Abstract
Background
Advanced glycation end products (AGE), one of the main factors causing diabetic end-organ damage, accumulate in long half-life proteins, such as skin and cartilage collagen. AGE measurement may offer additional evidence to predict diabetic vascular complications. Skin autofluorescence (SAF) is suggested as a non-invasive, quick, and reliable method to measure tissue AGE level. The aim of this study was to review and evaluate evidence on the clinical validation of SAF measurement in diabetes mellitus (DM) patients.
Methods
In this systematic review and meta-analysis, we searched "PubMed" (MEDLINE) and "Cochrane" databases from their inception to 10 August 2021 for observational studies concerning SAF measurement in diabetic patients. The following key terms were used in advanced searching: “Diabetes”, “Diabetes Mellitus”,” DM”, “Glycation “, “Advanced Glycation End product”, “AGE”, “skin autofluorescence”, “SAF”. Published studies that included DM patients and estimated their AGE using SAF were considered eligible for meta-analysis. Articles that were editorials, study proposals, congress posters, or case reports and were not on human subjects were excluded. We used a random-effect models for meta-analyzing the clinical validation of SAF in DM with particular emphasis on chronic diabetes complications.
Findings
We identified 881 records and twenty-nine records fulfilled our eligibility criteria and were included in the systematic review and meta-analysis. A statistically significant correlation was found between SAF and diabetes last HbA1c 0.21(0.13,0.28) in studies with substantial heterogeneity (I2=77.99%, p<0.05). Nevertheless, a significant positive association between SAF level and diabetic retinopathy (DR) [(OR= 1.05, 95% CI=1.03,1.08), (I2=63.78%, p<0.05)], diabetic peripheral neuropathy (DPN) [(OR= 1.11, 95%CI= 1.06,1.16), (I2=79.17%, p<0.05)], diabetic nephropathy (DNP) [(OR= 1.08, 95%CI: 1.05,1.11), (I2=65.36%, p<0.05)] and diabetic macrovascular events (D-MVE) [(OR=1.08, 95%CI=1.05,1.11) (I2=67.32, p<0.05)] were found.
Interpretation
Our study confirmed the significance of SAF measurement as a non-invasive surrogate marker of DM micro and macrovascular complications. Skin AGE estimation may be a useful factor for the prediction and early detection of irreversible DM complications. More studies with larger populations and longer follow-up periods are required.
Keywords: Diabetes Mellitus, Skin autofluorescence, Advanced glycation end product, Diabetic microvascular complication, Diabetic macrovascular complication
Research in context.
Evidence before this study
Several studies have informed the usefulness of SAF in screening DM, predicting DM microvascular and macrovascular long-term complications. This systematic review and meta-analysis aimed to review and assess the clinical validation of SAF as a non-invasive tool in DM, especially early measurement of the risk of DM complications. To our knowledge, there is no meta-analysis study concerning the significance of SAF measurement in DM. Therefore, we searched "PubMed" (MEDLINE) and "Cochrane" databases from their inception to 10 August 2021 for all observational studies concerning SAF measurement in DM patients.
Added value of this study
Our study confirmed the significance of SAF measurement as a non-invasive surrogate marker of DM micro and macrovascular complications. Skin AGE estimation, using SAF, may be a useful factor for the prediction and early detection of irreversible DM complications.
Implications of all the available evidence
Although some inconsistent results exist regarding the validation of SAF in DM different vascular complications, our meta-analysis results indicated a significant relationship between SAF and various diabetic vascular complications. More studies with larger populations and longer follow-up periods are required.
Alt-text: Unlabelled box
1. Introduction
Diabetes mellitus (DM), a common chronic disease, represents a significant public health problem. The incidence of DM is increasing throughout the world. DM can cause disability and complications in many organs of the body [1]. Knowledge about the pathophysiological mechanism of DM complications is crucial to prevent their development [2].
Advanced glycation end-products (AGE) are considered as one of the main pathogenic factors responsible for DM end-organ damage [3]. AGEs are produced from non-enzymatic reactions between glucose and tissue proteins, nucleic acid, and lipids by aging [3,4]. Long-standing hyperglycemia increases the rate of AGE accumulation in the body [4]. Accumulation of AGE on the body proteins is also associated with the development and worsening of oxidative-based diseases other than diabetes, including cardiovascular disease, chronic renal failure, and neurological disorders, and may expect future complications [5].
AGE accumulate mainly in proteins with a longer half-life, such as skin and cartilage collagen and lens crystalline [6]. They are cleared by the kidneys [7]. The lifetime of skin collagen is estimated to be 15-20 years [8]. Accumulation of AGE may cause functional and structural changes in body proteins that lead to impairment of the tissue protein functions [7].
Skin autofluorescence (SAF) is suggested as a novel, non-invasive, quick, and reliable method to measure tissue AGE level. An autofluorescence reader (AFR) illuminates a UV light to 4 cm2 of the normal skin of the lower arm. The emitted light source between 300-420nm with peak intensity at 370nm excites AGEs with autofluorescence properties. The spectrometer measures light from the skin in the range of 300-600 nm. To correct the effect of light absorption due to skin phototype on autofluorescence, the average light intensity per nanometer in the range of 420-600nm is divided by the average light intensity per nanometer in the range of 300-420nm. The mentioned ratio multiplies by 100 and represents autofluorescence in percentage (arbitrary units). Interestingly, SAF is correlated with both autofluorescent and non-fluorescent tissue AGE levels [9,10].
Collagen-linked fluorescence is significantly correlated with the quantity of AGE evaluated in the skin biopsy [11,12]. Moreover, SAF is correlated with plasma AGE. Interestingly, a higher association between SAF and tissue levels of AGE compared with plasma AGE was described [13].
Several studies have reported the usefulness of SAF in screening DM, predicting its microvascular and macrovascular long-term complications [13], [14], [15], [16], [17].
This systematic review and meta-analysis aimed to review and assess the clinical validation of SAF as a non-invasive tool in DM, especially early measurement of the risk of DM complications.
2. Methods
This systematic review and meta-analysis study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [18]. Our study was exempted from review by the institutional review board as it is based on published data.
2.1. Search strategy
We searched "PubMed" (MEDLINE) and "Cochrane" databases from their inception to 10 August 2021 for observational studies concerning SAF measurement in DM patients. The following key terms were used in advanced searching: “Diabetes”, “Diabetes Mellitus”,” DM”, “glycation”, “Advanced Glycation End product”, “AGE”, “skin autofluorescence”, “SAF” (Table 1). Furthermore, the reference list of the included observational studies and relevant review articles were searched for any missed survey.
Table 1.
