Abstract
Aperiodic activity contains important and meaningful physiological information that has been shown to dynamically change with age. However, no longitudinal studies have examined its development during early-to-mid adolescence. The current study closes this gap by investigating age- and sex-related longitudinal change in aperiodic activity across early-to-mid adolescence (N = 186; 54.3% female). Participants completed a resting state task and a Flanker task while EEG was record at age 13 years and again at age 15 years. Across different tasks and two time points, we observed significant age-related reductions in aperiodic offset and exponent. In addition, we observed significant sex-related differences in the aperiodic offset and exponent over time. We did not find any significant correlation between aperiodic activity and behavioral measures, nor did we find any significant condition-dependent change in aperiodic activity during the Flanker task. However, we did observe significant correlations between aperiodic activity across tasks and over time, suggesting that aperiodic activity may demonstrate stable trait-like characteristics. Collectively, these results may suggest a developmental parallelism between decreases in aperiodic components alongside adolescent brain development during this period; changes to cortical and subcortical brain structure and organization during early adolescence may have been responsible for the observed sex-related effects.
Keywords: EEG, Adolescence, Aperiodic activity, Development, Longitudinal, FOOOF
Highlights
-
•
Early adolescence is associated with changes in the aperiodic signal.
-
•
We observed significant sex-related differences in the aperiodic signal over time.
-
•
Aperiodic activity is significantly correlated within/between tasks and over time.
1. Introduction
Background aperiodic activity, often described as containing “1/f noise”, “scale-free”, or “fractal” dynamics (Donoghue et al., 2020a, He, 2014, He et al., 2010) contains important and meaningful physiological information that has been shown to dynamically change with age in the human brain. Background aperiodic activity present in the raw power spectrum can be characterized by two parameters, an aperiodic offset and an exponent. The offset denotes “the uniform shift in power across frequencies” (Donoghue et al., 2020a), and is believed to reflect neuronal population spiking (Manning et al., 2009, Miller et al., 2014). The exponent, measured as the positively signed X value in 1/fx formula, is related to the negative slope of the power spectrum in log-log space, that is, the inverse relationship between power and frequency. While prior work has demonstrated how aperiodic activity may change across age (Cellier et al., 2021, Dave et al., 2018, Chi et al., 2019, Schaworonkow and Voytek, 2021, Voytek and Knight, 2015a, Voytek et al., 2015b), prior work has not studied longitudinal change in aperiodic parameters during the early-to-mid adolescent period.
Findings from recent animal (Gao et al., 2017, Zhang et al., 2011) and human research (Donoghue et al., 2020a, Mamiya et al., 2021, Molina et al., 2020, Ostlund et al., 2021, Robertson et al., 2019, Voytek et al., 2015b) in addition to computational models (Gao et al., 2017) suggest that changes in exponent reflect changes in the ratio between excitatory (E) and inhibitory (I) currents in neural populations (Donoghue et al., 2020a, Gao et al., 2017). Specifically, reductions in exponent magnitude (flatter slopes) are thought to reflect increases in E:I ratios while increases in exponent magnitude (steeper slopes) are believed to reflect decreases in E:I ratios (Donoghue et al., 2020a, Gao et al., 2017). There is an increasing body of evidence to suggest that E:I imbalances are associated with neurological and psychiatric disorders such as attention-deficit/hyperactivity disorder (ADHD; Mamiya et al., 2021; Ostlund et al., 2021; Robertson et al., 2019) and schizophrenia (Molina et al., 2020) and Fragile X Syndrome (FXS; Wilkinson and Nelson, 2021). This is particularly evident in instances in which pharmaceutical interventions have led to changes in E:I ratios in schizophrenia (Molina et al., 2020) and possibly in ADHD (Mamiya et al., 2021, Robertson et al., 2019). These findings suggest that aberrant changes in the E:I balance found in particular disorders like ADHD and schizophrenia show that an optimal E:I balance may be of particular importance to typical and atypical brain development and function.
Prior studies have shown that changes in background aperiodic activity may reflect both task-related effects/state-like characteristics (Donoghue et al., 2020a, Ouyang et al., 2020; Podvalny et al., 2015) in addition to trait-like characteristics (Dave et al., 2018). For example, using intracranial recordings in six subjects with medication resistant epilepsy, Podvalny et al. (2015) found that there was a reduction in exponent magnitude during a visuomotor and object recognition task compared to the pre-stimulus baseline period. Ouyang et al. (2020) found that variability in 1/f-like activity during both eyes open and eyes closed conditions was predictive of cognitive processing speed as measured via reaction times (RTs). Donoghue et al. (2020a) showed that during a visual working memory task, aperiodic activity was a better predictor of performance than alpha activity whereas Dave et al. (2018) found that1/f -like activity significantly predicted N400 effects of successful lexical prediction and that whole head 1/f -like activity was highly correlated between tasks. The authors interpreted this finding as representing a “consistent and characteristic measure of the individual”. Ostlund et al. (2021) found that when controlling for ADHD status, adolescents with reduced exponent magnitudes (flatter spectral slope) demonstrated less RT variability and faster drift rates. In summary, these studies suggest that background aperiodic activity demonstrates both trait- and state-like characteristics. However, no prior work has tested whether longitudinal change in aperiodic activity predicts longitudinal changes in behavior. We address this question in the current study.
Age-related changes in aperiodic offset (Cellier et al., 2021, Donoghue et al., 2020a, Chi et al., 2019) and exponent (Cellier et al., 2021, Dave et al., 2018, Chi et al., 2019, Schaworonkow and Voytek, 2021, Voytek et al., 2015b, Zhang et al., 2011) have been observed in scalp EEG, electrocorticographic (ECoG) and MEG recordings during different stages of postnatal animal (Zhang et al., 2011) and human brain development (Cellier et al., 2021, Dave et al., 2018, Chi et al., 2019, Voytek et al., 2015b) including infants over the first year of life (Schaworonkow and Voytek, 2021). Although, in most of these studies sample sizes have been relatively small and age ranges relatively large, these studies show that brain development is associated with significant decreases in offset and exponent over time, indicating both a decrease in broadband power and a move away from cortical inhibition – a redistribution in the power spectrum from lower frequencies to higher frequencies (Table 1).
