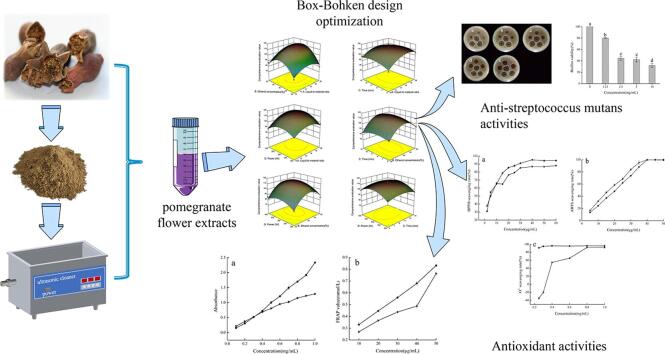
Graphical abstract
Keywords: Punica granatum L. flowers, Pomegranate flowers, Ultrasonic-assisted extraction, Box-Bohnken design, Streptococcus mutans, Antioxidant activities
Highlights
-
•
Unpetaled pomegranate flowers are usually discarded, rich in resources and valuable for exploitation.
-
•
Ultrasound-assisted extraction of active ingredients from pomegranate flowers.
-
•
Ultrasound-assisted extraction of active ingredients from pomegranate flowers.
Abstract
This study was designed to optimize the extraction rate of total polyphenols and ellagic acid from pomegranate flowers. Single factors were investigated for liquid-to-material ratio (5–25), ethanol concentration (20%–60%), sonication time (5–60 min), and sonication power (150–500 W). The level range of the Box-Bokhen design was determined with respect to the single-factor results. The components of each index were normalized using the entropy weighting method for obtaining the comprehensive evaluation value. Under the actual conditions, the final optimization results were 17 for liquid-to-material ratio, 43% for ethanol concentration, 10 min for ultrasonic time, and 300 W for ultrasonic power. The extracts obtained under optimal conditions were tested for the inhibition of Streptococcus mutans and its biofilm, and results showed that pomegranate flowers exerted some inhibitory effects on the bacterium. Phosphomolybdenum and FRAP assays were used, and DPPH, ABTS, and •O2− radical scavenging tests were conducted, indicating that pomegranate flower extracts have good antioxidant capacity.
1. Introduction
Pomegranate flowers are flowers of deciduous shrubs or small trees Pomegranate, a plant of the genus Pomegranate [1]. According to relevant literature [2], [3], [4], [5], pomegranates are rich in phenols, tannins, sugars, pigments, and trace elements, and polyphenols are the most abundant. Crude pomegranate extracts have good pharmacological effects, such as antioxidant, anti-aging, hypoglycemic, hypotensive, hypolipidemic, and anti-atherosclerosis [6], [7], [8]. In early summer, bell-shaped flowers that cannot bear fruit usually detaches when the pomegranate flowers first appear in the buds, and most of those flowers that fail to germinate properly during subsequent growth also drop off naturally. Hence, there is an abundance of available pomegranate flowers.
Currently, domestic and international studies [6], [9] are mainly focused on pomegranate peels and seeds, and little research has been done on pomegranate flowers, followed by research on the essential oil and red pigment of pomegranate flowers with petals as the research object [10], [11]. Pomegranate flower polyphenols have significant antioxidant activity [12] and functions, such as lowering blood pressure and blood lipids [13], preventing atherosclerosis and inhibiting cancer cell proliferation [14]. Ellagic acid is a polyphenol widely present in the form of free ellagic acid in the tissues of various soft fruits and nuts, along with its diverse derivatives. Therefore, this experiment was conducted to analyze polyphenols and ellagic acid in pomegranate flowers. Traditional extraction methods, such as leaching, percolation, and reflux usually have long extraction time and insufficient extraction yield [15], [16]. The ultrasound-assisted extraction of active ingredients is easy to operate and suitable for this experiment. In addition, Streptococcus mutansand and free radicals are associated with a variety of oral diseases. The production and scavenging of free radicals in a normal healthy organism shows a dynamic balance, while in pathological states, free radicals increase dramatically and the organism is damaged.
In a nutshell, two indicator components (polyphenols and ellagic acid) of pomegranate flowers were optimized for the determination of optimal extraction conditions, and the antibacterial and antioxidant effects of best conditions of the extracts were investigated.
2. Material and methods
2.1. Chemicals and reagents
Standards of gallic acid, ellagic acid, corilagin (HPLC grade, ≥98%) were purchased from Purify (Chengdu, China). Acetonitrile, methanol, ortho-phosphoric acid were obtained from and Fisher (Fair Lawn, U.S.A). DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt), TPTZ (2,4,6-Tri(2-pyridyl)-s-triazine) and ascorbic acid (Vitamin C) were obtained from Solarbio (Beijing, China). Folin-Ciocalteu agent was derived from Wobisen (Beijing, China). Sodium penicillin were purchased from Yuanye Bio-Technology (Shanghai, China). Ecko ultrapure water machine (Chengdu, China) prepared pure ultra-pure water. Other reagents of analytical purity are purchased from Fuyu (Tianjin, China).
2.2. Preparation of raw material
The samples of this experiment were un-petaled pomegranate flowers, collected from Hotan, Xinjiang, and identified by Chief Pharmacist Li Yonghe of the College of TCM, Xinjiang Medical University. The pistils and stamens were removed from the flowers, and then the samples were crushed and the powder was passed through a 50 mesh sieve and set aside and put into a desiccator for use.
Streptococcus mutans ATCC76100 were obtained from Collaborative Innovation Center, Xinjiang Medical University (Xinjiang, China).
2.3. Extraction procedure
A KQ5200DE (40 KHz) numerical control ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd., Kunshan, Jiangsu, China) was used for extraction. Approximately 0.5 g of herbal powder was placed in a 50 mL centrifuge tube, and ethanol was added according to the material-to-liquid ratio. Extraction was performed using specific ultrasonic power and ultrasonic time. After the extraction, the solution was centrifuged and filtered, and the supernatant was collected for the next operation.
2.4. Measurement method of indicator components
2.4.1. Folin-Ciocalteu method determination of total polyphenol content (TPC)
Total polyphenol content (TPC) was determined, and the method adopted was obtained from the literature and slightly modified [17], [18], [19]. Pomegranate flower extracts obtained with different process parameters were determined. Each solution was added to a volumetric flask as follows: 0.2 mL of test solution, 1 mL of Folin-Ciocalteu reagent, and 2 mL of 10% Na2CO3. A final volume of 25 mL was fixed. The solution was placed in a 75 °C water bath for 10 min, and then the absorbance was measured at 760 nm.. TPC was described as milligrams of gallic acid per gram of powder.
2.4.2. HPLC-DAD determination of ellagic acid content (EAC)
An Agilent 1200-DAD high-performance liquid chromatograph with a C18 reversed-phase column (WondaSil, 4.6 × 250 mm, 5 μm) was used in chromatographic analysis. The chromatographic procedure was adopted with the following conditions: flow rate, 0.8 mL/min; column temperature, 25 °C; detection wavelength, 254 nm; injection volume, 2 μL; mobile phase of acetonitrile (A) of 0.1% phosphoric acid aqueous solution (B), 0–25 min, 85% B-72% B, gradient elution [20], [21]. Ellagic acid content (EAC) was expressed as milligrams of ellagic acid per gram of powder.
