Abstract
Purpose
The purpose of this study was to construct and validate a simple model for the prediction of survival in patients with trauma‐related ARDS.
Methods
This is a single‐center, retrospective cohort study using MIMIC‐III Clinical Database.
Results
842 patients were included in this study. 175 (20.8%) died in‐hospital, whereas 215 (25.5%) died within 90 days. The deceased group had higher Acute Physiology Score (APS III), Sequential Organ Failure Assessment (SOFA), and Simplified Acute Physiology Score II (SAPS II). In multivariate logistic regression model, independent risk factors for mortality in ARDS patients included age ([odds ratio] OR, 1.035; 95% confidence interval [CI], 1.020–1.049), body mass index (OR, 0.957; 95% CI, 0.926–0.989), red blood cell distribution width (OR, 1.283; 95% CI, 1.141–1.443), hematocrit (OR, 1.055; 95% CI, 1.017–1.095), lactate (OR, 1.226; 95% CI, 1.127–1.334), blood urea nitrogen (OR, 1.025; 95% CI, 1.007–1.044), acute kidney failure (OR, 1.875; 95% CI, 1.188–2.959), sepsis (OR, 1.917; 95% CI, 1.165–3.153), type of admission (emergency vs. elective [OR, 2.822; 95% CI, 1.647–4.837], and urgent vs. elective [OR, 5.156; 95% CI, 1.896–14.027]). The area under the curve (AUC) of the model was 0.826, which was superior than the SAPS II (0.776), APS III (0.718), and SOFA (0.692). In the cross‐validation model, the accuracy of the test set was 0.823, the precision was 0.643, and the AUC was 0.813.
Conclusions
We established a prediction model using data commonly used in the clinic, which has high accuracy and precision and is worthy of use in clinical practice.
Keywords: acute respiratory distress syndrome, laboratory Inspection, predictive model, trauma
Acute respiratory distress syndrome is a heterogeneous disease. We established a prognostic model for in‐hospital death in patients with traumatic acute respiratory distress syndrome. So that we can timely identify high‐risk patients and formulate intervention strategies.

1. BACKGROUND
Acute respiratory distress syndrome (ARDS) refers to acute diffuse lung injury that may progress into acute respiratory failure. It is caused by various intrapulmonary and extrapulmonary pathogenic factors and is common in critically ill patients. Although protective pulmonary ventilation, limited fluid resuscitation, prone position ventilation, and other strategies are implemented for the treatment of ARDS, the mortality rate of patients with ARDS can be as high as 40%. 1 Trauma is a common predisposing factor for ARDS, accounting for about 10% of all ARDS cases. 2 Studies have shown that the mortality rate trauma‐related ARDS in adults ranges from 16% to 24% and can get as high as 35%–45% in critically ill patients. 3 Therefore, greater attention needs to be paid to trauma‐related ARDS.
Progress has been made in the understanding and management of ARDS. However, the treatment methods for ARDS are still limited. The strategic focus of ARDS has shifted to early identification of high‐risk patients and reduction in organ dysfunction and mortality. 4 The Glasgow Coma Score, Revised Trauma Score, Abbreviated Injury Score of the Thorax, and Injury Severity Score are used to assess the occurrence of ARDS in trauma patients. 5 The Simplified Acute Physiology Score (SAPS) and the Acute Physiology and Chronic Health Evaluation (APACHE II) score are also used to assess outcomes in critically ill patients and patients with ARDS. 6 , 7 However, these scores are not specific enough for the evaluation of prognosis in such cases. Thus, the aim of this study was to use conventional clinical parameters to construct a model for the prediction of in‐hospital survival in patients with ARDS after trauma or severe surgery.
2. MATERIALS AND METHODS
2.1. Patient population
This was a single‐center, retrospective cohort study conducted using data extracted from the Medical Information Mart for Intensive Care‐III Clinical Database, version 1.3 (mimic‐iii v1.3). All data extracted from the database are public and free. 8 The database contains more than 50,000 records of admissions to intensive care units (ICU) from 2001 to 2012, including details of 38,000 different patients and 49,000 admission records. In addition, nursing records, results of imaging examinations, and laboratory results can be obtained from the database. 9 To protect the patients’ privacy, all personal information is hidden before the database is accessed. The information is available to all researchers through the National Institutes of Health online course "Protecting human research participants."(Certification number: 9555299).
2.2. Selection of study participants
All the patients included in the database were screened. The inclusion criteria for this study were as follows: (1) age older than 18 years; (2) patients transferred to the ICU; (3) patients who underwent surgery or experienced trauma (International Classification of Diseases [ICD]‐9 code, 5185 [pulmonary insufficiency following trauma and surgery]); and (4) patients with ARDS that met the criteria in the Berlin definition for ARDS. 10 The criteria for ARDS included acute onset, partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg, bilateral infiltrates on chest radiograph, and absence of heart failure. Mild, moderate, and severe ARDS were classified based on the PaO2/FiO2 ratios of the patients. Pregnant women or perinatal patients transferred to the ICU due to trauma or surgery were excluded from this study. The main endpoint was in‐hospital mortality, whereas the secondary clinical outcome was 90‐day mortality. For patients with multiple ICU stays, only data related to the first ICU admission were considered.
