Abstract
Social integration and social status can substantially affect an individual’s health and survival. One route through which this occurs is by altering immune function, which can be highly sensitive to changes in the social environment. However, we currently have limited understanding of how sociality influences markers of immunity in naturalistic populations where social dynamics can be fully realized. To address this gap, we asked if social integration and social status in free-ranging rhesus macaques (Macaca mulatta) predict anatomical and physiological markers of immunity. We used data on agonistic interactions to determine social status, and social network analysis of grooming interactions to generate measures of individual variation in social integration. As measures of immunity, we included the size of two of the major organs involved in the immune response, the spleen and liver, and counts of three types of blood cells (red blood cells, platelets, and white blood cells). Controlling for body mass and age, we found that neither social status nor social integration predicted the size of anatomical markers of immunity. However, individuals that were more socially connected, i.e., with more grooming partners, had lower numbers of white blood cells than their socially isolated counterparts, indicating lower levels of inflammation with increasing levels of integration. These results build upon and extend our knowledge of the relationship between sociality and the immune system in humans and captive animals to free-ranging primates, demonstrating generalizability of the beneficial role of social integration on health.
Keywords: Sociality, Health, Immunity, Social networks, Rhesus macaques
1. Introduction
In primates and other mammals, social interactions between conspecifics (i.e., sociality) can have dramatic effects on an individual’s health and survival [1,2]. On the one hand, having more and stronger social connections has been related to extended lifespan [3,4]. On the other, aspects of the social structure of the species (e.g. dominance hierarchies, socioeconomic status) may determine individual differences in access to resources [5,6] that can lead to health disparities to those at a disadvantage [2]. However, precisely how the social environment ‘gets under the skin’ to alter health and survival remains an open and active area of inquiry.
One of the most susceptible systems to variation in the social environment is the immune system. For example, low socioeconomic status in humans has been linked to higher numbers of white blood cells, including lymphocytes and natural killer cells [7], which can be markers of chronic stress related inflammation [8]. Similarly, longitudinal studies have revealed that low socioeconomic status is associated with increases in markers of chronic inflammation, such as C-reactive protein and interleukin-6 [9]. Low socioeconomic status also affects the susceptibility of individuals to common chronic infections, such as Helicobacter pylori, cytomegalovirus, herpes simplex virus and hepatitis [10]. Similar effects have been found in animal models. Experimental manipulations of social status in captive female rhesus macaques (Macaca mulatta) found that low social status induced higher expression of genes related to interleukin signaling, T-cell activation, and inflammation [11,12]. Another study showed that the increase in T-cell activation in subordinate animals was followed by a decrease in T-cell numbers, which was attributed to a higher susceptibility to activation-induced T-cell death [13].
Opportunities for social support and the quality of these interactions (i.e., social integration) constitute the other major component of an individual’s social environment and can also affect the immune system. Studies in humans have found that social support- measured as the number of close contacts that can be of emotional help - enhanced the immunity of individuals infected with HIV, as evidenced by greater proliferation of T-helper cells [14]. Diversity of one’s support network-measured as the number of social roles that an individual experiences-might also reduce the susceptibility to infectious diseases, such as common colds [15]. Similarly, having a consistent social support network can lead to a reduction in the levels of interleukin-6 in elderly people [16]. Furthermore, a recent meta-analysis of studies on 73,037 individuals found that social integration and perceived social support significantly predicted lower levels of inflammatory cytokines, independent of the cytokine analyzed [17].
All these studies provide insight into the crucial role of social status and social integration in modulating markers of immunity, pointing to inflammatory processes as one of the possible underlying mechanisms by which the social environment affects an individual’s health and survival. However, there are many other markers of immunity about which we know very little when it comes to the social environment. Even more, our knowledge about the effects of sociality on the immunity of individuals living outside of WEIRD (western, educated, industrialized, rich, democratic) human societies [18], or of captivity in the case of animal models, where there is no access to medical healthcare and the pressure of environmental parasites on the immune system is probably higher, is very limited.
Free-ranging rhesus macaques on Cayo Santiago Island, Puerto Rico, are an excellent model for studying the effects of sociality on immune function. Monkeys in this population self-organize into groups, interact spontaneously with each other, and there is minimal medical intervention, thus individuals typically die of natural causes such as old age and disease. There are no predators on the island and the monkeys have ad-libitum access to food and water, which makes the social environment and rare ecological events, such as natural disasters [19], some of the major challenges with which the animals must contend. These features have made this population an ideal setting to test findings from laboratory animals about the stress response and immunity [20]. Since 2010, our group has collected behavioral data on social interactions on this population and detailed demographic records exist for all animals since the site’s foundation in 1938 [21]. Moreover, a large biobank of tissues and organs exists from individuals that were removed as part of population control implemented by the field station’s management, which allows behavioral information to be paired with rarely available and extremely valuable postmortem data.
Here we explored the association between an individual’s levels of social integration and social status with anatomical measures of immune function and physiological measures of immune activation. As measures of immune function, we considered the size of two of the major organs involved in immune defenses, the spleen and the liver [22,23]. The main role of the spleen is to filter blood-borne pathogens, store and produce white blood cells, and contribute to adaptive immunity through the production of antibodies [22]. The liver is a sentinel organ that filters gut-derived parasites, inducing immune tolerance for non-threats (i.e. microbiota) or, conversely, immunity in response to pathogens [23]. It also has the largest population of macrophages in the body, and thus plays a crucial role in innate immunity [23]. Changes in spleen size (i.e., splenomegaly) and liver size (i.e., hepatomegaly) are associated with environmental factors, such as parasite exposure [24,25], which can affect both organs concurrently [26]. As proxies for immune activation, we included standard measures of immunity and health: absolute counts of white blood cells, platelets and red blood cell [27]. We had two aims: to investigate 1) the relationship between sociality and immune organ sizes; and 2) the relationship between sociality and blood measures of immune activation. At the same time, we explored the effects of important demographic and morphological factors such as age, sex, body mass and group membership on our measures of immunity.
