Abstract
Introduction
differential diagnosis of tumor recurrence and radiation injury after stereotactic radiotherapy (SRT) is challenging. The advances in imaging techniques and feature-based radiomics could aid to discriminate radionecrosis from progression.
Methods
we performed a systematic review of current literature, key references were obtained from a PubMed query. Data extraction was performed by 3 researchers and disagreements were resolved with a discussion among the authors.
Results
we identified 15 retrospective series, one prospective trial, one critical review and one editorial paper. Radiomics involves a wide range of imaging features referred to necrotic regions, rate of contrast-enhancing area or the measure of edema surrounding the metastases. Features were mainly defined through a multistep extraction/reduction/selection process and a final validation and comparison.
Conclusions
feature-based radiomics has an optimal potential to accurately predict response and radionecrosis after SRT of BM and facilitate differential diagnosis. Further validation studies are eagerly awaited to confirm radiomics reliability.
Graphical abstract
Introduction
Brain metastases (BM) develop in up to 30% of patients with cancer and, once appeared, patient's outcome is generally dismal [1]. Notwithstanding every type of cancer can theoretically metastasize to the brain, three solid tumors such as lung and breast carcinomas and malignant melanoma, account for up to 75% of BM [2], [3], [4]. Survival of patients affected by BM is extremely variable, influenced by several factors and remarkably poor in absence of active treatment [2,3]. Neurologic sign and symptoms (including seizures, focal deficits, headache, cognitive or motor disorders) are common severely affecting patients' quality of life [2].
The current cornerstones of BM treatment are surgery (S), whole brain radiotherapy (WBRT), and stereotactic radiotherapy (SRT) [5]. Regarding radiotherapy, although whole brain radiotherapy (WBRT) has been for long considered the mainstay of the treatment [6], in recent years there was a progressive shift towards SRT due to equivalent OS with improved local control and reduced cognitive deficits [6], [7], [8], [9], [10]. Stereotact radiosurgery (SRS) and hypofractionated SRT are today standard BM treatments when target lesions are small in size and limited in number, in patients with controlled extra-cerebral disease and an overall good performance status [11]. SRT is usually performed for a restricted number and/or limited total volume of BM and the delivery of the entire prescription dose in one or a few fractions of 8 to 30 Gy per fraction is foreseen in this approach [11].
Although the suitability of the classic linear-quadratic model for large doses per fraction remains an open question, clinical and preclinical data obtained with SRT suggest that high dose per fraction SRT provides a cell killing efficacy superior to those predicted from standard fractionation [12]. Referring to the outcome, SRT can ensure excellent local control of disease for intracranial neoplastic localizations, even though the optimal radiological assessment of the response to SRT treatment can often be challenging [13]. Indeed, the persistence of non-viable residual tissue is common and pseudoprogression (a transitory apparent increase of the lesion due to treatment-induced benign changes, such as inflammation or edema) is frequently observed [14].
Radiology plays an essential role in oncology, remarkably concerning the diagnosis and evaluation of treatment response [15]. The importance of imaging is as well crucial in radiation oncology [16], as it underlies every step of the diagnostic-therapeutic workflow: from tumor staging to definition of target volumes and organs at risk (OARs), the image-guided delivery and the evaluation of the response and toxicity in short and long-term follow up.
Coherently, the evolution of radiotherapy has always been inextricably linked to the introduction of new imaging techniques [17]. Essential milestones in clinical practice and in the subsequent advance of radiation oncology were the advent of computed tomography (CT)-scan, magnetic resonance imaging (MRI) and positron emission tomography (PET)-CT [18].
Particularly, the implementation of MRI has represented a radical revolution in neuro-oncology, as the high contrast for soft tissues allowed the precise definition of lesions that are hardly visible or undetectable on CT-scan [18], [19], [20]. In addition to its central role in the anatomical definition of cerebral lesions and in the contouring of the gross tumor volume (GTV) and intra-cranial organs at risk (OARs), MRI provides multiple functional studies that investigate different properties of the tissues [18].
These techniques encompass Diffusion-Weighted Imaging (DWI), which allows the evaluation of cellularity, perfusion and necrosis, and Perfusion-Weighted Imaging (PWI), which enables a detailed analysis of the perfusion [20]. Although to date PET is not routinely performed for the diagnosis of brain lesions, the use of tracers that have tissue specificity (such as 11C-methyl-L-methionine-MET) or related with functional features (e.g. hypoxia or cell proliferation) could prove to be valuable to identify intra-tumoral heterogeneity and to characterize ill-defined lesions [21].
Innovative imaging techniques might be helpful to discriminate therapy induced modifications effects from actual disease progression, but the massive amount of information provided by modern anatomical and functional studies could be complex to be interpreted [22]. Indeed, the substantial increase in the volume and complexity of data limits the adoption of a large part of the studies to experimental settings, as validated systems to process them within a timing suitable for clinical routine are still limited [23].
In this framework, Radiomics has proven to be a particularly promising application. Radiomics is the field of artificial intelligence that allows the extraction of features from standard bioimaging and generation of predictive models [24]. Its potential applications are extensive, both for the interpretation of data resulting from modern imaging modalities and for the identification of new features undetectable through the human eyes, thus overcoming several limits of conventional imaging and fostering a further advance in radiation oncology [24,25].
Promising possible directions of radiomics include the definition of potentially radio-resistant tumoral regions, early identification of neoplastic lesions before they could be visible with conventional imaging, the discrimination of real and pseudo-progression, the evaluation of radiation-induced tissue damage and automated detection and contouring of OARs and targets [24], [25], [26].
Reliable and standardized criteria are needed in order to improve both therapeutic management and expectancy of life of patients with uncontrolled intracranial disease showing heterogeneous and unpredictable response to radiotherapy. Thanks to the above mentioned advances in imaging techniques and the increasing interest in MRI-based texture analysis, a wide range of quantitative measures of radiological response has been characterized. In this regard, many authors have focused their efforts on the identification of these features as prognostic factors of further cerebral progression after SRT or differential diagnosis between progression and radionecrosis. We here review radiomics and its clinical application in BM management, summarizing the main limitations and future perspectives of this innovative approach (Fig. 1).