The search strategy for finding eligible studies in the databases.
| “Diabetes” OR “Diabetes Mellitus” OR “Diabetic patients” OR “DM” | AND | “advanced glycation end products” OR “AGE” OR ‘Glycation” | AND | “skin autofluorescence” OR “SAF” | AND | “Microvascular complication” OR “Macrovascular complication” OR “Retinopathy” OR “Neuropathy” OR “Nephropathy” OR “Microalbuminuria” OR “Diabetic foot ulcer” OR “Clinical significance” OR “Clinical relevance” OR “Clinical value” |
2.2. Study inclusion
Observational surveys concerning the significance of skin autofluorescence in DM patients were included in the study. Inclusion criteria were: [1] Studies that included known DM patients [2]. Studies that used the SAF method for evaluating tissue AGE level [3]. DM patient's characteristics and complications were measured. Studies were excluded if: [1] Correlation values were not reported for study outcome, any association between SAF and DM characteristics or complications [2]. They were review articles, editorials, study proposals, congress posters, or case reports [3]. They were not human studies.
Two investigators (Dr. M. Hosseini and DR. Z. Razavi) independently assessed the title, abstract or full-body articles. Any disagreement was resolved by the judgment of the third author (Dr. A.H Ehsani). The authors excluded studies that did not fulfill the mentioned eligibility criteria. In case of overlap in the patient participants from the same center included in multiple studies, we selected the study considering MINOR scores with a special focus on a larger sample size and longer follow-up period.
2.3. Data extraction
If available. the following details were recorded: [1] first author name, [2] year of publication of the study, [3] country where a study was conducted [4] study design [4], study participants characteristics (number of the sample, male gender percentage, mean age, type of DM, mean BMI (Body Mass Index), duration of diabetes) [5]. Individual's HbA1c and max-IMT (maximum of carotid Intima Media Thickness) [6] patients’ diabetic complications (diabetic microvascular complications; retinopathy (DR), nephropathy (DNP), peripheral neuropathy (DPN); diabetic macrovascular events (D-MVE) and diabetic foot ulcer (DFU)) [7]. The mean of SAF [8]. The unadjusted OR/HR for SAF and each of DR, DPN, DNP, and D-MVE of each study were included [9]. The correlation coefficient between SAF and each of age, BMI, diabetic duration, max-IMT, and HbA1c.
2.4. Quality assessment and potential bias
We graded study strength using MINOR (Methodological Index for Non-Randomized Studies) criteria [19].. The following items were scored: [1] a clearly defined aim, [2] Inclusion of patients, [3] Collection of data, [4]. Endpoints were suitable to the goal of the study, [5] Unbiased assessment of the study outcomes, [6] Follow-up period proper to the object of the study, [7] Loss to follow up less than 5%, [8] Probable calculation of the study size.
In addition, for comparative observational studies additional four items were scored: [9] A satisfactory control group, [10] Contemporary groups, [11] Baseline similarity of groups, [12] Adequate statistical analyses.
The mentioned items were scored 0 if there was not reported, 1 if were inadequately reported and 2 if was adequately reported.
Two authors independently excreted the data for quality assessment, any disagreement was solved by discussion or intervention of the third author. It is worth mentioning that data regarding the authors' names, countries, and date of the publication were blinded.
2.5. Statistical analysis
We performed a meta-analysis if at least two studies reported correlation coefficient for SAF and age, BMI, diabetic duration, max-IMT or HbA1c in diabetes or otherwise, the OR/HR of SAF and DR, DPN, DNP, DFU and D-MVE.
Cochrane's Q statistics were used to express the heterogeneity among the included studies as percentages of I2. Moderate heterogeneity and substantial heterogeneity were defined if I2 was >40% and >60% respectively. Significant heterogeneity was expressed at p<0.05.
We conducted a random-effects model for analysis of the pooled effect sizes. The random-effects model declares that the true effect size may or may not vary from study to study, therefore it does not assume that either is the case.
Forest plots were applied to estimate correlations (Spearman and Pearson coefficients) for the association between SAF, and each of age, BMI, diabetic duration, IMT, and HbA1c.We used the DerSimonian and Laird method to analyze the pooled estimates of correlation coefficients with 95% confidence intervals. Besides, the pooled univariate OR/HRs with 95% confidence interval (CI) of DR, DPN, DNP, or D-MVE were used as effect sizes in the DerSimonian and Laird method.
Systemic re-analyses, sensitivity analyses, was assessed by removing a single study at a time and evaluating its impact on the overall effect size to evaluate the robustness of the summary results.
A sensitivity analysis was performed regarding diabetic patients’ outcomes. Moreover, additional sensitivity analyses including only case-control studies were investigated.
Funnel plots, as well as Eggers's test, were applied for assessing publication bias. A funnel plot is a visual tool for exploring publication bias. In-state, no publication bias in a Meta-analysis, funnel plot should represent scatterplots as an inverted funnel. An asymmetric funnel plot indicates the presence of publication bias. Furthermore, Eggers test by a value of p<0.05 suggests publication bias.
All the meta-analyses were performed with Stata version 16.0
3. Results
3.1. Search results and studies characteristics
A total of 881 records were primarily identified through database searching. Of them, 13 records were duplicated. Among the remaining 868 studies, 787 records did not report SAF in DM patients. The remaining 81 articles were assessed for eligibility. Finally, 29 records were included in this systematic review and meta-analysis [[11], [12], [13], [15], [16], [17], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]]. The PRISMA flowchart for this systematic review and meta-analysis is presented in Fig 1.
Fig 1.
The PRISMA flowchart for this systematic review and meta-analysis. The PRISMA flowchart for selecting eligible records to include in our study.
The characteristics of the included studies are described in Table 2. The included studies were published between 2008 and 2019 and included cross-sectional (25 studies), prospective longitudinal (three studies), and retrospective (one study) with patients aged 12.2 to 69.1 years (sample sizes from 16 to 881). The studies were conducted in Europe (13 studies), Asia (13 studies), Australia (two studies), and continental USA (one study). All studies included DM patients (type 1 or 2). All of the included studies assessed SAF by AGE-Reader™ technology (DiagnOptics, The Netherlands), and the SAF levels were evaluated from 1.23 to 3.03 AU in the included studies. Table 2 shows the characteristics of the included studies.
Table 2.
