Abstract
Denosumab is a monoclonal antibody that has been approved to treat osteoporosis, skeletal metastasis, and giant cell tumor of bone in skeletally mature patients. Due to its potential adverse effects on normal bone growth, its use has not yet been approved in skeletally immature patients; however, the use of this agent in such patients with overt or dysregulated bone resorptive conditions has been explored in recent years. While most studies have proven the effectiveness of denosumab in controlling the progression of various disorders in skeletally immature patients, they have also revealed that refractory hypercalcemia often follows the discontinuation of denosumab treatment, raising a concern over the use of this agent in these patients. Thus, this study was designed to better understand the pathology of this condition through a systematic review of the published literature. Our analysis suggests that this condition has a potential male predisposition, that there is a correlation between the duration of denosumab treatment and patient age, and that this condition often occurs within 3 months after the last administration of denosumab in skeletally immature patients but is significantly less likely in adults. These results may further underscore that high bone formation and bone turnover rates are critically associated with hypercalcemia after the discontinuation of denosumab. In contrast, given that not all skeletally immature patients develop hypercalcemia, it is probable that other unidentified factors are involved in the pathology of this condition.
Keywords: Denosumab, Hypercalcemia, Adverse event, Juvenile, Discontinuation, Rebound
Highlights
-
•
Rebound hypercalcemia often follows denosumab cessation in juveniles.
-
•
Although relatively rare, rebound hypercalcemia can occur in adults.
-
•
Rebound hypercalcemia may have a male predisposition in juveniles.
-
•
Treatment duration of denosumab required to trigger hypercalcemia correlates with age.
-
•
Onset of hypercalcemia is significantly earlier in juveniles than in adults.
1. Introduction
Osteoclasts are multinucleated bone-resorbing giant cells that play a major role in the pathology of several disorders, including osteoporosis, malignant bone metastasis, and giant cell tumor of bone (GCTB) 1, 2, 3. It has been thus considered that osteoclasts are a valid target for controlling the progression of these disorders. Past studies have identified various factors that are involved in the regulation of osteoclast differentiation and survival. Among these, the receptor activator of NFκB (RANK), and its ligand (RANKL), are probably the most critically involved in these processes 4, 5.
Denosumab is a fully-humanized antibody against RANKL, a membrane-bound molecule expressed on osteoblasts and stromal cells 6, 7. Denosumab specifically binds to RANKL and interferes with its binding to RANK, a receptor molecule expressed on osteoclasts and their precursors, thereby suppressing the formation of osteoclasts and bone resorption. The use of denosumab has been approved for the treatment of osteoporosis, bone metastasis, and GCTB in skeletally mature adult patients. While denosumab has been proven to be highly effective in suppressing bone resorption and alleviating the symptoms of these disorders, it also shares several side effects with bisphosphonates, another class of anti-bone-resorbing agents, including osteonecrosis of the jaw, atypical femoral fracture, and hypocalcemia. Furthermore, recent studies have shown that a sharp increase in the bone turnover occurs following the discontinuation of denosumab therapy, leading to a drastic bone loss and an increased risk of vertebrae fractures 8, 9, 10, 11. This sudden change in bone metabolism can ultimately result in multiple vertebrae fractures, a condition also known as “rebound-associated vertebral fractures”, in osteoporotic patients.
Because of the potential adverse effects on normal skeletal growth, the use of denosumab in skeletally immature patients has remained to be investigated. However, in recent years, the use of this agent has been explored in juveniles and adolescents with various disorders with overt or dysregulated osteoclastic bone resorption activity 12, 13, 14, 15, 16, 17. These studies have shown that denosumab is highly effective in controlling the progression of various disorders, including fibrous dysplasia, osteogenesis imperfecta, GCTB, central giant cell granuloma (CGCG), and aneurysmal bone cyst (ABC). These observations suggest the clinical benefit of denosumab for treating skeletally immature patients. However, unexpectedly, these clinical studies have also identified hypercalcemia as a previously unrecognized adverse event that occurs following the discontinuation of denosumab treatment. This adverse event, also referred to as “rebound hypercalcemia,” is often intractable and requires intensive treatments, raising a concern over the use of denosumab in skeletally immature patients.