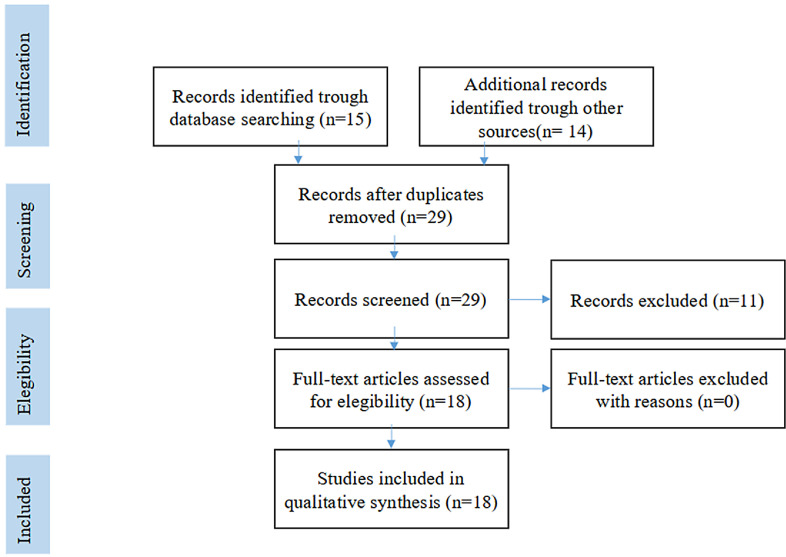
Fig. 1.
The PRISMA flow diagram.
Materials and methods - evidence acquisition
Between January and April 2021, key references were retrieved from a PubMed query, using the combination of the following keywords: <<radiomic>>AND <<stereotactic radiotherapy>>AND <<brain metastasis>>. The range of publication date was between 2015 and 2021.
Selection criteria included English language publications in humans. Hand searching included meeting proceedings of the European SocieTy for Radiotherapy & Oncology (ESTRO), European Society of Medical Oncology (ESMO), American Society of Clinical Oncology (ASCO) and American Society for Radiation Oncology (ASTRO). The website clinicaltrials.gov was also searched. Reference lists of the identified studies were explored and cross references were allowed. Data extraction was performed independently by three researchers (V.S, A.E.G and S.L) and disagreements were resolved on a case by case basis with a discussion among all the involved authors (C.G, L.B, V.N, F.D.F and I.D).
Results
Eighteen articles have been initially selected following the aforementioned inclusion criteria, as per the PRISMA flow diagram (Fig. 1). Of these, we identified 15 retrospective series, one prospective trial, one critical review and one editorial paper.
The radiological assessment of tumor response to SRT is often challenging, as tumor progression and radiation-induced damage might present with overlapping features. .In this context, feature-based radiomics can be applied to predict tumor recurrence (Table 1) and differential diagnosis between radiation injury and local progression (Table 2). We will discuss in the following paragraphs these two topics. Other papers retrieved are summarized in Table 3.
Table 1.
The current literature on the role of feature-based radiomics for predicting response after stereotactic radiation therapy for brain metastases.
| Prediction of local response after stereotactic radiotherapy of brain metastases |
| Study | Year | N° of pts | Type of study | Study population | Features | Outcomes | Results | Main conclusions |
|---|---|---|---|---|---|---|---|---|
| Karami et al. [35] | 2019 | 38 | Retrospective | BM treated withhypo-fractionated SRT | Quantitative MRI features of tumor and edema (T1w and T2-FLAIR images) | 6-month LC/LF | LF after SRT could be predicted with an AUC = 0.80 and an accuracy of82% | Biomarkers extracted from T1w and T2-FLAIR images have a good potential to predict the LC/LF outcome of SRT early after the treatment |
| Zheng et al.[36] | 2020 | 44 | Retrospective | BCBM treated with GKRS | Texture features | BCBM-specific progression-free survival | Age and CE-T1W-based kurtosis were the independent predictors. The combination of CE-T1W-based kurtosis and age displayed a higher C-index: 0.70 (95% CI 0.63–0.77) than did CE-T1W-based kurtosis or age alone. | Pretreatment CE-T1W-based kurtosis combined with age could improve prognostic ability in patients with BCBM undergoing GKRS. |
| Nardone et al. [37] | 2016 | 38 | Retrospective | 1, 2 BM (<3 cm) treated with SRT | ROI contoured by a radiation oncologist in consensus with a neuroradiologist, using the T2w sequences | Mean, standard deviation, skewness, kurtosis, entropy, and uniformity.OS, LF, L-TTP | A significant positive correlation (p = 0.013) was shown between L-TTP and entropy.A significant (p = 0.013) negative correlation of L-TTP for uniformity.Kurtosis had a significant negative correlation with both L-TTP and time to new BM (p = 0.046 and p = 0.023, respectively). | Entropy and uniformity could be an epiphenomenon of the radiosensibility and/or the vascularization of the BM and high kurtosis of the presence of a higher number of clonogenic cancer cells. |
| Della Seta et al. [38] | 2020 | 48 | Retrospective | Singular BM treated with SRT | quantitative tissue enhancement in pre-treatment cranial MRI | OS, iPFS | The enhancing tumor volume is significant for OS.Pts with high-level enhancement (>68.61% enhancing lesion volume) survived longer (4.9 vs. 10.2 months) and showed longer iPFS rates (univariable: P < 0.001). | Lesion enhancement may be a radiomic marker, useful in prognostic indices for survival prediction, in patients with singular BM. |
| Karami et al. [39] | 2019 | 100 | Retrospective | BM patients treated with SRT | Geometrical and textural features from T1w and T2-FLAIR images | Overall LC/LF6-month LC/LF12-month LC/LF | The optimal quantitative MRI biomarker consisted of 5 features that predict LF (AUC: 0.79), and a cross-validated sensitivity and specificity of 81% and 79%, respectively. The difference in LC (p<0.0001) and OS (p = 0.01)is statistically significant. | The majority of the features in the optimal quantitative MRI biomarkers characterize the heterogeneity in the surrounding regions of tumor including edema and tumor/lesion margins. |
| Mouraviev et al. [40] | 2020 | 87 | Retrospective | BM treated with GKRS | Radiomic features were extracted from the tumor core and the peritumoral regions, using the baseline pretreatment volumetric post-contrast T1 (T1c) and volumetric T2 fluid-attenuated inversion recovery (FLAIR) MRI sequences. | LF | An optimized combination of radiomic and clinical features resulted in a 19% higher resampled AUC (mean = 0.793; 95% CI = 0.792–0.795) than clinical features alone (0.669, 0.668–0.671). | The addition of radiomic features provides complementary information to standard routinely available clinical variables for the prediction of local failure in BM after SRS. A PM based on radiomic and clinical features shows promise for pretreatment outcome prediction. |
| Huang et al. [41] | 2020 | 161 | Retrospective | BM treated with GKRS | Shape features, first order features, and texture features | LC of BM after GKRS | Higher low GL zone emphasis (HR 0.757; P = 0.068) and higher zone percentage (HR 0.673; P= 0.005) of BMs are associated with a better LC.Higher zone percentage (HR 0.712; P = 0.022) is independently associated with the favorable LC of BMs. | Radiomic features indicate the biological basis and characteristics of tumors and could potentially be used as surrogate biomarkers for predicting tumor prognosis following GKRS. |