Characteristics of Included studies.
| Study, Country, Year | Study Design | Sample Size (DM)(n) | Age (Year) of DM | Type of Diabetes Population | Sex (%)Male | Diabetic Duration | Outcome evaluated* | SAF(AU) | MINORCriteria |
|---|---|---|---|---|---|---|---|---|---|
| Osawa, Japan, 2016 [11] | |||||||||
| Cross-sectional | 105 | 37.4 ± 12.4 | DM1 | 32.4 | 21.9 ± 9.2 | Age, diabetic duration, BMI, HbA1c, max-IMT | 2.07 ± 0.5 | 18 | |
| Škrha Jr, Czech Republic, 2013 [12] | |||||||||
| Cross-sectional | DM1:47 DM2:41 |
DM1:54.79±39.57 DM2:58±28.17 |
3.4%DM1 46.6%DM2 |
DM1:57 DM2:61 |
DM1:50.49±103.67 DM2:28.51±54.34 |
Age, diabetic duration, HbA1c | DM1: 2.39±0.54 DM2: 2.63±0.73 |
18 | |
| Hu, China, 2012 [13] | |||||||||
| Cross-sectional | 195 | 58.44 ± 3.74 | DM | 56.92 | 7.26 ± 1.45 | Age, BMI, diabetic duration, HbA1c, DFU | 2.35 ± 0.17 | 12 | |
| Cho, Australia, 2016 [15] | |||||||||
| Cross-sectional | 135 | 15.6 ± 2.1 | DM1 | 51 | 8.7 ± 3.5 | DR, Age, diabetic duration, HbA1c | 1.23 ± 0.27 | 13 | |
| Wan, China, 2019 [16] | |||||||||
| Cross-sectional | 820 | 60.72 ± 10.23 | DM2 | 52.43 | 12.77 ± 8.08 | DPN | 2.35 ± 0.25 | 12 | |
| Liu, China, 2015 [17] | |||||||||
| Cross-sectional | 118 | 64.6 ± 9.1 | 98.3% DM2 | 72.9 | 14.7 ± 7.5 | Age, BMI, diabetic duration, HbA1c | 2.8 ± 0.2 | 11 | |
| Uruska, Poland, 2019 # [20] | |||||||||
| Cross-sectional | 476 | 44.53 ± 16.09 | DM1 | 48.1 | 26.38 ± 10.8 | Age, BMI, diabetic duration, HbA1c | 2.37 ± 0.54 | 13 | |
| Li, China, 2017 [21] | |||||||||
| Cross-sectional | 362 | 50.5 ± 8.3 | DM2 | 49.44 | NA | BMI, HbA1c, | 2.72 ± 1.46 | 18 | |
| Vélayoudom Céphise, France, 2016 [22] | |||||||||
| Prospective1 | 243 | 51.2 ± 16.7 | DM1 | 58.9 | 21.4 ± 13.8 | DNP, D-MVE, | 2.13± 0.58 | 11 | |
| Vouillarmet, France, 2013 [23] | |||||||||
| Prospective2 | 150 | 63.3 ± 11.9 | 85% DM2 | 68 | 17 ± 12.4 | DFU | 3.03 ± 0.14 | 11 | |
| Stirban, Germany & Romania, 2018 [24] | |||||||||
| Cross-sectional | 497 | 61.08 ± 8.31 | 93.36% DM2 | 48.7 | 9 ± 5.93 | Age, BMI, HbA1c | 2.51 ± 0.06 | 10 | |
| Januszewski, Australia, 2011 [25] | |||||||||
| Cross-sectional | 69 | 36.47 ± 4.02 | DM1 | 55.07 | DM:20.13 ± 6.7 | Age, diabetic duration, HbA1c | 2.01 ± 0.04 | 16 | |
| Monami, Italy, 2008 [26] | |||||||||
| Cross-sectional | 92 | 69.1 ± 12.4 | DM2 | 60.9 | 12.3 ± 10.7 | Age, BMI, HbA1c | 2.5 ± 0.9 | 10 | |
| Sugisawa, Japan, 2013 [27] | |||||||||
| Cross-sectional | 241 | 36.7 ± 10.5 | DM1 | 54.77 | 18.2 ± 10.4 | Age, BMI, diabetic duration, HbA1c | 2.31 ± 0.5 | 17 | |
| Hangai, Japan, 2016 [28] | |||||||||
| Cross-sectional | 122 | 61 ± 13 | DM2 | 59 | 10.7 ± 9.3 | Age, diabetic duration, BMI, HbA1c, max-IMT | 2.42 ± 0.417 | 13 | |
| Hirano, Japan, 2013 [29] | |||||||||
| Cross-sectional | 138 | 63.7 ± 12.2 | DM2 | 44.2 | DM:13.2 ± 9.9 | Age | 2.48 ± 0.48 | 11 | |
| Osawa, Japan, 2018 [30] | |||||||||
| Cross-sectional | 193 | 61.1 ± 12.3 | DM2 | 55.4 | DM:13.7 ± 10.3 | DR, DPN, DNP, D-MVE, age, BMI, diabetic duration, HbA1c, max-IMT | 2.57±0.47 | 19 | |
| Tanaka, Japan, 2011 [31] | |||||||||
| Cross-sectional | 130 | 67.13 ± 12.72 | DM2 | 39.2 | 9.1 ± 7.64 | DR, DPN, DNP, D-MVE, age, BMI | 2.16 ± 0.49 | 12 | |
| Temma, Japan, 2015 [32] | |||||||||
| Cross-sectional | 61 | 66.6 ± 9.2 | DM2 | 62.29 | 10.4 ± 7.3 | Max-IMT, Age, diabetic duration, BMI, HbA1c, | 2.5 ± 0.5 | 12 | |
| Yasuda, Japan, 2014 [33] | |||||||||
| Cross-sectional | 67 | 61 ± 8.9 | DM2 | 56.71 | 13.42 ± 2.38 | Age | 2.5 ± 0.3 | 20 | |
| Yoshioka, Japan, 2018 [34] | |||||||||
| Cross-sectional | 162 | 61.2 ± 11.2 | DM2 | 55 | 14.6 ± 10 | Age, diabetic duration, HbA1c | 2.53 ± 0.45 | 19 | |
| Gerrits, The Netherland, 2008 [35] | |||||||||
| Prospective3 | 881 | 66 ± 11 | DM2 | 46 | 5.86 ± 6.07 | DR, DPN, DNP, diabetic any microvascular complication, D-MVE | 2.74 ± 0.7 | 10 | |
| Ahdi, The Netherland, 2015 [36] | |||||||||
| Cross-sectional | 810 | 59.67 ± 10.9 | DM2 | 52 | 14.17 ± 12.03 | DR,DPN,diabetic any microvascular complication, D-MVE, age, diabetic duration | 2.94 ± 0.68 | 12 | |
| van der Heyden, The Netherland, 2018 [37] | |||||||||
| Retrospective | 77 | 15.3 ± 2.52 | DM1 | 49.35 | DM: 6.53 ± 4.45 | Age, diabetic duration, HbA1c | 1.38 ± 0.23 | 16 | |
| Banser, The Netherland, 2015 [38] | |||||||||
| Cross-sectional | 144 | 12.2 ± 3.8 | DM1 | 56.94 | 4.1 ± 3.7 | Age, diabetic duration, HbA1c | 1.33 ± 0.36 | 11 | |
| Yozgatli, The Netherland, 2018 [39] | |||||||||
| Prospective4 | 514 | 65.01 ± 11.35 | DM2 | 48.4 | 14.12 ± 8.03 | diabetic any microvascular complication, D-MVE | 2.86 ± 0.65 | 11 | |
| Furst, USA, 2016 [40] | |||||||||
| Cross-sectional | 16 | 65.4 ± 2.4 | DM2 | NA | 14.3 ± 2 | HbA1c | 2.8 ± 0.1 | 10 | |
| Llaurado, Spain, 2014 [41] | |||||||||
| Cross-sectional | 68 | 35.3 ± 10.1 | DM1 | 50 | DM:13.1 ± 8.67 | Age, BMI, HbA1c | 2.05 ± 0.37 | 18 | |
| Rigalleau, France,2015 [42] | |||||||||
| Cross-sectional | 418 | 61.8 ± 10.3 | DM2 | 59.3 | 13.33 ± 9.78 | D-MVE | 2.53 ± 0.62 | 11 |
# This study was conducted at the same center of Araszkiewicz et al, study [43]. One hundred and forty DM patients were included in the later study, therefore only the odd ratio evaluations for SAF and DR, DPN, DNP and diabetic any microvascular complications were extracted from Araszkiewicz et al, study.
