Abstract
Helicobacter pylori (H. pylori) is a gram-negative bacterium that adapts to the gastric mucosa and provokes symptoms associated with gastritis. Chronic H. pylori infection in patients with a genetic predisposition can trigger autoimmune diseases due to the immune interaction of cellular and humoral responses. Infections are a triggering factor for systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren syndrome (SS), although the association between H. pylori and these diseases is unclear. Therefore, we reviewed this interaction and its clinical importance.
Keywords: Helicobacter pylori, Systemic lupus erythematosus, Rheumatoid arthritis, Sjögren, Syndrome, Infection
Highlights
-
•
Infections have been recognized as a triggering or exacerbating factor for autoimmune diseases.
-
•
Helicobacter pylori can generate autoimmunity through molecular mimicry, leading to the generation of autoantibodies.
-
•
There is a controversial relationship between Helicobacter pylori and some autoimmune diseases.
-
•
Clinical implications of Helicobacter pylori in autoimmune diseases should be investigated in large and longitudinal analysis.
1. Introduction
Helicobacter pylori (H.pylori) is a spiral-shaped gram-negative bacterium that can adapt to the acidic conditions of the human gastric mucosa. Currently, more than 4.4 billion individuals are infected, and the prevalence by country fluctuates from 18.9% to 87.7% [1].
Colonization of the gastric mucosa by H. pylori is associated with chronic active gastritis, chronic atrophic gastritis, peptic ulceration, gastric adenocarcinoma, and mucosa-associated lymphoid tissue (MALT) gastric lymphoma [2,3]. Recent studies suggest that some systemic diseases are related to H. pylori infection; these include iron deficiency anemia [4], vitamin B12 deficiency [5], non-alcoholic fatty liver disease [6], subclinical coronary atherosclerosis [7], insulin resistance, and metabolic syndrome [8]. Several studies describe a link between H. pylori infection and autoimmune disease [9].
H. pylori infections are of concern for patients with autoimmune diseases for several reasons. First, autoimmune diseases are related to low immune competence due to disease manifestations or pharmacologic treatment; thus, H. pylori infection risk increases [10]. Secondly, the side effects of non-steroidal anti-inflammatory drugs (NSAID) may cause additive damage [11]. Third, immune suppression increases the risk of neoplasm, including gastric cancer [12].
This paper reviews the evidence on these crucial pathogenic aspects related to systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren syndrome (SS).
2. Helicobater pylori and autoimmunity
Chronic infections constantly stimulate the immune system, which may generate an autoimmune environment in patients with a genetic predisposition [13]; chronic H. pylori infection could participate in the etiopathogenesis and maintenance of inflammatory activity in some autoimmune diseases [14,15].
One of the first trials of H. pylori in systemic autoimmune diseases was published in 1995 by Showji et al. [16]. They reported that patients with SS had a higher prevalence of serum antibodies against H. pylori than people without autoimmune diseases. Ram et al. [17] found that serum levels of IgG against H. pylori were more prevalent in patients with anti-phospholipid syndrome, giant cell arteritis, systemic sclerosis, and primary biliary cirrhosis, and anti-H. pylori antibodies were associated with a higher prevalence of anti-dsDNA and anti-Ro/SSA antibodies.
A link between H. pylori and autoimmune diseases such as immune thrombocytopenic purpura [18], IgA nephropathy [19], Devic disease [20], autoimmune pancreatitis [21], and autoimmune thyroid disease has been reported [22]. Moreover, H. pylori-related lymphomas of the gastrointestinal tract are associated with autoimmune diseases [23].
In contrast, evidence suggests H. pylori is a protective agent against disorders like asthma [24], allergic airway inflammation [25], and multiple sclerosis [26].
2.1. Pathways between Helicobacter pylori and autoimmunity
Genetic, epigenetic, and environmental factors such as infections lead to loss of immunological tolerance [27,28]. Molecular mimicry, bystander activation, epitope spreading and polyclonal activation are related to immune dysregulation in the presence of H. pylori infection [29]. Amedei et al. [30] showed that molecular mimicry produced by H. pylori antigens can activate self-reactive T lymphocytes by cross-immunity, and Yamanishi et al. [31] demonstrated that activation of B-1 cells mediated by H. pylori urease leads to the generation of autoreactive antibodies.
H. pylori may generate immune complexes [32] due to activation of cellular and humoral immune responses [33] since it increases the production of INF-gamma (Th1-mediated cellular response) [30,34], and the production of IgM and IgG3 antibodies. In vitro studies showed that B cells chronically stimulated with urease produced by H. pylori had the potential to generate autoantibodies such as IgM rheumatoid factor [35,36]. These mechanisms may cause loss of cell tolerance and the generation of more autoantibodies, such as anti-dsDNA and anti-phospholipids [37].
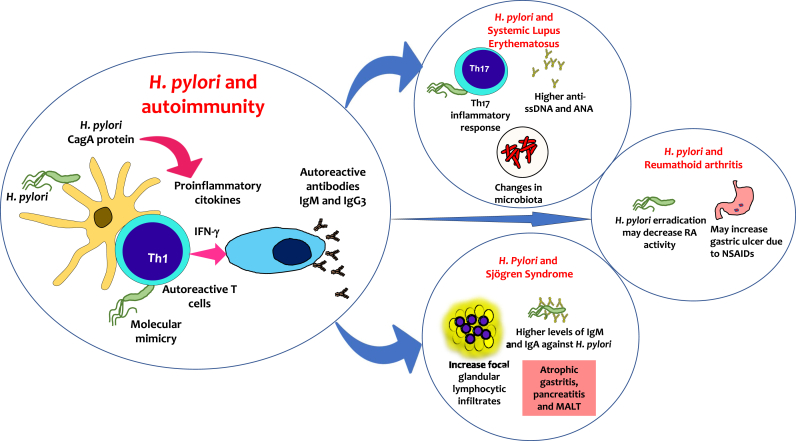
H. pylori strain coding for cytotoxin-associated gene A (CagA) has an enhanced capability to stimulate the secretion of pro-inflammatory cytokines, generating tissue injury, polarity, and host cell proliferation, leading to modulation of host immune responses [38] (Fig. 1).
Fig. 1.
Potential association of Helicobacter pylori and autoimmune diseases. H. pylori stimulates the secretion of pro-inflammatory cytokines and may generate immune complexes since it increases the production of INF-γ. These mechanisms may cause loss of cell tolerance and the generation of autoantibodies, such as anti-single-stranded DNA (ssDNA) and anti-nuclear antibodies (ANA).
In contrast, there is evidence of a symbiotic relationship between humans and H. pylori as a protective factor against chronic immune-mediated disorders [39]. H. pylori can induce a tolerogenic state in dendritic cells and stimulate the generation of regulatory T lymphocytes [[40], [41], [42]].
