Graphical abstract
Keywords: Infant meat puree, Ultrasound treatment, Texture, In vitro digestion
Highlights
-
•
Ultrasound treatment was applied to improve the quality of infant meat puree.
-
•
Ultrasound treatment modified the viscosity and hardness of infant meat puree.
-
•
Moderate ultrasound treatment increased protein digestibility of infant meat puree.
-
•
Infant pork puree was better under ultrasound treatment at 600 W for 15 min.
Abstract
Infant meat puree has an indispensable effect on the oral development and nutritional intake of infants. However, commercially available products have poor texture and relatively low digestibility. In this study, ultrasound (20 kHz and 200 W, 400 W, or 600 W) was applied to the pretreatment of raw meat for preparing infant meat puree for 15 min, 30 min, and 45 min. To assess the impact of ultrasound on infant meat puree, the viscosity, texture, water distribution, particle size and in vitro digestibility were determined. The results showed that, compared with control, viscosity and hardness of meat puree decreased and the texture was better in 400 W and 600 W groups. The content of immobilized water increased in comparison with the control. Ultrasound had no obvious effect on the digestibility of meat puree in gastric phase, but it increased the digestibility in intestinal phase with the highest digestibility (80.85%±3.33) in 600 W, 15 min group. Overall, the ultrasound parameters of 600 W for 15 min can be selected as the best condition to process infant meat puree. The findings provide a new perspective for the improvement of infant meat puree.
1. Introduction
Supplementary feeding is an important stage in the growth of infants and young children. Globally, malnutrition occurs at a high rate in children below five years old due to nutrient deficiencies. Meat is a good choice as raw material of supplementary foods for infants and young children because it contains high-quality protein, various minerals and vitamins [15], [26]. However, the coagulation and structural changes of myofibrillar proteins occur when meat is heated, which causes hard texture unfavorable for infants to eat [14], [28]. Consequently, it is of necessity to supply infants with meat products that are softer than commercial available ones.
Infant meat puree is a kind of supplementary food, which can not only assure supply of nutrition, but also assist infants in achieving the transition from liquid food to solid food. However, meat puree produced with traditional processing often has poor texture and low digestibility, which affects consumers’ desire to buy. Therefore, it is a challenge to ameliorate the texture and digestibility of infant meat puree. Ultrasound is an environmentally friendly and low-cost emerging technology, which has a positive application effect and promotion prospects in the meat processing industry [32]. The main applications of ultrasound to meat processing include pre-treatment, auxiliary processing and substitution of other processing links [7], [35]. One advantage of ultrasound treatment in meat industry is that it can modify the protein structure through the cavitation effect, thereby improving quality, taste and tenderness of the products [2]. Although lots of studies have demonstrated that ultrasound could effectively improve the tenderness of meat [18], [39], [29], some studies showed the opposite results. For example, Lyng et al. [19] reported that the tenderness of beef steak has not been effectively improved under the utilization of three ultrasonic baths of different intensities (0.29, 0.39, 0.62 W·cm−2). These indicated that the impact of ultrasound on the quality of products was not absolute, but mainly depended on suitable ultrasound conditions employed. The applications of ultrasound in increasing the digestibility of meat are mainly focused on protein level, but few data are available on its application to infant meat puree.
In this study, ultrasound was applied to the processing of infant meat puree, and the influence of ultrasound treatment on the quality of meat puree was explored, including texture, rheological properties, water distribution, in vitro digestibility and particle size. The results were expected to provide guidance for application of new techniques to process infant puree.
2. Materials and methods
2.1. Materials
Pork loin was obtained from the local company (Sushi, Huaian, Jiangsu, China). After removing the visible connective tissue and fat, samples were cut into small pieces (9 cm × 6 cm × 3 cm, approximately 110 g) for subsequent use. Pumpkins, potatoes, corn starch, and skimmed milk powder were all purchased from the local market. Pepsin and Pancreatin were ordered from Sigma-Aldrich Company (St. Louis, MO, USA). All other chemicals were analytical grade from local companies.
2.2. Preparation of infant meat puree
Infant meat puree was prepared according to the formulation as follows: 27% pork loin, 10% pumpkin, 5% potato, 50% water, 2% oat, 3.7% skimmed milk powder, and 2.3% corn starch. Pumpkins were peeled, heated in boiling water for 10 min and ground into puree. Potato were peeled, heated in boiling water for 15 min and ground into puree. Oat was dissolved in water (1:25, w/vol). Pork loin pieces were processed in an ultrasonic bath (Tianhua Ultrasonic Electronic Instrument, Jining, China) operating with frequency of 20 kHz and the water temperature was kept at 4 ± 1 °C by intermittently adding ice. A two-factor three-level design was implemented, that is, ultrasound power (200 W, 400 W and 600 W) and time (15 min, 30 min and 45 min). A control without ultrasound treatment was also applied.
After ultrasound treatment, the pork pieces were ground. The ground meat, pumpkin puree, potatoes puree, oats and water were mixed and heated in a magnetic stirring pot at 90 °C for 20 min. A mixture of starch and milk powder was poured into the hot mixing system, and homogenized at 10,000 rpm for 50 s in a homogenizer (PD500-TP, UK) that was repeated for four times. The system was completely mixed and pre-sterilized (100 °C, 30 min). The meat puree was hot-filled into thick glass containers (80 mL) and sealed using metal lids to yield about 71 g of product per container. After sterilization, all meat purees were naturally cooled to room temperature (25 °C).
