Abstract
Background
Mycotoxins are secondary fungal metabolites that are produced by some toxigenic fungi on foodstuffs which are poisoning and potentiate for human's health hazards. In coffee samples, ochratoxin A and fungal contamination were examined.
Methods
Immunoaffinity columns were used for treating of all 50 samples from four types of coffee, after that high‐performance liquid chromatography was used for determining the amount of ochratoxin. For the identification of fungi, all coffee samples were cultured in appropriated media.
Results
The results showed that all samples were contaminated by ochratoxin A but only up to 50% of them had toxins higher than acceptable level as detected in black beans (47%), green beans (33.3%), torch (33.3%), and espresso (25%). Black coffee had a higher mean concentration of ochratoxin A than green coffee.
Conclusion
Predominant fungi isolated from coffee samples were Aspergillus species. Finally, careful monitoring of mycotoxins in coffee samples is essential to improve the quality of this favorable beverage in future.
Keywords: Aspergillus, coffee, high‐performance liquid chromatography, mycotoxin, ochratoxin
Different steps of ochratoxin A extraction from coffee.
1. INTRODUCTION
Coffee is a member of Coffea genus and botanical Rubiaceae family. Coffee is a universal most wanted beverage that was consumed for its motivating affects caused by caffeine. Many people do not know about how the coffee is cultured or processed. 1 It is clear that for good quality of coffee production, processing of coffee beans is very important, after that suitable care needs to be taken for packaging and storage of coffee beans. 2 Different methods are used to prepare and consume coffee such as espresso, French press, and latte. Coffee beans contain more than 1500 chemical components. 3 These healthy compounds such as chlorogenic acids, caffeine, trigonelline, diterpenes, and tocopherols make coffee a beneficial beverage. 4 , 5 When beans of coffee have not roasted, they are named green coffee beans. 6 Chlorogenic acid that is in green coffee beans has many health benefit activity, and in green coffee beans, this component is higher compared with roasted coffee beans, and it seems that roasting process of coffee beans can decrease amount of the chlorogenic acid. 7 One of the major water‐soluble component of coffee is caffeine (1,3,7‐trimethylxanthine). Caffeine has psychoactive fragment and antioxidant properties. 8 Caffeine can increase mental performance, reduce type 2 diabetes mellitus, decrease incidence of cancer, protect against Parkinson´s disease, and protect premature neonates from bronchopulmonary dysplasia defect. 9 , 10 , 11 , 12 These beans can have significant effects on health, including increased wakefulness, reduced pain, lowered risk of Alzheimer's and Parkinson's disease, and reduced depression. 12 , 13 , 14 , 15 Recently, a study investigated that in elderly people, coffee consumption might be useful in those with hypertension, obesity, or diabetes. 16 One of the famous and top priority human's health hazard is mycotoxins that are produced by specific strains of fungi. 17 Mycotoxins are secondary toxic fungal metabolites which can cause minor‐to‐severe complications such as leukopenia, immunodeficiency, and even liver cancer. 18 One of these mycotoxins is ochratoxin A (OTA). 1 Ochratoxin A is the most secondary fungal compounds that is mainly produced by the genera of Aspergillus (e.g., Aspergillus ochraceus) and Penicillium (e.g., Penicillium verrucosum). 19 , 20 Ochratoxin A is categorized as a human carcinogen and teratogen; it has some toxic compounds which has harmful effect on kidney, liver, and immune system. 21 , 22 The acceptable concentration of OTA is 10 μg/kg that is recommended by FAO/WHO Expert Committee on Food Additives. 23 , 24 As these serious effects of OTA, many attempts need to be tested and adjusted for keeping among this mycotoxin below acceptable levels. Currently, many countries have documented maximum levels of OTA that can be present in foodstuffs. In European Union (EU), the legal maximum limitation for OTA that has determined in roasted and immediate coffee is 5 and 10 μg/kg, respectively. 25 Among many food products, beverages, including coffee fruits and beans, are highly susceptible and at risk of contamination by toxigenic fungi. 26 Determination of OTA concentration levels can be analyzed by different methods. The most commonly used methods are enzyme‐linked immunosorbent assay (ELISA), gas chromatography–mass spectrometry, thin‐layer chromatography (TLC), high‐performance liquid chromatography (HPLC), and ultra‐high‐performance liquid chromatography (UHPLC) with fluorescence detector (FLD). It is necessary to use sensitive, rapid, and repeatable assays to detect the presence of mycotoxins. Although using ELISA to determine OTA is a rapid method, the procedure did not work for some foodstuff because of the cross‐reactivity with related compounds. 27 Among these methods, HPLC and UHPLC are commonly used for analyzing OTA because of their high efficiency and also their detectors have low limits of quantification. UHPLC‐FLD provided a faster analysis, but this method needs FLD that are not available everywhere. HPLC method provides a high accuracy and sensitivity for rapid analysis of mycotoxins in the monitoring of food quality. 28 All of the coffee beans in Iran are imported, and there is less information about fungal contamination and mycotoxins level on coffee products. The aim of this study was to determine the concentration of OTA and deliberate the load of fungal contamination in green and black coffee based on HPLC method in Shiraz, Southern Iran.
