Abstract
This longitudinal cohort study aimed to determine whether circulating neurofilaments (NFs) can monitor response to molecular therapies in newborns with spinal muscular atrophy (SMA; NCT02831296). We applied a mixed-effect model to examine differences in serum NF levels among healthy control infants (n = 13), untreated SMA infants (n = 68), and SMA infants who received the genetic therapies nusinersen and/or onasemnogene abeparvovec (n = 22). Increased NF levels were inversely associated with SMN2 copy number. SMA infants treated with either nusinersen or onasemnogene abeparvovec achieved important motor milestones not observed in the untreated cohort. NF levels declined more rapidly in the nusinersen cohort as compared with the untreated cohort. Unexpectedly, those receiving onasemnogene abeparvovec monotherapy showed a significant rise in NF levels regardless of SMN2 copy number. In contrast, symptomatic SMA infants who received nusinersen, followed by onasemnogene abeparvovec within a short interval after, did not show an elevation in NF levels. While NF cannot be used as the single marker to predict outcomes, the elevated NF levels observed with onasemnogene abeparvovec and its absence in infants treated first with nusinersen may indicate a protective effect of co-therapy during a critical period of vulnerability to acute denervation.
Keywords: Neuromuscular diseases, Neurogenetics, Biomarkers, SMA, Gene Therapy
Graphical abstract
These findings show serum neurofilaments as biomarkers to monitor spinal muscular atrophy disease onset, progression, and treatment response early in life. The absence of elevated neurofilament levels in infants treated first with nusinersen may indicate a protective effect of co-therapy with nusinersen and gene therapy during a critical period.
Introduction
A major research gap in the neuromuscular field is the lack of simple, specific, and precise biological markers (biomarkers) to track disease progression and potential therapeutic response in pediatric neurodegenerative diseases. Spinal muscular atrophy (SMA) is a devastating neuromuscular disease and remains a leading genetic cause of infantile death worldwide.1 With the federal recommendation in the United States and many other countries for SMA newborn screening, and with the emergence of 3 recent U.S. Food and Drug Administration (FDA)-approved treatments for SMA,2, 3, 4, 5 identifying appropriate biomarkers to monitor SMA disease progression early in life and help guide treatment approach is now an urgent need.
Currently, the number of copies of the survival motor neuron 2 (SMN2) gene is the primary SMA disease modifier used to predict disease severity in newborns with SMA. While all patients with SMA have biallelic deletions or mutations in the survival motor neuron 1 (SMN1) gene, they also have at least one or more copies of SMN2, and SMN2 copy number inversely correlates with phenotypic severity at the population level. However, exceptions exist, and predicting disease onset and outcomes in infants with 4 or more copies remains a special challenge. Circulating SMN transcripts and protein concentrations demonstrate significant fluctuations and overlap between patients and healthy controls that have, thus far, precluded their use outside of the clinical trial setting.6,7 In addition, altered peripheral SMN levels are derived primarily from whole blood cells and platelets, and thus are not expected to be impacted by either onasemnogene abeparvovec (AVXS-101; Zolgensma, Novartis) or nusinersen (Spinraza, Biogen).6,8, 9, 10 We have previously demonstrated the usefulness of simple electrophysiologic measures of peripheral denervation such as maximum ulnar compound muscle action potential (CMAP) and motor unit number estimation (MUNE) to track the health and integrity of peripheral motor unit integrity and function.11,12 Both SMN2 copy number and ulnar CMAP and MUNE measurements are practical and useful tools that are associated with disease severity and can be used to monitor denervation status. However, additional biological indicators would be extremely useful in clinical practice to predict the earliest signs of impact on the health of motor neurons, skeletal muscle and other peripheral tissues, and to help monitor the response to available therapies.
Neurofilaments (NFs) are the main structural proteins of neurons and primarily expressed in long axons.13,14 Detection of high concentrations of NFs in the cerebrospinal fluid, serum, or plasma are indicators of neuronal damage and have been proposed as potential prognostic and treatment responsive biomarkers in several neurodegenerative diseases and disorders including SMA.15, 16, 17, 18, 19 NFs are composed of subunits with different molecular weights and functional properties. The largest NF subunit is the neurofilament heavy chain (Nf-H), consisting of 1020 amino acids. Phosphorylation of Nf-H regulates several protein-protein interactions. The smallest subunit is the neurofilament light chain (Nf-L), consisting of 543 amino acids. Both phosphorylated Nf-H (pNf-H) and total Nf-L levels can be precisely detected in the circulation with enzyme-linked immunosorbent assays, electrochemiluminescence immunoassays, or single-molecule arrays (Simoa).13
Since 2016, 3 novel therapies have been approved for clinical use in SMA patients by the U.S. FDA. Nusinersen is an antisense oligonucleotide that targets SMN2 splicing to produce more functional SMN protein. It is delivered to the central nervous system via repeated intrathecal injections, with limited exposure to peripheral tissues aside from elimination via kidneys. Onasemnogene abeparvovec is an adeno-associated virus vector-based gene therapy delivered via peripheral intravenous infusion, designed to deliver an intact SMN1 transgene to motor neurons. Transduction of cells in the central nervous system and peripheral tissues, including liver, spleen, and skeletal and cardiac muscle has been demonstrated in non-human primates, and thus likely impact outcomes in human patients that vary by age and manner of delivery,20,21 Caution and long-term follow-up data are still needed to attest to the safety of gene therapy in SMA patients, especially considering recent reports of gain of toxic function with SMN overexpression in SMA mouse models.22 Risdiplam (Evrysdi, Roche Genentech) is an orally bioavailable small molecule pre-mRNA SMN2 splicing modifier with enhanced specificity for SMN2 exon 7 splicing; however, since it was only recently FDA approved for clinical use, no patients received risdiplam during the course of this study. Circulating NF levels, specifically pNf-H, have been proposed as a pharmacodynamic response biomarker since SMA infants treated with nusinersen in both the ENDEAR (Efficacy and Safety of Nusinersen (ISIS 396443) in Infants With Spinal Muscular Atrophy) and NURTURE (A Study of Multiple Doses of Nusinersen (ISIS 396443) Delivered to Infants With Genetically Diagnosed and Presymptomatic Spinal Muscular Atrophy) studies demonstrated rapid decline in pNf-H levels.17 Changes in circulating NF levels after onasemnogene abeparvovec infusion or with nusinersen and onasemnogene abeparvovec co-therapy have not been previously reported. Based on the current evidence, more comprehensive longitudinal cohort studies are needed to establish circulating NFs as useful biomarkers for SMA.
The Project Cure SMA and SPOT SMA Longitudinal Pediatric Data Repositories (LPDRs) contain robust longitudinal genotype/phenotype data from untreated (natural history) and treated SMA patients (NBSTRN.org). Both LPDRs have linked biorepositories of samples from SMA patients as well as a subset of unaffected carrier and non-carrier siblings obtained during prospective longitudinal follow-up. In order to address key questions related to changes in circulating NFs, we examined data from all SMA and control newborns, infants, or toddlers between the ages of 0 and 3 years with samples suitable for analysis. First, we investigated whether serum pNf-H or Nf-L levels were increased in SMA patients when compared with age-matched control subjects. Second, we examined whether changes in serum pNf-H or Nf-L levels varied in association with age, SMN2 copy number, or ulnar CMAP values in SMA patients. Third, we analyzed all available data for patients who had received nusinersen, onasemnogene abeparvovec, or both (co-therapy) to examine the effects of these molecular or gene therapies on pNf-H or Nf-L levels. These novel findings have many implications for the potential value of circulating NFs for SMA and other early onset neurodegenerative disorders.
Results
Serum pNf-H and Nf-L levels are increased in SMA patients early in life and associated with the number of SMN2 copies
We investigated samples collected from 10 healthy control subjects, 47 SMA patients who had not received disease-modifying therapies (natural history cohort), and 22 treated SMA patients in this longitudinal cohort study. The treatment cohort includes 9 SMA patients receiving nusinersen therapy, 7 SMA patients who received intravenous onasemnogene abeparvovec therapy, and 6 SMA patients treated with both therapies. All available samples for subjects between 0 and 3 years old were analyzed. Longitudinal follow-up varied and ranged from 1 to 9 data points per subject within the specified age range. Table S2 presents the distribution of age, sex, race, ethnicity, SMN2 copies, SMA type, pNf-H levels, Nf-L levels, and ulnar CMAP values for this cohort.
