Abstract
We have previously shown that mutation of the two tyrosines in the cytoplasmic domain of integrin subunit β1 (Y783 and Y795) to phenylalanines markedly reduces the capability of β1A integrins to mediate directed cell migration. In this study, β1-dependent cell spreading was found to be delayed in GD25 cells expressing β1AY783/795F compared to that in wild-type GD25-β1A. Focal adhesion kinase (FAK) tyrosine phosphorylation and activation were severely impaired in response to β1-dependent adhesion in GD25-β1AY783/795F cells compared to that in wild-type GD25-β1A or mutants in which only a single tyrosine was altered (β1AY783F or β1AY795F). Phosphorylation site-specific antibodies selective for FAK phosphotyrosine 397 indicated that the defect in FAK phosphorylation via β1AY783/795F lies at the level of the initial autophosphorylation step. Indeed, β1A-dependent tyrosine phosphorylation of tensin and paxillin was lost in the β1AY783/795F cells, consistent with the impairment in FAK activation. In contrast, p130CAS overall tyrosine phosphorylation was unaffected by the β1 mutations. Despite the defect in β1-mediated FAK activation, FAK was still localized to focal adhesions. Taken together, the phenotype of the GD25-β1AY783/795F cells resembles, but is distinct from, the phenotype observed in FAK-null cells. These observations argue that tyrosines 783 and 795 within the cytoplasmic tail of integrin subunit β1A are critical mediators of FAK activation and cell spreading in GD25 cells.
Integrins are a family of adhesion receptors, each consisting of one α and one β subunit. Among the known integrins, 12 contain the β1 subunit, and ligands for this subfamily include collagens, laminins (LNs), and fibronectin (FN).
Integrin subunit β1, as well as all other integrin subunits except for β4, consists of a large extracellular domain, a single transmembrane stretch, and a short intracellular cytoplasmic domain. Although devoid of an intrinsic enzymatic activity, the cytoplasmic domain regulates the conformation and ligand binding activity of the extracellular domain (so-called “inside-out signaling”), as well as mediates interactions with the cytoskeleton and the transduction of intracellular signals (“outside-in” signaling). Specificity for this range of dynamic signaling events has been validated in many systems by using truncated and mutated integrin receptors (15, 16, 23, 26, 27, 29, 32, 33, 51).
Ligand binding to integrins triggers the assembly of a large number of cytoplasmic and transmembrane molecules into discrete regions referred to as focal adhesions (FAs) (5, 10). These complexes serve both as structural anchorage sites that connect the extracellular matrix with the intracellular actin cytoskeleton and as signaling complexes, which initiate signaling pathways in the cell cytoplasm and nucleus. The signaling molecules found in FAs include tyrosine kinases, serine/threonine kinases, phospholipid kinases, phosphatases, Ras superfamily proteins, and various adapter proteins (e.g., those containing SH2 and SH3 domains) (11, 28, 42, 49).
Although much has been learned about integrin-mediated signaling in recent years, the specific mechanisms by which the initial interactions between integrins and other FA proteins are controlled and how these interactions dictate the hierarchies of signaling pathways remain largely unresolved.
A critical component of integrin-FA signaling involves the formation of a complex between two soluble tyrosine kinases, FA kinase (FAK) and Src. The FAK-Src complex (7) has been shown to activate Ras, which via phosphotidylinositol (PI) 3-kinase→Akt/protein kinase B and the extracellular signal-regulated protein kinases ERK1 and -2 mediates cell survival and proliferation signals (20, 43). FAK-Src signaling also leads to phosphorylation of p130CAS, which positively regulates cell migration (8, 21). Although the activation of FAK and Src is a topic of much investigation, signaling from the FAK-Src complex is still poorly understood. Integrin ligation somehow leads to autophosphorylation of FAK at tyrosine 397, thereby creating a binding site for Src via its SH2 domain (40). This in turn, allows Src to further activate the complex by phosphorylating additional tyrosines in the FAK molecule, including tyrosines 576, 577, and 861 (6). Although this knowledge provides a basic understanding of receptor-FAK-Src signaling, several essential pieces of information are still required to fully understand the activation process. Major unresolved questions include the molecular events that trigger the autophosphorylation of FAK at tyrosine 397, the importance of positive and negative regulatory phosphorylation sites on Src for its binding to FAK, and finally the identity and role of the phosphatase or phosphatases responsible for dephosphorylating proteins involved in cell adhesion signaling.
To elucidate integrin-mediated signaling events, we have studied the role of the potential phosphorylation sites in the cytoplasmic domain of integrin subunit β1A. Previously, we demonstrated that the T777A, Y783F, S785A, and Y795F single substitutions had little effect on the regulation of extracellular conformation or intracellular signaling, indicating that phosphorylation of these residues is not essential for these integrin signaling events (38, 51). In contrast, a T788/789A double mutation resulted in a dramatic shift toward the inactive, non-ligand binding state (51). In addition, the Y783/795F double mutation markedly affected integrin β1A function, as indicated by the increased number of FAs, altered cytoskeletal architecture, and decreased cell migration (38). In the present study, we show that the β1AY783/795F mutation impairs β1A-mediated FAK autophosphorylation and activation. Moreover, this defect results in reduced tyrosine phosphorylation of paxillin and tensin, while the overall tyrosine phosphorylation of a third FAK-Src complex substrate, p130CAS, is unaffected.
MATERIALS AND METHODS
Proteins, peptides, and antibodies.
FN and vitronectin (VN) were purified from human plasma as previously described (46, 53). LN-1 was obtained from Gibco. GRGDS peptide was purchased from Bachem Feinchemikalien AG. Plasmids encoding the glutathione S-transferase (GST) protein fused to the SH2 domains of CrkII and Src, respectively, were provided by A. Sorokin (Milwaukee, Wis.) and S. Courtneidge (Redwood City, Calif.), and the fusion proteins were purified as described previously (22).