Table 1.
Summary of existing studies that have examined age-related effects in 1/f-like activity during human brain development.
| Developmental Research |
Imaging Method / Experimental Paradigm | Participants / age ranges | Sample size | Results | |
|---|---|---|---|---|---|
| Cellier et al. (2021) | Scalp EEG: Resting state, eyes open | Early childhood to young adulthood (ages 3 – 24 years) | N = 96 | Aperiodic offset and exponent decreased with age indicating decreases in broadband spectra power and a flattening of the slope | |
| Dave et al. (2018) | Scalp EEG: Comprehension/Prediction |
2 different age groups consisting of young adults (ages 18 – 28) and older adults (ages 64 – 79) | N = 96 | 1/f slopes became flatter with increasing age. Whole head 1/f slopes strongly correlated across tasks. The 1/f slope was a significant predictor of the effects of the N400, an ERP associated with language comprehension. | |
| Donoghue et al. (2020a) | Scalp EEG: resting state Scalp EEG: working memory task |
2 different groups consisting of young adults (ages 20 – 30 years) and older adults (ages 60 – 70 years) | N = 40 | Older adults had lower aperiodic offsets and exponents (flatter slopes). Aperiodic activity was a more consistent predictor of behavior than was periodic alpha band activity. | |
| Donoghue et al. (2020b) | Scalp EEG: resting state, eyes closed | Sample included children and adults (ages 6 – 44) | N = 111 | The aperiodic exponent was found to be highly correlated with age. θ/β ratio mostly reflective of changes in the aperiodic exponent and not θ power or β power fluctuations. | |
| He et al. (2019) | MEG resting state: eyes open | Sample included children (ages 8 +/- 2.5 years) and adults (ages 41 +/- 17 years) | N = 52 | Aperiodic offsets reduced and exponents decreased (flatter slopes) in adults compared to children. Only power in the β band was found to increase with age. | |
| Pathania et al. (2021) | Scalp EEG: resting state Cognitive assessments |
Young adults (ages 19 – 33 years) and older adults (ages 59 – 83 years) | N = 44 | Flatter spectral slopes in older compared to younger adults. Changes in spectral slopes mediated the relationships found between age-related differences in cognition. | |
| Schaworonkow and Voytek. (2021) | Longitudinal Scalp EEG: Baseline, reaching and non-reaching trials | Infants with 1 – 6 EEG recordings per infant with 1 month between sessions (ages from 1st recording to final recording 38 – 208 days) | N = 22 | Aperiodic exponent was found to decrease with increases in age. | |
| Voytek et al. (2015) | ECoG in preoperative patients with epilepsy: listening tasks Scalp EEG: visual working memory task |
Epilepsy patients (ages 15−53 years) and young adults (ages 20 – 30) and older adults (ages 60 – 70 years) | N = 39 | In both ECoG and EEG recordings aging was associated with flatter 1/f spectral slopes. Visual cortical 1/f slopes significantly mediated age-related effects found in visual working memory tasks. | |
Note: θ = theta periodic oscillations; β = beta periodic oscillations; EEG = electroencephalography; ECoG = electrocorticographic; This is not an exhaustive list and includes only studies that have examined changes in 1/f-like activity at different stages of brain development
However, little is known about how aperiodic activity changes during the adolescent period, a developmental period during which we know that subdivisions of the brain undergo substantial structural refinement, most notably protracted maturation trajectories in prefrontal cortical regions relative to cortical and subcortical limbic and striatal regions associated with affect and motivated states (Blakemore, 2012, Blakemore and Choudhury, 2006, Casey et al., 2000, Cao et al., 2017, Dosenbach et al., 2010, Goddings et al., 2014, Mills et al., 2016, Paus, 2005, Segalowitz et al., 2010). Sexual dimorphism in human brain development is also well documented, with girls showing earlier brain maturation patterns when compared to boys of similar age (Blakemore, 2012, Dennison et al., 2013, Giedd, 2004; Giedd et al., 1996; Lenroot et al., 2007; Neufang et al., 2008; Sowell et al., 2001). However, to the best of our knowledge, no longitudinal study has investigated how developmental trajectories in aperiodic activity may differ between male and female participants during early adolescence. Also, to the best of our knowledge pubertal status during early adolescence has been overlooked in this domain. Finally, most of these studies are cross-sectional and have not examined aperiodic activity across multiple tasks using longitudinal data.
The aims of the current study are therefore to 1) investigate age- and sex-related changes in the aperiodic offset and exponent in a longitudinal sample of early adolescents from whom we acquired resting state and Flanker task EEG at two time points, at approximately 13 and 15 years of age; 2) investigate whether changes in aperiodic activity are condition dependent; 3) investigate whether aperiodic activity is associated with Flanker RTs, Flanker RT variability and Flanker accuracy; 4) to examine whether aperiodic activity is correlated across tasks and over time. First, based upon prior age-related findings (Table 1) we hypothesized that adolescents would show decreases in offset and exponent magnitude over time. We also hypothesized that male participants would show greater offset and exponent magnitudes when compared to female participants. This hypothesis is based on prior research showing sexual dimorphism in human brain development (Blakemore, 2012, Dennison et al., 2013, Giedd, 2004; Giedd et al., 1996; Lenroot et al., 2007; Neufang et al., 2008; Sowell et al., 2001). As an additional exploratory step, we also investigated whether a measure of pubertal development explained any interactions found between sex and age. Second, based on prior findings we hypothesized that we would see differences in aperiodic activity between tasks (Podvalny et al., 2015). Third, based on prior studies (Ostlund et al., 2021, Ouyang et al., 2020) we hypothesized that reduced exponent magnitudes would be associated with shorter RTs, less RT variability and increases in accuracy. Finally, based on the Dave et al. (2018) study we hypothesized that measures of aperiodic activity would be significantly correlated between task and over time.
