Highlights
-
•
Tendon and ligament collagens differ in their post-translational lysine and cross-linking chemistry.
-
•
In ligament collagen, hydroxylysyl aldehyde, permanent cross-linking dominates.
-
•
Tendon collagen has a mix of cross-links based on lysyl and hydroxylysyl aldehydes.
-
•
The profile in tendon appears more adapted to facilitating growth, structural remodeling and repair of the fibrillar matrix.
Abbreviations: ACL, Anterior cruciate ligament; PCL, posterior cruciate ligament; LCL, lateral collateral ligament; MCL, medial collateral ligament; QT, quadriceps tendon; HP, hydroxylysine pyridinoline; LP, lysine pyridinoline; HLNL, hydroxylysinonorleucine; DHLNL, dehydrohydroxylysinonorleucine; HHL, histidinohydroxylysinonorleucine; HHMD, histidinohydroxymerodesmosine; P3H1, prolyl 3-hydroxylase 1; P3H2, prolyl 3-hydroxylase 2; LC, liquid chromatography; MS, mass spectrometry
Keywords: Collagen, Cross-linking, Tendon, Ligament, Post-translational modifications, Mass spectrometry
Abstract
Tendons and ligaments tend to be pooled into a single category as dense elastic bands of collagenous connective tissue. They do have many similar properties, for example both tissues are flexible cords of fibrous tissue that join bone to either muscle or bone. Tendons and ligaments are both prone to degenerate and rupture with only limited capacity to heal, although tendons tend to heal faster than ligaments. Type I collagen constitutes about 80% of the dry weight of tendons and ligaments and is principally responsible for the core strength of each tissue. Collagen synthesis is a complex process with multiple steps and numerous post-translational modifications including proline and lysine hydroxylation, hydroxylysine glycosylation and covalent cross-linking. The chemistry, placement and quantity of intramolecular and intermolecular cross-links are believed to be key contributors to the tissue-specific variations in material strength and biological properties of collagens. As tendons and ligaments grow and develop, the collagen cross-links are known to chemically mature, strengthen and change in profile. Accordingly, changes in cross-linking and other post-translational modifications are likely associated with tissue development and degeneration. Using mass spectrometry, we have compared tendon and ligaments from fetal and adult bovine knee joints to investigate changes in collagen post-translational properties. Although hydroxylation levels at the type I collagen helical cross-linking lysine residues were similar in all adult tissues, ligaments had significantly higher levels of glycosylation at these sites compared to tendon. Differences in lysine hydroxylation were also found between the tissues at the telopeptide cross-linking sites. Total collagen cross-linking analysis, including mature trivalent cross-links and immature divalent cross-links, revealed unique cross-linking profiles between tendon and ligament tissues. Tendons were found to have a significantly higher frequency of smaller diameter collagen fibrils compared with ligament, which we suspect is functionally associated with the unique cross-linking profile of each tissue. Understanding the specific molecular characteristics that define and distinguish these specialized tissues will be important to improving the design of orthopedic treatment approaches.
Introduction
Tendons and ligaments are collagenous connective tissues that are often grouped as the tough, flexible cords that hold together the musculoskeletal system [1]. Indeed, the only consistent distinction made between the two tissues in much of the literature is that tendons join muscle-to-bone whereas ligaments join bone-to-bone. Tendons and ligaments do have many general similarities including biomechanics (high tensile strength) and composition (predominantly type I collagen) and the common public health issue that half of all musculoskeletal injuries are the result of ligament or tendon damage [2]. Despite the prevalence of tendon and ligament injuries and the economic cost of surgical repair, there remains a fundamental lack of understanding of tendon and ligament biology as it relates to development and healing [3]. For example, it is not fully known why injured intra-articular ligaments, such as anterior cruciate ligament (ACL) and posterior cruciate ligament (PCL), have no significant healing capacity and often require surgery [4], whereas vascularized tendons and ligaments, such as medial collateral ligament (MCL) and quadriceps tendon (QT), have a limited ability to heal [5], [6]. Although some tendon and ligament injuries such as strains and sprains may not require surgery, the healing process involves the formation of scar tissue that may require days, months and even years to remodel [5]. Furthermore, remodeled MCL has been shown to be weaker, less stiff and absorb less energy compared to normal MCL [7]. Even after surgical repair, ligaments often fail to regain their native structure and function, which can lead to re-tear events [8]. Unlike surgical repair, which is defined as any surgical procedure attempting to restore the original injured tissue; surgical reconstruction is a surgical procedure that replaces the injured tissue with a graft [4]. For example, in the case of ACL reconstruction, the autografted tissue is often obtained from a tendon source. However, it is unclear if mature tendon is the best substitute for ligament reconstruction. Recognizing tendons and ligaments as distinct specialized tissues with distinguishing features could help inform future applications and improvements in orthopedic outcomes.
Interestingly, it has been shown that injured fetal tendons have the capacity to regenerate fully thus restoring native structure and function [9]. This has been correlated to embryonic tendons having a higher cell density and activity, both of which decrease as tendons develop and mature [10]. Yet the specific conditions, which enable fetal regeneration and scarless healing, have not been fully explored. Furthermore, it is unknown if this capacity for healing in fetal tendons can be applied to adult tendons. Defining any consistent collagen post-translational variances between fetal and adult tendons and ligaments could help explain differences in their capacity to remodel and heal. For example, during growth and development, both tendons and ligaments have immature and reversible collagen cross-links that, with age, increase in strength and become permanent cross-links [7].
Both tendons and ligaments are predominantly composed of type I collagen (approximately 80% by dry weight). Type I collagen biosynthesis includes many post-translational hydroxylations (4-hydroxyproline, 3-hydroxyproline, 5-hydroxylysine), glycosylation (galactosyl-hydroxylysine, glucosylgalactosyl-hydroxylysine) and cross-linking (e.g. hydroxylysine pyridinoline) [11]. The importance of these post-translational modifications is clear from the many heritable disorders that cause altered collagen biosynthesis [12], [13]. A detailed profile of the tissue-specific phenotype of type I collagen post-translational modifications is essential for understanding the functional character of connective tissues. For example, tendon type I collagen is known to contain more sites of 3-hydroxyproline [14], [15] and less of hydroxylysine glycoside than type I collagen of skin or bone [16]. Only one site in tendon type I collagen appears ever to be significantly glycosylated (at K87 of the helical domain [16], [17]). The K87 residue is of unique importance as a key participant in cross-link formation with α1(I) C-telopeptide aldehydes along with K930(α1) and K933(α2) with N-telopeptide aldehydes. Although, the functional effect of glycosylation in collagens is not fully understood, a broad spectrum of roles have been proposed including facilitating matrix remodeling [18], [19], collagen fibrillogenesis [20], [21], [22], bone mineralization [23], in addition to participating in collagen cross-linking [24], [25], [26], [27].
In the current study, we set out to define distinct tissue specific features that may distinguish tendon and ligament during development. Recent studies focusing on collagen post-translational modifications in ligaments have been carried out on periodontal ligament [28], [29], [30]. In this study, we characterize the collagen profile of multiple ligament tissues isolated from adult and fetal bovine knee joints. Our focus was to compare type I collagen post-translational variances between ligaments (ACL, PCL, LCL, MCL) and tendon (QT). We propose that modifications unique to tendon type I collagen, including collagen cross-links, have evolved to enable the matrix to adapt and function in a long-lived tissue environment.