The rabbit anti-β1 serum was prepared in our laboratories as described previously (3). Phosphorylation site-specific rabbit anti-FAK antibodies selective for either phosphotyrosine 397 (the major autophosphorylation site of FAK) or phosphotyrosine 861 (the major site phosphorylated by Src) were obtained from BioSource International. These antibodies are highly selective for the targeted phosphorylation site, as demonstrated by peptide competition studies and through use of site-directed mutants possessing a tyrosine-to-phenylalanine substitution at the phosphorylation site (E. M. Schaefer, unpublished data). Generation of these antibody preparations involves extensive epitope-specific affinity chromatography, including negative preadsorption using (i) a nonphosphopeptide corresponding to the site of phosphorylation to remove antibody that is reactive with nonphosphorylated FAK enzyme; (ii) a generic tyrosine-phosphorylated peptide to remove antibody that is reactive with phosphotyrosine, irrespective of the sequence; and, for the anti-pY397 antibody, (iii) a phosphopeptide derived from the corresponding region of Pyk2 (also known as CAKβ, a FAK-related enzyme) to remove antibody that is reactive with the phosphorylated Pyk2/CAKβ enzyme. The final product is generated by affinity chromatography with a FAK-derived peptide that is phosphorylated at the corresponding position (i.e., tyrosine 397 or tyrosine 861). The targeted sequence in each case is conserved in human, mouse, rat, chicken, and frog.
The following antibodies were purchased: hamster anti-rat β1 monoclonal antibody (MAb) Ha2/5 and hamster anti-mouse β1 MAb HMβ1-1 from Pharmingen; mouse anti-human vinculin MAb and antitalin MAb from Sigma Immunochemicals; mouse anti-FAK MAb, mouse antitensin MAb, mouse antipaxillin MAb, mouse anti-p130CAS MAb, mouse antiphosphotyrosine MAb PY20, and horseradish peroxidase-conjugated recombinant antiphosphotyrosine MAb RC20 from Transduction Laboratories; rabbit anti-mouse immunoglobulin G (IgG) from Southern Biotechnology; horseradish peroxidase-conjugated antimouse and antirabbit IgG from Amersham Pharmacia Biotech; and fluorescein-goat anti-rabbit IgG, Cy3–goat anti-rabbit IgG, fluorescein-goat anti-mouse IgG, and Cy3–goat anti-mouse IgG from Jackson ImmunoResearch Laboratories, Inc. (all of multiple labeling quality).
Cell culture.
The cell lines GD25, GD25-β1A, GD25-β1AY783F, GD25-β1AY795F, and GD25-β1AY783/795F have been described previously (13, 38, 51, 52). The GD25 cells lack the β1 family of integrins due to a disrupted β1 gene. The GD25-β1A and the GD25-β1A mutant cell lines were derived from GD25 upon stable transfection with cDNAs encoding the wild-type and mutated murine integrin subunit β1A, respectively. Clones with similar levels of β1 integrins expressed on their surface, as determined by fluorescence-activated cell sorter analysis (38, 51), were chosen for these studies. In addition, expression levels of β1 integrins of these cell lines were verified throughout the duration of this study.
GD25 cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum, l-glutamine (2 mM), penicillin-streptomycin, and amphotericin B (Fungizone), while the GD25-β1A, GD25-β1AY783F, GD25-β1AY795F, and GD25-β1AY783/795F cells were grown in the same medium containing 20 μg of puromycin per ml.
Cell spreading assay.
The wells of 96-well microtiter plates were coated with extracellular matrix (ECM) proteins (2 μg of VN, 10 μg of FN, or 10 μg of LN-1 per ml) in DMEM for 1 h at 37°C. The wells were washed twice with phosphate-buffered saline (PBS) and blocked with 1% heat-treated bovine serum albumin (BSA) in PBS for 1 h at 37°C. After being washed with PBS, 104 cells suspended in DMEM were added to each well and were allowed to attach for 30, 60, 120, or 240 min at 37°C. In experiments in which GRGDS peptide (0.5 mg/ml) was used, the cells were preincubated with the peptide for 15 min. Unattached cells were removed by washing with PBS twice, and the remaining cells were fixed in 96% ethanol for 10 min and stained with 0.1% crystal violet in H2O for 30 min. The percentage of the cells in three microscopic fields (50 to 100 cells/field) that had a spread morphology was calculated.
Phosphoprotein blotting assay.
Petri dishes were coated with either VN (2 μg/ml), FN (10 μg/ml), LN-1 (10 μg/ml), or MAb Ha2/5 (25 μg/ml) for 1 h at 37°C and blocked with 1 mg of heat-treated BSA per ml for 1 h at 37°C. Cells (5 × 106) suspended in DMEM (attachment to VN, LN-1, or Ha2/5) or DMEM plus 0.5 mg of GRGDS peptide per ml (β1-dependent attachment to FN) were seeded in each dish and allowed to attach for the indicated periods of time. As a negative control, cells were kept in suspension at 37°C. Subsequently, the cells were lysed with ice-cold radioimmunoprecipitation assay buffer (150 mM NaCl, 20 mM Tris-HCl [pH 7.5], 1% NP-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]) containing 50 mM NaF, 30 mM Na4P2O7, 200 μM Na3VO4, 2 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM N-ethylmaleimide (NEM), 2 mM EDTA, and 1 μg of pepstatin A per ml. The lysates were aspirated three times through an injection needle and centrifuged at 21,000 × g for 10 min. The supernatant was precleared with protein A-Sepharose for 1 h at 4°C by end-over-end rotation. The precleared supernatants were incubated with 1 μg of MAb (directed against FAK, tensin, paxillin, or p130CAS) for 2 h at 4°C. The antibodies were precipitated with rabbit anti-mouse IgG and protein A-Sepharose. The pellet was washed three times with lysis buffer, and the immunoprecipitated proteins were separated on an SDS-polyacrylamide gel electrophoresis (PAGE) gel followed by transfer to nitrocellulose membrane. The membranes were blocked with 1% BSA in Tris-buffered saline–Tween (150 mM NaCl, 10 mM Tris [pH 7.4], and 0.1% Tween 20) and incubated with horseradish peroxidase-conjugated antiphosphotyrosine antibodies. Alternatively, the filters were incubated with anti-FAK pY397 or anti-FAK pY861 antibodies followed by a horseradish peroxidase-labeled anti-rabbit IgG antibody. Reactive bands were detected with enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech). Controls for equal loading were performed either by running half of the immunoprecipitated material on a separate gel followed by transfer to nitrocellulose membranes and blotting for the immunoprecipitated protein (phosphotyrosine blots) or by stripping the membrane followed by blotting for the immunoprecipitated protein (FAK pY397 and pY861 blots).
FAK activity assay.