2.5. Optimization of processes
2.5.1. Single-factor experimental design
The default extraction conditions [22], [23] were as follows: liquid-to-material ratio, 15; ethanol concentration, 40%; ultrasonic time, 10 min; and ultrasonic power, 300 W. The other conditions were kept constant when one of the factors was examined. The effects of four factors: liquid-to-material ratio (5, 10, 15, 20, and 25), ethanol concentration (20%, 30%, 40%, 50%, and 60%), sonication time (5, 10, 20, 40, and 60 min), and sonication power (150, 200, 300, 400, and 500 W), on the yield of total polyphenols and ellagic acid from pomegranate flowers were investigated. A circulating water unit was set up to prevent the influence of sonication temperature on the experiment and ensure a constant temperature.
2.5.2. Response surface design
A Box-Bohnken (B-B) design was used in optimizing the pomegranate flower extraction parameters [24], [25], [26]. Different factors with high and low levels are shown in Table S1. A four-factor three-level experimental design was performed. Liquid-to material-ratio (A), ethanol concentration (B), sonication time (C), and power (D) were selected as investigating factors. High level was represented by 1, whereas low level was represented by − 1. TPC and EAC were used as evaluation indexes.
2.5.3. Calculation of comprehensive evaluation value (CEV) by entropy weighting method (EWM)
The entropy weighting method (EWM) is an objective weighting method for determining target weights, and information entropy, as a measure of the confusion of a system, can be used in identifying the weights of each target in a multi-index evaluation system and the amount of information contained in data [27], [28], [29]. The content data of each index component from the B-B test were normalized (index component = [measured value − minimum value]/[maximum value − minimum value]), and the weight coefficients of each component were calculated with EWM after the influence of scale and magnitude among the indexes were eliminated, so as to obtain the comprehensive evaluation value (CEV).
2.6. Inhibitory activity of pomegranate flower extracts against Streptococcus mutans
The dried extracts were extracted and dried for antibacterial and antioxidant tests with the optimal process of B-B test.
2.6.1. Inhibition circle test
The antibacterial activities of pomegranate flower extracts at different concentrations were assayed by using the agar diffusion method [30], [31]. Exactly 100 μL of bacterial solution at a concentration of 1 × 106CFU/mL was added to BHI agar plates. The extracts were dissolved in BHI and solubilized with an appropriate amount of Tween 80 to obtain 50–3.15 mg/mL with the twofold dilution method. Six wells were punched on each plate with a puncher, and 40 μL of pomegranate flower extracts were added. The plates were placed at an environment with temperature of 37 °C and 5% CO2.
The plates were observed after 24 h. Penicillin sodium was considered as the positive reference, and the negative reference was BHI. An inhibition circle with a diameter of <6 mm indicated absence of inhibition activity because the perforator aperture was 6 mm. The sensitivity of the inhibition circles were categorized according to their diameters as follows: greater than 20 mm, extremely sensitive; <10 mm, resistant; 15–20 mm, highly sensitive; and 10–15 mm, moderately sensitive.
2.6.2. Evaluation of bacterial biofilm formation
In the 96-well plate, 20 μL of each pomegranate flower extract was added, which was mixed with 80 μL of bacterial solution (1 × 106 CFU/mL). The culture solution was used as the negative control, and three wells in each group were parallel. The 96-well plates were placed in an apparatus with constant temperature (37 °C) and 5% CO2 concentration. The biofilm was analyzed with the crystalline violet staining method [32], [33], [34]. After 48 h, planktonic bacteria in the well plates were discarded. The well plates were washed with PBS or sterile water. Then, 100 μL of 2% methylcellulose was added, and the solution was allowed to set for 15 min. The plates were washed and dried, and 100 μL of 1% crystal violet solution was added to stain the biofilm. It was repeated to wash off and dry after 15 min. Finally, 2% sodium deoxycholate was used to ablate the biofilm. After 30 min, OD600 were measured. Biofilm viability was calculated as follows: Biofilm(%) = exacts group/negative group × 100.
IBM SPSS statistics 21 was selected for data analysis of bacteria and bacterial biofilms, and Tukey’s test (p < 0.05) was chosen to analyze the variability of different extracts concentration groups.
2.7. Antioxidant capacity
The phosphomolybdenum method was used in determining the total antioxidant capacity (TAC) according to a previously described method with slight modification [35], [36]. The working solution consisted of 0.6 mol/L sulfuric acid, 28 mmol/L sodium phosphate, and 4 mmol/L ammonium molybdate. Each extract (0.4 mL) was mixed with 4 mL of working solution in a test tube. A constant temperature water bath was used in the subsequent steps and set to 95 °C. The test tubes were allowed to set for 90 min before being removed. The reaction solutions were cooled, and 200 µL of each reaction solution was transferred to a 96-well plate. The data of the extracts were recorded at 695 nm, and absorbance was used to express TAC.
Ferric ion reducing antioxidant power (FRAP) was used according to the methods in the literature [37], [38]. In this assay, exactly 180 μL of the FRAP working solution (prepareded with reference [37]) was gently mixed with 5 μL of each extract and left for 5 min. The data of the extracts were recorded at 593 nm. A standard curve was plotted with the FeSO4 solution instead of the sample solution for the test. The fitted equation was y = 0.3607x + 0.4385 (r = 0.9990), indicating that 0.15–1.5 mmol/L FeSO4 solution showed a good linear correlation with absorbance. The FRAP value was expressed as the concentration of the standard substance FeSO4 solution.
The DPPH radical scavenging assay was used according to the literature [39], [40], [41]. After the preparation of a 0.1 mmol/L DPPH solution, 100 μL of each sample was mixed with 100 μL of DPPH, and the solution was stored away from light for 30 min. The detection wavelength was set at 517 nm for the reaction solution.
The method was based on previous tests and modified for the ABTS free radical scavenging assay [39], [42]. An ABTS stock solution was made by mixing 10 mmol/L ABTS solution and 10 mmol/L K2S2O8 and stored away from light for 16 h. The working solution was made by diluting the stock solution to an absorbance of 0.70 ± 0.02. Then, 100 μL of each gradient sample solution and 100 μL of ABTS working solution were thoroughly mixed, and the mixture was set for 15 min at room temperature away from light. The detection wavelength was set at 734 nm for reaction solution.
The •O2− radical scavenging test was performed according to a previously described method [43], [44]. The reaction was carried out by mixing 2.5 mL of 50 mmol/L Tris-HCl buffer with 1 mL of sample solution in a test tube and placing the resulting solution in a water bath at 25 °C for 20 min. Then, 3 mmol/L o-triphenol (dissolved in 10 mmol/L HCl) was added for 6 min. The reaction was discontinued by adding 0.1 mL of 8 mol/L HCl. A UV spectrophotometer was used in the measurement and set at 320 nm.