Since our report on one of the first cases of rebound hypercalcemia [17], the number of reported cases has been increasing in recent years. However, these are mainly sporadic case reports and currently, no comprehensive overview on this condition exists. Thus, this study was designed to better understand the pathology of rebound hypercalcemia following denosumab discontinuation through a systematic review of the published literature. Specifically, we aimed to identify the factors or conditions that are potentially associated with the onset of this adverse event.
2. Materials and methods
2.1. Eligibility criteria
We followed the recommendations of the Preferred Reporting Items for Systematic Review and Meta-Analysis statement [18]. Published reports in the English language, including clinical studies, case series, case reports, and meeting abstracts, describing cases with hypercalcemia after the termination of denosumab treatment were included.
2.2. Literature search and study selection
We searched PubMed, Scopus, Web of Science, Reaction Weekly (through ProQuest Health & Medical Collection), and the Cochrane Library on July 12, 2021 using “denosumab” and “hypercalcemia” as search terms and with the language restricted to English. Bibliographical data were imported into EndNote X9 (Clarivate, London, UK), and duplicates were removed. The title and abstract of all publications were reviewed by one author (KH), and publications that do not describe cases with hypercalcemia after the use of denosumab were excluded. Next, the full text of potentially relevant publications was reviewed. Publications that describe identical cases with other publications and those that do not provide clinical information of each case were excluded from this study.
2.3. Data extraction
We used a data collection sheet and extracted the data items, including patient age (at the initiation of denosumab therapy), sex, indication for the use of denosumab, dosage and frequency of denosumab treatment, treatment duration, cumulative denosumab dose, duration from the last denosumab treatment to the onset of hypercalcemia, serum calcium level at the time of the diagnosis of hypercalcemia, and treatments for hypercalcemia. In cases where hypercalcemia occurred in more than one occasion, the data from the first episode were extracted. When the durations were described in years or weeks, they were converted into months. If cumulative doses were not shown, they were deduced based on the information provided in the reports when possible. The calcium levels were converted to mg/dL when they were provided in mmol/L by dividing by 0.2495.
2.4. Statistical analysis
Cases were excluded from a given analysis when the corresponding value was unavailable. Based on the patient population distribution, we arbitrarily divided the cases into two groups: the skeletally immature group (18-year-old or less; henceforth referred to as Group I) and the skeletally mature group (more than 18-year-old; henceforth referred to as Group M). The Mann–Whitney test (two-tailed) (Fig. 2A (right panel), B, C, D, and F) was performed using Prism 6 (GraphPad Software, San Diego, CA). Linear regression analysis (Fig. 2E and G) and the chi-square test (Table 3) were performed using Excel 2019 (Microsoft, Redmond, WA). P values smaller than 0.05 were considered statistically significant.
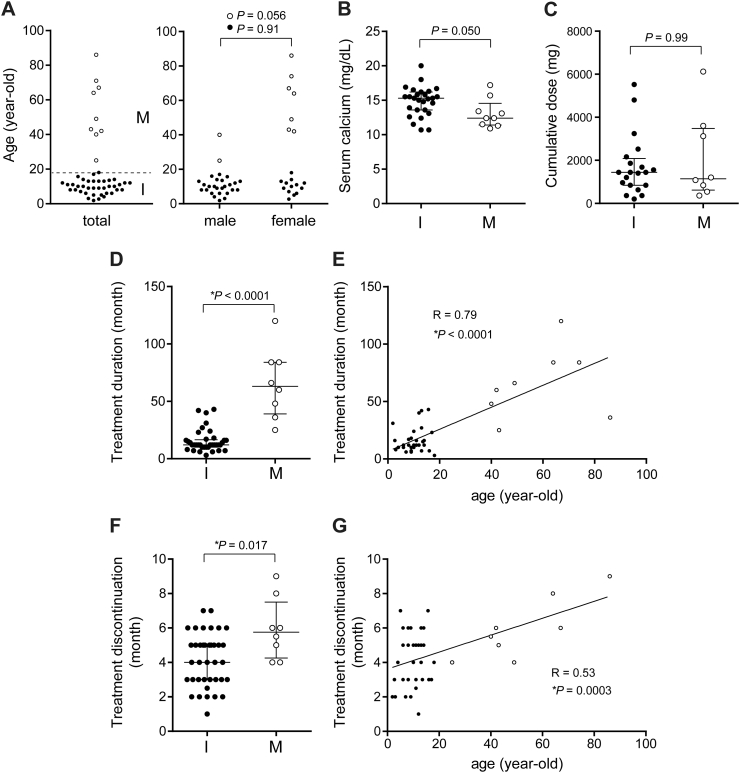
Fig. 2.