3. Helicobacter pylori and systemic lupus erythematosus
Systemic lupus erythematosus is a chronic disease characterized by the production of autoantibodies that generate tissue damage [43]. Infections are a trigger for SLE, and disease activity is linked to Mycoplasma spp. [44], human papillomavirus [45] and H. pylori [46]. The main microorganisms associated with SLE development are Epstein-Barr virus, parvovirus B 19, human T-lymphotropic virus-1, and endogenous retroviruses [47]. Bacteria are also probable causal microorganisms of SLE; the most studied are Vibrio cholerae and H. pylori [48].
3.1. Epidemiological relationship between Helicobacter pylori and SLE
Studies of the epidemiological relationship between SLE and H. pylori have yielded contradictory results. For example, Sawalha et al. [49] showed that African-American women with SLE are less frequently seropositive for H. pylori than controls, and H. pylori infection was associated with a later SLE onset. However, Showji et al. [16] reported that anti-H. pylori antibody concentrations in SLE Japanese patients are similar to those in healthy controls and even lower than in other connective tissue diseases such as SS.
These results contrast with a nationwide cohort study in Taiwan by Wu et al. [50], who reported that the incidence of SLE is higher in the H. pylori-infected population compared with controls (1.17 vs. 0.72 per 100,000 person-months) and H. pylori infection increases 1.63 times the risk of SLE, especially in women aged <30 years.
A subsequent study conducted by Wu et al. [51] showed that eradication of H. pylori within three months of diagnosis decreased the risk of SLE (aHR = 0.16, p = 0.0013) in the first three years of follow-up. This suggests that more prolonged exposure to H. pylori confers an increased risk of SLE. Nevertheless, the time to the start of eradication therapy did not significantly influence the long-term SLE risk [51].
Youssefi et al. [52] conducted a recent meta-analysis to resolve this issue; they found no significant relationship between SLE and H. pylori infection (OR: 0.97; 95%CI: 0.76–1.23; p-value: 0.82). However, there is an association between H. pylori CagA positive strains and autoimmune diseases such as autoimmune gastritis and SLE (ORs: 2.65 with 95% CI, p-value: 0.001) [52].
3.2. Helicobacter pylori and SLE-related markers
In FcγRIIb−/− mice, a polymorphism associated with SLE development, H. pylori infection was associated with increased SLE severity, higher levels of anti-H. pylori and anti-dsDNA antibodies, and increased production of splenic autoimmune cells compared with wild-type mice [53]. Urease induced the production of anti-ssDNA antibodies in animal models [31].
Additionally, Ram et al. [17] found a higher prevalence of anti-dsDNA antibodies in a population with different autoimmune diseases and seropositive for H. pylori (21 vs. 16.2%, p < 0.05). At the same time, the presence of anti-dsDNA was related to a higher concentration of anti-H. pylori antibodies (1.9 vs. 1.7, p < 0.05). Despite this, no significant relationship was found between SLE and anti-H. pylori antibodies. However, even in healthy adults, a positive association was found between higher concentrations of anti-ANA antibodies and H. pylori seropositivity, despite other factors such as age, sex, and race [54].
3.3. Medication and gastric lesions in SLE patients
Reshetnyak et al. [55] found significant differences in the detection rate of H. pylori with respect to the drugs used to treat SLE; the rate is higher with anticoagulant therapy (P = 0.038; OR = 2.96; 95% CI, 1.01–8.68) and low-dose acetylsalicylic acid (P = 0.031; OR = 3.58; 95% CI, 1.05–12.17) than for glucocorticoid and NSAID users. Nevertheless, Mendoza-Pinto et al. [46] reported that immunosuppressive or glucocorticoid therapy did not increase the prevalence of H. pylori infection in patients with SLE.
Furthermore, there is no higher prevalence of gastroduodenal mucosal lesions or reflux disease in SLE patients with H. pylori infection than in the general population [46,55].
3.4. Potential links between Helicobacter pylori and SLE
Multiple studies have been conducted on the relationship between the alteration of the intestinal microbiota and autoimmune diseases such as SLE [56,57] through ways such as the stimulation of regulatory B cells [58], enhanced exposure to extracellular nuclear autoantigens, molecular mimetism [59], and the translocation of gut pathobiont to systemic organs in SLE-prone hosts [60]. H. pylori may play a role in this because it produces changes in the gut microbiota through hypochlorhydria, hypergastrinemia, and by the action of the CagA factor [61]; in addition, H. pylori eradication treatment affects bacterial diversity [62]. However, to the best of our knowledge, there are no studies on the relationship between H. pylori, dysbiosis, and SLE.
H. pylori infection induces a systemic Th-17 inflammatory response, which plays an essential role in the pathophysiology of SLE [63,64]. Moreover, H. pylori can increase the expression of ETS1, through the CagA-activated NF-κB pathway [65], a negative regulatory transcription factor for Th17 cell and B cell differentiation involved in the pathogenesis of SLE [66]. Therefore, further investigations are required to comprehend the role of H. pylori in these pathways and the risk of SLE.
In sum, the few reports on the relationships between H. pylori and SLE have contradictory results. However, there is evidence that H. pylori is a dynamic factor with two possible roles: a triggering and protective factor; depending on race, age and the organs affected. It is necessary to consider H. pylori in the comprehensive approach to patients with SLE, mainly because the clinical and immunological implications are yet to be elucidated.
4. Helicobacter pylori and rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic synovial membrane inflammation damage to articular cartilage and juxta-articular bone [67]. Microorganisms such as Porphyromonas gingivalis, gut microbiota, and the herpes simplex virus are associated with the etiopathogenesis of RA [68]; in addition, there are antecedents that H. pylori eradication worsens RA activity [69]. However, the association between H. pylori infection and RA is not well-understood [35,70].
4.1. Epidemiological relationship between Helicobacter pylori and RA
Recently, Bartels et al. [27] studied 56,000 patients diagnosed as H. pylori-positive or H. pylori-negative for a median of 8 years, both groups with similar comorbidities. No relationship between H. pylori and RA was found, and there was no difference in the prevalence of RA.
Youssefi et al. [29] found no significant association between H. pylori infection and RA (OR 1.18; 95% CI: 0.91–1.52, p-value: 0.19), and the authors concluded that H. pylori infection had no significant effect on RA pathogenesis [29]. Meron et al. [71] found no significant differences in the rates of antibodies against H. pylori between RA patients and healthy controls.
4.2. Helicobacter pylori and RA-related markers
Zentilin et al. [72] evaluated the effects of H. pylori eradication on disease activity in RA patients after four months of follow-up. They found that the resolution of H. pylori infection reduced the chronic inflammatory stimulus, improving both serological and clinical abnormalities. Similarly, Seriolo et al. [73] found that patients with eradicated H. pylori infection significantly improved clinical and laboratory findings compared with H. pylori-negative and positive (unresponsive) patients. The authors suggested that H. pylori infection was associated with the pathogenesis of RA, and H. pylori eradication may induce a significant improvement in disease activity over 24 months [74].