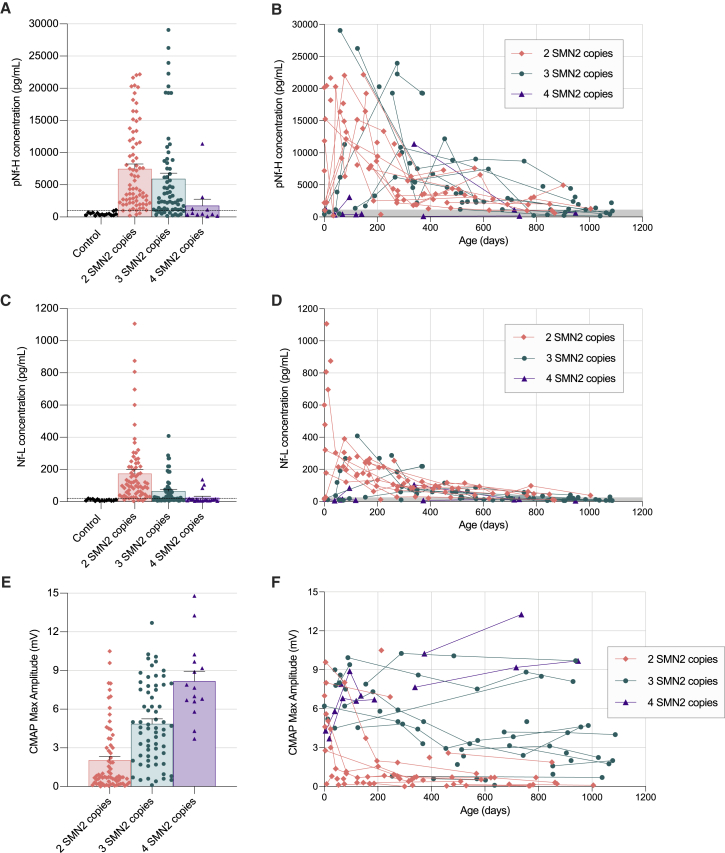
To determine whether serum pNf-H levels are increased in SMA patients when compared with age-matched control subjects, and whether pNf-H levels vary according to the number of SMN2 copies, we compared all time points from SMA subjects not receiving disease-modifying therapies with healthy control subjects. The highest pNf-H value observed in a healthy control subject was ∼1,000 pg/mL, while wide-ranging pNf-H levels were observed in SMA patients up to 30,000 pg/mL (Figures 1A and 1B). Subjects with 2 or 3 SMN2 copies had higher pNf-H levels than healthy controls or SMA patients with 4 SMN2 copies (Figure 1A). Peak pNf-H levels in infants with 2 SMN2 copies were mostly observed from the earliest possible time point post birth to 6 months of age, while infants with 3 SMN2 copies had a more variable time course with peak levels occurring between 3 months and 1 year of age (Figure 1B; Figure S1). We next compared Nf-L levels between SMA patients and healthy control subjects. The highest Nf-L value observed in a healthy control subject was ∼20 pg/mL, while up to 1,100 pg/mL was observed in SMA patients (Figures 1C and 1D). SMA patients with 2 SMN2 copies also had higher Nf-L levels than healthy controls and those SMA patients with 4 SMN2 copies or 3 SMN2 copies (Figure 1C). SMA patients with 3 SMN2 copies also had higher Nf-L levels than healthy controls (Figure 1C), and those SMA patients with 4 SMN2 copies (Figure 1C). As complementary information, we plotted all available maximum ulnar CMAP data for these cohorts. This data clearly demonstrate an association between SMN2 copy number and maximum ulnar CMAP amplitude (Figures 1E and 1F), indicating that patients with lower SMN2 copies are more likely to demonstrate objective evidence of denervation early in life. Altogether, these findings validate previous evidence showing that serum pNf-H and Nf-L levels are increased in SMA patients and clearly demonstrate that these changes are associated with SMN2 copy number and inversely associated with maximum ulnar CMAP amplitude in the first 3 years of life.
Figure 1.
Natural history data comparing serum pNf-H levels, Nf-L levels, and ulnar CMAP values among healthy controls and SMA patients with 2, 3, and 4 SMN2 copies early in life
Sample size includes healthy controls (20 samples from 13 subjects), SMA subjects with 2 SMN2 copies (78 samples from 34 subjects), SMA subjects with 3 SMN2 copies (62 samples from 30 subjects), and SMA subjects with 4 SMN2 copies (11 samples from 4 subjects) not receiving any molecular therapy. pNf-H (A and B) and Nf-L (C and D) data collected in the 3 first years of life. All maximum ulnar CMAP amplitude (E and F) data available for this cohort in the 3 first years of life. Dashed lines or gray area in these plots indicate highest concentrations observed in healthy control subjects.
SMA patients treated with nusinersen and/or onasemnogene abeparvovec therapies achieve important motor milestones in the 3 first years of life
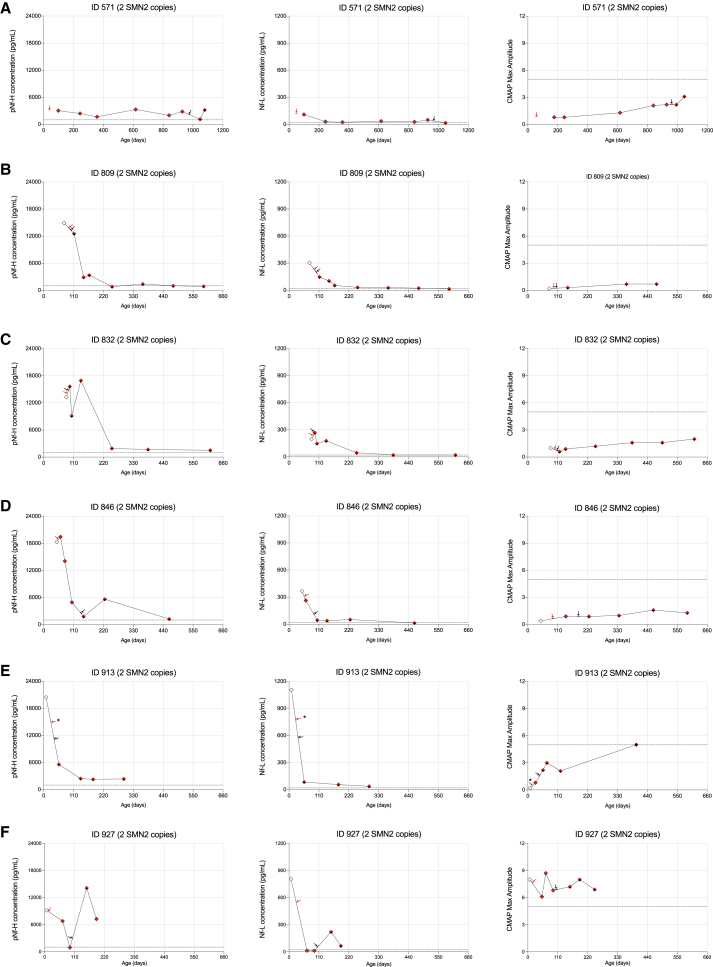
We next investigated cohorts of SMA patients receiving nusinersen, onasemnogene abeparvovec therapy, or co-therapy early in life. Of the 9 cases receiving nusinersen, 7 were symptomatic patients with 2 or 3 SMN2 copies, and 2 were presymptomatic patients with 3 SMN2 copies at the time of initiation of nusinersen therapy (Table S2). Of the 7 cases receiving onasemnogene abeparvovec therapy, all were considered clinically presymptomatic at the time of initial evaluation, including 1 patient with 2 SMN2 copies, 4 patients with 3 SMN2 copies, and 2 patients with 4 SMN2 copies; however, more subtle signs of disease activity, including the presence of tongue fasciculations, modest head lag, and lower than expected CMAP values led to the decision to proceed with treatment in both infants with 4 SMN2 copies (Table 2). The third cohort consists of 6 symptomatic infants with early infantile onset and 2 SMN2 copies who received co-therapy with nusinersen (1 or more doses) and onasemnogene abeparvovec early in life (Table 2).
