Abstract
Using a set of genomic TY1A-lacZ fusions, we show that Ste12 and Tec1, two transcription factors of the Kss1 mitogen-activated protein kinase (MAPK) cascade activate Ty1 transcription in Saccharomyces cerevisiae. This result strongly suggests that the invasive-filamentous pathway regulates Ty1 transcription. Since this pathway is active in diploid cells, we suspected that Ty1 transposition might occur in this cell type, despite the fact that this event has been never reported before (unless activated by heterologous promoters such as that of GAL1). We demonstrate here that constitutive activation of the invasive-filamentous pathway by the STE11-4 allele or by growth in low-nitrogen medium induces Ty1 transcription and retrotransposition in diploid cells. We show that Ty1 retrotransposition can be activated by STE11-4 in haploid cells as well. Our findings provide the first evidence that Ty1 retrotransposition can be activated by environmental signals that affect differentiation. Activation of the Kss1 MAPK cascade by stress is known to cause filament formation that permits the search for nutrients away from the colonization site. We propose that activation of Ty1 retrotransposition by this cascade could play a role in adaptive mutagenesis in response to stress.
Retrotransposons are a class of mobile genetic elements that move via an RNA intermediate. They are structurally and functionally related to retroviruses. Five different families, Ty1 to Ty5, have been identified in Saccharomyces cerevisiae (6). The Ty1 copia-like retrotransposon is the most abundant (31). Thirty-two copies of this element are present in the sequenced genome of the strain S288C (33).
Ty1 contains an internal coding region of 5.3 kb, flanked by two long terminal repeats (LTR) of 0.33 kb (6). The internal domain contains two overlapping open reading frames, TY1A and TY1B, analogous to the retroviral gag and pol genes, respectively. Ty1 is transcribed from LTR to LTR by RNA polymerase II, the resulting transcript serving as a template for both translation and reverse transcription. Ty1 preferentially integrates next to RNA polymerase III promoters (18), but less-frequent insertions into or upstream of genes transcribed by RNA polymerase II have been reported.
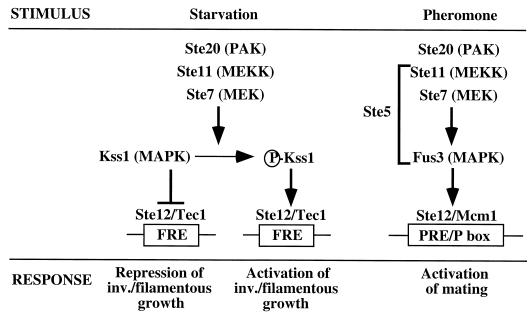
Ty1 insertions have been shown to activate the expression of genes such as ADH2, CYC7, CAR1, CAR2, DUR1, and DUR2 (6). In this case, Ty1 transcription is always divergent from the activated gene, and the transcription start site of the target gene is generally not modified upon Ty1 insertion. These mutant alleles, called ROAM (for regulated overproducing alleles responding to mating) behave as haploid-specific genes, since their mRNA levels decrease in a/α diploid cells (24). Their expression is reduced in ste7, ste11, ste12, and tec1 mutants (19, 25, 34). The Ste11 and Ste7 proteins are a mitogen-activated protein kinase (MAPK) kinase kinase (MEKK), and a MAPK kinase (MEK), respectively, and Ste12 and Tec1 are transcription factors acting downstream of Ste7 and Ste11. These four proteins are involved in an invasive-filamentous developmental pathway that has been described in both haploid and diploid cells (41) (Fig. 1). In diploid cells, nitrogen starvation induces a switch from growth as a single-cell form to chains of elongated cells (called filaments or pseudohyphae) which invade the agar surface (30). In haploid cells, activation of this pathway leads to agar invasion, but the inducing environmental signals are not known (49). This mode of growth is believed to enable cells to forage for nutrients at a distance from their initial colonization site, under adverse conditions. The invasive-filamentous pathway shares several signaling components with the mating pathway (38). However, their specificity of action is ensured by two distinct MAPKs that limit cross talk between these pathways. The Kss1 MAPK acts in the invasive-filamentous pathway, and the Fus3 MAPK is dedicated to the mating pathway (15, 42). Tec1 ensures another level of specificity (40, 41). In the invasive-filamentous pathway, Tec1 binds cooperatively with Ste12 to a DNA site called FRE (filamentation- and invasion-responsive element). In the mating pathway, Ste12 binds to DNA, as a homomultimer or as a heteromultimer with Mcm1.
FIG. 1.
MAPK pathways regulating invasive-filamentous and mating developments. The invasive-filamentous pathway is depicted on the left, and the mating pathway is shown on the right. Ste5 binds Ste11, Ste7, and Fus3 proteins and is required for pheromone-induced signaling (41). FRE, filamentation- and invasion-responsive element; PRE, pheromone-responsive element; Pbox, Mcm1 binding site. In the invasive-filamentous pathway, Ste12 binds to FRE cooperatively with Tec1. Ste12 acts in the mating pathway by binding to DNA as a homomultimer or as a heteromultimer with Mcm1.
Two regions of Ty1 are responsible for haploid-specific and STE-dependent regulation of ROAM mutant expression (12). The first region, encompassing nucleotides 384 to 433, is responsive to Ste7 and Ste12 activation (11). It contains an FRE site to which Tec1 and Ste12 have been shown to bind in vitro (2, 40). The second region (nucleotides 815 to 927) contains both Mcm1 and a1/α2 repressor complex binding sites that confer enhancer and diploid control activities, respectively (26). Some findings suggest that Ty1 expression is regulated as in ROAM mutants: mRNA levels decrease 10-fold in diploid cells (21) and are reduced in ste7, ste11, or tec1 mutants (19, 34). The lower levels of Ty1 mRNA in these mutants, along with the presence of an FRE site in the Ty1 sequence, suggest that Ty1 transcription might be regulated by the invasive-filamentous pathway. The use of Ty1 FRE as a tool to identify components of the invasive-filamentous pathway strengthens this hypothesis (40, 42). However, for this identification, Ty1 FRE was used as an upstream activating sequence of reporter genes, while in the Ty1 retrotransposon, FRE is located downstream of the TATA box, in the TY1A coding sequence. This unusual location might prevent activation of Ty1 transcription by the invasive-filamentous cascade. Consistent with an abnormal regulation, the Ste12 activator was proposed to act as a repressor of Ty1 expression (10). Thus, we decided to analyze further the regulation of Ty1 expression and retrotransposition by the invasive-filamentous pathway.
In this study we show that the transcription of most native Ty1 elements is activated by Ste12 and Tec1 and is regulated by the invasive-filamentous pathway. Our results also indicate that Ty1 transcription and retrotransposition can be activated in diploid cells by this pathway. Since invasive-filamentous growth is a response to environmental stress, we propose that activation of Ty1 retrotransposition by this pathway may help survival of stress conditions by creating adaptive mutations.