Results
Tendon and ligament collagens have similar prolyl 3-hydroxylation characteristics
Tendon (QT) and ligaments (ACL, PCL, LCL and MCL) were harvested from hindlimb knee joints of 18-month (adult; n = 3) and fetal bovine animals (n = 3). Collagens were 3%(v/v) acetic acid extracted from adult and fetal bovine tendons and ligaments. SDS-PAGE sample loads were normalized to the lyophilized dry weight of tissue. Although all tissues produced a similar banding pattern of collagen α-chains on SDS-PAGE, the adult tissue collagens were consistently less extractable compared to fetal tissue (Fig. 1). Type I collagen was the main type extracted from all tissues. Mass spectrometric analysis of the collagen α-chains revealed distinct developmental post-translational features between adult and fetal tendons and ligaments. We initially focused on the known prolyl 3-hydroxylase 2 (P3H2)-catalyzed modifications, which in type I collagen are at α1(I)P707 and the C-terminally located (GPP)n motifs. Type I collagen isolated from adult tendon is known to contain more 3-hydroxyproline than other adult connective tissues, such as bone and skin [15], [31]. This characteristic, which initially was thought to be unique to tendon, was also observed across all investigated adult bovine ligaments (Fig. 2, Table 1). Another feature common to the two connective tissues was an increase in prolyl 3-hydroxylation during development. Fetal tendons were previously shown to lack the high levels of 3-hydroxyproline in type I collagen that characterized adult tendons [31], [32], [33], [34]. The P3H2-catalyzed sites were minimally occupied in both fetal tendons and ligaments (Table 1). Fig. 2 highlights the difference in prolyl 3-hydroxylation of the (GPP)n motif between adult and fetal ACL as quantified by MS analysis. In contrast, the known prolyl 3-hydroxylase 1 (P3H1)-modified 3-hydroxyproline sites (α1(I)P986 and α2(I)P707) were almost fully occupied in both adult and fetal ligaments and tendons (Table 1).
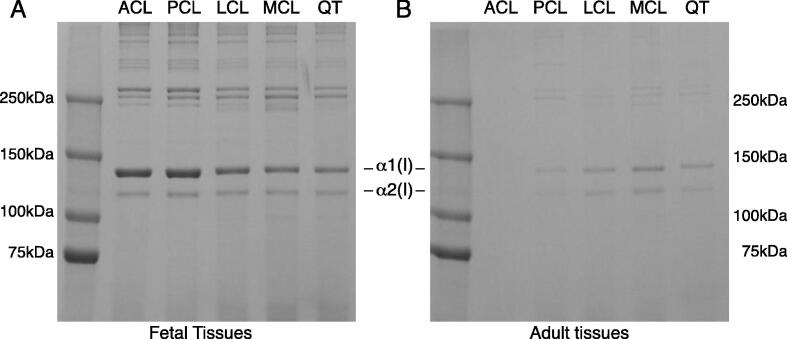
Fig. 1.
SDS-PAGE reveals a decrease in collagen extractability in adult tendons and ligaments compared to fetal tissue. Acid labile aldimine cross-links are broken with mild acetic acid treatment, allowing native type I collagen monomers to be extracted from the tissue. Collagen was more acid extractable from fetal tissues (A) than adult tissues (B). Anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), lateral collateral ligament (LCL), medial collateral ligament (MCL), quadriceps tendon (QT). SDS-PAGE sample loads were normalized to the dry weight of lyophilized tissues. The reduction in collagen extractability from adult tissues is consistent with an increase in mature collagen cross-links with development.
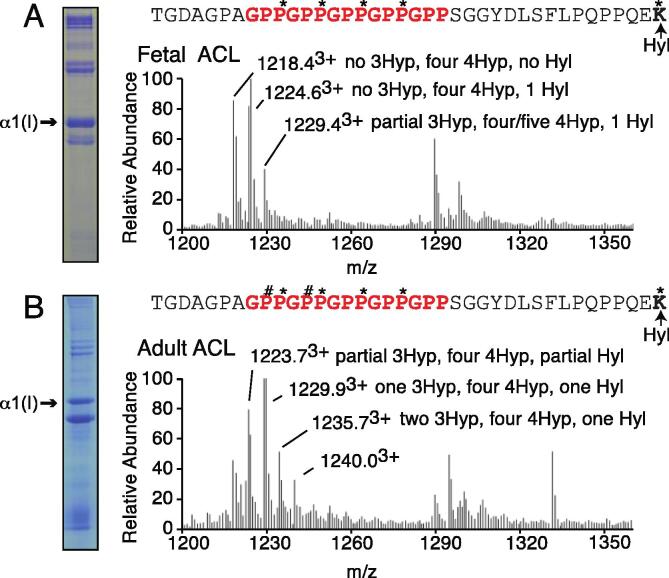
Fig. 2.
Type I collagen from ligaments exhibits age-dependent 3-hydroxyproline profile. LC-MS profiles of in-gel trypsin digests of the collagen α1(I) chain from fetal and adult bovine ACL. (A) MS profile of fetal bovine ACL shows only partial 3-hydroxylation (~8%) at the α1(I) C-terminal GPP motif; (B) MS profile at the same site of adult bovine ACL shows a hydroxylation like bovine tendon 3-hydroxylation (~60%). The trypsin digested peptide is shown with P# indicating 3Hyp, P* indicating 4Hyp and K* indicating Hyl. See Table 1 for more details.
Table 1.
Comparison of 3Hyp occupancy in type I collagens from adult and fetal bovine tendon and ligaments. Mean percentage of 3Hyp at each major substrate site in type I collagen from tendons and ligament tissues (n = 3). The percentages were determined based on the ratios between the ion-current yields of each post-translational variant as previously described.
| α1(I)986 |
α1(I)707 |
α2(I)707 |
α1(I)GPP |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QT | ACL | LCL | MCL | QT | ACL | LCL | MCL | Q | ACL | LCL | MCL | QT | ACL | LCL | MCL | |
| Adult | 95 ± 1% | 96 ± 1% | 95 ± 0% | 95 ± 0% | 60 ± 15% | 43 ± 14% | 52 ± 10% | 62 ± 13% | 92 ± 3% | 90 ± 6% | 99 ± 2% | 96 ± 3% | 57 ± 12% | 50 ± 10% | 53 ± 15% | 62 ± 8% |
| Fetal | 96 ± 0% | 96 ± 2% | 96 ± 1% | 96 ± 1% | 19 ± 5% | 21 ± 5% | 21 ± 5% | 25 ± 2% | 91 ± 2% | 98 ± 3% | 97 ± 6% | 98 ± 3% | 8 ± 3% | 7 ± 3% | 7 ± 3% | 8 ± 3% |
Ligament type I collagen helical domain cross-linking lysines (K87) are highly glycosylated in contrast with tendon
Using in-gel trypsin digestion we investigated the post-translational quality of the type I collagen sites, including the C-telopeptide cross-linking residue (α1(I)K1030) and helical cross-linking residues (α1(I)K87 and α2(I)K87). The cross-linking K87 residues from both α-chains of type I collagen from fetal ligament and tendon were fully hydroxylated and subsequently glycosylated (glucosylgalactosyl-hydroxylysine) (Fig. 3). Adult tendon had significantly less glycosylation at the K87 residues compared to ligament tissues (~25% compared to ~80%), denoting a developmental difference between the tissues (Fig. 3). The adult ligaments investigated in this study varied significantly in the degree of modification at the K87 residues. It appears that the intra-articular ligaments (ACL) retain a higher level of glycosylation in adulthood compared to vascularized connective tissues (QT, LCL, MCL). These results are summarized in Table 2. Collagen from the intra-articular ligaments, ACL (Fig. 3) and PCL (not shown), had comparable post-translational modification profiles at α1(I)K87 and α2(I)K87. The post-translational quality of α1(I)K930 and α2(I)K933 could only be detected using bacterial collagenase digestion followed by resolution of peptide fragments by reverse phase chromatography. Although this approach is not as quantitative as in-gel-trypsin, our data support almost 100% hydroxylation of both α1(I)K930 and α2(I)K933 in fetal and adult tendons and ligaments (Table 2).
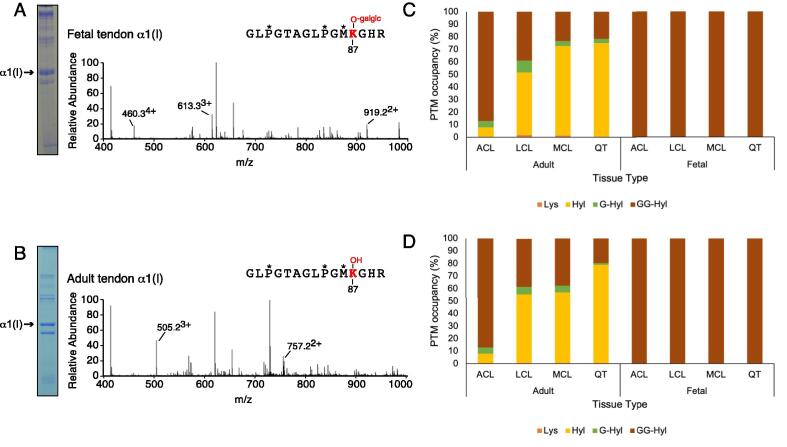
Fig. 3.