2. MATERIALS
2.1. Samples
A total of 50 coffee samples including 18 black coffee beans, 8 green coffee beans, 12 coffee espresso powder, and 12 coffee torch powder samples from different brands were randomly purchased from supermarkets around Shiraz City, Iran, during 2018.
2.2. Methods
2.2.1. Fungal detection and load of contamination
2.2.1.1. Direct plating test
Culture was used for the detection of fungal isolates. Five coffee beans and 0.2 g of each coffee powder (5 beans per plate) were inoculated in Sabouraud dextrose agar containing chloramphenicol (SC) and SC with 6% NaCl (Sigma Chemicals, USA). For evaluating the load of fungal contamination, 5 g of each coffee samples was added to 45 ml of sterile distilled water that contained 0.05% Tween 80. Then, 100 µl of suspension was inoculated to SC after vortexing for 2 min. The plates were incubated at 25℃ for 7 days. The fungal colonies were counted, calculated, and reported as colony‐forming unit (CFU) per gram based on International Commission on Microbiological Safety for Foods (ICSMF) protocol. Finally, for identification of fungi's genus, the colonies were subcultured. 29 , 30 , 31
2.2.2. Analysis of OTA by HPLC method
OTA standard was obtained from Supelco (Bellefonte, USA). Solvents for mobile phase such as sodium chloride, acetic acid glacial, and acetonitrile and all chemicals were of the analytical grade (Merck Darmstadt, Germany). A standard working solution of OTA (100 μg kg−1) was prepared in methanol water (50:50, v/v). Ten grams of sample was extracted with 200 ml of 84% acetonitrile in 3 min in a blender with low speed. One hundred milliliters of this solution was diluted, and the pH was adjusted with NOH (pH =7.4). HPLC apparatus consisted of a binary pump equipped with microvacuum degasser, thermostated autosampler, column compartment, and fluorescence 152 detectors (model G1321A). The amount of injection was 20 μl, and the column temperature was deposited at 40℃. Detector wavelengths were set at λexc160 and λem of 365 and 435 nm. For data acquisition and processing, the system was interfaced to the computer (Agilent ChemStation).
2.2.3. Sample treatment and purification
In accordance with the Institute of Standards and Industrial Research of Iran (ISIRI no. 9238, 2011), 5 grams of the ground coffee samples, 1 g NaCl, and 20 cc methanol (80%) were blended and homogenized for 3 min by using a warring blender (Model MX1050XTX). The mixture was filtered with Whatman filter paper, and then, 10ml of the filtrated solution was added to 40ml of phosphate‐buffered saline (PBS). Then, the solution was centrifuged (1372 g, 15 min) and filtered again. For purification of OTA from coffee samples, 50 ml of the filtrated solution was passed through the IAC column (Libios) at a flow rate of 1 ml min−1. Then, OTA was eluted with 1.5ml of methanol acetic acid (98:2) from the column and collected in a glass vial, and 1.5 ml of sterile, deionized water was added. As a final point, for injection into the HPLC, 100 μl of the eluted solution was used. 32
2.2.4. Validation and quality assurance method
For the OTA, calibration curve was built with concentrations of 0.5, 2, 5, 10, 30 μg kg−1. The limits of detection (LOD) and limits of quantification (LOQ) were established by using the signal‐to‐noise approach, described as per the concentration, resulting in a signal‐to‐noise ratio of approximately 3:1 for LOD and 10:1 for LOQ. Twenty‐five grams of different OTA‐free coffee samples was spiked with OTA at the levels of 1, 5, and 20 μg kg−1 for accuracy of the method applied. All the tests were done three times, and then, the recovery and standard deviation (SD) were calculated. 32 , 33
2.2.5. Quality Control
By internal quality control, the precision and exactness of the methods were confirmed. For this purpose, OTA retrievals were recorded by analyzing a blank sample and spiked at 5 μg kg−1 for OTA. The recovery rate for OTA was 93 μg kg−1. The OTA level was corrected, according to the recovery value. The LOD and LOQ for OTA were 1 μg kg−1. For calculating method linearity, the measurement of determination (R2) was used. A sequence of working standard solutions was prepared. Calibration curves were created by distinctly conspiracy area against concentration. R 2 values were considered using the regression equations. In Iran, the acceptable concentration of OTA is 2 μg kg−1.