MATERIALS AND METHODS
Yeast strains and media.
Yeast strains used in this work are described in Table 1. All derivatives of S288C contains the flo8-1 recessive allele that impairs filamentous growth (39). Since the FLO8 gene is a target of the cyclic AMP signaling pathway that regulates pseudohyphal growth independently from the Kss1 MAPK cascade (47), the flo8-1 mutation will not affect activation of the Kss1 MAPK in S288C. Although some derivatives of S288C strains were reported to be kss1− (22), we found that the FYBL1-23D and FY839 strains, both isogenic to the S288C strain used as the source of DNA for the European Union Yeast Genome Sequencing Program (55), were wild types for KSS1. The evidence came from the ability of the STE11-4 allele to activate FUS1-lacZ fusion in FYBL1-23D, FYBL1-23D fus3Δ and FYBL1-23D kss1Δ strains but not in FYBL1-23D fus3Δ kss1Δ (A. Morillon and P. Lesage, unpublished data). Identical results were obtained by Elion et al. with strain W303 after activation with pheromones instead of STE11-4 (22). These results were interpreted as meaning that the strain is wild type for both FUS3 and KSS1.
TABLE 1.
Strains used in this studya
| Strain | Genotype | Background | Source or reference |
|---|---|---|---|
| FYBL1-23D | MATα flo8-1 KSS1 ura3-Δ851 trp1Δ63 his3Δ200 | S288C | B. Dujon |
| FY839 | MATa flo8-1 KSS1 ura3-52 his3Δ200 leu2Δ1 | S288C | F. Winston |
| FY1679-28C | MATa flo8-1 KSS1 ura3-52 trp1Δ63 leu2Δ1 his3Δ200 | S288C | B. Dujon |
| JC297 | MATα FLO8 (?) KSS1 ura3-167 trp1::hisG his3Δ200 Ty1his3AI-270 | J. Curcio (13) | |
| JC242 | MATα FLO8 (?) KSS1 ura3-167 his3Δ200 Ty1his3AI-242 | J. Curcio (16) | |
| L5684 | MATa FLO8 KSS1 ura3-52 leu2::hisG | Σ1278b | G. Fink |
| L5685 | MATa FLO8 KSS1 ura3-52 trp1::hisG | Σ1278b | G. Fink |
| LV50 | MATa flo8-1 KSS1 ura3-Δ851 his3Δ200 trp1Δ63 leu2Δ1 lys2Δ202 | S288C | This study |
| LV69 | MATα flo8-1 KSS1 lys2::his3Δ4 ura3-Δ851 trp1Δ63 his3Δ200 | S288C | This study |
| LV101 | MATα flo8-1 KSS1 ste12Δ1::TRP1 ura3-Δ851 trp1Δ63 his3Δ200 | S288C | This study |
| LV105 | MATa flo8-1 KSS1 ste12Δ1::TRP1 ura3-52 trp1Δ63 leu2Δ1 his3Δ200 | S288C | This study |
| LV126 | MATα flo8-1 KSS1 tec1Δ1::TRP1 ura3-Δ851 trp1Δ63 his3Δ200 | S288C | This study |
| LV128 | MATa flo8-1 KSS1 tec1Δ1::TRP1 ura3-Δ851 his3Δ200 trp1Δ63 leu2Δ1 lys2Δ202 | S288C | This study |
| LV148 | MATa FLO8 KSS1 ura3-52 trp1::hisG his3Δ::TRP1 | Σ1278b | This study |
| LV150 | MATα flo8-1 KSS1 lys2::ROAM-his3Δ4-1 ura3-Δ851 trp1Δ63 his3Δ200 | S288C | This study |
| LV237 | MATα flo8-1 kss1::KanMX ura3-Δ851 trp1Δ63 his3Δ200 | S288C | This study |
| LV247 | MATa flo8-1 kss1::KanMX ura3-52 his3Δ200 leu2Δ1 | S288C | This study |
The S288C strains are isogenic to the S288C strain used as a source of DNA for the European Union Yeast Genome Sequencing program. The TY1A-lacZ fusions have been introduced in FYBL1-23D. S288C diploid strains are unable to form filaments due to the flo8-1 mutation. Diploid strains obtained by mating Σ1278b haploid strains with S288C haploid strains or with JC242 or JC297 haploid strains form filaments well.
The Σ1278b derivatives L5684 and LV148 (obtained by disrupting the HIS3 open reading frame with TRP1 in L5685) were used to make diploid cells when filamentous growth needed to be checked (39). LV101 and LV105 were obtained by disrupting the STE12 open reading frame in FYBL1-23D and FY1679-28C, respectively. LV126 and LV128 were obtained by disrupting TEC1 in FYBL1-23D and LV50, respectively. Null alleles of STE12 and TEC1 were obtained by one-step gene replacement using PCR fragments of the TRP1 gene amplified with long primers containing 5′ and 3′ sequences of STE12 and TEC1, respectively (1). Gene replacements were checked by PCR analysis. Similarly, LV237 and LV247 were obtained by one-step gene replacement of KSS1 in FYBL1-23D and FY839, respectively, using a PCR fragment of the KanMX gene with 5′ and 3′ sequences of KSS1 (43). All gene replacements were checked by PCR analysis.
Strain LV150, which carries a ROAM-his3Δ4-1 allele was constructed in two steps. First, we constructed strain LV69 by replacing the LYS2 locus in FYBL1-23D with a lys2::his3Δ4 allele as described by Z. Qian (48). In a second step, LV150 was selected as an His+ prototroph upon galactose induction of pGTy1-H3 (kindly provided by J. Boeke) in LV69, as described previously (5). PCR analysis indicated that a Ty1 element was integrated approximately 150 bp upstream of his3Δ4 in LV150 and that Ty1 and his3Δ4 were oriented divergently.
Strains JC297 and JC242, used in the transposition assays, were kindly provided by M. J. Curcio (13, 16).
Yeast transformations were performed by the LiAc method (1). Yeast cells were grown in rich (YPD), synthetic complete (HC), synthetic minimum (SD), and synthetic low-ammonium (SLAD) media (1, 30). Unless otherwise stated, 2% glucose was used as the carbon source.
Construction of 31 strains each carrying a TY1A-lacZ fusion at a different Ty1 locus.