Cells were stimulated by adhesion as described for the phosphotyrosine blotting assay, and the kinase activity of FAK was measured as previously described (35). Briefly, cells were solubilized in lysis buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 0.5% NP-40, 1% Triton X-100, 1 mM EGTA, 1 mM EDTA, 1 mM PMSF, and 200 μM Na3VO4), FAK was immunoprecipitated, and the pellet was washed twice with lysis buffer and twice with kinase buffer (10 mM Tris [pH 7.5], 10 mM MnCl2, 2 mM MgCl2, and 0.05% Triton X-100). In vitro phosphorylation was performed for 15 min at room temperature in 15 μl of kinase buffer containing 5 μCi of [γ-32P]ATP. The samples were subjected to SDS-PAGE, fixed with methanol-acetic acid, treated with 1 M KOH at 55°C for 1 h, fixed again, dried, and exposed on X-ray film.
SH2 domain pull-down assays.
Cells were either kept in suspension or allowed to adhere to anti-β1 antibodies as described in the phosphotyrosine blotting assay and lysed in a mixture of 20 mM HEPES (pH 7.5), 3 mM MgCl2, 2 mM EDTA, 40 mM NaF, 40 mM Na4P2O7, 1% Triton X-100, 10% glycerol, 200 μM Na3VO4, 1 mM NEM, 1 mM PMSF, 10 μg of leupeptin per ml, and 10 μg of pepstatin A per ml. The lysates were precleared by end-over-end rotation with glutathione-Sepharose beads (Amersham Pharmacia Biotech) for 1 h at 4°C. The precleared samples were incubated with glutathione-Sepharose and 10 μg of either GST-Crk SH2 or GST-Src SH2 for 2 h at 4°C. The pellets were washed three times with lysis buffer and subsequently boiled in sample buffer. The precipitated material was separated on SDS-PAGE (8% polyacrylamide) gels and transferred to nitrocellulose membranes, and blotting was performed as described for the phosphotyrosine blotting assay. To ensure that equal amounts of protein were subjected to the pull-down in each sample, the protein concentration of the cell lysates was determined by the bicinchoninic acid method (data not shown).
Immunofluorescent staining of cells.
Cells were allowed to attach and spread for 1 h in DMEM on glass coverslips coated with ECM proteins. Coating with FN or LN-1 was done by incubating the coverslips overnight at 4°C with 10 μg of FN or 100 μg of LN-1 per ml in PBS. The coated coverslips were blocked with 1% BSA in PBS. Cells were fixed with 2% paraformaldehyde in PBS for 10 min, washed three times for 10 min each with PBS, and immunostained as described previously (52).
RESULTS
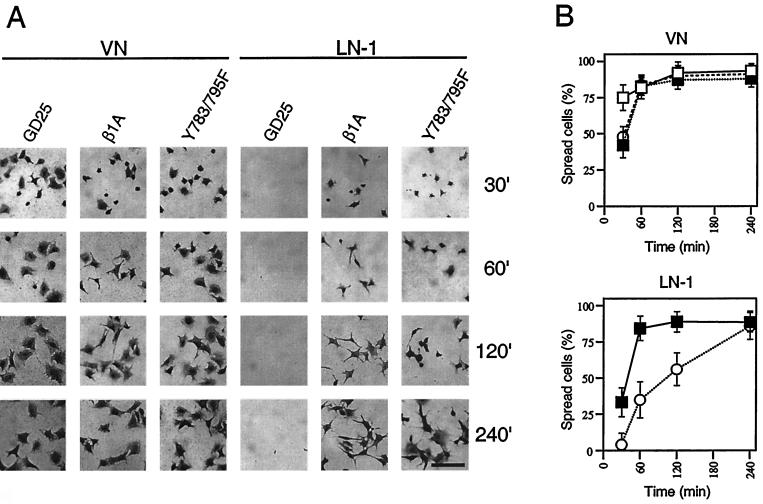
GD25-β1AY783/795F cells exhibit delayed spreading.
To assess the effect of the Y783/795F double mutant on cell spreading, GD25, GD25-β1A, and GD25-β1AY783/795F cells were allowed to attach and spread on VN (an αVβ3- and αVβ5-dependent substrate), on LN-1 (α6β1 dependent), and on FN in the presence of GRGDS peptide (α5β1 dependent) for 30 to 240 min. As illustrated in Fig. 1, the β1-expressing cell lines spread at a similar rate on VN. In contrast, on LN-1 (Fig. 1), the GD25-β1AY783/795F cells exhibited a marked delay in spreading compared to the GD25-β1A cells, while the GD25 cells (lacking β1 integrins) did not attach at all. After 4 h, the mutant cells were able to attain the same degree of spreading as the wild-type cells. The same difference in rate of spreading between the wild-type and the double tyrosine mutant cells was obtained when the cells were plated on FN in the presence of 0.5 mg of GRGDS peptide per ml (data not shown), which completely blocks αVβ3- and αVβ5-mediated adhesion to FN, while the α5β1-mediated adhesion is largely unaffected (52).
FIG. 1.
Effects of wild-type and mutant β1 integrins on spreading of GD25 cells. Phase-contrast images of cells (A) and calculated percentages (B) of spread cells of GD25 (□), GD25-β1A (■), and GD25-β1AY783/795F (○) cells on VN and LN-1 after different times of attachment (30, 60, 120, and 240 min). The spreading assay was performed as described in Materials and Methods. Bar, 100 μm.
The β1AY783/795F mutant is defective in signaling to FAK.
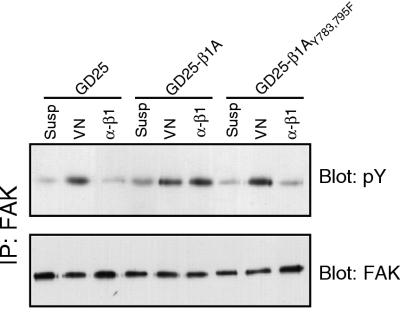
FAK has been implicated in the regulation of integrin-mediated spreading and cell migration (18). To investigate the molecular mechanisms for the slow spreading and nonmigratory phenotype of the GD25 cells expressing the double tyrosine mutation Y783/795F in β1A, we tested the ability of these cells to mediate activation of FAK as monitored by measuring tyrosine phosphorylation of FAK following adhesion to an appropriate matrix. Plating of GD25, GD25-β1A, and GD25-β1AY783/795F cells on anti-β1 antibodies resulted in a pronounced increase in tyrosine phosphorylation of FAK in the wild-type β1A cells, but not in the GD25 cells, which failed to attach under these conditions (Fig. 2). The GD25-β1AY783/795F cells attached equally well as the GD25-β1A cells, as previously reported (38), but only a very small increase in FAK tyrosine phosphorylation was observed compared to the level for cells in suspension. Identical results were obtained when the wild-type and mutant cells were plated on FN plus GRGDS and on LN-1. However, we feel that plating cells on anti-β1 antibodies is a more specific way to look at signals exclusively derived from β1 integrins than plating on ECM proteins, where other cell surface molecules could be involved in binding to the substrate. To demonstrate that the impairment in FAK tyrosine phosphorylation in response to adhesion via the β1AY783/795F mutant was not due to a general FAK defect in these cells, we plated the three cell lines on VN and monitored αVβ3- and αVβ5-induced FAK phosphorylation (Fig. 2). All three cell lines exhibited similar levels of tyrosine phosphorylation of FAK on this substrate, illustrating that the defect in FAK phosphorylation in the GD25-β1AY783/795F cells plated on β1-dependent substrates was a consequence of the mutant integrin.