2.3. Statistical Analysis
Normal distribution of toxin contents, means, standard error and variances were calculated by the SPSS software (Statistical Package for the Social Sciences). Chi‐square test was used for the determination of statistical significance of means differences. p value <0.05 was considered as significant.
3. RESULT
After the optimization of the analytical conditions, the linearity in the working standard solutions of five concentration levels after triplicate and duplicate injections for each concentration level was analyzed, and finally, 0.9991 and 0.9997 were reliable for OTA, respectively. (Figure 1, Table 1).
FIGURE 1.
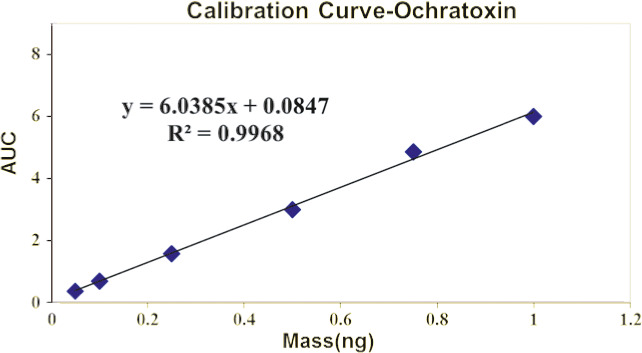
Standard curve of ochratoxin A
TABLE 1.
Validation parameters for the HPLC determination of OTA
| Validation parameters | OTA |
|---|---|
| LOD (μg kg−1) | 0.47 |
| LOQ (μg kg−1) | 1.23 |
| Correlation coefficient (R 2) | 0.9968 |
| Recovery (%) | 83.7–99.6 |
| RSD (%, n = 20) | 1.64 |
| RME (%, n = 20) | 2.6 |
More information about the quantity of OTA in the coffee samples is demonstrated in Table 2. Among 50 samples, all of them were contaminated with OTA, in which 40% of these samples had more OTA than standard levels of this toxin (2 μg kg−1). According to Table 2, the mean concentration of this toxin was higher in the black bean samples. There was no significant difference in amount of OTA in four types of coffee samples (p‐value =0.69). There were different genus of molds that isolated from coffee samples. In our study, 3 samples of black bean (17.6%), 7 samples of torch coffee (59.8%), 4 samples of espresso (33.3%), and 6 samples of green bean (66.6%) had fungal contamination in which Penicillium spp were the most fungal genus isolated from all coffee type except green bean. (Table 3). The fungal species identified from the culture medium in all samples included the following: Aspergillus flavus 2 (10%), Aspergillus niger 4 (20%), Penicillium spp 12 (60%), Cladosporium spp 1 (5%), and Aspergillus fumigatus 1 (5%). There was no significant difference between amount of OTA and culture result in four types of coffee samples (p‐value =0.14). Out of the 50 coffee samples, 20 (40%) samples had fungal contamination. Among samples with positive fungal culture, only 4 (22.2%) samples contaminated with more than the allowed OTA level.
TABLE 2.
Concentration of OTA in coffee samples
| Samples type | No. of samples | ≥2 μg kg−1 | <2 μg kg−1 | Mean (range) ± SD |
|---|---|---|---|---|
| Black bean | 18 | 11 (47%) | 7 (52/9%) | 4.3 (0.81–15.7) ±3.71 |
| Green bean | 8 | 2 (33/3%) | 6 (66/7%) | 1.32 (0.48–2.69) ±0.96 |
| Espresso | 12 | 3 (25%) | 9 (75%) | 3.4 (1.29–7.55) ±2.8 |
| Torch | 12 | 4 (33/3%) | 8 (66/7%) | 1.68 (0.84–2.04) ±0.31 |
TABLE 3.