Post-translational variances in linear cross-linking α1(I)K87 and α2(I)87 from type I collagens. (A, B) LC-MS profiles of in-gel trypsin digests of the collagen α1(I) chain from fetal and adult bovine tendon. (A) Fetal bovine tendon shows complete glycosylation of α1(I)K87 (613.33+). (B) The α1(I)K87 residue from adult bovine tendon is hydroxylated but not subsequently glycosylated (505.23+). The trypsin-digested peptide is shown with P* indicating 4Hyp, M* indicating oxidized methionine. Summary of modifications on lysine 87 from (C) α1(I) and (D) α2(I) chains of type I collagen. Lysine modifications include unmodified lysine (Lys), hydroxylysine (Hyl), galactosyl-hydroxylysine (G-Hyl) and glucosylgalactosyl-hydroxylysine (GG-Hyl). The percentages were determined based on the ratios between the ion-current yields of each post-translational variant as previously described. See Table 2 for more details.
Table 2.
Summary of post-translational variances in linear helical cross-linking lysines from type I collagens. Modifications on α1(I)K87, α2(I)K87, α1(I)K930 and α2(I)K933 from both α-chains of type I collagen were measured in adult and fetal bovine tissues using mass spectrometry. Lysine modifications include unmodified lysine (Lys), hydroxylysine (Hyl), galactosyl-hydroxylysine (G-Hyl) and glucosylgalactosyl-hydroxylysine (GG-Hyl). Mean percentages for K87 (n = 3) and K930/933 (n = 1–2) were determined based on the ratio the m/z peaks of each post-translational variant as previously described.
| K87 hydroxylation (%) | ||||
|---|---|---|---|---|
| Adult α1(I) K87 | ||||
| Lys | Hyl | G-Hyl | GG-Hyl | |
| QT | 0 | 75 ± 13 | 3 ± 6 | 22 ± 16 |
| ACL | 0 | 10 ± 17 | 6 ± 10 | 88 ± 21 |
| LCL | 2.0 ± 3 | 50 ± 29 | 9 ± 10 | 39.0 ± 20 |
| MCL | 1 ± 2 | 71 ± 24 | 4 ± 7 | 23 ± 15 |
| Adult α 2(I) K87 | ||||
| Lys | Hyl | G-Hyl | GG-Hyl | |
| QT | 0 | 79 ± 12 | 1 ± 2 | 20 ± 10 |
| ACL | 0 | 17 ± 14 | 5 ± 4 | 79 ± 18 |
| LCL | 0 | 55 ± 22 | 6 ± 7 | 38 ± 16 |
| MCL | 0 | 57 ± 27 | 5 ± 4 | 38 ± 23 |
| Fetal α1(I) K87 | ||||
| QT | 0 | 0 | 0 | 100 |
| ACL | 0 | 0 | 0 | 100 |
| LCL | 0 | 0 | 0 | 100 |
| MCL | 0 | 0 | 0 | 100 |
| Fetal α 2(I) K87 | ||||
| QT | 0 | 0 | 0 | 100 |
| ACL | 0 | 0 | 0 | 100 |
| LCL | 0 | 0 | 0 | 100 |
| MCL | 0 | 0 | 0 | 100 |
| K930/K933 hydroxylation (%) | ||
|---|---|---|
| Adult α 1(I) 930 | ||
| Lys | Hyl | |
| QT | 0 | 100 |
| ACL | 0 | 100 |
| LCL | 0 | 100 |
| MCL | 0 | 100 |
| Adult α 2(I) 933 | ||
| Lys | Hyl | |
| QT | 0 | 100 |
| ACL | 0 | 100 |
| LCL | 0 | 100 |
| MCL | 0 | 100 |
| Fetal α 1(I) 930 | ||
| QT | 0 | 100 |
| ACL | 0 | 100 |
| LCL | 0 | 100 |
| MCL | 0 | 100 |
| Fetal α 2(I) 933 | ||
| QT | 0 | 100 |
| ACL | 0 | 100 |
| LCL | 0 | 100 |
| MCL | 0 | 100 |
Stable hydroxylysine-aldehyde cross-links dominate in ligament more than tendon
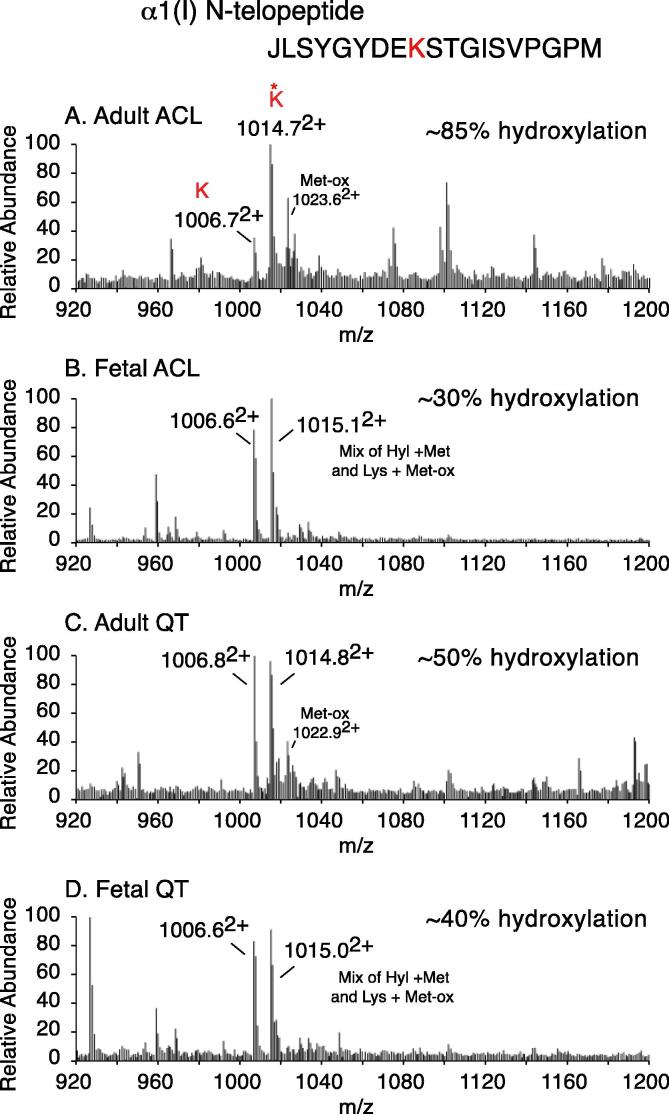
The collagen C-telopeptide cross-linking residue (α1(I)K1030) was found to be partially hydroxylated in adult tendons (60% hydroxylation) as well as all fetal tissues (~65% hydroxylation) (Fig. 4). In contrast, α1(I)K1030 was almost fully hydroxylated in adult ligaments (~90%) (Fig. 2, Fig. 4). Ligaments from older fetuses were observed to have higher levels of C-telopeptide lysine hydroxylation than younger fetuses. This variability between fetal tissues is evident from the high standard deviation in the calculated mean percent hydroxylation (Fig. 4). Similar to K930/K933, the hydroxylation state of the N-telopeptide cross-linking lysines is only detectable using bacterial collagenase digests (Fig. 5). Fetal tissues were found to contain approximately 30–40% hydroxylysine at the N-telopeptide cross-linking site. Even in adulthood, this site remained about 50% hydroxylated in tendon (QT) (Fig. 5C). However, the N-telopeptide cross-linking lysine was about 85% hydroxylated in adult ligaments (Fig. 5A).
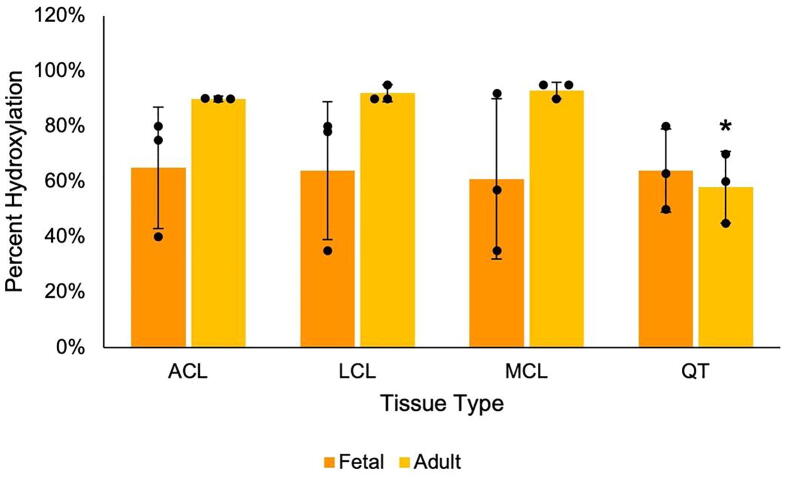
Fig. 4.
