaki M, & Kubzansky LD (2014). Taking the tension out of hypertension: a prospective study of psychological well being and hypertension. Journal of Hypertension, 32(6), 1222–1228. 10.1097/HJH.0000000000000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MJ, Jones MV, Sheffield D, Barker JB, & Coffee P (2014). Manipulating cardiovascular indices of challenge and threat using resource appraisals. International journal of Psychophysiology : Official journal of the International Organization of Psychophysiology, 94(1), 9–18. 10.1016/j.ijpsycho.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Vitality. (n.d.) In Merriam-Webster’s collegiate dictionary. Retrieved from http://www.merriam-webster.com/dictionary/vitality
- Whitworth JA, Williamson PM, Mangos G, & Kelly JJ (2005). Cardiovascular consequences of cortisol excess. Vascular Health and Risk Management, 1(4), 291–299. 10.2147/vhrm.2005.1.4.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Cumming J, & Balanos GM (2010). The use of imagery to manipulate challenge and threat appraisal States in athletes. Journal of Sport & Exercise Psychology, 32(3), 339–358. 10.1123/jsep.32.3.339 [DOI] [PubMed] [Google Scholar]
- Williams SE, Veldhuijzen van Zanten J, Trotman GP, Quinton ML, & Ginty AT (2017). Challenge and threat imagery manipulates heart rate and anxiety responses to stress. International Journal of Psychophysiology : Official journal of the International Organization of Psychophysiology, 117, 111–118. 10.1016/j.ijpsycho.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Wright BJ, O’Brien S, Hazi A, & Kent S (2014). Increased systolic blood pressure reactivity to acute stress is related with better self-reported health. Scientific Reports, 4, 6882. 10.1038/srep06882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanstra YJ, & Johnston DW (2011). Cardiovascular reactivity in real life settings: measurement, mechanisms and meaning. Biological Psychology, 86(2), 98–105. 10.1016/j.biopsycho.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, & Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Authors have a data set which can be shared upon reasonable request.