The frequency of fungal species contamination in coffee samples
| Coffee type | Culture results | Species (n) | |
|---|---|---|---|
| Negative | Positive | ||
| Black bean | 3 | 15 | Aspergillus niger (1) |
| Penicillium (2) | |||
| Green seed | 6 | 2 | A.flavi complex (1) |
| Aspergillus niger (3) | |||
| Penicillium (2) | |||
| Espresso | 4 | 8 | Penicillium (3) |
| Cladosporium (1) | |||
| Torch | 7 | 5 | A.flavi complex (1) |
| Aspergillus fumigatus (1) | |||
| Penicillium (5) | |||
3.1. Load of fungal spore
The lowest loads of fungal contamination in coffee bean samples were 33 CFU/gr and seen in Cladosporium spp and the highest fungal contamination was 134 CFU/gr and belonged to Penicillium spp. In coffee powder samples, the lowest load of fungal contamination was 35 CFU/gr (Aspergillus spp) and highest load of fungal contamination was 99 CFU/gr (Penicillium spp), in which all spore levels were below the standard level of food safety (10,000 CFU/gr‐ICSMF). There was no significant difference between amount of OTA and four types of coffee (p‐value =0.69).
4. DISCUSSION
Nowadays, drinking coffee became one of the most popular habits in the world. Arabica coffee and Robusta coffee are the two main of coffee type which was farmed around the world that included 98% of the world coffee production. 34 More than 450 million cups of coffee are drunk in the United States every day. 35 In Iran, a developing country, coffee is one of the highly demanded products after water and tea. All of the coffee products are imported to Iran, so they can be contaminated with toxigenic fungi during pre‐ or post‐harvesting, storage, processing, and packing stages. 36 Mycotoxins are obvious contaminants in foodstuffs, and occurrence of mycotoxins is worldwide. These toxic substances are responsible for a big challenge in food safety, and also, economies and agricultural organization of the developing countries were affected a lot by these toxins, and every year, they suffered a huge damage. 37 A successful detection method for mycotoxin concentration should be sensitive, flexible, and specific, and the results must be relevant and easy to analyze; nowadays, many methods such as TLC, ELISA, and HPLC were used for achieving a reliable result. Among these methods, HPLC was more suitable for the determination of mycotoxin concentration compared with other methods. 38 According to previous studies, OTA is a powerful toxin that causes kidney damage in animals. 39 , 40 The in vivo study in pig showed that OTA has ability to cause mycotoxic nephropathy and expressed major effect in proximal tubules in pig kidneys. 41 Although all coffee samples were contaminated with OTA but only up to 50% of samples had OTA higher than hazardous range. In 47% of black beans, 33.3% of green bean, 33.3% of Torch and 25% of espresso, the amount of OTA was higher than standard. Previous study showed that OTA levels decrease during the roasting process of coffee beans. 42 Several studies have demonstrated that some mycotoxins such as aflatoxins in contaminated coffee beans have to be degraded by heat treatment, 43 but on the other hand, it is known that OTA preserved a certain stability during most thermal food processing stage. 44 In accordance with this result, roasting process in black coffee bean has no effect in decreasing mycotoxin compared with green coffee samples. According to our knowledge, there was no information about OTA level in coffee beans in Iran. Further studies need in order to confirm our findings. However, in our study, 100% of samples were contaminated with OTA (0.48–2.69). Noonim et al in Thailand reported that out of 64 coffee bean samples analyzed, 98% of samples were contaminated with OTA in levels of <0.6–5.5 μg kg−1 (Arabica) and 1–27 μg kg−1 (Robusta). 45 Vaclavik ‘s et al in the United States and Viegas et al in Brazil reported that 36% and 64.3% of samples were contaminated, respectively. 25 , 46 The potential to contaminate the coffee beans depends on several factors such as species of fungi, storage, transportation, and processing stages. Producing toxin in Aspergillus spp should be under special conditions. 47 In some samples, although the fungal growth but mycotoxin was not detected, and also in some samples mycotoxin was detected without fungal growing, these results obtained by comparison of fungal growth and mycotoxin production. Mycotoxin could be produced during processing in samples that have less fungal contamination but high toxin. Also, some kind of Aspergillus species are non‐toxicogenic and could not produce any mycotoxin after growing. On the other hand, if the conditions are not desirable, the fungi may be removed but the toxins will mostly remain. In our study, the genus Aspergillus, Penicillium, Cladosporium were detected as fungal contamination. Aspergillus spp were predominant species for fungal contamination which is known as a major cause of mycotoxigenic fungi. The most load of fungal contamination was in torch coffee. According to standard level of food safety (10,000 CFU/gr‐ICSMF), fungal contamination in our samples was at permissible level of food safety.