| Cha et al. [43] | 2018 | 89 | Retrospective | BM treated with SRS | Models including10 individual NN | SRS response | No significant differences between responders and non-responders in terms of MTD, tumor's intracranial position, age, sex, KPS, total dose of radiation, BED, or treatment platform.AUC range: 0.761–0.856 | A NN-based ensemble radiomics model that learned SRSfrom planning CT images for BMs and known earlyresponses predicted the SRS responses of unlearned imagesof BMs with high accuracy. |
| Kawahara et al. [42] | 2021 | 88 | Retrospective | Patients with BM | 700 features; NN prediction model | LC | 7 features with the accuracy and sensitivity of 44 and 54%.The accuracy and sensitivity of the proposed NN model were 78 and 87%, AUC: 0.87. | The proposed NN model using the radiomics features can help physicians to gain a more realistic expectation of the treatment outcome than the traditional method |
Abbreviations: patients (pts), stereotactic radiotherapy (SRT), stereotactic radiosurgery (SRS), area under the curve (AUC), brain metastasis (BM), Magnetic Resonance Imaging (MRI), contrast-enhanced T1-weighted (CE T1w), T2-weighted (T2w), Gamma Knife radiosurgery (GKRS), Breast cancer BM (BCBM), region of interest (ROI), overall survival (OS), time to local progression (L-TTP), local failure (LF), local control (LC), predictive model (PM), hazard ratio (HR), confidence interval (CI), maximum tumor diameter (MTD), Karnofsky performance status (KPS), biologically.
effective dose (BED), progression disease (PD), gray level (GL), gray level run length matrix (GLRLM), neighborhood gray tone difference matrix (NGTDM), gray level co-occurrence matrix (GLCM) not specified (NS), not applicable (NA), Least absolute shrinkage and selection operator (LASSO), Neural network (NN), intracranial progression-free survival (iPFS).
Table 2.
The current literature on the role of feature-based radiomics for differential diagnosis between tumor progression and radiation injury after stereotactic radiation therapy for brain metastasis.
| Differentiation of radiation injury from local brain metastasis relapse |
| Study | Year | N° of pts | Type of study | Study population | Features | Outcomes | Results | Main conclusions |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. [46] | 2018 | 87 | Retrospective | Pathologically confirmed necrosis or progression BM | 285 Textural features:- GL co-occurrence matrix- GLRLM- geometric shape;- NGTDM- histogram of oriented gradients2280 radiomic features1140 delta radiomic features | Radionecrosis vs PD | Delta radiomic features with a RUSBoost ensemble classifier had an overall predictive accuracy of 73.2%, AUC: 0.73 in leave-one-out cross-validation. | Delta radiomic features extracted from MRI images have potential for distinguishing radiation necrosis from PD after SRS for BM |
| Hettal et al.[47] | 2020 | 20 | Retrospective | BM | Quantitative imaging | Radionecrosis vs PD | Clinical relevance of 1766 radiomics features (T1-w MRI) after SRT showing a lesion modification: 7 feature-selection methods and 12 classification methods in terms of respective predictive performance. 75% of prediction accuracy for radionecrosis, and 91% for PD. | Radiomics method is able to discriminate radionecrosis from progression in an accurate, early and noninvasive way. |
| Peng et al. [48] | 2018 | 66 | Retrospective | BM treated with SRS | Radiomic features from T1c or FLAIR imaging sequence.5 categories:- first-order statistics (14)- GLCM (18)- GLRLM (11)- NGTDM (5)- morphologic features (3) | Treatment effect after SRS | An optimized Iso SVM classifier based on top-ranked radiomic features had sensitivity and specificity of 65.38% and 86.67%, respectively, AUC: 0.81 on leave-one-out cross-validation. 73% of cases were classifiable by the neuroradiologist, with a sensitivity of 97% and specificity of 19%. | Radiomics holds promise for differentiating between treatment effect and true progression in BM treated with SRS. A predictive model built on radiomic features from an institutional cohort performed well on cross-validation testing. |
| Tiwari et al. [49] | 2016 | 21 | Retrospective | Brain tumor MR imaging performed 9 months post-radiochemotherapy | Radiomic features from MRI imaging sequence: CE T1w, T2w, and FLAIR | Radionecrosis vs PD | AUC was highest for FLAIR (0.79) | Radiomic features may provide complementary diagnostic information on routine MR imaging sequences that may improve the distinction of radiation necrosis from recurrence for both primary and BM. |
| Lohmann et al. [51] | 2018 | 47 | Retrospective | New or progressive contrast-enhancing brain lesions on MRI after radiotherapy(predominantly SRS) of BM | Textural featuresextracted from CE-MRIFET PET texturalfeatures | Radionecrosis vs PD | Textural features from CE-MRI had a diagnostic accuracy of 81% (sensitivity, 67%; specificity, 90%).FET PET textural had a slightly higher diagnostic accuracy of 83% (sensitivity, 88%; specificity, 75%).The combination of CE-MRI and FET PET features: accuracy, 89%;sensitivity, 85%; specificity, 96%. | Findings suggest that combined FET PET/CE-MRI radiomics using textural feature analysisoffers a great potential to contribute significantly to the management of pts with BM. |
| Larroza et al. [52] | 2015 | 73 | Retrospective | Patients with BM treated with SRS | Texture features were extracted from CE T1w | Radionecrosis vs PD | 7 features with an AUC of 0.94 | High classification accuracy was obtained using texture features and a support vector machine classifier in an approach based on conventional MRI to differentiate between BM and radiation necrosis. |
| Takami et al. [53] | 2020 | 30 | Prospective | Patient with BM treated with neoadjuvant SRS | NS | LC and radiation toxicity | Clinical trial Protocol | Assessment of toxicity associated with SRS and provide additional quantitative metrics of efficacy for future comparative trials |
| Others | ||||||||
Abbreviations: patients (pts), stereotactic radiotherapy (SRT), stereotactic radiosurgery (SRS), area under the curve (AUC), brain metastasis (BM), Magnetic Resonance Imaging (MRI), contrast-enhanced T1-weighted (CE T1w), T2-weighted (T2w), Gamma Knife radiosurgery (GKRS), Breast cancer BM (BCBM), region of interest (ROI), overall survival (OS), time to local progression (L-TTP), local failure (LF), local control (LC), predictive model (PM), hazard ratio (HR), confidence interval (CI), maximum tumor diameter (MTD), Karnofsky performance status (KPS), biologically.