2.3. Data sources
The PostgreSQL tool (version 9.5) was used to extract data. The original data of the same variable were missing <5%. We extracted information on patients’ age, sex, body mass index (BMI), ICU length of stay, and other indicators. Laboratory results were all derived from the first recorded within 24 h of transfer to the ICU. Missing values were filled by the mean or median. The laboratory results extracted included oxygenation index (OI), white blood cell (WBC), red blood cell (RBC), hemoglobin, red blood cell distribution width (RDW), platelet (PLT), hematocrit (HCT), international normalized ratio (INR), prothrombin time (PT), activated partial prothrombin time (APTT), lactate, blood glucose, blood urea nitrogen (BUN), and blood creatinine. To avoid the influence of maximum or minimum values on the research results, winsorization was applied to process the laboratory results, with 1% and 99% as the cutoff points. We also used ICD‐9 codes to define the main disease complications, including pneumonia, hypertension, infection, acute kidney failure, and diabetes. Acidosis was defined as a blood pH <7.35. Acute kidney failure (now acute kidney injury) refers to increase in serum creatinine level by ≥0.3 mg/dl within 48 h, or urine volume <0.5 ml/kg/h for 6 h or more, or a ≥1.5‐fold increase from the baseline serum creatinine level within the seven days prior. 11 Shock refers to systolic blood pressure (SBP) ≤90 mmHg. The treatment methods included mechanical ventilation (MV) and continuous renal replacement therapy. Several scoring systems, such as the Acute Physiology Score (APS III), Sequential Organ Failure Assessment (SOFA), and Simplified Acute Physiology Score II (SAPS II), were also used to assess the severity of the patients’ conditions and their mortality rates during hospitalization. The type of admission is related to the prognosis of trauma patients; thus, it was also included in the analysis.
2.4. Statistical analysis
Statistical data were analyzed using SPSS version 26, and relevant figures were drawn using GraphPad prism8. Continuous variables are presented as means and standard deviations or medians and interquartile ranges. A two‐tailed independent Student's t test or Wilcoxon rank–sum test was used for the assessment of continuous variables in the two groups. Categorical variables are presented as percentages, and the differences between the two groups were compared using the chi‐square test. Multivariable logistic regression was performed to identify the determinant risk factors for in‐hospital death. Variables that were significant between the survival and death groups were included in the model. Acidosis was defined as a blood pH. Infection, which is a relatively long‐term complication, increases gradually with the extension of hospital stay. Therefore, they were not included in the multivariable regression model. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were reported as well. We calculated the area under the curve (AUC) of the prediction model, APS III, SAPS II, and SOFA scores to evaluate the predictive efficacy of the model. To avoid overfitting or poor fitting, the data were randomly divided into 7:3 groups for 10 times cross‐validation to evaluate the mean accuracy and precision of the model.
3. RESULTS
A total of 842 patients with trauma‐related ARDS were included in this study. Of these, 667 were included in the survivor group and 175 in the deceased group. Males accounted for 57.72% of the participants. The average age of the participants was 61.97 ± 17.40 years old, whereas their average BMI was 29.11 ± 7.01 kg/㎡. The median length of stay in the ICU was 9.28 (4.13–17.39) days, and the hospital mortality rate was 20.8%. The deceased group had lower pH, OI, higher red blood cell distribution width, hematocrit level, INR, PT, APPT, lactate level, blood glucose level, BUN level, and blood creatinine level than the survivor group. The differences between the APS III (p = 0.001), SOFA (p = 0.001), and SAPS II (p = 0.001) scores of the two groups were statistically significant. Type of admission had a significant impact on the in‐hospital mortality of the patients. The mortality rate at the time of emergency admission was 24.18%. In addition, there was no significant difference in MV between the deceased and survivor groups (98.29% vs. 95.65%, p = 0.162). Continuous renal replacement therapy was significantly different between the two groups (21.71% vs. 4.2%, p = 0.001). The results are presented in Table 1.
TABLE 1.