2. Methods
2.1. Study subjects and location
Study subjects were free-ranging rhesus macaques (Macaca mulatta) living at the Cayo Santiago field station, Puerto Rico, administered by the Caribbean Primate Research Center (CPRC). Mean annual population growth rates of the Cayo Santiago macaques are higher than those of wild rhesus populations, which have forced management efforts towards live capture and removal of individuals since 1956 [28]. From 2016 onward, our group started collecting postmortem data on animals removed by the CPRC. In 2016, 2018 and 2019, one entire social group of animals was scheduled for removal per year. In the year leading up to their removal, we collected behavioral data on subadult and adult macaques (i.e., 4 years old or more) from those groups. Animals were removed between October and November in the respective year, yet not all individuals planned for removal were successfully captured. In this study we included only those that were removed from the population. Our final data set comprised 142 animals (95 females and 47 males of known ages) from the three different social groups, each group representing a single year of data (group ID-year: HH-2016, KK-2018, S-2019). Subjects’ ages and maternal relatedness were extracted from the CPRC demographic database (detailed composition of groups and datasets in Table S1 and Fig. S1).
2.2. Behavioral data collection
Behavioral data were collected using two data collection protocols [29]: 5-min focal animal samples for group S and HH, and group-wide scan sampling for group KK. The data collection was done by a single experienced observer on Group S (group size: 149 adults) from February to October 2019, on group HH (group size: 95 adults and 13 subadults) from August to October 2016, and on group KK (group size: 124 adults) from January to October 2018. The use of scan sampling in group KK was due to the impact of Hurricane Maria, which made landfall in Puerto Rico in September 2017. Damage that resulted in inconsistent access to electricity in Puerto Rico posed challenges to the use of our power reliant data collection computers (Psion Work About Pro ©), so we switched to basic tablets, that could be more easily charged from other sources (e.g., car battery). This limitation, combined with the dangerous post-hurricane terrain that affected our ability to safely follow a focal animal, forced us to switch to a scan sampling protocol. Focal sampling was done following a previously established protocol [30]. Briefly, we recorded state behaviors (i.e., resting, feeding, travelling) along with all affiliative grooming interactions and agonistic encounters along with the identity of the focal animal’s adult or subadult social partners. Agonistic interactions included threat and submissive behaviors, along with contact and non-contact aggression. For scan sampling, we recorded state behaviors and all affiliative and agonistic interactions between all visible adults and subadults at 15 min intervals. We collected 1.46 ± 0.08 and 3.66 ± 0.64 h of behavioral data per individual in HH and S, respectively. For the scan samples in group KK, we collected 548.1± 161.3 behavioral events per individual.
2.3. Dominance hierarchy
We computed dominance hierarchies by group and separately for males and females [5,31–33]. Our approach is based on the literature in this species supporting sex-differences in how social status is acquired and on prior evidence that opposite-sex agonistic interactions have no impact on health measures [34]. Females are philopatric and form maternally inherited stable linear dominance hierarchies, where daughters acquire rank just below their mothers [35]. In contrast, males typically disperse from the natal group and acquire rank in the new group by physical contest and tenure [36]. We built both hierarchies using the outcomes of win-loss agonistic encounters from focal/scan sampling and ad-libitum observations, with known maternal relatedness used to resolve behavioral gaps in the female hierarchy [37]. To account for variation in group sizes, dominance rank was defined as the percentage of group mates from a subject’s sex that they outranked, where 100% corresponded to the highest-ranking animal [38].
2.4. Social networks
Social integration was quantified using a network approach applied to grooming interactions. As proxies for social integration, we considered an individual’s number of grooming partners (degree) and a measure that quantifies the quality of an individual’s grooming partners (weighted eigenvector centrality). Grooming degree is a measure of an individual’s direct social connections that provides insight into the opportunities for social support that an individual has. Eigenvector centrality quantifies indirect social connections and provides information on how well individuals are integrated into the network as a whole [39]. We decided to examine direct and indirect measures of social integration as both can significantly influence an individual’s health outcomes [4].
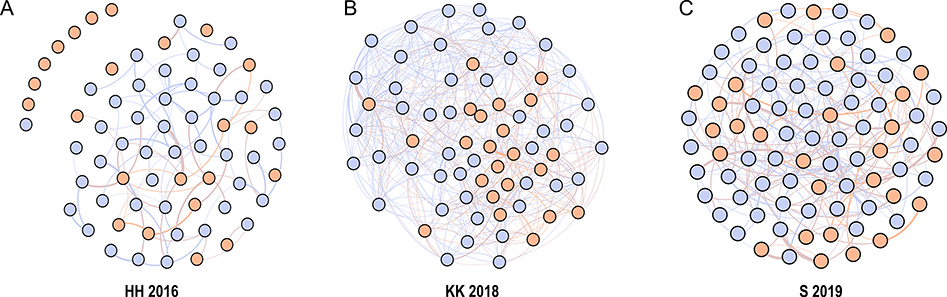
We generated weighted social networks for each behavioral group using the R package ‘igraph’ (Fig. 1) [40]. Social networks were built including all adult animals from a group, with the addition of subadults to group HH, thus network metrics reflect an individual’s score relative to all other members of their group. For groups in which focal sampling was used, edge weight was computed as the amount of time (secs) a pair engaged in grooming relative to the total observation time (hours) for each individual in the dyad [30]. Given the limitations of scan sampling to quantify the duration of behaviors, for group KK we used the number of occurrences of behavioral events instead of using time to compute edge weights. Behavioral events included all social interactions and state behaviors. Specifically, we computed edge weight as the number of times a pair of individuals (A,B) engaged in grooming relative to the total number of behavioral events observed for individual A plus the number of behavioral events for individual B. Our edge weights in KK thus represent how often a pair of individuals were observed grooming relative to how often they were observed in social and other maintenance behaviors. Grooming degree does not consider edge weights in its computation, thus to control for sampling effort in the group where behavioral data was collected by scan sampling (KK), an individual’s grooming degree was divided by the total number of behavioral events recorded for that animal. For groups where animals were sampled with focal observations, sampling was evenly distributed across individuals. Additionally, group differences attributed to sampling methods can be neglected as social integration levels were determined by comparing an individual to others within their group. We considered animals to be part of the group if they were observed for a period of time (or number of occurrences for KK) equal or higher than the mean - 2SD for the group. For statistical analyses, we standardized both measures of social integration within groups by dividing an individual’s value for a given metric by the mean value of that metric for the group.