effective dose (BED), progression disease (PD), gray level (GL), gray level run length matrix (GLRLM), neighborhood gray tone difference matrix (NGTDM), gray level co-occurrence matrix (GLCM) not specified (NS), not applicable (NA), Least absolute shrinkage and selection operator (LASSO), Neural network (NN), intracranial progression-free survival (iPFS).
Table 3.
Other papers regarding potential roles of feature-based radiomics in the treatment of brain metastases or central nervous system primary tumors.
| Study | Year | N° of pts | Type of study | Study population | Features | Outcomes | Results | Main conclusions |
|---|---|---|---|---|---|---|---|---|
| Kocher et al. [26] | 2020 | NA | Critical review | Malignant glioma or BM | Feature-based radiomics and deep learning-based machine learning methods | LC after SRTRadiation necrosis vs recurrent BM | Radiomics can detect smaller BM, accurate segmentation of multiple larger BM, prediction of LC after SRS, and differentiate radiation injury from LF.High diagnostic accuracies of 80–90% can be achieved by most approaches. | Clinical application of radiomics and artificial intelligence has a great potential for improving radiotherapy in patients with malignant brain tumors. A common problem is the large variability and the lack of standardization. |
| Hu et al. [54] | 2020 | NA | Editorial paper | Glioblastoma or multiple BM | Machine-learning algorithms | NS | NS | Radiomics models run the risk of extrapolation which underscores the importance of quantifying the uncertainty of each model prediction |
Abbreviations: not applicable (NA), brain metastasis (BM), local control (LC), stereotactic radiotherapy (SRT), stereotactic radiosurgery (SRS), local failure (LF), not specified (NS).
Analysis of response to stereotactic radiotherapy for brain metastasis
As previously described, the main factors implicated in the response to SRT include size of lesions and prescription dose of radiotherapy [27,28]. However, the radiological assessment of BM treated with ablative radiotherapy is still complex, mainly due to specific radiobiological determinants [29].
It is generally accepted that chronic hypoxic cancer cells localizing on the edge of necrotic regions of the metastasis may be characterized by radioresistance [30]. Moreover, the delivery time of the single-fraction radiosurgery presumably does not allow for sufficient reoxygenation during treatment. Coherently, the presence of tumor necrosis should strongly influence the local control rates and survival in patients affected by BM treated by SRT [31]. Considering that the biological aspects of BM contribute to determine the radiological appeareance of the tumor and surrounding tissues, feature-based radiomics can help to identify features that are not clearly visible by the naked eye, and thus accurately predict BM specific recurrence and local control.
Radiomics involves a wide range of imaging techniques and heterogeneous features referred to radiological determinants such as the evidence of necrotic regions, the rate of contrast-enhancing area, or the measure of edema surrounding the secondary lesion [32]. According to literature, radiomic features are mainly defined through a multistep extraction/reduction/selection process and a final validation and comparison resulting in optimal quantitative biomarkers [33]. After processing image and defining regions of interest of tumor and/or surrounding edema regions, algorithms are applied to generate multiscale filter features. The geometrical and textural radiomic features can be extracted using different time points, such as the baseline and follow-up, and their modifications are used to develop prognostic models of outcome, in the framework of delta radiomics analysis [34].
With respect to this, Karami et al. [35] analyzed a retrospective series of 38 patients with BM treated with hypofractionated SRT. Extrapolating quantitative MRI features from T1-weighted (T1w) and T2-FLAIR images, the authors reported that 6 month local control after treatment could be predicted with an area under the curve (AUC) of 0.80 and an accuracy of 82%. Similarly, an analysis of MRI texture features from 44 BM of breast cancer patients undergoing SRT highlighted the prognostic ability of pretreatment contrast-enhanced T1-weighted (CE T1w) based kurtosis combined with age [36]. Nardone et al. [37] used an in-house developed software to perform a first-order texture analysis on pretreatment MRI images of consecutive non-small-cell lung cancer (NSCLC) patients with 1, 2 brain metastases who underwent SRT (median total dose 20 Gy, range 18–24 Gy in 3–5 fractions) or SRS (median dose 18 Gy, range 14–23 Gy).
A significant positive correlation was observed between time to local progression (L-TTP) and entropy, with better results for patients with values over the median value. Conversely, a significant negative correlation of L-TTP was obtained for uniformity. Kurtosis had a significant negative correlation with both L-TTP and time to new BM. Although no defined biological correlation has been established, authors speculated that entropy and uniformity could be an epiphenomenon of the radiosensibility and/or the vascularization of the BM and high kurtosis of the presence of a higher number of clonogenic cancer cells [37]. Moreover, the quantitative tissue enhancement in pretreatment cranial MRI was evaluated also in a series of singular brain secondary lesions treated with SRT [38]. Patients with high-level enhancement (>68.61% enhancing lesion volume) survived significantly longer and showed significantly longer intracranial progression-free survival (iPFS) rates. Patients with lesions that showed a higher percentage of enhancement in pre-treatment MRI demonstrated improved intracranial progression-free survival (iPFS) and overall survival (OS) compared to those with mainly hypo-enhancing lesion [38]. Additionally, data from 133 BM treated with SRT were retrospectively analyzed by Karami et al. [39]; the most represented tumor types were NSCLC (48.9%), breast cancer (23.3%) and melanoma (9%).