Baseline between survivors and non‐survivors in‐hospital mortality
| Variable | Total (n = 842) | Survivors (n = 667) | Non‐survivors (n = 175) | p Value |
|---|---|---|---|---|
| Age (year) | 61.97 ± 17.40 | 60.52 ± 17.70 | 67.52 ± 15.00 | 0.001 |
| BMI (kg/㎡) | 29.11 ± 7.01 | 29.49 ± 7.04 | 27.63 ± 6.70 | 0.002 |
| ICU LOS | 9.28 (4.13–17.39) | 10.10 (4.62–18.19) | 6.86 (2.43–13.38) | 0.001 |
| Male (n%) | 486 (57.72%) | 387 (58.02%) | 99 (56.57%) | 0.730 |
| Laboratory Inspection | ||||
| PH value | 7.34 ± 0.10 | 7.35 ± 0.10 | 7.32 ± 0.12 | 0.001 |
| OI (mmHg) | 185.66 ± 72.31 | 189.03 ± 71.37 | 172.82 ± 74.62 | 0.008 |
| WBC (k/ul) | 13.76 ± 7.54 | 13.58 ± 6.99 | 14.48 ± 9.34 | 0.235 |
| RBC (m/ul) | 3.53 ± 0.67 | 3.51 ± 0.66 | 3.60 ± 0.71 | 0.145 |
| HB (g/l) | 10.70 ± 1.95 | 10.64 ± 1.92 | 10.94 ± 2.04 | 0.068 |
| RDW (%) | 14.81 ± 1.73 | 14.62 ± 1.57 | 15.52 ± 2.11 | 0.001 |
| PLT (k/ul) | 211.51 ± 113.59 | 214.36 ± 110.05 | 200.63 ± 125.92 | 0.189 |
| HCT (%) | 31.53 ± 5.89 | 31.22 ± 5.75 | 32.68 ± 6.28 | 0.004 |
| INR | 1.44 ± 0.49 | 1.40 ± 0.44 | 1.60 ± 0.64 | 0.001 |
| PT (seconds) | 15.36 ± 3.47 | 15.08 ± 3.00 | 16.39 ± 4.73 | 0.001 |
| APTT (seconds) | 39.94 ± 24.37 | 38.48 ± 22.50 | 45.49 ± 29.90 | 0.004 |
| Lactate (mmol/l) | 3.05 ± 2.51 | 2.69 ± 2.01 | 4.43 ± 3.54 | 0.001 |
| Glucose (mg/dl) | 150.06 ± 58.52 | 147.59 ± 53.79 | 159.46 ± 73.25 | 0.046 |
| BUN (mg/dl) | 21.65 ± 14.42 | 19.80 ± 12.33 | 28.69 ± 18.97 | 0.001 |
| Creatinine (mg/l) | 0.90 (0.70–1.20) | 0.90 (0.70–1.20) | 1.00 (0.80–1.60) | 0.001 |
| ICU Severity Score | ||||
| APS III | 42.37 ± 14.98 | 46.71 ± 19.28 | 66.81 ± 29.07 | 0.001 |
| SOFA | 6.62 ± 3.50 | 6.06 ± 3.03 | 8.75 ± 4.28 | 0.001 |
| SAPS II | 42.37 ± 14.98 | 39.17 ± 12.76 | 54.55 ± 16.51 | 0.001 |
| Admission Type | 0.001 | |||
| Elective | 231 (27.77%) | 209 (31.33%) | 22 (12.57%) | |
| Emergency | 579 (68.76%) | 439 (65.82%) | 140 (80.00%) | |
| Urgent | 32 (3.80%) | 19 (2.85%) | 13 (7.43%) | |
| Treatment Method | ||||
| MV (n%) | 810 (96.2%) | 638 (95.65%) | 172 (98.29%) | 0.162 |
| CRRT (n%) | 66 (7.84%) | 28 (4.20%) | 38 (21.71%) | 0.001 |
Continuous variables were presented using mean and standard deviation or median and interquartile range. A two‐tailed independent student's t test or Wilcoxon rank‐sum test was used for continuous variables in two groups. Categorical variables were presented as percentages, and differences between the two groups were compared using the chi‐square test.
Abbreviations: APS III, Acute Physiology Score III; APTT, activated partial prothrombin time; BMI, body mass index; BUN, blood urea nitrogen; CRRT, continuous renal replacement therapy; HB, hemoglobin; HCT, hematocrit; ICU LOS, intensive care unit length of stay; INR, international normalized ratio; MV, mechanical ventilation; OI, oxygenation index; PLT, platelets; PT, prothrombin time; RBC, red blood cells; RDW, red blood cell distribution width; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment; WBC, white blood cells.
We investigated the major complications and comorbidities that were recorded during hospitalization (Table 2). The occurrence of post‐traumatic secondary pneumonia was not statistically significant in either group (p = 0.086), whereas the occurrence of infection, acute renal failure, sepsis, shock, acute posthemorrhagic anemia, and acidosis were statistically significant. The significant variables of the multivariate logistic regression analysis Table 3 were included in the nomogram (Figure 1).
TABLE 2.
Major complications of two group
| Comorbidities, n(%) | Total (n = 842) | Survivors (n = 667) | Non‐survivors (n = 175) | p Value |
|---|---|---|---|---|
| Pneumonia | 356 (42.3) | 292( 43.8) | 64 (36.6) | 0.086 |
| Hypertension | 336 (39.9) | 281 (42.1) | 55 (31.4) | 0.01 |
| Infection | 295 (35.0) | 246 (36.9) | 49 (28.0) | 0.028 |
| Acute kidney failure | 208 (24.7) | 130 (19.5) | 78 (44.6) | 0.001 |
| Diabetes | 173 (20.5) | 147 (22.0) | 26 (14.9) | 0.036 |
| Sepsis | 170 (20.2) | 101 (15.1) | 69 (39.4) | 0.001 |
| Shock | 163 (19.4) | 110 (16.5) | 53 (30.3) | 0.001 |
| Acute posthemorrhagic anemia | 138 (16.4) | 119 (17.8) | 19 (10.9) | 0.026 |
| Acidosis | 117 (13.9) | 76 (11.4) | 41 (23.4) | 0.001 |
TABLE 3.