2.3. Texture profile analysis (TPA)
A texture analyzer (TA-XT2i, Stable Micro Systems Ltd., Surrey, UK) was used for measuring the texture profile of meat puree. The samples were filled in 10 mL beakers to make the surface flat. A probe (P 0.5) was selected, and the operation parameters were tested at a speed of 2 mm/s. The pre-test and post-test speeds were set to 2 mm/s. There was an interval of 3 s between two-cycle compression tests. According to the characteristics of infant meat puree, hardness, adhesiveness and cohesiveness were selected as the measured variables.
2.4. Rheological measurements
Rheological measurements of infant meat puree was tested on an advanced dynamic rheometer (MCR301, Anton Paar Ltd., Austria) fitted with a parallel plate (50 mm diameter, 1 mm gap) and each sample was placed on the parallel plate. Apparent viscosity was tested over shear rate range of 0.01–100 s−1 at 25 ± 1 °C and fitted to a power-law model indicated as below:
≥ 0where τ is the apparent viscosity (Pa·s); K is the consistency index (Pa·sn); γ is the shear rate (s−1); and n is the flow behavior index.
2.5. Particle size
A Mastersizer 3000 laser particle size analyzer (Malvern Instruments, Malvern, UK) was applied to measure particle size of meat puree. Deionized water was served as the dispersion medium, and the refractive index of the dispersed phase was set to 1.33. The laser obscuration level was set from 8% to 20%. The refractive index and absorptivity of the samples were set to 1.54 and 0.001, respectively.
2.6. In vitro protein digestibility
The in vitro protein digestion model used in this study was in view of the digestive conditions of infants. The experimental method was carried out according to Brodkorb et al. [6] and Zou et al. [38] with modification and simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were prepared as previously described by David et al. [9].
Pork puree (2.50 g) was mixed with SGF (4.50 mL) and homogenized at 6,000 rpm for 30 s, and the pH was adjusted to 3.5 ± 0.1 with 1.0 M HCl. The 5 μL of CaCl2·2H2O (0.3 M) and 0.5 mL of pepsin solution (0.16 g, 25 mL SGF) were added to each sample solution, respectively, and then digested for 2 h in a shaker at 37 °C. Gastric digestion was suspended by raising the pH to 7.0 using 1 M NaOH.
To simulate the intestinal phase, SIF (6.4 mL) was added to the digested product of the gastric stage and the pH was adjusted to 7.5 with 1 M NaOH. The sample solution was mixed with 0.5 mL of bile juice, 2.5 μL of CaCl2·2H2O (0.3 M) and 1.0 mL of pancreatin solution (0.144 g: 20 mL SIF), and then digested for 2 h in the shaker at 37 °C. After 2 h of digestion, the mixture was heated in a 95 °C water bath for 5 min to stop the reaction.
All digestive solutions were mixed with 3 volumes of ethanol and maintained at 4 °C for 12 h, and then subjected to centrifugation (10,000 g, 20 min, 4 °C). The supernatant was removed and the precipitate was dried to constant weight at 50 °C. The total protein contents of pork puree and drying residue were determined using the Kjeldahl method. The in vitro protein digestibility was calculated as follows:
Where W0 is the initial protein of meat puree (g), and W1 is the final protein of drying residue (g).
2.7. Low-field NMR measurement
The water distribution and migration of meat puree were measured using a NMR analyzer (Niumag Electric Corporation, Shanghai, China) with operating frequency of 40.0 MHz and magnet temperature of 32 °C. NMR relaxation measurements were performed in accordance with the procedures of Li et al. [17].
Approximately 5.00 g samples were first stuffed into a specific sample tube, and the whole was placed in the center of the radio frequency coil of the magnet box. T2 was measured with the Carr-Purcell-Meiboom-Gill (CPMG) sequence. Typical pulse parameters were set as follows: spectral width, 100 kHz; time domain, 89,990; radio frequency delay time, 0.080 s; waiting time, 2000 ms; number of scans, 4; number of echoes, 3000; and TE, 0.3 ms. Distributed exponential fitting of CPMG decay curves was performed in MultiExp Inv Analysis software.
2.8. Statistical analysis
All data were analyzed using the SAS 9.1.2 program (SAS Institute Inc., Cary, NC, USA). The effects of ultrasound power and time on the texture, water distribution, in vitro digestibility and particle size of infant pork purees were analyzed by multiple analysis of variance (ANOVA). The CT group was a non-ultrasound group and was not included in the ANOVA but set as a reference. Figures were made using the Origin 8.0. For all statistical tests, significant level was set to 0.05 and the data were presented as means ± standard deviations.