Fig. 1.
Social networks generated from grooming interactions for all groups included in the study. Females are in blue and males in orange. The lines connecting the nodes represent edge weights, where line thickness indicates the frequency with which a pair of individuals engage in grooming behavior.
2.5. Physiological and anatomical markers of immunity
All procedures related to the removal of animals were conducted by the CPRC following standard protocols approved by the IACUC (Institutional Animal Care and Use Committee). Sedation was performed by the administration of ketamine (100 mg/Kg body mass) using the squeeze cage method [41]. Immediately after, blood was drawn via femoral venipuncture and 4 mL was collected using BD Vacutainer® K2-EDTA collection tubes (approximately 4 mL was collected per animal). A veterinarian then performed euthanasia by administering ketamine (100 mg/Kg) with Xylazine (10 mg/Kg), followed by heparin (100 mg/Kg) and barbiturate (500 mg/Kg). A dual confirmation of death was required before necropsies began, whereby spleen, liver and other organs were extracted and weighed. Time elapsed between when the animals were trapped and when they were euthanized ranged from 1 to 11 days with an average time of 2.3 days. Spleens, livers, and blood samples were collected from each study subject.
Blood cell counts were obtained through a complete blood count (CBC) performed on the VetScan® HM5 analyzer (Abaxis, Inc.). This system uses a combination of chemical differentiation and impedance technology to detect different types of blood cells and compute other relevant blood parameters. We report the absolute count of white blood cells (WBC × 106/mL), red blood cells (RBC × 109/mL) and platelets (PLT × 106/mL). Differentiation between specific types of white blood cells (e. g. monocytes, lymphocytes, neutrophils) was not possible.
Liver and spleen weights were obtained from 128 animals (85 females, 43 males) across the three behavioral groups. CBC data was obtained from 83 individuals (55 females, 28 males) belonging to groups S and KK. Sampling protocols including the collection of blood measures were added after 2016, thus one entire social group (HH), which was removed in 2016, did not have blood measures. Only sixty-nine individuals in total (45 females, 24 males) had both types of data collected. To maximize our sample size and power for each analysis, we ran separate analyses for the organ weights and for the CBC data.
2.6. Statistical analyses
All statistical analyses were done in R version 4.0.3 [42]. To test for associations between social integration and social status with organ weights and with CBC, we opted for a Bayesian approach with Markov chain Monte Carlo simulations (MCMCglmm R package [43]), which allowed us to run multivariate (i.e., multiple-response) linear mixed models. Before fitting our models, we identified outliers for our immunity measures by computing the Mahalanobis distance for each dataset, which helps to identify outliers in more than one dimension [44]. Individuals that were significantly (p < 0.01) isolated in the multivariate space were removed. This resulted in eight animals (3 males, 5 females) being removed from the organ weight data and two females from the CBC data.
To test if social status and social integration predict organ sizes, we ran two bivariate models with spleen and liver size as response variables. Model 1 included grooming degree as measure of social integration and rank as a measure of social status. Model 2 included eigenvector centrality, as a measure of social integration, and rank. We expected organ sizes to vary with body size, thus body mass was added to the models to control for allometry [45]. Additionally, we included a quadratic term for age to control for a possible non-linear relationship between age and our immunity parameters. Behavioral group and sex were also included as fixed effects.
To assess whether social status and social integration influenced activation of the immune system we ran two multivariate models with the absolute count of red blood cells, white blood cells, and platelets as response variables. The model structure was similar to the liver and spleen models, where grooming degree and rank were included as predictors for one model and eigenvector centrality and rank as predictors for the other. In both of these models we controlled for sex, behavioral group, body mass and age by including them as fixed effects.
In all of our models, we included social status and social integration together because their separate effects are not easily distinguishable in rhesus macaques [12,33] and not necessarily correlated (Fig. S3). We included animal identity as a covariate in all the models, to account for the non-independence on the response variables obtained from the same individual (e.g., spleen and liver weights from the same animal). We explored interaction terms between all predictors and retained them only if statistically significant to preserve statistical power. Details for all the models can be found in Table 1.
Table 1.
Specifications for the four multivariate MCMCglmm models.
| Model | Dependent variables | Covariate | Predictors of sociality | Fixed effects |
|---|---|---|---|---|
| Organs size 1 | Spleen, Liver | Animal ID | Degree, Rank | Sex, Group, Body mass, Age, Age2 |
| Organs size 2 | Spleen, Liver | Animal ID | Eigenvector*, Rank | Sex, Group*, Body mass, Age, Age2 |
| Blood cells 1 | RBC, WBC, PTL | Animal ID | Degree, Rank | Sex, Group, Body mass, Age, Age2 |
| Blood cells 2 | RBC, WBC, PTL | Animal ID | Eigenvector, Rank | Sex, Group, Body mass, Age, Age2 |
Interaction term included. Age2: quadratic term for age.
To build our models, first, we z-scored all continuous predictors and response variables in both datasets to improve model fit and to have a direct estimate of effect size from the regression coefficients [46]. Then, we fit multivariate models with Gaussian distributions. We used weakly informative priors for variance components, with degree of belief equal to 2 for random effects fitted to organ weights or 3 for random effects fitted to CBC. Given the absence of repeated measures for the same trait across individuals, the residual (‘within-individual’) variance was not estimated and fixed to 0.0001 for all the models [47]. For all the models we ran 300,000 iterations, dropping the first 2000 iterations and recording every 100th iteration. We assessed the goodness of fit of our models by checking changes in the estimates of fixed effects using different priors, examining the variance component plots, the levels of autocorrelation (< 0.1 for each run) and the effective sample size (> 2000 per variable)[46,47]. To estimate the covariance between response variables, we fit the models by allowing free variation in the estimated variances. We determined the inter-individual correlation between response variables by dividing the corresponding covariance by the product of the square root of their variances [47]. We reported correlation coefficients and credible intervals (CI) for variance components and posterior means with CI for regression estimates.
All density plots were generated using the supplementary R-code shared by Timothee Bonnet [48]. We used the open source software Gephi [49] to plot the social networks and Inkscape v1.0.1 for minor esthetic modifications of the plots.