Data for the age of acquisition of 4 critical gross motor milestones are presented in Table 2. While all SMA patients, regardless of treatment, achieved clinical outcomes beyond those expected in an untreated SMA cohort, the data clearly demonstrate that presymptomatic status and baseline ulnar CMAP at the time of treatment initiation are critically important factors that determine whether patients acquire age-expected gross motor milestones. Of special interest are the 6 infants who received nusinersen prior to or concurrent with treatment with onasemnogene abeparvovec. Excluding subjects 913 and 927, who were identified via newborn screening, the remaining 4 infants presented with typical onset of SMA symptoms and signs prior to 6 weeks of age, and all had ulnar CMAP amplitude ≤1.0 mV (normal ≥5 mV) at the time of treatment initiation. While all have achieved independent sitting and continue to acquire additional motor skills, such as assisted standing during the follow-up period, 3 of 6 children have disease-related complications including persistent lower extremity weakness and contractures, hip dysplasia, and scoliosis.
Changes in serum pNf-H levels, serum Nf-L levels, and ulnar CMAP values over time in individual SMA patients treated with nusinersen and/or onasemnogene abeparvovec
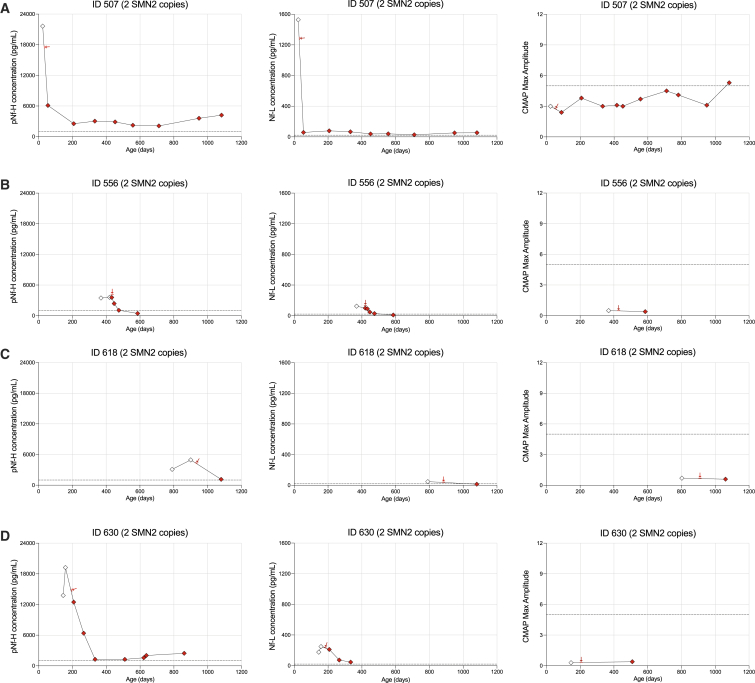
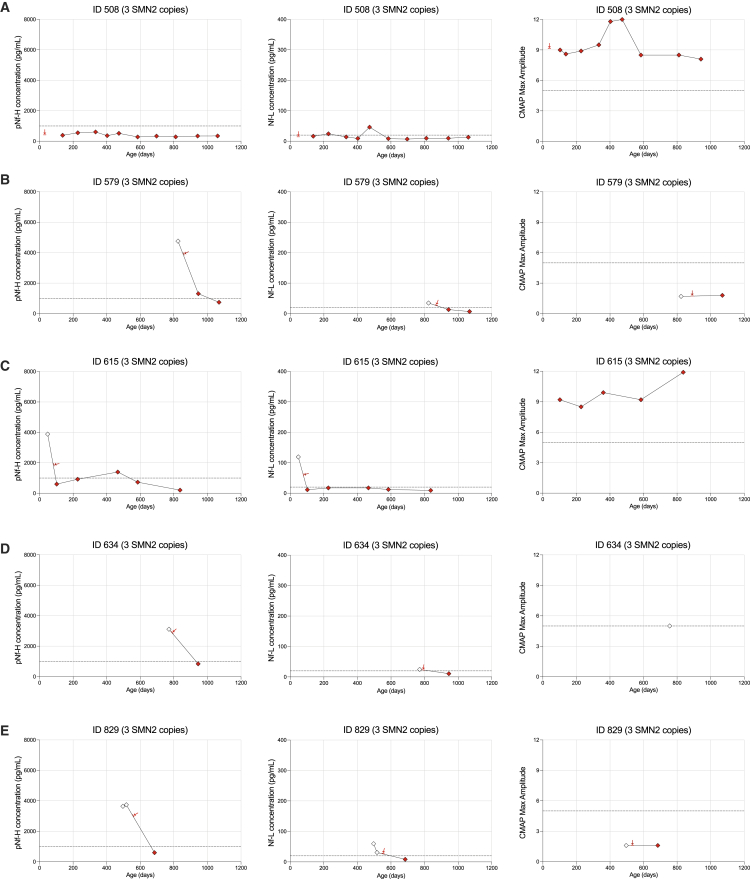
Individual pNf-H and Nf-L data are presented for patients treated with nusinersen and/or onasemnogene abeparvovec in Figures 2 and 3. All 4 SMA patients with 2 SMN2 copies who received nusinersen monotherapy demonstrated a rapid decrease in pNf-H and Nf-L after the first dose (Figures 2A–2D). Importantly, 1 infant identified and dosed early demonstrated a progressive increase in ulnar CMAP amplitude over time (Figure 2A), while 3 patients much more severely denervated at treatment initiation (Table 2) had no significant recovery of CMAP values over time (Figures 2B–2D). Among SMA patients with 3 SMN2 copies, we observed a rapid decrease in pNf-H or Nf-L levels in 4 of 5 subjects with documented elevated baseline levels; a baseline sample for 1 patient was not available (Figures 3B–3E).
Figure 2.
Cohort of 4 SMA patients with 2 SMN2 copies receiving nusinersen early in life
Longitudinal plots for pNf-H concentrations, Nf-L concentrations, and CMAP values for each individual case (A–D) in this cohort. Red arrows indicate the first nusinersen dose.
Figure 3.
Cohort of 5 SMA patients with 3 SMN2 copies receiving nusinersen early in life
Longitudinal plots for pNf-H concentrations, Nf-L concentrations, and CMAP values for each individual case (A–E) in this cohort. Red arrows indicate the first nusinersen dose.