Ebrahimi et al. [38] investigated the relationship between H. pylori infection and clinical outcomes in RA patients. They measured anti-H. pylori IgG antibodies, fecal H. pylori antigen, and CagA protein, and found that several serum inflammatory biomarkers were significantly higher in H. pylori-positive patients than in negative patients, and in CagA positive patients than in CagA negative patients. However, they found no differences in the DAS-28 score regarding H. pylori status, although the DAS-28 score and VAS were significantly higher in CagA positive patients than in CagA negative patients.
In contrast, Steen et al. [75] demonstrated a limited and transient effect of H. pylori eradication therapy on the lipid profile and no impact on C-reactive protein concentrations in patients with rheumatic diseases on chronic NSAID treatment.
4.3. Medication and gastric lesions in RA patients
Almost three decades ago, Janssen et al. [76] reported that RA patients receiving intramuscular gold had decreased H. pylori seropositivity and hypothesized that intramuscular gold could be a protective factor against peptic ulcer disease (PUD). However, Wolde et al. [77] and Paimela et al. [78] showed that gold therapy in RA patients does not influence the serological parameters of H. pylori infection.
Later, Grigoriadou et al. [79] studied a possible role of NSAIDs and colonization of the gastric antrum with H. pylori in the development of PUD in RA patients and found that NSAID increased the relative risk (RR) of ulceration (RR 8.67 (1.19–62.87)), while the presence of H. pylori is associated with ulcers in RA patients (RR 3.71 (0.37–37.35)). The RR for the combination of NSAID consumption and H. pylori colonization was 14.44 (2.05–101). The authors concluded that H. pylori infection increased the risk of NSAID-induced ulceration [79].
Moriyama et al. [80] showed that H. pylori infection was not associated with gastroduodenal lesions or disease activity in RA patients under long-term NSAID treatment. In addition, spontaneous remission of H. pylori infection in RA patients was documented. The authors concluded that routine H. pylori eradication might not be necessary for RA patients under long-term NSAID treatment.
Lin et al. [39] conducted a study based on 79,181 patients classified as having PUD and treatment against H. pylori infection (PUD + HPRx), patients with PUD without treatment against H. pylori (PUD–HPRx), and patients without PUD (controls), and compared the effects of treatment for H. pylori infection on the risk of autoimmunity. They found that PUD + HPRx had the highest adjusted hazard risk (aHR) for autoimmunity, including RA (aHR, 2.44; 95% CI, 2.01–2.95; P < 0.001). The authors hypothesized that resident gut microbes play a role in modulating self-susceptibility to systemic immune-mediated disorders, but more research is needed.
In conclusion, H. pylori is considered one of the infectious agents linked to RA, but the association remains unclear.
5. Helicobacter pylori infection and Sjögren's syndrome
Sjögren's syndrome (SS) is a systemic autoimmune disease characterized by sicca syndrome due to lymphoplasmacytic infiltration of the exocrine glands [81]. Infections are considered a risk factor for SS, especially viral infections, such as those caused by the Epstein-Barr virus (EBV), cytomegalovirus (CMV), hepatitis C (HCV), coxsackievirus and human T-lymphocyte virus type I (HTLV-1) [82]. Additionally, bacteria have been proposed to be involved in the pathogenesis of SS, including commensal bacteria, mycobacteria, and H. pylori [87].
5.1. Epidemiological relationship between Helicobacter pylori and SS
H. pylori infection triggers an immune response against bacterial antigens, including the heat-shock protein of 60 kDa (HSP60), which has homology to a human protein, partially explaining the relationship between H. pylori and autoimmune diseases [83].
In a study that included 118 persons divided into four groups (primary SS, secondary SS, other autoimmune diseases, and healthy controls), an increase in the prevalence of serum antibodies against H. pylori and HSP60 was in primary SS patients compared with the rest of the groups [83].
Showji et al. [84] found that SS patients had much higher serum IgG anti-H. pylori titers than patients with chronic lung disease, age-matched controls, and those with other connective tissue diseases.
Saghafi et al. [85] compared serum IgM and IgA anti-H. pylori antibodies levels in 43 SS patients and 95 healthy controls, these antibodies were found to be significantly higher in SS patients (34.9% vs. 10.5% %, p = 0.001). El Miedany et al. [86] also showed that the prevalence of H. pylori is higher in groups with SS than other connective tissue diseases and healthy controls. Caporali R et al. [87] evaluated the presence of H. pylori antibodies in Italian patients with anti-Ro positivity antibodies. They found a higher prevalence of H. pylori antibodies in confirmed SS patients compared with those without SS (Odds ratio OR- 15.67, 95% CI:4.5–54.8, P-value < 0.001).
Chen et al. [88] in a meta-analysis of nine studies with 1958 participants, including 619 patients with SS, found that patients with primary SS had a small but significantly-higher rate (63.6% vs. 49.3%) of H. pylori infection than controls (OR = 1.19, 95% CI: 1.01–1.41, P = 0.033), and a significantly-higher H. pylori infection rate in patients with primary SS than controls (OR = 1.24, 95% CI: 1.03–1.50, P = 0.026). Of the nine studies included, seven evaluated positivity for H. pylori through serum and two through tissue specimens [89]. In addition, Banno et al. [90] demonstrated a highly positive association between H. pylori infection and SS (OR = 2.33).
Studies have linked H. pylori and SS with the development of multiple diseases, such as MALT lymphoma [[91], [92], [93], [94], [95]] and autoimmune pancreatitis [96,97], which could be incidental clinical findings, although they may share pathogenic molecular pathways such as the expression of CXCL13 and its CXCR5 receptor [98,99]. For example, in a study conducted in 9 SS patients with gastric MALT lymphoma, MALT lymphoma translocation 1 gene (MALT1) rearrangement was present in 78% of cases, and MALT1 is associated with resistance to H. pylori eradication therapy [100].
5.2. Helicobacter pylori and SS-related markers
H. pylori infection is also associated with SS due to shared histological findings, such as the destruction of exocrine glands, lymphocytic infiltration, and activation of CD8 cells [101,102].
Irani et al. [88] demonstrated through immunohistochemical tissue stuides a higher presence of H. pylori in patients with inflammatory oral mucosa lesions than oral mucosa from healthy patients. Moreover, H. pylori possibly interacts with surface of epithelial cells, developing direct cell damage or producing pro-inflammatory mediators [103].
Theander et al. [103] found that H. pylori seropositivity was not associated with the presence of immunological markers of SS such as circulating autoantibodies or a lip biopsy with abnormal focus score. However, Caporali et al. [87] found a significant association between seropositivity for H. pylori and focal glandular lymphocytic infiltrates (OR 14.17, 95% CI 4.1–48.7, P-value < 0.001).