2. Methods
2.1. Participants
The current study included adolescents who were recruited as part of a larger longitudinal study investigating individual differences in temperament and socio-emotional development. This study was conducted in the Washington DC area beginning in 2001 (Hane et al., 2008). Here, we focus on 186 adolescents (female = 101, Caucasian = 125, Multi-ethnic = 29, African American = 23, Hispanic = 5, Asian = 3, Other = 1) for whom we had usable EEG data collected in at least one of the two separate time points during resting state or during the Flanker task. The first EEG recordings were conducted when the participants were approximately thirteen years of age (N = 145, M age = 13.14, SD = 0.62, age range = 12 – 15) and the second when the participants were approximately 15 years of age (N = 163, M age = 15.41, SD = 0.61, age range = 15–17). Of the 186 participants, 106 had data points for all measures collected at both time point 1 and time point 2. All procedures were approved by the University of Maryland College Park institutional review board. All parents provided written informed consent and all participants provided assent.
2.2. Resting state and Flanker task EEG
All EEG recordings were conducted in a dimly lit and sound-attenuated room and all participants were alone in the room during each recording session. The experimenters monitored the experiment from an adjacent room. Resting state EEG data were recorded during alternating one-minute eyes open and one-minute eyes closed conditions. In total six minutes of resting state data were collected. During the eyes open condition participants were instructed to fixate on a small white cross in the center of a computer screen. Following the resting state recordings, each participant completed a Flanker task (Eriksen and Eriksen, 1974). The Flanker task always came after the resting state task. The Flanker task included 12 blocks each consisting of 32 trials. Each trial began with the presentation of a fixation cross which was then followed by the presentation of a central arrowhead flanked on each side by two additional arrowheads facing either in the same direction (congruent) or in the opposite direction (incongruent). Participants were instructed to ignore the flanking arrowheads and to indicate the direction of the central arrowhead by pressing a button on an EGI response pad button box (Model: 4608150–50). At the end of each block, participants received feedback regarding their performance. If they performed at or below 75%, text was presented on the screen indicating that they need to be more accurate. If they performed at 90% or higher, text was presented on the screen indicating that they need to respond faster. If performance was between 75% and 90%, text was presented on the screen indicating that they were doing a good job. At all times participants were seated approximately 1 m from a 17″ LCD monitor. The stimuli were presented using E-Prime 2.0.874 (Psychology Software Tools, Pittsburg, PA).
2.3. EEG acquisition and preprocessing
For each visit, EEG data were collected using a 128-channel HydroGel Geodesic Sensor Net using EGI software (Electrical Geodesic, Inc., Eugene, OR). EEG preprocessing and analyses were conducted using the EEGLAB toolbox (Delorme and Makeig, 2004) with custom MATLAB scripts (The MathWorks, Natick, MA). EEG data were preprocessed following the procedures described in the Maryland Analysis of Developmental EEG (MADE) pipeline (Debnath et al., 2020, https://github.com/ChildDevLab/MADE-EEG-preprocessing-pipeline). Details of the EEG processing steps are presented in Supplement 2.
2.4. Parameterizing the power spectra
First, a Fast Fourier Transformation (FFT) was performed on the epoched data sets using a Hanning taper, which resulted in a frequency resolution of 0.33 Hz. The power spectra for each electrode were calculated for each epoch and then averaged across all of the epochs. This resulted in a power x channel matrix for each participant from which we averaged across all electrodes to create a single power spectrum for each participant in each condition. Finally, prior to parameterizing the power spectra, group matrices were created for each condition (resting state eyes open and resting state eyes closed, Flanker all conditions (congruent and incongruent together), Flanker congruent and Flanker incongruent separately). These group matrices encompassed all of the participants power spectra for session 1 and session 2 separately. The Fitting Oscillations and One-Over-F (FOOOF)1 algorithm (Donoghue et al., 2020a) – an open source Python package (https://github.com/fooof-tools/fooof/) - was then applied to the group x power matrices in Python (v3.7.0) from which the aperiodic offset and exponent were extracted. Both the aperiodic offset and exponent were extracted and entered into RStudio (Version 1.2.5001) for further analyses using R Version 3.6.1(R Core Team (2019). An overview of the FOOOF algorithm alongside a figure depicting the steps taken when parametrizing the power spectra is presented in Supplement 2.
2.5. Statistical analyses
To examine age- and sex-related change in aperiodic offset and exponent, linear mixed effects models (LME) were performed in RStudio using the nlme package (Pinheiro et al., 2007). For each analysis, Mahalanobis distance was used to investigate the presence of multivariate outliers. If outliers were found (χ2 < 0.001), these were removed before the LME model was run. In addition, Q-Q plots, histograms showing the standardized residuals, and scatter plots showing fitted versus standardized residuals were used to assess model assumptions. Each of the dependent variables (aperiodic offset/exponent resting state data and aperiodic offset/exponent Flanker data) were entered into separate LME models. For each LME model subject identifier was entered as a random effect. Condition, sex, and age in months were entered as fixed effects. In addition, we controlled for the number of trials per condition by adding this as an additional covariate in each model. Successive difference contrast coding was applied to the factors in the model. The method used for the LMEs was Maximum Likelihood. For participants who had missing data (i.e. if they only had data for one of the two data collection time points) these participants were still included in the analysis. See Supplement 1 for additional exploratory analyses with pubertal development added to the LME models for both aperiodic offset and exponent. To investigate whether log transformed Flanker RT, Flanker RT variability measured as SDRT (standard deviation reaction time) and Flanker accuracy were significant predictors of aperiodic activity (offset and exponent) we added these as covariates in our LME models. For each participant, anticipation RTs (RTs shorter than 150 ms) were removed prior to averaging or calculating SDRT. This approach is similar to that taken by Ostlund et al. (2021). All RT measures were based on correct responses only. We took additional steps to investigate non-linear relationships between aperiodic activity and log-transformed Flanker RT, Flanker SDRT and Flanker accuracy by conducting polynomial regression analyses in R with squared Flanker RT, squared Flanker SDRT and squared Flanker accuracy measures predicting aperiodic offset and exponent derived from each of the conditions. Finally, we conducted Pearson product-moment correlations to investigate the relationship between offset and exponent in each of the conditions and the correlation between offset and exponent across both time points (13 and 15 years of age). See Supplement 3 for the results of these analyses.
