Abstract
The combination of hydroxyapatite and the herbal extract ellagic acid is expected to accelerate the bone healing process (osteogenesis) due to the extract's anti-inflammatory and antioxidant properties. The osteogenesis process is closely associated with angiogenesis markers, such as fibroblast growth factor 2 (FGF-2), vascular endothelial growth factor (VEGF) and alkali phosphatase (ALP). The objective of this study is to analyse the combination of ellagic acid and hydroxyapatite to promote FGF-2, VEGF and ALP expression as angiogenesis markers in a bone defect model. The research sample comprised 30 male Wistar rats with a defect introduced on the left femur; these were divided into three groups for treatment with ellagic acid and hydroxyapatite, hydroxyapatite and polyethylene glycol (PEG) (control). On days 7 and 14 days after treatment, the Wistar rats were euthanised, and the femoral bone tissue was removed for the immunohistochemical analysis of FGF-2, VEGF and ALP expression. FGF-2 and ALP expression increased in the group treated with ellagic acid and hydroxyapatite on days 7 and 14 post treatment (p < 0.05), and there was an increase in VEGF expression on day 7 post treatment (p < 0.05). The combination of ellagic acid and hydroxyapatite promoted FGF-2, VEGF and ALP expression as angiogenesis markers in the bone defect model.
Keywords: Angiogenesis, Ellagic acid, Hydroxyapatite, Human & health
Graphical abstract
1. Introduction
In the biomedical field, one of the problems caused by the increasing trauma in dentistry is alveolar defects. This can be caused by several factors, e.g. infection, tumours, surgery and the presence of congenital abnormalities.1 The healing process in alveolar bone and bone defects involves interactions between osteoblasts, osteoclasts and osteocytes as well as other factors, such as hormones, nutrients, growth factors and inflammatory cytokines. In the process of bone healing (osteogenesis), bone tissue undergoes a regeneration process in which new bone tissue is formed.2
Bone graft material is used to overcome the problem in both alveolar and bone defects. This material is divided into several types, namely, autografts, allografts and xenografts, each with their respective advantages and disadvantages.3, 4, 5 Hydroxyapatite is a bovine-derived xenograft and an inorganic material with the formula Ca5(PO4)3(OH). Recently, it has been widely studied in the biomedical field because it has a mineral composition that is similar to that of bone and teeth. In terms of properties, hydroxyapatite has the biological advantages of biocompatibility, bio affinity, bioactivity, osteo-conduction, osteointegration and osteo-induction. This material contains only calcium and phosphate, so there is no local or systemic toxicity.6
Optimal osteogenesis can occur when there is a balance between bone formation and bone resorption by osteoblasts, osteoclasts and osteocytes.7 The first phase of the process is the inflammatory phase in which monocyte cells differentiate into osteoclast cells, which play a role in bone resorption.8 However, the use of xenografts has a disadvantage in that they can cause an intense inflammatory reaction. Efforts to suppress inflammation so that it does not become chronic require natural ingredients to be combined with hydroxyapatite to accelerate the bone healing process. One natural ingredient that has been proven to have this ability and is non-toxic is ellagic acid.9,10 Ellagic acid can reduce the expression of pro-inflammatory cytokines, including interleukin 1β (IL-1β), tumour necrosis factor (TNF) and interleukin 6 (IL-6). Also, it inhibits the activation of nuclear factor kappa B (NF-kB) to increase interleukin 10 (IL-10) and interleukin 4 (IL-4), which stimulates the production of collagen and fibroblasts and triggers protein synthesis.11, 12, 13
Fibroblast growth factor-2 (FGF-2) is a potent mitogenic factor for various cell types, including fibroblasts and osteoblasts. FGF-2 induces the process of angiogenesis through autocrine and paracrine factors that stimulate endothelial cell proliferation and migration along with the expression of proteases, growth factors and integrins involved in angiogenesis processes, which are important in the proliferative phase of bone repair mechanisms.14,15 Vascular endothelial growth factor (VEGF) is stimulated by FGF-2 and plays a role in increasing the expression of bone morphogenic protein 2 (BMP-2) and osteoblast differentiation for the bone healing process.16 Additionally, the role of VEGF increases the activity of alkaline phosphatase (ALP) in the bone remodelling process, thereby increasing the mineralization of the extracellular matrix.17
Based on the above, it is necessary to research bone defect treatments using a combination of ellagic acid and hydroxyapatite. The objective of this study is to analyse the combination of ellagic acid and hydroxyapatite to promote FGF-2, VEGF and ALP expression as angiogenesis markers in bone defects.