Analysis of the extracted data from cases with rebound hypercalcemia. A. Patient age (left panel, total cases; right panel, male and female cases) at the initiation of denosumab treatment. The dotted line in the left panel indicates 18-year-old. Group I (I), n = 40; Group M (M), n = 9. B. Serum calcium level at the diagnosis of hypercalcemia. Group I, n = 27; Group M, n = 9. C. Cumulative dose of denosumab. Group I, n = 20; Group M, n = 8. D and E. The duration of denosumab treatment. Group I, n = 37; Group M, n = 8. E and F. The duration from the last denosumab injection to the onset of hypercalcemia. Group I, n = 38; Group M, n = 8. Statistical analysis was performed using the Mann-Whitney test (B, C, D, and F) and linear regression analysis (E and G). Black and white dots represent Group I cases and Group M cases, respectively. Data are presented as the median and interquartile range (B, C, D, and F). *, statistically significant.
Table 3.
Treatments for hypercalcemia, besides hydration and administration of diuretics. B + D, bisphosphonate and denosumab; NA, not available. Data are n.
| Group I | Group M | Total | |
|---|---|---|---|
| Bisphosphonate | 23 | 4 | 27 |
| Denosumab | 5 | 4 | 9 |
| B + D | 2 | 0 | 2 |
| Other/NA | 10 | 2 | 12 |
3. Results
3.1. Search results
Our search yielded 500 unique hits. Among these, 48 records were identified as potentially relevant, and the full text of these publications was further reviewed. We excluded 11 publications describing identical cases with other publications (these were mostly meeting abstracts) and 2 publications that lack clinical information of individual cases. Finally, 35 publications, including 12 case series, 16 case reports, and 7 meeting abstracts, were subjected to analysis. The list of all publications and cases is shown in Supplementary Table S1 and Supplementary References. The flowchart of the search procedure is presented in Fig. 1.
Fig. 1.
Publication selection flowchart based on the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines.
3.2. Patient characteristics
A total of 49 cases were included in the study. The mean age of the cases was 18.3 years with a standard deviation of 19.7 years. The patient population was highly skewed toward juveniles and adolescents (median age, 11.0 years) (Fig. 2A, left panel). Given that this population (18-year-old or less) mostly represents skeletally immature patients, we divided the cases into two groups: the skeletally immature group (Group I, 40 cases) and the skeletally mature group (Group M, 9 cases). The patient age and sex of each group are shown in Table 1. The number of male cases in Group I was noticeably higher than that of female cases (25 vs. 13 cases; P = 0.052, the chi-square test), indicating a potential male predisposition of this condition in this population. There was no difference in the mean age between male cases (9.7 years) and female cases (9.9 years) in this group (Table 1 and Fig. 2A, right panel, P = 0.91). On the other hand, the number and the mean age of female cases in Group M were markedly higher than those of male cases (7 vs. 2 cases; P = 0.096, the chi-square test; 60.7 vs. 32.5 years, P = 0.056), but neither reached a statistical significance (Table 1 and Fig. 2A, right panel).
Table 1.