*SAF correlation was analyzed for age, BMI, diabetic duration, HbA1c, max-IMT. Odd ratio was measured for SAF (independent variable) and each of DR, DPN, DNP, diabetic microvascular complication, D-MVE, and DFU. In Yozgatli et al. study HR was estimated for SAF and diabetic vascular complications relation.
1 prospective study with 4 years follow-up. 2two months follow-up.
3follow-up for 3.1 years. 4A media follow-up for 5.1 years.
Abbreviations: SAF, skin autofluorescence; AU, Arbitrary unit; MINOR, Methodological Index for Non-Randomized Studies; DM, Diabetes Mellitus; BMI, Body Mass Index; max-IMT, max carotid Intima Media Thickness; NA, Not Available; DR, Diabetic Retinopathy; DPN, Diabetic Peripheral Neuropathy; DNP, Diabetic Nephropathy; D-MVE, Diabetic Macrovascular Event; DFU, Diabetic Foot Ulcer.
3.2. Meta-analysis
3.2.1. Diabetes characteristics
Twenty-nine articles with substantially heterogeneous DM participants were included in this meta-analysis.
3.2.2. The correlation between SAF and other variables
A significant correlation was observed between SAF and age [0.38 (0.33, 0.43)], [I2=64.5%, p<0.05]. However, no correlation was described between SAF and BMI [0.003(-0.08, 0.09), (I2=79%, p<0.05)]. (supplementary figures S1A, S1B). In addition, a significant correlation described for SAF and diabetes duration [0.33 (0.25, 0.42), (I2=84.06%, p<0.05)] (supplementary figure S1C). Similarly, a statistically significant relation between SAF and HbA1c was evaluated [0.21(0.13,0.28), I2=77.99%, p<0.05] (supplementary figure S1D). Only four substantially heterogenous articles (I2 =69.01%, p<0.05) reported the association of SAF and max-IMT level. The pooled correlation coefficient for SAF and the max-IMT level was 0.29 (0.14, 0.44), which also indicates a significant correlation statistically (supplementary figure S1E).
3.2.3. The association for SAF and diabetic micro and macrovascular complications
3.3.1. Diabetic retinopathy (DR)
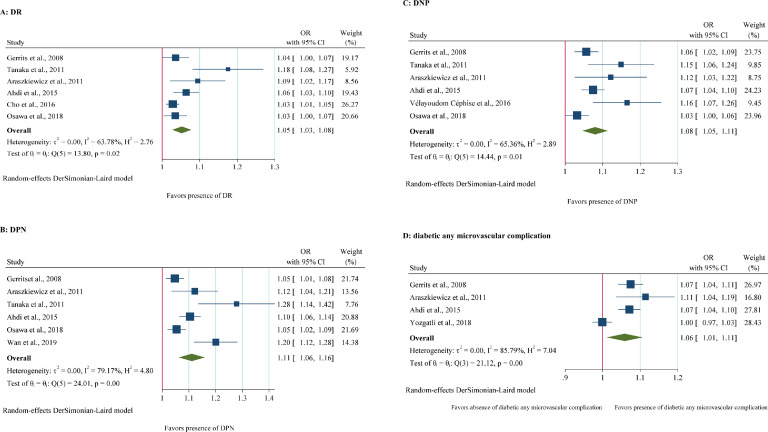
Six of the included studies reported the relation between SAF and DR. A significant substantial heterogeneity was revealed across these studies (I2=63.78%, p<0.05). A random-effects model showed a positive significant relation between SAF level and DR, meaning that 0.1 unit increase in SAF level, is related with greater odds of DR by 5% (OR= 1.05, 95%CI=1.03,1.08), (Fig 2A).
Fig 2.
Forest Plots of OR of SAF for diabetic microvascular complications. A: Forest plot of pooled unadjusted OR of SAF for DR (Diabetic Retinopathy). B: Pooled unadjusted OR and HR of SAF for DPN (Diabetic Peripheral Neuropathy). C: Pooled unadjusted OR of SAF for DNP (Diabetic Nephropathy). D: Pooled unadjusted OR and HR of SAF for diabetic any microvascular complication. 95% CI, 95% Confidence Interval; I2 represents the quantity of heterogeneity (between 0 and 100%). T2 is the inter-study variance. H: Heterogeneity. p is the p-value of the heterogeneity test.
3.3.2. Diabetic peripheral neuropathy (DPN)
There were six articles regarding the association between SAF and DPN. Substantial heterogeneity was observed among these included studies (I2=79.17%, p<0.05), a random-effects meta-analysis demonstrated that higher levels of SAF were significantly associated with DPN (OR= 1.11, 95%CI= 1.06,1.16). (Fig 2B). In other words, by increasing 0.1 units in the SAF level, the odds of DPN would be 11% in DM patients.
3.3.3. Diabetic nephropathy (DNP)
Six included studies were concerning the relation between SAF and DNP. With a substantial heterogeneity (I2=65.36%, p<0.05) a random-effects model for meta-analysis revealed a remarkable association between higher levels of SAF with DNP (OR= 1.08, 95%CI: 1.05,1.11). (Fig 2C). therefore, a 0.1 unit increase in the SAF is associated with odds of DNP by 8%.
3.3.4. Diabetic any microvascular complication (DR, DPN, DNP)
Four heterogeneous articles concerning the relationship between SAF and diabetic microvascular complications were included (I2=85.79%, p<0.05). Therefore, a random-effects model described that higher levels of SAF were significantly associated with diabetic any microvascular complication (OR=1.06, 95%CI=1.01,1.11) (Fig 2D). Thus, a 0.1 unit increase of SAF is expected to be related to 6% greater odds of any microvascular complication.