3. Results
3.1. Condition-, age- and sex-related change in aperiodic offset and exponent during resting state EEG
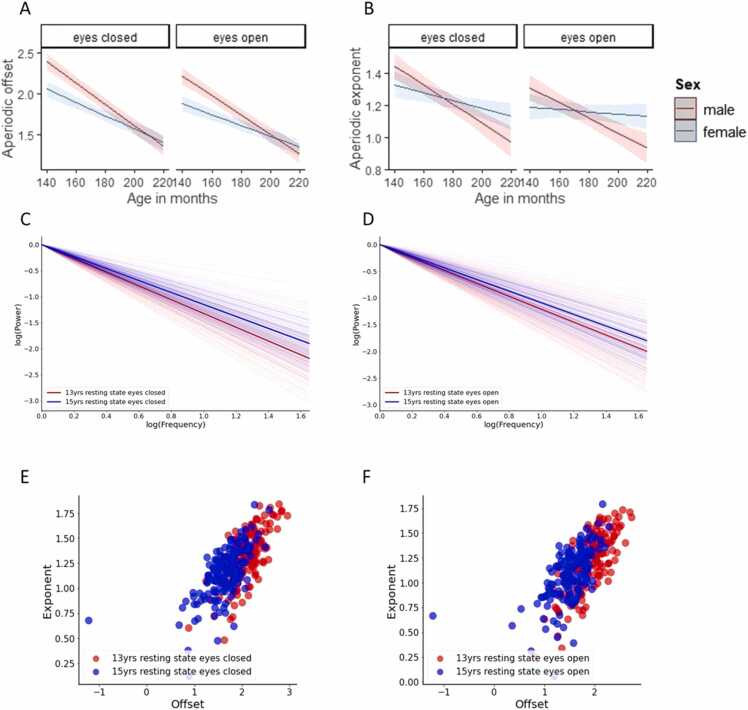
For the aperiodic offset during the eyes closed versus the eyes open condition, the results of LME model showed that there were significant main effects of condition (b = −0.35, t(358) = −2.38, p = 0.01, CI[− 0.64, −0.06]), sex (b = −1.04, t(177) = −5.37, p < 0.0001, CI[−1.42, −0.66]) and age (b = −0.01, t(358) = −15.21, p < 0.0001, CI[−1.42, −0.66]) showing that aperiodic offsets were significantly reduced during the eyes open condition compared to the eyes closed condition, significantly reduced in females compared to males, and significantly reduced with age. A significant sex*age interaction was also observed (b = 0.005, t (358) = 4.78, p < 0.0001, CI[0.003, 0.007]) showing that although male participants had higher offsets at earlier ages compared to females, the rate of change (reduction in aperiodic offset over time) was significantly greater for male participants than female participants (Fig. 1, A).
Fig. 1.
A) age- and sex-related change in aperiodic offset during resting state EEG B) age- and sex-related change in aperiodic exponent during resting state C) resting state eyes closed age group average aperiodic fits at 13 years and 15 years of age showing exponents plotted in log-log space while controlling for offset D) resting state eyes open age group average aperiodic fits at 13 years and 15 years of age showing exponents plotted in log-log space while controlling for offset E) resting state eyes closed exponent and offset values at 13 years and 15 years of age F) resting state eyes open exponent and offset values at 13 years and 15 years of age.
For the aperiodic exponent during the eyes closed condition versus the eyes open condition, again the results of LME model showed that there were significant main effects of condition (b = −0.33, t(358) = −2.60, p = 0.009, CI[−0.59, −0.08]), sex (b = −0.64, t(177) = −3.93, p = 0.0001, CI[− 0.96, −0.32]) and age (b = −0.003, t(358) = −6.30, p < 0.0001, CI[− 0.004, −0.002]), showing that aperiodic exponents were significantly reduced during the eyes open condition compared to the eyes closed condition, significantly reduced in females compared to males, and significantly reduced with age (i.e., flattening over time). A significant sex*age interaction was also observed (b = 0.003, t(358) = 4.14, p < 0.0001, CI[0.001, 0.005]) showing that while male participants had larger exponents at earlier ages compared to females, the rate of change (flattening in spectral slope over time) was significantly greater for male participants than female participants (Fig. 1, B).
3.2. Condition-, age- and sex-related changes in aperiodic offset and exponent during Flanker EEG
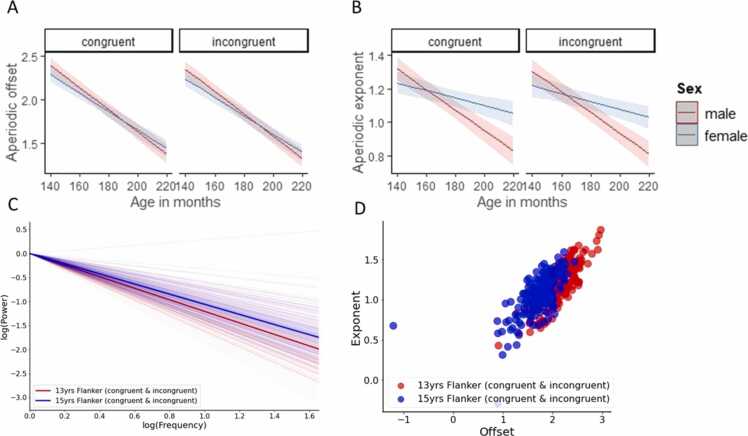
For the aperiodic offset during the Flanker congruent versus incongruent conditions, the results of LME model showed that there was no significant main effect of condition (b = −0.03, t(383) = −0.28, p = 0.77, CI[− 0.27, 0.20]) but there were significant main effects of sex (b = −0.41, t(182) = −2.64, p = 0.008, CI[− 0.72, −0.10]) and age (b = −0.011, t(383) = −23.75, p < 0.0001, CI[−0.012, − 0.010]) showing that although the aperiodic offset did not differ significantly between conditions (congruent vs incongruent), again male participants had a higher offset compared to female participants and that the aperiodic offset decreases with increases in age. A significant sex*age interaction was also observed (b = 0.002, t(383) = 2.61, p = 0.009, CI[0.001, 0.003]) again showing, similar to the resting state EEG data, that male participants started off with higher offsets at earlier ages compared to female participants but showing that the rate of change (reductions in aperiodic offset over time) was significantly greater for male participants compared to female participants (Fig. 2, A).