Multivariate logistic regression models in‐hospital mortality
| Predictor | OR (95% CI) | p Value |
|---|---|---|
| Age (year) | 1.035 (1.020–1.049) | 0.001 |
| BMI (kg/㎡) | 0.957 (0.926–0.989) | 0.008 |
| PH value | 0.754 (0.099–5.764) | 0.786 |
| OI (mmHg) | 0.998 (0.995–1.000) | 0.096 |
| RDW (%) | 1.283 (1.141–1.443) | 0.001 |
| HCT (%) | 1.055 (1.017–1.095) | 0.004 |
| INR | 1.691 (0.735–3.892) | 0.217 |
| PT (seconds) | 0.953 (0.842–1.077) | 0.44 |
| APTT (seconds) | 0.998 (0.989–1.008) | 0.731 |
| Lactate (mmol/l) | 1.226 (1.127–1.334) | 0.001 |
| Glucose (mg/dl) | 1.003 (1.000–1.006) | 0.07 |
| BUN (mg/dl) | 1.025 (1.007–1.044) | 0.007 |
| Creatinine (mg/l) | 0.881 (0.700–1.108) | 0.279 |
| Hypertension | 0.689 (0.442–1.074) | 0.1 |
| Diabetes | 0.602 (0.340–1.064) | 0.081 |
| Acute posthemorrhagic anemia | 0.572 (0.310–1.054) | 0.073 |
| Acute kidney failure | 1.875 (1.188–2.959) | 0.007 |
| Sepsis | 1.917 (1.165–3.153) | 0.01 |
| Shock | 0.818 (0.471–1.422) | 0.477 |
| Admission type | ||
| Emergency VS elective | 2.822 (1.647–4.837) | 0.001 |
| Urgent VS elective | 5.156 (1.896–14.027) | 0.001 |
Abbreviations: APTT, activated partial prothrombin time; BMI, body mass index; BUN, blood urea nitrogen; HCT, hematocrit; INR: international normalized ratio; OI, oxygenation index; PT, prothrombin time; RDW, red blood cell distribution width.
FIGURE 1.
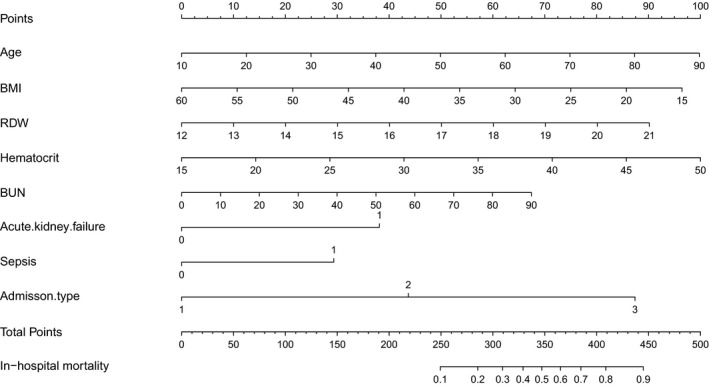
Construction of a nomogram with clinical indices to predict traumatic ARDS in‐hospital mortality (based on the model). The score for each value is assigned by drawing a line upward to the points line, and the sum of the nine scores is plotted on the total points line. ARDS, acute respiratory distress syndrome
In Figure 2, we show the calibration curves of survival group and death group .The relationship between continuous variables and in‐hospital death in the model is shown in Figure 3 using the Locally Weighted Scatterplot Smoothing (Lowess). Survival curves were generated by analyzing the relationship between the categorical variables and 90‐day mortality using a univariate Cox regression model (Figure 4). The AUCs and 95% CIs of the logistic regression model and the SAPS II, APS III, and SOFA scores are shown in Figure 5 Table 4. In the cross‐validated model, the test set achieved a mean accuracy of 0.823, a precision of 0.643, and an AUC of 0.813, which shows good predictive value (Table 5).
FIGURE 2.