Patients' clinical data. ABC, aneurysmal bone cyst; CGCG, central giant cell granuloma; GCTB, giant cell tumor of bone; FD, fibrous dysplasia; McCune-Albright syndrome; OI, osteogenesis imperfecta; SJX, solitary juvenile xanthogranuloma; JPD, juvenile Paget's disease; NA, not available. Data are n (mean age).
| Group I | Group M | Total | |
|---|---|---|---|
| Sex | |||
| Male | 25 (9.7) | 2 (32.5) | 27 (11.4) |
| Female | 13 (9.9) | 7 (60.7) | 20 (27.7) |
| NA | 2 (NA) | 0 (NA) | 2 (NA) |
| Total | 40 (9.8) | 9 (54.4) | 49 (18.3) |
| Indications for denosumab treatment | |||
| ABC | 11 (9.1) | 0 (NA) | 11 (9.1) |
| CGCG | 10 (10.2) | 0 (NA) | 10 (10.2) |
| GCTB | 8 (11.8) | 1 (40) | 9 (15.0) |
| FD / MAS | 3 (13.3) | 2 (34.0) | 5 (21.6) |
| OI | 2 (2.3) | 0 (NA) | 2 (2.3) |
| Cherubism | 3 (7.7) | 0 (NA) | 3 (7.7) |
| Breast cancer | 0 (NA) | 2 (45.5) | 2 (45.5) |
| Osteoporosis | 1 (13.5) | 3 (68.3) | 4 (54.6) |
| SJX | 1 (5) | 0 (NA) | 1 (5) |
| JPD | 1 (7) | 0 (NA) | 1 (7) |
| Hyperparathyroidism | 0 | 1 | 1 (86) |
The indications for denosumab treatment are summarized in Table 1. The most frequent disorders were ABC, CGCG (including cases with Noonan syndrome), GCTB, and fibrous dysplasia (including cases with McCune-Albright syndrome), comprising approximately two-thirds of all cases. No significant differences in any item values (including patient age, treatment duration, and duration from the last denosumab treatment to the onset of hypercalcemia) were observed between these disorder groups (data not shown).
3.3. Denosumab dosage and frequency
The dosage and frequency of denosumab administration differed significantly among cases in Group I (Supplementary Table S1). Some cases followed the standard regimen recommended for skeletally mature adults with GCTB (120 mg per 4 weeks with loading doses on days 8 and 15), whereas in other cases, the dose was adjusted for body weight (most frequently, 1 mg/kg) or body surface area with varying dose frequencies (e.g., once weekly for the first month and once monthly thereafter, once per month, and thrice per month). In Group M, the dosage and frequency varied from the standard regimens in 5 cases. Three cases with osteoporosis and one case with GCTB were treated with standard regimens (60 mg per 6 months or 120 mg per 4 weeks with loading doses, respectively).
3.4. Hypercalcemia and clinical features
The serum calcium level at the onset of hypercalcemia tended to be higher in Group I (median, 15.3 mg/dL; interquartile (IQR), 13.6–16.2 mg/dL) than in Group M (median, 12.4 mg/dL; IQR, 11.4–14.6 mg/dL) (Fig. 2B). There was no difference in the cumulative dose of denosumab between Groups I and M (Fig. 2C). The duration of denosumab treatment (the duration from the first to the last injection of denosumab) was significantly shorter in Group I (median, 12 months; IQR, 10–16.5 months) than in Group M (median, 60 months; IQR, 39.0–84 months) (Fig. 2D). Of interest, we found a significant correlation between patient age and the duration of denosumab treatment (Fig. 2E), indicating that the duration of denosumab treatment required to trigger hypercalcemia is shorter in juveniles and adolescents than in adults. There was a significant difference in the duration from the last denosumab injection to the onset of hypercalcemia between Groups I (median, 4 months; IQR 3–5 months) and M (median, 5.75 months; IQR 4.25–7.5 months) (Fig. 2F). Accordingly, a relatively weak but statistically significant correlation was observed between age and the duration from the last denosumab injection to the onset of hypercalcemia (Fig. 2G). Furthermore, the percentage of cases who had hypercalcemia within 3 months after the last denosumab injection was significantly higher in Group I (13 of 35 cases, 37%) than in Group M (0 of 9 cases) (P = 0.027, the chi-square test) (Table 2).
Table 2.