3. Results and discussion
3.1. Ultrasound treatment changed the rheological properties of infant meat puree
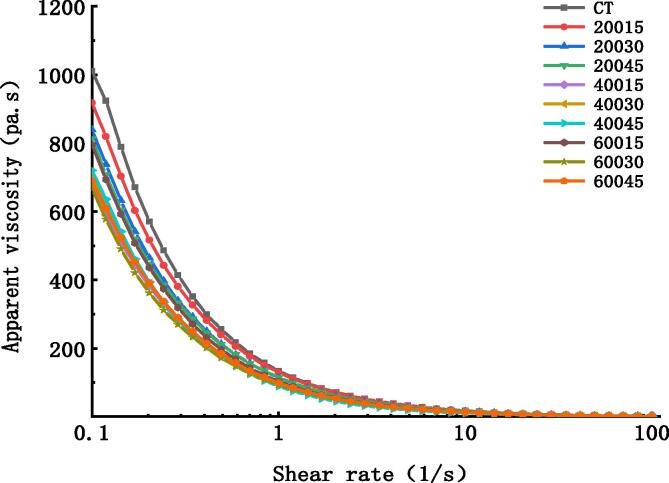
The taste and texture of liquid and semi-solid foods are closely related to rheology. Infant meat puree is a semi-solid food, which is easy for infants and young children to swallow because of its viscous state. Stokes, Boehm and Baier [27] propose that the textural features sensed at early stages of oral processing are those mostly dominated by rheology, whereas those sensed at a later stage of oral processing are related to oral tribology. Viscosity reflects the fluid resistance to flow and relies on the interactions between components [31]. Fig. 1 showed apparent viscosity of the samples at different ultrasonic powers and times. It can be seen that apparent viscosity of infant meat puree decreased with the increase in shearing rate, which indicates that the infant meat puree was non-Newtonian fluid and had shear thinning characteristics. This property was not changed under ultrasound treatment. The viscosity value of the samples decreased after ultrasonic treatment. The fitting equation ( = K ) was the best-fit model for the flow curves on the basis of estimated errors (Table 1). Compared with CT group, the ultrasound groups had lower consistency coefficient and higher flow index (P < 0.05), which demonstrated that the ultrasound-treated samples exhibited less resistance to flow, resulting in lower viscosity. The reduction in viscosity under ultrasound treatment was mainly relevant to the physical forces triggered by cavitation. There is also a certain correlation between particle size and viscosity of the sample. The sample with a larger particle size is easy to form an interface film, resulting in high viscosity [1]. The reduction in viscosity of the samples could be partly ascribed to the smaller particle size [5].
Fig. 1.
Effects of ultrasound power and time on rheological characteristic of pork puree.
Table 1.
Flow parameters of meat puree.
| K | n | R2 | |
|---|---|---|---|
| CT | 136.45 | 0.079 | 0.944 |
| 20,015 | 123.03 | 0.078 | 0.969 |
| 20,030 | 114.31 | 0.100 | 0.973 |
| 20,045 | 114.71 | 0.120 | 0.988 |
| 40,015 | 96.70 | 0.122 | 0.990 |
| 40,030 | 97.79 | 0.118 | 0.991 |
| 40,045 | 89.38 | 0.107 | 0.980 |
| 60,015 | 105.53 | 0.108 | 0.986 |
| 60,030 | 93.52 | 0.129 | 0.992 |
| 60,045 | 98.57 | 0.114 | 0.989 |
CT, non-ultrasound; 20015, 200 W for 15 min; 20030, 200 W for 30 min; 20045, 200 W for 45 min; 40015, 400 W for 15 min; 40030, 400 W, 30 min; 40045, 400 W for 45 min; 60015, 600 W for 15 min; 60030, 600 W for 30 min; and 60045, 600 W for 45 min.
Proper texture can extend the fillable time of the weaker flavor of food in the mouth. If infants eat foods that are too viscous, they may be at risk of suffocation. However, if the food is too thin, the taste disappears faster, and there is a risk of respiratory inhalation [24]. Therefore, it is very important to control the consistency of infant meat puree.
3.2. Ultrasound treatment changed the textural properties of infant meat puree
Texture is a crucial index for comprehensive sensory evaluation of meat product [25]. The food palatability depends mainly on infants’ acceptance of food texture and development of infants’ masticatory apparatus [8]. It can assist infants in achieving the transition from liquid food to solid food if the comfortable texture of meat puree was provided. As can be seen from Table 2, compared with the CT group, hardness and adhesiveness were lower for the ultrasound groups. All the ultrasound treatments for 30 min decreased hardness and adhesiveness as the ultrasound power increased. The ultrasound-treated groups for 45 min exhibited similar change. Under the ultrasound power of 200 W and 400 W, ultrasound time did not show significant effect on the hardness, adhesiveness and cohesiveness of samples (P > 0.05). When the ultrasound power was 600 W, hardness, adhesiveness and cohesiveness of the samples decreased with the increase of the ultrasound time.
Table 2.
Effect of ultrasound power and time on textural properties of pork puree.
| CT | Time min |
Power, W | |||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| Hardness (g) | 38.67 ± 2.47 | 15 | 36.82 ± 3.72x | 31.92 ± 3.51y | 33.61 ± 2.88xy |
| 30 | 35.88 ± 2.92x | 33.43 ± 3.12x | 33.05 ± 4.71x | ||
| 45 | 36.53 ± 5.62x | 34.22 ± 5.73xy | 30.15 ± 3.94y | ||
| Adhesiveness (g.sec) | 141.07 ± 6.63 | 15 | 135.89 ± 12.30x | 117.55 ± 11.52y | 127.06 ± 14.12xy |
| 30 | 135.83 ± 16.34x | 130.63 ± 12.89x | 125.84 ± 25.21x | ||
| 45 | 132.29 ± 7.29x | 118.63 ± 15.29xy | 112.37 ± 16.38y | ||
| Cohesiveness | 0.83 ± 0.09 | 15 | 0.86 ± 0.03x | 0.86 ± 0.03x | 0.87 ± 0.02x |
| 30 | 0.87 ± 0.01x | 0.87 ± 0.01x | 0.86 ± 0.02x | ||
| 45 | 0.85 ± 0.04x | 0.83 ± 0.02x | 0.85 ± 0.04x | ||
Notes: “x-z”, different letters in each row indicate significant difference between different ultrasound power under the same ultrasound time (P < 0.05).