3. Results
3.1. Sociality and organ sizes
Liver (mean = 206.5 gr, SD = 59.7) and spleen weight (mean = 7.0 gr, SD = 2.4) were positively correlated within individuals (r = 0.4, CI = 0.24 – 0.54). Liver size was positively associated to social status (estimate = 0.18, CI = 0.019 – 0.34) when controlling for age, sex, body mass and group; high ranking animals had bigger livers than low ranking ones. However, this association was only significant in the model that included our direct measure of social integration; the number of grooming social partners (degree) (Table S2). In the main effect model including the indirect social integration measure (eigenvector centrality), the variation explained by rank was not significant (estimate = 0.06, CI = −0.11 – 0.24; Fig. S2A). Instead, indirect social connections had a positive significant relationship with liver size (estimate = 0.19, CI = 0.01 – 0.61), probably due to the moderate correlation between eigenvector centrality and rank (Fig. S3). The only significant interaction term in any of our organ size models was between eigenvector centrality and group, showing that the effect of indirect social connections was not the same across groups. Indirect social connections significantly predicted bigger livers and spleens in group S (Fig. S2B, Table S4). However, a single high-ranking female with an extreme eigenvector centrality drove the effects of eigenvector centrality on liver and spleen sizes (Fig. S4, Tables S7 and S8), and therefore, also the effect of social status on liver size (Table S7).
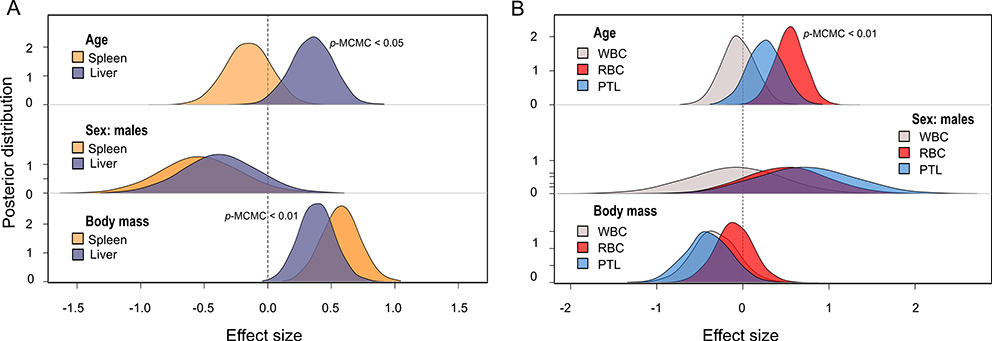
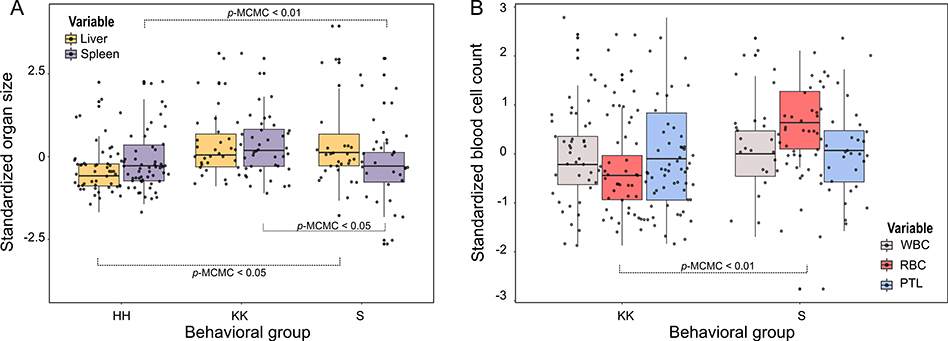
Body mass predicted organ sizes; heavier individuals had bigger spleens (estimate = 0.59, CI = 0.28 – 0.88) and livers (estimate = 0.38, CI = 0.1 – 0.64). Spleen size was not influenced by the age or sex of individuals after controlling for body mass. However, when accounting for body mass, liver size varied with age (estimate = 0.35, CI = 0.02 – 0.67); older monkeys had bigger livers for their body size (Fig. 3A). Differences in organ size attributed to group were significant for both organs; monkeys from group S had bigger livers than monkeys from HH (estimate = 0.45, CI = 0.01 – 0.88), while the spleens of monkeys from S were smaller than those of HH (estimate = −0.67, CI = −1.1 - −0.2) and KK (estimate = −0.73, CI = −1.2 - −0.28; Fig. 4A; Tables S2 and S4).
Fig. 3.
Posterior distributions from the MCMC models for the effect of individual attributes on A) organ sizes and B) blood cells. Statistically significant effects are indicated by p-MCMC values. Sex effect considered females as the intercept. Only results from model 1 are plotted, but results of model 2 are qualitatively similar. WBC: white blood cells, RBC: red blood cells, PTL: platelets.
Fig. 4.
Differences between groups in standardized (z-scored): A) organ sizes and B) blood cells. Statistically significant effects are indicated by p-MCMC values. WBC: white blood cells, RBC: red blood cells, PTL: platelets.
3.2. Sociality and blood measures
There was no significant covariance among red blood cells (mean = 5.1 × 109/mL, SD= 0.68), platelets (mean= 343.2 × 106/mL, SD = 112.2), and white blood cells (mean = 9.86 × 106/mL, SD = 3.04; Table S10). Social integration (degree) was negatively associated with the number of white blood cells after controlling for sex, group, body mass, and age. Individuals with more social partners had fewer WBCs (estimate = −0.27, CI = −0.5 - −0.04; Fig. 2). No interaction terms were significant. We did not find a significant relationship between social integration or social status with other blood cell types. No effect of sex or body mass was detected on blood cells counts (Tables S9 and S11). However, the number of red blood cells was significantly predicted by an individual’s age (estimate = 0.53, CI = 0.21 – 0.87, Fig. 3B) and by the quadratic of age (estimate = −0.005, CI = −0.008 - −0.001). RBC numbers initially increased with age and then declined in older individuals. There were also significant group differences in blood measures; monkeys belonging to group S had more red blood cells than monkeys from KK (estimate = 1.16, CI = 0.73 – 1.58; Fig. 4B).
Fig. 2.
Posterior distributions from the MCMC models for the effect of sociality on blood cells. Statistically significant effects are indicated by p-MCMC values. Sex effect considered females as the intercept. WBC: white blood cells, RBC: red blood cells, PTL: platelets.