The number of cases that had hypercalcemia after 3 months (>3 M) and within 3 months (≤3 M) from the last denosumab injection in each group. Data are n.
| >3 M | ≤3 M | |
|---|---|---|
| Group I | 22 | 13 |
| Group M | 9 | 0 |
3.5. Treatments for hypercalcemia
In most symptomatic cases, hydration and administration of diuretics (e.g., furosemide) were performed as a basic treatment. In several cases, calcitonin and/or corticosteroids were administered; however, their efficacy was marginal in most cases. Bisphosphonates, including zoledronate, pamidronate, and neridronate, were the most frequently used agents in controlling hypercalcemia (Table 3). Denosumab was readministered in 9 cases and combination of bisphosphonates and denosumab were used in 2 cases.
4. Discussion
The awareness of rebound hypercalcemia after the discontinuation of denosumab therapy has recently risen, as evidenced by the increasing number of reported cases in the literature. However, the etiology of this condition and preventive methods remain to be elucidated. To gain insight into this condition, we systematically reviewed the published literature and analyzed the extracted data. Our results suggest that this condition has a potential male predisposition in juveniles and adolescents, that treatment duration of denosumab that is required to trigger hypercalcemia correlates with patient age, and that this condition often occurs within 3 months after the last administration of denosumab in juveniles and adolescents but later than 3 months in skeletally mature adults.
The mechanisms underlying hypercalcemia after denosumab withdrawal are not fully understood; however, it is most likely caused by the rapid recovery of osteoclastic activity, triggering the surge of bone resorption and the release of calcium from the calcified tissue into the circulation 17, 19. This concept indicates that bone turnover rate and the amount of overstored calcium determine the severity of the increase in serum calcium following denosumab discontinuation. Theoretically, this may also explain the reason for the potential male predisposition in Group I (males tend to have a higher bone mass than females [20], and thus store more calcium while on denosumab treatment during the growth period) and for the early onset of hypercalcemia after the discontinuation of denosumab in Group I compared with Group M. Whereas, the reason for the higher number and the higher mean age of female cases in Group M could be due to the higher prevalence of osteoporosis (post-menopausal osteoporosis), which often necessitates the use of denosumab, in women than in man. Nevertheless, further studies with more subjects will be necessary to address these issues. In addition, considering that the release of calcium from the calcified tissue should precede the actual bone mass loss, it is reasonable that the duration from the discontinuation of denosumab to the onset of hypercalcemia (median, 5.75 months in Group M) is shorter than that of rebound-associated vertebral fractures, which usually occurs 7–20 months (median, 11 months) after denosumab cessation in adult patients [9].
The correlation between the treatment duration and patient age (Fig. 2E) indicates that rebound hypercalcemia occurs following a relatively short treatment duration in juveniles and adolescents compared with adults. It is possible that the patients in Group I were treated for a shorter period with denosumab than those in Group I for medical reasons. However, considering that a substantial number of adult patients had GCTB to whom denosumab was preoperatively used for a relatively short duration and that there is no reported case of rebound hypercalcemia in these patients [21], this assumption seems implausible. Instead, it would be more rational to assume that calcium accumulation required to incite hypercalcemia is reached much faster in juveniles and adolescents than in adults. Additionally, this result also suggests that hypercalcemia occurs even in skeletally mature patients, although rarely, if they are treated with denosumab long enough to reach the potential threshold.
Although rebound hypercalcemia after discontinuing denosumab occurs at a significantly higher rate in skeletally immature patients than in adults, the exact occurrence rate of this adverse event remains unclear. Our database search revealed only 9 adult cases (Group M), contrary to 40 juvenile and adolescent cases (Group I), even though denosumab has been widely used in adult patients in the past decade. According to reports of consecutive case series of skeletally immature patients treated with denosumab, hypercalcemia occurred in 14 of 31 cases (4 of 4 cases with Noonan syndrome and CGCG [22], 3 of 6 cases with CGCG [23], 2 of 4 cases with osteogenesis imperfecta [24], 2 of 9 cases with ABC [25], 2 of 5 cases with ABC [26], one of 3 cases (ABC, CGCG, and cherubism) [27]), indicating that this event occurs as frequently as 45% in juvenile and adolescent patients. In contrast, phase 2 clinical study of denosumab in patients with GCTB (>12-year-old) showed that the frequency of hypercalcemia was <1% (4 of 526 cases) [28]. Although the details of the clinical data of these patients were not provided, it will be reasonable to assume that this condition is markedly rare in adult patients or that most such cases are asymptomatic and overlooked in adults.