Increased hydroxylation of the linear C-telopeptide cross-linking lysine residue in adult ligaments. The C-telopeptide cross-linking lysine of type I collagen is a useful predictor of cross-linking quality across tissues. Percent hydroxylation is calculated from tryptic peptides using mass spectrometry (n = 3). Note that lysine hydroxylation in adult QT is significantly less than adult ligaments; * p < 0.05 by t-test assuming unequal variance.
Fig. 5.
Ligament type I collagen has elevated N-telopeptide cross-linking lysine hydroxylation compared to tendon. LC-MS profiles of collagenase-digested adult ACL (A), Fetal ACL (B), Adult QT (C) and Fetal QT (D). (A) Adult ligaments had the highest level of hydroxylysine (~10152+) at the N-telopeptide cross-linking site of all tissues tested. A small population of peptide containing hydroxylysine + oxidized methionine (~10232+) was observed in adult tissues.
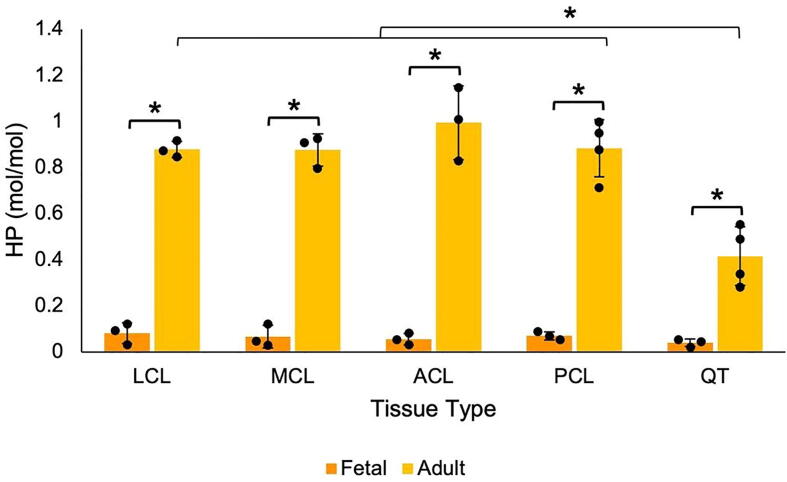
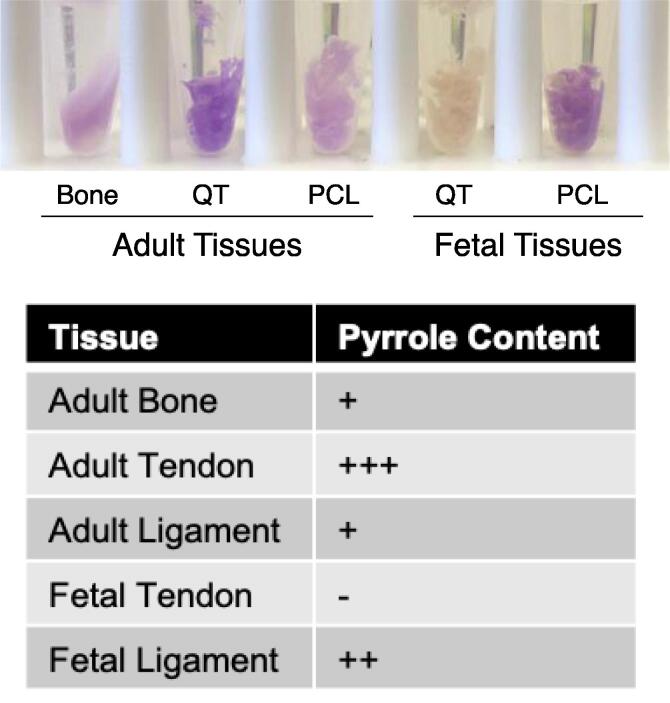
Despite more than half of the C-telopeptide cross-linking residues being hydroxylysine in fetal tendons and ligaments, pyridinoline cross-linking analysis revealed only trace levels of HP in all fetal tissues tested (~0.06 mol/mole of collagen each; Fig. 6). The relatively high levels of C-telopeptide hydroxylysine in fetal tissues with low levels of HP cross-links is consistent with fetal collagens containing mostly divalent ketoimine cross-links that have yet to mature to HP. A partial C-telopeptide lysine hydroxylation profile is also consistent with fetal tendon and ligament collagens containing a mix of cross-links based on hydroxylysine-aldehyde and lysine-aldehyde precursors, and so fewer chemically stable cross-links than adult tissues [35], [36]. The increase in telopeptide lysine hydroxylation in adult ligaments explains the increase in pyridinoline cross-links in mature ligament. Adult tendons, which had ~50% hydroxylation at α1(I)K1030, had ~10-fold higher levels of pyridinoline than fetal tendon (Fig. 6). Mature ligaments uniformly had higher levels of HP (~1.0 mol/mole of collagen) than mature tendon (0.5 mol/mole of collagen), consistent with their higher hydroxylation at α1(I)K1030 (Fig. 4, Fig. 6). Measurable levels of LP were not detected in any of the analyzed tissues. Since trivalent pyrrole cross-links are formed in bone collagen because it has partially hydroxylated telopeptide lysines, the presence (or absence) of pyrroles in adult and fetal tendon was assessed by direct reaction of tissue samples with Ehrlich’s reagent (p-dimethylaminobenzaldehyde). Of all the tissues tested, only fetal tendon failed to turn pink after a 5-minute incubation in Ehrlich’s reagent. Based on color intensity, fetal and adult ligament both contain pyrroles. Adult tendon produced the darkest pink color, indicating the highest level of pyrroles of the tissues tested (Fig. 7) consistent with its 50% telopeptide lysine hydroxylation (Fig. 4, Fig. 5). In this respect, the tendon resembles bone [37].
Fig. 6.
Increased pyridinoline cross-linking with increased tissue maturity. Concentration of hydroxylysine pyridinoline cross-linking residues in fetal and adult bovine tendon and ligaments expressed as moles/mole of collagen (n = 3). (HP, hydroxylysine pyridinoline). None of the tissues contained measurable levels of LP (lysine pyridinoline). *p < 0.05 by t-test assuming unequal variance.
Fig. 7.
Tissue pyrrole content determined from Ehrlich reagent color change. Bovine tissues were minced and incubated in Ehrlich’s reagent for 5 min. No color change was detected in fetal tendon (n/d). The color reactions (n = 2) were measured using online color detection software.
Immature cross-links dominate in fetal tendon and ligament
Total collagen cross-links were quantified in acid hydrolysates of borohydride-reduced whole tissues. Cross-linking amino acid products were separated from bulk amino acids by organic solvent partition on a hydrated cellulose column and resolved by normal phase HPLC [38]. Amounts of Individual cross-links are presented as ratios of their ion-current yields (Table 3). The moles/mole of collagen value of HP was calibrated independently by HPLC fluorescence quantitation (Fig. 6). Although this gives relative, not absolute moles/mole of collagen amounts for the other cross-links, the ratios are useful for comparing tissues. It is evident from the cross-link ratios that histidinohydroxymerodesmosine (HHMD), a tetravalent cross-link formed by borohydride reduction from an N-telopeptide aldol in aldimine linkage to hydroxylysine at α1(I)K930 (and neighboring α1(I)H932 by Michael addition) [38], is a prevalent cross-link in both the fetal tissues as well as adult tendon. As previously reported, both fetal tendons and ligaments are comprised of higher levels of immature cross-links hydroxylysinonorleucine (HLNL) and dehydrohydroxylysinonorleucine (DHLNL) than mature cross-links (HP) [39]. Significant differences were also observed between adult tendon and ligaments. For example, the predominant cross-link in adult ligament was HP; however, adult tendon has less HP and more HHMD and pyrrole cross-links than adult ligament. The pyrrole cross-link content is not measurable by this method due to its acid lability.