5.3. Medication and gastric lesions in RA patients
Collin et al. [104] evaluated the frequency of gastritis in patients with SS compared with controls, finding a higher rate of atrophic antral gastritis in SS patients but with no difference in the prevalence of H. pylori. However, Banno et al. [90] showed that atrophic gastritis in SS patients may occur due to H. pylori infection.
In contrast, Sorrentino et al. [105] found no differences in the prevalence of IgG anti-H. pylori in patients with dyspepsia with SS (57%) or without SS (62%) or in anti-CagA antibodies among patients with SS and the control group, so SS was not associated with more virulent strains.
Small series and non-randomized trials show conflicting results regarding the benefit of H. pylori eradication in autoimmune diseases, including SS [106]. Lin et al. [39] found that patients with PUD and treatment of H. pylori infection had an aHR (3.15; 95% CI, 2.57–3.87; P < 0.001) for SS compared with patients with PUD without H. pylori infection treatment.
6. Conclusion
Although infectious agents are essential triggers in both the induction and perpetuation of autoimmunity, the role of H. pylori infection in this process remains unclear, so further research is required to evaluate this association and its clinical significance, with a focus on when H. pylori eradication should be recommended in patients with an autoimmune disease or a high risk of developing them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank David Buss for technical assistance and Jose Luis Galvez-Romero for artwork.
Contributor Information
Ivet Etchegaray-Morales, Email: ivetcheg@gmail.com.
Erick Alejandro Jiménez-Herrera, Email: alejimenez_2790@hotmail.com.
Claudia Mendoza-Pinto, Email: cmp_26@yahoo.com.mx.
Adriana Rojas-Villarraga, Email: sarojas@fucsalud.edu.co.
Salvador Macías-Díaz, Email: drsalvadormd@gmail.com.
Ángel David Osorio-Peña, Email: angeldav37@gmail.com.
Pamela Munguía-Realpozo, Email: pamela.munguia@yahoo.com.mx.
Mario García-Carrasco, Email: mgc30591@yahoo.com.
References
- 1.Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., Chan F.K.L., Sung J.J.Y., Kaplan G.G., Ng S.C. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Abadi A.T.B. Strategies used by helicobacter pylori to establish persistent infection. World J. Gastroenterol. 2017;23:2870–2882. doi: 10.3748/wjg.v23.i16.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravina A.G., Zagari R.M., De Musis C., Romano L., Loguercio C., Romano M. Helicobacter pylori and extragastric diseases: a review. World J. Gastroenterol. 2018;24:3204–3221. doi: 10.3748/wjg.v24.i29.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudak L., Jaraisy A., Haj S., Muhsen K. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter. 2017;22 doi: 10.1111/hel.12330. [DOI] [PubMed] [Google Scholar]
- 5.Yang G.-T., Zhao H.-Y., Kong Y., Sun N.-N., Dong A.-Q. Correlation between serum vitamin B12 level and peripheral neuropathy in atrophic gastritis. World J. Gastroenterol. 2018;24:1343–1352. doi: 10.3748/wjg.v24.i12.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wijarnpreecha K., Thongprayoon C., Panjawatanan P., Manatsathit W., Jaruvongvanich V., Ungprasert P. Helicobacter pylori and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. J. Clin. Gastroenterol. 2018;52:386–391. doi: 10.1097/MCG.0000000000000784. [DOI] [PubMed] [Google Scholar]
- 7.Lee M., Baek H., Park J.S., Kim S., Kyung C., Baik S.J., Lee B.K., Kim J.-H., Ahn C.W., Kim K.R., Kang S. Current Helicobacter pylori infection is significantly associated with subclinical coronary atherosclerosis in healthy subjects: a cross-sectional study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsay F.-W., Hsu P.-I. Pylori infection and extra-gastroduodenal diseases. J. Biomed. Sci. 2018;25:65. doi: 10.1186/s12929-018-0469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyk D.S., Koutsoumpas A.L., Mytilinaiou M.G., Rigopoulou E.I., Sakkas L.I., Bogdanos D.P. Helicobacter pylori and autoimmune disease: cause or bystander. World J. Gastroenterol. 2014;20 doi: 10.3748/wjg.v20.i3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reshetnyak T.M., Doroshkevich I.A., Seredavkina N.V., Nasonov E.L., Maev I.V., Reshetnyak V.I. The contribution of drugs and Helicobacter pylori to gastric mucosa changes in patients with systemic lupus erythematosus and antiphospholipid syndrome. Int. J. Rheumatol. 2019 doi: 10.1155/2019/9698086. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford A.C., Mahadeva S., Carbone M.F., Lacy B.E., Talley N.J. Functional dyspepsia. Lancet (Lond., Engl.) 2020;396:1689–1702. doi: 10.1016/S0140-6736(20)30469-4. [DOI] [PubMed] [Google Scholar]
- 12.Song M., Latorre G., Ivanovic-Zuvic D., Camargo M.C., Rabkin C.S. Cancer Research and Treatment; 2019. Autoimmune Diseases and Gastric Cancer Risk: A Systematic Review and Meta-Analysis; p. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamriz O., Shoenfeld Y. Infections: a double-edge sword in autoimmunity. Curr. Opin. Rheumatol. 2018;30 doi: 10.1097/BOR.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 14.Magen E., Delgado J.S. Helicobacter pylori and skin autoimmune diseases. World J. Gastroenterol. 2014;20 doi: 10.3748/wjg.v20.i6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radić M. Role of Helicobacter pylori infection in autoimmune systemic rheumatic diseases. World J. Gastroenterol. 2014;20 doi: 10.3748/wjg.v20.i36.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Showji Y., Nozawa R., Sato K., Suzuki H. Seroprevalence of Helicobacter pylori infection in patients with connective tissue diseases. Microbiol. Immunol. 1996;40 doi: 10.1111/j.1348-0421.1996.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 17.Ram M., Barzilai O., Shapira Y., Anaya J.M., Tincani A., Stojanovich L., Bombardieri S., Bizzaro N., Kivity S., Levin N.A., Shoenfeld Y. Helicobacter pylori serology in autoimmune diseases - fact or fiction? Clin. Chem. Lab. Med. 2013:51. doi: 10.1515/cclm-2012-0477. [DOI] [PubMed] [Google Scholar]