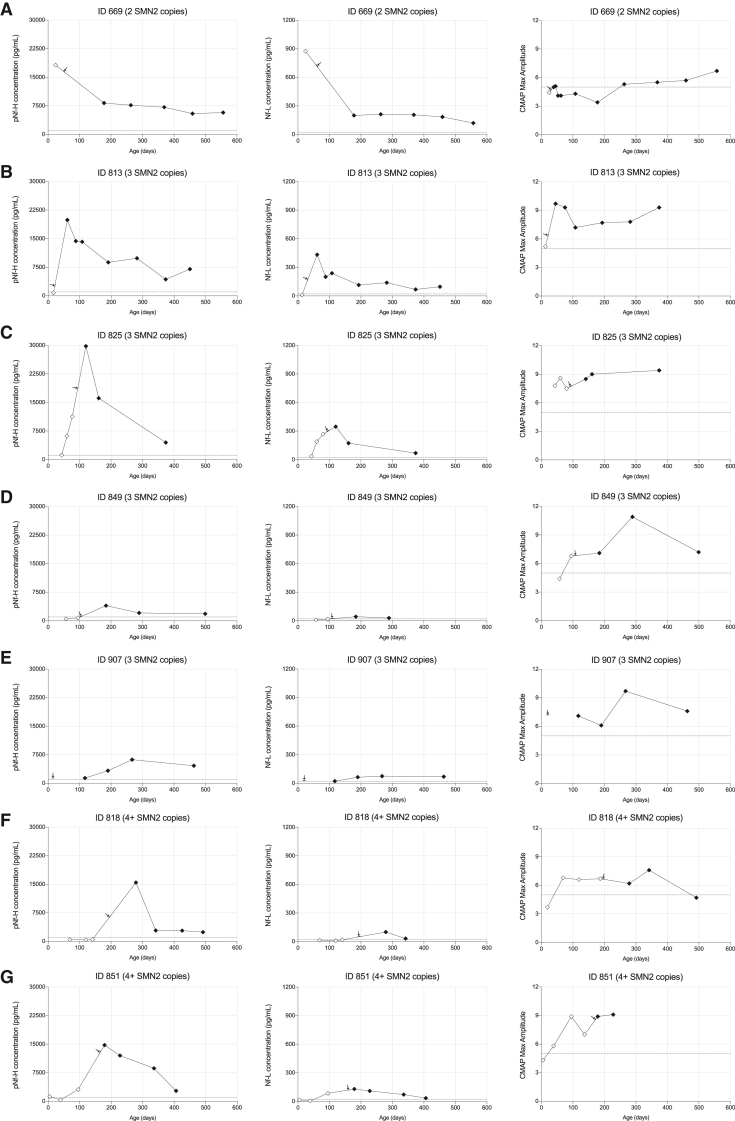
Of 7 patients receiving onasemnogene abeparvovec monotherapy, all were presymptomatic at time of dosing and demonstrated significantly higher baseline CMAP values (Table 2). Only 1 patient demonstrated decreased pNf-H and Nf-L levels after treatment (Figure 4A), while 6 patients demonstrated significantly increased pNf-H and Nf-L levels post intravenous infusion (Figures 4B–4G). While elevated levels subsequently decreased in the weeks following the infusion, they remained above normal in 5 of 6 patients (Figures 4B–4G). Importantly, all patients who received onasemnogene abeparvovec demonstrated a progressive increase in CMAP values indicating clinically relevant reinnervation, likely in part due to significantly higher baseline CMAP values than the nusinersen cohort (Figures 4A–4G). Only 1 SMA patient had 2 SMN2 copies in this cohort. She was identified prior to symptom onset as a neonate because an older affected sibling had presented with respiratory failure by 6 months of age. NF levels and maximum ulnar CMAP were performed prior to onasemnogene abeparvovec infusion by 1 month of age (Figure 4A). Although she sat independently by 6 months of age, she developed tongue fasciculations, upper extremity polyminimyoclonus, and absent patellar reflexes in the setting of a reduced ulnar CMAP amplitude. She manifested proximal lower extremity weakness by ∼12 months of age but ultimately walked independently by 18 months, consistent with an SMA type 3a phenotype (Table 2, subject ID 669). This case highlights the potential value of elevated NF levels and reduced ulnar CMAP at baseline to predict a less than ideal outcome despite early onasemnogene abeparvovec monotherapy. Identifying such infants early to ensure optimal clinical support and consider adjunct therapeutic approaches could result in improved clinical outcomes.
Figure 4.
Cohort of 7 SMA patients receiving onasemnogene abeparvovec early in life
Longitudinal plots for pNf-H concentrations, Nf-L concentrations, and CMAP values for each individual case (A–G) in this cohort. Arrows indicate the onasemnogene abeparvovec injection time point for each case.
We analyzed data from 6 infants who received co-therapy with 1 or more intrathecal loading doses of nusinersen followed by a single intravenous infusion of onasemnogene abeparvovec. All patients in this cohort had 2 SMN2 copies and 5 of 6 showed significant denervation at the time of treatment initiation as indicated by reduced baseline ulnar CMAP values ranging from 0.2 to 1.0 mV (Figures 5A–5F). Of note, 3 of 6 infants received the onasemnogene abeparvovec infusion within 1 to 2 weeks of the initial nusinersen loading dose and demonstrated a significant decrease in pNf-H and Nf-L levels. In contrast, elevated pNf-H and Nf-L levels were observed post onasemnogene abeparvovec infusion in the 2 infants who were dosed 3 to 4 weeks following completion of the Spinraza loading series, but to a lesser degree than that observed in the onasemnogene abeparvovec monotherapy cohort.
Figure 5.
Cohort of 6 SMA patients receiving co-therapy with nusinersen and onasemnogene abeparvovec early in life
Longitudinal plots for pNf-H concentrations, Nf-L concentrations, and CMAP values for each individual case (A–F) in this cohort. Red arrows indicate the first nusinersen dose and dark arrows indicate the onasemnogene abeparvovec injection. ∗Subject 913 received only a single loading dose of nusinersen (please see materials and methods for details).
Association of serum pNf-H and Nf-L levels with SMN2 copy number and effects of therapies after correcting for age effects
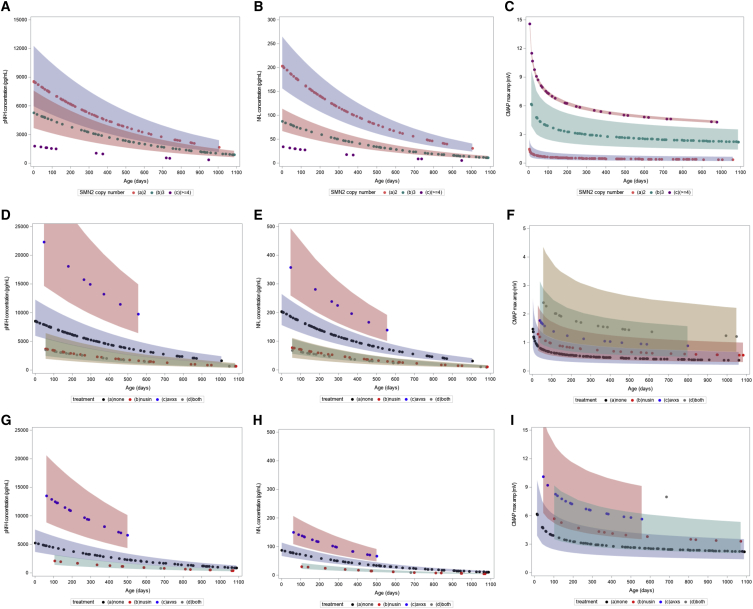
We performed mixed-effect models considering age as a cofounding factor since NF levels decrease over time. There was a significant association between pNf-H and Nf-L measurements (95% confidence interval [CI] 1.97–2.26; p < 0.0001). Sex was neither a significant nor a confounding factor in our model. After correction for age and treatment status, the mixed-effect model revealed significant differences in pNf-H (Figure 6A; p < 0.0001) and Nf-L levels (Figure 6B; p < 0.0001) among SMA patients with different SMN2 copy number. On average, patients with 2 SMN2 copies had 19.3-fold higher pNf-H levels (95% CI 10.9–34.1; p < 0.0001), patients with 3 SMN2 copies had 12.3-fold higher pNf-H levels (95% CI 7.0–22.9; p < 0.0001) and patients with 4 SMN2 copies had 4.8-fold higher pNf-H levels (95% CI 2.1–10.8; p = 0.0002) as compared with healthy controls. For Nf-L levels, patients with 2 SMN2 copies had 20.1-fold higher Nf-L levels (95% CI 12.4–32.9; p < 0.0001), patients with 3 SMN2 copies had 9.1-fold higher Nf-L levels (95% CI 2.5–15.2; p < 0.0001), and patients with 4 SMN2 copies had 3.7-fold higher Nf-L levels (95% CI 1.9–7.2; p < 0.0001) as compared with healthy controls. In addition, there were significant differences in CMAP values among SMA patients with different SMN2 copies after correction for age (Figure 6C; p < 0.0001), where patients with 4 SMN2 copies had higher CMAP maximum amplitude as compared with patients with 2 or 3 SMN2 copies, and patients with 3 SMN2 copies had higher CMAP maximum amplitude as compared with patients with 2 SMN2 copies (p < 0.0001 for all comparisons). Therefore, these models confirmed that serum pNf-H and Nf-L levels are increased in SMA patients when compared with healthy controls and are associated with SMN2 copy number as well as inversely associated with CMAP maximum amplitude in the first 3 years of life.