2. Materials and methods
2.1. Animals and ethical approval
The study was conducted on 30 healthy male Wistar rats (Rattus novergicus) weighing 200–250 g. They were divided into three groups, each containing five rats. The protocol of this research was approved by the ethics committee at the Faculty of Dental Medicine at Airlangga University (registration number: 360/HRECC.FDODM/VIII/2020).
3. Material and methods
This study used ellagic acid 90% (Xi'an Biof-Technology Co., Ltd. China), hydroxyapatite (BATAN, Indonesia) and polyethylene glycol (PEG, 202398, Sigma-Aldrich) in addition to antibody FGF-2 (recombinant anti-FGF2, rabbit monoclonal ab92337, Abcam), VEGF (anti-VEGFA, rabbit polyclonal, ab46154, Abcam) and ALP (anti-ALP, mouse polyclonal, ab67228, Abcam).
3.1. Preparation of ellagic acid and hydroxyapatite
This study used hydroxyapatite and ellagic acid with hydroxyapatite in gel form. The hydroxyapatite gel was formed by mixing with polyethylene glycol at a ratio of 1:0.25. The ellagic acid and hydroxyapatite (ratio 97:3) gel was formed by mixing with polyethylene glycol at a ratio of 1:0.25.9
3.2. Bone defect creation
The Wistar rats were acclimatised in individual cages for seven days. They were given food and water and were fasted for 12 h before the introduction of bone defects. The animals were anaesthetised with ketamine hydrochloride (Ketalar, Warner Lambert, Ireland) (100 mg/kg/body weight) and xylazine (X1126, Sigma-Aldrich) (4 mg/kg/body weight).
A round bur (801G-018, Meisinger, Germany) was used to create bone defects of 2 mm in diameter and 2 mm in depth on the lateral side of the left femur. The bone defects were irrigated with saline solution during the procedure. After irrigation, each animal was treated as described in Table 1. The treated bone defects were sutured with nylon sutures (Nylus nylon nonabsorbable sutures, Lotus Surgical, India) and were given the topical antibiotic gentamicin sulphate (2–4 mg/kg body weight/24 h). Seven and 14 days after therapy, the animals were euthanised for bone tissue biopsy.
Table 1.
The animal distribution.
| Group | Treatment |
|---|---|
| A | PEG gel (control) |
| B | Hydroxyapatite gel |
| C | Hydroxyapatite and ellagic acid gel |
3.3. FGF2, VEGF and ALP expression
Indirect immunohistochemical analysis was conducted by observing the number of macrophages in the bone defect area expressing FGF-2 and VEGF. Additionally, ALP expression was conducted on osteoblasts. Observations were made using a light microscope with ×400 and ×1000 magnification.
3.4. Statistical analysis
The data were analysed using the Shapiro–Wilk normality test, while Levene's test was used for homogeneity. One-way analysis of variance and high significant difference tests were used to determine the differences in the expression of FGF-2, VEGF and ALP between the groups.