MRI features T1w and T2-FLAIR images were acquired before SRT and every 2, 3 months. A total of 3072 imaging features were extracted and selected by machine learning algorithms and the relative change from the baseline was calculated for each feature. A support vector machine (SVM) classifier was used to predict outcomes of SRT in terms of local control or failure. The optimal quantitative MRI biomarkers resulted in an AUC of 0.80, 0.81, and 0.79 for the 6-month, 12-month and overall local control, with a cross-validated accuracy of 80%, 82% and 80%, respectively. Texture features mainly characterizing the heterogeneity from the edema and lesion margin showed higher prognostic performances compared to those extracted from the tumor itself, suggesting that this variety could reflect the presence of malignant cells that are not yet enough to result in an evident image [39]. Mouraviev et al. [40] also investigated pretreatment T1-w and T2-FLAIR MRI sequences of BM treated with Gamma Knife. A total of 440 features were compared in their ability to predict local lesion control and selected using a machine learning algorithm by resampled random forest (RF) feature importance. The addition of any one of the top 10 radiomic features to the set of clinical features resulted in a statistically significant increase of approximately 19% in the AUC. Conversely, according to Karami et al. [39], 9 out of the top 10 radiomic features were T1-w based. This could be partly correlated with the automated method used to contour the region of interest on the T2-FLAIR sequences that may have reduced its accuracy and incremented image noise.
The two radiomic features that emerged as significant on univariate analysis were related to T1-w core volume (a surrogate of tumor size, known predictor of local failure) and sphericity (generally correlated with higher probability of local failure for low values). Patients affected by NSCLC who underwent Gamma Knife radiosurgery (GKRS) for BM were retrospectively analyzed by Huang et al. [41]: 107 radiomic features (14 shape, 18 first order and 75 texture features) of each brain metastasis were extracted and selected to reduce redundancy using consensus clustering with dedicated software. The zone percentage of BM, derived from pre-GKRS CE-T1w MRI, was found to be an independent prognostic factor of local tumor control. Higher zone percentage, indicating a finer texture and thus a more homogeneous enhancement pattern, was independently associated with superior LC on univariate analysis, multivariate Cox proportional hazards model and multivariate cause-specific proportional hazards model [41].
A recent experience [42] provided a model to predict the response of BM treated by GKRS using a machine learning process with radiomics features. Using MRI sequence data extracted from CET1w, the local response of 157 brain metastases was classified into two groups (Group I: responder and Group II: non-responder). The authors used the least absolute shrinkage and selection operator (LASSO) regression to reduce the total number of extracted features (700) in order to build a neural network predictive model. The accuracy, sensitivity and the AUC of the prediction model of local recurrence (LR) were analyzed. The performance of the machine learning process was compared with a visual evaluation method. Seven radiomic features were found to be useful for the classification through the LASSO analysis of the training data. The accuracy and sensitivity of the visual evaluation approach were 44 and 54%, respectively, while they were 78 and 87% in the neural network predictive model that achieved an AUC of 0.87 [42]. Adopting a different approach, Cha et al. [43] utilized planning CT-scan images of 89 patients with 110 BM to develop a convolutional neural network (CNN)-based radiomics model. The lesions had a maximum diameter of 1–3.5 cm and SRT was performed with CyberKnife or RapidArc at a median dose of 23 Gy. The CNN model was able to predict response to SRT with an AUC ranging from 0.60 to 0.82. The AUC of ensemble models, which averaged prediction results of 10 individual models within the same group, ranged from 0.76 to 0.85, with a sensitivity and specificity for response prediction of, respectively, 82% and 83%.
Differential diagnosis between radiation necrosis and brain metastasis recurrence
Radiation damage of the brain tissue after radiosurgery may occur in approximately 5–20% of BM treatments [44]. The diagnosis of radiation induced injury can be suspected when the new contrast-enhancing lesion is within the GTV or close to its edge. The differential diagnosis between radiation induced necrosis and disease recurrence is often hard to define using conventional MRI alone. The increasing integration of radiomic frameworks in the radiological diagnostics of BM may therefore improve the appropriate detection of radionecrosis after SRT and successfully support clinicians in their decision making process [45].
With regards to this, Zhang et al. [46] retrospectively analyzed the feature-based radiomic profile of 87 patients with pathologically confirmed necrosis or progression after SRT for brain metastasis. “Delta” radiomic features, defined as the change in features from baseline to the next time points, were extracted for each MRI sequence. The combination of 5 radiomic features from both CE-T1w and T2 MR images were demonstrated to be helpful in differential diagnosis between radiation injury and progression lesions, More specifically, delta radiomic features was found to have an overall predictive accuracy of 73.2% and AUC value of 0.73. Coherently, a promising model for assessing the appropriate radiomic framework to support decision-making in brain oligometastases was recently reported by Hettal et al. [47]. 1766 features were selected using IBEX software from CE T1-W MRI sequences after SRT comparing them with baseline radiomic determinants. The authors analyzed 7 feature-selection processes and 12 classification methods in order to identify the respective predictive power. The classification with the best predictive ability, measured by Cohen's kappa, was found to have a score of 0.68 with an accuracy of 85%. Radionecrosis and progression of lesions were predicted with an accuracy of 75% and 91%, respectively. Similarly, Peng et al. [48] developed a method to distinguish true progression from post-treatment changes after SRT for BM in patients affected from different histology of primary tumor (i.e. NSCLC, melanoma and breast cancer). Most lesions were treated with Cyberknife at a median dose of 20 Gy (range 14–25 Gy) delivered in one fraction. Among 82 identified lesions with suspect progression after treatment, 77 underwent surgical resection for histopathologic diagnosis resulting in 63% of progression confirmed by histopathology and the remaining labeled as treatment effect. 51 radiomic features were extracted from T1/T2-FLAIR MRI sequences. A hybrid machine-learning selection/classification algorithm was assessed by 10-fold cross-validation with 100 repeats. All the cases were independently reviewed by an expert board-certified neuroradiologist for comparison.
The radiomic-based machine learning algorithm improved differentiation between true progression and treatment effect: sensitivity and specificity were, respectively, 65.4% and 86.7% (machine learning) versus 97% and 19% (neuroradiologist assessment). Moreover, non-uniformity parameter was higher in true progression cases, highlighting the possible use of tissue heterogeneity as a biomarker for malignancy.
A preliminary study by Tiwari et al. [49] investigated a small sample ‘training’ cohort’ of 43 patients with either primary or metastatic brain lesions recurring after radiotherapy, developing a machine-learning algorithm based on radiomic texture features from CE-T1w, T2w, and FLAIR MRI sequences. All the patients underwent resection or biopsy of the recurrent lesion to discriminate progression from radionecrosis. Among patients of the metastatic group, 12 lesions were true progression and 9 radionecrosis. 119 2-D radiomic textural features were extracted using in-house developed software. The minimum redundancy and maximum relevance (mRmR) were used to select the top 5 most discriminative features that were included in a SVM classifier algorithm. The best performing radiomic framework was obtained for FLAIR with accuracy and AUC values of 0.75 and 0.79, respectively. This model was validated in a cohort of 15 ‘test’ patients enrolled in a second institution, unfortunately including only 4 metastatic lesions, that were as well evaluated by two board-certified neuro-radiologists. Accuracy for these four lesions was 50% for both the two radiologists and the evaluated algorithm.