FIG. 2.
Tyrosine phosphorylation of FAK in cells expressing wild-type or Y783/795F mutant β1A. GD25, GD25-β1A, and GD25-β1AY783/795F cells were either kept in suspension (Susp) or were attached to VN (VN) or to anti-β1 antibody (α-β1) for 60 min. FAK phosphorylation was detected by immunoprecipitation (IP) of FAK followed by an immunoblotting for total phosphotyrosine (pY [upper panel]). The loading control was performed by blotting for FAK (lower panel).
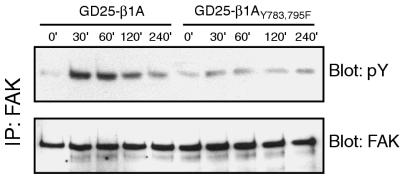
Since the GD25-β1AY783/795F cells show a reduced rate of spreading via their β1 integrins, it was possible that this contributed to the defect in FAK phosphorylation. However, this explanation is unlikely, since neither the wild-type β1A nor the β1AY783/795F cells adhering to β1 antibodies spread efficiently (data not shown), and yet we observed the same difference in FAK phosphorylation seen when the cells were plated on FN plus GRGDS or LN-1. To exclude delayed activation as a cause for the reduced tyrosine phosphorylation of FAK in the mutant cells at 60 min after plating, different times of attachment to anti-β1 antibodies (Fig. 3) were tested. In the GD25-β1A cells, tyrosine phosphorylation of FAK peaked around 30 to 60 min after seeding and slowly declined to the background level during the following hours. In the GD25-β1AY783/795F cells, no obvious peak in FAK phosphorylation was seen at any time point up to 4 h after plating. Identical results were obtained on FN plus GRGDS and LN-1 (data not shown).
FIG. 3.
Kinetics of FAK tyrosine phosphorylation. GD25-β1A and GD25-β1AY783/795F cells were plated on anti-β1 antibodies for 0, 30, 60, 120, and 240 min. Immunoprecipitates (IP) were run on SDS-PAGE, transferred to nitrocellulose membranes, and blotted for phosphotyrosine (pY [upper panel]) and FAK (lower panel).
Single tyrosine substitutions do not dramatically affect FAK activation or cell spreading.
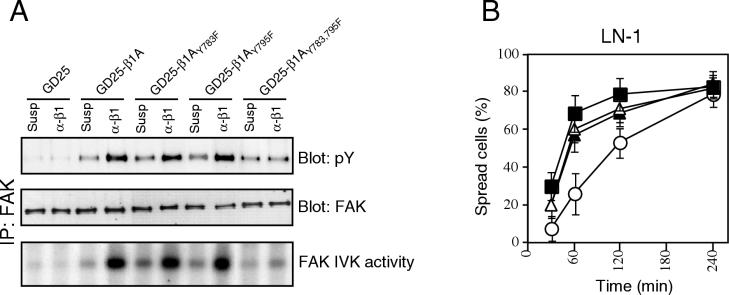
To test if the effect seen on β1-dependent FAK tyrosine phosphorylation and cell spreading with the β1AY783/795F cells was the result of substituting both tyrosines within the tail or whether this would result from single-tyrosine substitutions, we also tested the ability of GD25-β1AY783F and GD25-β1AY795F cells to mediate FAK activation and cell spreading via β1 integrins. In this system, neither of the single-tyrosine mutations had a dramatic effect on β1-dependent FAK tyrosine phosphorylation and activation (Fig. 4A) or β1-dependent cell spreading (Fig. 4B) compared to that in wild-type cells. Only minor differences were observed compared to the level in the wild-type cells, and no obvious hierarchy of the importance of the two tyrosines could be detected, showing that the defects seen in the GD25-β1AY783/795F cells required mutation of both tyrosines to phenylalanine.
FIG. 4.
Comparison of single versus double tyrosine mutations on FAK activation and cell spreading. (A) FAK was immunoprecipitated (IP) from GD25, GD25-β1A, GD25-β1AY783F, GD25-β1AY795F, and GD25-β1AY783/795F cells either kept in suspension (Susp) or attached to anti-β1 antibody (α-β1) for 60 min. The precipitates were analyzed by blotting for phosphotyrosine (pY [upper panel]) and FAK (middle panel) and for in vitro kinase (IVK) activity (lower panel, FAK band is shown). (B) Percentages of spread cells were calculated for the cell lines GD25-β1A (■), GD25-β1AY783F (▵), GD25-β1AY795F (▴), and GD25-β1AY783/795F (○) after different times of attachment to LN-1.
FAK autophosphorylation is defective in the GD25- β1AY783/795F cells.
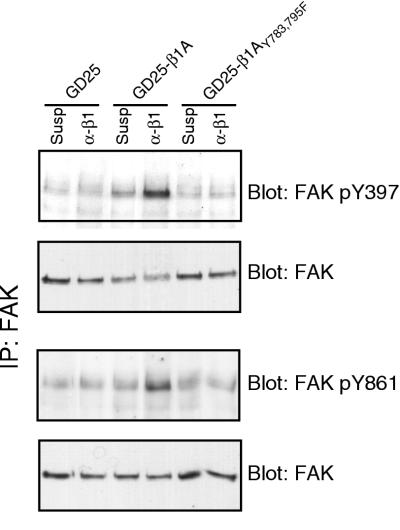
FAK activation involves both autophosphorylation and phosphorylation by Src family kinases at several different sites (6, 34). To elucidate at which level the defect of FAK phosphorylation in the double tyrosine mutant occurs, we used antibodies specifically directed to either the autophosphorylation site, pY397, or the major Src phosphorylation site, pY861, in FAK. In GD25-β1A cells, both tyrosines 397 and 861 were phosphorylated in response to adhesion to β1 MAb (Fig. 5). In contrast, negligible phosphorylation levels of either Y397 or Y861 were detected in the β1A double tyrosine mutant cells in response to β1-mediated attachment (Fig. 5). These data strongly suggest that the reduced phosphotyrosine content of FAK in the Y783/795F mutant cells is due to a defect in β1-dependent autophosphorylation of FAK at tyrosine 397, which presumably also blocks the ability of Src to efficiently phosphorylate FAK on additional sites such as tyrosine 861.