4. Result
4.1. FGF2 expression
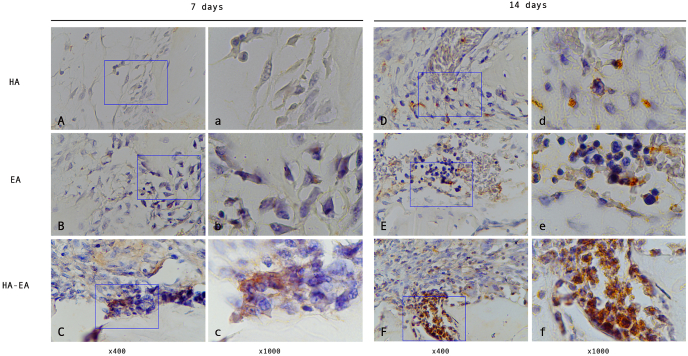
FGF2 expression was observed to increase on days 7 and 14 (see Fig. 1, Table 2). Observations on day 7 showed that the group treated with ellagic acid and hydroxyapatite had higher FGF2 expression than the group treated with hydroxyapatite and the control (p = 0.005 and p = 0.000, respectively).
Fig. 1.
The FGF-2 expression in the bone defect. (A, B, C) observation after 7 days treatment. (D, E, F) observation after 14 days treatment.
Table 2.
The expression of FGF-2, VEGF dan ALP.
| Days observation | Groups | Expression (Mean ± SD)* |
||
|---|---|---|---|---|
| FGF-2 | VEGF | ALP | ||
| 7 days | A | 2.60 ± 0.89a | 4.80 ± 1.48a | 4.60 ± 1.34a |
| B | 5.80 ± 1.64b | 7.20 ± 2.05b | 7.80 ± 2.39b | |
| C | 10.40 ± 1.67a,b | 11.80 ± 2.59a,b | 13.40 ± 1.52a,b | |
| 14 days | A | 6.40 ± 2.07a,b | 8.00 ± 2.24a | 7.80 ± 1.31a |
| B | 11.40 ± 1.14a,c | 12.40 ± 2.70 | 11.40 ± 3.36b | |
| C | 15.40 ± 2.70a,b,c | 15.80 ± 2.78a | 15.80 ± 2.17a,b | |
SD = Standard deviation.
*Same character in each marker expression and each days means significant different with Tukey HSD with significant value as p < 0.05.
Observations on day 14 were similar to day 7; the group treated with ellagic acid and hydroxyapatite had higher FGF2 expression than the group treated with hydroxyapatite and the control (p = 0.019 and p = 0.000, respectively). The group treated with hydroxyapatite also showed higher FGF2 expression compared with the control (p = 0.002).
4.2. VEGF expression
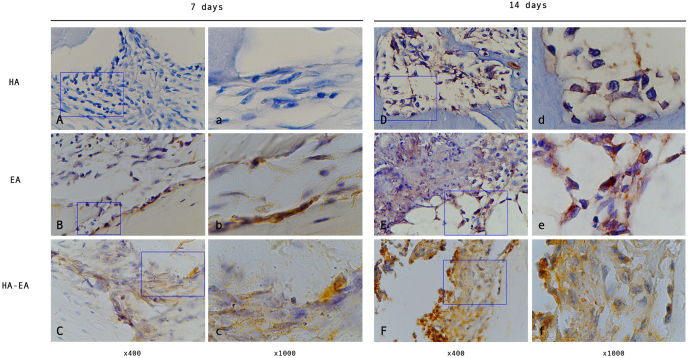
Higher VEGF expression was observed in the group treated with ellagic acid and hydroxyapatite than in the group treated with hydroxyapatite and the control on day 7 (p = 0.049 and p = 0.001, respectively) (see Fig. 2, Table 2).
Fig. 2.
The VEGF expression in the bone defect. (A, B, C) observation after 7 days treatment. (D, E, F) observation after 14 days treatment.
VEGF expression was also higher in the group treated with ellagic acid and hydroxyapatite than with the control on day 14 (p = 0.000).