Figure 6.
Mixed-effect model comparing serum pNf-H, Nf-L levels and CMAP values in SMA patients
(A–C) Comparison of pNf-H levels (A), Nf-L levels (B), and CMAP values (C) among SMA patients with 2, 3, and 4 SMN2 copies not receiving molecular therapies. (D–F) Comparison of pNf-H levels (D), Nf-L levels (E), and CMAP values (F) among SMA patients with 2 SMN2 copies receiving or not nusinersen and/or onasemnogene abeparvovec. (G–I) Comparison of pNf-H levels (G), Nf-L levels (H), and CMAP values (I) among SMA patients with 3 SMN2 copies receiving or not nusinersen and/or onasemnogene abeparvovec early in life.
We next applied a mixed-effect model to determine the effects of nusinersen and/or onasemnogene abeparvovec on SMA patients after correction for age and SMN2 copy number. SMA subjects with 4 SMN2 copies were not included in this analysis due to limited sample size. The mixed-effect model revealed significant treatment effects on pNf-H levels (Figures 6D and 6G; p < 0.0001), Nf-L levels (Figures 6E and 6H; p < 0.0001), and CMAP maximum amplitude (Figures 6F and 6L; p < 0.0001) in SMA patients with 2 and 3 SMN2 copies.
On average, patients receiving only nusinersen had 0.47-fold lower pNf-H levels compared with untreated patients (95% CI 0.32–0.69; p = 0.0002) and patients receiving co-therapy with nusinersen and onasemnogene abeparvovec had 0.34-fold lower pNf-H levels compared with patients not receiving these therapies (95% CI 0.17–0.69; p = 0.009) after correcting for age and SMN2 copy number. Remarkably, patients receiving only onasemnogene abeparvovec had 2.8-fold higher pNf-H levels compared with untreated patients (95% CI 1.8–4.3; p < 0.0001) after correcting for age and SMN2 copy number. Similar effects were observed for Nf-L data, where patients receiving only nusinersen had 0.43-fold lower Nf-L levels compared with untreated patients (95% CI 0.32–0.69; p = 0.0002) and patients receiving co-therapy with nusinersen and onasemnogene abeparvovec had 0.19-fold lower Nf-L levels compared with untreated patients (95% CI 0.10–0.39; p = 0.01). Patients receiving only onasemnogene abeparvovec had 1.9-fold higher Nf-L levels compared with untreated patients (95% CI 1.33–2.76; p = 0.0005). However, when applying the same model for the CMAP values, we observed that all therapies increased ulnar CMAP maximum amplitude as compared with untreated SMA subjects with 2 (Figure 6F; p < 0.0001) or 3 (Figure 6I; p < 0.0001) SMN2 copies. There were no available data for co-therapy in SMA patients with 3 SMN2 copies.
Taken together, these models suggest that nusinersen treatment was associated with decreased NF levels, while onasemnogene abeparvovec was associated with increased NF levels in SMA subjects in the 3 first years of life. However, both nusinersen and onasemnogene abeparvovec therapies were associated with increased CMAP maximum amplitude, suggesting less denervation in treated patients when compared with untreated SMA patients with either 2 or 3 SMN2 copies.
Discussion
Identifying specific biomarkers to help guide treatment in newborns and infants genetically confirmed with SMA is urgent. Such biomarkers will ideally include indicators of the health of motor neurons and other peripheral tissues and help detect onset and status of ongoing denervation and monitor responses to treatment(s). In the present study, in a real-world clinical setting, we demonstrate that circulating pNf-H and Nf-L concentrations are elevated in the first months of life in SMA patients, and that these increased levels are inversely correlated to the number of SMN2 copies and to maximum ulnar CMAP negative peak amplitude values. We also present for the first time NF data with correlated clinical data for SMA infants and children receiving either nusinersen or onasemnogene abeparvovec monotherapy or co-therapy in the 3 first years of life in the real-world clinical setting. These data confirm prior observations that (1) initiation of nusinersen treatment is associated with a rapid decrease in pNf-H levels and Nf-L levels, (2) baseline ulnar CMAP is reflective of overall status of denervation, correlates with SMN2 copy number, and is associated with the capacity for reinnervation in response to therapy, (3) timing for treatment intervention as early as possible in the neonatal period is critical to ensure the best possible clinical outcomes, especially for the 2 SMN2 copy cohort but in all infants with borderline CMAP amplitudes or elevated NF levels, and confirms that (4) infants and children early in life receiving either or both therapies are achieving milestones beyond that expected from natural history data.
In addition, we report the following novel observations: (1) intravenous gene therapy with onasemnogene abeparvovec is associated with an increase in serum NF levels, (2) elevated NF levels in the setting of a declining ulnar CMAP following onasemnogene abeparvovec in an infant with 2 SMN2 copies predicted the emergence of a later-onset SMA phenotype, (3) pretreatment of infants with nusinersen therapy appears to prevent or reduce the impact of elevated NFs temporally associated with onasemnogene abeparvovec infusion, and (4) infants with ≥4 SMN2 copies can present with signs of denervation in early infancy, and the use of ulnar CMAP and NFs can help guide when and if treatment is indicated. These novel findings have important implications for the field and provide a foundation for using serum NFs and maximum ulnar CMAP amplitude measures in longitudinal follow-up in the clinic for monitoring SMA newborns to help guide therapeutic decisions.
SMA patients had elevated serum pNf-H and Nf-L levels in our natural history cohort. On one hand, the highest value observed for healthy control newborns was ∼1000 pg/mL for pNf-H and ∼20 pg/mL for Nf-L. On the other hand, SMA patients display a huge range in both pNf-H and Nf-L, with pNf-H values up to ∼30,000 pg/mL and Nf-L values up to 1,100 pg/mL. By comparing these absolute values with reports from previous studies (NURTURE NCT02386553; ENDEAR NCT02193074, and EMBRACE NCT02462759 [A Study to Assess the Safety and Tolerability of Nusinersen (ISIS 396443) in Participants With Spinal Muscular Atrophy]), we noticed that SMA newborns with 2 SMN2 copies can show even higher pNf-H levels than those observed in our current natural history cohort.17,23,24 Of note, plasma pNf-H levels were elevated up to 45,000 pg/mL in SMA newborns with 2 SMN2 copies in the NURTURE study24 and up to 50,000 pg/mL in SMA patients with 2 SMN2 copies in the ENDEAR study.17 Importantly, in the current study, we clearly demonstrate dynamic changes in circulating NFs and it is important to consider the longitudinal time course of these changes. For example, we observed high pNf-H and Nf-L values in SMA patients with 3 SMN2 copies, but the peak values were observed later than those with 2 SMN2 copies. This notably parallels differences observed in timing of ulnar CMAP decline observed in our STOP SMA cohort: Infants with 2 SMN2 copies who manifested an SMA type 1 phenotype had lower CMAP values from the earliest possible time point post birth and demonstrated a precipitous decline by 3 months of age, whereas infants with 3 SMN2 copies who ultimately manifested an SMA type 2 phenotype most often have relatively preserved CMAP amplitudes in the first few months, followed by a plateau or decline in amplitude between 3 and 12 months of age (NCT00528268, Study of sodium phenylbutyrate in presymptomatic infants with SMA).