5. CONCLUSIONS
The focus of this study was to compare the levels of OTA in different brands of coffee samples. Recently, consumption of coffee is common among Iranian people. In our study, all of the coffee samples were contaminated in low level of OTA; therefore, the standardization of coffee processing and quality control criteria are recommended. Also, level of toxicity will increase by continuous daily consumption of coffee because of the capability of this toxin to accumulate in human body. Finally, coffee is imported in our country so it is suggested that adequate controls and monitoring on transferring, packaging, and maintenance process of this product are needed. The results of such studies can be useful for coffee customer that these beverage were safe, and the concentration of toxin in coffee was below standard level and not harmful for their healthy.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
ETHICAL APPROVAL
This project was found to be in accordance with the ethical principles and the national norms and standards for conducting medical research in Iran and has been approved by the research ethics committee.
ACKNOWLEDGMENTS
The data of this study were extracted from MSc thesis of Andishe Dehghani and were financially supported by the Vice‐Chancellor for Research of Shiraz University of Medical Sciences grant no 16726.
Pakshir K, Dehghani A, Nouraei H, Zareshahrabadi Z, Zomorodian K. Evaluation of fungal contamination and ochratoxin A detection in different types of coffee by HPLC‐based method. J Clin Lab Anal. 2021;35:e24001. 10.1002/jcla.24001
Funding information
This work was financially supported by the Vice‐Chancellor for Research of Shiraz University of Medical Sciences [grant numbers: 16726]
Data Availability Statement
The data used to support the findings of this study were supplied by Shiraz University of Medical Sciences under license and so cannot be made freely available. Requests for access to these data should be made to Keyvan Pakshir, pakshirk@gmail.com.
REFERENCES
- 1. Onaolapo O, Onaolapo A. Caffeinated Beverages, Behavior, and Brain Structure. Elsevier; 2019:163‐207. [Google Scholar]
- 2. Sobri NFNM, Nazri NFM, Sumani NS, Shah NAA, Mohamad N, Hajar N. Effects of different roasting parameters on selected physicochemical properties and sensory evaluation of coffee beans. J Acad. 2019;7(2):130‐7. [Google Scholar]
- 3. Poole R, Ewings S, Parkes J, Fallowfield JA, Roderick P. Misclassification of coffee consumption data and the development of a standardised coffee unit measure. BMJ Nutr Prev Health. 2019;2(1):11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uppal D, Sharma A, Tewari K. Method for extracting high content of chlorogenic acids from green coffee beans. Google Patents. 2019.
- 5. Hu G, Wang X, Zhang L, Qiu M. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019;10(6):3113‐26. [DOI] [PubMed] [Google Scholar]
- 6. Zhang SJ, De Bruyn F, Pothakos V, Torres J, Falconi C, Moccand C, et al. Following coffee production from cherries to cup: microbiological and metabolomic analysis of wet processing of Coffea arabica. Appl Environ Microbiol. 2019;85(6):e02635‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mubarak A, Croft KD, Bondonno CP, Din NS. Comparison of liberica and arabica coffee: Chlorogenic acid, caffeine, total phenolic and DPPH radical scavenging activity. 2019.
- 8. Leitão AL. Occurrence of ochratoxin A in coffee: Threads and solutions—A mini‐review. Beverages. 2019;5(2):36. [Google Scholar]
- 9. Caffeine Kumar VH. Bronchopulmonary Dysplasia and Neurodevelopmental Outcomes in Premature Infants. 2018.