4. Discussion
Here we examined the effect of social status and social integration on several markers of immunity and health in a free-ranging population of rhesus macaques. After controlling for age, sex, group membership, and body mass, we found no evidence that either social status or social integration predicted variation in immune organ sizes. However, social integration affected one physiological marker of immune activation, the absolute count of white blood cells. Individuals with more direct social partners had fewer WBCs. Our results add to the growing evidence of a relationship between sociality and health, and demonstrate that social integration can influence the immunity of animals living outside captivity.
Animals with more social partners had lower counts of white blood cells. WBCs are one of the main components of inflammatory processes, thus our results are consistent with current literature in humans and captive animals linking the social environment with inflammatory pathways [12,17]. Our results specifically indicate that social integration could play a role in reducing inflammation markers in animals from this population. Previous studies on humans have shown a positive correlation between WBCs and several pathologies, such as hypertension, insulin resistance, cardiovascular disease and stress [8,50–52], that can eventually be modulated by an individual’s level of social integration [53]. These results together suggest that the low levels of WBCs observed in socially integrated animals may be favorable for the individual’s health, supporting previous findings on the beneficial role of social partners on the longevity of animals from this population [3,33]. Precisely how social integration influences inflammation levels, however, remains an open question. On one side, inflammatory processes due to infection or other diseases can increase social withdrawal as part of what is known as ‘sickness behavior’ [54]. On the other, positive social relationships can reduce stress-induced inflammation via social buffering [55,56]. The lack of effect on other blood measures provide some evidence that neither recent wounds - which would increase platelet count [57]- nor blood borne pathogens - affecting red blood cell count [58] - account for our results. However, given the correlational nature of our data and the limitation of having a single sample per individual, we cannot establish the directionality of the relationship between sociality and WBCs.
No effect of social status on white blood cell count was detected. Several studies have demonstrated a positive relationship between low social status and inflammation levels in humans and animal models [7,9, 12]. The lack of evidence of an effect in our study can be probably attributed to differences in the immune markers examined and the population under analysis. Here, we quantified all types of white blood cells (e.g. monocytes, lymphocytes, neutrophils) in a single category, while other studies have explored specific changes in T-helper lymphocytes, natural killer cells or in acute-phase proteins [7,12,13]. Differences in the immune role of the distinct populations of white blood cells could account for the lack of effect of social status on inflammation. Alternatively, previous studies showing an effect of social status on inflammation have been done in captive animals or WEIRD societies, where individuals have readily available access to medical health care. In such a context it is likely that inflammatory processes are mostly driven by psychosocial stress [11,59], in contrast to more naturalistic settings like Cayo Santiago, where parasites may play an important role and differences in stress susceptibility explained by social status alone were not found [20,37].
We found no evidence that social status or social integration are correlated with immune organ sizes at the population level. Only a single high ranking female showed an effect of indirect social connections on the size of both liver and spleen. Literature linking sociality to these markers of immunity is scarce and inconsistent. In humans, morphological changes in spleen and liver size as a consequence of infection have been described [25,26,60], yet no relationship with sociality has been explored to our knowledge. An association between social status and spleen size was found in a study of captive Brandt’s voles, which was attributed to higher immune function in low ranking animals [61]. Our results in individual rhesus macaques showed no relationship between immune organ sizes and sociality. Although it has been shown that socially-integrated individuals [62] and those of higher social status [63,64] are typically at higher risk of acquiring socially transmitted parasites, we did not find any evidence to support this relationship in the Cayo Santiago population. Alternatively, it is possible that liver and spleen size are not good markers of socially induced changes in immune function.
Individual attributes contributed to the variation in immunity markers in this study. As expected, liver and spleen size correlated positively with an individual’s body mass. The variation of organ size relative to body size has been frequently documented in primates and other mammals [65]. Similarly, we expected that organ size would also be influenced by an individual’s age [66]. Yet, this was only the case for the liver but not the spleen. One possible explanation is that compared to the liver, spleen size may be more susceptible to rapid changes in environmental factors such as parasite exposure [24], while changes in liver size could also reflect differences in metabolic status with age [67]. Despite potential differences in how sensitive to change spleen and liver sizes may be, the size of these organs did covary among individual rhesus macaques, providing some support to their coordinated role in immune function [68,69]. Of all individual attributes examined, only age predicted blood cell count. RBC initially increased with age, followed by a reduction in older animals. Anemia (i.e., reduction in RBC) has been reported previously in elderly humans [70]. Our results may therefore reflect immuno-senescence in the older monkeys of this population.
We also found variation at the group level. Monkeys from group S significantly differed from monkeys from the other two groups, having bigger livers than HH, and smaller spleens than HH and KK. Additionally, S individuals had higher RBC counts in relation to KK monkeys. The interpretation of these differences is not straightforward as they could be related to intrinsic properties of each group, but also to the impact of Hurricane Maria on Cayo Santiago island [71], and consequently, on the social dynamics [19] and/or health of the individuals. While group HH was removed prior to the Hurricane, group KK was sampled 1 year after the storm and group S sampled 2 years after. Differences in the size of the liver in monkeys from this population could therefore reflect a delayed effect of the hurricane on the immune function [23] or metabolism [67] of these animals. However, the smaller spleens of monkeys from group S may also suggest that localized group-level events may account for these findings. In this regard, we have anecdotal information on the social hierarchy between groups. Among the three groups, S seemed to be the highest ranking and HH the lowest, placing individuals from S in a privileged spot for accessing resources that could contribute to differences in nutritional and metabolic status.
In sum, the results of our study provide the first evidence from free-ranging primates of variation in physiological measures of immunity with an individual’s level of social integration. Our findings reinforce prior evidence from humans and captive model organisms showing that one way the social environment can affect health is via the immune system.
Supplementary Material
Acknowledgments
We thank the Caribbean Primate Research Center (CPRC) for the permission to undertake research on Cayo Santiago, along with Daniel Phillips and many interns who assisted in behavioral data collection. Additionally, we thank members of the Brent lab and CRAB for their helpful suggestions, especially Delphine De Moor, Jordan Hart and Andre Pereira.