There are many factors that affect the texture of infant meat puree, including the composition of raw materials, external processing, and interactions among various substances. Complete structure of muscle fibers is beneficial to maintain stability of meat products. One of the reasons for the decrease in hardness of meat is that the cavitation effect of ultrasound causes cavities between the muscle fibers, the pores increase, and the muscle structure get loose [3]. Another reason for the decrease is that the cell structure is destroyed by ultrasound [2]. In this study, the changes of adhesiveness of meat puree measured using TPA was similar to apparent viscosity of rheological measurement, indicating that ultrasound treatment of raw meat can improve the consistency of infant meat puree.
3.3. Ultrasound treatment changed the water holding capacity of infant meat puree
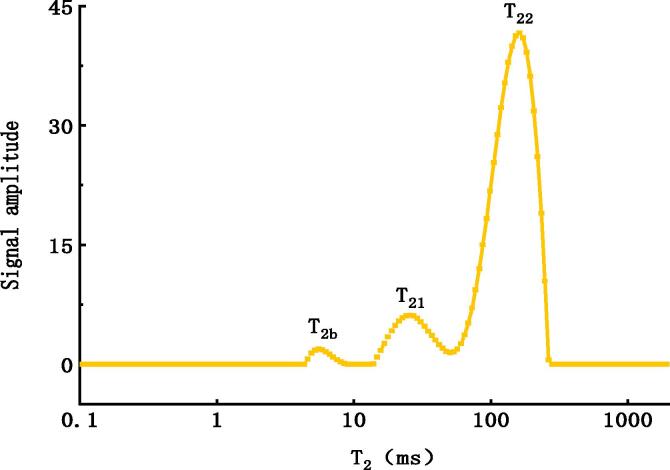
Low-field NMR generally uses nuclear spin characteristics to detect foods with high water content, which can provide information regarding the distribution and mobility of water molecules [21]. The analysis of magnetization decay curves of meat puree was inverted to a spectrum (Fig. 2), and the abscissa was the relaxation time (T2), which indicated the liquidity of water. Three peaks were identified in the infant pork puree according to the multi-exponential fitting of T2 distribution. The peak with the shortest relaxation time, T2b (0–10 ms) represents bound water. The intermediate peak of T21 (10–60 ms) is known as immobilized water, and T22 (60–300 ms) represents free water, which accounted for 85% signal amplitude. This showed that the main state of water in infant pork puree was free water, which mainly because infant pork puree is the semi-solid food, water is the main ingredient.
Fig. 2.
Typical transverse relaxation time (T2) spectrum of infant pork puree.
The peak ratios of samples were shown in Table 3. P2b, P21, and P22 are the peak ratio of bound water, immobilized water, and free water, respectively. Compared with the CT group, the ultrasound treatment groups had higher P21 and lower P22, which indicates that ultrasound treatment can increase the content of immobilized water of samples. When the ultrasound time was 15 min, P21 of 600 W group was higher than 200 W and 400 W groups, while P22 in 600 W was lower than 200 W and 400 W (P < 0.05). This change was consistent with the ultrasound groups of 30 min.
Table 3.
Effect of ultrasound power and time on water distribution of infant pork puree.
| CT | Time min |
Power, W | |||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| P2b/% | 1.50 ± 0.19 | 15 | 1.19 ± 0.16ay | 1.55 ± 0.55axy | 2.05 ± 0.61ax |
| 30 | 1.20 ± 0.10ay | 1.69 ± 0.12axy | 1.99 ± 0.79ax | ||
| 45 | 1.52 ± 0.17ax | 1.57 ± 0.38ax | 1.34 ± 0.36ax | ||
| P21/% | 10.02 ± 0.88 | 15 | 12.04 ± 0.57axy | 10.84 ± 0.03by | 12.79 ± 1.85ax |
| 30 | 10.90 ± 0.24ay | 12.74 ± 1.68ax | 12.84 ± 0.77ax | ||
| 45 | 12.15 ± 0.15ax | 10.82 ± 0.35bx | 10.63 ± 0.64bx | ||
| P22/% | 88.47 ± 1.20 | 15 | 86.69 ± 0.66abx | 87.57 ± 0.52ax | 84.35 ± 0.80by |
| 30 | 87.68 ± 0.14ax | 85.56 ± 1.71by | 85.17 ± 1.36by | ||
| 45 | 85.92 ± 0.02by | 87.61 ± 0.74ax | 88.01 ± 0.48ax | ||
Notes: “a-c”, different letters in each column indicate significant difference between different ultrasound time under the same ultrasound power (P < 0.05); “x-z”, different letters in each row indicate significant difference between different ultrasound power under the same ultrasound time (P < 0.05).