Fig. 2.
A) age- and sex-related change in aperiodic offset during Flanker EEG B) age- and sex-related change in aperiodic exponent during Flanker EEG C) age group average aperiodic fits at 13 years and 15 years of age showing exponents plotted in log-log space while controlling for offset collapsing across Flanker tasks D) flanker exponent and offset values at 13 years and 15 years of age.
For the aperiodic exponent during the Flanker congruent versus incongruent conditions, the results of LME model showed that there was no significant main effect of condition (b = −0.002, t(383) = −0.02, p = 0.97, CI[−0.20, 0.20]) but there were again significant main effects of sex (b = −0.61, t(182) = −4.73, p < 0.0001, CI[− 0.87, −0.36]) and age (b = −0.004, t(383) = −10.17, p < 0.0001, CI[− 0.005, −0.003]) showing that although the aperiodic exponent did not differ significantly between Flanker conditions, male participants again had a steeper slope compared to female participants and that the aperiodic exponent decreases (i.e., becomes flatter) with older age. A significant sex*age interaction was again observed (b = 0.003, t(383) = 5.33, p < 0.0001, CI[0.002, 0.005]) and again this interaction showed that while male participants had larger exponents at earlier ages they showed a more significant flattening of the slope over time compared to female participants (see Fig. 2, A).
3.3. Additional exploratory analyses examining condition-, puberty - and sex-related changes in aperiodic offset and exponent
The results of these analyses largely mirrored the results we found for age. However, when both age and pubertal status were entered into the same models, pubertal status was no longer a significant predictor of aperiodic activity whereas age remained a significant predictor (Supplement 1).
3.4. Aperiodic offset, exponent and behavior
No significant main effects were found for Flanker RT, Flanker SDRT nor Flanker accuracy for offset or exponent for either eyes open or eyes closed conditions (ps > 0.05). We applied the same models to aperiodic activity during the Flanker task and again we failed to find any significant relationships between aperiodic activity and behavior (ps > 0.05). Additional polynomial regression analyses were conducted to investigate non-linear relationships between these measures. However, these analyses yielded non-significant findings (ps > 0.05).
3.5. Correlations between offset and exponent
The correlation analyses investigating the relationship between offset and exponent during tasks yielded moderate to strong significant correlations between aperiodic activity during resting state conditions and during the Flanker task for each time point i.e. when participants were approximately 13- and 15-years of age. Correlations remained significant when looking at the relationship between aperiodic components over time (Supplement 3).
4. Discussion
4.1. Summary of main findings
We leveraged a longitudinal dataset to investigate changes in aperiodic activity during early-to-mid adolescence. As predicted, we observed significant age-related reductions in aperiodic offset and exponent between the age 13 and age 15 assessments, across all conditions. Furthermore, we observed significant sex-related differences in the aperiodic offset and exponent, with male participants starting off with higher offsets and larger exponent magnitudes, and over time, showing greater decreases in both measures when compared to their female counterparts. In addition, we also observed significant differences in both the aperiodic offset and exponent across eyes closed and eyes open conditions, with reduced offsets and exponent magnitudes during the eyes open condition. However, contrary to our hypothesis that behavior (Flanker RT, Flanker SDRT and Flanker accuracy) would predict aperiodic activity, we did not observe any significant effects. Moreover, we did not find any significant non-linear relationships between behavior and aperiodic activity. We did find that aperiodic activity was moderate-to-strongly correlated across conditions, at both time points. We also observed significant correlations between aperiodic measures over time.
4.2. Longitudinal change in aperiodic activity
In the current longitudinal study, age-related changes in the aperiodic offset were observed across all conditions for both male and female participants. Age-related decreases in offset have been previously reported in cross-sectional studies that compared early childhood to young adulthood (Cellier et al., 2021, Chi et al., 2019) and when comparing young adults to older adults (Dave et al., 2018). Our results are consistent with these findings, indicating broadband power reductions over time – decreases in the uniform shift in power across frequencies - appear to be stable measures of brain maturation. Increases and/or decreases in broadband power may represent a measure of the overall neuronal population firing rate (Manning et al., 2009, Miller et al., 2014). Indeed, Whitford et al. (2007) reported that age-related changes in absolute EEG power in slow-range frequency bands during resting state EEG in 138 participants aged 10–30 years of age, showed a similar curvilinear decline to gray matter volume as measured using MRI. In addition, decreases in gray matter volume in frontal and parietal cortices were the greatest during adolescence. Therefore, it is possible that maturational changes in the aperiodic offset as measured in scalp EEG may well reflect underlying morphological change, with such changes in cortical thickness/volume occurring during early- to mid-adolescence. In fact, total cerebral grey matter volume appears to decline from its maximal level between approximately 7–12 years of age (Giedd et al., 1999, Mills et al., 2016, Sowell et al., 2001) and continues to decrease post-puberty into late adolescence and young adulthood (Giedd et al., 1999, Giedd, 2004, Gogtay et al., 2004, Lenroot et al., 2007, Paus, 2005).