- 18.Zain M.A., Zafar F., Ashfaq A., Jamil A.R., Ahmad A. Helicobacter pylori: an underrated cause of immune thrombocytopenic purpura. A comprehensive review. Cureus. 2019;11 doi: 10.7759/cureus.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X.-Z., Zhang Y.-M., Jia N.-Y., Zhang H. Helicobacter pylori infection is associated with elevated galactose-deficient IgA1 in IgA nephropathy. Ren. Fail. 2020;42:539–546. doi: 10.1080/0886022X.2020.1772295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kira J.-I., Isobe N. Helicobacter pylori infection and demyelinating disease of the central nervous system. J. Neuroimmunol. 2019;329:14–19. doi: 10.1016/j.jneuroim.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Kunovsky L., Dite P., Jabandziev P., Dolina J., Vaculova J., Blaho M., Bojkova M., Dvorackova J., Uvirova M., Kala Z., Trna J. Helicobacter pylori infection and other bacteria in pancreatic cancer and autoimmune pancreatitis. World J. Gastrointest. Oncol. 2021;13:835–844. doi: 10.4251/wjgo.v13.i8.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuan-Baltazar Y., Soto-Vega E. Microorganisms associated to thyroid autoimmunity. Autoimmun. Rev. 2020;19:102614. doi: 10.1016/j.autrev.2020.102614. [DOI] [PubMed] [Google Scholar]
- 23.Guitart J., Deonizio J., Bloom T., Martinez-Escala M.E., Kuzel T.M., Gerami P., Kwasny M., Rosen S.T. High incidence of gastrointestinal tract disorders and autoimmunity in primary cutaneous marginal zone b-cell lymphomas. JAMA Dermatol. 2014;150 doi: 10.1001/jamadermatol.2013.9223. [DOI] [PubMed] [Google Scholar]
- 24.Melby K.K., Carlsen K.L., Håland G., Samdal H.H., Carlsen K.-H. Helicobacter pylori in early childhood and asthma in adolescence. BMC Res. Notes. 2020;13:79. doi: 10.1186/s13104-020-04941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engler D.B., Reuter S., van Wijck Y., Urban S., Kyburz A., Maxeiner J., Martin H., Yogev N., Waisman A., Gerhard M., Cover T.L., Taube C., Müller A. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc. Natl. Acad. Sci. U. S. A. 2014;111:11810–11815. doi: 10.1073/pnas.1410579111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranjbar R., Karampoor S., Jalilian F.A. The protective effect of Helicobacter Pylori infection on the susceptibility of multiple sclerosis. J. Neuroimmunol. 2019;337:577069. doi: 10.1016/j.jneuroim.2019.577069. [DOI] [PubMed] [Google Scholar]
- 27.Bartels L.E., Pedersen A.B., Kristensen N.R., Jepsen P., Vilstrup H., Stengaard-Pedersen K., Dahlerup J.F. Helicobacter pylori infection is not associated with rheumatoid arthritis. Scand. J. Rheumatol. 2019;48:24–31. doi: 10.1080/03009742.2018.1464205. [DOI] [PubMed] [Google Scholar]
- 28.Anaya J.M., Ramirez-Santana C., Alzate M.A., Molano-Gonzalez N., Rojas-Villarraga A. The autoimmune ecology. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youssefi M., Tafaghodi M., Farsiani H., Ghazvini K., Keikha M. Helicobacter pylori infection and autoimmune diseases; Is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? A systematic review and meta-analysis study. J. Microbiol. Immunol. Infect. 2021;54:359–369. doi: 10.1016/j.jmii.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Amedei A., Bergman M.P., Appelmelk B.J., Azzurri A., Benagiano M., Tamburini C., van der Zee R., Telford J.L., Vandenbroucke-Grauls C.M.J.E., D'Elios M.M., del Prete G. Molecular mimicry between Helicobacter pylori antigens and H +,K+-Adenosine triphosphatase in human gastric autoimmunity. J. Exp. Med. 2003;198 doi: 10.1084/jem.20030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamanishi S., Iizumi T., Watanabe E., Shimizu M., Kamiya S., Nagata K., Kumagai Y., Fukunaga Y., Takahashi H. Implications for induction of autoimmimity via activation of B-1 cells by Helicobacter pylori urease. Infect. Immun. 2006;74 doi: 10.1128/IAI.74.1.248-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uemura N., Okamoto S., Yamamoto S., Matsumura N., Yamaguchi S., Yamakido M., Taniyama K., Sasaki N., Schlemper R.J. Helicobacter pylori Infection and the development of gastric cancer. N. Engl. J. Med. 2001;345:784–789. doi: 10.1056/nejmoa001999. [DOI] [PubMed] [Google Scholar]
- 33.Graham D.Y. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J. Gastroenterol. 2014;20 doi: 10.3748/wjg.v20.i18.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miendje Deyi V.Y., van den Borre C., Fontaine V. Comparative evaluation of 3 selective media for primary isolation of Helicobacter pylori from gastric biopsies under routine conditions. Diagn. Microbiol. Infect. Dis. 2010;68 doi: 10.1016/j.diagmicrobio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Hasni S.A., Ippolito A., Illei G.G. Helicobacter pylori and autoimmune diseases. Oral Dis. 2011;17:621–627. doi: 10.1111/j.1601-0825.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamanishi S., Iizumi T., Watanabe E., Shimizu M., Kamiya S., Nagata K., Kumagai Y., Fukunaga Y., Takahashi H. Implications for induction of autoimmunity via activation of B-1 cells by Helicobacter pylori. Urease. 2006;74:248–256. doi: 10.1128/IAI.74.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wotherspoon A.C., Ortiz-Hidalgo C., Falzon M.R., Isaacson P.G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338 doi: 10.1016/0140-6736(91)92035-Z. [DOI] [PubMed] [Google Scholar]
- 38.Ebrahimi A., Soofizadeh B., Ebrahimi F., Moaadab S.Y., Bonyadi M., Gojazadeh M., Malek Mahdavi A. Relationship between Helicobacter pylori cytotoxin-associated gene A protein with clinical outcomes in patients with rheumatoid arthritis. Immunol. Lett. 2019;211:49–52. doi: 10.1016/j.imlet.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Der Lin K., Chiu G.F., Waljee A.K., Owyang S.Y., El-Zaatari M., Bishu S., Grasberger H., Zhang M., Wu D.C., Kao J.Y. Effects of anti–Helicobacter pylori therapy on incidence of autoimmune diseases, including inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2019;17:1991–1999. doi: 10.1016/j.cgh.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kao J.Y., Zhang M., Miller M.J., Mills J.C., Wang B., Liu M., Eaton K.A., Zou W., Berndt B.E., Cole T.S., Takeuchi T., Owyang S.Y., Luther J. Helicobacter pylori immune escape is mediated by dendritic cell-induced treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138 doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oertli M., Sundquist M., Hitzler I., Engler D.B., Arnold I.C., Reuter S., Maxeiner J., Hansson M., Taube C., Quiding-Järbrink M., Müller A. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori - specific immune tolerance, and asthma protection. J. Clin. Invest. 2012;122 doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano C., Diaz M.I., Valdivia A., Godoy A., Peña A., Rollan A., Kirberg A., Hebel E., Fierro J., Klapp G., Venegas A., Harris P.R. Relationship between Helicobacter pylori virulence factors and regulatory cytokines as predictors of clinical outcome. Microb. Infect. 2007;9 doi: 10.1016/j.micinf.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dörner T., Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. 2019;393 doi: 10.1016/S0140-6736(19)30546-X. [DOI] [PubMed] [Google Scholar]