Construction of these strains will be described in detail elsewhere (unpublished data). Briefly, strain FYBL1-23D was transformed with a DNA fragment carrying lacZ fused in frame to TY1A (at coordinate 1571 of Ty1-H3 [4]) and located upstream of the URA3 gene followed by sequences of TY1B. In the ′ty1a′-′lacZ-URA3-′ty1b′ construct, the ′ty1a′ sequence comes from Ty1-H3 (coordinates 1144 to 1571). Since the first 1 kb of Ty1 is sufficient to produce high levels of Ty1 transcripts (6), the use of a ty1a fragment starting at coordinate 1144 should not modify the transcriptional level of the Ty1 element fused to lacZ in the recombinants. To identify which Ty1 element carried the fusion, Ura+ recombinants obtained upon transformation were analyzed by PCR using 32 oligonucleotides, each specific to the region upstream of one Ty1 locus and an oligonucleotide specific to lacZ. The synthesis of a DNA fragment by PCR amplification, using one oligonucleotide specific to a Ty1 element and the lacZ oligonucleotide, allowed us to identify which Ty1 element carried the TY1A-lacZ fusion in the recombinant. In parallel, the DNA of the recombinants was digested with several restriction enzymes that do not cut in TY1A but do cut downstream of lacZ to perform Southern blot analysis. The hybridization of a single fragment to a probe specific to lacZ indicated a single integration event. The size of the labeled fragment, compared to the size predicted with the restriction map of the 32 Ty1 loci, allowed us to confirm which Ty1 element carried the TY1A-lacZ fusion in these strains.
Null alleles of STE12 and TEC1 were obtained in these strains by one-step gene replacement using PCR fragments of the TRP1 gene as described above with LV101 and LV126. Similarly, a null allele of KSS1 was obtained in these strains by one-step gene replacement using PCR fragments of KanMX, as described for LV247.
Construction of GAL1p-TY1A(PR1)-lacZ fusion.
The GAL1p-TY1A(PR1)-lacZ fusion is a TY1A-lacZ fusion at Ty1-PR1 in which the U3 region of 5′ LTR has been replaced by the GAL1 promoter. This construct was done in several steps. First, a BamHI-EcoRI fragment containing the TRP1 sequence (coordinates −80 to 728) and obtained by PCR amplification was ligated with the 756-bp EcoRI-XhoI fragment of pGTy1-H3 (5) containing the GAL1 promoter and with pRS426 digested with BamHI and XhoI (9). In the resulting pAM3 plasmid, TRP1 and GAL1p are divergent. In parallel, a fragment encompassing the −606 to −58 region upstream of Ty1(PR1) in the genome of S288C was PCR amplified, and a BamHI site was created adjacent to position −58 (position +1 corresponds to the first nucleotide of 5′ LTR). This PCR product was ligated to the 2.9-kb BamHI-NcoI fragment of pAM3 in order to place the fragment homologous to the region upstream of Ty1(PR1) adjacent to TRP1-GAL1p. The final construct, upstream Ty1(PR1)-TRP1-GAL1p-R, was then obtained by high-fidelity PCR amplification using a primer specific to the region upstream of Ty1(PR1) (coordinates −403 to −383) and a primer specific to GAL1p with a 5′ extension homologous to the R region of Ty1 LTR. This construct was then integrated in the FYBL1-23D derivative containing the TY1A(PR1)-lacZ fusion by homologous recombination. Finally, the replacement at Ty1(PR1) was checked by PCR. The GAL1p-TY1A(DR3)-lacZ fusion was obtained by a similar strategy.
Plasmids.
The pAM7 plasmid (DPS1-lacZ URA3 CEN) was constructed in two steps. First, pAM6 was constructed by cloning in Yep353 (45), a 2-kb BamHI-HindIII fragment carrying the first 400 bp of DPS1, as well as 1.6 kb of upstream regulatory sequence (kindly provided by G. Eriani). Then, the 5.9-kb BamHI-NcoI fragment of pAM6 carrying the DPS1-lacZ fusion was ligated with the corresponding 3.5-kb fragment of pRS316 (52), yielding pAM7. Plasmids pSL974 (FUS1-lacZ URA3, 2μm; kindly provided by G. Sprague), p11-4/HIS3 (STE11-4 HIS3 CEN), pSL1509 (STE11-4 URA3 CEN), and pFRE(Ty1)-lacZ [FRE(Ty1)-lacZ URA3, 2μm; generously provided by G. Fink] have already been described (32, 40, 42).
Northern blot analysis.
Total RNA was extracted from 10-ml cultures grown at 22°C to mid-log phase as described earlier (3) without the 70% ethanol RNA washing step. For each strain, 2 μg of RNA was loaded onto a 1% agarose–0.5× TBE gel, unless otherwise stated. The size-fractionated RNA was blotted onto a Hybond-N membrane (Amersham), and hybridization was performed as recommended by the supplier. Probes against Ty1, Ty1his3AI (i.e., HIS3), lacZ, and ACT1 were generated by random priming. The Ty1 probe was derived from a region of Ty1 nonhomologous to Ty2 (coordinates 3137 to 3682 in Ty1-H3). Results were quantified on a Molecular Dynamics PhosphorImager with ImageQuant software.
β-Galactosidase assays.
For filter lift assays, patches of strains grown at 30°C on HC-URA plates were replica plated on a fresh HC-URA plate covered with a 1MM Whatman sterile paper. Replica were incubated overnight at 30°C. Cells were lysed on filters by three successive cycles of freezing (10 min at −80°C) and defreezing (10 min at 37°C). Finally, filters were soaked at room temperature in Z buffer plus X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 100 μg/ml) to detect β-galactosidase activity. After 2 h, the reaction was stopped in 1 M NA2CO3.
For liquid assays, precultures were grown at 30°C overnight in HC medium lacking the appropriate nutrients to maintain selection for plasmids. Precultures were diluted 100-fold in selective medium and grown at 22°C to mid-log phase. Whole-cell extracts were prepared as described earlier (50) except that Z buffer without β-mercaptoethanol was used as the extraction buffer. Protein concentration was determined by the Bio-Rad assay, and β-galactosidase activity was measured as described elsewhere (44). β-Galactosidase units are expressed in nanomoles of 2-nitrophenyl-β-d-galactopyranoside hydrolyzed per minute per nanogram of protein. Values are the averages of at least three independent measurements. Standard deviations are <10%.
Transposition assay.
To determine the transposition frequency, cells were grown at 30°C overnight in HC medium lacking the appropriate nutrients to maintain selection for plasmids. Precultures were diluted 50-fold into selective medium and grown for three generations at 22°C. For each culture, aliquots were plated on YPD and HC medium lacking histidine to determine the number of His+ prototrophs.