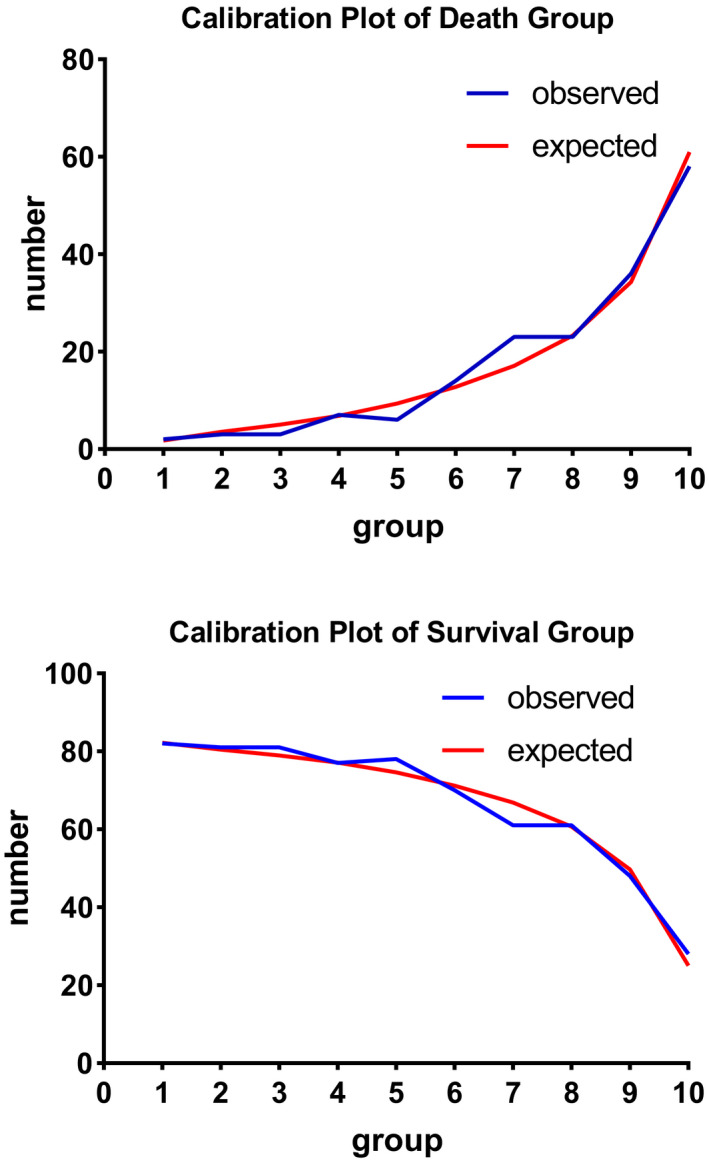
Calibration Plot of Death Group and Survival Group show the relationship between the observed outcome frequencies and the predicted frequencies; model is adjusted by age, BMI, red blood cell distribution width, hematocrit, lactate, blood urea nitrogen, admission type, acute renal failure, and sepsis
FIGURE 3.
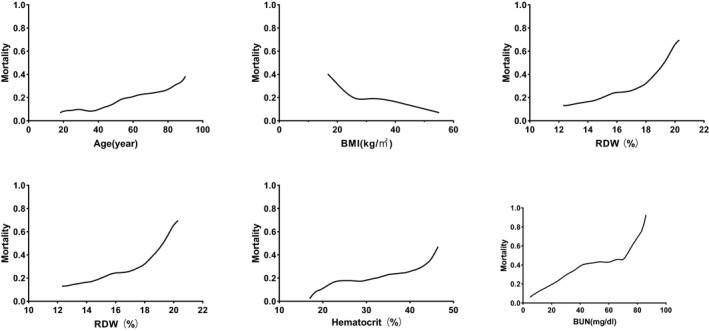
LOWESS Smooth Curve shows continuous variables and in‐hospital mortality in logistic regression model
FIGURE 4.
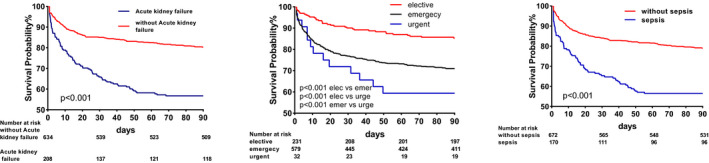
Univariate Cox regression model showed the relationship between categorical variables and 90‐day mortality
FIGURE 5.
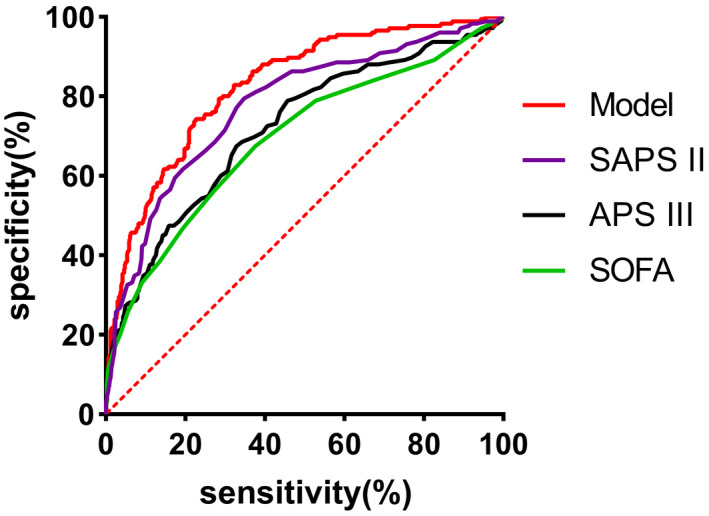
ROC shows area under curve of model, APS III, SOFA, SAPS II. APS III, acute physiology score III; SAPS II, simplified acute physiology score II; SOFA, sequential organ failure assessment
TABLE 4.