During the heating process of meat puree, proteins may form complex network structure because of gelation. Ultrasound treatment can destroy the structure of myofibrillar proteins, which helps form stronger network structure and accommodate more immobilized water, thereby further improving the water retention of meat puree. Zhang et al. [36] reported that the increase in immobilized water after ultrasound treatment was consistent with the increase in water retention. Free water is easily lost and used by microorganisms during storage. Ultrasound treatment is beneficial to improve water retention of the product.
3.4. Ultrasound treatment changed the in vitro protein digestibility of infant meat puree
The intake and digestion of protein are crucial for infants. Incomplete digestion of protein for infants tends to cause adverse consequences, such as diarrhea, intolerance, allergy, malabsorption, and constipation [11], [16]. For adults, meat is easy to digest, while for infants, meat is not easily digested because of the higher pH of gastric juice and the lower pepsin concentration compared to adults [15], [23].
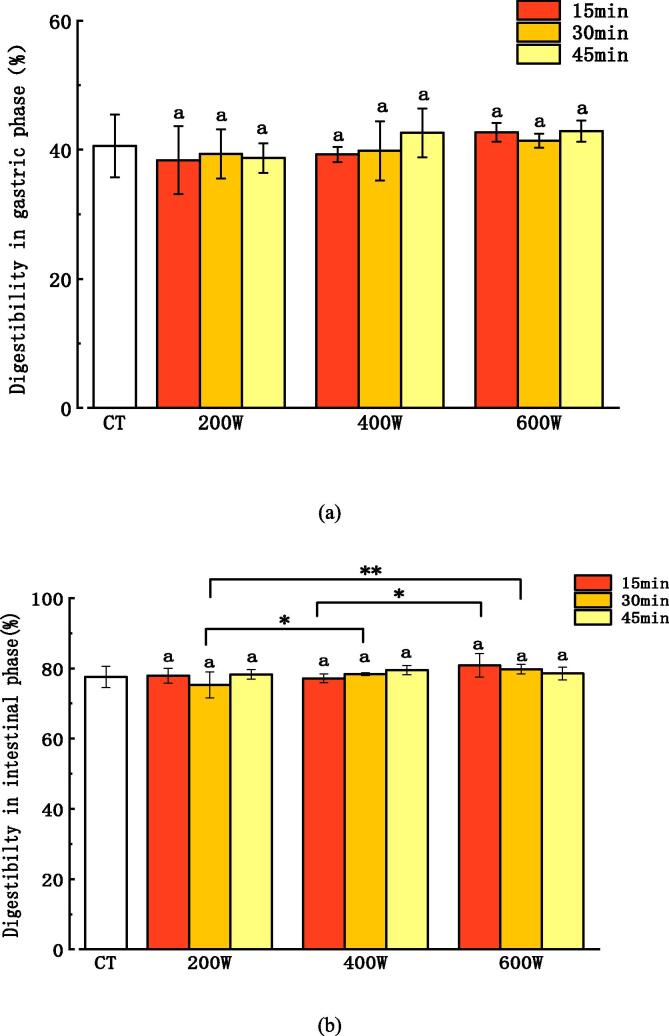
Some processing methods usually were used to make meat easier to digest by infants. Lee et al. [16] improved the protein digestibility of beef by aging or freezing to promote the degradation of myofibrillar proteins. As shown in Fig. 3, ultrasound power and time had no significant effect on the digestibility of meat puree in the gastric phase (P > 0.05). In the intestinal phase (Fig. 3), the digestibility of the 600 W ultrasound power groups were higher than that of the CT group, 200 W groups and 400 W groups. When ultrasound power was 400 W, protein digestibility of the samples increased with the increase of the ultrasound time. While the ultrasound power was 600 W, digestibility of the samples decreased with increasing ultrasound time. It indicates that moderate ultrasound treatment can increase the digestibility of protein, but excessive ultrasound treatment can bring adverse effect to the digestibility of the products.
Fig. 3.
Effect of ultrasound power and time on one-step and two-step digestion of pork puree. Notes: “*, **”, indicate significant differences between the same ultrasound time and different power groups (*P < 0.05; **P < 0.01).
Among all treatment groups, the group (600 W, 15 min) had the highest digestibility (80.85%±3.33%, P < 0.05). Several studies have shown that the rate and degree of protein digestion depended on the local flexibility of the substrate molecule, which determined the quantity of exposed and available cleavage sites for hydrolysis, and how easily digestive enzymes can combine with cleavage sites on the protein [13], [20]. As we all know, structure of proteins are tightly folded and cannot fully bind to enzymes. Ultrasound can induce the changes of protein conformation, resulting in the exposure of enzyme cleavage sites, which is conducive to protein hydrolysis, thereby improving the protein digestibility [30]. Meanwhile, the effect of ultrasound causes disruption of myofibrils or gaps between myofibrils and rupture of collagen fibrils, which also enhances the accessibility of digestive enzymes. Ultrasound treatment induces cavitation effect in which micro bubbles are distributed throughout the aqueous protein system, and violently collapsed when the bubbles reach the maximum critical interfacial tension [33], [34]. Dong et al. [10] showed that shrimp samples were processed with ultrasonic equipment (20 kHz, 400 W). The result shown that in vitro digestibility of shrimp proteins had a significant improvement after 20 min ultrasound processing, which increased from 76.42% to 83.95%. The main reason was perhaps that ultrasound-induced conformational changes of proteins and aggregates led to the unfolding of active sites and increase of digestibility. However, an opposite result was revealed by Martínez-Velasco et al. [22]. The digestibility of faba bean protein was decreased by 3.6% after ultrasound treatment. Variability of results may be due to the different protein structures of different substances.