3.3.5. Diabetic macrovascular event (D-MVE)
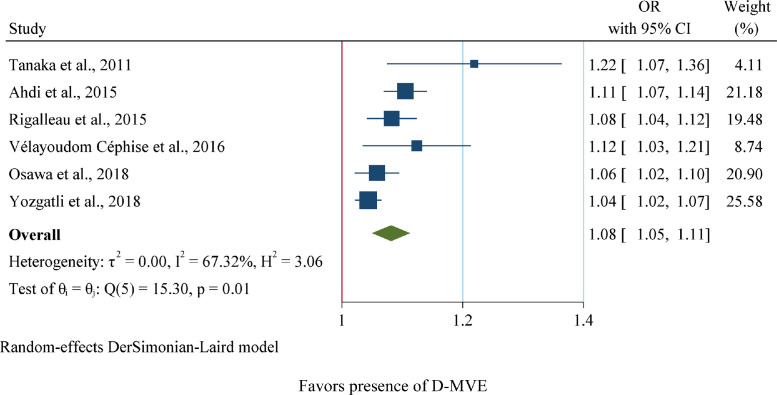
As shown in Fig 3, six articles regarding the association of SAF and D-MVE with substantial heterogeneity were included (I2=67.32%, p<0.05). Henceforward, the random-effects model represented that higher SAF may significantly increase the odd of D-MVE development (OR=1.08, 95%CI=1.05,1.11). This means that a 0.1 unit increase in SAF is correlated with 8% more odds of D-MVE.
Fig 3.
Forest plot of pooled unadjusted OR and HR of SAF for D-MVE (Diabetic Macrovascular Event). 95% CI, 95% Confidence Interval; I2 represents the quantity of heterogeneity (between 0 and 100%). T2 is the inter-study variance. H: Heterogeneity. p is the p-value of the heterogeneity test.
3.3.6. Diabetic foot ulcer (DFU)
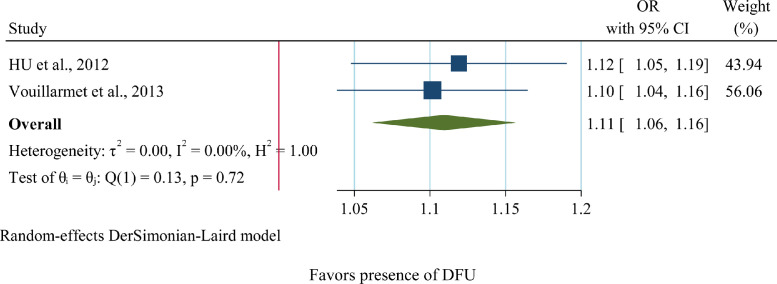
Regarding the relation between SAF and DFU, only two articles with no heterogeneity were found (I2=0, p=0.72). Henceforward, by using the random-effects model a positive significant association was revealed between SAF and DFU (OR=1.11, 95%CI= 1.06,1.16) (Fig 4). Therefore, a 0.1 unit increase in SAF is linked with 11% greater odds of DFU.
Fig 4.
Forest plot of pooled unadjusted OR of SAF for DFU (Diabetic Foot Ulcer). 95% CI, 95% Confidence Interval; I2 represents the quantity of heterogeneity (between 0 and 100%). T2 is the inter-study variance. H: Heterogeneity. p is the p-value of the heterogeneity test.
3.4. Sensitivity analysis and publication bias
After removing one article at a time, the estimated pooled correlation coefficients and pooled unadjusted OR were not significantly different in magnitude and direction. In this re-analysis, we removed every single article at a time starting from articles with a higher risk of bias considering MINOR criteria. Moreover, additional sensitivity analyses including only case-control studies were investigated. In addition, the weight of each study which is mentioned in every figure represents the impact of the study in the meta-analysis.
The Eggers test and Funnel plots for publication bias in articles reporting the validation of SAF for each of DR, DPN, DNP, and D-MVE yielded significant (supplementary figures S2A-S2D). While no publication bias was found among studies reporting the association between SAF and each of DFU and diabetic any microvascular complications (supplementary figures S2E, S2F). Likewise, no publication bias was described among articles that assessed the relation between SAF and other variables (diabetes age, BMI, diabetic duration, max-IMT, HbA1c) (p>0.05). (supplementary figures S3A-S3E)
4. Discussion
This systematic review and meta-analysis provide evidence suggesting that higher SAF levels could be a predictor of chronic micro and macrovascular complications of DM. Our data confirm the association between chronic deposits of AGEs produced by enzymatic glycation in the skin and diabetic neuro-vascular complications. Notably, considering the limited number of eligible studies and large heterogeneity, the outcomes should be interpreted with caution.
DM patients are predisposed to long-term irreversible vascular complications, consequently, DM complications not only cause morbidity but also reduce their life expectancy to two-thirds of that of non-diabetic populations [43]. Several studies have been introduced hyperglycemia as the most significant factor responsible for the incidence and advancement of diabetic vascular complications. Although the exact role of hyperglycemia in the pathological mechanism of diabetic complications is unclear until now. The hypothesis of the role of protein glycation in DM complications is introduced and received remarkable attention [7].
Today, more than 20 known AGE have been introduced. In diabetes AGEs accumulating in long life proteins, and the degrees of protein modification is not reduced even after optimal glycemic control is restored, in contrast to e.g. HbA1c, which is not an AGE. Although AGE accumulate in the proteins by aging, a hyperglycemic state accelerates the AGE formation. Therefore, DM patients have more AGEs bounded proteins than age-matched non-diabetic individuals [44]. AGE accumulation not only represent hyperglycemia but also shows growing metabolic load (hyperlipidemia and hyperglycemia), inflammation, and oxidative stress [45].
AGEs cause cross-linkage of long-lasting proteins which lead to vascular stiffness, changing vascular structures, and eventually altering vascular functions. Interaction between AGE and its receptor initiates intracellular signaling which promotes oxidative stress and stimulates proinflammatory and prosclerotic cytokines [46].
AGE measurement may offer additional evidence to expect diabetic vascular complications [44]. Several studies have shown that the tissue levels of AGEs, assessed by skin biopsies of DM patients, were more compatible than HbA1c with the incidence of retinopathy and nephropathy and disease progression [44]. A review of seven studies established that SAF was positively correlated with nephropathy, neuropathy, cardiovascular events, and mortality [7].
Some studies estimated a significant association between SAF and HbA1c, while others could not obtain similar results [24,38]. The inconsistency between results might be due to differences in the type of diabetes of the patients, duration of diabetes, duration of studies, percentage of complications in participants, and outcomes. Lastly, the probable difference in pathological inducers and pathways between different diabetic vascular complications should be further investigated [7].