AUC and 95% CI of model and ICU severity score
| Score | AUC | 95% CI | p Value |
|---|---|---|---|
| Model | 0.826 | 0.793–0.861 | 0.001 |
| APS III | 0.718 | 0.673–0.763 | 0.001 |
| SOFA | 0.692 | 0.645–0.739 | 0.001 |
| SAPS II | 0.776 | 0.736–0.817 | 0.001 |
Model is adjusted by age, BMI, red blood cell distribution width, hematocrit, lactate, blood urea nitrogen, admission type, acute renal failure, and sepsis.
Abbreviations: APS III, Acute Physiology Score III; SAPS II, simplified acute physiology score II; SOFA, sequential organ failure assessment.
TABLE 5.
Cross‐validation model of 10 times random groups
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy | 0.815 | 0.802 | 0.825 | 0.838 | 0.823 | 0.840 | 0.829 | 0.847 | 0.799 | 0.802 |
| Precision | 0.700 | 0.666 | 0.640 | 0.621 | 0.576 | 0.576 | 0.653 | 0.772 | 0.551 | 0.724 |
| AUC | 0.831 | 0.803 | 0.816 | 0.807 | 0.811 | 0.845 | 0.789 | 0.844 | 0.771 | 0.828 |
Model is adjusted by age, age, BMI, red blood cell distribution width, hematocrit, lactate, blood urea nitrogen, admission type, acute renal failure, and sepsis. Data were randomly divided into 7:3 groups for 10 times cross‐validation. The table shows the accuracy, precision, and AUC (area under curve) of the test set. In this article, we report the average of 10 results (accuracy: 0.823; precision: 0.643; AUC: 0.813).
4. DISCUSSION
Post‐traumatic ARDS is a systemic inflammatory response syndrome (SIRS) and compensatory anti‐inflammatory response (CARS) that is pathologically characterized by diffuse lung injury, pulmonary edema, and inflammatory cell infiltration. 12 Reduced lung compliance and disproportionate ventilatory flow after thoracic trauma, neurogenic pulmonary edema after head trauma, 13 fat embolism after fracture of the extremities, and second surgery are the most important predisposing factors for post‐traumatic ARDS. 14 The incidence rate of ARDS increases significantly within 24 h when the infusion of red blood cells is more than 5U. 15 It is believed that trauma‐induced SIRS is a major cause of post‐traumatic ARDS. 1 In severe trauma, the body can activate and release inflammatory cytokines, including tumor necrosis factor (TNF) and interleukin (IL). ARDS occurs when SIRS is out of balance with CARS.
Previous studies have confirmed that many patients with ARDS die from sepsis and multiple organ failure, with only about 9% dying from respiratory failure. 16 , 17 The findings of an ARDS study conducted over three years suggested that the incidence of trauma‐related ARDS is decreasing (3%–1.1%), whereas its mortality rate is increasing (18%‐21%). 18 Therefore, it is important to establish a prognostic model to facilitate timely identification of high‐risk patients and reduction in hospital mortality.
In the study, age, BMI, red blood cell distribution width, hematocrit, lactate, BUN, type of admission, acute renal failure, and sepsis were independently associated with the prognoses of patients. The similarities and differences in the prognoses of patients with trauma‐related ARDS, sepsis‐related ARDS, and trauma patients were also assessed in this study. These results suggest that ARDS is a heterogeneous group of disorders and that its clinical features vary according to its different types.
The results of previous trauma studies suggest that obese elderly patients have poor prognoses. 19 , 20 The findings of a few studies suggest that obesity is not associated with mortality. 21 , 22 In short, obesity tends to be a non‐protective factor in trauma patients. However, in an ARDS study, Gong et al. and Anzueto et al. 23 , 24 reported that obesity increases the incidence rate of ARDS and has no significant effect on mortality. The results of some previous studies suggest that BMI is negatively correlated with mortality due to ARDS, 25 , 26 a finding that is similar to our results. There are also some hypotheses about the protective effects of moderate obesity. For example, fat can provide energy and fat‐soluble nutrients under high metabolic conditions. Adipocytes also secrete immunomodulatory substances, such as leptin and IL‐10, which attenuate inflammatory responses and improve survival in critically ill patients. 27 However, poor distribution of fat, muscle loss‐related obesity, 28 and obesity are easily misdiagnosed as ARDS. In addition, these factors may affect the interpretation of examination results. The relationship between obesity and the prognosis of patients with traumatic ARDS still needs further research.