- 44.Méndez-Martínez S., García-Carrasco M., Cedillo-Ramírez M.L., Mendoza-Pinto C., Etchegaray-Morales I., Gil-Juárez C., Montiel-Jarquín Á.J., Taboada-Cole A., Jiménez-Herrera E.A., Muñóz-Guarneros M., Cervera R. Genital Mycoplasma infection among Mexican women with systemic lupus erythematosus. Int. J. Gynecol. Obstet. 2017;138 doi: 10.1002/ijgo.12154. [DOI] [PubMed] [Google Scholar]
- 45.Méndez-Martínez S., García-Carrasco M., Jiménez-Herrera E.A., Mendoza-Pinto C., Etchegaray-Morales I., Barahona-Rubio P.W., Gálvez-Romero J.L., Munguía-Realpozo P., Muñóz-Guarneros C.O., Cedillo-Ramírez M.L., Silva-Gómez S.E., Linares-Fleites G., Rojas-Vallaraga A. Factors of the epidemiological triad that influence the persistence of human papilloma virus infection in women with systemic lupus erythematosus. Lupus. 2018;27 doi: 10.1177/0961203318773176. [DOI] [PubMed] [Google Scholar]
- 46.Mendoza-Pinto C., García-Carrasco M., Méndez-Martínez S., Mogollán-Delfín T., Munguía-Realpozo P., Herrera-Robles E., Etchegaray-Morales I., Gálvez-Romero J.L., Montiel-Jarquín Á., López-Colombo A. Helicobacter pylori infection and gastroduodenal lesions in patients with systemic lupus erythematosus. Clin. Rheumatol. 2020;39 doi: 10.1007/s10067-019-04805-w. [DOI] [PubMed] [Google Scholar]
- 47.Pan Q., Liu Z., Liao S., Ye L., Lu X., Chen X., Li Z., Li X., Xu Y.Z., Liu H. Current mechanistic insights into the role of infection in systemic lupus erythematosus. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109122. [DOI] [PubMed] [Google Scholar]
- 48.Francis L., Perl A. Infection in systemic lupus erythematosus: friend or foe? Int. J. Clin. Rheumatol. 2010;5 doi: 10.2217/ijr.09.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawalha A.H., Schmid W.R., Binder S.R., Bacino D.K., Harley J.B. Association between systemic lupus erythematosus and Helicobacter pylori seronegativity. J. Rheumatol. 2004;31 [PubMed] [Google Scholar]
- 50.Wu M.C., Leong P.Y., Chiou J.Y., Chen H.H., Huang J.Y., Wei J.C.C. Increased risk of systemic lupus erythematosus in patients with Helicobacter pylori infection: a nationwide population-based cohort study. Front. Med. 2020;6 doi: 10.3389/fmed.2019.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu M.C., Huang J.Y., Chen H.H., Wei J.C.C. Effect of early eradication therapy on systemic lupus erythematosus risk in patients with Helicobacter pylori infection: a nationwide population-based cohort study. Lupus. 2020;29 doi: 10.1177/0961203320923393. [DOI] [PubMed] [Google Scholar]
- 52.Youssefi M., Tafaghodi M., Farsiani H., Ghazvini K., Keikha M. Helicobacter pylori infection and autoimmune diseases; Is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? A systematic review and meta-analysis study. J. Microbiol. Immunol. Infect. 2021;54 doi: 10.1016/j.jmii.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Surawut S., Panpetch W., Makjaroen J., Tangtanatakul P., Thim-Uam A., Wongphoom J., Tumwasorn S., Leelahavanichkul A. Helicobacter pylori infection increased anti-dsDNA and enhanced lupus severity in symptomatic FcγRIIb-deficient lupus mice. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meier H.C.S., Miller F.W., Dinse G.E., Weinberg C.R., Cho C.C., Parks C.G. 2020. Helicobacter pylori Seropositivity Is Associated with Antinuclear Antibodies in US Adults, NHANES 1999-2000, Epidemiology and Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reshetnyak T.M., Doroshkevich I.A., Seredavkina N.v., Nasonov E.L., Maev I.v., Reshetnyak V.I. The contribution of drugs and Helicobacter pylori to gastric mucosa changes in patients with systemic lupus erythematosus and antiphospholipid syndrome. Int. J. Rheumatol. 2019 doi: 10.1155/2019/9698086. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vieira S.M., Pagovich O.E., Kriegel M.A. Diet, microbiota and autoimmune diseases. Lupus. 2014;23 doi: 10.1177/0961203313501401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo X.M., Edwards M.R., Mu Q., Yu Y., Vieson M.D., Reilly C.M., Ahmed S.A., Bankole A.A. Gut microbiota in human systemic lupus erythematosus and a mouse model of lupus. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.02288-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mu Q., Edwards M.R., Swartwout B.K., Cabana Puig X., Mao J., Zhu J., Grieco J., Cecere T.E., Prakash M., Reilly C.M., Puglisi C., Bachali P., Grammer A.C., Lipsky P.E., Luo X.M. Gut microbiota and bacterial DNA suppress autoimmunity by stimulating regulatory B cells in a murine model of lupus. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.593353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu C.C., Caricchio R., Gallucci S. Triggers of autoimmunity: the role of bacterial infections in the extracellular exposure of lupus nuclear autoantigens. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manfredo Vieira S., Hiltensperger M., Kumar V., Zegarra-Ruiz D., Dehner C., Khan N., Costa F.R.C., Tiniakou E., Greiling T., Ruff W., Barbieri A., Kriegel C., Mehta S.S., Knight J.R., Jain D., Goodman A.L., Kriegel M.A. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359 doi: 10.1126/science.aar7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tao Z.H., Han J.X., Fang J.Y. Helicobacter pylori infection and eradication: exploring their impacts on the gastrointestinal microbiota. Helicobacter. 2020;25 doi: 10.1111/hel.12754. [DOI] [PubMed] [Google Scholar]