Ascorbic acid was used as the positive control in all the above tests, and DPPH, ABTS, •O2− radical scavenging rate (%) = [1 − (A2 − A1)]/A0 × 100. A2 was the extract solution with the blank solvent, A1 was the sample solution with the working solution, and A0 was the blank solvent with the free radical working solution.
3. Results and discussion
3.1. Determination of indicator components
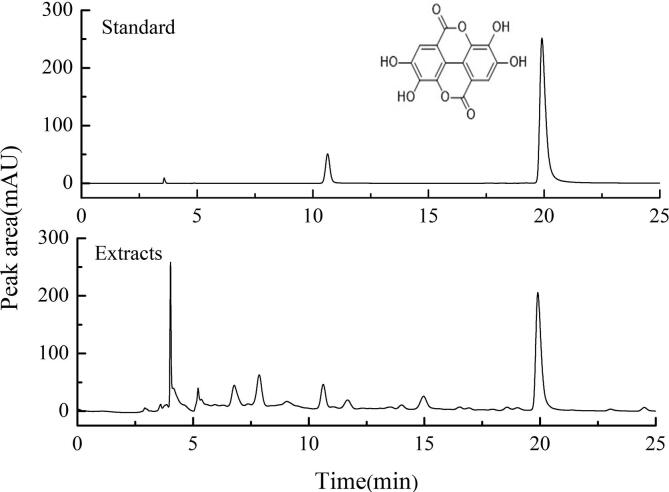
The standard curve of gallic acid is shown in Fig. S1 while that of ellagic acid is in Fig. S2. And the HPLC chromatograms are shown in Fig. 1.
Fig. 1.
HPLC chromatograms of standard (ellagic acid) and extracts (pomegranate flower).
3.2. Single-factor test
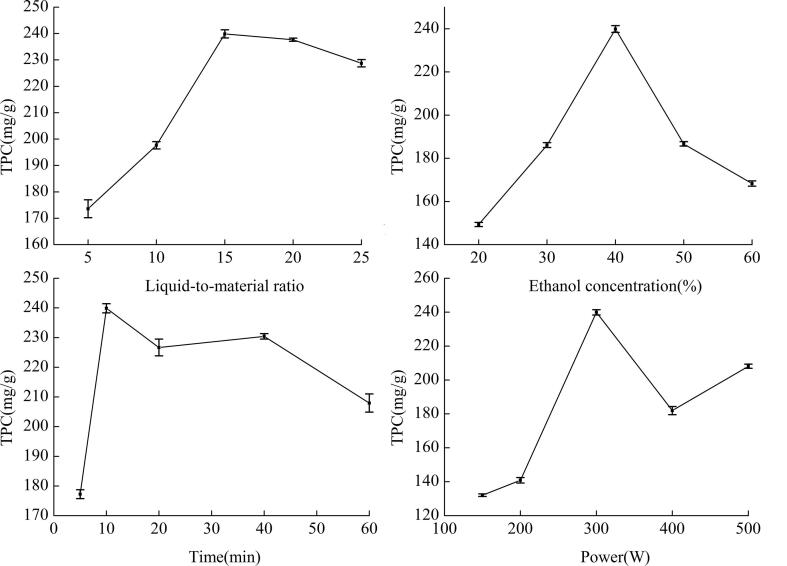
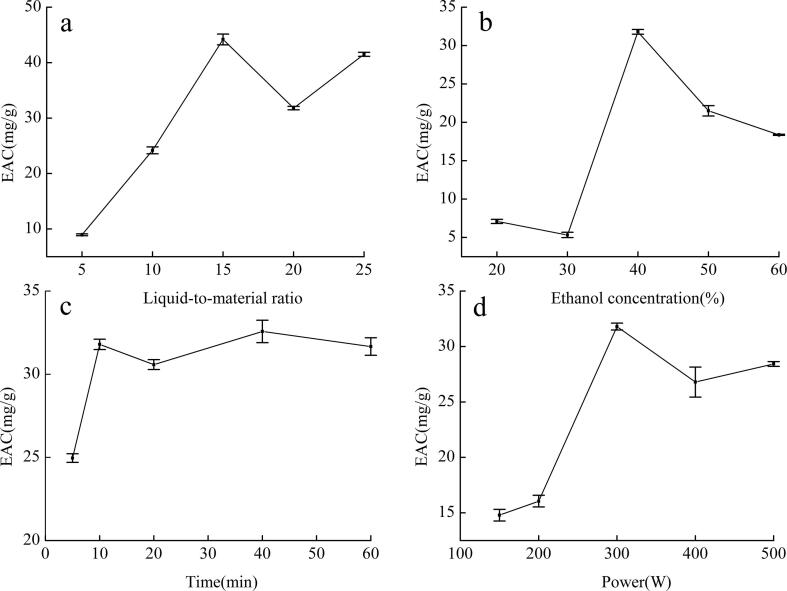
The effect of each factor on extraction rate was elucidated with single-factor tests. The results are shown in Fig. 2, Fig. 3. The trend of pomegranate flower extract yields under different conditions was visualized.
Fig. 2.
Effect of different factors on extraction rate of total polyphenols (TPC: total polyphenol content).
Fig. 3.
Effect of different factors on extraction rate of ellagic acid (EAC: ellagic acid content).
As shown in Figs. 2a and 3a, the liquid-to-material ratio significantly influenced the extraction rate. The extraction rate showed an increasing trend as the liquid-to-material ratio gradually increased. The reasons were increases in the amount of powder to be wetted and in the contact area between the liquid and material as the amount of solvent increased. Increase in concentration difference in the inner and outer cells contributed to the dissolution of the active substance. However, the contribution of this factor to the extraction rate was nearly negligible when the liquid-to-material ratio of TPC exceeded 15 and EAC exceeded 25. In this case, most of the effective substances had been extracted after the liquid-to-material ratio reached a certain level. Therefore, further increase in the amount of the solvent would cause waste.
As shown in Figs. 2b and 3b, the extraction rate reached its highest value when the ethanol concentration was 40%. According to the principle of similar solubility, the higher the concentration of ethanol is, the less polar it is. The extraction rate of the effective substances decreased when the ethanol concentration was above or below 40%.
The extraction rate increased between 5 and 10 min. The maximum value was reached at 10 min, during which the maximum extraction speed was observed. When the time exceeded 10 min, TPC decreased slightly, whereas EAC tended to fluctuate within the error range, and their extraction rates became flat. These effects may be due to the strong mechanical shearing effect of ultrasound. These effect caused the destruction of polyphenol structures, the dissolution of impurities, and decrease in active ingredients.