Overall, increases in serum NF levels were inversely associated with the number of SMN2 copies. This has high clinical relevance since both pNf-H and Nf-L measurements could be additional tools to determine onset of first symptoms in SMA patients, or to predict the onset of denervation in some cases. Previous6,24 and ongoing (NURTURE and SPRINT [Pre-Symptomatic Study of Intravenous Onasemnogene Abeparvovec-xioi in Spinal Muscular Atrophy (SMA) for Patients With Multiple Copies of SMN2] trials: NCT02386553 and NCT03505099) studies have demonstrated the importance of initiating treatment as early as possible in SMA newborns with 2 or 3 SMN2 copies. Challenges that delay initiation of treatment include the high costs of these therapies and the complex logistics involving medical insurance coverage for newborns in the U.S. The use of disease-activity-related biomarkers like pNf-H and/or Nf-L could be additional tools to help justify the urgency for early intervention in specific settings, such as higher SMN2 copy number. Currently, phenotypic indicators of disease onset are limited to clinical examination, motor function assessments, and electrophysiologic studies including ulnar CMAP in SMA newborns and infants. While straightforward, pediatric neurophysiologic studies are not readily available on short notice in all settings. The use of maximum ulnar CMAP for monitoring status of denervation in infants with SMA requires adherence to a protocol using consistent equipment, electrodes, and ideally, staff. While peroneal CMAP recorded from the tibialis anterior muscle can be a useful adjunct, median or ulnar CMAP studies are critical to help ascertain that therapies delivered via lumbar intrathecal infusion are effectively impacting motor neurons in the cervical spinal cord. While useful, CMAP studies might be expected to be less sensitive than an increase in circulating NF concentrations to indicate the earliest onset of acute neuronal damage in an infant with higher SMN2 copy number. Circulating NF levels in SMA patients during the chronic phase of denervation may reflect damage that occurred much earlier; some authors have speculated that pNf-H half-life as long as 8 months.17,25 However, in patients during the chronic much more slowly progressive phase of denervation, preserved CMAP levels can occur for prolonged periods of time if compensatory reinnervation is sufficient. Thus, elevated NF levels could prove a much more sensitive biomarker in SMA patients with later-onset phenotypes, and allow much earlier treatment intervention (for example, the infant with >4 SMN2 copies in Figure 4G). However, NF levels should not be used as a single marker to predict outcomes.
Data from the natural history cohort demonstrate that changes in pNf-H levels are well correlated with changes in Nf-L levels. However, Nf-L distinguished SMA patients with 2 and 3 SMN2 copies in the first months of life better than pNf-H. Differences in the biology of these structural proteins and/or assay-dependent variability could explain these differences.13,14 Importantly, both pNf-H and Nf-L showed similar responses to treatment intervention, indicating they are equally valid candidates as treatment response biomarkers.
NF levels rapidly decreased in SMA patients treated with nusinersen alone or in combination with onasemnogene abeparvovec therapy. In 8 of 9 patients with available baseline levels, all showed a rapid decrease in NF levels following initiation of dosing. Moreover, all 6 patients who received co-therapy showed a rapid decrease following the first nusinersen dose. These findings corroborate prior clinical trial data demonstrating that nusinersen treatment decreases pNf-H concentration in SMA subjects.17,23,24 Taken together, these observations indicate that circulating NFs decrease over time in the natural history of the disease following the period of most acute neuronal damage, although chronic ongoing denervation likely contributes to persistently elevated levels over the first 3 years of life. Nusinersen treatment is associated with a faster decrease in circulating NF levels than that observed in natural history subjects. The degree of clinical response and impact on ulnar CMAP values is clearly highly dependent on initiating nusinersen treatment sufficiently early in the disease course when it can most effectively limit the greatest neuronal damage. However, the continued normalization of levels during months of longitudinal follow-up in the setting of ongoing maintenance therapy are in contrast to the persistently elevated NF levels following a single onasemnogene abeparvovec infusion, indicating an ongoing neuroprotective effect. Clinically, all of the infants who received nusinersen treatment in this study prior to 6 months of age achieved important motor milestones not expected for untreated SMA patients with similar age and SMN2 dosage. However, those children with 2 SMN2 copies with pre-existing significant distal denervation and older age at treatment initiation, while improved, had much less favorable outcomes.
One of the most unexpected findings in this study was the observation of significantly increased pNf-H and Nf-L levels in a cohort of 7 SMA infants who were treated with onasemnogene abeparvovec monotherapy between 20 and 190 days of age. All infants were presymptomatic at the time of initial evaluation; however, in 2 infants with ≥4 SMN2 copies, treatment was initiated in response to subtle clinical signs indicative of possible onset of denervation. In spite of the significant elevation in NF levels in the immediate post-treatment period, 6 of 7 children are developing normally and continue to acquire age-appropriate motor milestones. However, 1 infant with 2 SMN2 copies had an incomplete benefit in spite of treatment initiation in the neonatal period; notably, she demonstrated chronically elevated NF levels that failed to normalize during the subsequent 18 months. While the interpretation of these findings is speculative and caution is needed, we hypothesize a direct effect of the gene therapy treatment regimen may be causing acute collateral damage to vulnerable neurons during a critical period of vulnerability in the setting of an enhanced cellular stress response. Interestingly, exogenous SMN1 gene replacement using non-integrating vectors such as onasemnogene abeparvovec presents many challenges, including caution regarding possible toxic effects of long-term uncontrolled SMN overexpression.22 A recent study provided evidence that long-term SMN overexpression interferes with RNA regulation and triggers pathogenic events.22 In our patients, the transduction of cells by the viral vector is thought to occur over a period of days to just a few weeks. The persistently elevated levels in some subjects could be related to ongoing death of non-transduced cells. However, the observation that nusinersen treatment administered prior to the administration of gene therapy seemed to prevent or moderate this response indicates the increasing SMN protein levels prior to gene therapy delivery may provide a neuroprotective effect, and a role for adjunct co-therapy in infants with 2 SMN2 copies. Further studies are needed to better understand the mechanisms underlying these observations.
In summary, a major challenge in the SMA field has been to identify biomarkers to track disease status, particularly in newborns. Our novel observations provide evidence to support the use of serum NFs and maximum ulnar CMAP as biomarkers to monitor SMA disease onset, progression, and treatment response early in life in the clinical setting. More studies investigating SMA patients with ≥4 SMN2 copies are needed since we have limited data in the present cohort. We encourage future studies to analyze circulating NFs in additional SMA cohorts who receive gene therapy alone or in combination with other treatments. We advocate for additional studies analyzing different strategies of combined therapies since nusinersen could be administrated before or after dosing with onasemnogene abeparvovec. Last, further studies evaluating co-therapy with risdiplam will be critical for the SMA community, since many patients are currently receiving combinations of risdiplam with either nusinersen or onasemnogene abeparvovec or both in the clinical setting.
Materials and methods
Study approval and subjects
Written informed consent and parental consent were obtained from all participants under Institutional Ethics Review Boards at the University of Utah (protocol 8751) and Massachusetts General Hospital (protocol 2016P000469). We queried the Project Cure SMA and SPOT SMA LPDRs housed within the Research Electronic Data Capture Web Application at the Newborn Screening Translational Research Network for all serum samples available from SMA subjects under 3 years of age not receiving any SMN-targeted molecular or gene therapy at the time of sample collection or receiving nusinersen and/or onasemnogene abeparvovec. Thus, follow-up included all samples available from 0 to 1,095 days old. Samples and visits from subjects receiving risdiplam were not included in this analysis (Evrysdi, Roche). We queried the database for all available CMAP data, and all fields containing pertinent data regarding aspects of medical history, physical and neurological examinations, motor function assessments, and motor milestones. Table 1 presents the distribution of age, sex, race, ethnicity, SMN2 copies, SMA type, pNf-H levels, Nf-L levels, and CMAP values for this cohort.
Table 1.
Clinical milestones for individual SMA patients receiving nusinersen and/or onasemnogene abeparvovec early in life
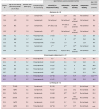 |
Background colors indicate SMN2 copy number for each case. Red: 2 SMN2 copies. Green: 3 SMN2 copies. Purple: 4 SMN2 copies. All milestones achieved were documented to be maintained during the follow-up of this cohort study to the age of last evaluation unless otherwise specified. Regarding Subjects 818 and 927 ∗Adjusted for prematurity. ∗∗Parent reported.