Assays under nitrogen starvation conditions were done as follows. Patches were replica plated from SD medium supplemented with histidine (high nitrogen) to SD medium supplemented with histidine (high nitrogen) and SLAD medium supplemented with histidine (low nitrogen). After 7 days of growth at 22°C, patches were replica plated again on SD medium plus histidine (for patches on the SD-plus-His plates) and on SLAD medium plus histidine (for patches on the SLAD-plus-His plates) to ensure nitrogen starvation in the latter case. After 7 days of growth at 22°C, replica plates were done on SD medium (lacking histidine) to score retrotransposition events of Ty1his3AI-270 that result in His+ prototrophs. Plates were incubated for 4 days at 30°C.
RESULTS
Native Ty1 elements are poorly transcribed in diploid cells.
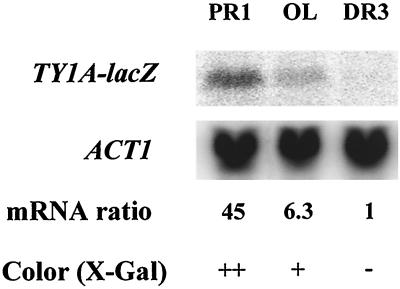
There are 32 native Ty1 elements in the genome of S. cerevisiae S288C. To study the relative expression level of each endogenous Ty1 element, we constructed haploid strains each expressing lacZ under the transcriptional controls of a different Ty1 element at its original locus. Since sequences located in TY1A are required for Ty1 transcription, lacZ was fused in frame to the 3′ end of the TY1A gene of each native Ty1 element (see Materials and Methods for details). Ty1A-lacZ fusions were named according to the Ty1 sequence annotation by MIPS (http://www.mips.biochem.mpg.de/), i.e., TY1A(PR1)-lacZ corresponds to lacZ fused to the first Ty1 element of chromosome XVI. Thirty-one different strains were constructed; one native Ty1 copy (Ty1-H) was not successfully fused to lacZ. The expression of the 31 TY1A-lacZ fusions was determined in haploid cells using filter lift assays. Most patches of haploid cells were blue or light blue in the presence of X-Gal, and five remained white (Table 2). Northern blot analysis was performed to determine whether the β-galactosidase levels reflected the steady-state level of TY1A-lacZ mRNA in these strains. Total RNA extracted from three strains yielding blue, light blue, and white patches, respectively, in the presence of X-Gal was hybridized to a lacZ probe (Fig. 2). The results indicated a clear correlation between steady-state levels of TY1A-lacZ transcripts and the β-galactosidase activity estimated in these strains.
TABLE 2.
Expression of native Ty1 elements in haploid and diploid cellsa
| TY1A-lacZ fusion(s) | Color in the presence of X-Gal
|
|
|---|---|---|
| Haploid | Diploid | |
| DR3, DR5, ER2, LR2, and ML1 | − | − |
| DR1, GR2, GR3, JR1, LR1, MR1, MR2, NL1, OL, and PR2 | + | − |
| A, NL2, and OR | + | + |
| DR4, DR6, LR3, ML2, PL, and PR3 | ++ | − |
| BL, BR, ER1, GR1, JR2, and PR1 | ++ | + |
| LR4 | ++ | ++ |
TY1A-lacZ expression was determined on patches of cells by filter assays. −, white; +, light blue; ++, blue. The nomenclature of the Ty1 elements follows the sequence annotation established by MIPS (http://www.mips.biochem.mpg.de/), e.g., ER2 is the second element on the right arm of chromosome V). On chromosome IV, the Ty1 element previously identified as DR2 by MIPS is a Ty2 element (http://www.public.iastate.edu/∼voytas/). Haploid, TY1A-lacZ fusions introduced into FYBL1-23D by recombination (see Materials and Methods); diploid, FYBL1-23D derivatives carrying the TY1A-lacZ fusions mated with FY839.
FIG. 2.
Northern blot analysis of TY1A-lacZ transcripts in haploid cells. For each strain, 10 μg of RNA was loaded on gel. Steady-state levels of TY1A(PR1)-lacZ, TY1A(OL)-lacZ, and TY1A(DR3)-lacZ mRNA were normalized with ACT1 mRNA. The ratios are given relative to TY1A(DR3)-lacZ. Details on β-galactosidase activity are given in Table 2. Strains were grown in HC medium lacking uracil. Strains carrying the TY1A-lacZ fusions are derivatives of FYBL1-23D.
To determine whether the β-galactosidase activity reflects the promoter activity of each fusion, we replaced the promoter of TY1A(PR1)-lacZ and TY1A(DR3)-lacZ with the inducible GAL1 promoter, such that transcription of these fusions becomes regulated by galactose availability (see Materials and Methods). The β-galactosidase activities of TY1A(PR1)-lacZ and TY1A(DR3)-lacZ fused to the GAL1 promoter were similar (150 and 151 U, respectively), while TY1A(PR1)-lacZ β-galactosidase activity was 50-fold higher than TY1A(DR3)-lacZ β-galactosidase activity when these fusions were expressed from their own promoters (26 and 0.5 U, respectively). Thus, the monitoring of β-galactosidase activity in these strains allowed us to assess the promoter activity of each native Ty1 element.
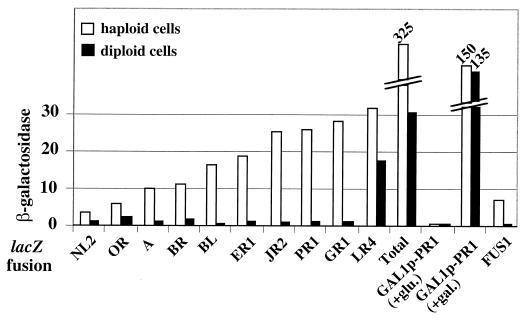
In diploid cells, the decrease of total Ty1 mRNA (21) suggests that Ty1 expression is repressed, but it is not known if all Ty1 elements are regulated identically. To address this issue, the 31 haploid strains carrying the different TY1A-lacZ fusions in FYBL1-23D were crossed with FY839 to obtain diploid cells. By comparing the expression of the 31 TY1A-lacZ fusions in haploid and diploid cells with filter lift assays, we observed a general decrease of β-galactosidase activity in diploid cells (Table 2). Most patches of diploid cells were white. We performed a quantitative β-galactosidase assay for the 10 TY1A-lacZ fusions still expressed in diploid cells on plates. Except for the TY1A(LR4)-lacZ fusion, which was only slightly repressed in diploid cells, the β-galactosidase activity was close to background for all fusions, indicating that expression of the corresponding Ty1 elements was repressed in diploid cells (Fig. 3). As expected, a similar decrease was observed for the haploid specific FUS1-lacZ fusion. Finally, we compared the sum of the β-galactosidase activities of the 10 fusions expressed to a measurable level in diploid cells to the sum of specific activities obtained with the 31 TY1A-lacZ fusions expressed in haploid cells. A 10-fold decrease of total β-galactosidase activity was observed in diploid cells (Fig. 3). The decrease is similar to that of Ty1 mRNA previously measured in diploid cells (21). These results indicate that expression of all native Ty1 copies, with the noticeable exception of Ty1(LR4), is strongly repressed in diploid cells.