In most cases, treatments for hypercalcemia followed the standard protocol, consisting of hydration and administration of diuretics, corticosteroids, and/or calcitonin. However, in severe cases, repeated use of bisphosphonates or reinjection of denosumab was often required to repress the surge of calcium released into the circulation 14, 16, 17, 19, 22, 24, 29, 30, 31, 32, 33, 34. Although no established treatment modality exists to prevent this condition, tapering of the dosage of denosumab and prophylactic use of bisphosphonates were attempted in several cases 22, 23, 31. However, due to the small number of cases, the clinical benefit of these methods is currently inconclusive.
Several limitations should be acknowledged in this study. First, given that the present study is based on the data extracted from case reports and case series, and that there are various inherent biases in such studies, the risk of bias in each report was not systematically assessed. Most critically, due to publication bias, it is likely that symptomatic cases were more frequently published than asymptomatic cases, resulting in the underestimation of cases with mild or no clinical manifestations. Second, because the data items presented in the publications were not fully consistent, not all cases were included in each analysis. Furthermore, when considered appropriate, we have calculated or deduced the missing item values based on the information provided in the publication (the values with asterisk in Supplementary Table S1). It is possible that these values did not fully reflect the actual values and could have skewed the results. Finally, although we have thoroughly searched the databases available to us, the number of cases was insufficient to perform more stringent statistical analyses.
Despite these limitations, we believe that the results of the present study further support the idea that high bone formation and bone turnover rates are associated with hypercalcemia after the discontinuation of denosumab. Moreover, the results may explain why this condition occurs predominantly in skeletally immature patients and why the duration of denosumab treatment and the onset after the discontinuation of denosumab are shorter in skeletally immature patients than in adult patients. On the other hand, considering that not all skeletally immature patients are inflicted with this condition even if they were treated with denosumab for a certain duration, there will remain unidentified factors that are necessary to trigger this event. Clinically, we believe that a close follow-up would be mandatory not only in skeletally immature patients but also in skeletally mature patients when long-term use of denosumab is discontinued. Furthermore, establishing a protocol based either on the tapering of denosumab dosage or prophylactic administration of bisphosphonates will be critical to circumvent this undesired condition and fully optimize the potential benefit of denosumab in treating patients with overt or dysregulated bone resorption disorders.
The following are the supplementary data related to this article.
Summary of the reported cases with hypercalcemia following denosumab withdrawal.
Declaration of competing interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Author names: Keisuke Horiuchi, Eisuke Kobayashi, Tsukasa Mizuno, Michiro Susa, and Kazuhiro Chiba.
Acknowledgments
None.