FIG. 5.
Phosphorylation of FAK on tyrosines 397 and 861. GD25-β1A and GD25-β1AY783/795F cells were kept in suspension (Susp) or attached to anti-β1 antibodies (α-β1) for 60 min. FAK was immunoprecipitated (IP), and blots were performed with antibodies specific for FAK phosphotyrosines (pY) 397 and 861, respectively. Loading controls for each precipitation are shown.
Differential phosphorylation of paxillin, tensin, and p130CAS by β1 integrins.
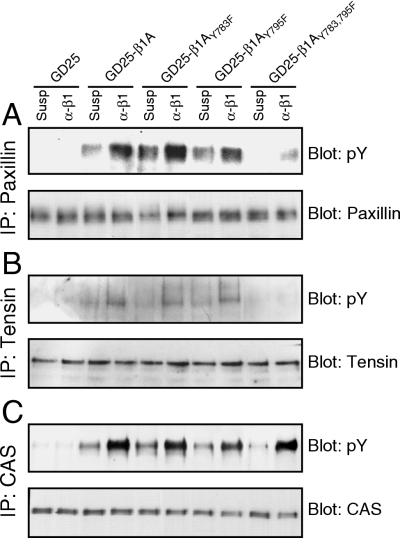
The downstream effects of impaired FAK activation in the GD25-β1AY783/795F cells were studied by analyzing the adhesion-dependent tyrosine phosphorylation of paxillin, tensin, and p130CAS, three substrates for FAK or the FAK-Src complex (2, 31, 41). When plated on VN, all three proteins became tyrosine phosphorylated in both GD25-β1A and GD25-β1AY783/795F cells (data not shown). Attachment to anti-β1 antibodies induced phosphorylation of all three proteins in the wild-type β1A, β1AY783F, and β1AY795F cells (Fig. 6). In contrast, with GD25-β1AY783/795F cells, there was an absence of β1-dependent tyrosine phosphorylation of paxillin and tensin, consistent with the observed defect in integrin β1-mediated FAK activation in the double tyrosine mutant. On the other hand, p130CAS was strongly tyrosine phosphorylated in the GD25-β1AY783/795F cells in response to β1-mediated adhesion. The same results were obtained when the cells were allowed to attach to FN plus GRGDS or LN-1 (data not shown). These data indicate that β1-mediated p130CAS tyrosine phosphorylation can occur through a signaling pathway that is distinct from that used for tyrosine phosphorylation of paxillin and tensin.
FIG. 6.
Integrin β1A-dependent phosphorylation of paxillin, tensin, and p130CAS. GD25, GD25-β1A, GD25-β1AY783F, GD25-β1AY795F, and GD25-β1AY783/795F cells were either kept in suspension (Susp) or attached to anti-β1 antibodies (α-β1) for 60 min. Immunoprecipitates (IP) of paxillin (A), tensin (B), and p130CAS (C) were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and blotted for phosphotyrosine (pY [upper panels]) and the precipitated protein (lower panels).
p130CAS is phosphorylated by FAK-dependent and -independent pathways.
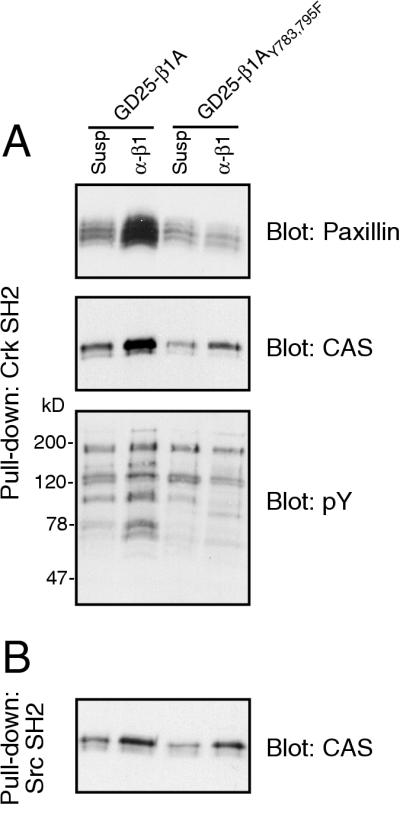
To further elucidate the role of the defective FAK activation by the β1AY783/795F mutant, we used a fusion protein consisting of GST coupled to the SH2 domain of Crk to pull down binding proteins from the lysates of GD25-β1A and GD25-β1AY783/795F cells (Fig. 7). Paxillin was clearly precipitated from adherent wild-type-expressing cells, but not from the double tyrosine mutant cells. More surprisingly, the amount of p130CAS precipitated from adherent cells was strongly reduced in the Y783/795F cells compared to that in the wild-type cells. This result suggests that although no change in the adhesion-dependent overall tyrosine phosphorylation of p130CAS was detected by using broad-specificity reagents in GD25-β1AY783/795F cells, the specific phosphorylation site(s) responsible for binding of p130CAS to Crk may be less efficiently generated. In pull-downs blotted for phosphotyrosine, we could detect reduced precipitation of proteins with approximate sizes of 100, 150, and 190 kDa in the GD25-β1AY783/795F cells, in addition to bands likely to correspond to paxillin and p130CAS (70 to 80 and 130 kDa, respectively). We also tested the ability of the SH2 domain of Src to pull down p130CAS (Fig. 7). In contrast to the Crk SH2 domain, the adhesion-dependent binding of p130CAS to Src SH2 was only slightly reduced in the GD25-β1AY783/795F cells compared to that in the GD25-β1A cells, indicating that these two SH2 domains bound to different phosphorylation sites. In this respect, the Crk SH2-binding site most likely represents a FAK-Src-dependent phosphorylation site, while the Src SH2-binding site might represent a FAK-independent site.
FIG. 7.