The effect of power is shown in Figs. 2d and 3d. The extraction rate reached its highest value when the power was 300 W. Ultrasonic action on a liquid can produce a large number of small bubbles. Among the reasons are the local tensile stress in the liquid and the formation of negative pressure. Decrease in pressure leads to the oversaturation of gas originally dissolved in the liquid. The gas eventually escapes from the liquid and forms a small bubble. Another reason, known as the cavitation effect, is the strong tensile stress of the liquid “torn” into a cavity. When ultrasonic power increases, ultrasonic energy increases, and the “ultrasonic cavitation” phenomenon occurs. When energy reaches a threshold, the sharp collapse of small bubbles in the liquid generates a high local temperature and pressure, which destroy plant cells and thereby promote the release of active substances. When power is lower than 300 W, extraction rate is low because power density is small at a low ultrasound power, the sound pressure is below the cavitation threshold cavitation effect, and the cavitation effect is not obvious. Therefore, an active substance cannot be completely dissolved. When power exceeds 300 W, extraction rate decreases slightly possibly because of an excessive cavitation effect at an extremely high power, which increases the temperature near the probe, and the dissolution of more impurities. As a result, extraction rate decreases.
3.3. Box-Bokhen design
The results of the single-factor test showed that all four factors had significant effects on TPC and EAC. A four-factor three-level B-B test was designed according to analysis results of the single-factor test. The factor level table is shown in Table S1. Weighting values of 0.4274 (TPC) and 0.5726 (EAC) were obtained through EWM. The overall scores under different test numbers were calculated according to CEV = 0.4274 TPC + 0.5726 EAC. The test results are shown in Table 1.
Table 1.
Box-Behnken Response Surface Test Design and Results.
| Run | A | B(%) | C(min) | D(W) | Y1(mg/g) | Y2(mg/g) | CEV |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 218.53 | 31.54 | 111.46 |
| 2 | 1 | −1 | 0 | 0 | 176.77 | 7.10 | 79.62 |
| 3 | −1 | 0 | 0 | −1 | 183.06 | 21.98 | 90.83 |
| 4 | 0 | 0 | 0 | 0 | 229.02 | 30.62 | 115.42 |
| 5 | 1 | 0 | 1 | 0 | 208.33 | 32.54 | 107.67 |
| 6 | 0 | 0 | −1 | −1 | 188.75 | 30.94 | 98.39 |
| 7 | 0 | −1 | 0 | −1 | 176.03 | 6.29 | 78.84 |
| 8 | −1 | −1 | 0 | 0 | 169.40 | 6.55 | 76.16 |
| 9 | 0 | 1 | 0 | 1 | 202.52 | 21.28 | 98.75 |
| 10 | −1 | 0 | 0 | 1 | 179.53 | 20.13 | 88.26 |
| 11 | 0 | 0 | 0 | 0 | 223.72 | 32.37 | 114.16 |
| 12 | −1 | 1 | 0 | 0 | 172.70 | 22.44 | 86.67 |
| 13 | 1 | 1 | 0 | 0 | 209.74 | 28.04 | 105.70 |
| 14 | 0 | 1 | 1 | 0 | 201.11 | 24.28 | 99.86 |
| 15 | 0 | 1 | −1 | 0 | 193.69 | 21.22 | 94.94 |
| 16 | 0 | 0 | 0 | 0 | 219.83 | 29.95 | 111.11 |
| 17 | 1 | 0 | 0 | 1 | 221.52 | 24.07 | 108.46 |
| 18 | 1 | 0 | −1 | 0 | 206.45 | 29.34 | 105.04 |
| 19 | 0 | 0 | 1 | 1 | 201.82 | 24.82 | 100.47 |
| 20 | 0 | −1 | −1 | 0 | 195.46 | 9.26 | 88.85 |
| 21 | 1 | 0 | 0 | −1 | 187.13 | 29.10 | 96.65 |
| 22 | 0 | 0 | −1 | 1 | 203.23 | 22.86 | 99.96 |
| 23 | −1 | 0 | 1 | 0 | 191.07 | 28.85 | 98.18 |
| 24 | 0 | −1 | 0 | 1 | 190.87 | 7.92 | 86.11 |
| 25 | 0 | 0 | 1 | −1 | 190.16 | 26.94 | 96.70 |
| 26 | 0 | 1 | 0 | −1 | 171.08 | 21.58 | 85.48 |
| 27 | 0 | −1 | 1 | 0 | 186.98 | 8.32 | 84.68 |
| 28 | 0 | 0 | 0 | 0 | 227.25 | 32.37 | 115.67 |
| 29 | −1 | 0 | −1 | 0 | 170.34 | 26.85 | 88.18 |
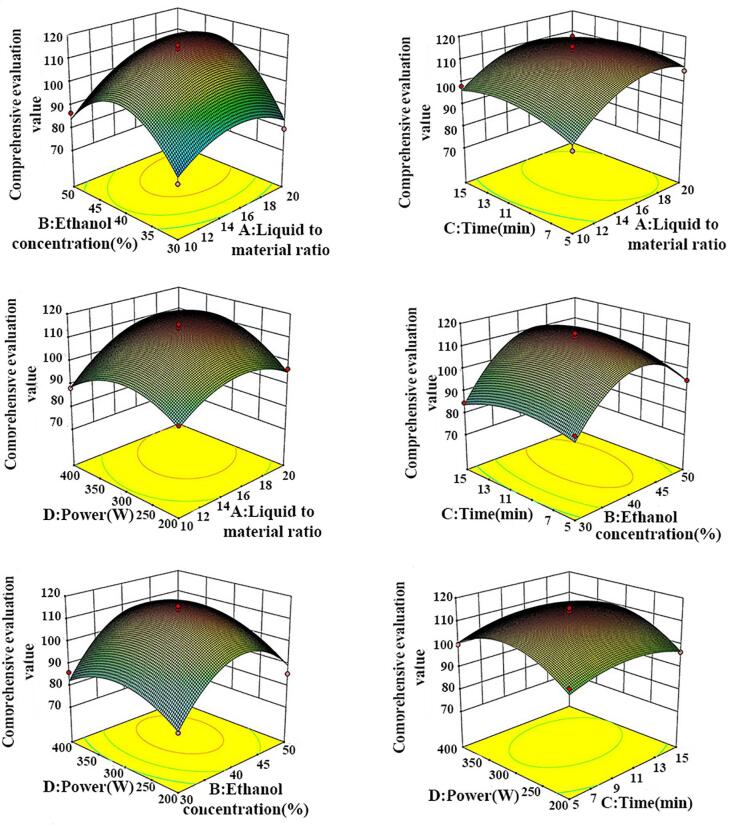
Design-Expert 10. 0. 7 software was used in the response surface experimental design in Table 1. Analysis of variance (ANOVA) was applied in the model. The equation was as follows: CEV = 113.56 + 6.24 A + 6.43B + 1.02C + 2.93 D + 3.89 AB − 1.84 AC + 3.60 AD + 2.27 BC + 1.50 BD + 0.55CD − 8.87 A2 − 17.09 B2 − 4.93 C2 − 9.19 D2 (R2 = 0.9644) after the multiple quadratic regression. The ANOVA result is presented in Table 2. The difference between R2Pred (0.8166) and R2Adj (0.9289) indicated that the two were in reasonable agreement, which was <0.2 [16]. The F-value was 27.12, which indicated that the model was significant. The lack of fit term was not significant, indicating good fit and predictive power. A, B, D, AB, AD, A2, B2, C2, and D2 were significant model terms, suggesting the absence of a simple linear relationship between the factors and CEV. Three-dimensional surface diagrams of the change in CEV with factors are shown in Fig. 4, which illustrates the impression of each factor and any two factor interactions on CEV. After comparing the F-values of different factors and the curvature of the 3D response surface, we concluded that the most influential factor on the extraction rate was ethanol concentration, followed by the liquid-to-material ratio, power, and time.