3.5. Ultrasound treatment changed the particle size of infant meat puree
The changes in particle size of infant pork puree under ultrasound treatment were displayed in the Table 4, which showed that in comparison with CT group, the ultrasound groups had smaller particle size. When the ultrasound time was constant, the particle size decreased as the ultrasound power increased. When the ultrasound power was 600 W, the Dx(50) of the ultrasound treatment group (15 min) was significantly lower than that of the 30 min and 45 min groups.
Table 4.
Effect of ultrasound power and time on particle size of infant pork puree.
| CT | Time min |
Power, W | |||
|---|---|---|---|---|---|
| 200 | 400 | 600 | |||
| DX(10) (μm) | 14.58 ± 0.84 | 15 | 14.17 ± 0.36abx | 14.46 ± 0.29ax | 13.63 ± 0.30ay |
| 30 | 14.48 ± 0.30ax | 13.68 ± 0.32by | 13.64 ± 0.55ay | ||
| 45 | 13.76 ± 0.43by | 14.35 ± 0.40ax | 13.75 ± 0.42ay | ||
| DX(50) (μm) | 51.85 ± 2.64 | 15 | 49.22 ± 1.78by | 50.88 ± 1.46ax | 44.14 ± 1.35bz |
| 30 | 51.35 ± 1.92ax | 46.62 ± 1.44by | 46.45 ± 2.84ay | ||
| 45 | 46.79 ± 1.72cy | 50.04 ± 1.81ax | 46.90 ± 2.39ay | ||
| DX(90) (μm) | 183.10 ± 6.00 | 15 | 173.90 ± 12.62ax | 176.84 ± 7.92ax | 139.55 ± 8.93by |
| 30 | 170.30 ± 14.00ax | 157.12 ± 6.59by | 151.11 ± 12.27ay | ||
| 45 | 156.69 ± 10.56bx | 153.43 ± 8.77bx | 146.15 ± 10.76aby | ||
Notes: “a-c”, different letters in each column indicate significant difference between different ultrasound time under the same ultrasound power (P < 0.05); “x-z”, different letters in each row indicate significant difference between different ultrasound power under the same ultrasound time (P < 0.05).
During 15 min ultrasound treatment, the Dx(10), DX(50) and Dx(90) of 600 W treatment groups were significantly lower than those of the 200 W and 400 W groups (P < 0.05). The similar changes were seen in the 30 min ultrasound-treated groups. The decrease of particle size may be due to the fact that the ultrasonic turbulence inhibited protein aggregation, and the non-covalent bonds between proteins were damaged by the micro-streaming and turbulent forces [12]. Zhao et al. [37] discovered smaller particles in goat milk processed with ultrasound, compared with the non-ultrasound group. A positive effect in digestion occurred because of the reduction of particle size. Amiri et al. [4] reported ultrasound caused an obvious decrease in the particle size of myofibrillar proteins, because of the cavitation forces and turbulence forces produced by ultrasound.
4. Conclusion
In this study, ultrasound treatment of raw meat can improve the quality of infant meat puree. Through ultrasound treatment, the hardness and viscosity of infant meat puree decreased, the content of immobilized water increased, and the protein digestibility improved. The optimal parameter (600 W, 15 min) for ultrasound pretreatment of raw meat in the processing of infant meat puree was obtained. The results showed that ultrasound is suitable for soft food processing. This technology can not only be used in foods for infants, but also in food processing for the elderly or people with dysphagia in the future.
CRediT authorship contribution statement
Mingyang Luo: Data curation, Formal analysis, Writing – original draft. Kai Shan: Data curation, Formal analysis. Miao Zhang: Data curation, Formal analysis. Weixin Ke: Data curation, Formal analysis. Di Zhao: Data curation, Formal analysis. Yingqun Nian: Data curation, Formal analysis. Juqing Wu: Data curation, Formal analysis. Chunbao Li: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by Ministry of Science and Technology (10000 Talent Project), and China Agriculture Research System of MOF and MARA (CARS 35).