In addition to the structural changes that occur during brain development, the balance between excitatory and inhibitory neurotransmitters may be of particular importance (Cohen Kadosh et al., 2015). As noted in the introduction, researchers have proposed that changes in exponent reflect changes in the ratio between excitatory and inhibitory currents in neural populations (Donoghue et al., 2020a, Gao et al., 2017, He, 2014, Voytek et al., 2015b). These excitatory and inhibitory currents in neural populations are largely driven by the relative expression of excitatory (glutamate) and inhibitory γ-aminobutyric acid (GABA) neurotransmitter levels. It has been proposed that an increase in glutamate (E) to GABA (I) ratio may be critical for acquiring new cognitive abilities and that the levels of these neurochemical ratios are important for neuroplasticity during important developmental periods (Cohen Kadosh et al., 2015). For example, using single-voxel-proton magnetic resonance spectroscopy (1H-MRS), Cohen Kadosh et al. (2015) found that higher levels of glutamate in the inferior frontal gyrus (IFG) in children (ages 7–10 years), but not adults (ages 20–23 years), correlated positively with face processing proficiency. This suggests that increased excitation during late childhood, but not adulthood, may be important for acquiring new skills. When comparing young adults to older adults, Voytek et al. (2015) found that age-related changes in the spectral slope for older adults (flattening of the slope) was associated with cognitive decline as measured during a working memory task. Taken together, these studies suggest that age-related E:I ratios may be important for optimal brain functioning and plasticity.
Here we show that longitudinal changes in aperiodic activity appears to be a stable measure of maturational trajectories and these findings are supported by previous studies that have also shown age-related changes in background aperiodic activity across different age groups, from the first year of life to older age (Cellier et al., 2021; Dave et al., 2018; He et al., 2019; Schaworonkow and Voytek, 2021; Voytek et al., 2015). Additionally, our results show that both the offset and exponent were moderate-to-highly correlated across conditions. Our longitudinal findings suggest that aperiodic measures are robust across conditions and may index individual patterns of changes in brain morphology and neuronal activation. These findings and conclusion support those suggested by Dave et al. (2018).
Studies investigating normative and divergent development of spectral parameters in EEG have reported significant differences between typical and atypical neurodevelopment (Mamiya et al., 2021, Ostlund et al., 2021, Robertson et al., 2019). If changes in background aperiodic activity over time in part reflect E:I developmental patterns, then these findings may point to critical neurodevelopmental periods during which time these E:I ratios become unbalanced, potentially leading to maladaptive brain function (Mamiya et al., 2021). Where possible, future studies should adopt longitudinal approaches to specifically investigate changes in aperiodic activity over time when comparing clinical and non-clinical cohorts.
4.3. Sex-related differences in aperiodic offset and exponent
In the current study, we observed sex-related differences in the aperiodic offset and exponent over time. These sex-related differences in the aperiodic activity may be partly explained by well-documented sex-specific developmental changes in the brain. For example, total cerebral volume appears to peak at roughly 10.5 years of age in females and 14.5 years of age in males (Lenroot et al., 2007). Male brains are approximately 7–12% larger than female brains (Giedd, 2004; Giedd et al., 1996; Lenroot et al., 2007; Sowell et al., 2001) and this remains statistically significant when weight and height are taken into account. Region-specific sex differences in subcortical grey matter volume have also been reported in the right hippocampus, left amygdala, caudate, thalamus, and putamen (Dennison et al., 2013, Giedd et al., 1996, Giedd, 2004, Sowell et al., 2001). It has been suggested that sex-differences in the developmental trajectories of white matter, cortical and subcortical gray matter in the human brain during adolescence may reflect differential increases in and expression of gonadal hormones (testosterone, oestrogen, and progesterone) in males and females (Blakemore, 2012). For example, greater amygdala volume during puberty has been found in males compared to females while greater hippocampal volume has been found in females compared to males (Lenroot et al., 2007, Neufang et al., 2008). In addition, maturational change in these brain regions has been associated with different stages of pubertal development in both males and females (Neufang et al., 2008). Taken together, these findings suggest that changes during child and adolescent development may result in significant sex-related changes to cortical and subcortical brain structure and organization. The sex-related effects that we observed may in part be the result of the aforementioned changes in underlying neurophysiology.
However, it should be noted that when both age and puberty were entered into the same model, age over and above puberty remained a significant predictor of aperiodic activity whereas puberty did not. This suggests that other factors during adolescent development may be of more relevance to the changes in aperiodic activity that we observed. Future studies specifically dedicated to pubertal development may be better equipped to address the question of whether pubertal developmental stages predict aperiodic activity. For example, clinical observation by trained physicians in addition to self-report measures and the collection of salivary assays to assess hormone levels would give a much better measure of pubertal development. In addition, the age range in the current study may not be ideal for such an investigation with age ranges spanning 10 – 13/15 years of age being better suited for this type of study.
Nonetheless, the findings presented here show that significant sex-related differences in aperiodic activity is present across early-to-mid adolescence. If the underlying neurophysiology were indeed more mature in females compared to males in our early adolescent sample, we would expect that females would show reductions in offset and decreases in exponent magnitude (flatter spectral slope) when compared to males, which is what we found. In fact, one study conducted by Lenroot et al. (2007) showed that male brains consistently showed more substantial change during adolescence than female brains and that this rate of change appeared to converge as both males and females reached the late teens and early twenties. It may be that the male participants in the current study were still undergoing more substantial developmental change compared to female participants. However, it must be noted here that these hypotheses are speculative. It is not possible to definitively state that changes in aperiodic activity in the EEG signal are the result of underlying brain structure and organization or function without further investigation into the structural and functional brain changes that occur during adolescence (e.g., with the use of structural MRI or fMRI). Future studies should investigate whether changes in brain structure and function during early adolescence correlate with changes in aperiodic activity.