FIG. 3.
Expression of native Ty1 elements in haploid and diploid cells. The figure shows the β-galactosidase activity of the TY1A-lacZ fusions that gave a blue or light-blue color in filter lift assays of diploid cells. The total is the sum of the specific activities obtained with the 31 TY1A-lacZ fusions expressed in haploid cells and in diploid cells. GAL1p-PR1 is a TY1A-lacZ fusion, in which the U3 region of 5′ LTR has been replaced by the GAL1 promoter at Ty1(PR1) (see Materials and Methods). +glu., in the presence of glucose; +gal., in the presence of galactose. FUS1-lacZ is carried on pSL974. Diploid cells were obtained by mating the FYBL1-23D derivatives carrying different TY1A-lacZ fusions with FY839.
To determine whether the dramatic decrease in Ty1 expression was promoter dependent, we compared the β-galactosidase activity of TY1A(PR1)-lacZ, fused to the GAL1 promoter, in haploid and diploid cells grown in the presence of galactose. The β-galactosidase activity was 300-fold higher in the presence of galactose than in the presence of glucose (Fig. 3). However, no difference in β-galactosidase activity was observed between the haploid and diploid cells. A similar result was obtained when the promoter of TY1A(DR3)-lacZ was replaced by the GAL1 promoter (data not shown). These results indicate that repression of the promoter activity of Ty1 is responsible for the decrease of Ty1 expression in diploid cells.
Ste12 and Tec1 activate Ty1 transcription in haploid cells.
An increase of Ty1 mRNA in ste12Δ haploid cells has been previously reported, suggesting that Ste12 might act as a repressor of Ty1 transcription (10). This observation is surprising, since Ste12, which binds in association with Tec1 to Ty1 FRE in vitro, is known to activate FRE-driven expression (40). Therefore, we decided to analyze the role of STE12 on Ty1 transcription further, by monitoring the β-galactosidase activity of several TY1A-lacZ fusions in wild-type and ste12Δ strains.
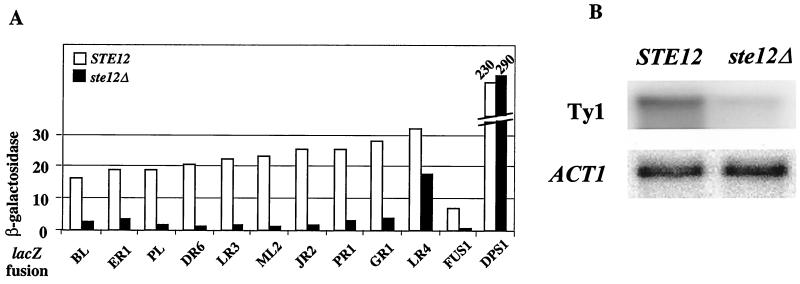
STE12 was disrupted in 10 derivatives of FYBL1-23D carrying the best-expressed TY1A-lacZ fusions. The sum of the expression of these fusions accounts for 75% of the total in wild-type haploid cells (A. Morillon and P. Lesage, unpublished data). In the absence of STE12, β-galactosidase activity diminished significantly for all fusions (5- to 17-fold compared to the wild type), except for the TY1A(LR4)-lacZ fusion, where only a 2-fold decrease was observed (Fig. 4A). Interestingly, the TY1A(LR4)-lacZ fusion was also not fully repressed in diploid cells (Fig. 3). In control experiments, expression of FUS1-lacZ, which is known to depend on STE12 (32), was dramatically reduced in the presence of the deletion, whereas expression of DPS1-lacZ remained constant (DPS1 encodes the aspartyl-tRNA synthetase and is constitutively expressed). By comparing the sum of the β-galactosidase activities obtained with the 10 TY1A-lacZ fusions in the ste12Δ strain (i.e., 38.6 U) with the total activity of the 31 fusions expressed in wild-type haploid cells (325 U [Fig. 3]), we calculate an eightfold decrease in TY1A-lacZ expression in the absence of STE12. A Northern blot analysis, performed in parallel to compare the total level of Ty1 mRNA in wild-type and ste12Δ haploid cells, indicated a similar decrease of total Ty1 transcripts levels in the absence of STE12 (ninefold, Fig. 4B). The reduction of β-galactosidase activity for the 10 fusions analyzed, as well as the lower level of Ty1 mRNA in a ste12Δ strain, demonstrate that STE12 enhances Ty1 transcription in haploid cells.
FIG. 4.
Ste12 activates Ty1 transcription. (A) β-Galactosidase activity determined in haploid STE12 and ste12Δ cells grown in HC medium lacking uracil. Details on lacZ fusions are given in the legends to Fig. 2 and 3. DPS1-lacZ is carried on the centromeric plasmid pAM7. Plasmids were introduced in FYBL1-23D (STE12) and LV101 (ste12Δ). (B) Northern blot analysis of steady-state level of Ty1 mRNA normalized with ACT1 mRNA in FY1679-28C (STE12) and LV105 (ste12Δ) strains grown in YPD. The ratios of total Ty1 mRNA to ACT1 mRNA relative to the STE12 strain were as follows: STE12, 1.0; ste12Δ, 0.11.
We also deleted TEC1 in three strains carrying the TY1A(JR2)-lacZ, TY1A(ML2)-lacZ, and TY1A(PR1)-lacZ fusions, respectively. As observed in the absence of STE12, the β-galactosidase activity of these fusions decreased 3- to 10-fold in the absence of TEC1 (data not shown). This result is in agreement with already-published data indicating a reduction of Ty1 mRNA levels in tec1Δ mutant (34). Taken together, these results indicate that both STE12 and TEC1 activate Ty1 transcription in haploid cells.
Activation of the Kss1 MAPK cascade by the STE11-4 allele induces Ty1 transcription in diploid cells.
The positive role of both Ste12 and Tec1 on Ty1 transcription shown above and the previous evidence that Ty1 transcription requires Ste7 and Ste11 strongly suggest that Ty1 expression is under the control of the Kss1 invasive-filamentous pathway (references 19 and 25 and Fig. 1). Since this pathway can be activated in diploid cells, we predicted that Ty1 transcription could be similarly stimulated in this cell type. Previous experiments have shown that FRE-dependent gene expression and filamentation can be induced in diploid cells by the hypermorphic STE11-4 allele that leads to constitutive phosphorylation of Kss1 (42). Therefore, we decided to test whether activation of Ste12 and Tec1 by the invasive-filamentous pathway in the presence of STE11-4 would activate Ty1 transcription. Since Fus3 is not expressed in diploid cells, STE11-4 does not activate the mating pathway in these experiments (23, 29).