Most studies indicate that patients with diabetes have an increased risk of infection compared to the general population, especially in the skin, soft tissue, urogenital system, gastrointestinal tract, and respiratory system. The prognosis of patients with diabetes is worse than that of patients without diabetes. 29 , 30 , 31 However, in most ARDS cohorts, diabetes significantly increases the incidence rate of ARDS, especially sepsis or pneumonia‐related ARDS, but does not increase its long‐term mortality rate. In the present study, diabetes (OR, 0.602; 95% CI, 0.340–1.064) was not correlated with the prognoses of the patients. This may be mediated by reduced peroxide production, decreased TNF‐1 β and IL‐10, and other mechanisms involved in bronchoalveolar fluid. 32 In addition, leptin resistance confers protection against lung injury in diabetes. 33
The prognostic factors of trauma‐related ARDS are different from those of sepsis‐related ARDS. Urea nitrogen, an end product of protein metabolism in humans, has been infrequently reported in to be associated with septic ARDS. Further, elevation of BUN level occurs in chronic renal failure and trauma after severe surgery. A urea nitrogen/creatinine ratio greater than 10:1 between the two groups in the present study suggests that patients with traumatic ARDS have predominantly prerenal azotemia. The results of studies by Brown et al. and Komara et al. 34 , 35 also suggest that high urea nitrogen level is associated with poor outcomes in patients with ARDS. We also observed that the hematocrit level of the deceased group in the present study was higher than that of the survivor group; however, both were lower than the normal value. Even after adjustment, the hematocrit level remained statistically significant (OR = 1.055, p = 0.004). This may be related to the higher blood concentration caused by capillary leakage. 36 In addition, pneumonia is regarded as a risk factor for ARDS in most studies. 1 However, we observed no significant difference in post‐traumatic pneumonia between the two groups (48.8% vs. 36.6%, p = 0.086).
As previously mentioned, ARDS is a heterogeneous disease; thus, the APACHE II score does not have high accuracy (0.623–0.777) for the prediction of ARDS. 37 , 38 The APS III, SOFA, and SAPS II scores analyzed in this study also have poor prediction effects. Therefore, we developed a model to predict in‐hospital mortality in patients with ARDS using simple and easily available variables. The combined use of multiple data provides a more accurate prediction of the probability of survival. The results of the present study show that the model can be used as an economical and effective tool for predicting the prognosis of ARDS and assisting in clinical decision‐making.
This study has several limitations. First, although many studies have confirmed that immune response and cardiovascular overload caused by blood transfusion can increase the incidence of ARDS and mortality in critically ill patients, we did not evaluate the correlation between blood transfusion and mortality in this study. Second, different aspects of trauma that affect the mortality of patients with ARDS may impact the results of the present study. Third, we did not study the mechanism of trauma on patient mortality. At last, this was a single‐center, retrospective study with a long study period; thus, the research results may have some deviation and may need more external data for verification.
5. CONCLUSION
We used clinical variables to develop a new model for the prediction of in‐hospital mortality in patients with ARDS. The results of this study can aid the timely identification of high‐risk patients and the development intervention strategies.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGEMENT
None.
Tang R, Wang H, Peng J, Wang D. A trauma‐related survival predictive model of acute respiratory distress syndrome. J Clin Lab Anal. 2021;35:e24006. 10.1002/jcla.24006
DATA AVAILABILITY STATEMENT
Data available on request.
REFERENCES
- 1. Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788‐800. [DOI] [PubMed] [Google Scholar]
- 2. Pfeifer R, Heussen N, Michalewicz E, Hilgers RD, Pape HC. Incidence of adult respiratory distress syndrome in trauma patients: a systematic review and meta‐analysis over a period of three decades. J Trauma Acute Care Surg. 2017;83(3):496‐506. [DOI] [PubMed] [Google Scholar]
- 3. Killien EY, Mills B, Vavilala MS, et al. Association between age and acute respiratory distress syndrome development and mortality following trauma. J Trauma Acute Care Surg. 2019;86(5):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yadav H, Thompson BT, Gajic O. Fifty years of research in ARDS. is acute respiratory distress syndrome a preventable disease? Am J Respir Crit Care Med. 2017;195(6):725‐736. [DOI] [PubMed] [Google Scholar]
- 5. Afshar M, et al. Trauma indices for prediction of acute respiratory distress syndrome. J Surg Res. 2016;201(2):394‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bajwa EK, Malhotra CK, Thompson BT, Christiani DC, Gong MN. Statin therapy as prevention against development of acute respiratory distress syndrome: an observational study. Crit Care Med. 2012;40(5):1470‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faust HE, Reilly JP, Anderson BJ, et al. Plasma mitochondrial DNA levels are associated with ARDS in trauma and sepsis patients. Chest. 2020;157(1):67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng M, McSparron JI, Kien DT, et al. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC‐III database. Intensive Care Med. 2018;44(6):884‐892. [DOI] [PubMed] [Google Scholar]