Table 3.
Total collagen cross-links in bovine ligament and tendon. Borohydride reduced total collagen cross-links were determined from LC-MS profiles of tissue acid hydrolysates. Enriched cross-linking amino acids were resolved and analyzed by electrospray LC-MS on the LTQ XL using a Cogent 4 diamond hydride column (values based on the intensity of each cross-link (m/z) in the total ion current; n = 2). Cross-link populations are presented as ratio of HP.
| Tissue | HHMD/HP | HHL/HP | HLNL/HP | DHLNL/HP |
|---|---|---|---|---|
| Adult QT | 4.1 | 0.7 | 0.5 | 0.2 |
| 1.5 | 0.3 | 0.3 | 0.2 | |
| Average | 2.8 | 0.5 | 0.4 | 0.2 |
| Adult MCL | 0.6 | 0.2 | 0.3 | 0.2 |
| 0.5 | 0.1 | 0.3 | 0.3 | |
| Average | 0.6 | 0.1 | 0.3 | 0.2 |
| Adult ACL | 0.4 | 0.2 | 0.3 | 0.2 |
| 0.3 | 0.1 | 0.3 | 0.2 | |
| Average | 0.4 | 0.1 | 0.3 | 0.2 |
| Fetal QT | 4.8 | 0.8 | 2.4 | 4.5 |
| 7.9 | 0.8 | 4.9 | 4.8 | |
| Average | 6.3 | 0.8 | 3.6 | 4.7 |
| Fetal MCL | 6.6 | 0.6 | 2.7 | 5.2 |
| 9.6 | 0.7 | 3.0 | 5.0 | |
| Average | 8.1 | 0.6 | 2.8 | 5.1 |
| Fetal ACL | 2.9 | 0.5 | 1.6 | 4.1 |
| 2.9 | 0.3 | 1.0 | 2.7 | |
| Average | 2.9 | 0.4 | 1.3 | 3.4 |
Ultrastructural differences between tendon and ligament
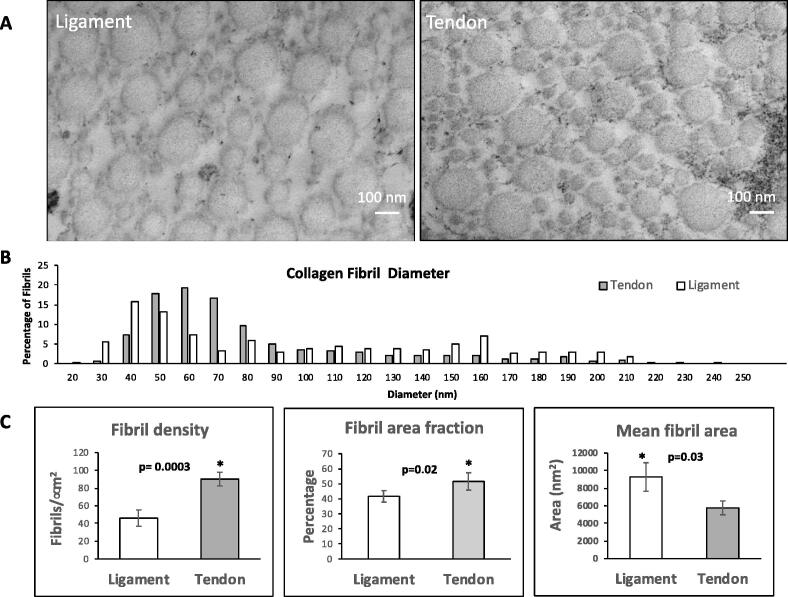
Tendon (QT) and ligament (ACL) collagen fibrils were investigated by transmission electron microscopy (TEM) to assess potential ultrastructural differences associated with the unique cross-linking profile of each tissue. Thin sections of tendon and ligament were cut perpendicular to the long axis of the tissue in order to yield transverse collagen fibril transections. Overall, collagen fibril structure from tendon and ligament were quite similar as determined by TEM; however, several subtle distinctions were identified between the tissues (Fig. 8A). For example, it was immediately apparent that ACL had more interfibrillar spacing than QT. This is consistent with ACL having a lower fibril density (number of fibrils/μm2) and fibril area fraction (percentage of ECM occupied by collagen fibrils) than QT (Fig. 8C). A bimodal distribution of collagen fibril diameters was found in both tendons and ligaments, which were defined as small (30–90 nm) and large (140–200 nm) diameter fibril populations (Fig. 8B). A similar pattern in the bimodal distribution of fibril diameters was first observed in horse tendons and ligaments [40]. Tendon was found to have a higher frequency of the small fibril population compared to ligament, whereas ligament had a higher frequency of the larger population compared to tendon (Fig. 8B).
Fig. 8.
Transmission electron microscopy of collagen fibrils from bovine ligament and tendon. Representative transmission electron microscopy images showing slight distinctions between collagen fibrils between ligament (ACL) and tendon (QT) (A). Scale bar is 100 nm. Collagen fibril diameter plot illustrates bimodal distribution in both tissues (B). Fibril density, mean fibril area and fibril area fraction graphs illustrate several subtle distinctions between the tissues (C).
Discussion
Tendons and ligaments both largely consist of type I collagen, but each has distinct evolutionary origins and functions. Tendons evolved early, before bone, from myosepta, sheet-like structures that transmitted primitive muscle force [41]. Although ligaments may have evolved from a similar origin, the primary function of most tendons is to transmit force needed for joint movement, whereas the primary function of most ligaments is joint stabilization and proprioception [7]. In this study, we attempted to distinguish between tendon and ligament with a focus on type I collagen post-translational modifications. It is becoming increasingly evident that the post-translational quality of fibril-forming collagens is central to understanding the functional and structural properties of specific tissues [42]. Defining the unique molecular collagen phenotypes of tendons and ligaments may reveal how these tissues evolved to function within their distinct environments.
Similarities between tendon and ligament
Type I collagen from tendon and ligaments was surveyed for prolyl 3-hydroxylation content, as specific substrate sites for this rare modification have been uniquely localized to tendons [15], [31]. Type I collagen from both tendon and ligaments shared a similar 3-hydroxyproline profile attributable to P3H1 [43] and P3H2 [33], [44] activity. Another common feature between tissues was that fetal tissues have only minimal levels of prolyl 3-hydroxylation in type I collagen sites compared to adult tissues. The increase in 3-hydroxyproline previously associated with developing tendon [34], was also observed in all bovine knee joint ligament collagens. It is clear from this work that fetal tendon and ligament cells have active P3H1, lysyl hydroxylase-1 (LH1), lysyl hydroxylase-2 (LH2) and glycosyltransferase enzyme networks. Fetal tissue has full lysyl 5-hydroxylation and glycosylation (glucosylgalactosyl-hydroxylysine) at cross-linking K87 residues from both α-chains. P3H2 and glycosyltransferase also appear to be uniquely regulated in ligament and tendon. Adult tendon cells, in particular, seem to experience a distinct decrease in glycosyltransferase activity, in addition to the accompanying increase in P3H2 activity. The result is adult tissues that have different collagen cross-linking profiles and likely unique structural and functional properties.
Differences between tendon and ligament
Differences in post-translational modifications between tissues were first observed in the linear cross-linking K87 residues (Fig. 3). For example, adult ACL contained significantly more K87 glycosylation (predominantly glucosylgalactosyl-hydroxylysine) than any other adult ligament collagen. Of all the adult tissues tested, QT collagen had the lowest level of glycosylation at these cross-linking residues. It is not clear if the differences in glycosylation between intra-articular tissue (ACL, PCL) and more vascularized tissues (MCL, LCL, QT) imparts an advantage to these unique specialized tissues. Only negligible levels of non-modified lysine at K87 were detected in any adult tissue. In contrast, K87 from both α-chains was 100% glycosylated with glucosylgalactosyl-hydroxylysine in all fetal tissues tested. Although a specific role for hydroxylysine glycosylation in collagen cross-linking has yet to be fully elucidated, it has been proposed that the post-translational state of the helical-domain cross-linking lysines may serve as a regulator for normal collagen divalent aldimine cross-link chemistry [16]. In a mouse model, it was shown that reduction of hydroxylation and glycosylation at K87 led to a decrease in the level of intermolecular aldimine cross-links with C-telopeptide lysine aldehyde, which resulted in increased intramolecular aldol cross-links relative to intermolecular aldol cross-links [16], [45]. Glucosylgalactosyl hydroxylysine at α1(I)K87 has also been shown to be required for HHL formation on acid hydrolysis of skin and cornea collagens from species that have a histidine in the α1(I) C-telopeptide two residues from the cross-linking lysine aldehyde [38]. It is notable therefore, that HHL is detected in those tissues with partial C-telopeptide lysine hydroxylation and glucosylgalactosyl-hydroxylysine at α1K87, for example QT (Table 3, Fig. 5).