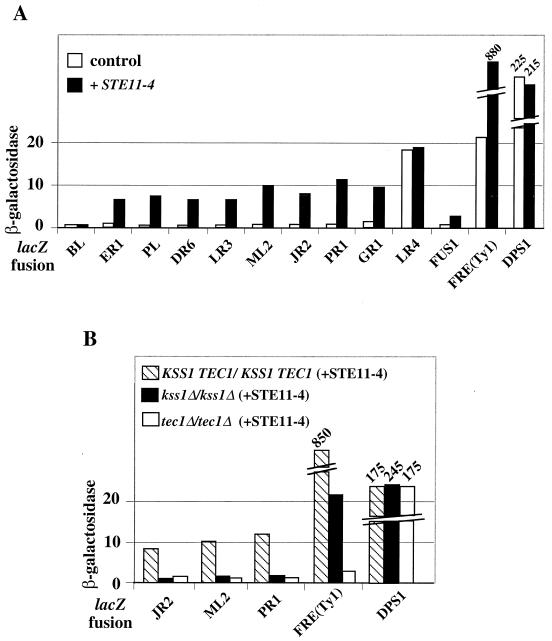
Ten derivatives of FYBL1-23D carrying different TY1A-lacZ fusions, chosen because they are well expressed in haploid cells but repressed in diploid cells [except for the TY1A(LR4)-lacZ fusion], were crossed with FY839. The resulting diploid strains were transformed with a plasmid expressing STE11-4 and a plasmid vector, respectively. Little β-galactosidase activity was detected in the absence of STE11-4, except for the TY1A(LR4)-lacZ fusion, whose expression is not fully repressed in diploid cells. However, in the presence of STE11-4, a significant increase in β-galactosidase activity was observed for most fusions (Fig. 5A). The sum of the individual β-galactosidase values for the 10 tested diploid strains indicates that the STE11-4 plasmid causes a 3.8-fold increase in Ty1 expression (compare 88.5 U obtained in the presence of STE11-4 with 23 U obtained in the absence of STE11-4).
FIG. 5.
Induction of the Kss1 MAPK cascade in the presence of STE11-4 activates Ty1 transcription. (A) β-Galactosidase activity determined in diploid cells obtained by mating the FYBL1-23D derivatives carrying different TY1A-lacZ fusions with FY839. Diploid cells were transformed with the pRS313 vector (control) (52) or with p11-4/HIS3 (STE11-4) and grown in HC medium lacking uracil and histidine. Details on lacZ fusions are given in the legends to Fig. 3 and 4. In FRE(Ty1)-lacZ, the 27-bp Ty1 FRE is located upstream of an enhancerless CYC1-lacZ reporter gene (40). pFRE(Ty1)-lacZ and pAM7 (DPS1-lacZ) were introduced in diploid cells obtained by crossing FYBL1-23D with FY839. (B) β-Galactosidase activity determined in wild-type, kss1Δ/kss1Δ, and tec1Δ/tec1Δ diploid cells transformed with p11-4/HIS3 (plus STE11-4) and grown in HC medium lacking uracil and histidine. Wild-type diploid cells were obtained by crossing the FYBL1-23D derivatives carrying the different TY1A-lacZ fusions with LV50. pFRE(Ty1)-lacZ was introduced in diploid cells obtained by mating FYBL1-23D with LV50. The kss1Δ/kss1Δ diploid cells were obtained by disrupting KSS1 in the FYBL1-23D derivatives carrying the different TY1A-lacZ fusions and by crossing the resulting strains with LV247. Similarly, the tec1Δ/tec1Δ diploid cells were obtained by disrupting TEC1 in the FYBL1-23D derivatives carrying the different TY1A-lacZ fusions and by crossing the resulting strains with LV128. pFRE(Ty1)-lacZ was introduced in diploid cells obtained by crossing LV126 with LV128 (for tec1Δ/tec1Δ diploid cells) and LV237 with VL247 (for kss1Δ/kss1Δ diploid cells).
To verify that the activation of Ty1 transcription occurred via the Kss1 invasive-filamentous pathway, we deleted KSS1 in three diploid strains carrying different TY1A-lacZ fusions and measured their β-galactosidase activities in the presence of a plasmid expressing STE11-4. The three fusions were not activated by STE11-4 in the absence of KSS1 (Fig. 5B). Similarly, the three fusions were not activated by STE11-4 when TEC1 was deleted (Fig. 5B). Taken together, our results indicate that STE11-4 acts on Tec1 and Ste12, via the Kss1 MAPK cascade, to activate Ty1 transcription. The β-galactosidase activity observed with Ty1(FRE)-lacZ in the absence of Kss1 (Fig. 5B) is due to the fact that Kss1 has two antagonistic activities (Fig. 1) (42); thus, deletion of KSS1 leads to an intrinsic activity of Tec1 and Ste12. The β-galactosidase activity measured in diploid cells transformed with STE11-4 was lower than in haploid cells (see Fig. 4A), suggesting that activation of Ty1 transcription by Ste12 and Tec1 does not completely overcome the repression of haploid specific genes by the a1/α2 complex in diploid cells.
The expression of two fusions, TY1A(BL)-lacZ and TY1A(LR4)-lacZ, did not increase in the presence of STE11-4 (Fig. 5A). Whereas the Ste12 binding-site of most native Ty1 elements (CGTTTCA) differs from the consensus site by one nucleotide (TGTTTCA [40]), that of Ty1(BL) contains two differences (CATTTCA) (Saccharomyces Genome Database, http://genome-www.stanford.edu/). Ty1(BL) belongs with Ty1(MR1) to a subtype of Ty1 elements (33). Ty1(MR1) presents the same modified Ste12 binding site. As for TY1A(BL)-lacZ, TY1A(MR1)-lacZ expression was not activated by STE11-4 in diploid cells (data not shown). In haploid cells, TY1A(BL)-lacZ expression is high and requires Ste12 (Fig. 4A), indicating that Ste12 is still able to bind to Ty1(BL) DNA despite the two differences in the consensus binding site. However, the lack of activation of TY1A(BL)-lacZ and TY1A(MR1)-lacZ fusions by STE11-4 in diploid cells suggests a less-efficient binding of Ste12 to its DNA target which, in combination with the lower amount of Ste12 present in diploid cells, could severely reduce Ste12-mediated activation. With respect to TY1A(LR4)-lacZ, the lack of activation by STE11-4 was expected since, despite the presence of a normal Ste12 binding-site, its expression is only partially dependent on Ste12 (Fig. 4A). We suggest that the specific location of Ty1(LR4), which overlaps the HAP1 open reading frame (Saccharomyces Genome Database), may impair its regulation by Ste12: RNA polymerase II molecules transcribing HAP1 may facilitate transcription of Ty1(LR4) without a need for additional transcriptional activators.