4.3. ALP expression
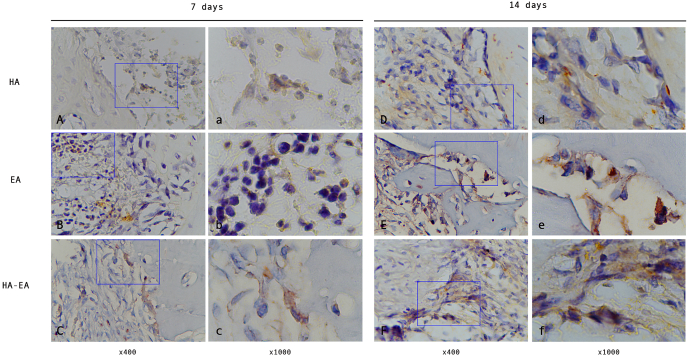
Higher ALP expression was observed in the group treated with ellagic acid and hydroxyapatite than in the group treated with hydroxyapatite and the control on days 7 and 14 (p = 0.004, p = 0.000, p = 0.035 and p = 0.000, respectively) (see Fig. 3, Table 2).
Fig. 3.
The ALP expression in the bone defect. (A, B, C) observation after 7 days treatment. (D, E, F) observation after 14 days treatment.
5. Discussion
Hydroxyapatite is an inorganic compound that is reported to support the bone healing process because it has good osteoinduction and osteoconduction properties18
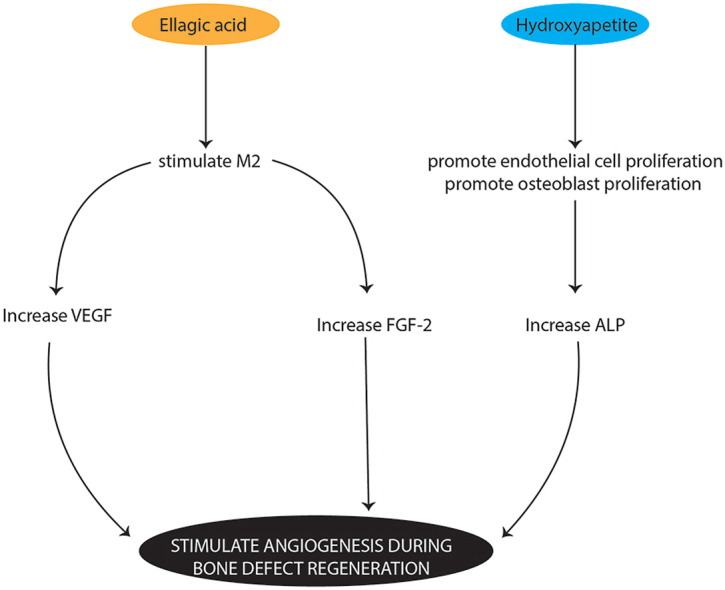
Ellagic acid has anti-inflammatory, antioxidant, antibacterial, antiviral and anti-allergic properties.19 This combination enhances the properties of hydroxyapatite in bone remodelling through its anti-inflammatory properties. It was shown that there was an increase in the expression of FGF-2 and VEGF with this combination on days 7 and 14 post treatment. The mechanism involved ellagic acid as an anti-oxidant, which inhibits reactive oxygen species or free radicals due to stress conditions on osteoblast cells from trauma. The inhibition of reactive oxygen species that causes the translocation of NF-kB to the nucleus is also inhibited so that pro-inflammatory cytokines, such as TNF-, IL-1β, IL-8, and IL-6.20, 21, 22, 23 As an anti-inflammatory, ellagic acid, which is a flavonoid compound, can also stimulate M2 phenotype macrophages; this stimulates the release of IL-10, IL-4, VEGF, FGF-2, BMP-2, TGF-β and PDGF to reduce inflammation and support the differentiation of mesenchymal stem cells, resulting in an increase in vascular tissue, fibroblasts and osteoblasts. Vascular tissue stimulates the migration of mesenchymal stem cells to bone defect areas to provide appropriate oxygen and nutrients. Fibroblasts and osteoblasts stimulate the formation of collagen, which functions as bone matrix in the process of osteogenesis.24 Research conducted by Ioyah et al. (2019) revealed that ellagic acid derived from the skin of Garcinia mangostana L. also has anti-inflammatory and antioxidant properties. Ellagic acid positively increases and accelerates the bone healing process, which can be seen from the reduction in the diameter of defects in the femur of rats.25
VEGF is a strong angiogenic factor that can stimulate and initiate the function of endothelial cells to migrate, proliferate and differentiate to increase the formation of new blood vessels. Clinically, this affects the bone remodelling process.26 VEGF also initiates the process of angiogenesis through neovascularization, thereby stimulating the migration and recruitment of mesenchymal stem cells in the area around bone defects. VEGF does not promote bone regeneration directly, but it interacts synergistically with BMP-2 to trigger the recruitment of mesenchymal stem cells to the defect site and to induce osteoblast differentiation.16