- 9. Johnson AE, Pollard TJ, Shen L, et al. MIMIC‐III, a freely accessible critical care database. Sci Data. 2016;3:160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307(23):2526‐2533. [DOI] [PubMed] [Google Scholar]
- 11. Kellum JA, Bellomo R, Ronco C. Definition and classification of acute kidney injury. Nephron Clin Pract. 2008;109(4):c182‐c187. [DOI] [PubMed] [Google Scholar]
- 12. Wang HE, Brown SP, MacDonald RD, et al. Association of out‐of‐hospital advanced airway management with outcomes after traumatic brain injury and hemorrhagic shock in the ROC hypertonic saline trial. Emerg Med J. 2014;31(3):186‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17(2):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duggal A, Perez P, Golan E, Tremblay L, Sinuff T. Safety and efficacy of noninvasive ventilation in patients with blunt chest trauma: a systematic review. Crit Care. 2013;17(4):R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duggal A, Mireles‐Cabodevila E, Krishnan S, Arroliga AC. Acute respiratory distress syndrome: implications of recent studies. Cleve Clin J Med. 2014;81(11):683‐690. [DOI] [PubMed] [Google Scholar]
- 16. Frutos‐Vivar F, Ferguson ND, Esteban A. Epidemiology of acute lung injury and acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):327‐336. [DOI] [PubMed] [Google Scholar]
- 17. Bersten AD, Edibam C, Hunt T, et al. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian states. Am J Respir Crit Care Med. 2002;165(4):443‐448. [DOI] [PubMed] [Google Scholar]
- 18. Kasotakis G, Stanfield B, Haines K, et al. Acute Respiratory Distress Syndrome (ARDS) after trauma: improving incidence, but increasing mortality. J Crit Care. 2021;64:213‐218. [DOI] [PubMed] [Google Scholar]
- 19. Liu T, Chen JJ, Bai XJ, Zheng GS, Gao W. The effect of obesity on outcomes in trauma patients: a meta‐analysis. Injury. 2013;44(9):1145‐1152. [DOI] [PubMed] [Google Scholar]
- 20. Clement ND, Tennant C, Muwanga C. Polytrauma in the elderly: predictors of the cause and time of death. Scand J Trauma Resusc Emerg Med. 2010;18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Newell MA, Bard MR, Goettler CE, et al. Body mass index and outcomes in critically injured blunt trauma patients: weighing the impact. J Am Coll Surg. 2007;204(5):1056‐1061. discussion 1062–4. [DOI] [PubMed] [Google Scholar]
- 22. Duane TM, Dechert T, Aboutanos MB, Malhotra AK, Ivatury RR. Obesity and outcomes after blunt trauma. J Trauma. 2006;61(5):1218‐1221. [DOI] [PubMed] [Google Scholar]
- 23. Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65(1):44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anzueto A, Frutos‐Vivar F, Esteban A. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax. 2011;66(1):66‐73. [DOI] [PubMed] [Google Scholar]
- 25. Soto GJ, Frank AJ, Christiani DC, Gong MN. Body mass index and acute kidney injury in the acute respiratory distress syndrome. Crit Care Med. 2012;40(9):2601‐2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Brien JM Jr, Phillips GS, Ali NA, Lucarelli M, Marsh CB, Lemeshow S. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34(3):738‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schetz M, De Jong A, Deane AM, et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757‐769. [DOI] [PubMed] [Google Scholar]
- 28. Neeland IJ, Poirier P, Després JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018;137(13):1391‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guvener M, Pasaoglu I, Demircin M, Oc M. Perioperative hyperglycemia is a strong correlate of postoperative infection in type II diabetic patients after coronary artery bypass grafting. Endocr J. 2002;49(5):531‐537. [DOI] [PubMed] [Google Scholar]
- 30. Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010;145(9):858‐864. [DOI] [PubMed] [Google Scholar]
- 31. Yong PH, Weinberg L, Torkamani N, et al. The presence of diabetes and higher HbA(1c) are independently associated with adverse outcomes after surgery. Diabetes Care. 2018;41(6):1172‐1179. [DOI] [PubMed] [Google Scholar]
- 32. Honiden S, Gong MN. Diabetes, insulin, and development of acute lung injury. Crit Care Med. 2009;37(8):2455‐2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singla A, Turner P, Pendurthi MK, Agrawal V, Modrykamien A. Effect of type II diabetes mellitus on outcomes in patients with acute respiratory distress syndrome. J Crit Care. 2014;29(1):66‐69. [DOI] [PubMed] [Google Scholar]
- 34. Brown LM, Calfee CS, Matthay MA, et al. A simple classification model for hospital mortality in patients with acute lung injury managed with lung protective ventilation. Crit Care Med. 2011;39(12):2645‐2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Komara NL, Paragomi P, Greer PJ, et al. Severe acute pancreatitis: capillary permeability model linking systemic inflammation to multiorgan failure. Am J Physiol Gastrointest Liver Physiol. 2020;319(5):G573‐G583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191‐1198. [DOI] [PubMed] [Google Scholar]
- 37. Hwang H, Choi SM, Lee J, et al. Validation of age, PaO(2)/FlO(2) and plateau pressure score in Korean patients with acute respiratory distress syndrome: a retrospective cohort study. Respir Res. 2020;21(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Polita JR, Gomez J, Friedman G, Ribeiro SP. Comparison of APACHE II and three abbreviated APACHE II scores for predicting outcome among emergency trauma patients. Rev Assoc Med Bras. 2014;60(4):381‐386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request.