Motor milestones
Motor function was assessed at each visit by a physical therapist with SMA clinical trial training26 using 1 or more of the following outcome measures: Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND),27,28 Bayley Scales of Infant and Toddler Development III (BSID-III),29 Hammersmith Infant Neurological Examination Section 2: Motor Milestones (HINE-2),30 Hammersmith Functional Motor Scale Expanded (HFMSE),31 and World Health Organization motor milestones (WHO-MM).32 Frequency of assessment was every 3 months for type I SMA and every 4 to 6 months for type II SMA. The primary outcome measure for determining gross motor milestone achievement for children with type I SMA who were less than 2 years old and unable to sit was the CHOP-INTEND. The primary outcome measure for those with type II SMA who were 2 to 3 years old and able to sit was the HFMSE. Secondary outcomes used to document motor milestone achievement for children under 3 years of age were the HINE-2, WHO-MM, and BSID-III. Parent report of motor milestone achievement corroborated, when possible, by date-stamped home video recording was used to supplement data collected at clinical evaluations for children who achieved milestones in-between clinic visits. Rolling was defined as the ability to roll from supine to prone either elicited (CHOP-INTEND items 6/7) or independently (HFMSE items #8/9 ≥ 1; HINE-2 rolling item = 3; BSID-III gross motor item #25 = 1). Sitting was defined as the ability to sit without support indexed by the following: HFMSE item #1 = 1; HINE-2 sitting item ≥3; WHO-MM sitting without support; or BSID-III gross motor item #26 = 1. Standing was defined as standing with support indexed by: HFMSE item #18 ≥ 1; HINE-2 standing ≥2; WHO-MM standing with assistance; BSID-III gross motor item = #1. Walking was defined as the ability to walk independently indexed by: HFMSE item #20 = 2; HINE-2 walking item = 3; WHO-MM walking alone; or BSID-III gross motor item #43 = 2. Motor assessments were video recorded and used for clarification or confirmation of source document data. Table S1 shows test sources used to determine motor milestones for each child with SMA receiving molecular and/or gene therapy.
SMN2 copy number and ulnar CMAP maximum amplitude
SMN1 and SMN2 copy numbers were determined using qPCR as previously described.33 Maximum ulnar CMAP amplitude was obtained by recording from the abductor digiti minimi muscle after ulnar nerve stimulation at the wrist as previously described.11,34
Neurofilament levels
Serum phosphorylated neurofilament heavy chain (pNf-H) concentrations were measured using a pNF-H enzyme-linked lectin assay (ProteinSimple, CA, USA) according to the manufacturer's instruction and as previously described.17 This Simple Plex Ella immunoassay was performed in a microfluidic cartridge using the 72-well assay plate format. All reagents were provided in the immunoassay kit and all samples were run using Lot #9056. The assay was further qualified using quality control (QC) samples generated in-house. Multiple QC samples were prepared, including buffered and endogenous samples, and included in the ELLA analyzer at Biogen (Cambridge, MA). We tested multiple parameters, including precision and accuracy, parallelism, short-term and long-term stability, freeze/thaw stability, bench top stability, and specificity. During the qualification steps, we used 3 different buffered QC samples (high, medium, and low levels) and endogenous QC samples. They were prepared as stocks and aliquoted to single uses. In each run, 2 sets of 4 QC samples were included. All human samples were thawed on ice, and 1:2 diluted with sample diluent provided in the assay kit. For each diluted sample, 30 μL was pipetted into each port of the assay cartridge plate. Washing buffer was added into the plate and the plate was loaded into the ELLA machine. The curves were generated by plotting relative fluorescence units versus concentration using a 5-parameter logistical curve-fit. A 1/y2 weighted regression was applied. The calibration curve range was 7.5 to 28,480 pg/mL. The software automatically generated the calibration curve and interpolated all data points to calculate concentrations for each sample. All these procurements were automated, and this assay is intended to be highly reliable and reproducible.
Neurofilament light chain (Nf-L) were determined at Quanterix (Billerica, MA) using a single-molecule array (Simoa) NF-light Advantage Kit (Quanterix). This is a commercially available measurement and details can be obtained at https://www.quanterix.com.
Statistical analysis
Generalized linear mixed model (GLMM) with random intercept was applied using SAS 9.4 (SAS Institute Inc, Cary, NC). To match the assumptions of the GLMM, log transformation was applied to some continuous variables. Data were controlled for age. Sex was neither a significant nor a confounding factor in our model. To compare pNf-H, Nf-L, or CMAP values among healthy control subjects and SMA patients with 2, 3, or ≥4 SMN2 copies and to compare patients treated with nusinersen and/or onasemnogene abeparvovec, the model was Ln(pNf-H or Nf-L or CMAP) versus age, SMN2 copy number, nusinersen, and onasemnogene abeparvovec. Differences in pNf-H and Nf-L levels were reported as fold-change, 95% CIs, and p values. Adjustment for SMN2 copy number was also performed, with SMN2 copies treated as a categorical variable. Critical value was set as p < 0.05.
Acknowledgments
We thank Kendall Trautman, Maria Soledad Herrmann, Alec J. Johnstone, and Salomé Da Silva Duarte Lepez for helpful technical support during this study. We are grateful to all the patients and families who participated in this study. C.R.R.A. received a fellowship from the MGH ECOR. This study was funded by NIH NICHD R01HD054599, NIH NINDS R21NS108015, Biogen, and Cure SMA.
Author contributions
C.R.R.A. and K.J.S. conceived and designed the study. M.P., J.S., and W.F. performed and analyzed neurofilaments experiments. C.R.R.A., R.S., R.G., and E.J.E. processed human samples for this cohort study. E.R., E.A.A., M.K., E.L.T., S.S., and K.J.S. collected patient data. R.Z. performed statistical analysis. K.J.S. provided laboratory support and supervised the experiments. C.R.R.A. and K.J.S. drafted the manuscript. All authors interpreted the data, participated in manuscript review, and approved the final manuscript.
Declaration of interests
C.R.R.A. and K.J.S. are inventors on a patent filed by Mass General Brigham that describes genome engineering technologies to treat SMA. C.R.R.A. holds stocks in CRISPR Therapeutics. K.J.S. is the recipient of a grant from Biogen and receives clinical trial funding from AveXis and Biogen. E.L.T. serves on a physical therapy advisory board for Biogen. M.P., J.S., and W.F. are current or former employees of Biogen and hold stock/stock options in Biogen. R.S., R.G., R.Z., E.J.E., E.R., E.A.A., S.S., and M.K. report no conflict of interest.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2021.10.011.