References
- 1.Coleman R.E., Croucher P.I., Padhani A.R., Clezardin P., Chow E., Fallon M., Guise T., Colangeli S., Capanna R., Costa L. Bone metastases. 2020;6(1):83. doi: 10.1038/s41572-020-00216-3. [DOI] [PubMed] [Google Scholar]
- 2.Rachner T.D., Khosla S., Hofbauer L.C. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowan R.W., Singh G. Giant cell tumor of bone: a basic science perspective. Bone. 2013;52(1):238–246. doi: 10.1016/j.bone.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Rao S., Cronin S.J.F., Sigl V., Penninger J.M. RANKL and RANK: from mammalian physiology to cancer treatment. Trends Cell Biol. 2018;28(3):213–223. doi: 10.1016/j.tcb.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Asagiri M., Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Lacey D.L., Boyle W.J., Simonet W.S., Kostenuik P.J., Dougall W.C., Sullivan J.K., San Martin J., Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 2012;11(5):401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 7.Baron R., Ferrari S., Russell R.G. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Tsourdi E., Langdahl B., Cohen-Solal M., Aubry-Rozier B., Eriksen E.F., Guanabens N., Obermayer-Pietsch B., Ralston S.H., Eastell R., Zillikens M.C. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Lamy O., Stoll D., Aubry-Rozier B., Rodriguez E.G. Stopping denosumab. Curr. Osteoporos. Rep. 2019;17(1):8–15. doi: 10.1007/s11914-019-00502-4. [DOI] [PubMed] [Google Scholar]
- 10.Cummings S.R., Ferrari S., Eastell R., Gilchrist N., Jensen J.B., McClung M., Roux C., Törring O., Valter I., Wang A.T., Brown J.P. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J. Bone Miner. Res. 2018;33(2):190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]
- 11.Bone H.G., Bolognese M.A., Yuen C.K., Kendler D.L., Miller P.D., Yang Y.C., Grazette L., San Martin J., Gallagher J.C. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J. Clin. Endocrinol. Metab. 2011;96(4):972–980. doi: 10.1210/jc.2010-1502. [DOI] [PubMed] [Google Scholar]
- 12.Polyzos S.A., Makras P., Tournis S., Anastasilakis A.D. Off-label uses of denosumab in metabolic bone diseases. Bone. 2019;129 doi: 10.1016/j.bone.2019.115048. [DOI] [PubMed] [Google Scholar]
- 13.Boyce A.M. Denosumab: an emerging therapy in pediatric bone disorders. 2017;15(4):283–292. doi: 10.1007/s11914-017-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce A.M., Chong W.H., Yao J., Gafni R.I., Kelly M.H., Chamberlain C.E., Bassim C., Cherman N., Ellsworth M., Kasa-Vubu J.Z., Farley F.A., Molinolo A.A., Bhattacharyya N., Collins M.T. Denosumab treatment for fibrous dysplasia. J. Bone Miner. Res. 2012;27(7):1462–1470. doi: 10.1002/jbmr.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naidu A., Malmquist M.P., Denham C.A., Schow S.R. Management of central giant cell granuloma with subcutaneous denosumab therapy. J. Oral Maxillofac. Surg. 2014;72(12):2469–2484. doi: 10.1016/j.joms.2014.06.456. [DOI] [PubMed] [Google Scholar]
- 16.Gossai N., Hilgers M.V., Polgreen L.E., Greengard E.G. Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr. Blood Cancer. 2015;62(6):1078–1080. doi: 10.1002/pbc.25393. [DOI] [PubMed] [Google Scholar]
- 17.Setsu N., Kobayashi E., Asano N., Yasui N., Kawamoto H., Kawai A., Horiuchi K. Severe hypercalcemia following denosumab treatment in a juvenile patient. J. Bone Miner. Metab. 2016;34(1):118–122. doi: 10.1007/s00774-015-0677-z. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deodati A., Fintini D., Levtchenko E., Rossi M., Ubertini G., Segers H., Battafarano G., Cappa M., Del Fattore A. Mechanisms of acute hypercalcemia in pediatric patients following the interruption of denosumab. J. Endocrinol. Investig. 2021 doi: 10.1007/s40618-021-01630-4. [DOI] [PubMed] [Google Scholar]
- 20.Nieves J.W., Formica C., Ruffing J., Zion M., Garrett P., Lindsay R., Cosman F. Males have larger skeletal size and bone mass than females, despite comparable body size. J. Bone Miner. Res. 2005;20(3):529–535. doi: 10.1359/JBMR.041005. [DOI] [PubMed] [Google Scholar]