Differences between adult and fetal collagen cross-linking were first visualized by SDS-PAGE (Fig. 1), as collagen α-chain extractability was significantly less in adult tissues compared to fetal tissues. This decrease in extractability is consistent with the maturation of collagen cross-links from acid labile aldimines in fetal tissues to stable ketoimines and pyridinolines in adult tissues. An increase in pyridinoline content associated with tissue maturation has also been observed during cartilage [36] and bone development [46].
Fetal cross-links
HP and pyrrole cross-links were notably absent from fetal bovine tendon, consistent with the time-scale for maturation from initial, divalent to mature, trivalent intermolecular cross-links in collagens [46]. Borohydride-reduced fetal tendon was found to contain more HLNL, DHLNL and HHMD than fetal ligament. These findings are consistent with early amino acid analyzer studies, which found fetal tendon to contain elevated levels of ketoimines, particularly DHLNL [39]. Unlike fetal tendon, fetal ligament also appears to contain some pyrrole cross-links. It should be noted that HLNL and borohydride-reduced lysinoketonorleucine are indistinguishable by mass (292 Da) using LC-MS. However, the high levels of hydroxylation of helical cross-linking lysines in fetal tissues combined with partial telopeptide lysine hydroxylation supports a prevalence of HLNL over the latter cross-link.
Adult cross-links
Adult tendon and ligaments could be distinguished by their degree of telopeptide cross-linking lysine hydroxylation. The N- and C-telopeptide cross-linking lysine residues were over 80% hydroxylated in all adult ligament tissues, whereas adult and fetal tendon were only partially hydroxylated. This is consistent with adult ligaments having more mature HP cross-links than adult tendon. Partial hydroxylation at the telopeptide cross-linking lysines in adult tendon would also support tendon having higher levels of pyrrole cross-links, which is consistent with the Ehrlich’s reagent assay. In this respect tendon is similar to bone in having roughly 50% hydroxylation of telopeptide lysines [36], which may reflect a similar evolutionary origin. No significant differences were found between intra-articular ligaments and vascularized ligaments in their C-telopeptide hydroxylysine or HP content. From HP analysis (Fig. 4) and the total cross-link profiles (Table 3), HP dominated in both intra-articular and vascularized adult ligaments. In contrast, adult tendon had about half the HP content and more HHMD than any other cross-link, consistent with tendon forming a more equal mix of cross-links based on both lysyl and hydroxylysyl aldehydes than ligament. HHMD is an artifact of borohydride reduction, which naturally in situ, is a relatively labile aldimine cross-link between an N-telopeptide aldol and a hydroxylysine side-chain [38]. Similarly, the Ehrlich reactive pyrroles in adult tendon are predictably the reaction product of N-telopeptide to α2(I)K933 ketoimine cross-links with an additional N-telopeptide lysine aldehyde, based on bone collagen chemistry [36], [37], [47]
Concluding remarks
Intermolecular collagen cross-linking is the definitive post-translational modification that imparts tensile strength to connective tissues. The unique cross-link profile of tendon (e.g., pyridinolines, pyrroles and aldols) may be an adaptation that evolved to support biomechanical properties throughout growth and remodeling phases. For example, the high levels of labile intermolecular cross-links may allow newly synthesized collagen molecules to fuse laterally to the growing fibril with accompanying labile cross-link breakages and reformations without compromising overall mechanical function [38]. A possible illustration of this cross-linking phenomenon is seen in the TEM images by the high frequency of small diameter fibrils in QT compared to ACL. Lateral fusion of these small fibrils to larger fibrils during tissue growth and remodeling would be facilitated by collagen molecules that accrue through less permanent cross-links. In other words, labile collagen cross-linking should enable the continual state of equilibrium needed on the surface of collagen fibrils to support tissue remodeling and growth, even in mature tendons. Based on this study, as well as our unpublished findings on human Achilles and anterior tibial tendons, we conclude that, in general, tendons have a mixed profile of labile and permanent cross-links. Indeed, Achilles tendons have been shown to contain high levels of pyridinoline [48], whereas in rodent tail tendons, which almost exclusively use the lysine aldehyde cross-linking pathway, pyridinoline is nearly undetectable [46], [49]. It is also likely that the distinct collagen cross-linking profile of tendon (and possibly ligament) is important for specialized tendon functions, such as interfibrillar tendon sliding to allow length growth with skeletal growth [50], [51]. It is becoming increasingly clear that as connective tissues develop and mature, their collagen molecular phenotype also changes. Defining tissue-specific variances in collagen post-translational modifications and cross-linking may be useful diagnostically to predict how healthy tendons and ligaments will respond to injury and during healing.
Experimental procedures
Collagen extraction
Ligaments (ACL, PCL, MCL, LCL) and tendon (QT) were isolated from 18-month steer (adult) and fetal bovine knees purchased from Sierra for Medical Science (Whittier, CA). Intact type I collagen was solubilized from the tissues by acid extraction in 3% acetic acid for 24 h at 4 °C. Collagen α-chains were resolved by SDS-PAGE and stained with Coomassie Blue R-250. Total collagenase digests were resolved into peptide fractions on a C8 column by reverse-phase HPLC and monitored at 220 nm.
Pyridinoline cross-linking analysis
The pyridinoline cross-link content of collagen preparations was determined by HPLC after acid hydrolysis in 6 N HCl for 24 hrs at 108 °C. Dried samples were dissolved in 1% (v/v) n-heptafluorobutyric acid and their reverse-phase HPLC lysine pyridinoline (LP) and hydroxylysine pyridinoline (HP) contents quantified by fluorescence monitoring as previously described [46].
Pyrrole cross-linking analysis
The pyrrole cross-link content of collagen preparations was determined based on established protocols [37]. Briefly, equal amounts of tissues were finely minced (15 mg each) and resuspended in 6:1 (v/v)) Milli-Q water: 5% (w/v) p-dimethylaminobenzaldehyde in 4 M HClO4. The color reactions (n = 2) were recorded after 5 min and measured using online color detection software (ColorMeter Free – color picker, vistech.projects).
LC-MS peptide analysis
Lysine hydroxylation, glycosylation (glucosylgalactosyl-hydroxylysine and galactosyl-hydroxylysine), and prolyl 3-hydroxylation were quantified at specific sites in collagen α-chains as previously described [5], [18]. Collagen α-chains were cut from SDS-PAGE gels and subjected to in-gel trypsin digestion. Samples were also digested with bacterial collagenase, with and without sodium borohydride reduction, and resolved by C8 reverse-phase HPLC prior to analysis by MS. Electrospray mass spectrometry was carried out on the trypsin and collagenase-digested peptides using an LTQ XL linear quadrapole ion-trap mass spectrometer equipped with in-line Accela 1250 liquid chromatography and automated sample injection [14]. Thermo Xcalibur software and Proteome Discoverer software (ThermoFisher Scientific) were used for peptide identification. Tryptic peptides were also identified manually by calculating the possible MS/MS ions and matching these to the actual MS/MS spectrum. Glycosylation and hydroxylation content in collagen α-chains differences were determined manually by averaging the full scan MS over several minutes to include all the post-translational variations of a given peptide. Protein sequences used for MS analysis were obtained from the Ensembl genome database.
LC-MS cross-link analysis
Freeze-dried bovine tendon and ligament samples were reduced with sodium borohydride and hydrolyzed in 6 N HCl at 108 °C for 24 h. Cross-linking amino acids were enriched from hydrolysates by partition on a hydrated cellulose column as previously described [38], [52]. Briefly, the hydrolyzed sample was loaded on a cellulose (CF1, Whatman) column in butanol/ water/acetic acid (4:1:1, v/v/v). Free amino acids were removed by washing the column with butanol/water/acetic acid solution. Cross-linking amino acids were eluted with water and freeze-dried for mass spectral analysis. Electrospray LC-MS/MS was performed on the LTQ XL using a Cogent 4 diamond hydride column (15 cm × 1 mm; Microsolv Technology, 70000-15P-1) eluted at 50 μl min. The LC mobile phase consisted of buffer A (0.1% formic acid in Milli-Q water) and buffer B (0.1% formic acid in 80% acetonitrile) [38].