Activation of the Kss1 MAPK cascade by STE11-4 induces Ty1 retrotransposition.
We wondered whether activation of Ty1 transcription by the Kss1 MAPK cascade in diploid cells would be followed by retrotransposition events. Transposition of a genomic Ty1 element under the control of its native promoter can be detected when the element is tagged with the retrotransposition indicator his3AI. The transposition frequency of this element is proportional to the frequency of His+ prototroph formation (13, 16). We measured the frequency of His+ colony formation in diploid cells obtained by crossing FY839 with JC297 and JC242, respectively. These diploid strains containing the endogenous Ty1his3AI-270 or Ty1his3AI-242 elements were transformed by the pRS316 vector or by the pSL1509 plasmid carrying STE11-4. While no His+ colonies were detected with pRS316 transformants, His+ prototrophs arose in the presence of STE11-4. The frequency of Ty1his3AI-270 or Ty1his3AI-242 retrotransposition was similar to that found in haploid cells in the absence of STE11-4 (Table 3). These results indicate that activation of the invasive-filamentous Kss1 MAPK cascade induces Ty1 retrotransposition in diploid cells.
TABLE 3.
Induction of the Kss1 MAPK cascade activates Ty1his3AI-270 and Ty1his3AI-242 retrotranspositiona
| Strain | Presence (+) or absence (−) of STE11-4 | Ty1his3AI-270
|
Ty1his3AI-242
|
||
|---|---|---|---|---|---|
| Transposition frequency | Fold effect | Transposition frequency | Fold effect | ||
| Diploid | − | (≤1.2 ± 0.2) × 10−8 | ≥16.5 | (≤8.7 ± 0.8) × 10−9 | ≥16.1 |
| + | (2.1 ± 0.8) × 10−7 | (1.4 ± 0.4) × 10−7 | |||
| Haploid | − | (2.3 ± 0.9) × 10−7 | 15.5 | (2.5 ± 0.4) × 10−7 | 6.4 |
| + | (3.6 ± 1.7) × 10−6 | (1.6 ± 0.2) × 10−6 | |||
The transposition frequency was determined as the fraction of total colonies that are His+. Strains were transformed either with the pRS316 vector (−STE11-4) (52) or with pSL1509 (+STE11-4) and grown in HC medium lacking uracil. Haploid strain, JC297 (Ty1his3AI-270) and JC242 (Ty1his3AI-242); diploid strain, JC297 and JC242 crossed with FY839. For each strain, values are the averages of measurements made with three independent transformants.
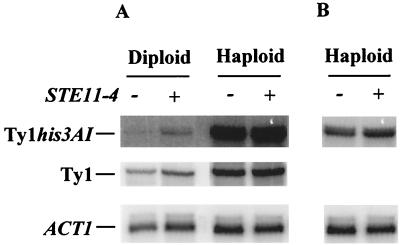
We also performed a Northern analysis to compare the amount of Ty1his3AI-270 and Ty1 transcripts in the absence and in the presence of STE11-4 (Fig. 6). A fivefold increase in Ty1his3AI-270 mRNA was detected in the presence of STE11-4 in diploid cells, strongly suggesting that activation of Ty1his3AI-270 retrotransposition is a direct effect of transcriptional activation of this element in the presence of STE11-4 (Fig. 6A, top panel). Total Ty1 mRNA levels were also twofold higher in the presence of STE11-4 (Fig. 6A, center panel). The increase in Ty1his3AI-270 and Ty1 mRNA levels in the presence of STE11-4 confirms that induction of the invasive-filamentous pathway by STE11-4 activates Ty1 transcription in diploid cells, as shown above with the activation of TY1A-lacZ expression in the presence of STE11-4.
FIG. 6.
Northern blot analysis of total RNA from diploid and haploid cells in the presence or in the absence of STE11-4. (A) Strains were transformed with either the pRS316 vector (−STE11-4) or with pSL1509 (+STE11-4) and grown in HC medium lacking uracil. Haploid and diploid strains are described in Table 3. Probes against HIS3 and Ty1 were used to detect Ty1his3AI-270 and Ty1 mRNA, respectively. Ty1his3AI-270 and Ty1 mRNA levels were normalized with ACT1 mRNA. The increase of Ty1his3AI-270 mRNA levels in the presence of STE11-4 was 5-fold in diploid cells and 1.6-fold in haploid cells; that of Ty1 mRNA levels was 2-fold in diploid cells and 1.4-fold in haploid cells. (B) Segment of a shorter exposure of the same Northern blot to show the slight increase of Ty1his3AI mRNA in haploid cells transformed with pSL1509 (+STE11-4).
Interestingly, we found that STE11-4 significantly enhances the retrotransposition frequency of the endogenous Ty1his3AI-270 and Ty1his3AI-242 elements in haploid cells as well (15.5- and 6.4-fold, respectively [Table 3]). An increase in Ty1his3AI-270 and Ty1 transcript levels was detected by Northern analysis in haploid cells in the presence of STE11-4 (Fig. 6). Although minor (1.6- and 1.4-fold, respectively), this increase suggests that STE11-4 also activates Ty1 transcription in haploid cells. Taken together, these results show that STE11-4 activates Ty1 transcription and retrotransposition in diploid and in haploid cells.
Ty1 transcription and transposition are activated in diploid cells starved for nitrogen.
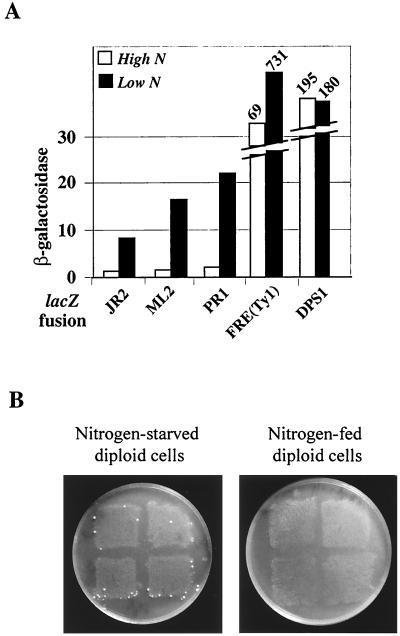
As nitrogen starvation induces filamentation of diploid cells (30), we asked whether Ty1 transcription and transposition would be activated in diploid cells grown in low-nitrogen medium. Since the flo8-1 recessive mutation impairs filamentous growth in S288C derivatives, we constructed diploid cells able to filament by crossing the Σ1278b derivative L5684, which is FLO8, with three derivatives of FYBL1-23D carrying different TY1A-lacZ fusions. Production of filaments on low-nitrogen medium confirmed that nitrogen starvation induces the invasive-filamentous pathway in these diploid strains (data not shown). To determine if Ty1 transcription was activated upon nitrogen starvation, we measured the β-galactosidase activity in the three diploid strains grown either in high- or low-nitrogen medium. In nitrogen-starved cultures, expression of the three TY1A-lacZ fusions [as well as the FRE(Ty1)-lacZ control] increased severalfold (Fig. 7A), whereas expression of DPS1-lacZ did not. We conclude that Ty1 transcription is activated in diploid cells upon nitrogen starvation.