In addition to MRI, some authors focused their efforts on the extraction of radiomic features from amino acid PET, aiming to improve the diagnosis of treatment-related injury from BM progression [50]. With this regard, Lohmann et al. [51] retrospectively evaluated 47 patients (mainly affected by lung or breast cancer) with BM treated with SRT alone (16–25 Gy) or in combination with WBRT performing a dynamic O-(2-[18F]fluoroethyl)-L-tyrosine (18F-FET) PET. 56% of lesions were classified as recurrent BM and 44% as radiation injury. Tumor-to-brain ratios (TBRs) of 18F-FET uptake and 62 textural parameters were determined, as well as kinetic parameters. Textural parameters resulted in a slight increase of diagnostic accuracy in combination with TBRs, while there was no improvement of diagnostic accuracy in combination with kinetic PET parameters. The accuracy of TBR mean and max were found to be 81% and 83%, increased to 85% after combining with the textural parameters Coarseness or Short-zone emphasis. On the other hand, an increase of specificity up to 100% was observed when the standard parameters were combined with textural parameters. Consistently to the previous experience, Larroza et al. [52] retrospectively analyzed a cohort of 73 patients with a total of 115 BM treated with SRT. Texture features were extracted from CE-T1w images, to determine the differential diagnosis between radiation necrosis and metastatic progression. A subset of 7 optimal features were found and the Receiver operating characteristic (ROC) curves provided high classification accuracy (AUC > 0.9).
Lastly, Takami H et al. designed a multi-center, non-randomized, open phase II clinical trial which investigated a novel strategy using neoadjuvant SRS (NaSRS) followed by resection in a small series of 30 patients with up to 10 BM. Interestingly, authors reported a lower rate of postoperative leptomeningeal dissemination and symptomatic radiation injury for the NaSRS group compared to the cohort of patients treated with postoperative SRS. Among the study's outcomes reported in the clinical trial protocol, the tertiary analyses will assess the correlation between local control and radiation toxicity with pretreatment clinical factors, serum markers, radiomic features and molecular analysis of the resected BM [53]. Main limitations of the studies analyzed in our work are the retrospective nature of the series, the non-standardized pool of selected radiomic features, the common absence of an external validation cohorts, and the relatively small sample size. Although the obtained results seem promising, remarkably in terms of specificity, the above mentioned pitfalls can limit the performance of classification algorithms and the replicability using independent datasets [54].
Conclusion
Despite the remarkable progress in the field of diagnostic imaging, differential diagnosis between tumor progression and treatment-related effects is often challenging. Moreover, clinical parameters might be sub-optimal to predict response to treatment and prognosis. Multiple experiences highlighted the strong correlation between quantitative imaging features and outcomes after radiation therapy, with the potential to generate predictive models that could improve the diagnostic and therapeutic process of personalized cancer care. Hence, a multidisciplinary approach involving clinicians, medical physicists, and computer scientists becomes necessary to integrate feature-based radiomics and deep learning-based machine learning methods in the medical decision-making and radiation therapy workflow of BM. The imaging biomarkers extracted from MRI or PET images show an optimal potential to predict with high classification accuracy the local control/failure after SRT and as well to aid in the differential diagnosis between radiation necrosis and tumor progression after SRT treatment.
In this respect, further validation studies of the existing predictive models are strongly awaited to confirm radiomics reliability.
CRediT authorship contribution statement
Viola Salvestrini: Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing. Carlo Greco: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Supervision. Andrea Emanuele Guerini: Methodology, Data curation, Writing – original draft, Writing – review & editing, Visualization. Silvia Longo: Data curation, Writing – original draft, Writing – review & editing. Valerio Nardone: Conceptualization, Methodology, Validation, Formal analysis, Writing – review & editing, Visualization, Supervision. Luca Boldrini: Conceptualization, Validation, Formal analysis, Writing – review & editing, Visualization, Supervision. Isacco Desideri: Conceptualization, Validation, Formal analysis, Writing – review & editing, Visualization, Supervision. Francesca De Felice: Conceptualization, Validation, Formal analysis, Writing – review & editing, Visualization, Supervision.
CRediT authorship contribution statement
Viola Salvestrini: Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing. Carlo Greco: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Supervision. Andrea Emanuele Guerini: Methodology, Data curation, Writing – original draft, Writing – review & editing, Visualization. Silvia Longo: Data curation, Writing – original draft, Writing – review & editing. Valerio Nardone: Conceptualization, Methodology, Validation, Formal analysis, Writing – review & editing, Visualization, Supervision. Luca Boldrini: Conceptualization, Validation, Formal analysis, Writing – review & editing, Visualization, Supervision. Isacco Desideri: Conceptualization, Validation, Formal analysis, Writing – review & editing, Visualization, Supervision. Francesca De Felice: Conceptualization, Validation, Formal analysis, Writing – review & editing, Visualization, Supervision.
Acknowledgment
The Authors thank the Scientific Committee and Board of the AIRO for the critical revision and final approval of the manuscript (Nr. 36/2021)
Contributor Information
Carlo Greco, Email: c.greco@unicampus.it.
Andrea Emanuele Guerini, Email: a.e.guerini@gmail.com.
Silvia Longo, Email: silvia.longo@guest.policlinicogemelli.it.
Valerio Nardone, Email: v.nardone@hotmail.it.
Luca Boldrini, Email: luca.boldrini@policlinicogemelli.it.
Isacco Desideri, Email: isacco.desideri@unifi.it.
Francesca De Felice, Email: francesca.defelice@uniroma1.it.