The significant VEGF expression in this study was observed only with hydroxyapatite and ellagic acid on day 7 post treatment. This condition arose because during bone regeneration, VEGF expression began to appear on day 3 and reached its peak on days 5–10.27 In addition, on days 7–9, which was the soft callus formation phase, the angiogenesis process was stimulated by several growth factors, including VEGF, BMP, TGF-β and FGF. VEGF expression played an important role at the beginning of the angiogenesis process for endothelial migration and proliferation in the formation of a strong blood vessel structure.5 When reaching the final phase of proliferation on day 14, the number of macrophages decreased, and VEGF expression decreased accordingly. The decrease in VEGF expression was also influenced by a decrease in the number of blood vessels because on day 14, the blood vessels already appeared to be stable.26,28
In addition, VEGF also plays a role in the bone remodelling process by inhibiting the apoptotic process of osteoblast cells and increasing the activity of osteocalcin (OSC) and ALP. This results in the stimulation, migration and proliferation of osteoblast cells and increases extracellular matrix mineralization. This study also showed that ALP expression increased after the administration of hydroxyapatite and ellagic acid on days 7 and 14 post treatment.
ALP is an enzyme attached to the outer membrane of osteoblasts and is the main regulator of bone mineralization. ALP is considered to be an important biomarker for bone formation.29 The ellagic acid and hydroxyapatite treatment produced an increase in ALP expression in the bone defect on days 7 and 14. Previous studies have confirmed that the combination of ellagic acid and hydroxyapatite can increase new bone formation by increasing OSC, osteoprotegerin and osteoblasts while decreasing the osteoclast activation receptor activator of the nuclear factor kappa β ligand during bone defect healing on days 7 and 14.9 Ellagic acid showed a significant effect on increasing ALP expression. This is because ellagic acid acts as an anti-inflammatory that can control inflammation by reducing pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α, and inducing anti-inflammatory cytokines, such as IL-10.30,31 IL-10 inhibits the formation of osteoclasts and increases osteoblast differentiation.32
During bone formation, osteoblasts form type-I collagen and proteoglycans as bone matrix (osteoid) and secrete large amounts of ALP. The role of ALP in mineralization is to prepare an alkaline atmosphere in the formed osteoid tissue so that calcium can be deposited easily. In addition, ALP can break down phosphate bonds into free phosphate ions that will react with calcium ions to form calcium–phosphate bonds in the form of hydroxyapatite crystals. The formed hydroxyapatite crystals then settle or are buried in the bones and accelerate bone calcification in the mineralization process. ALP secretion decreases if the osteoid mineralization process has been completed. Therefore, it can be concluded that if there is an increase in ALP, then bone calcification during the mineralization phase for new bone formation occurs faster, and healing of the bone defects occurs.