- 62.Alarcón T., Llorca L., Perez-Perez G. Current Topics in Microbiology and Immunology. 2017. Impact of the microbiota and gastric disease development by Helicobacter pylori. [DOI] [PubMed] [Google Scholar]
- 63.Arachchi P.S., Fernando N., Weerasekera M.M., Senevirathna B., Weerasekera D.D., Gunasekara C.P. Proinflammatory cytokine IL-17 shows a significant association with Helicobacter pylori infection and disease severity. Gastroenterol. Res. Prac. 2017:2017. doi: 10.1155/2017/6265150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koga T., Ichinose K., Kawakami A., Tsokos G.C. The role of IL-17 in systemic lupus erythematosus and its potential as a therapeutic target. Expet Rev. Clin. Immunol. 2019;15 doi: 10.1080/1744666X.2019.1593141. [DOI] [PubMed] [Google Scholar]
- 65.Teng Y., Cang B., Mao F., Chen W., Cheng P., Peng L., Luo P., Lu D., You N., Zou Q., Zhuang Y. Expression of ETS1 in gastric epithelial cells positively regulate inflammatory response in Helicobacter pylori-associated gastritis. Cell Death Dis. 2020;11 doi: 10.1038/s41419-020-2705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leng R.X., Pan H.F., Chen G.M., Feng C.C., Fan Y.G., Ye D.Q., Li X.P. The dual nature of Ets-1: focus to the pathogenesis of systemic lupus erythematosus. Autoimmun. Rev. 2011;10 doi: 10.1016/j.autrev.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 67.Aletaha D., Smolen J.S. Diagnosis and management of rheumatoid arthritis. J. Am. Med. Assoc. 2018;320 doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
- 68.Arleevskaya M., Takha E., Petrov S., Kazarian G., Novikov A., Larionova R., Valeeva A., Shuralev E., Mukminov M., Bost C., Renaudineau Y. Causal risk and protective factors in rheumatoid arthritis: a genetic update. J. Transl. Autoimmun. 2021;4:100119. doi: 10.1016/j.jtauto.2021.100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsukawa Y., Asai Y., Kitamura N., Sawada S., Kurosaka H. Exacerbation of rheumatoid arthritis following Helicobacter pylori eradication: disruption of established oral tolerance against heat shock protein? Med. Hypotheses. 2005;64:41–43. doi: 10.1016/j.mehy.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 70.Radić M. Role of Helicobacter pylori infection in autoimmune systemic rheumatic diseases. World J. Gastroenterol. 2014;20:12839–12846. doi: 10.3748/wjg.v20.i36.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meron M.K., Amital H., Shepshelovich D., Barzilai O., Ram M., Anaya J.-M., Gerli R., Nicola B., Shoenfeld Y. Infectious aspects and the etiopathogenesis of rheumatoid arthritis. Clin. Rev. Allergy Immunol. 2010;38:287–291. doi: 10.1007/s12016-009-8158-6. [DOI] [PubMed] [Google Scholar]
- 72.Zentilin P., Savarino V., Garnero A., Accardo S., Seriolo B. Is Helicobacter pylori infection a risk factor for disease severity in rheumatoid arthritis? [1] Gastroenterology. 1999;116:503–504. doi: 10.1016/S0016-5085(99)70161-7. [DOI] [PubMed] [Google Scholar]
- 73.Seriolo B., Cutolo M., Zentilin P., Savarino V. Helicobacter pylori infection in rheumatoid arthritis. J. Rheumatol. 2001;28:1195–1196. [PubMed] [Google Scholar]
- 74.Zentilin P., Seriolo B., Dulbecco P., Caratto E., Iiritano E., Fasciolo D., Bilardi C., Mansi C., Testa E., Savarino V. Eradication of Helicobacter pylori may reduce disease severity in rheumatoid arthritis. Aliment Pharmacol. Therapeut. 2002;16:1291–1299. doi: 10.1046/j.1365-2036.2002.01284.x. [DOI] [PubMed] [Google Scholar]
- 75.Steen K., Lems W., Visman I., De Koning M.R., Van De Stadt R., Twisk J., De Leest H., Dijkmans B., Nurmohamed M. The effect of Helicobacter pylori eradication on C-reactive protein and the lipid profile in patients with rheumatoid arthritis using chronic NSAIDs. Clin. Exp. Rheumatol. 2009;27:170. [PubMed] [Google Scholar]
- 76.Janssen M., Dijkmans B.A.C., Vandenbroucke J.P., Van Duijn W., Pefna A.S., Lamers C.B.H.W. Decreased level of antibodies against Helicobacter pylon in patients with rheumatoid arthritis receiving intramuscular gold. Ann. Rheum. Dis. 1992;51:1036–1038. doi: 10.1136/ard.51.9.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ten Wolde S., Janssen M., Van Duijn W., Lamers C.B.H.W., Hermans J., Dijkmans B.A.C. No effect of intramuscular gold therapy on serological parameters of Helicobacter pylori infection in patients with rheumatoid arthritis: a 12 month prospective study. Ann. Rheum. Dis. 1994;53:400–402. doi: 10.1136/ard.53.6.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paimela L., Leirisalo-Repo M., Kosunen T.U. Effect of long term intramuscular gold therapy on the seroprevalence of Helicobacter pylori in patients with early rheumatoid arthritis. Ann. Rheum. Dis. 1995;54:437. doi: 10.1136/ard.54.5.437-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grigoriadou S., Quraishi A., Saravanna J., Saravanan V., Heycock C., Kelly C. What effect does Helicobacter pylori infection have on the risk of peptic ulceration in patients receiving NSAIDs for rheumatoid arthritis? Eur. J. Intern. Med. 2002;13:269–273. doi: 10.1016/s0953-6205(02)00039-0. [DOI] [PubMed] [Google Scholar]
- 80.Moriyama T., Matsumoto T., Fuchigami T., Nakamura S., Ishikawa N., Takubo N., Yamamoto S., Oshiro Y., Nakanishi M., Tomioka K., Iida M. Changes in Helicobacter pylori status in patients with rheumatoid arthritis under non-steroidal anti-inflammatory drugs. Scand. J. Gastroenterol. 2004;39:111–118. doi: 10.1080/00365520310008089. [DOI] [PubMed] [Google Scholar]
- 81.Brito-Zerón P., Baldini C., Bootsma H., Bowman S.J., Jonsson R., Mariette X., Sivils K., Theander E., Tzioufas A., Ramos-Casals M. Sjögren syndrome. Nat. Rev. Disease Primers. 2016;2 doi: 10.1038/nrdp.2016.47. [DOI] [PubMed] [Google Scholar]
- 82.Björk A., Mofors J., Wahren-Herlenius M. Environmental factors in the pathogenesis of primary Sjögren’s syndrome. J. Intern. Med. 2020;287:475–492. doi: 10.1111/joim.13032. [DOI] [PubMed] [Google Scholar]