Pull-down of tyrosine-phosphorylated proteins with SH2 domains. GD25-β1A and GD25-β1AY783/795F cells were kept in suspension (Susp) or attached to anti-β1 antibodies (α-β1) for 60 min, lysed, and incubated with GST-Crk SH2 (A) or GST-Src SH2 (B) fusion proteins bound to glutathione-Sepharose. Precipitates were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and blotted for the indicated proteins. pY, phosphotyrosine; CAS, p130CAS.
FAK localizes to FAs in GD25-β1AY783/795F cells.
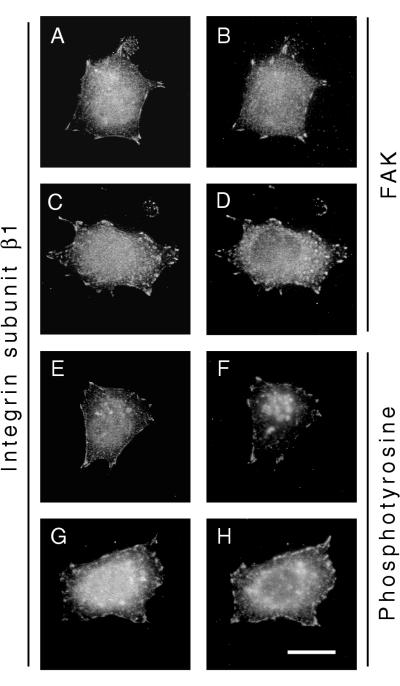
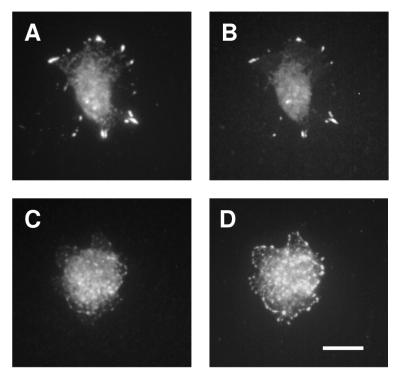
When cells were allowed to attach for 60 min either to FN in the presence of GRGDS peptide or to LN-1, immunostaining of FAK revealed that it still localized to FAs in both GD25-β1AY783/795F cells and GD25-β1A cells (Fig. 8A to D). The phosphotyrosine content of the FAs of GD25-β1AY783/795F cells looked similar to that of GD25-β1A cells, indicating that there are other active tyrosine kinases in the FAs of these cells (Fig. 8E to H). In addition, vinculin, talin, paxillin, and tensin localized to FAs in both wild-type β1A- and β1AY783/795F-expressing cells (data not shown). In contrast, when stained for FAK pY397 (Fig. 9) and pY861 (data not shown), a prominent signal in FAs was observed in GD25-β1A cells (Fig. 9A), whereas only weak staining was detected in FAs of GD25-β1AY783/795F cells (Fig. 9C). Thus, although localization of FAK to FAs has been shown to be an essential step for integrin-dependent activation of the enzyme (44), our results emphasize that additional regulation of FAK signaling occurs at these sites.
FIG. 8.
Subcellular localization of FAK and phosphotyrosine-containing proteins. Immunofluorescent stainings of GD25-β1A (A, B, E, and F) and GD25-β1AY783/795F (C, D, G, and H) cells attached to FN plus GRGDS for 1 h are shown. Double staining for integrin subunit β1 (A and C) and FAK (B and D) and integrin subunit β1 (E and G) and phosphotyrosine (F and H) was performed as described in Materials and Methods. Bar, 20 μm.
FIG. 9.
Detection of FAK tyrosine phosphorylation in FAs. FAK pY397 was detected by immunofluorescence in GD25-β1A (A and B) and GD25-β1AY783/795F (C and D) cells attached to FN plus GRGDS for 1 h. The cells were double stained for FAK pY397 (A and C) and FAK (B and D). Bar, 20 μm.
DISCUSSION
In this study, we have shown that GD25 cells expressing integrin subunit β1A containing the Y783/795F mutation are defective in activating FAK, most likely due to a loss in autophosphorylation of the tyrosine 397. These cells were previously shown to be able to attach and spread via the mutated β1 integrins, but they contained an increased number of FAs, had an altered cytoskeletal architecture, and were defective in migration (38). In this report, we have also now demonstrated that the β1AY783/795F mutant cells exhibit delayed spreading compared to cells expressing wild-type integrin. Thus, the phenotype of GD25 cells expressing integrin subunit β1AY783/795F is in several ways similar to that of the FAK knockout fibroblasts (18, 34). The effects of the FAK knockout suggested that FAK is involved in the turnover of FA and thereby promotes cell spreading and migration. Cell spreading has been suggested to be triggered by FAK-Src phosphorylation of paxillin (37), while the role of FAK in cell migration was reported to be dependent on PI 3-kinase, which can bind to pY397 in FAK via its SH2 domain (36), and p130CAS, which can bind via its SH3 domain to the FAK-Src complex and become phosphorylated (8, 21).
In the GD25-β1AY783/795F cells, no β1-dependent tyrosine phosphorylation of paxillin and tensin occurred. Contrary to our results, phosphorylation of these proteins was not reduced in FAK-null cells compared to FAK-expressing fibroblasts (18, 50). This discrepancy may be due to upregulation of the FAK-related kinase Pyk2 (also known as CAKβ, CADTK, or RAFTK) in the FAK-null cells, which in this context can partially compensate by carrying out FAK functions (45).
Although GD25-β1AY783/795F cells clearly have a defect in β1 integrin-dependent FAK activation, FAK is still present in FAs, indicating that the defect is not at the level of gross cellular localization. As a model to explain the impaired FAK activation in the GD25-β1AY783/795F cells, we therefore suggest that the mutation of the two tyrosines in the cytoplasmic tail of integrin β1A prevents the interaction with one or more other proteins, which are required to create a functional complex that stimulates FAK autophosphorylation and activation. When single Y-to-F mutants were tested (β1AY783F and β1AY795F), only minor effects on β1-dependent FAK phosphorylation and activation, paxillin phosphorylation, tensin phosphorylation, and cell spreading were observed. These results emphasize that the defect seen in the GD25-β1AY783/795F cells is an effect of removing both tyrosines in the carboxyl-terminal part of the cytoplasmic domain.