4.4. Aperiodic measures and behavior
Contrary to our hypothesis that we would find associations between aperiodic activity and behavioral measures, we failed to find any significant relationship between aperiodic activity and measures of Flanker RT, Flanker SDRT or Flanker accuracy. One might expect that if changes in aperiodic activity is associated with changes in information processing efficiency we would find that changes in the aperiodic signal translate into changes in behavior. This was not the case in the current study. Previous studies have uncovered putative brain-behavior correlates specific to the shape of EEG power spectra (Donoghue et al., 2020a, Ostlund et al., 2021, Ouyang et al., 2020). In particular, Ostlund et al. (2021) showed that a flatter spectral slope during resting state EEG was predictive of better performance on a dual-task “Stopping task” as measured by SDRT and drift diffusion parameters. Ouyang et al. (2020) showed that resting state “1/f” activity was associated with cognitive processing speed. Although we adopted a similar approach in terms of behavioral measures (RT, SDRT) and the use of resting state EEG as well as Flanker EEG, we failed to find similar associations. However, there is currently a limited number of studies that have shown associations between aperiodic activity and behavior. Future studies should attempt to replicate aperiodic related brain-behavior findings using similar tasks, population characteristics and statistical methods as those used in prior work.
4.5. Limitations
The current study has a number of limitations. Each participant completed resting state EEG recordings prior to the Flanker task (i.e., the resting state and Flanker task EEG recordings were not counterbalanced). A further limitation relates to the representativeness of our sample, as participants were majority white and affluent, and therefore not representative of the general population. Future studies should attempt to recruit more diverse populations to improve the generalizability of findings. A final limitation, which is common to many studies examining aperiodic activity, is that changes in exponent magnitude may lead to concomitant changes in offset. This can occur when there is a rotation in the power spectrum around a non-zero frequency, which results in the two measures being highly correlated. We have proposed that changes in the aperiodic offset measured via scalp EEG may reflect changes in underlying cortical, and likely subcortical, structures of the brain and that changes in aperiodic exponent may reflect changes in excitatory and inhibitory ratios. While these two measures may reflect distinct properties, it remains unclear how to best control for offset when looking at the exponent and vice versa. Future work should attempt to better disentangle offset and exponent in order to improve the validity of inferences drawn from each measure.
5. Conclusions
In this longitudinal study we demonstrate that significant age-related reductions in aperiodic activity are detectable using scalp EEG recordings in a cohort of participants spanning early-to-mid adolescence. We also demonstrate that these changes differed significantly for male and female participants. To the best of our knowledge, this is also the first study to report sex-related differences in aperiodic activity during early-to-mid adolescence in a longitudinal data set. We also show that aperiodic activity is highly correlated across conditions and as such may prove to be a robust measure of patterns of neural activation. These findings also highlight the importance of conducting additional studies that attempt to disentangle these two aperiodic parameters that is ubiquitous in EEG recordings.
Funding
This work was supported by the National Institute of Mental Health [grant number U01MH093349] to NAF.
CRediT authorship contribution statement
Marco McSweeney: Conceptualization, Writing – original draft, Formal analysis, Writing – review & editing. Santiago Morales: Conceptualization, Writing – review & editing. Emilio A. Valadez: Writing – review & editing. George A. Buzzell: Conceptualization, Writing – review & editing. Nathan A. Fox: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgments
We would very much like to thank all of the families involved without whom our research would not be possible. In addition, we would like to thank all of the Research Assistants who worked tirelessly to collect the data that is presented in the current work..
Footnotes
The name of this toolbox has been recently updated to specparam (https://github.com/fooof-tools/fooof/issues/193).
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2021.101035.
Appendix A. Supplementary material
Supplementary material
.
Data availability
We have a data sharing agreement with the National Institute of Mental Health (NIMH) whereby we will make the data available by uploading the data to NDAR (data repository). In addition, the data that support the findings of this study are available on request from the corresponding author, [MM].
References
- Blakemore S.J. Imaging brain development: the adolescent brain. Neuroimage. 2012;61(2):397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J. Child Psychol. Psychiatry. 2006;47(3–4):296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cao B., Mwangi B., Passos I.C., Wu M.J., Keser Z., Zunta-Soares G.B., Xu D., Hasan K.M., Soares J.C. Lifespan gyrification trajectories of human brain in healthy individuals and patients with major psychiatric disorders. Sci. Rep. 2017;7(1):511. doi: 10.1038/s41598-017-00582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier D., Riddle J., Petersen I., Hwang K. The development of theta and alpha neural oscillations from ages 3 to 24 years. Dev. Cogn. Neurosci. 2021;50 doi: 10.1016/j.dcn.2021.100969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K., Krause B., King A.J., Near J., Cohen Kadosh R. Linking GABA and glutamate levels to cognitive skill acquisition during development. Human Brain Mapp. 2015;36(11):4334–4345. doi: 10.1002/hbm.22921. https://psycnet.apa.org/doi/10.1002/hbm.229Debnath, R., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave S., Brothers T.A., Swaab T.Y. 1/f neural noise and electrophysiological indices of contextual prediction in aging. Brain Res. 2018;1691:34–43. doi: 10.1016/j.brainres.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath R., Buzzell G.A., Morales S., Bowers M.E., Leach S.C., Fox N.A. The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology. 2020;57(6) doi: 10.1111/psyp.13580. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dennison M., Whittle S., Yücel M., Vijayakumar N., Kline A., Simmons J., Allen N.B. Mapping subcortical brain maturation during adolescence: evidence of hemisphere‐and sex‐specific longitudinal changes. Dev. Sci. 2013;16(5):772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Donoghue T., Haller M., Peterson E.J., Varma P., Sebastian P., Gao R., Voytek B. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 2020;23(12):1655–1665. doi: 10.1038/s41593-020-00744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue T., Dominguez J., Voytek B. Electrophysiological frequency band ratio measures conflate periodic and aperiodic neural activity. Eneuro. 2020;7(6) doi: 10.1523/ENEURO.0192-20.2020. https://dx.doi.org/10.1523%2FENEURO.0192-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Nardos B., Cohen A.L., Fair D.A., Power J.D., Church J.A., Barnes K.A. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16:143–149. [Google Scholar]