Our findings found a correlation between HbA1c and SAF levels. The lack of association between HbA1c and AGEs in some included studies might be due to the shorter turn-over time of HbA1c comparing to SAF, the difference in handling the hyperglycemic state, and the resultant oxidative stress by individuals [15]. SAF, representing a cumulative metabolic control, might be more correlated with HbA1c of previous years than the present [47]. Also, hyperglycemia maybe not be the only one responsible for AGE accumulation in diabetes [48].
Numerous pathological mechanisms for AGE that can cause D-MVE and even mortality are introduced. They consequently result in endothelial dysfunction, arterial stiffness, vasoconstriction, proliferation and thickness of vessel wall, atherosclerotic plaque progression, thrombosis, and fibrosis [49,50]. Although max-IMT is a powerful marker for atherosclerosis and predicting cardiovascular events, the results of previous studies regarding the association of SAF and max-IMT were inconsistent [11,28]. This highlights the various unknown pathological mechanisms for D-MVE development and progression, Although, our findings provided a significant relation for SAF and max-IMT
SAF measurement may not represent the precise tissue AGEs burden. Most AGEs are non-fluorescent such as carboxymethyl-lysine (CML), carboxyethyl-lysine (CEL) which have been introduced to be significant in predicting cardiovascular events. Moreover, several skin proteins fluorescence within wavelengths overlap with AGEs spectra, thus SAF measurement is not specific [44]. However, investigators have established that AGE reader measures tissue AGE that is highly positively correlated with fluorescent and nonfluorescent tissue AGE measures in skin biopsies [51].
Several factors may affect SAF measurements such as dark pigmentation of the skin (skin phototype 4-6), skin product applications (particularly sunscreens and skin tanners), the fasting or postprandial state (about 5% variation of SAF in a day), extreme local hyperemia and vasoconstriction. These potential confounders should be kept in mind and avoided if possible when measuring SAF [52], [53], [54]. Increased formation or absorption of AGEs through foods or smoking may enhance AGE accumulation, as well as reduced clearance of AGE in renal failure conditions, which may eventually lead to more AGE accumulation [45]. SAF is independently higher in premenopausal females comparing males, which may be due to estrogen-related effects. Sex hormones impact AGE tissue deposition by changing the collagen turn-over time. Postmenopausal women have a reduced amount of skin collagen, therefore the SAF level difference between male and female sexes becomes not significant in advanced ages [48].
Several limitations in this systematic review and meta-analysis must be mentioned. First, some of the included studies were cross-sectional while others were prospective or retrospective. Second, studies measured different multiple outcomes. Using a variety of diagnostic tools in studies led to different investigated outcomes. Third, adjusted complication risk factors were not investigated in this study because of diverse confounding factors in studies. Fourth, heterogeneous cohort groups, adults and adolescence, were included, and difference because clinical heterogeneity exists. Due to rare existing studies concerning SAF relation with DFU, we conduct a meta-analysis on SAF association with DFU in diabetes including only two studies. Publication bias was found in the included articles, by Egger´s test for several outcomes such as DR, DPN, DNP and D-MVE, therefore unpublished studies could change the results of this meta-analysis. Last, the majority of the studies provided neither clarification of the validation of the required sample size nor the blinding of the assessor which may have caused bias.
Our study confirms the evidence that SAF measurement could be a non-invasive surrogate marker of DM micro and macrovascular complications. Skin AGE accumulation estimation may be a useful factor for the prediction and early detection of irreversible diabetic complications. Thus, various anti-AGE therapies might be needed. More studies with larger populations and longer follow-up are required.
Declaration of Competing Interest
None.
Acknowledgments
Contributors
Z. Razavi: Researched the database and wrote the manuscript.
M. Hosseini: Researched the database and edited the manuscript.
AH Ehsani: Contributed in data interpretation, preparing the figures and Tables, reviewing the manuscript.
A. Firooz: Contributed in study design, data analysis, editing the article.
S. Afazeli: Contributed in study design, data analysis.
Role of the funding source
There was no funding source for this study. The corresponding author had access to the dataset and was responsible for the decision to submit for publication
Data sharing
Detailed review data can be provided by the corresponding author upon reasonable request.
Funding
The authors declare any funding source.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101194.
Appendix. Supplementary materials
References
- 1.Liu J., Ren Z.H., Qiang H., Wu J., Shen M., Zhang L., Lyu J. Trends in the incidence of diabetes mellitus: results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC public health. 2020;20:1–12. doi: 10.1186/s12889-020-09502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dörhöfer L., Lammert A., Krane V., Gorski M., Banas B., Wanner C., Krämer B.K., Heid I.M., Böger C.A. DIACORE Study Group. Study design of DIACORE (DIAbetes COhoRtE)–a cohort study of patients with diabetes mellitus type 2. BMC med genetics. 2013;14(1):25. doi: 10.1186/1471-2350-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krul-Poel Y.H., Agca R., Lips P., van Wijland H., Stam F., Simsek S. Vitamin D status is associated with skin autofluorescence in patients with type 2 diabetes mellitus: a preliminary report. Cardiovascular diabet. 2015;14(1):89. doi: 10.1186/s12933-015-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori H., Kuroda A., Araki M., Suzuki R., Taniguchi S., Tamaki M., Akehi Y., Matsuhisa M. Advanced glycation end-products are a risk for muscle weakness in Japanese patients with type 1 diabetes. J diabetes invest. 2017;8(3):377–382. doi: 10.1111/jdi.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghazaryan A., Omar M., Tserevelakis G.J., Ntziachristos V. Optoacoustic detection of tissue glycation. Biomed optics express. 2015;6(9):3149–3156. doi: 10.1364/BOE.6.003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad M.S., Damanhouri Z.A., Kimhofer T., Mosli H.H., Holmes E. A new gender-specific model for skin autofluorescence risk stratification. Scient reports. 2015;5:10198. doi: 10.1038/srep10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos D.C., de Ranitz-Greven W.L., de Valk H.W. Advanced glycation end products, measured as skin autofluorescence and diabetes complications: a systematic review. Diabetes tech therap. 2011;13(7):773–779. doi: 10.1089/dia.2011.0034. [DOI] [PubMed] [Google Scholar]