Funding
This work was supported by CONICYT-Chilean scholarship [number 72190290], NIH grant [number R01AG060931] to N.S-M., L.J.N.B. and J.P.H., NIH grant [number R00AG051764] to N.S-M., NIH grant [number MH118203] to L.J.N.B.. and M.L.P, and NSF grant [number 1800558] to J.P.H. and Susan Anton. The CPRC is supported by the National Institutes of Health. An Animal and Biological Material Resource Center Grant [P40OD012217] was awarded to UPR from the Office of Research Infrastructure Programs (ORIP), and a Research Facilities Construction Grant [C06OD026690] was awarded for the renovation of CPRC facilities after Hurricane Maria.
Footnotes
Ethical statement
This research complied with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Puerto Rico (protocol no. A6850108) and by the University of Exeter School of Psychology’s Ethics Committee. The CPRC’s Animal Care and Use Program is evaluated and approved by the IACUC. Pain and distress are assessed as part of the program. Every protocol used in research, teaching, testing or as part of the daily management of the Center, is evaluated by the IACUC using USDA pain and distress categories.
Declaration of Competing Interest
None.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.physbeh.2021.113560.
Data availability:
R- code used for models and plots available at https://github.com/MPavFox/Sociality-and-Immunity-rhesus.git
References
- [1].Snyder-Mackler N, Burger JR, Gaydosh L, Belsky DW, Noppert GA, Campos FA, Bartolomucci A, Yang YC, Aiello AE, O’Rand A, Harris KM, Shively CA, Alberts SC, Tung J, Social determinants of health and survival in humans and other animals, Science 368 (2020) eaax9553, 10.1126/science.aax9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sapolsky RM, The Influence of Social Hierarchy on Primate Health, Science 308 (2005) 648–652, 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- [3].Ellis S, Snyder-Mackler N, Ruiz-Lambides A, Platt ML, Brent LJN, Deconstructing sociality: the types of social connections that predict longevity in a group-living primate, Proc. R. Soc. B 286 (2019), 20191991, 10.1098/rspb.2019.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Holt-Lunstad J, Smith TB, Layton JB, Social relationships and mortality risk: a meta-analytic review, PLoS Med. 7 (2010), e1000316, 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chancellor RL, Isbell LA, Punishment and competition over food in captive rhesus macaques, Macaca mulatta, Anim. Behav. 75 (2008) 1939–1947, 10.1016/j.anbehav.2007.11.007. [DOI] [Google Scholar]
- [6].Klerman LV, Alive and well?. A Research and Policy Review of Health Programs For Poor Young Children Columbia University, 1991, p. 137 [Online]. Available, https://eric.ed.gov/?id=ED331624. [Google Scholar]
- [7].Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A, Socioeconomic status C -reactive protein, immune factors, and responses to acute mental stress, Brain Behav. Immun. 17 (2003) 286–295, 10.1016/S0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- [8].Black PH, Stress and the inflammatory response: a review of neurogenic inflammation, Brain Behav. Immun. 16 (2002) 622–653, 10.1016/s0889-1591(02,00021-1. [DOI] [PubMed] [Google Scholar]
- [9].Steptoe A, Socioeconomic Status, Inflammation, and Immune Function, Oxford University Press, 2012, pp. 1–40, 10.1093/oxfordhb/9780195394399.013.0013. [DOI] [Google Scholar]
- [10].Dowd JB, Zajacova A, Aiello A, Early origins of health disparities: burden of infection, health, and socioeconomic status in U.S. children, Soc. Sci. Med. 68 (2009) 699–707, 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y, Social environment is associated with gene regulatory variation in the rhesus macaque immune system, Proc. Natl. Acad. Sci. 109 (2012) 6490–6495, 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Snyder-Mackler N, Sanz J, Kohn JN, Brinkworth JF, Morrow S, Shaver AO, Grenier J−C, Pique-Regi R, Johnson ZP, Wilson ME, Barreiro LB, Tung J, Social status alters immune regulation and response to infection in macaques, Science 354 (2016) 1041–1045, 10.1126/science.aah3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paiardini M, Hoffman J, Cervasi B, Ortiz AM, Stroud F, Silvestri G, Wilson ME, T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys, Brain Behav. Immun. 23 (2009) 286–293, 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Theorell T, Blomkvist V, Jonsson H, Schulman S, Berntorp E, Stigendal L, Social support and the development of immune function in human immunodeficiency virus infection, Psychosom. Med. 57 (1995) 32–36, 10.1097/00006842199501000-00005. [DOI] [PubMed] [Google Scholar]
- [15].Cohen S, Brissette I, Skoner D, Doyle W, Social integration and health: the case of the common cold, J. Social Struct. 1 (2000) [Online]. Available, http://www.cmu.edu/joss/content/articles/volume1/cohen.html. [Google Scholar]
- [16].Lutgendorf SK, Russell D, Ullrich P, Harris TB, Wallace R, Religious participation, interleukin-6, and mortality in older adults, Health Psychol. 23 (2004) 465–475, 10.1037/0278-6133.23.5.465. [DOI] [PubMed] [Google Scholar]
- [17].Uchino BN, Trettevik R, Kent de Grey RG, Cronan S, Hogan J, Baucom BRW, Social support, social integration, and inflammatory cytokines: a meta-analysis, Health Psychol. 37 (2018) 462–471, 10.1037/hea0000594. [DOI] [PubMed] [Google Scholar]