Transmission electron microscopy (TEM)
Bovine ACL and QT samples were dissected from the knee joint and prepared for TEM using a modification of the procedure we have published before [53]. Mid-section samples from both tissues were rinsed 3X in PBS pH 7.4 at 4 °C for 2 h. Samples were fixed in 2.5% paraformaldehyde and 0.25% glutaraldehyde in 0.2 M sodium phosphate buffer pH 7.4 for 24 h. Post-fixation was carried out using 2% osmium tetraoxide in 0.2 M sodium phosphate buffer, pH 7.4. The samples were then dehydrated and embedded in plastic and cured overnight. Ultrathin transverse sections (70–90 nm) were cut, placed on 150-mesh copper grids, and stained with 4% uranyl acetate and Reynold’s lead citrate. All sections were examined and photographed on a FEI Tecnai Spirit Bio-Twin transmission electron microscope, fitted with a Hamamatsu ORCS HR camera. For each sample a minimum of 4 different regions were viewed. An unbiased sampling of each region was performed. A magnification of 30,000× was used for collagen fibril area, collagen fibril area fraction and collagen fibrils per μm2. All measurements were made using ImageJ software (NIH freeware, http://rsb.info.nih.gov/nih-image) [54]. Areas of collagen fibrils in 7.28 μm2 of ligament and tendon was measured. Mean fibril area was calculated from average collagen area and number of fibrils counted. Collagen area fraction was calculated using total collagen area measured in the total area sample. Fibril diameter was calculated from fibril area assuming circularity (1/4 × π × diameter2). Data are presented as mean ± SD. For statistics, the two-sample assuming unequal variances t-Test was used and alpha was set to 0.05. p values less than 0.05 were considered to be significant.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This work was supported by the National Institutes of Health (NIH) Grants: (NIAMS) AR037318 and (NICHD) HD070394 to DRE; (NIAMS) AR057025 to RJF; (NIA) AG065605 to DMH. The authors would like to thank Jennifer K. Swicord, Electron Microscopy Supervisor, Department of Laboratory Medicine and Pathology, University of Washington Medical Center for expert technical assistance.
Author contributions
Conceptualization, D.M.H., D.R.E.; Supervision, D.M.H., D.R.E.; Data Collection and Analysis, D.M.H., M.A., M.A.W. R.J.F.; Formal analysis; D.M.H., R.J.F., D.R.E.; Roles/Writing - original draft, D.M.H.; Writing - review & editing D.M.H., R.J.F., D.R.E.; Funding acquisition, D.M.H., R.J.F., D.R.E. All authors intellectually contributed and provided approval for publication.
References
- 1.Provenzano P.P., Vanderby R. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84. doi: 10.1016/j.matbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Wu F., Nerlich M., Docheva D. Tendon injuries: basic science and new repair proposals. EFORT Open Rev. 2017;2:332–342. doi: 10.1302/2058-5241.2.160075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connizzo B.K., Yannascoli S.M., Soslowsky L.J. Structure-function relationships of postnatal tendon development: a parallel to healing. Matrix Biol. 2013;32:106–116. doi: 10.1016/j.matbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.P. Mahapatra, S. Horriat, B.S. Anand, Anterior cruciate ligament repair - past, present and future, J Exp Orthop. 5 (2018) 20–10. doi:10.1186/s40634-018-0136-6. [DOI] [PMC free article] [PubMed]
- 5.Leong N.L., Kator J.L., Clemens T.L., James A., Enamoto‐Iwamoto M., Jiang J. Tendon and ligament healing and current approaches to tendon and ligament regeneration. J. Orthop. Res. 2020;38:7–12. doi: 10.1002/jor.v38.110.1002/jor.24475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andarawis-Puri N., Flatow E.L., Soslowsky L.J. Tendon basic science: development, repair, regeneration, and healing. J. Orthop. Res. 2015;33:780–784. doi: 10.1002/jor.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank C.B. Ligament structure, physiology and function. J. Musculoskelet. Neuronal. Interact. 2004;4:199–201. [PubMed] [Google Scholar]
- 8.Duquin T.R., Buyea C., Bisson L.J. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am. J. Sports Med. 2010;38:835–841. doi: 10.1177/0363546509359679. [DOI] [PubMed] [Google Scholar]
- 9.Schweitzer R., Zelzer E., Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development. 2010;137:2807–2817. doi: 10.1242/dev.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa Y., Majima T., Nagashima K. Effect of ageing on ultrastructure of slow and fast skeletal muscle tendon in rabbit Achilles tendons. Acta Physiol. Scand. 1994;152:307–313. doi: 10.1111/j.1748-1716.1994.tb09810.x. [DOI] [PubMed] [Google Scholar]
- 11.Salo A.M., Myllyharju J. Prolyl and lysyl hydroxylases in collagen synthesis. Exp. Dermatol. 2021;30:38–49. doi: 10.1111/exd.14197. [DOI] [PubMed] [Google Scholar]
- 12.Forlino A., Marini J.C. Osteogenesis imperfecta. Lancet. 2016;387:1657–1671. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marini J.C., Forlino A., Bächinger H.P., Bishop N.J., Byers P.H., Paepe A.D., et al. Osteogenesis imperfecta. Nat. Rev. Dis. Primers. 2017;3 doi: 10.1038/nrdp.2017.52. [DOI] [PubMed] [Google Scholar]
- 14.Weis M.A., Hudson D.M., Kim L., Scott M., Wu J.-J., Eyre D.R. Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly. J. Biol. Chem. 2010;285:2580–2590. doi: 10.1074/jbc.M109.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson D.M., Eyre D.R. Collagen prolyl 3-hydroxylation: a major role for a minor post-translational modification? Connect. Tissue Res. 2013;54:245–251. doi: 10.3109/03008207.2013.800867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudson D.M., Weis M., Rai J., Joeng K.S., Dimori M., Lee B.H., et al. P3h3-null and Sc65-null mice phenocopy the collagen lysine under-hydroxylation and cross-linking abnormality of Ehlers-Danlos Syndrome Type VIA. J. Biol. Chem. 2017;292:3877–3887. doi: 10.1074/jbc.M116.762245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pokidysheva E., Zientek K.D., Ishikawa Y., Mizuno K., Vranka J.A., Montgomery N.T., et al. Posttranslational modifications in type I collagen from different tissues extracted from wild type and prolyl 3-hydroxylase 1 null mice. J. Biol. Chem. 2013;288:24742–24752. doi: 10.1074/jbc.M113.464156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C.L., Rui H., Mosler S., Notbohm H., Sawaryn A., Müller P.K. Collagen II from articular cartilage and annulus fibrosus. Structural and functional implication of tissue specific posttranslational modifications of collagen molecules. Eur. J. Biochem. 1993;213:1297–1302. doi: 10.1111/j.1432-1033.1993.tb17881.x. [DOI] [PubMed] [Google Scholar]
- 19.Jürgensen H.J., Madsen D.H., Ingvarsen S., Melander M.C., Gårdsvoll H., Patthy L., et al. A novel functional role of collagen glycosylation: interaction with the endocytic collagen receptor uparap/ENDO180. J. Biol. Chem. 2011;286:32736–32748. doi: 10.1074/jbc.M111.266692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Notbohm H., Nokelainen M., Myllyharju J., Fietzek P.P., Müller P.K., Kivirikko K.I. Recombinant human type II collagens with low and high levels of hydroxylysine and its glycosylated forms show marked differences in fibrillogenesis in vitro. J. Biol. Chem. 1999;274:8988–8992. doi: 10.1074/jbc.274.13.8988. [DOI] [PubMed] [Google Scholar]
- 21.Batge B., Winter C., Notbohm H., Acil Y., Brinckmann J., Muller P.K. Glycosylation of human bone collagen I in relation to lysylhydroxylation and fibril diameter. J. Biochem. 1997;122:109–115. doi: 10.1093/oxfordjournals.jbchem.a021717. [DOI] [PubMed] [Google Scholar]