Table 2.
ANOVA for Response Surface Quadratic model
| Source | Squares | df | Square | Value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 3520.91 | 14 | 251.49 | 27.12 | <0.0001 | significant |
| A | 467.1 | 1 | 467.10 | 50.38 | <0.0001 | significant |
| B | 495.82 | 1 | 495.82 | 53.47 | <0.0001 | significant |
| C | 12.44 | 1 | 12.44 | 1.34 | 0.2661 | |
| D | 102.84 | 1 | 102.84 | 11.09 | 0.0050 | significant |
| AB | 60.64 | 1 | 60.64 | 6.54 | 0.0228 | significant |
| AC | 13.57 | 1 | 13.57 | 1.46 | 0.2464 | |
| AD | 51.71 | 1 | 51.71 | 5.58 | 0.0332 | significant |
| BC | 20.64 | 1 | 20.64 | 2.23 | 0.1579 | |
| BD | 8.97 | 1 | 8.97 | 0.97 | 0.3420 | |
| CD | 1.21 | 1 | 1.21 | 0.13 | 0.7229 | |
| A2 | 510.68 | 1 | 510.68 | 55.08 | <0.0001 | significant |
| B2 | 1895.5 | 1 | 1895.50 | 204.43 | <0.0001 | significant |
| C2 | 157.96 | 1 | 157.96 | 17.04 | 0.0010 | significant |
| D2 | 547.58 | 1 | 547.58 | 59.06 | <0.0001 | significant |
| Residual | 129.81 | 14 | 9.27 | |||
| Lack of Fit | 111.17 | 10 | 11.12 | 2.38 | 0.2087 | not significant |
| Pure Error | 18.65 | 4 | 4.66 | |||
| Cor Total | 3650.72 | 28 |
Fig. 4.
Three-dimensional response surface diagrams of the influence of different factors on the composite evaluation value.
The best conditions for the extraction process were A = 17.268, B = 42.581, C = 10.463, D = 327.235, and CEV = 116.25. The optimal extraction parameters were adjusted to A = 17, B = 43, C = 10, and D = 300 for practical purposes. On the bsis of these conditions, three validation tests were conducted. The average CEV was 114.70 (RSD < 1%) with a deviation of 0.34% from the model prediction. This value indicated that the extraction process had good reproducibility and operability.
3.4. Antibacterial activities
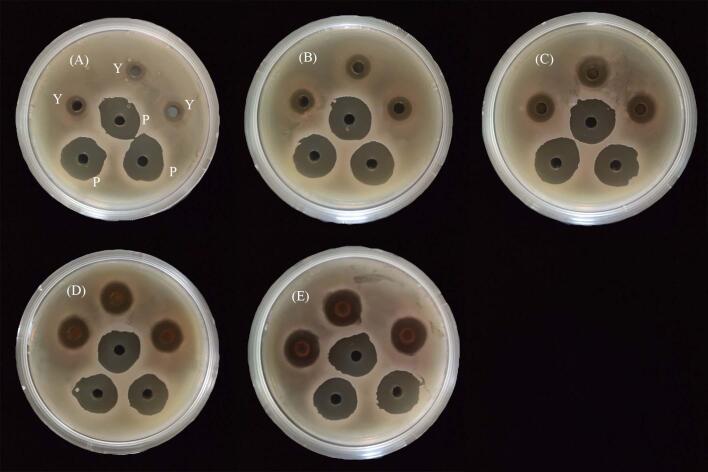
According to the method in Section 2.6.1, the sizes of the inhibition circles of the extracts acting on S. mutans at different concentrations were obtained. The results are shown in Fig. 5 and Table 3. The inhibition circle increased with extract concentration, indicating that the concentration of the pomegranate flower extracts was correlated with inhibition ability. As indicated in Table 3, the positive drug was extremely sensitive, the 50 mg/mL extract was highly sensitive, the 6.25–25 mg/mL extracts were moderately sensitive, and the 3.125 mg/mL extract was resistant. Tukey's test showed significant differences among the groups (p < 0.05), indicating that a correlation existed between concentration and the sensitivity of S. mutans to the extracts.
Fig. 5.
Inhibition circle of Streptococcus mutans ((A): 3.125 mg/mL extracts plus positive control; (B): 6.25 mg/mL extracts plus positive control; (C): 12.5 mg/mL extracts plus positive control; (D): 25 mg/mL extracts plus positive control; (E): 50 mg/mL extracts plus positive control; Y: different concentration exacts; P: positive control).
Table 3.
Diameter of inhibition circle of extracts.
| Concentration | Diameter (mm) |
|---|---|
| Sodium penicillin | 20.23 ± 0.15a |
| 50 mg/mL | 16.50 ± 0.10b |
| 25 mg/mL | 14.43 ± 0.15c |
| 12.5 mg/mL | 12.6 ± 0.30d |
| 6.25 mg/mL | 10.73 ± 0.15e |
| 3.125 mg/mL | 9.37 ± 0.20f |
Note: identical letters indicate no significant difference at p < 0.05.
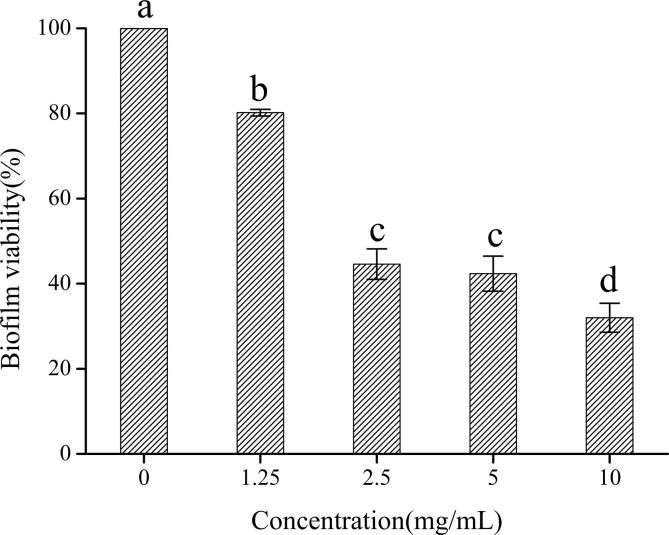
The S. mutans biofilm was tested according to the method in Section 2.6.2. The growth of the bacterial biofilm was significantly inhibited after the addition of 10, 5, 2.5, or 1.25 mg/mL solution. The results are shown in Fig. 6. The different concentrations of the extracts had inhibitory effects on the biofilm, and the overall showed some concentration correlation between extracts concentrations and biofilm viability by Tukey's test. However, no significant difference was found between the inhibitory effects of the 2.5 and 5 mg/mL extracts on the biofilm. We speculated that this result may be due to the plateauing of biofilm inhibition at a medium concentration and that concentration should be increased to achieve a significant effect.