- 10. Kumar VH, Lipshultz SE. Caffeine and clinical outcomes in premature neonates. Children. 2019;6(11):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stefanello N, Schmatz R, Pereira LB, Cardoso AM, Passamonti S, Spanevello RM, et al. Effects of chlorogenic acid, caffeine and coffee on components of the purinergic system of streptozotocin‐induced diabetic rats. J Nutr Biochem. 2016;38:145‐53. [DOI] [PubMed] [Google Scholar]
- 12. Ascherio A, Weisskopf MG, O’Reilly EJ, McCullough ML, Calle EE, Rodriguez C, et al. Coffee consumption, gender, and Parkinson’s disease mortality in the cancer prevention study II cohort: The modifying effects of estrogen. Am J Epidemiol. 2004;160(10):977‐84. [DOI] [PubMed] [Google Scholar]
- 13. Vignoli J, Bassoli D, Benassi M. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011;124(3):863‐8. [Google Scholar]
- 14. O'Keefe JH, Bhatti SK, Patil HR, DiNicolantonio JJ, Lucan SC, Lavie CJ. Effects of habitual coffee consumption on cardiometabolic disease, cardiovascular health, and all‐cause mortality. J Am Coll Cardiol. 2013;62(12):1043‐51. [DOI] [PubMed] [Google Scholar]
- 15. Butt MS, Sultan MT. Coffee and its consumption: Benefits and risks. Crit Rev Food Sci Nutr. 2011;51(4):363‐73. [DOI] [PubMed] [Google Scholar]
- 16. Machado‐Fragua MD, Struijk EA, Graciani A, Guallar‐Castillon P, Rodríguez‐Artalejo F, Lopez‐Garcia E. Coffee consumption and risk of physical function impairment, frailty and disability in older adults. Eur J Nutr. 2019;58(4):1415‐27. [DOI] [PubMed] [Google Scholar]
- 17. Pakshir K, Mirshekari Z, Nouraei H, Zareshahrabadi Z, Zomorodian K, Khodadadi H, et al. Mycotoxins detection and fungal contamination in black and green tea by HPLC‐based method. J Toxicol. 2020;2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perdoncini MRFG, Sereia MJ, Scopel FHP, Formigoni M, Rigobello ES, Benetti SC, et al. Growth of fungal cells and the production of mycotoxins. Cell Growth. IntechOpen; 2019. [Google Scholar]
- 19. Khan Achakzai AK, Yaqoob M, Khan Barozai MY. Chemical structure, occurrence and health hazard status of ochratoxin A (OTA) in cereal food and feeds of Pakistan: A review. J Chem Soc Pak. 2017;39(5):867‐878. [Google Scholar]
- 20. Zareshahrabadi Z, Bahmyari R, Nouraei H, Khodadadi H, Mehryar P, Asadian F, et al. Detection of aflatoxin and ochratoxin A in spices by high‐performance liquid chromatography. J Food Qual. 2020;2020:1‐8. [Google Scholar]
- 21. Fuchs R, Peraica M. Ochratoxin A in human kidney diseases. Food Addit Contam. 2005;22(s1):53‐7. [DOI] [PubMed] [Google Scholar]
- 22. Reddy L, Bhoola K. Ochratoxins—Food contaminants: Impact on human health. Toxins. 2010;2(4):771‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malir F, Ostry V, Pfohl‐Leszkowicz A, Malir J, Toman J. Ochratoxin A: 50 years of research. Toxins. 2016;8(7):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joint FAO/WHO Expert Committee on Food Additives. Meeting, World Health Organization . Evaluation of Certain Contaminants in Food: Eighty‐third report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization. 2017. [Google Scholar]
- 25. Vaclavik L, Vaclavikova M, Begley TH, Krynitsky AJ, Rader JI. Determination of multiple mycotoxins in dietary supplements containing green coffee bean extracts using ultrahigh‐performance liquid chromatography–tandem mass spectrometry (UHPLC‐MS/MS). J Agric Food Chem. 2013;61(20):4822‐30. [DOI] [PubMed] [Google Scholar]
- 26. Velmourougane K, Bhat R. Improvement of coffee quality through prevention of moulds. Frontiers in Fungal Ecology, Diversity and Metabolites: IK. International Publishing House Pvt. Ltd.; 2009:204‐20. [Google Scholar]
- 27. Kaya EMO. Development and validation of a SPE‐UHPLC‐fluorescence method for the analysis of ochratoxin‐a in certain Turkish wines. Maced J Chem Chem Eng. 2019;38(2):161‐70. [Google Scholar]
- 28. Monaci L, Palmisano F. Determination of ochratoxin A in foods: state‐of‐the‐art and analytical challenges. Anal Bioanal Chem. 2004;378(1):96‐103. [DOI] [PubMed] [Google Scholar]
- 29. Klich MA. Identification of Common Aspergillus Species. Centraalbureau Voor Schimmelcultures Utrecht. Cambridge University Press. 2002. [Google Scholar]
- 30. Orlowski M. Mucor dimorphism. Microbiol Mol Biol Rev. 1991;55(2):234‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zaini F, Mehbod A, Emami M. Compr Med Mycol Tehran. 2013;1377:330‐48. [Google Scholar]
- 32. Zhang L, Dou X‐W, Zhang C, Logrieco AF, Yang M‐H. A review of current methods for analysis of mycotoxins in herbal medicines. Toxins. 2018;10(2):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li W, Xu K, Xiao R, Yin G, Liu W. Development of an HPLC‐based method for the detection of aflatoxins in Pu‐erh tea. Int J Food Prop. 2015;18(4):842‐8. [Google Scholar]
- 34. Etana BOFMB, Aga MC. Review on Post‐harvest and Green Bean Coffee Processing in Ethiopia. 2019.