- 22.Torre-Blanco A., Adachi E., Hojima Y., Wootton J.A., Minor R.R., Prockop D.J. Temperature-induced post-translational over-modification of type I procollagen. Effects of over-modification of the protein on the rate of cleavage by procollagen N-proteinase and on self-assembly of collagen into fibrils. J. Biol. Chem. 1992;267:2650–2655. [PubMed] [Google Scholar]
- 23.Sricholpech M., Perdivara I., Yokoyama M., Nagaoka H., Terajima M., Tomer K.B., et al. Lysyl hydroxylase 3-mediated glucosylation in type I collagen: molecular loci and biological significance. J. Biol. Chem. 2012;287:22998–23009. doi: 10.1074/jbc.M112.343954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.H. Ruotsalainen, L. Sipilä, M. Vapola, R. Sormunen, A.M. Salo, L. Uitto, et al., Glycosylation catalyzed by lysyl hydroxylase 3 is essential for basement membranes, J. Cell Sci. 119 (2006) 625–635. doi:10.1242/jcs.02780. [DOI] [PubMed]
- 25.Rautavuoma K., Takaluoma K., Sormunen R., Myllyharju J., Kivirikko K.I., Soininen R. Premature aggregation of type IV collagen and early lethality in lysyl hydroxylase 3 null mice. Proc. Natl. Acad. Sci. U. S. A. 2004;101:14120–14125. doi: 10.1073/pnas.0404966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C., Risteli M., Heikkinen J., Hussa A.-K., Uitto L., Myllylä R. Identification of amino acids important for the catalytic activity of the collagen glucosyltransferase associated with the multifunctional lysyl hydroxylase 3 (LH3) J. Biol. Chem. 2002;277:18568–18573. doi: 10.1074/jbc.M201389200. [DOI] [PubMed] [Google Scholar]
- 27.Moro L., Romanello M., Favia A., Lamanna M.P., Lozupone E. Posttranslational modifications of bone collagen type I are related to the function of rat femoral regions. Calcif. Tissue Int. 2000;66:151–156. doi: 10.1007/s002230010030. [DOI] [PubMed] [Google Scholar]
- 28.Kaku M., Yamauchi M. Mechano-regulation of collagen biosynthesis in periodontal ligament. J. Prosthodont. Res. 2014;58:193–207. doi: 10.1016/j.jpor.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudson D.M., Garibov M., Dixon D.R., Popowics T., Eyre D.R. Distinct post-translational features of type I collagen are conserved in mouse and human periodontal ligament. J. Periodont. Res. 2017;52:1042–1049. doi: 10.1111/jre.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamauchi M., Shiiba M. Post-Translational Modifi Cations of Proteins. Humana Press; Totowa, NJ: 2008. Lysine hydroxylation and cross-linking of collagen; pp. 95–108. doi:10.1007/978-1-60327-084-7_7. [Google Scholar]
- 31.Eyre D.R., Weis M., Hudson D.M., Wu J.-J., Kim L. A novel 3-hydroxyproline (3Hyp)-rich motif marks the triple-helical C terminus of tendon type I collagen. J. Biol. Chem. 2011;286:7732–7736. doi: 10.1074/jbc.C110.195768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudson D.M., Werther R., Weis M., Wu J.-J., Eyre D.R., Ruggiero F. Evolutionary origins of C-terminal (GPP)n 3-hydroxyproline formation in vertebrate tendon collagen. PloS One. 2014;9:e93467. doi: 10.1371/journal.pone.0093467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hudson D.M., Joeng K.S., Werther R., Rajagopal A., Weis M., Lee B.H., et al. Post-translationally abnormal collagens of prolyl 3-hydroxylase-2 null mice offer a pathobiological mechanism for the high myopia linked to human LEPREL1 mutations. J. Biol. Chem. 2015;290:8613–8622. doi: 10.1074/jbc.M114.634915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taga Y., Kusubata M., Ogawa-Goto K., Hattori S. Developmental stage-dependent regulation of prolyl 3-hydroxylation in tendon type I collagen. J. Biol. Chem. 2016;291:837–847. doi: 10.1074/jbc.M115.686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eyre D.R., Wu J.-J. Collagen, Springer Berlin Heidelberg; Berlin, Heidelberg: 2005. Collagen Cross-Links; pp. 207–229. doi:10.1007/b103828. [Google Scholar]
- 36.Eyre D.R., Weis M.A., Wu J.-J. Advances in collagen cross-link analysis. Methods. 2008;45:65–74. doi: 10.1016/j.ymeth.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanson D.A., Eyre D.R. Molecular site specificity of pyridinoline and pyrrole cross-links in type I collagen of human bone. J. Biol. Chem. 1996;271:26508–26516. doi: 10.1074/jbc.271.43.26508. [DOI] [PubMed] [Google Scholar]
- 38.Eyre D.R., Weis M., Rai J. Analyses of lysine aldehyde cross-linking in collagen reveal that the mature cross-link histidinohydroxylysinonorleucine is an artifact. J. Biol. Chem. 2019;294:6578–6590. doi: 10.1074/jbc.RA118.007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robins S.P., Shimokomaki M., Bailey A.J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem. J. 1973;131:771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parry D.A., Barnes G.R., Craig A.S. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc. R. Soc. Lond. B. Biol. Sci. 1978;203:305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- 41.Summers A.P., Koob T.J. The evolution of tendon–morphology and material properties. Comp. Biochem. Physiol. a. Mol. Integr. Physiol. 2002;133:1159–1170. doi: 10.1016/s1095-6433(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 42.Eyre D.R., Weis M.A. Bone collagen: new clues to its mineralization mechanism from recessive osteogenesis imperfecta. Calcif. Tissue Int. 2013;93:338–347. doi: 10.1007/s00223-013-9723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabral W.A., Chang W., Barnes A.M., Weis M., Scott M.A., Leikin S., et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat. Genet. 2007;39:359–365. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandes R.J., Farnand A.W., Traeger G.R., Weis M.A., Eyre D.R. A role for prolyl 3-hydroxylase 2 in post-translational modification of fibril-forming collagens. J. Biol. Chem. 2011;286:30662–30669. doi: 10.1074/jbc.M111.267906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heard M.E., Besio R., Weis M., Rai J., Hudson D.M., Dimori M., et al. Sc65-null mice provide evidence for a novel endoplasmic reticulum complex regulating collagen lysyl hydroxylation. PLoS Genetics. 2016;12:e1006002. doi: 10.1371/journal.pgen.1006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eyre D. Collagen cross-linking amino acids. Methods Enzymol. 1987;144:115–139. doi: 10.1016/0076-6879(87)44176-1. [DOI] [PubMed] [Google Scholar]
- 47.D.M. Hudson, M. Weis, D.R. Eyre, Collagen cross-linking and bone pathobiology, in: Principles of Bone Biology, Academic Press, 2020: pp. 339–358. doi:10.1016/B978-0-12-814841-9.00014-2.
- 48.Eyre D.R., Koob T.J., Van Ness K.P. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal. Biochem. 1984;137:380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 49.Eyre D.R., Paz M.A., Gallop P.M. Cross-linking in collagen and elastin. Annu. Rev. Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 50.Szczesny S.E., Elliott D.M. Interfibrillar shear stress is the loading mechanism of collagen fibrils in tendon. Acta Biomater. 2014;10:2582–2590. doi: 10.1016/j.actbio.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Screen H.R.C. Investigating load relaxation mechanics in tendon. J. Mech. Behav. Biomed. Mater. 2008;1:51–58. doi: 10.1016/j.jmbbm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Naffa R., Holmes G., Ahn M., Harding D., Norris G. Liquid chromatography-electrospray ionization mass spectrometry for the simultaneous quantitation of collagen and elastin crosslinks. J. Chromatogr. A. 2016;1478:60–67. doi: 10.1016/j.chroma.2016.11.060. [DOI] [PubMed] [Google Scholar]
- 53.Fernandes R.J., Schmid T.M., Eyre D.R. Assembly of collagen types II, IX and XI into nascent hetero-fibrils by a rat chondrocyte cell line. Eur. J. Biochem. 2003;270:3243–3250. doi: 10.1046/j.1432-1033.2003.03711.x. [DOI] [PubMed] [Google Scholar]
- 54.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]