- 83.Aragona P., Magazzù G., Macchia G., Bartolone S., Di Pasquale G., Vitali C., Ferreri G. Presence of antibodies against Helicobacter pylori and its heat-shock protein 60 in the serum of patients with Sjögren’s syndrome - PubMed. J. Rheumatol. 1999;26:1306–1311. [PubMed] [Google Scholar]
- 84.Showji Y., Nozawa R., Sato K., Suzuki H. Seroprevalence of Helicobacter pylori infection in patients with connective tissue diseases. Microbiol. Immunol. 1996;40:499–503. doi: 10.1111/j.1348-0421.1996.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 85.Saghafi M., Abdolahi N., Orang R., Hatef M., Molseghi M. Helicobacter pylori infection in sjögren’s syndrome: Co-incidence or causality? Curr. Rheumatol. Rev. 2019;15:238–241. doi: 10.2174/1573397114666181113102427. [DOI] [PubMed] [Google Scholar]
- 86.El Miedany Y.M., Baddour M., Ahmed I., Fahmy H. Sjogren's syndrome: concomitant H. Pylori infection and possible correlation with clinical parameters. Joint Bone Spine. 2005;72:135–141. doi: 10.1016/j.jbspin.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Caporali R., Epis O., Negrini R., Scirè C., Solcia E., Montecucco C. Salivary gland lymphocytic infiltrates and Helicobacter pylori serology in anti-SSA/Ro positive patients in Italy. Clin. Exp. Rheumatol. 2003;21:266–267. [PubMed] [Google Scholar]
- 88.Irani S., Monsef Esfahani A., Bidari Zerehpoush F. Detection of Helicobacter pylori in oral lesions. J. Dent. Res. Dent. Clin. Dent. Prospects. 2013;7:230–237. doi: 10.5681/joddd.2013.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Q., Zhou X., Tan W., Zhang M. Association between Helicobacter pylori infection and Sjögren syndrome A meta-analysis. Medicine (Baltim.) 2018;97 doi: 10.1016/j.jocn.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banno S., Matsumoto Y., Sugiura Y., Yoshinouch T., Shibata H., Ueda R. Seroprevalence of Helicobacter pylori and association with atrophic gastritis in patients with Sji gren ’ s syndrome. Jpn. J. Rheumatol. 1999;9:353–363. [Google Scholar]
- 91.Ferraccioli G.F., Sorrentino D., De Vita S., Casatta L., Labombarda A., Avellini C., Dolcetti R., Di Luca D., Beltrami C.A., Boiocchi M., Bartoli E. B cell clonality in gastric lymphoid tissues of patients with Sjögren’s syndrome. Ann. Rheum. Dis. 1996;55:311–316. doi: 10.1136/ard.55.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guitart J., Deonizio J., Bloom T., Martinez-Escala M.E., Kuzel T.M., Gerami P., Kwasny M., Rosen S.T. High incidence of gastrointestinal tract disorders and autoimmunity in primary cutaneous marginal zone b-cell lymphomas. JAMA Dermatol. 2014;150:412–418. doi: 10.1001/jamadermatol.2013.9223. [DOI] [PubMed] [Google Scholar]
- 93.Zucca E., Bertoni F., Vannata B., Cavalli F. Emerging role of infectious etiologies in the pathogenesis of marginal zone B-cell lymphomas. Clin. Cancer Res. 2014;20:5207–5216. doi: 10.1158/1078-0432.CCR-14-0496. [DOI] [PubMed] [Google Scholar]
- 94.McFarlane M., Wong J.L.H., Paneesha S., Rudzki Z., Arasaradnam R., Nwokolo C. Synchronous upper and lower gastrointestinal mucosa-associated lymphoid tissue lymphomas, case reports in gastroenterology. 2016. 10, 241, 247. [DOI] [PMC free article] [PubMed]
- 95.Cerhan J.R., Habermann T.M. Epidemiology of marginal zone lymphoma. Ann. Lymphoma. 2021;5 doi: 10.21037/aol-20-28. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guarneri F., Guarneri C., Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J. Cell Mol. Med. 2005;9:741–744. doi: 10.1111/j.1582-4934.2005.tb00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kountouras J., Zavos C., Chatzopoulos D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J. Cell Mol. Med. 2005;9:196–207. doi: 10.1111/j.1582-4934.2005.tb00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hjelmström P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J. Leukoc. Biol. 2001;69:331–339. doi: 10.1189/jlb.69.3.331. [DOI] [PubMed] [Google Scholar]
- 99.Winter S., Loddenkemper C., Aebischer A., Räbel K., Hoffmann K., Meyer T.F., Lipp M., Höpken U.E. The chemokine receptor CXCR5 is pivotal for ectopic mucosa-associated lymphoid tissue neogenesis in chronic Helicobacter pylori-induced inflammation. J. Mol. Med. 2010;88:1169–1180. doi: 10.1007/s00109-010-0658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Streubel B., Huber D., Wöhrer S., Chott A., Raderer M. Frequency of chromosomal aberrations involving MALT1 in mucosa-associated lymphoid tissue lymphoma in patients with sjögren’s syndrome. Clin. Cancer Res. 2004;10:476–480. doi: 10.1158/1078-0432.CCR-0873-03. [DOI] [PubMed] [Google Scholar]
- 101.Fisher B.A., Brown R.M., Bowman S.J., Barone F. A review of salivary gland histopathology in primary Sjögren’s syndrome with a focus on its potential as a clinical trials biomarker. Ann. Rheum. Dis. 2015;74:1645–1650. doi: 10.1136/annrheumdis-2015-207499. [DOI] [PubMed] [Google Scholar]
- 102.Mezache L., Magro C., Hofmeister C., Pichiorri F., Sborov D., Nuovo G.J. Modulation of PD-L1 and CD8 activity in idiopathic and infectious chronic inflammatory conditions. Appl. Immunohistochem. Mol. Morphol. AIMM. 2017;25:100–109. doi: 10.1097/PAI.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Theander E., Nilsson I., Manthorpe R., Jacobsson L.T.H., Wadström T. Seroprevalence of Helicobacter pylori in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2001;19:633–638. [PubMed] [Google Scholar]
- 104.Collin P., Karvonen A.L., Korpela M., Laippala P., Helin H. Gastritis classified in accordance with the Sydney System in patients with primary Sjogren's syndrome. Scand. J. Gastroenterol. 1997;32:108–111. doi: 10.3109/00365529709000179. [DOI] [PubMed] [Google Scholar]
- 105.Sorrentino D., Faller G., DeVita S., Avellini C., Labombarda A., Ferraccioli G., Kahlow-Toussaint S. Helicobacter pylori associated antigastric autoantibodies: role in sjögren’s syndrome gastritis. Helicobacter. 2004;9:46–53. doi: 10.1111/j.1083-4389.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 106.Raderer M., Osterreicher, Machold K., Formanek M., Fiebiger W., Penz M., Dragosics B., Chott A. Impaired response of gastric MALT-lymphoma to Helicobacter pylori eradication in patients with autoimmune disease. Ann. Oncol. 2001;12:937–939. doi: 10.1023/A. [DOI] [PubMed] [Google Scholar]