- 8.Gerrits E.G., Lutgers H.L., Kleefstra N., Groenier K.H., Smit A.J., Gans R.O., Bilo H.J. Skin advanced glycation end product accumulation is poorly reflected by glycemic control in type 2 diabetic patients (ZODIAC-9. J Diabetes Scien and Tech. 2008;2(4):572–577. doi: 10.1177/193229680800200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meerwaldt R., Links T., Graaff R., Thorpe S.R., Baynes J.W., Hartog J., Gans R., Smit A. Simple noninvasive measurement of skin autofluorescence. Ann New York Acad Scien. 2005;1043(1):290–298. doi: 10.1196/annals.1333.036. [DOI] [PubMed] [Google Scholar]
- 10.Fernando M.E., Crowther R.G., Lazzarini P.A., Sangla K.S., Wearing S., Buttner P., Golledge J. Within-and between-body-site agreement of skin autofluorescence measurements in people with and without diabetes-related foot disease. J diabetes scien tech. 2019;13(5):836–846. doi: 10.1177/1932296819853555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osawa S., Katakami N., Kuroda A., Takahara M., Sakamoto F., Kawamori D., Matsuoka T., Matsuhisa M., Shimomura I. Skin autofluorescence is associated with early-stage atherosclerosis in patients with type 1 diabetes. J atherosclerosis and thrombosis. 2016:35592. doi: 10.5551/jat.35592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Škrha J., Šoupal J., Loni Ekali G., Prázný M., Kalousová M., Kvasnička J., Landová L., Zima T. Skin autofluorescence relates to soluble receptor for advanced glycation end-products and albuminuria in diabetes mellitus. J diabetic research. 2013;1 doi: 10.1155/2013/650694. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu H., Han C.M., Hu X.L., Ye W.L., Huang W.J., Smit A.J. Elevated skin autofluorescence is strongly associated with foot ulcers in patients with diabetes: a cross-sectional, observational study of Chinese subjects. J Zhejiang University SCIENCE B. 2012;13(5):372–377. doi: 10.1631/jzus.B1100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Sala L., Tagliabue E., de Candia P., Prattichizzo F., Ceriello A. One-hour plasma glucose combined with skin autofluorescence identifies subjects with pre-diabetes: the DIAPASON study. BMJ Open Diabetes Research and Care. 2020;8(1) doi: 10.1136/bmjdrc-2020-001331. e001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho Y.H., Craig M.E., Januszewski A.S., Benitez-Aguirre P., Hing S., Jenkins A.J., Donaghue K.C. Higher skin autofluorescence in young people with Type 1 diabetes and microvascular complications. Diabetic Med. 2017;34(4):543–550. doi: 10.1111/dme.13280. [DOI] [PubMed] [Google Scholar]
- 16.Wan L., Qin G., Yan W., Sun T. Skin autofluorescence is associated with diabetic peripheral neuropathy in chinese patients with type 2 diabetes: A cross-sectional study. Genetic test molecul biomark. 2019;23(6):387–392. doi: 10.1089/gtmb.2018.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C., Xu L., Gao H., Ye J., Huang Y., Wu M., Lu S. The association between skin autofluorescence and vascular complications in Chinese patients with diabetic foot ulcer: an observational study done in Shanghai. The internat J lower extremity wounds. 2015;14(1):28–36. doi: 10.1177/1534734614568375. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Slim K., Nini E., Forestier D., Kwiatkowski F. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 20.Uruska A., Gandecka A., Araszkiewicz A., Zozulinska-Ziolkiewicz D. Accumulation of advanced glycation end products in the skin is accelerated in relation to insulin resistance in people with Type 1 diabetes mellitus. Diabetic Medicine. 2019;36(5):620–625. doi: 10.1111/dme.13921. [DOI] [PubMed] [Google Scholar]
- 21.Li Z., Wang G., Zhu Y.J., Li C.G., Tang Y.Z., Jiang Z.H., Niu W.Y. The relationship between circulating irisin levels and tissues AGE accumulation in type 2 diabetes patients. Bioscience reports. 2017;37(3) doi: 10.1042/BSR20170213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vélayoudom-Céphise F.L., Rajaobelina K., Helmer C., Nov S., Pupier E., Blanco L., Rigalleau V. Skin autofluorescence predicts cardio-renal outcome in type 1 diabetes: a longitudinal study. Cardiovascular diabetology. 2016;15(1):1–7. doi: 10.1186/s12933-016-0448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vouillarmet J., Maucort-Boulch D., Michon P., Thivolet C. Advanced glycation end products assessed by skin autofluorescence: a new marker of diabetic foot ulceration. Diabet technol therap. 2013;15(7):601–605. doi: 10.1089/dia.2013.0009. [DOI] [PubMed] [Google Scholar]
- 24.Stirban A.O., Bondor C.I., Florea B., Veresiu I.A., Gavan N.A. Skin autofluorescence: Correlation with measures of diabetic sensorimotor neuropathy. J Diabetes Complications. 2018;32(9):851–856. doi: 10.1016/j.jdiacomp.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Januszewski A.S., Sachithanandan N., Karschimkus C., O'Neal D.N., Yeung C.K., Alkatib N., Jenkins A.J. Non-invasive measures of tissue autofluorescence are increased in Type 1 diabetes complications and correlate with a non-invasive measure of vascular dysfunction. Diabet med. 2012;29(6):726–733. doi: 10.1111/j.1464-5491.2011.03562.x. [DOI] [PubMed] [Google Scholar]
- 26.Monami M., Lamanna C., Gori F., Bartalucci F., Marchionni N., Mannucci E. Skin autofluorescence in type 2 diabetes: beyond blood glucose. Diabet research and clin practice. 2008;79(1):56–60. doi: 10.1016/j.diabres.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Sugisawa E., Miura J., Iwamoto Y., Uchigata Y. Skin autofluorescence reflects integration of past long-term glycemic control in patients with type 1 diabetes. Diabetes care. 2013;36(8):2339–2345. doi: 10.2337/dc12-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hangai M., Takebe N., Honma H., Sasaki A., Chida A., Nakano R., Ishigaki Y. Association of advanced glycation end products with coronary artery calcification in Japanese subjects with type 2 diabetes as assessed by skin autofluorescence. J atherosclerosis and thrombosis. 2021;6:30155. doi: 10.5551/jat.30155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirano T., Iesato Y., Toriyama Y., Imai A., Chiba D., Murata T. Correlation between diabetic retinopathy severity and elevated skin autofluorescence as a marker of advanced glycation end-product accumulation in type 2 diabetic patients. J Diabet Complicat. 2014;28(5):729–734. doi: 10.1016/j.jdiacomp.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Osawa S., Katakami N., Sato I., Ninomiya H., Omori K., Yamamoto Y., Shimomura I. Skin autofluorescence is associated with vascular complications in patients with type 2 diabetes. J Diabetes Complicat. 2018;32(9):839–844. doi: 10.1016/j.jdiacomp.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka K., Tani Y., Asai J., Nemoto F., Kusano Y., Suzuki H., Watanabe T. Skin autofluorescence is associated with severity of vascular complications in Japanese patients with Type 2 diabetes. Diabet med. 2012;29(4):492–500. doi: 10.1111/j.1464-5491.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- 32.Temma J., Matsuhisa M., Horie T., Kuroda A., Mori H., Tamaki M., Matsumoto T. Non-invasive measurement of skin autofluorescence as a beneficial surrogate marker for atherosclerosis in patients with type 2 diabetes. J Medical Invest. 2015;62(3.4):126–129. doi: 10.2152/jmi.62.126. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda M., Shimura M., Kunikata H., Kanazawa H., Yasuda K., Tanaka Y., Nakazawa T. Relationship of skin autofluorescence to severity of retinopathy in type 2 diabetes. Current eye research. 2015;40(3):338–345. doi: 10.3109/02713683.2014.918152. [DOI] [PubMed] [Google Scholar]