Supplemental information
References
- 1.Groen E.J.N., Talbot K., Gillingwater T.H. Advances in therapy for spinal muscular atrophy: promises and challenges. Nat. Rev. Neurol. 2018;14:214–224. doi: 10.1038/nrneurol.2018.4. [DOI] [PubMed] [Google Scholar]
- 2.Finkel R.S., Mercuri E., Darras B.T., Connolly A.M., Kuntz N.L., Kirschner J., Chiriboga C.A., Saito K., Servais L., Tizzano E., et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 2017;377:1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri E., Darras B.T., Chiriboga C.A., Day J.W., Campbell C., Connolly A.M., Iannaccone S.T., Kirschner J., Kuntz N.L., Saito K., et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 2018;378:625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 4.Sturm S., Gunther A., Jaber B., Jordan P., Al Kotbi N., Parkar N., Cleary Y., Frances N., Bergauer T., Heinig K., et al. A phase 1 healthy male volunteer single escalating dose study of the pharmacokinetics and pharmacodynamics of risdiplam (RG7916, RO7034067), a SMN2 splicing modifier. Br. J. Clin. Pharmacol. 2019;85:181–193. doi: 10.1111/bcp.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K., et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 6.Alves C.R.R., Zhang R., Johnstone A.J., Garner R., Eichelberger E.J., Lepez S., Yi V., Stevens V., Poxson R., Schwartz R., et al. Whole blood survival motor neuron protein levels correlate with severity of denervation in spinal muscular atrophy. Muscle Nerve. 2020;62:351–357. doi: 10.1002/mus.26995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaworski P., von Herrmann K.M., Taylor S., Sunshine S.S., McCarthy K., Risher N., Newcomb T., Weetall M., Prior T.W., Swoboda K.J., et al. SMN protein can Be reliably measured in whole blood with an electrochemiluminescence (ECL) immunoassay: implications for clinical trials. PLoS One. 2016;11:e0150640. doi: 10.1371/journal.pone.0150640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkel R.S., Crawford T.O., Swoboda K.J., Kaufmann P., Juhasz P., Li X., Guo Y., Li R.H., Trachtenberg F., Forrest S.J., et al. Candidate proteins, metabolites and transcripts in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS One. 2012;7:e35462. doi: 10.1371/journal.pone.0035462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otsuki N., Arakawa R., Kaneko K., Aoki R., Arakawa M., Saito K. A new biomarker candidate for spinal muscular atrophy: identification of a peripheral blood cell population capable of monitoring the level of survival motor neuron protein. PLoS One. 2018;13:e0201764. doi: 10.1371/journal.pone.0201764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi D.T., Decker D., Zaworski P., Klott K., McGonigal J., Ghazal N., Sly L., Chung B., Vanderlugt J., Chen K.S. Evaluation of peripheral blood mononuclear cell processing and analysis for Survival Motor Neuron protein. PLoS One. 2012;7:e50763. doi: 10.1371/journal.pone.0050763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewelt A., Krosschell K.J., Scott C., Sakonju A., Kissel J.T., Crawford T.O., Acsadi G., D'Anjou G., Elsheikh B., Reyna S.P., et al. Compound muscle action potential and motor function in children with spinal muscular atrophy. Muscle Nerve. 2010;42:703–708. doi: 10.1002/mus.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swoboda K.J., Prior T.W., Scott C.B., McNaught T.P., Wride M.C., Reyna S.P., Bromberg M.B. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann. Neurol. 2005;57:704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusnierova P., Zeman D., Hradilek P., Cabal M., Zapletalova O. Neurofilament levels in patients with neurological diseases: a comparison of neurofilament light and heavy chain levels. J. Clin. Lab. Anal. 2019;33:e22948. doi: 10.1002/jcla.22948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant P., Pant H.C. Neurofilament protein synthesis and phosphorylation. J. Neurocytol. 2000;29:843–872. doi: 10.1023/a:1010999509251. [DOI] [PubMed] [Google Scholar]
- 15.Winter B., Guenther R., Ludolph A.C., Hermann A., Otto M., Wurster C.D. Neurofilaments and tau in CSF in an infant with SMA type 1 treated with nusinersen. J. Neurol. Neurosurg. Psychiatry. 2019;90:1068–1069. doi: 10.1136/jnnp-2018-320033. [DOI] [PubMed] [Google Scholar]
- 16.Ackerley S., Thornhill P., Grierson A.J., Brownlees J., Anderton B.H., Leigh P.N., Shaw C.E., Miller C.C. Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments. J. Cell Biol. 2003;161:489–495. doi: 10.1083/jcb.200303138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darras B.T., Crawford T.O., Finkel R.S., Mercuri E., De Vivo D.C., Oskoui M., Tizzano E.F., Ryan M.M., Muntoni F., Zhao G., et al. Neurofilament as a potential biomarker for spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2019;6:932–944. doi: 10.1002/acn3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wurster C.D., Steinacker P., Gunther R., Koch J.C., Lingor P., Uzelac Z., Witzel S., Wollinsky K., Winter B., Osmanovic A., et al. Neurofilament light chain in serum of adolescent and adult SMA patients under treatment with nusinersen. J. Neurol. 2020;267:36–44. doi: 10.1007/s00415-019-09547-y. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y., Lee B.H., Yip W., Chou P., Yip B.S. Neurofilament proteins as prognostic biomarkers in neurological disorders. Curr. Pharm. Des. 2020;25:4560–4569. doi: 10.2174/1381612825666191210154535. [DOI] [PubMed] [Google Scholar]
- 20.Bevan A.K., Duque S., Foust K.D., Morales P.R., Braun L., Schmelzer L., Chan C.M., McCrate M., Chicoine L.G., Coley B.D., et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol. Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pattali R., Mou Y., Li X.J. AAV9 Vector: a Novel modality in gene therapy for spinal muscular atrophy. Gene Ther. 2019;26:287–295. doi: 10.1038/s41434-019-0085-4. [DOI] [PubMed] [Google Scholar]
- 22.Van Alstyne M., Tattoli I., Delestree N., Recinos Y., Workman E., Shihabuddin L.S., Zhang C., Mentis G.Z., Pellizzoni L. Gain of toxic function by long-term AAV9-mediated SMN overexpression in the sensorimotor circuit. Nat. Neurosci. 2021 doi: 10.1038/s41593-021-00827-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acsadi G., Crawford T.O., Muller-Felber W., Shieh P.B., Richardson R., Natarajan N., Castro D., Ramirez-Schrempp D., Gambino G., Sun P., Farwell W. Safety and efficacy of nusinersen in spinal muscular atrophy: the EMBRACE study. Muscle Nerve. 2021 doi: 10.1002/mus.27187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vivo D.C., Bertini E., Swoboda K.J., Hwu W.L., Crawford T.O., Finkel R.S., Kirschner J., Kuntz N.L., Parsons J.A., Ryan M.M., et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019;29:842–856. doi: 10.1016/j.nmd.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan A., Rao M.V., Veeranna, Nixon R.A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 2017;9 doi: 10.1101/cshperspect.a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glanzman A.M., Mazzone E.S., Young S.D., Gee R., Rose K., Mayhew A., Nelson L., Yun C., Alexander K., Darras B.T., et al. Evaluator training and reliability for SMA global nusinersen Trials1. J. Neuromuscul. Dis. 2018;5:159–166. doi: 10.3233/JND-180301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glanzman A.M., Mazzone E., Main M., Pelliccioni M., Wood J., Swoboda K.J., Scott C., Pane M., Messina S., Bertini E., et al. The children's hospital of Philadelphia infant test of neuromuscular disorders (CHOP INTEND): test development and reliability. Neuromuscul. Disord. 2010;20:155–161. doi: 10.1016/j.nmd.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glanzman A.M., McDermott M.P., Montes J., Martens W.B., Flickinger J., Riley S., Quigley J., Dunaway S., O'Hagen J., Deng L., et al. Validation of the children's hospital of Philadelphia infant test of neuromuscular disorders (CHOP INTEND) Pediatr. Phys. Ther. 2011;23:322–326. doi: 10.1097/PEP.0b013e3182351f04. [DOI] [PubMed] [Google Scholar]
- 29.Bayley N. Pearson; 2006. Bayley Scales of Infant and Toddler Development. [Google Scholar]
- 30.De Sanctis R., Coratti G., Pasternak A., Montes J., Pane M., Mazzone E.S., Young S.D., Salazar R., Quigley J., Pera M.C., et al. Developmental milestones in type I spinal muscular atrophy. Neuromuscul. Disord. 2016;26:754–759. doi: 10.1016/j.nmd.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Hagen J.M., Glanzman A.M., McDermott M.P., Ryan P.A., Flickinger J., Quigley J., Riley S., Sanborn E., Irvine C., Martens W.B., et al. An expanded version of the Hammersmith functional motor Scale for SMA II and III patients. Neuromuscul. Disord. 2007;17:693–697. doi: 10.1016/j.nmd.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 32.WHO Multicentre Growth Reference Study Group Reliability of motor development data in the WHO multicentre growth reference study. Acta Paediatr. Suppl. 2006;450:47–55. doi: 10.1111/j.1651-2227.2006.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 33.Prior T.W., Krainer A.R., Hua Y., Swoboda K.J., Snyder P.C., Bridgeman S.J., Burghes A.H., Kissel J.T. A positive modifier of spinal muscular atrophy in the SMN2 gene. Am. J. Hum. Genet. 2009;85:408–413. doi: 10.1016/j.ajhg.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bromberg M.B., Swoboda K.J. Motor unit number estimation in infants and children with spinal muscular atrophy. Muscle Nerve. 2002;25:445–447. doi: 10.1002/mus.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.