- 21.Palmerini E., Staals E.L., Jones L.B., Donati D.M., Longhi A., Randall R.L. Role of (neo)adjuvant denosumab for giant cell tumor of bone. Curr. Treat. Options in Oncol. 2020;21(8):68. doi: 10.1007/s11864-020-00766-4. [DOI] [PubMed] [Google Scholar]
- 22.Ferriero K., Shah B., Yan Y., Khatri S., Caccamese J., Napoli J.A., Bober M.B., Crane J.L. Case report: safety and efficacy of denosumab in four children with Noonan syndrome with multiple Giant cell lesions of the jaw. Front. Pediatr. 2020;8:515. doi: 10.3389/fped.2020.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choe M., Smith V., Okcu M.F., Wulff J., Gruner S., Huisman T., Venkatramani R. Treatment of central giant cell granuloma in children with denosumab. Pediatr. Blood Cancer. 2021;68(3) doi: 10.1002/pbc.28778. [DOI] [PubMed] [Google Scholar]
- 24.Trejo P., Rauch F., Ward L. Hypercalcemia and hypercalciuria during denosumab treatment in children with osteogenesis imperfecta type VI. J. Musculoskelet. Neuronal Interact. 2018;18(1):76–80. [PMC free article] [PubMed] [Google Scholar]
- 25.Kurucu N., Akyuz C., Ergen F.B., Yalcin B., Kosemehmetoglu K., Ayvaz M., Varan A., Aydin B., Kutluk T. Denosumab treatment in aneurysmal bone cyst: evaluation of nine cases. Pediatr. Blood Cancer. 2018;65(4) doi: 10.1002/pbc.26926. [DOI] [PubMed] [Google Scholar]
- 26.Raux S., Bouhamama A., Gaspar N., Brugieres L., Entz-Werle N., Mallet C., Dijoud F., Gouin F., Marec-Berard P. Denosumab for treating aneurysmal bone cysts in children. 2019;105(6):1181–1185. doi: 10.1016/j.otsr.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Upfill-Brown A., Bukata S., Bernthal N.M., Felsenfeld A.L., Nelson S.D., Singh A., Wesseling-Perry K., Eilber F.C., Federman N.C. Use of denosumab in children with osteoclast bone dysplasias: report of three cases. JBMR Plus. 2019;3(10) doi: 10.1002/jbm4.10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chawla S., Blay J.Y., Rutkowski P., Le Cesne A., Reichardt P., Gelderblom H., Grimer R.J., Choy E., Skubitz K., Seeger L., Schuetze S.M., Henshaw R., Dai T., Jandial D., Palmerini E. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. 2019;20(12):1719–1729. doi: 10.1016/S1470-2045(19)30663-1. [DOI] [PubMed] [Google Scholar]
- 29.Foertsch A., Hunt L., Homans A. Recurrent, severe hypercalcemia upon discontinuation of long-term denosumab for treatment of pediatric metastatic giant cell tumor. Pediatr. Blood Cancer. 2016;63 S88-S88. [Google Scholar]
- 30.Uday S., Gaston C.L., Rogers L., Parry M., Joffe J., Pearson J., Sutton D., Grimer R., Hogler W. J. Clin. Endocrinol. Metab. 2018;103(2):596–603. doi: 10.1210/jc.2017-02025. [DOI] [PubMed] [Google Scholar]
- 31.Sydlik C., Durr H.R., Pozza S.B., Weissenbacher C., Roeb J., Schmidt H. Hypercalcaemia after treatment with denosumab in children: bisphosphonates as an option for therapy and prevention? World J. Pediatr. 2020;16(5):520–527. doi: 10.1007/s12519-020-00378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harcus M., Aldridge S., Abudu A., Jeys L., Senniappan S., Morgan H., Pizer B. The efficacy of denosumab in the management of a tibial paediatric aneurysmal bone cyst compromised by rebound hypercalcaemia. 2020;2020:8854441. doi: 10.1155/2020/8854441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roux S., Massicotte M.H., Huot Daneault A., Brazeau-Lamontagne L., Dufresne J. Acute hypercalcemia and excessive bone resorption following anti-RANKL withdrawal: case report and brief literature review. Bone. 2019;120:482–486. doi: 10.1016/j.bone.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Mariz B., Migliorati C.A., Alves F.A., Penteado F.M., Carvalho N.P.F., Santos-Silva A.R., Rocha A.C. Successful denosumab treatment for central giant cell granuloma in a 9-year-old child. 2021;41(4):519–525. doi: 10.1111/scd.12588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the reported cases with hypercalcemia following denosumab withdrawal.