- 35. Maamoun A. Coca‐Cola brews a hot acquisition. Costa coffee. 2020.
- 36. Esfehani M, Ghasemzadeh S, Mirzadeh M. Comparison of fluoride ion concentration in black, green and white tea. Int J Ayurvedic Med. 2018;9(4):263‐5. [Google Scholar]
- 37. Alshannaq A, Yu J‐H. Occurrence, toxicity, and analysis of major mycotoxins in food. Inte J Environ Res Pub Health. 2017;14(6):632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peng H, Chang Y, Baker RC, Zhang G. Interference of mycotoxin binders with ELISA, HPLC and LC‐MS/MS analysis of aflatoxins in maize and maize gluten. Food Addit Contam: Part A. 2020;37(3):496‐506. [DOI] [PubMed] [Google Scholar]
- 39. Hard GC. Critical review of renal tubule karyomegaly in non‐clinical safety evaluation studies and its significance for human risk assessment. Crit Rev Toxicol. 2018;48(7):575‐95. [DOI] [PubMed] [Google Scholar]
- 40. Szczech G, Carlton W, Tuite J, Caldwell R. Ochratoxin A toxicosis in swine. Vet Pathol. 1973;10(4):347‐64. [DOI] [PubMed] [Google Scholar]
- 41. Stoev SD, Gundasheva D, Zarkov I, Mircheva T, Zapryanova D, Denev S, et al. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and fumonisin B1. Exp Toxicol Pathol. 2012;64(7–8):733‐41. [DOI] [PubMed] [Google Scholar]
- 42. Vanesa D, Ana P. Occurrence of Ochratoxin A in coffee beans, ground roasted coffee and soluble coffee and method validation. Food Control. 2013;30(2):675‐8. [Google Scholar]
- 43. Bokhari F, Aly M. Evolution of traditional means of roasting and mycotoxins contaminated coffee beans in Saudi Arabia. Adv Biol Res. 2009;3(3–4):71‐8. [Google Scholar]
- 44. Copetti MV, Iamanaka BT, Pereira JL, Lemes DP, Nakano F, Taniwaki MH. Co‐occurrence of ochratoxin A and aflatoxins in chocolate marketed in Brazil. Food Control. 2012;26(1):36‐41. [Google Scholar]
- 45. Noonim P, Mahakarnchanakul W, Nielsen KF, Frisvad JC, Samson RA. Isolation, identification and toxigenic potential of ochratoxin A‐producing Aspergillus species from coffee beans grown in two regions of Thailand. Int J Food Microbiol. 2008;128(2):197‐202. [DOI] [PubMed] [Google Scholar]
- 46. Viegas C, Pacífico C, Faria T, de Oliveira AC, Caetano LA, Carolino E, et al. Fungal contamination in green coffee beans samples: a public health concern. J Toxicol Env Health, Part A. 2017;80(13–15):719‐28. [DOI] [PubMed] [Google Scholar]
- 47. Mahtabani A, Bayat M, Hosseini S, Aminafshar M, Tavakoli H. Assessment of ochratoxin A and aflatoxin b1, b2, g1, g2 rates in breakfast grains of supermarkets in tehran using hplc method in 2010. Hakim Res J. 2011;14:10‐15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study were supplied by Shiraz University of Medical Sciences under license and so cannot be made freely available. Requests for access to these data should be made to Keyvan Pakshir, pakshirk@gmail.com.