References
- 1.Akbulut M., Saricoban C., Ozcan M.M. Determination of rheological behavior, emulsion stability, color, and sensory of sesame pastes (Tahin) blended with pine honey. Food Bioprocess Technol. 2012;5(5):1832–1839. doi: 10.1007/s11947-011-0668-6. [DOI] [Google Scholar]
- 2.Alarcon-Rojo A.D., Janacua H., Rodriguez J.C., Paniwnyk L., Mason T.L. Power ultrasound in meat processing. Meat Sci. 2015;107:86–93. doi: 10.1016/j.meatsci.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Ali S., Zhang W.G., Rajput N., Khan M.A., Li C.B., Zhou G.H. Effect of multiple freeze-thaw cycles on the quality of chicken breast meat. Food Chem. 2015;173:808–814. doi: 10.1016/j.foodchem.2014.09.095. [DOI] [PubMed] [Google Scholar]
- 4.Amiri A., Sharfian P., Soltanizadeh N. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Int. J. Biol. Macromol. 2018;111:139–147. doi: 10.1016/j.ijbiomac.2017.12.167. [DOI] [PubMed] [Google Scholar]
- 5.Arzeni C., Martínez K., Zema P., Arias A., Pérez O.E., Pilosof A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. Food Eng. 2012;108(3):463–472. doi: 10.1016/j.jfoodeng.2011.08.018. [DOI] [Google Scholar]
- 6.Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S., Bohn T., Bourlieu-Lacanal C., Boutrou R., Carrière F., Clemente A., Corredig M., Dupont D., Dufour C., Edwards C., Golding M., Karakaya S., Kirkhus B., Le Feunteun S., Lesmes U., Macierzanka A., Mackie A.R., Martins C., Marze S., McClements D.J., Ménard O., Minekus M., Portmann R., Santos C.N., Souchon I., Singh R.P., Vegarud G.E., Wickham M.S.J., Weitschies W., Recio I. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019;14(4):991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- 7.Caraveo O., Alarcon-Rojo A.D., Renteria A., Santellano E., Paniwnyk L. Physicochemical and microbiological characteristics of beef treated with high-intensity ultrasound and stored at 4 °C. J. Sci. Food Agric. 2015;95(12):2487–2493. doi: 10.1002/jsfa.2015.95.issue-1210.1002/jsfa.6979. [DOI] [PubMed] [Google Scholar]
- 8.Chen J. Food oral processing-A review. Food Hydrocolloids. 2009;23(1):1–25. doi: 10.1016/j.foodhyd.2007.11.013. [DOI] [Google Scholar]
- 9.David S., Wojciechowska A., Portmann R., Shpigelman A., Lesmes U. The impact of food-grade carrageenans and consumer age on the in vitro proteolysis of whey proteins. Food Res. Int. 2020;130:108964. doi: 10.1016/j.foodres.2019.108964. [DOI] [PubMed] [Google Scholar]
- 10.Dong X., Wang J., Raghavan V. Effects of high-intensity ultrasound processing on the physiochemical and allergenic properties of shrimp. Innovative Food Sci. Emerg. Technol. 2020;65:102441. doi: 10.1016/j.ifset.2020.102441. [DOI] [Google Scholar]
- 11.Gan J., Bornhorst G.M., Henrick B.M., German J.B. Protein digestion of baby foods: Study approaches and implications for infant health. Mol. Nutr. Food Res. 2018;62(1):1–11. doi: 10.1002/mnfr.201700231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S., Zhao D.i., Nian Y., Wu J., Zhang M., Li Q., Li C. Ultrasonic treatment increased functional properties and in vitro digestion of actomyosin complex during meat storage. Food Chem. 2021;352:129398. doi: 10.1016/j.foodchem.2021.129398. [DOI] [PubMed] [Google Scholar]
- 13.Joye, I. (2019). Protein digestibility of cereal products. Foods, 8(6), 199. http://doi.org/10.3390/foods8060199. [DOI] [PMC free article] [PubMed]
- 14.Karastogiannidou C., Ryley J. The formation of water-soluble antioxidants in chicken held at 80 °C. Food Chem. 1994;51(2):215–220. doi: 10.1016/0308-8146(94)90260-7. [DOI] [Google Scholar]
- 15.Lee S., Jo K., Hur S.J., Choi Y.S., Kim H.J., Jung S. Low protein digestibility of beef puree in infant in vitro digestion model. Food Sci. Animal Resour. 2019;39(6):1000–1007. doi: 10.5851/kosfa.2019.e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S., Jo K., Lee H.J., Jo C., Yong H.I., Choi Y.-S., Jung S. Increased protein digestibility of beef with aging in an infant in vitro digestion model. Meat Sci. 2020;169:108210. doi: 10.1016/j.meatsci.2020.108210. [DOI] [PubMed] [Google Scholar]
- 17.Li C., Liu D., Zhou G., Xu X., Qi J., Shi P., Xia T. Meat quality and cooking attributes of thawed pork with different low field NMR T21. Meat Sci. 2012;92(2):79–83. doi: 10.1016/j.meatsci.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Li X., Wang Y., Sun Y.Y., Pan D.D., Cao J.X. The effect of ultrasound treatments on the tenderizing pathway of goose meat during conditioning. Poult. Sci. 2018;97(8):2957–2965. doi: 10.3382/ps/pey143. [DOI] [PubMed] [Google Scholar]
- 19.Lyng J.G., Allen P., Mckenna B.M. The influence of high intensity ultrasound bath on aspects of beef tenderness. J. Muscle Foods. 1997;8(3):237–249. doi: 10.1111/j.1745-4573.1997.tb00630.x. [DOI] [Google Scholar]
- 20.Mackie A., Macierzanka A. Colloidal aspects of protein digestion. Curr. Opin. Colloid Interface Sci. 2010;15(1–2):102–108. doi: 10.1016/j.cocis.2009.11.005. [DOI] [Google Scholar]