References
- 1.Wen P.Y., Black P.M., Loeffler J.S. In: Cancer: Principles and Practice of Oncology. DeVita V., Hellman S., Rosenberg S.A., editors. Lippincott, Williams & Wilkins; Philadelphia, PA: 2001. Metastatic brain cancer; pp. 2655–2670. [Google Scholar]
- 2.Stelzer K.J. Epidemiology and prognosis of brain metastases. Surg. Neurol. Int. 2013;4(Suppl 4):S192–S202. doi: 10.4103/2152-7806.111296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cagney D.N., Martin A.M., Catalano P.J., et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro. Oncol. 2017;19(11):1511–1521. doi: 10.1093/neuonc/nox077. Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brastianos P.K., Carter S.L., Santagata S., et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5:1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller-Riemenschneider F., Bockelbrink A., Ernst I., et al. Stereotactic radiosurgery for the treatment of brain metastases. Radiother. Oncol. 2009;91:67–74. doi: 10.1016/j.radonc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Borghetti P., Pedretti S., Spiazzi L., et al. Whole brain radiotherapy with adjuvant or concomitant boost in brain metastasis: dosimetric comparison between helical and volumetric IMRT technique. Radiat. Oncol. 2016;11:59. doi: 10.1186/s13014-016-0634-6. Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinde A., Akhavan D., Sedrak M., et al. Shifting paradigms: whole brain radiation therapy versus stereotactic radiosurgery for brain metastases. CNS Oncol. 2019;8(1):CNS27. doi: 10.2217/cns-2018-0016. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahgal A., Aoyama H., Kocher M., et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015;91(4):710–717. doi: 10.1016/j.ijrobp.2014.10.024. Mar 15. [DOI] [PubMed] [Google Scholar]
- 9.Buglione M., Jereczek-Fossa B.A., Bonù M.L., et al. Radiosurgery and fractionated stereotactic radiotherapy in oligometastatic/oligoprogressive non-small cell lung cancer patients: results of a multi-institutional series of 198 patients treated with "curative" intent. Lung Cancer. 2020;141:1–8. doi: 10.1016/j.lungcan.2019.12.019. Mar. [DOI] [PubMed] [Google Scholar]
- 10.Brown P.D., Jaeckle K., Ballman K.V., et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. doi: 10.1001/jama.2016.9839. Jul 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soliman H., Das S., Larson D.A., et al. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016;7(11):12318–12330. doi: 10.18632/oncotarget.7131. Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown J.M., Carlson D.J., Brenner D.J. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int. J. Radiat. Oncol. Biol. Phys. 2014;88(2):254–262. doi: 10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galldiks N., Kocher M., Ceccon G., et al. Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: response, progression, and pseudoprogression. Neuro. Oncol. 2020;22(1):17–30. doi: 10.1093/neuonc/noz147. Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruzevick J., Kleinberg L., Rigamonti D. Imaging changes following stereotactic radiosurgery for metastatic intracranial tumors: differentiating pseudoprogression from tumor progression and its effect on clinical practice. Neurosurg. Rev. 2014;37(2):193–201. doi: 10.1007/s10143-013-0504-8. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins L.J., Pomper M.G. The evolution of imaging in cancer: current state and future challenges. Semin. Oncol. 2011;38(1):3–15. doi: 10.1053/j.seminoncol.2010.11.010. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira G.C., Traughber M., Muzic R.F. The role of imaging in radiation therapy planning: past, present, and future. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/231090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaton L., Bandula S., Gaze M.N., Sharma R.A. How rapid advances in imaging are defining the future of precision radiation oncology. Br. J. Cancer. 2019;120(8):779–790. doi: 10.1038/s41416-019-0412-y. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo M. History and evolution of brain tumor imaging: insights through radiology. Radiology. 2014;273(2 Suppl):S111–S125. doi: 10.1148/radiol.14140130. Nov. [DOI] [PubMed] [Google Scholar]
- 19.Langen K.J., Galldiks N., Hattingen E., Shah N.J. Advances in neuro-oncology imaging. Nat. Rev. Neurol. 2017;13(5):279–289. doi: 10.1038/nrneurol.2017.44. May. [DOI] [PubMed] [Google Scholar]
- 20.Benzakoun J., Robert C., Legrand L., et al. Anatomical and functional MR imaging to define tumoral boundaries and characterize lesions in neuro-oncology. Cancer Radiother. 2020;24(5):453–462. doi: 10.1016/j.canrad.2020.03.005. Aug. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann P., Kocher M., Ruge M.I., et al. PET/MRI radiomics in patients with brain metastases. Front. Neurol. 2020;11:1. doi: 10.3389/fneur.2020.00001. Feb 7eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soares J.M., Magalhães R., Moreira P.S., et al. A Hitchhiker's guide to functional magnetic resonance imaging. Front. Neurosci. 2016;10:515. doi: 10.3389/fnins.2016.00515. Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguirre G.K. Functional neuroimaging: technical, logical, and social perspectives. Hastings Cent. Rep. 2014 doi: 10.1002/hast.294. Mar-AprSpec No:S8-18. [DOI] [PubMed] [Google Scholar]
- 24.Bibault J.E., Xing L., Giraud P., et al. Radiomics: a primer for the radiation oncologist. Cancer Radiother. 2020;24(5):403–410. doi: 10.1016/j.canrad.2020.01.011. Aug. [DOI] [PubMed] [Google Scholar]
- 25.Boldrini L., Bibault J.E., Masciocchi C., et al. Deep Learning: a review for the radiation oncologist. Front. Oncol. 2019;9:977. doi: 10.3389/fonc.2019.00977. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kocher M., Ruge M.I., Galldiks N., Lohmann P. Applications of radiomics and machine learning for radiotherapy of malignant brain tumors. Strahlenther. Onkol. 2020;196(10):856–867. doi: 10.1007/s00066-020-01626-8. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamel-Perreault E., MathieuD L., Masson-Cote L. Factors influencing the outcome of stereotactic radiosurgery in patients with five or more brain metastases. Curr. Oncol. 2019;26(1):e64–e69. doi: 10.3747/co.25.4244. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minniti G., Scaringi C., Paolini S., et al. Single-fraction versus multifraction (3 × 9Gy) stereotactic radiosurgery for large (>2cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2016;95(4):1142–1148. doi: 10.1016/j.ijrobp.2016.03.013. Jul 15. [DOI] [PubMed] [Google Scholar]