The fact that overall tyrosine phosphorylation levels of the FAK-Src substrate p130CAS are not affected by the Y783/795F double mutation in β1 is intriguing. We do not know the identity of the kinase responsible for carrying out this integrin-dependent phosphorylation. However, p130CAS is considered to be phosphorylated mainly by Src (14, 47, 50), and it has been reported that Src family kinases can become activated by integrin ligation independently of FAK activation (9, 43). The p130CAS phosphorylation detected by generic phosphotyrosine-directed antibodies in the GD25-β1AY783/795F cells might therefore depend on integrin-mediated activation of Src molecules, which are not in complex with activated FAK. However, by using two different SH2 domains to bind p130CAS, we were able to detect differential phosphorylation of p130CAS. Interestingly, the binding site of Crk SH2 domain seems to largely coincide with FAK activation, and the binding site of Src SH2 seems to be independent of the activation of the FAK-Src complex. Our observations illustrate the complex nature of integrin-mediated signaling and support previous reports, which indicate that tyrosine phoshorylation of p130CAS can occur through several independent pathways (4, 12, 30).
Detection of tyrosine-phosphorylated β1 has so far only been made in transformed cells (17, 19). Integrin subunit β3, on the other hand, is tyrosine phosphorylated at residues corresponding to Y783 and Y795 in β1 after ligating αIIbβ3 and αVβ3 integrins (1, 25), and these two tyrosines have been shown to play an important role in platelet function (24). Various results illustrating the effect on FAK activation by single Y-to-F mutations in β3 have been reported (39, 48), while the effect on FAK activation of double Y-to-F mutants has not been studied for β3. Our results strongly indicate that the hydroxyl moieties of the two tyrosines in the integrin subunit β1A are of critical importance, possibly by serving as phosphate acceptors. The roles of tyrosine phosphorylation of the β1A cytoplasmic tail in relation to FAK activation and FA dynamics in transformed and nontransformed cells are pressing questions that are being pursued.
ACKNOWLEDGMENTS
We thank Helena Larsson and Ivan Dikic, Uppsala, Sweden, for support and helpful discussions.
This work was supported by grants from Polysackaridforskning AB, Uppsala, Sweden (K.W.), the Swedish Medical Research Council (no. 7147) (S.J.), Swedish Cancer Foundation (no. 4158) (S.J.), King Gustaf V:s 80-års Fond (S.J. and R.F.), the National Institutes of Health (HL 21644 and HL 56396) (D.M.), and the Swedish Natural Science Research Council (R.F.).
REFERENCES
- 1.Blystone S D, Lindberg F P, Williams M P, McHugh K P, Brown E J. Inducible tyrosine phosphorylation of the β3 integrin requires the αV integrin cytoplasmic tail. J Biol Chem. 1996;271:31458–31462. doi: 10.1074/jbc.271.49.31458. [DOI] [PubMed] [Google Scholar]
- 2.Bockholt S M, Burridge K. Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. J Biol Chem. 1993;268:14565–14567. [PubMed] [Google Scholar]
- 3.Bottger B A, Hedin U, Johansson S, Thyberg J. Integrin-type fibronectin receptors of rat arterial smooth muscle cells: isolation, partial characterization and role in cytoskeletal organisation and control of differentiated properties. Differentiation. 1989;41:158–167. doi: 10.1111/j.1432-0436.1989.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 4.Bouton A H, Burnham M R. Detection of distinct pools of the adapter protein p130CAS using a panel of monoclonal antibodies. Hybridoma. 1997;16:403–411. doi: 10.1089/hyb.1997.16.403. [DOI] [PubMed] [Google Scholar]
- 5.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 6.Calalb M B, Polte T R, Hanks S K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cary L A, Guan J L. Focal adhesion kinase in integrin-mediated signaling. Front Biosci. 1999;4:D102–D113. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- 8.Cary L A, Han D C, Polte T R, Hanks S K, Guan J L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark E A, King W G, Brugge J S, Symons M, Hynes R O. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig S W, Johnson R P. Assembly of focal adhesions: progress, paradigms, and portents. Curr Opin Cell Biol. 1996;8:74–85. doi: 10.1016/s0955-0674(96)80051-2. [DOI] [PubMed] [Google Scholar]
- 11.Dedhar S, Hannigan G E. Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Curr Opin Cell Biol. 1996;8:657–669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- 12.Eisenmann K M, McCarthy J B, Simpson M A, Keely P J, Guan J L, Tachibana K, Lim L, Manser E, Furcht L T, Iida J. Melanoma chondroitin sulphate proteoglycan regulates cell spreading through Cdc42, Ack-1 and p130cas. Nat Cell Biol. 1999;1:507–513. doi: 10.1038/70302. [DOI] [PubMed] [Google Scholar]
- 13.Fässler R, Pfaff M, Murphy J, Noegel A A, Johansson S, Timpl R, Albrecht R. Lack of β1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamasaki K, Mimura T, Morino N, Furuya H, Nakamoto T, Aizawa S, Morimoto C, Yazaki Y, Hirai H, Nojima Y. Src kinase plays an essential role in integrin-mediated tyrosine phosphorylation of Crk-associated substrate p130Cas. Biochem Biophys Res Commun. 1996;222:338–343. doi: 10.1006/bbrc.1996.0745. [DOI] [PubMed] [Google Scholar]
- 15.Hibbs M L, Jakes S, Stacker S A, Wallace R W, Springer T A. The cytoplasmic domain of the integrin lymphocyte function-associated antigen 1 β subunit: sites required for binding to intercellular adhesion molecule 1 and the phorbol ester-stimulated phosphorylation site. J Exp Med. 1991;174:1227–1238. doi: 10.1084/jem.174.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hibbs M L, Xu H, Stacker S A, Springer T A. Regulation of adhesion of ICAM-1 by the cytoplasmic domain of LFA-1 integrin β subunit. Science. 1991;251:1611–1613. doi: 10.1126/science.1672776. [DOI] [PubMed] [Google Scholar]
- 17.Hirst R, Horwitz A, Buck C, Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci USA. 1986;83:6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 19.Johansson M W, Larsson E, Luning B, Pasquale E B, Ruoslahti E. Altered localization and cytoplasmic domain-binding properties of tyrosine-phosphorylated β1 integrin. J Cell Biol. 1994;126:1299–1309. doi: 10.1083/jcb.126.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klemke R L, Leng J, Molander R, Brooks P C, Vuori K, Cheresh D A. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klint P, Kanda S, Claesson-Welsh L. Shc and a novel 89-kDa component couple to the Grb2-Sos complex in fibroblast growth factor-2-stimulated cells. J Biol Chem. 1995;270:23337–23344. doi: 10.1074/jbc.270.40.23337. [DOI] [PubMed] [Google Scholar]