As an anti-inflammatory, ellagic acid can also help the function of VEGF in endothelial cells to increase and accelerate the vascularization process.33 The application of hydroxyapatite can promote endothelial cell proliferation and maintain the morphology and biochemistry related to endothelial cell function. In other conditions, the application of hydroxyapatite with osteoconductive properties induces the growth of new blood vessels and induces mesenchymal stem cells to proliferate and differentiate into osteoblasts; this facilitates osteoblast migration at the surface, which accelerates the bone healing process.34 The pore structure in hydroxyapatite is an important aspect related to pore interconnectivity because interconnected pores increase blood vessel vascularity, which increases the supply of oxygen and nutrients.35 Additionally, hydroxyapatite can induce the angiogenesis process by increasing VEGF expression and enhancing the bone healing process. The presence of calcium and phosphorus ions in hydroxyapatite can induce osteoblast differentiation.36
6. Conclusion
This study used a combination of ellagic acid and hydroxyapatite to promote FGF-2, VEGF and ALP expression as angiogenesis markers in a bone defect model. These three markers are the main markers in angiogenesis which are the main determinants of bone regeneration. The use of this combination of ingredients promises to be used after tooth extraction to prevent bone damage and regenerate bone tissue.
Source of funding
This study was funded by the Faculty of Dental Medicine at Universitas Airlangga 2021 in the schema Penelitian Unggulan Fakultas (PUF) with grant number 219/UN3.1.2/PT/2021.
Declaration of competing interest
The authors declare no conflicts of interest.
References
- 1.Fernandez de Grado G., Keller L., Idoux-Gillet Y., et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng. 2018;9 doi: 10.1177/2041731418776819. http://journals.sagepub.com/doi/10.1177/2041731418776819 [Internet] 204173141877681. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domazetovic V., Marcucci G., Iantomasi T., Brandi M.L., Vincenzini M.T. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab. 2017;14:209–216. doi: 10.11138/ccmbm/2017.14.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oryan A., Alidadi S., Moshiri A., Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9:18. doi: 10.1186/1749-799X-9-18. https://josr-online.biomedcentral.com/articles/10.1186/1749-799X-9-18 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seyler T.M., Bracey D.N., Plate J.F., et al. The development of a xenograft-derived scaffold for tendon and ligament reconstruction using a decellularization and oxidation protocol. Arthrosc - J Arthrosc Relat Surg. 2017;33:374–386. doi: 10.1016/j.arthro.2016.07.016. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 5.Oryan A., Alidadi S., Moshiri A. Current concerns regarding healing of bone defects. Hard Tissue. 2013;2:1–12. http://www.oapublishinglondon.com/article/374 [Internet] Available from: [Google Scholar]
- 6.Kattimani V.S., Kondaka S., Lingamaneni K.P. Hydroxyapatite–-Past, present, and future in bone regeneration. Bone Tissue Regen Insights. 2016;7 http://journals.sagepub.com/doi/10.4137/BTRI.S36138 [Internet] BTRI.S36138. Available from: [Google Scholar]