- [18].Jaeggi AV, Blackwell AD, von Rueden C, Trumble BC, Stieglitz J, Garcia AR, Kraft T, Beheim BA, Hooper PL, Kaplan H, Gurven MD, Do wealth and inequality associate with health in a small-scale subsistence society?”, eLife 10 (2021) 10.7554/eLife.59437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Testard C, Larson SM, Watowich MM, Kaplinsky CH, Bernau A, Faulder M, Marshall HH, Lehmann J, Ruiz-Lambides A, Higham JP, Montague MJ, Snyder-Mackler N, Platt ML, and Brent LJ, “Rhesus macaques build new social connections after a natural disaster,” Curr. Biol, pp. 1–11, 2021. doi: 10.1016/j.cub.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hoffman CL, Higham JP, Heistermann M, Coe CL, Prendergast BJ, Maestripieri D, Immune function and HPA axis activity in free-ranging rhesus macaques, Physiol. Behav. 104 (2011) 507–514, 10.1016/j.physbeh.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kessler MJ, Rawlins RG, A 75-year pictorial history of the Cayo Santiago rhesus monkey colony, Am. J. Primatol. 78 (2016) 6–43, 10.1002/ajp.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lewis SM, Williams A, Eisenbarth SC, Structure and function of the immune system in the spleen, Sci. Immunol. 4 (2019) eaau6085, 10.1126/sciimmunol.aau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kubes P, Jenne C, Immune Responses in the Liver, Annu. Rev. Immunol. 36 (2018) 247–277, 10.1146/annurev-immunol-051116-052415. [DOI] [PubMed] [Google Scholar]
- [24].Henning LN, Miller SM, Pak DH, Lindsay A, Fisher DA, Barnewall RE, Briscoe CM, Anderson MS, Warren RL, Pathophysiology of the Rhesus Macaque Model for Inhalational Brucellosis, Infect. Immun. 80 (2012) 298–310, 10.1128/IAI.05878-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Terzic D, Brmbollic B, Jevtovic D, Dupanovic B, Korac M, Selemovic D, Svirtlih N, Draskovic N, Mugosa B, Boricic I, Terzic Z, Liver enlargement associated with opportunistic infections in patients with human immunodeficiency virus infection, J. Gastroint. Liver Dis. 17 (2008) 401–404 [Online]. Available: https://europepmc.org/article/med/19104700. [PubMed] [Google Scholar]
- [26].Wilson S, Vennervald BJ, Dunne DW, Chronic hepatosplenomegaly in African school children: a common but neglected morbidity associated with schistosomiasis and malaria, PLoS Negl. Trop. Dis. 5 (2011) e1149, 10.1371/journal.pntd.0001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Merino KM, Slisarenko N, Taylor JM, Falkenstein KP, Gilbert MH, Bohm RP, Blanchard JL, Ardeshir A, Didier ES, Kim W−K, Kuroda MJ, Clinical and immunological metrics during pediatric rhesus macaque development, Front. Pediatr. 8 (2020) 1–26, 10.3389/fped.2020.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hernandez-Pacheco R, Delgado DL, Rawlins RG, Kessler MJ, Ruiz-Lambides AV, Maldonado E, Sabat AM, Managing the Cayo Santiago rhesus macaque population: the role of density, Am. J. Primatol. 78 (2016) 167–181, 10.1002/ajp.22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Altmann J, Observational study of behavior: sampling methods, Behaviour 49 (1974) 227–266, 10.1163/156853974X00534. [DOI] [PubMed] [Google Scholar]
- [30].Brent LJN, MacLarnon A, Platt ML, Semple S, Seasonal changes in the structure of rhesus macaque social networks, Behav. Ecol. Sociobiol. 67 (2013) 349–359, 10.1007/s00265-012-1455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kulik L, Amici F, Langos D, Widdig A, Sex Differences in the development of social relationships in Rhesus Macaques (Macaca mulatta, Int. J. Primatol. 36 (2015) 353–376, 10.1007/s10764-015-9826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].MacIntosh AJJ, Jacobs A, Garcia C, Shimizu K, Mouri K, Huffman MA, Hernandez AD, Monkeys in the middle: parasite transmission through the social network of a wild primate, PLoS ONE 7 (2012) e51144, 10.1371/journal.pone.0051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brent LJN, Ruiz-Lambides A, Platt ML, Family network size and survival across the lifespan of female macaques, Proc. R. Soc. B 284 (2017), 20170515, 10.1098/rspb.2017.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vandeleest JJ, Winkler SL, Beisner BA, Hannibal DL, Atwill ER, McCowan B, Sex differences in the impact of social status on hair cortisol concentrations in rhesus monkeys (Macaca mulatta, Am. J. Primatol. 82 (1) (2020) 1–10, 10.1002/ajp.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chikazawa D, Gordon TP, Bean CA, Bernstein IS, Mother-daughter dominance reversals in rhesus monkeys (Macaca mulatta, Primates 20 (1979) 301–305, 10.1007/BF02373382. [DOI] [Google Scholar]
- [36].Manson JH, Do female rhesus macaques choose novel males? Am. J. Primatol. 37 (1995) 285–296, 10.1002/ajp.1350370403. [DOI] [PubMed] [Google Scholar]
- [37].Brent L, Semple S, Dubuc C, Heistermann M, MacLarnon A, Social capital and physiological stress levels in free-ranging adult female rhesus macaques, Physiol. Behav. 102 (2011) 76–83, 10.1016/j.physbeh.2010.09.022. [DOI] [PubMed] [Google Scholar]
- [38].Madlon-Kay S, Brent L, Montague M, Heller K, Platt M, Using machine learning to discover latent social phenotypes in free-ranging macaques, Brain Sci. 7 (2017) 91, 10.3390/brainsci7070091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brent LJ, Friends of friends: are indirect connections in social networks important to animal behaviour? Anim. Behav. 103 (2015) 211–222, 10.1016/j.anbehav.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Csardi G, Nepusz T, The igraph software package for complex network research, InterJournal Complex Systems (2006) 1695 [Online]. Available, http://igraph.org. [Google Scholar]
- [41].Wienker WR, Giraffe squeeze cage procedure, Zoo Biol. 5 (1986) 371–377, 10.1002/zoo.1430050408. [DOI] [Google Scholar]
- [42].R Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing,, Vienna, Austria, 2020. [Online]. Available, http://www.R-project.org/. [Google Scholar]
- [43].Hadfield JD, MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package, J. Stat. Softw. 33 (2010) 1–22, 10.18637/jss.v033.i02.20808728 [DOI] [Google Scholar]
- [44].Filzmoser P, Ruiz-Gazen A, Thomas-Agnan C, Identification of local multivariate outliers, Stat. Pap. 55 (2014) 29–47, 10.1007/s00362-013-0524-z. [DOI] [Google Scholar]