- 23.LaFlamme S E, Thomas L A, Yamada S S, Yamada K M. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law D A, DeGuzman F R, Heiser P, Ministri-Madrid K, Killeen N, Phillips D R. Integrin cytoplasmic tyrosine motif is required for outside-in αIIbβ3 signalling and platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 25.Law D A, Nannizzi-Alaimo L, Phillips D R. Outside-in integrin signal transduction. αIIbβ3-(GP IIb IIIa) tyrosine phosphorylation induced by platelet aggregation. J Biol Chem. 1996;271:10811–10815. doi: 10.1074/jbc.271.18.10811. [DOI] [PubMed] [Google Scholar]
- 26.Leong L, Hughes P E, Schwartz M A, Ginsberg M H, Shattil S J. Integrin signaling: roles for the cytoplasmic tails of αIIbβ3 in the tyrosine phosphorylation of pp125FAK. J Cell Sci. 1995;108:3817–3825. doi: 10.1242/jcs.108.12.3817. [DOI] [PubMed] [Google Scholar]
- 27.Lewis J M, Schwartz M A. Mapping in vivo associations of cytoplasmic proteins with integrin β1 cytoplasmic domain mutants. Mol Biol Cell. 1995;6:151–160. doi: 10.1091/mbc.6.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longhurst C M, Jennings L K. Integrin-mediated signal transduction. Cell Mol Life Sci. 1998;54:514–526. doi: 10.1007/s000180050180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcantonio E E, Guan J L, Trevithick J E, Hynes R O. Mapping of the functional determinants of the integrin β1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990;1:597–604. doi: 10.1091/mbc.1.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murakami H, Iwashita T, Asai N, Iwata Y, Narumiya S, Takahashi M. Rho-dependent and -independent tyrosine phosphorylation of focal adhesion kinase, paxillin and p130Cas mediated by Ret kinase. Oncogene. 1999;18:1975–1982. doi: 10.1038/sj.onc.1202514. [DOI] [PubMed] [Google Scholar]
- 31.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y, et al. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 32.O'Toole T E, Katagiri Y, Faull R J, Peter K, Tamura R, Quaranta V, Loftus J C, Shattil S J, Ginsberg M H. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Toole T E, Mandelman D, Forsyth J, Shattil S J, Plow E F, Ginsberg M H. Modulation of the affinity of integrin αIIbβ3 (GPIIb-IIIa) by the cytoplasmic domain of αIIb. Science. 1991;254:845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- 34.Owen J D, Ruest P J, Fry D W, Hanks S K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol Cell Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi J H, Ito N, Claesson-Welsh L. Tyrosine phosphatase SHP-2 is involved in regulation of platelet-derived growth factor-induced migration. J Biol Chem. 1999;274:14455–14463. doi: 10.1074/jbc.274.20.14455. [DOI] [PubMed] [Google Scholar]
- 36.Reiske H R, Kao S C, Cary L A, Guan J L, Lai J F, Chen H C. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J Biol Chem. 1999;274:12361–12366. doi: 10.1074/jbc.274.18.12361. [DOI] [PubMed] [Google Scholar]
- 37.Richardson A, Malik R K, Hildebrand J D, Parsons J T. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakai T, Zhang Q, Fässler R, Mosher D F. Modulation of β1A integrin functions by tyrosine residues in the β1 cytoplasmic domain. J Cell Biol. 1998;141:527–538. doi: 10.1083/jcb.141.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaffner-Reckinger E, Gouon V, Melchior C, Plancon S, Kieffer N. Distinct involvement of β3 integrin cytoplasmic domain tyrosine residues 747 and 759 in integrin-mediated cytoskeletal assembly and phosphotyrosine signaling. J Biol Chem. 1998;273:12623–12632. doi: 10.1074/jbc.273.20.12623. [DOI] [PubMed] [Google Scholar]
- 40.Schaller M D, Hildebrand J D, Shannon J D, Fox J W, Vines R R, Parsons J T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaller M D, Parsons J T. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlaepfer D D, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- 43.Schlaepfer D D, Jones K C, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen Y, Schaller M D. Focal adhesion targeting: the critical determinant of FAK regulation and substrate phosphorylation. Mol Biol Cell. 1999;10:2507–2518. doi: 10.1091/mbc.10.8.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sieg D J, Ilic D, Jones K C, Damsky C H, Hunter T, Schlaepfer D D. Pyk2 and src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but pyk2 does not fully function to enhance FAK-cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smilenov L, Forsberg E, Zeligman I, Sparrman M, Johansson S. Separation of fibronectin from a plasma gelatinase using immobilized metal affinity chromatography. FEBS Lett. 1992;302:227–230. doi: 10.1016/0014-5793(92)80447-o. [DOI] [PubMed] [Google Scholar]
- 47.Tachibana K, Urano T, Fujita H, Ohashi Y, Kamiguchi K, Iwata S, Hirai H, Morimoto C. Tyrosine phosphorylation of Crk-associated substrates by focal adhesion kinase. A putative mechanism for the integrin-mediated tyrosine phosphorylation of Crk-associated substrates. J Biol Chem. 1997;272:29083–29090. doi: 10.1074/jbc.272.46.29083. [DOI] [PubMed] [Google Scholar]
- 48.Tahiliani P D, Singh L, Auer K L, LaFlamme S E. The role of conserved amino acid motifs within the integrin β3 cytoplasmic domain in triggering focal adhesion kinase phosphorylation. J Biol Chem. 1997;272:7892–7898. doi: 10.1074/jbc.272.12.7892. [DOI] [PubMed] [Google Scholar]
- 49.Vuori K. Integrin signaling: tyrosine phosphorylation events in focal adhesions. J Membr Biol. 1998;165:191–199. doi: 10.1007/s002329900433. [DOI] [PubMed] [Google Scholar]
- 50.Vuori K, Hirai H, Aizawa S, Ruoslahti E. Introduction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for Src family kinases. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wennerberg K, Fässler R, Wärmegåard B, Johansson S. Mutational analysis of the potential phosphorylation sites in the cytoplasmic domain of integrin β1A. Requirement for threonines 788–789 in receptor activation. J Cell Sci. 1998;111:1117–1126. doi: 10.1242/jcs.111.8.1117. [DOI] [PubMed] [Google Scholar]
- 52.Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fässler R. β1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]