- 7.Florencio-Silva R., Sasso GR. da S., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed res int. 2015:1–17. doi: 10.1155/2015/421746. https://linkinghub.elsevier.com/retrieve/pii/S0923253205801826 [internet]. 2015. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baht G.S., Vi L., Alman B.A. The role of the immune cells in fracture healing. Curr Osteoporos Rep. 2018;16:138–145. doi: 10.1007/s11914-018-0423-2. http://link.springer.com/10.1007/s11914-018-0423-2 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardhana A.S., Nirwana I., Budi H.S., Surboyo M.D.C. Role of hydroxyapatite and ellagic acid in the osteogenesis. Eur J Dent. 2020:1–6. doi: 10.1055/s-0040-1714039. http://www.thieme-connect.de/DOI/DOI?10.1055/s-0040-1714039 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nirwana I., Munadziroh E., Yogiartono R., Thiyagu C., Ying C., DInaryanti A. Cytotoxicity and proliferation evaluation on fibroblast after combining calcium hydroxide and ellagic acid. "J Adv Pharm Technol Research"" (JAPTR)". 2021;12:27–31. doi: 10.4103/japtr.JAPTR_154_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Obaidi M.M.J., Al-Bayaty F.H., Al Batran R., Hussaini J., Khor G.H. Impact of ellagic acid in bone formation after tooth extraction: an experimental study on diabetic rats. Sci world J. 2014:1–14. doi: 10.1155/2014/908098. http://www.hindawi.com/journals/tswj/2014/908098/ [Internet]. 2014. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asgary S., Javanmard S., Zarfeshany A. Potent health effects of pomegranate. Adv Biomed Res. 2014;3:100. doi: 10.4103/2277-9175.129371. http://www.advbiores.net/text.asp?2014/3/1/100/129371 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee A., Chatterjee S., Das S., Saha A., Chattopadhyay S., Bandyopadhyay S.K. Ellagic acid facilitates indomethacin-induced gastric ulcer healing via COX-2 up-regulation. Acta Biochim Biophys Sin (Shanghai) 2012;44:565–576. doi: 10.1093/abbs/gms034. https://academic.oup.com/abbs/article-lookup/doi/10.1093/abbs/gms034 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 14.Fei Y., Xiao L., Doetschman T., Coffin D.J., Hurley M.M. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J Biol Chem. 2011;286:40575–40583. doi: 10.1074/jbc.M111.274910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romeo U., Rocchetti F., Montori A. Criticisms and controversies in the diagnosis of cheilitis. Proceedings. 2019;35:8. https://www.mdpi.com/2504-3900/35/1/8 [Internet] Available from: [Google Scholar]
- 16.Yang Y.Q., Tan Y.Y., Wong R., Wenden A., Zhang L.K., Rabie A.B.M. The role of vascular endothelial growth factor in ossification. Int J Oral Sci. 2012;4:64–68. doi: 10.1038/ijos.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan Y., Yang Y.-Q., Chai L., Wong R., Rabie A. Effects of vascular endothelial growth factor (VEGF) on MC3T3-E1. Orthod Craniofac Res. 2010;13:223–228. doi: 10.1111/j.1601-6343.2010.01498.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1601-6343.2010.01498.x [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 18.Sotto-Maior B.S., Senna P.M., Aarestrup B.J.V., Ribeiro R.A., De Souza Picorelli Assis N.M., Del Bel Cury A.A. Effect of bovine hydroxyapatite on early stagesof bone formation. Rev Odonto Ciência. 2011;26:198–202. [Google Scholar]
- 19.Ginwala R., Bhavsar R., Chigbu D.I., Jain P., Khan Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants. 2019;8:35. doi: 10.3390/antiox8020035. http://www.mdpi.com/2076-3921/8/2/35 [internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [Internet] Available from: [DOI] [Google Scholar]
- 21.Karunaweera N., Raju R., Gyengesi E., Mã¼nch G. Plant polyphenols as inhibitors of NF-kB induced cytokine production - potential anti-inflammatory treatment for Alzheimer's disease? Front Mol Neurosci. 2015;8:1–5. doi: 10.3389/fnmol.2015.00024. http://journal.frontiersin.org/Article/10.3389/fnmol.2015.00024/abstract [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surboyo M.D.C., Arundina I., Rahayu R.P., Mansur D., Bramantoro T. Potential of distilled liquid smoke derived from coconut (cocos nucifera L) shell for traumatic ulcer healing in diabetic rats. Eur J Dent. 2019;13:271–279. doi: 10.1055/s-0039-1693527. http://www.thieme-connect.de/DOI/DOI?10.1055/s-0039-1693527 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surboyo M.D.C., Ernawati D.S., Radithia D., et al. Distilled liquid smoke coconut shell attenuates the cytokine profile of macrophages in oral ulcer in experimental model of diabetes mellitus. J Appl Pharm Sci. 2021;11:62–69. https://www.japsonline.com/abstract.php?article_id=3454&sts=2 [Internet] Available from: [Google Scholar]