Fig. 6.
Streptococcus mutans biofilm viability under different concentrations of extracts (Note:identical letters indicate no significant difference at p < 0.05.).
S. mutans is a major caries-causing bacterium that synthesizes insoluble extracellular polysaccharides by producing glucosyltransferases, which adhere to the tooth surface to form a bacterial biofilm that inhibits bacterial metabolic activity and protects the plaque flora against the harsh environment of the mouth. The ability of S. mutans to form biofilms is therefore more decisive in the caries-causing process than its ability to produce acid. The inhibitory effect on S. mutans and its biofilm increased with extract concentration. TPC and EAC were positively correlated with its extracts concentration, suggesting that the inhibitory effect of the extracts on bacteria was related to the polyphenolic component.
3.5. Antioxidant activities
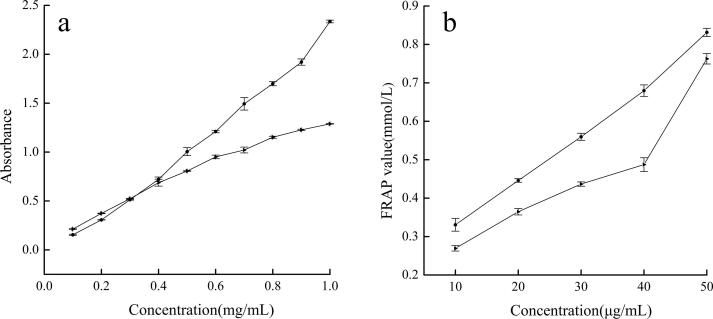
The results of the phosphomolybdenum method are presented in Fig. 7a. At a concentration of 0.4 mg/mL or lower, the TAC of ascorbic acid was not significantly different from that of an extract. As concentration increased, a large difference in the antioxidant capacity was observed between the two. The absorbance of ascorbic acid at 1.0 mg/mL was 2.33, whereas that of each extract was only 1.29, which was 0.55 times that of ascorbic acid.
Fig. 7.
the Results of total antioxidant capacity (a) and FRAP (b) of extracts (►) and ascorbic acid (●).
As shown in Fig. 7b, the FRAP values of the extracts and ascorbic acid increased with concentration, showing a positive correlation. The FRAP value of the extracts at 50 μg/mL was 0.76, which was 0.91 times that of ascorbic acid (0.83), indicating that their FRAP values were relatively close at 50 μg/mL.
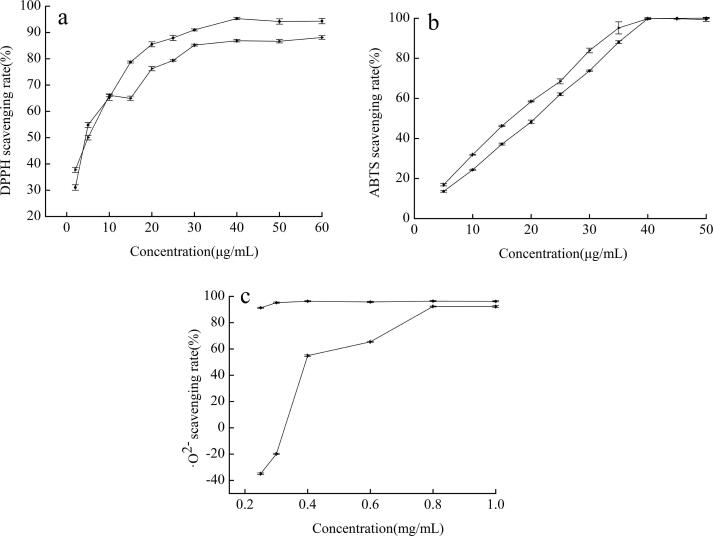
As shown in Fig. 8a, the DPPH scavenging rate increased and then stabilized with increasing concentration, and the DPPH radical scavenging rate was quantitatively related in a range of 0–30 μg/mL. When the concentrations of the extracts reached 40 μg/mL, the DPPH scavenging rate reached an inflection point, and the scavenging rate reached 86.87%. The DPPH scavenging rate of ascorbic acid reached an inflection point of 95.31% at 40 μg/mL, and the scavenging rate did not increase when the concentration increased. The maximum free radical scavenging rate of the extracts was 91.14% of that of ascorbic acid.
Fig. 8.
the Results of three free radical scavenging ability of extracts (►) and ascorbic acid (●) (a:DPPH, b:ABTS, c, •O2−).
As shown in Fig. 8b, the scavenging rate of ABTS by extracts and ascorbic acid increased and then stabilized, and the scavenging rate of ABTS radicals showed a certain quantitative-effect relationship in a concentration range of 0–40 μg/mL. The scavenging rate stabilized after the concentration of the extract increased. Notably, the IC50 of the extracts was slightly lower than that of ascorbic acid.
As shown in Fig. 8c, the •O2− radical scavenging rate of ascorbic acid reached 95.14% at a concentration of 0.3 mg/mL, and the increasing trend of the scavenging rate was not obvious when the extract concentration was increased. By contrast, the •O2− radical scavenging rate of the extracts only reached 92.26% at 0.8 mg/mL. This result indicated that the scavenging capacities of the extracts for •O2− were lower than the scavenging capacity of ascorbic acid.
4. Conclusion
The fruiting rate of pomegranate flowers is not high, and a large proportion of pomegranate flowers do not bear fruit. Un-petaled pomegranate flowers are not well utilized, and most of them are directly discarded. In this experiment, un-petaled pomegranate flowers were used, and the amounts of polyphenols and ellagic acid of their ethanol extracts were optimized with an ultrasonic process. Entropy weight method was used in determining the optimal process parameters. The ultrasonic extraction of un-petaled pomegranate flowers was superior to other traditional extraction methods, requiring a shorter time of consumption and offering a higher extraction rate. The presence of S. mutans and a large number of free radicals have adverse effects on the oral environment and increase the incidence of dental caries. The antibacterial test showed that the extracts had good inhibitory effects on S. mutans and its biofilm activity, and the antioxidant test showed that the extracts had good scavenging ability for various free radicals. This study provides some reference for the development and utilization of un-petaled pomegranate flowers.
CRediT authorship contribution statement
Wenxia Wu: Conceptualization, Methodology, Data curation, Writing – original draft. Shan Jiang: Investigation, Formal analysis, Visualization. Mengmeng Liu: Investigation, Methodology. Shuge Tian: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Financial support of this work was from Key Discipline of the 13th Five – Year Plan of Xinjiang Uygur Autonomous Region – Discipline of Integrated Traditional Chinese and Western Medicine of Xinjiang Medical University – 1006 and the Xinjiang Uygur Autonomous Region of the major science and technology projects in China (Grant Numbers 2017A03005-2).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105833.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Editorial Committee of the Flora of China, Chinese Academy of Sciences. Flora of China.