- Gao R., Peterson E.J., Voytek B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage. 2017;158:70–78. doi: 10.1016/j.neuroimage.2017.06.078. [DOI] [PubMed] [Google Scholar]
- Giedd J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N.Y. Acad. Sci. 2004;1021(1):77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Rumsey J.M., Castellanos F.X., Rajapakse J.C., Kaysen D., Vaituzis A.C., Rapoport J.L. A quantitative MRI study of the corpus callosum in children and adolescents. Dev. Brain Res. 1996;91(2):274–280. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goddings A.L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.J. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. https://dx.doi.org/10.1016%2Fj.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Rapoport J.L. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane A.A., Fox N.A., Henderson H.A., Marshall P.J. Behavioral reactivity and approach-withdrawal bias in infancy. Dev. Psychol. 2008;44(5):1491–1496. doi: 10.1037/a0012855. https://psycnet.apa.org/doi/10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J. Scale-free brain activity: past, present, and future. Trends Cogn. Sci. 2014;18(9):480–487. doi: 10.1016/j.tics.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J., Zempel J.M., Snyder A.Z., Raichle M.E. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010;66(3):353–369. doi: 10.1016/j.neuron.2010.04.020. https://dx.doi.org/10.1016%2Fj.neuron.2010.04.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L., Cheng X., He X., Sun J., Liang F., Pei Z., Teng W. Co-increasing neuronal noise and beta power in the developing brain. BioRxiv. 2019;30:839258–839433. doi: 10.1101/839258. [DOI] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S., Thompson P.M. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. https://dx.doi.org/10.1016%2Fj.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya P.C., Arnett A.B., Stein M.A. Precision medicine care in ADHD: the case for neural excitation and inhibition. Brain Sci. 2021;11(1):91. doi: 10.3390/brainsci11010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J.R., Jacobs J., Fried I., Kahana M.J. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J. Neurosci. 2009;29(43):13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.J., Honey C.J., Hermes D., Rao R.P., Ojemann J.G. Broadband changes in the cortical surface potential track activation of functionally diverse neuronal populations. Neuroimage. 2014;85:711–720. doi: 10.1016/j.neuroimage.2013.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.L., Herting M.M., Meuwese R., Blakemore S.J., Crone E.A., Tamnes C.K. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage. 2016;141:273–281. doi: 10.1016/j.neuroimage.2016.07.044. https://dx.doi.org/10.1016%2Fj.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J.L., Voytek B., Thomas M.L., Joshi Y.B., Bhakta S.G., Talledo J.A., Light G.A. Memantine effects on electroencephalographic measures of putative excitatory/inhibitory balance in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2020;5(6):562–568. doi: 10.1016/j.bpsc.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S., Specht K., Hausmann M., Güntürkün O., Herpertz-Dahlmann B., Fink G.R., Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex. 2008;19(2):464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Ostlund B.D., Alperin B.R., Drew T., Karalunas S.L. Behavioral and cognitive correlates of the aperiodic (1/f-like) exponent of the EEG power spectrum in adolescents with and without ADHD. Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang G., Hildebrandt A., Schmitz F., Herrmann C.S. Decomposing alpha and 1/f brain activities reveals their differential associations with cognitive processing speed. NeuroImage. 2020;205 doi: 10.1016/j.neuroimage.2019.116304. [DOI] [PubMed] [Google Scholar]
- Pathania, A., Clark, M., Cowan, R., Euler, M.J., Duff, K., Lohse, K.R., 2021. Relating resting EEG power spectra to age-related differences in cognitive performance: An observational pilot study. medRxiv. https://doi.org/10.1101/2021.02.12.21251655.
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., Team, R.C. , 2007. Linear and nonlinear mixed effects models. R package version, 3(57), 1–89.
- Podvalny E., Noy N., Harel M., Bickel S., Chechik G., Schroeder C.E., Malach R. A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J. Neurophys. 2015;114(1):505–519. doi: 10.1152/jn.00943.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M.M., Furlong S., Voytek B., Donoghue T., Boettiger C.A., Sheridan M.A. EEG power spectral slope differs by ADHD status and stimulant medication exposure in early childhood. J. Neurophys. 2019;122(6):2427–2437. doi: 10.1152/jn.00388.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R. Core Team, 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.https://www.R-project.org/.
- Schaworonkow N., Voytek B. Longitudinal changes in aperiodic and periodic activity in electrophysiological recordings in the first seven months of life. Dev. Cogn. Neurosci. 2021;47 doi: 10.1016/j.dcn.2020.100895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalowitz S.J., Santesso D.L., Jetha M.K. Electrophysiological changes during adolescence: a review. Brain Cogn. 2010;72(1):86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Tessner K.D., Toga A.W. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during post adolescent brain maturation. J. Neurosci. 2001;21(22):8819–8829. doi: 10.1523/jneurosci.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B., Knight R.T. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol. Psychiatry. 2015:1089–1097. doi: 10.1016/j.biopsych.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B., Kramer M.A., Case J., Lepage K.Q., Tempesta Z.R., Knight R.T., Gazzaley A. Age-related changes in 1/f neural electrophysiological noise. J. Neurosci. 2015;35(38):13257–13265. doi: 10.1523/JNEUROSCI.2332-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford T.J., Rennie C.J., Grieve S.M., Clark C.R., Gordon E., Williams L.M. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Human Brain Mapp. 2007;28(3):228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson C.L., Nelson C.A. Increased aperiodic gamma power in young boys with Fragile X Syndrome is associated with better language ability. Molecul. Autism. 2021;12(1):1–15. doi: 10.1186/s13229-021-00425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Jiao Y.Y., Sun Q.Q. Developmental maturation of excitation and inhibition balance in principal neurons across four layers of somatosensory cortex. Neuroscience. 2011;174:10–25. doi: 10.1016/j.neuroscience.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
We have a data sharing agreement with the National Institute of Mental Health (NIMH) whereby we will make the data available by uploading the data to NDAR (data repository). In addition, the data that support the findings of this study are available on request from the corresponding author, [MM].