- 24.Saran U., Gemini Piperni S., Chatterjee S. Role of angiogenesis in bone repair. Arch biochem biophys [internet] 2014. Available from: 561, 109-117. [DOI] [PubMed]
- 25.Ioyah B.R., Djohan W., Idrus E. Effect of mangosteen peel extract on bone fracture healing. Int J Appl Pharm. 2019;11:100–102. [Google Scholar]
- 26.Johnson K.E., Wilgus T.A. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv wound care. 2014;3:647–661. doi: 10.1089/wound.2013.0517. http://www.liebertpub.com/doi/10.1089/wound.2013.0517 [internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B., Wang H., Qiu G., Su X., Wu Z. Synergistic effects of vascular endothelial growth factor on bone morphogenetic proteins induced bone formation in vivo: influencing factors and future research directions. Biomed res int. 2016:1–11. doi: 10.1155/2016/2869572. https://www.hindawi.com/journals/bmri/2016/2869572/ [internet]. 2016. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayuningtyas N.F., Surboyo M.D.C, Ernawati D.S., Parmadiati A.E. The role of liquid smoke coconut shell in the proliferation phase of an oral traumatic ulcer. J Pharm Pharmacogn Res. 2020;8:549–557. [Google Scholar]
- 29.Szulc P., Bauer D.C. Osteoporosis [Internet]. Fourth Edi. Elsevier; 2013. Biochemical markers of bone turnover in osteoporosis; pp. 1573–1610. Available from: [DOI] [Google Scholar]
- 30.Allam G., Mahdi E.A., Alzahrani A.M., Abuelsaad A.S. Ellagic acid alleviates adjuvant induced arthritis by modulation of pro- and antiinflammatory cytokines. Cent Eur J Immunol. 2016;41:339–349. doi: 10.1016/B978-0-12-415853-5.00067-4. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson K.C., Teuber S.S. Annals of the New York Academy of Sciences. 2010. Ellagic acid and polyphenolics present in walnut kernels inhibit in vitro human peripheral blood mononuclear cell proliferation and alter cytokine production; pp. 86–96. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q., Chen B., Yan F., et al. Interleukin-10 inhibits bone resorption: a potential therapeutic strategy in periodontitis and other bone loss diseases. Biomed res int. 2014:1–5. doi: 10.1155/2014/284836. [internet]. 2014. Available from: http://www.hindawi.com/journals/bmri/2014/284836/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong H., Tian X.Y. The role of macrophages in vascular repair and regeneration after ischemic injury. Int J Mol Sci. 2020;21:1–12. doi: 10.3390/ijms21176328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granito R.N., Renno A.C.M., Yamamura H., de Almeida M.C., Ruiz P.L.M., Ribeiro D.A. Hydroxyapatite from fish for bone tissue engineering: a promising approach. Int J Mol Cell Med. 2018;7:80–90. doi: 10.22088/IJMCM.BUMS.7.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corrales L.P., Esteves M.L., Vick J aime E. Scaffold design for bone regeneration. J Nanoscience Nanotechnology. J Nanosci Nanotechnol. 2014;14:15–56. doi: 10.1166/jnn.2014.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Souza Nunes L.S., De Oliveira R.V., Holgado L.A., Nary Filho H., Ribeiro D.A., Matsumoto M.A. Use of bovine hydroxyapatite with or without biomembrane in sinus lift in rabbits: histopathologic analysis and immune expression of core binding factor 1 and vascular endothelium growth factor. J Oral Maxillofac Surg. 2011;69:1064–1069. doi: 10.1016/j.joms.2010.02.057. [Internet] Available from: [DOI] [PubMed] [Google Scholar]