- 2.Vini R., Sreeja S. Punica granatum and its therapeutic implications on breast carcinogenesis: a review: Punica granatum and breast carcinogenesis. BioFactors. 2015;41(2):78–89. doi: 10.1002/biof.1206. [DOI] [PubMed] [Google Scholar]
- 3.Yisimayili Z., Abdulla R., Tian Q., Wang Y., Chen M., Sun Z., Li Z., Liu F., Aisa H.A., Huang C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samplesby high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A. 2019;1604:460472. doi: 10.1016/j.chroma.2019.460472. [DOI] [PubMed] [Google Scholar]
- 4.Wu W., Wang L., Tian S. Simultaneous qualitative and quantitative analyses of ursolic acid and oleanolicacid in Punica granatum L. (Pomegranate) flowers by high-performance thin-layer chromatography. JPC-J. Planar Chromatogr.-Mod. TLC. 2021;34(2):165–172. doi: 10.1007/s00764-021-00092-x. [DOI] [Google Scholar]
- 5.Suručić R., Travar M., Petković M., Tubić B., Stojiljković M.P., Grabež M., Šavikin K., Zdunić G., Škrbić R. Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 Receptor: In silico and in vitro studies. Bioorganic Chem. 2021;114:105145. doi: 10.1016/j.bioorg.2021.105145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vahid-Dastjerdi E., Monadi E., Khalighi H.R., Torshabi M. Down-Regulation of Glycosyl transferase genes in Streptococcus mutans by Punica Granatum L. flower and Rhus Coriaria L. fruit water extracts. Iran. J. Pharm. Res. 2016;15(2):513–519. [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Q., Zhang L., Cheng N., Jia M., Zhang Y. Extraction optimization of oleanolic and ursolic acids from pomegranate(Punica granatum L.) flowers. Food Bioprod. Process. 2014;92(3):321–327. doi: 10.1016/j.fbp.2012.12.006. [DOI] [Google Scholar]
- 8.Khan I., Rahman H., Abd El-Salam N.M., Tawab A., Hussain A., Khan T.A., Khan U.A., Qasim M., Adnan M., Azizullah A., Murad W., Jalal A., Muhammad N., Ullah R. Punica granatum peel extracts: HPLC fractionation and LC MS analysis to quest compoundshaving activity against multidrug resistant bacteria. BMC Complement. Altern. Med. 2017;17(1) doi: 10.1186/s12906-017-1766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva L.d.O., Garrett R., Monteiro M.L.G., Conte-Junior C.A., Torres A.G. Pomegranate (Punica granatum) peel fractions obtained by supercritical CO2 increase oxidative and colour stability of bluefish (Pomatomus saltatrix) patties treated by UV-C irradiation. Food Chem. 2021;362:130159. doi: 10.1016/j.foodchem.2021.130159. [DOI] [PubMed] [Google Scholar]
- 10.Jia X., Zhang L., Li X., Meng J., Ai Z., Li X. Analysis of the chemical constituents of essential oil from pomegranate flower and evaluation of Its free radical scavenging ability. Food Sci. 2015;36(24):152–155. https://doi.org/10.75061spkxl002-6630-201524027. [Google Scholar]
- 11.Li T., Zhang L., Jin C., Xiong Y., Cheng Y., Chen K. Pomegranate flower extract bidirectionally regulates the proliferation, differentiation and apoptosis of 3T3-L1 cells through regulation of PPARy expression mediated by PI3K-AKT signaling pathway. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110769. [DOI] [PubMed] [Google Scholar]
- 12.Yang K., Che Y., Huang S., Zhao L., Luo Y. Antioxidant activity of free and bound polyphenols from pomegranate flowers. Sci. Tech. Food Ind. 2021 doi: 10.13386/j.issn1002-0306.2021030110. [DOI] [Google Scholar]
- 13.Bekir J., Cazaux S., Mars M., Bouajila J. In vitro anti-cholinesterase and anti-hyperglycemic activities of flowers extracts from seven pomegranate varieties. Ind. Crop. Prod. 2016;81:176–179. doi: 10.1016/j.indcrop.2015.11.066. [DOI] [Google Scholar]
- 14.Zhao Y., Liu C., Ge D., Yan M., Ren Y., Huang X., Yuan Z. Genome-wide identification and expression of YABBY genes family during flower development in Punica granatum L. Gene. 2020;752:144784. doi: 10.1016/j.gene.2020.144784. [DOI] [PubMed] [Google Scholar]
- 15.Nie J., Chen D., Ye J., Lu Y., Dai Z. Optimization and kinetic modeling of ultrasonic-assisted extraction offucoxanthin from edible brown algae Sargassum fusiforme using green solvents. Ultrason. Sonochem. 2021;77:105671. doi: 10.1016/j.ultsonch.2021.105671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ismail B.B., Guo M., Pu Y., Wang W., Ye X., Liu D. Liu, Valorisation of baobab (Adansonia digitata) seeds by ultrasound assisted extraction of polyphenolics. Optimisation and comparison with conventional methods. Ultrason. Sonochem. 2019;52:257–267. doi: 10.1016/j.ultsonch.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Behbahani B.A., Shahidi F., Yazdi F.T., Mortazavi S.A., Mohebbi M. Antioxidant activity and antimicrobial effect oftarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J. Food Meas. Charact. 2017;11(2):847–863. doi: 10.1007/s11694-016-9456-3. [DOI] [Google Scholar]
- 18.Peláez-Acero A., Cobos-Velasco J.E., González-Lemus U., Espino-Manzano S.O., Aguirre-Álvarez G., González-Montiel L., Figueira A.C., Campos-Montiel R.G. Bioactive compounds and antibacterial activities in crystallized honey liquefied with ultrasound. Ultrason. Sonochem. 2021;76:105619. doi: 10.1016/j.ultsonch.2021.105619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alara O.R., Abdurahman N.H., Olalere O.A. Ethanolic extraction of flavonoids, phenolics and antioxidants fromVernonia amygdalina leaf using two-level factorial design. J. King Saud Univ. Sci. 2020;32(1):7–16. doi: 10.1016/j.jksus.2017.08.001. [DOI] [Google Scholar]
- 20.Huerga-González V., Lage-Yusty M.A., Lago-Crespo M., López-Hernández J. Comparison of methods for the study of ellagic acid in pomegranate juice beverages. Food Anal. Methods. 2015;8:2286–2293. doi: 10.1007/s12161-014-9997-1. [DOI] [Google Scholar]
- 21.Williams D.J., Edwards D., Chaliha M., Sultanbawa Y. Measuring the three forms of ellagic acid: suitability of extraction solvents. Chem. Pap. 2016;70:144–152. doi: 10.1515/chempap-2015-0193. [DOI] [Google Scholar]
- 22.Chouaibi M., Rigane K., Ferrari G. Extraction of Citrullus colocynthis L. seed oil by supercritical carbon dioxideprocess using response surface methodology (RSM) and artificial neural network (ANN) approaches. Ind. Crop. Prod. 2020;158:113002. doi: 10.1016/j.indcrop.2020.113002. [DOI] [Google Scholar]