- [45].Nakagawa S, Kar F, O’Dea RE, Pick JL, Lagisz M, Divide and conquer? Size adjustment with allometry and intermediate outcomes, BMC Biol. 15 (2017) 107, 10.1186/s12915-017-0448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hadfield J, “MCMCglmm Course Notes,” Tech. Rep, 2019. [Online]. Available: http://cran.us.r-project.org/web/packages/MCMCglmm/vignettes/CourseNotes.pdf. [Google Scholar]
- [47].Houslay TM, Wilson AJ, Avoiding the misuse of BLUP in behavioural ecology, Behav. Ecol. 28 (2017) 948–952, 10.1093/beheco/arx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bonnet T, Morrissey MB, Morris A, Morris S, Clutton-Brock TH, Pemberton JM, Kruuk LEB, The role of selection and evolution in changing parturition date in a red deer population, PLoS Biol. 17 (2019), e3000493, 10.1371/journal.pbio.3000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bastian M, Heymann S, and Jacomy M, “Gephi: an open source software for exploring and manipulating networks,” Icwsm, pp. 361–362, 2009. [Google Scholar]
- [50].Karthikeyan VJ, Lip GYH, White blood cell count and hypertension, J. Hum. Hypertens 20 (2006) 310–312, 10.1038/sj.jhh.1001980. [DOI] [PubMed] [Google Scholar]
- [51].Nakanishi N, Yoshida H, Matsuo Y, Suzuki K, Tatara K, White blood-cell count and the risk of impaired fasting glucose or Type II diabetes in middle-aged Japanese men, Diabetologia 45 (2002) 42–48, 10.1007/s125-002-8243-1. [DOI] [PubMed] [Google Scholar]
- [52].Sabatine MS, Morrow DA, Cannon CP, Murphy SA, Demopoulos LA, DiBattiste PM, McCabe CH, Braunwald E, Gibson C, Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes, J. Am. Coll. Cardiol. 40 (2002) 1761–1768, 10.1016/S0735-1097(02)02484-1. [DOI] [PubMed] [Google Scholar]
- [53].Yang YC, Boen C, Gerken K, Li T, Schorpp K, Harris KM, Social relationships and physiological determinants of longevity across the human life span, Proc. Natl. Acad. Sci. 113 (2016) 578–583, 10.1073/pnas.1511085112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, From inflammation to sickness and depression: when the immune system subjugates the brain, Nat. Rev. Neurosci. 9 (2008) 46–56, 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mezuk B, Diez AV, Seeman T, Brain, behavior, and immunity evaluating the buffering vs. direct effects hypotheses of emotional social support on inflammatory markers: the multi-ethnic study of atherosclerosis, Brain Behav. Immun. 24 (2010), 10.1016/j.bbi.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Uchino BN, Social Support and Physical Health: Understanding the Health Consequences of Relationships, Yale University Press, New Haven: CT, 2004. [Google Scholar]
- [57].Eisinger F, Patzelt J, Langer HF, The platelet response to tissue injury, Front. Med. 5 (2018) 1–15, 10.3389/fmed.2018.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McCullough J, RBCs as targets of infection, Hematology 2014 (2014) 404–409, 10.1182/asheducation-2014.1.404. [DOI] [PubMed] [Google Scholar]
- [59].Snyder-Mackler N, Sanz J, Kohn JN, Voyles T, Pique-Regi R, Wilson ME, Barreiro LB, Tung J, Social status alters chromatin accessibility and the gene regulatory response to glucocorticoid stimulation in rhesus macaques, Proc. Natl. Acad. Sci. 116 (2019) 1219–1228, 10.1073/pnas.1811758115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Talwani R, Gilliam BL, Howell C, Infectious diseases and the liver, Clin. Liver Dis. 15 (2011) 111–130, 10.1016/j.cld.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Li F−H, Zhong W−Q, Wang Z, Wang D−H, Rank in a food competition test and humoral immune functions in male Brandt’s voles (Lasiopodomys brandtii), Physiol. Behav. 90 (2007) 490–495, 10.1016/j.physbeh.2006.10.009, issn: 00319384. [DOI] [PubMed] [Google Scholar]
- [62].Balasubramaniam KN, Beisner BA, Hubbard JA, Vandeleest JJ, Atwill ER, McCowan B, Affiliation and disease risk: social networks mediate gut microbial transmission among rhesus macaques, Anim. Behav. 151 (2019) 131–143, 10.1016/j.anbehav.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Habig B, Archie EA, Social status, immune response and parasitism in males: a metaanalysis, Philos. Trans. R. Soc. B 370 (2015), 20140109, 10.1098/rstb.2014.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Habig B, Doellman MM, Woods K, Olansen J, Archie EA, Social status and parasitism in male and female vertebrates: a meta-analysis, Sci Rep 8 (2018) 3629, 10.1038/s41598-018-21994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Stahl WR, Organ weights in primates and other mammals, Science 150 (1965) 1039–1042, 10.1126/science.150.3699.1039. [DOI] [PubMed] [Google Scholar]
- [66].He Q, Heshka S, Albu J, Boxt L, Krasnow N, Elia M, Gallagher D, Smaller organ mass with greater age, except for heart, J Appl. Physiol. 106 (2009) 1780–1784, 10.1152/japplphysiol.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Trefts E, Gannon M, Wasserman DH, The liver, Curr. Biol. 27 (2017) R1147–R1151, 10.1016/j.cub.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lang K, Lang P, Balancing viral replication in spleen and liver determines the outcome of systemic virus infection, Z. Gastroenterol. 53 (2015) 1432–1435, 10.1055/s-0041-109631. [DOI] [PubMed] [Google Scholar]
- [69].Conlan JW, Early host-pathogen interactions in the liver and spleen during systemic murine listeriosis: an overview, Immunobiology 201 (1999) 178–187, 10.1016/S0171-2985(99)80057-6. [DOI] [PubMed] [Google Scholar]
- [70].Halawi R, Moukhadder H, Taher A, Anemia in the elderly: a consequence of aging? Expert Rev. Hematol. 10 (2017) 327–335, 10.1080/17474086.2017.1285695. [DOI] [PubMed] [Google Scholar]
- [71].Morcillo DO, Steiner UK, Grayson KL, Ruiz-Lambides AV, HernándezPacheco R, Hurricane-induced demographic changes in a non-human primate population: demographic effects of hurricanes, R. Soc. Open Sci 7 (2020), 10.1098/rsos.200173rsos200173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
R- code used for models and plots available at https://github.com/MPavFox/Sociality-and-Immunity-rhesus.git