- 21.Marigheto N., Duarte S., Hills B.P. NMR relaxation study of avocado quality. Appl. Magn. Reson. 2005;29(4):687–701. doi: 10.1007/BF03166344. [DOI] [Google Scholar]
- 22.Martínez-Velasco A., Lobato-Calleros C., Hernández-Rodríguez B.E., Román-Guerrero A., Alvarez-Ramirez J., Vernon-Carter E.J. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: Effect on surface properties, foaming ability and structural changes. Ultrason. Sonochem. 2018;44:97–105. doi: 10.1016/j.ultsonch.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen T., Bhandari B., Julie C., Sangeeta P. A comprehensive review on in vitro digestion of infant formula. Food Res. Int. 2015;76(3):373–386. doi: 10.1016/j.foodres.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Park J., Lee S., Yoo B., Nam K. Effects of texture properties of semi-solid food on the sensory test for pharyngeal swallowing effort in the older adult. BMC Geriatrics. 2020;20(1):493. doi: 10.1186/s12877-020-01890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salcedo-Sandoval L., Cofrades S., Pérez C., Solas M.T., Jiménez-Colmenero F. Healthier oils stabilized in konjac matrix as fat replaces in n-3 PUFA enriched frankfurters. Meat Sci. 2013;93(3):757–766. doi: 10.1016/j.meatsci.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 26.De Smet S., Vossen E. Meat: The balance between nutrition and health. A review. Meat Sci. 2016;120:145–156. doi: 10.1016/j.meatsci.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Stokes J.R., Boehm M.W., Baier S.K. Oral processing, texture and mouthfeel: From rheology to tribology and beyond. Curr. Opin. Colloid Interface Sci. 2013;18(4):349–359. doi: 10.1016/j.cocis.2013.04.010. [DOI] [Google Scholar]
- 28.Tokifuji A., Matsushima Y., Hachisuka K., Yoshioka K. Texture, sensory and swallowing characteristics of high-pressure-heat-treated pork meat gel as a dysphagia diet. Meat Sci. 2013;93(4):843–848. doi: 10.1016/j.meatsci.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 29.Wang A., Kang D., Zhang W., Zhang C., Zou Y., Zhou G. Changes in calpain activity, protein degradation and microstructure of beef M. semitendinosus by the application of ultrasound. Food Chem. 2018;245:724–730. doi: 10.1016/j.foodchem.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Wang B., Meng T.T., Ma H.L., Zhang Y., Li Y., Jin J., Ye X. Mechanism study of dual-frequency ultrasound assisted enzymolysis on rapeseed protein by immobilized Alcalase. Ultrason. Sonochem. 2016;32:307–313. doi: 10.1016/j.ultsonch.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Westphalen A.D., Briggs J.L., Lonergan S.M. Influence of muscle type on rheological properties of porcine myofibrillar protein during heat-induced gelation. Meat Sci. 2006;72(4):697–703. doi: 10.1016/j.meatsci.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Xiong G.-Y., Zhang L.-L., Zhang W., Wu J. Influence of ultrasound and proteolytic enzyme inhibitors on muscle degradation, tenderness, and cooking loss of hens during aging. Czech J. Food Sci. 2012;30(No. 3):195–205. [Google Scholar]
- 33.Xu L.e., Xia Q., Cao J., He J., Zhou C., Guo Y., Pan D. Ultrasonic effects on the headspace volatilome and protein isolate microstructure of duck liver, as well as their potential correlation mechanism. Ultrason. Sonochem. 2021;71:105358. doi: 10.1016/j.ultsonch.2020.105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L.e., Zheng Y., Zhou C., Pan D., Geng F., Cao J., Xia Q. Kinetic response of conformational variation of duck liver globular protein to ultrasonic stimulation and its impact on the binding behavior of n-alkenals. LWT. 2021;150:111890. doi: 10.1016/j.lwt.2021.111890. [DOI] [Google Scholar]
- 35.Zhang J., Zhang Y., Wang Y., Xing L., Zhang W. Influences of ultrasonic-assisted frying on the flavor characteristics of fried meatballs. Innovative Food Sci. Emerg. Technol. 2020;62:102365. doi: 10.1016/j.ifset.2020.102365. [DOI] [Google Scholar]
- 36.Zhang Z.Y., Regenstein J.M., Zhou P., Yang Y.L. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017;34:960–967. doi: 10.1016/j.ultsonch.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Zhao L., Zhang S., Uluko H., Liu L., Lu J., Xue H., Kong F., Lv J. Effect of ultrasound pretreatment on rennet-induced coagulation properties of goat’ s milk. Food Chem. 2014;165(15):167–174. doi: 10.1016/j.foodchem.2014.05.081. [DOI] [PubMed] [Google Scholar]
- 38.Zou X.Y., Zhou G.H., Yu X.B., Bai Y., Wang C., Xu X.L., Dai C., Li C.B. In vitro protein digestion of pork cuts differ with muscle type. Food Res. Int. 2018;106:344–353. doi: 10.1016/j.foodres.2017.12.070. [DOI] [PubMed] [Google Scholar]
- 39.Zou Y., Xu P., Wu H., Zhang M., Sun Z., Sun C., Wang D., Cao J., Xu W. Effects of different ultrasound power on physicochemical property and functional performance of chicken actomyosin. Int. J. Biol. Macromol. 2018;113:640–647. doi: 10.1016/j.ijbiomac.2018.02.039. [DOI] [PubMed] [Google Scholar]