- 34.Yoshioka K. Skin autofluorescence is associated with high-sensitive cardiac troponin T, a circulating cardiac biomarker, in Japanese patients with diabetes: A cross-sectional study. Diabetes and Vascular Disease Research. 2018;15(6):559–566. doi: 10.1177/1479164118785314. [DOI] [PubMed] [Google Scholar]
- 35.Gerrits E.G., Lutgers H.L., Kleefstra N., Graaff R., Groenier K.H., Smit A.J., Bilo H.J. Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes care. 2008;31(3):517–521. doi: 10.2337/dc07-1755. [DOI] [PubMed] [Google Scholar]
- 36.Ahdi M., Gerdes V.E., Graaff R., Kuipers S., Smit A.J., Meesters E.W. Skin autofluorescence and complications of diabetes: does ethnic background or skin color matter? Diabet technol therap. 2015;17(2):88–95. doi: 10.1089/dia.2013.0374. [DOI] [PubMed] [Google Scholar]
- 37.van der Heyden J.C., Birnie E., Mul D., Bovenberg S., Veeze H.J., Aanstoot H.J. Increased skin autofluorescence of children and adolescents with type 1 diabetes despite a well-controlled HbA1c: results from a cohort study. BMC endocrine disorders. 2016;16(1):1–8. doi: 10.1186/s12902-016-0129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banser A., Naafs J.C., Hoorweg-Nijman J.J., van de Garde E.M., van der Vorst M.M. Advanced glycation end products, measured in skin, vs. HbA1c in children with type 1 diabetes mellitus. Pediatric diabetes. 2016;17(6):426–432. doi: 10.1111/pedi.12311. [DOI] [PubMed] [Google Scholar]
- 39.Yozgatli K., Lefrandt J.D., Noordzij M.J., Oomen P.H.N., Brouwer T., Jager J., Smit A.J. Accumulation of advanced glycation end products is associated with macrovascular events and glycaemic control with microvascular complications in Type 2 diabetes mellitus. Diabetic Medicine. 2018;35(9):1242–1248. doi: 10.1111/dme.13651. [DOI] [PubMed] [Google Scholar]
- 40.Furst J.R., Bandeira L.C., Fan W.W., Agarwal S., Nishiyama K.K., McMahon D.J., Rubin M.R. Advanced glycation endproducts and bone material strength in type 2 diabetes. J Clin Endocrinol Metabolism. 2016;101(6):2502–2510. doi: 10.1210/jc.2016-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llaurado G., Ceperuelo-Mallafre V., Vilardell C., Simo R., Gil P., Cano A., Gonzalez-Clemente J.M. Advanced glycation end products are associated with arterial stiffness in type 1 diabetes. J Endocrinol. 2014;221(3):405–413. doi: 10.1530/JOE-13-0407. [DOI] [PubMed] [Google Scholar]
- 42.Rigalleau V., Cougnard-Gregoire A., Nov S., Gonzalez C., Maury E., Lorrain S. Association of advanced glycation end products and chronic kidney disease with macroangiopathy in type 2 diabetes. J diabetes its complications. 2015;29(2):270–274. doi: 10.1016/j.jdiacomp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Araszkiewicz A., Naskret D., Niedzwiecki P., Samborski P., Wierusz-Wysocka B., Zozulinska-Ziolkiewicz D. Increased accumulation of skin advanced glycation end products is associated with microvascular complications in type 1 diabetes. Diabetes technology & therapeutics. 2011;13(8):837–842. doi: 10.1089/dia.2011.0043. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabet research clin practice. 2005;67(1):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Welsh K.J., Kirkman M.S., Sacks D.B. Role of glycated proteins in the diagnosis and management of diabetes: research gaps and future directions. Diabetes care. 2016;39(8):1299–1306. doi: 10.2337/dc15-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meerwaldt R., Links T., Zeebregts C., Tio R., Hillebrands J.L., Smit A. The clinical relevance of assessing advanced glycation endproducts accumulation in diabetes. Cardiovascular Diabetology. 2008;7(1):1–8. doi: 10.1186/1475-2840-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma Y., Saxena S., Mishra A., Saxena A., Natu S.M. Advanced glycation end products and diabetic retinopathy. J ocular biology, diseases, and informatics. 2012;5(3-4):63–69. doi: 10.1007/s12177-013-9104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Genevieve M., Vivot A., Gonzalez C., Raffaitin C., Barberger-Gateau P., Gin H., Rigalleau V. Skin autofluorescence is associated with past glycaemic control and complications in type 1 diabetes mellitus. Diabet metabolism. 2013;39(4):349–354. doi: 10.1016/j.diabet.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Lutgers H.L., Graaff R., Links T.P., Ubink-Veltmaat L.J., Bilo H.J., Gans R.O., Smit A.J. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes care. 2006;29(12):2654–2659. doi: 10.2337/dc05-2173. [DOI] [PubMed] [Google Scholar]
- 50.Varikasuvu S.R., Sulekar H., Aloori S., Thangappazham B. The association of non-invasive skin autofluorescence measurements with cardiovascular and all-cause mortality in hemodialysis patients: a meta-analysis. International Urology and Nephrology. 2020;52(9):1757–1769. doi: 10.1007/s11255-020-02543-6. [DOI] [PubMed] [Google Scholar]
- 51.Saz-Lara A., Álvarez-Bueno C., Martínez-Vizcaíno V., Notario-Pacheco B. Sequí-Dominguez I, Cavero-Redondo I. Are advanced glycation end products in skin associated with vascular dysfunction markers? A meta-analysis. Inter J environ research public health. 2020;17(18):6936. doi: 10.3390/ijerph17186936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perrone A, Giovino A, Benny J, Martinelli F. Advanced glycation end products (AGEs): biochemistry, signaling, analytical methods, and epigenetic effects. Oxidative med cellular longevity, 2020 [DOI] [PMC free article] [PubMed]
- 53.Fokkens B.T., Smit A.J. Skin fluorescence as a clinical tool for non-invasive assessment of advanced glycation and long-term complications of diabetes. Glycoconjugate J. 2016;33(4):527–535. doi: 10.1007/s10719-016-9683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noordzij M.J., Lefrandt J.D., Graaff R., Smit A.J. Dermal factors influencing measurement of skin autofluorescence. Diabet technol therapeut. 2011;13(2):165–170. doi: 10.1089/dia.2010.0123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.