- 23.Sharayei P., Azarpazhooh E., Zomorodi S., Einafshar S., Ramaswamy H.S. Optimization of ultrasonic- assistedextraction of astaxanthin from green tiger (Penaeus semisulcatus) shrimp shell. Ultrason. Sonochem. 2021;76:105666. doi: 10.1016/j.ultsonch.2021.105666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciric A., Krajnc B., Heath D., Ogrinc N. Response surface methodology and artificial neural network approachfor the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020;135:110976. doi: 10.1016/j.fct.2019.110976. [DOI] [PubMed] [Google Scholar]
- 25.Yu Z.-X., Zhang Y.-Y., Zhao X.-X., Yu L.i., Chen X.-B., Wan H.-T., He Y.u., Jin W.-F. Simultaneous optimization of ultrasonic-assisted extraction of Danshen for maximal tanshinone IIA andsalvianolic acid B yields and antioxidant activity: a comparative study of the response surface methodology andartificial neural network. Ind. Crop. Prod. 2021;161:113199. doi: 10.1016/j.indcrop.2020.113199. [DOI] [Google Scholar]
- 26.Palma A., Díaz M.J., Ruiz-Montoya M., Morales E., Giráldez I. Ultrasound extraction optimization for bioactive molecules from Eucalyptus globulus leaves through antioxidant activity. Ultrason. Sonochem. 2021;76:105654. doi: 10.1016/j.ultsonch.2021.105654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S., Dao T. Multi-objective optimal design of a 2-DOF flexure based mechanism using hybrid approach of grey-Taguchi coupled response surface methodology and entropy measurement. Arab. J. Sci. Eng. 2016;41(12):5215–5231. doi: 10.1007/s13369-016-2242-z. [DOI] [Google Scholar]
- 28.Fu Y., Cheng S., Chen H., Yuan T., Zhang Y., Yang J., Luo X. Study on the optimization of the extraction technology of Valeriana jatamansi rhizomes and roots based on response surface method combined with entropy weight method. J. Chin. Med. Mater. 2021;44(2):404–408. doi: 10.13863/j.issn1001-4454.2021.02.028. [DOI] [Google Scholar]
- 29.Zhao G., Hong L., Wang N., He L., Zhang S., Chen W. Optimization of Naoluo Xintong decotion extraction based on Box-Behnken response surface methodology and entropy weight method. Central South Phar. 2021:1–6. [Google Scholar]
- 30.Yan L., Wu W., Tian S. Antibacterial and antibiofilm activities of Trollius altaicus C. A. Mey. On Streptococcusmutans. Microb. Pathog. 2020;149:104265. doi: 10.1016/j.micpath.2020.104265. [DOI] [PubMed] [Google Scholar]
- 31.Jiang M., Yan L., Li K., Ji Z., Tian S. Evaluation of total phenol and flavonoid content and antimicrobial and antibiofilm activities of Trollius chinensis Bunge extracts on Streptococcus mutans. Microsc. Res. Tech. 2020;83:1471–1479. doi: 10.1002/jemt.23540. [DOI] [PubMed] [Google Scholar]
- 32.Doll K., Jongsthaphongpun K.L., Stumpp N.S., Winkel A., Stiesch M. Quantifying implant-associated biofilms: comparison of microscopic, microbiologic and biochemical methods. J. Microbiol. Methods. 2016;130:61–68. doi: 10.1016/j.mimet.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z., Liang Y., Lin S., Chen D., Li B., Li L., Deng Y. Crystal violet and XTT Assays on Staphylococcus aureus biofilm quantification. Curr. Microbiol. 2016;73(4):474–482. doi: 10.1007/s00284-016-1081-1. [DOI] [PubMed] [Google Scholar]
- 34.Alshahrani A.M., Gregory R.L. In vitro Cariostatic effects of cinnamon water extract on nicotine-inducedStreptococcus mutans biofilm. BMC Complement. Med. Ther. 2020;20:45. doi: 10.1186/s12906-020-2840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mocan A., MBabotă, Pop A., Fizeșan I., Diuzheva A., Locatelli M., Carradori S., Campestre C., Menghini L., Sisea C.R., Sokovic M., Zengin G., Păltinean R., Bădărău S., Vodnar D.C., Crisan G. Chemical constituents and biologic activities of sage species: a comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur ex Griseb. & Schenk) Schur. Antioxidants. 2020;9(6):480. doi: 10.3390/antiox9060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aktumsek A., Zengin G., Guler G.O., Cakmak Y.S., Duran A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013;55:290–296. doi: 10.1016/j.fct.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Wang R., Luo Q., Feng Y. Determination of antioxidant effects of Kaempferol by micro-model of DPPH, ABTS and FRAP assay, Guangzhou. Chem. Ind. 2021;49:58–63. [Google Scholar]
- 38.Sinan K.I., Mahomoodally M.F., Eyupoglu O.E., Etienne O.K., Sadeer N.B., Ak G., Behl T., Zengin G. HPLC-FRAP methodology and biological activities of different stem bark extracts of Cajanus cajan (L.) Millsp. J. Pharm. Biomed. Anal. 2021;192:113678. doi: 10.1016/j.jpba.2020.113678. [DOI] [PubMed] [Google Scholar]
- 39.Chouaibi M., Rezig L., Hamdi S., Ferrari G. Chemical characteristics and compositions of red pepper seed oils extractedby different methods. Ind. Crop. Prod. 2019;128:363–370. doi: 10.1016/j.indcrop.2018.11.030. [DOI] [Google Scholar]
- 40.Sobuj M.K.A., Islam M.A., Haque M.A., Islam M.M., Alam M.J., Rafiquzzaman S.M. Evaluation of bioactive chemical composition, phenolic, and antioxidant profiling of different crude extracts of Sargassum coriifolium and Hypnea pannosa seaweeds. J. Food Meas. Charact. 2021;15(2):1653–1665. doi: 10.1007/s11694-020-00758-w. [DOI] [Google Scholar]
- 41.Silva V., Falco V., Dias M.I., Barros L., Silva A., Capita R., Alonso-Calleja C., Amaral J.S., Igrejas G., Ferreira I.C.F.R., Poeta P. Evaluation of the phenolic profile of Castanea sativa Mill. by-products and their antioxidant and antimicrobial activity against multiresistant bacteria. Antioxidants. 2020;9:87. doi: 10.3390/antiox9010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sundaram V., Sadhasivam S., Chandrasekaran S., Nanjian R., Pandian A. Strobilanthes heyneanus root extract as a potential source for antioxidant and antimicrobial activity. Futur. J. Pharm. Sci. 2021;7:91. doi: 10.1186/s43094-021-00242-2. [DOI] [Google Scholar]
- 43.Tewari R.K., Kumar P., Sharma P.N. Antioxidant responses to enhanced generation of superoxide anion radical andhydrogen peroxide in the copper-stressed mulberry plants. Planta. 2006;223(6):1145–1153. doi: 10.1007/s00425-005-0160-5. [DOI] [PubMed] [Google Scholar]
- 44.Lanez T., Henni M. Antioxidant activity and superoxide anion radical interaction with 2-(ferrocenylmethylamino) benzonitrile and 3-(ferrocenylmethylamino) benzonitrile. J. Iran. Chen. Soc. 2016;13(9):1741–1748. doi: 10.1007/s13738-016-0891-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.