- 29.Song C.W., Kim M.S., Cho L.C., et al. Radiobiological basis of SBRT and SRS. Int. J. Clin. Oncol. 2014;19(4):570–578. doi: 10.1007/s10147-014-0717-z. [DOI] [PubMed] [Google Scholar]
- 30.Sørensen B.S., Horsman M.R. Tumor hypoxia: impact on radiation therapy and molecular pathways. Front. Oncol. 2020;10:562. doi: 10.3389/fonc.2020.00562. Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kocher M., Voges J., Treuer H., Kondziolka D., et al. Vol. 3. Karger; 2000. Reduced response rate of necrotic brain metastases to radiosurgery; pp. 240–246. (Radiosurgery 1999). Radiosurgery. Basel. [Google Scholar]
- 32.Kocher M., Ruge M.I., Galldiks N., Lohmann P. Applications of radiomics and machine learning for radiotherapy of malignant brain tumors. Strahlenther. Onkol. 2020;196(10):856–867. doi: 10.1007/s00066-020-01626-8. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwanenburg A., Vallières M., Abdalah M.A. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295(2):328–338. doi: 10.1148/radiol.2020191145. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peeken J.C., Nüsslin F., Combs S.E. "Radio-oncomics": the potential of radiomics in radiation oncology. Strahlenther. Onkol. 2017;193(10):767–779. doi: 10.1007/s00066-017-1175-0. Oct. [DOI] [PubMed] [Google Scholar]
- 35.Karami E., Ruschin M., Soliman H., et al. An MR radiomics framework for predicting the outcome of stereotactic radiation therapy in brain metastasis. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019;2019:1022–1025. doi: 10.1109/EMBC.2019.8856558. Jul. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y., Geng D., Yu T., et al. Prognostic value of pretreatment MRI texture features in breast cancer brain metastasis treated with Gamma Knife radiosurgery. Acta Radiol. 2020 doi: 10.1177/0284185120956296. Sep 10284185120956296. [DOI] [PubMed] [Google Scholar]
- 37.Nardone V., Tini P., Biondi M., et al. Prognostic value of MR imaging texture analysis in brain non-small cell lung cancer oligo-metastases undergoing stereotactic irradiation. Cureus. 2016;8(4):e584. doi: 10.7759/cureus.584. Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Della Seta M., Collettini F., Chapiro J., et al. A 3D quantitative imaging biomarker in pre-treatment MRI predicts overall survival after stereotactic radiation therapy of patients with a singular brain metastasis. Acta Radiol. 2019;60(11):1496–1503. doi: 10.1177/0284185119831692. Nov. [DOI] [PubMed] [Google Scholar]
- 39.Karami E., Soliman H., Ruschin M., et al. Quantitative MRI biomarkers of stereotactic radiotherapy outcome in brain metastasis. Sci. Rep. 2019;9(1):19830. doi: 10.1038/s41598-019-56185-5. Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouraviev A., Detsky J., Sahgal A., et al. Use of radiomics for the prediction of local control of brain metastases after stereotactic radiosurgery. Neuro. Oncol. 2020;22(6):797–805. doi: 10.1093/neuonc/noaa007. Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C.Y., Lee C.C., Yang H.C., et al. Radiomics as prognostic factor in brain metastases treated with Gamma Knife radiosurgery. J. Neurooncol. 2020;146(3):439–449. doi: 10.1007/s11060-019-03343-4. Feb. [DOI] [PubMed] [Google Scholar]
- 42.Kawahara D., Tang X., Lee C.K., et al. Predicting the local response of metastatic brain tumor to gamma knife radiosurgery by radiomics with a machine learning method. Front. Oncol. 2021;10 doi: 10.3389/fonc.2020.569461. Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cha Y.J., Jang W.I., Kim M.-.S., et al. Prediction of response to stereotactic radiosurgery for brain metastases using convolutional neural networks. Anticancer Res. 2018;38(9):5437–5445. doi: 10.21873/anticanres.12875. Sep. [DOI] [PubMed] [Google Scholar]
- 44.Vellayappan B., Tan C.L., Yong C., et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front. Oncol. 2018;8:395. doi: 10.3389/fonc.2018.00395. Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondziolka D., Shin S.M., Brunswick A., et al. The biology of radiosurgery and its clinical applications for brain tumors. Neuro. Oncol. 2015;17(1):29–44. doi: 10.1093/neuonc/nou284. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z., Yang J., Ho A., et al. A predictive model for distinguishing radiation necrosis from tumour progression after gamma knife radiosurgery based on radiomic features from MR images. Eur. Radiol. 2018;28(6):2255–2263. doi: 10.1007/s00330-017-5154-8. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hettal L., Stefani A., Salleron J., et al. Radiomics method for the differential diagnosis of radionecrosis versus progression after fractionated stereotactic body radiotherapy for brain oligometastasis. Radiat. Res. 2020;193(5):471–480. doi: 10.1667/RR15517.1. May. [DOI] [PubMed] [Google Scholar]
- 48.Peng L., Parekh V., Huang P., et al. Distinguishing true progression from radionecrosis after stereotactic radiation therapy for brain metastases with machine learning and radiomics. Int. J. Radiat. Oncol. Biol. Phys. 2018;102(4):1236–1243. doi: 10.1016/j.ijrobp.2018.05.041. Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiwari P., Prasanna P., Wolansky L., et al. Computer-extracted texture features to distinguish cerebral radionecrosis from recurrent brain tumors on multiparametric MRI: a feasibility study. AJNR Am. J. Neuroradiol. 2016;37(12):2231–2236. doi: 10.3174/ajnr.A4931. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ceccon G., Lohmann P., Stoffels G., et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol. 2017;19(2):281–288. doi: 10.1093/neuonc/now149. Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lohmann P., Stoffels G., Ceccon G., et al. Radiation injury vs. recurrent brain metastasis: combining textural feature radiomics analysis and standard parameters may increase (18)F-FET PET accuracy without dynamic scans. Eur. Radiol. 2017;27(7):2916–2927. doi: 10.1007/s00330-016-4638-2. Jul. [DOI] [PubMed] [Google Scholar]
- 52.Larroza A., Moratal D., Paredes-Sánchez A., et al. Support vector machine classification of brain metastasis and radiation necrosis based on texture analysis in MRI. J. Magn. Reson. Imaging. 2015;42(5):1362–1368. doi: 10.1002/jmri.24913. Nov. [DOI] [PubMed] [Google Scholar]
- 53.Takami H., Nassiri F., Moraes F.Y., et al. A phase II study of neoadjuvant stereotactic radiosurgery for large brain metastases: clinical trial protocol. Neurosurgery. 2020;87(2):403–407. doi: 10.1093/neuros/nyz442. Aug 1. [DOI] [PubMed] [Google Scholar]
- 54.Hu L.S., Swanson K.R. Roadmap for the clinical integration of radiomics in neurooncology. Neuro. Oncol. 2020;22(6):743–745. doi: 10.1093/neuonc/noaa078. Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]