FIG. 7.
Nitrogen starvation activates Ty1 transcription and transposition. (A) β-Galactosidase activity determined in diploid cells obtained by crossing the FYBL1-23D derivatives carrying different TY1A-lacZ fusions with L5684. Cells were either grown in SD medium (High N, high nitrogen) or in SLAD medium (Low N, low nitrogen). Details on lacZ fusions are given in the legends to Fig. 4 and 5. (B) Retrotransposition of the endogenous Ty1his3AI-270 element. Patches correspond to four different diploid strains obtained by crossing JC297 with LV148. SD plates are shown after 4 days of incubation at 30°C. On the left are shown diploid cells replica plated onto the SD plate after growth on SLAD plus histidine (nitrogen-starved diploid cells), and on the right are shown diploid cells replica plated onto an SD plate after growth on SD plus histidine (nitrogen-fed diploid cells). The nonrandom localization of His+ colonies probably results from higher growth at the edge of the patches.
We also analyzed the transposition frequency of the endogenous Ty1his3AI-270 element in nitrogen-starved diploid cells. We constructed a diploid strain containing the endogenous Ty1his3AI-270 element and a complete deletion of HIS3 on both chromosomes by mating JC297 with the Σ1278b derivative LV148. This strain was unable to grow in the absence of histidine. Patches of diploid cells containing the Ty1his3AI-270 element were grown at 22°C either on high (SD)- or low (SLAD)-nitrogen plates supplemented with histidine. Filaments were detected after several days of growth on low-nitrogen plates, indicating that the invasive-filamentous pathway was activated upon nitrogen starvation. Patches were subsequently replica plated on SD medium lacking histidine to identify retrotransposition events. His+ prototrophs appeared only with the patches of cells grown on low-nitrogen plates, indicating that Ty1his3AI-270 retrotransposition is activated in nitrogen-starved diploid cells (Fig. 7B). In conclusion, the activation of TY1A-lacZ transcription in nitrogen-starved diploid cells along with the transposition of Ty1his3AI-270 shows that stimulation of the invasive-filamentous pathway by nitrogen starvation induces Ty1 transcription and retrotransposition in diploid cells.
Ty1 insertions can confer regulation by the invasive-filamentous pathway to adjacent genes.
ROAM mutants can easily be selected using the promoterless his3Δ4 allele, which is activated in haploid cells by upstream Ty1 insertions (7). Because of the presence of the FRE sequence in Ty1 and based on the experiments described above, we predict that expression of ROAM-his3Δ4 alleles should be under the control of the invasive-filamentous pathway. As a consequence, their expression, which is constitutive in haploid cells and repressed in diploid cells, should be induced in diploid cells when the invasive-filamentous pathway is activated.
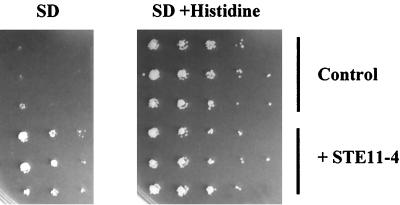
We failed to detect retrotransposition events upstream of his3Δ4 in diploid cells upon activation of Ty1 retrotransposition by the invasive-filamentous pathway in the presence of STE11-4, presumably because his3Δ4 is not a preferential target for Ty1 integration. Thus, we decided to first construct a haploid strain carrying a ROAM-his3Δ4 mutation by transpositional induction using pGTy1-H3 (see Materials and Methods). Using this approach, His+ prototrophs of LV69 that arise at a frequency of 5 × 10−7 per cell (data not shown) were obtained. One such strain, LV150, was crossed with FY839 to obtain a diploid strain carrying ROAM–his3Δ4-1. This strain was then transformed with a plasmid expressing STE11-4 or with a plasmid vector as a control. Transformants containing the plasmid vector were unable to grow in the absence of histidine, whereas His+ diploid cells grew in the presence of STE11-4 (Fig. 8). In conclusion, the activation of ROAM–his3Δ4-1 expression in the presence of STE11-4 indicates that, in diploid cells, induction of the invasive-filamentous pathway can activate the expression of genes located in the proximity of a Ty1 element, as is the case in ROAM mutants.
FIG. 8.
Activation of ROAM–his3Δ4-1 allele in diploid cells in the presence of STE11-4. Diploid cells containing the ROAM–his3Δ4-1 allele were transformed either with the pRS316 vector (control) or with pSL1509 (+STE11-4) and grown to saturation in SD medium supplemented with histidine liquid cultures. Transformants were assayed for His expression by spotting serial dilutions on SD medium and for growth by spotting on SD-plus-histidine plates. Haploid and diploid cells containing the promoterless his3Δ4 allele were unable to grow in the absence of histidine (data not shown). Diploid cells were obtained by crossing LV150 with FY839.
DISCUSSION
To study the transcriptional regulation of the Ty1 elements in their native location, we constructed 31 strains, each carrying a lacZ chromosomal fusion expressed from the transcriptional signals of a different Ty1 element (Morillon and Lesage, unpublished data). We focused our analysis on the 10 best-expressed TY1A-lacZ fusions since their global expression amounts to 75% of the total, thus giving a representative picture of the regulation of Ty1 expression. Our experiments support the view that the Kss1 MAPK invasive-filamentous pathway activates Ty1 transcription and that stress-like conditions, i.e., constitutive activation of this cascade or nitrogen starvation, can induce Ty1 transcription and retrotransposition in diploid cells.
The Kss1 invasive-filamentous pathway activates Ty1 transcription.
First, we found that TY1A-lacZ transcription in haploid cells requires Ste12 and Tec1, two transcriptional activators acting downstream of the invasive-filamentous pathway and that bind cooperatively to the FRE site (2, 40). In agreement with the activation of TY1A-lacZ transcription by Ste12, our Northern blot analysis indicated that Ty1 mRNA levels decrease severalfold in the absence of STE12 (Fig. 4). In ROAM mutant alleles, in Ty1 FRE reporter gene constructs, as well as in the TEC1 and FLO11 genes (6, 46, 51), FRE is located upstream of the TATA box. In the case of Ty1, it is located 157 bp downstream of the transcription start site, in the TY1A open reading frame. Our results indicate that Ty1 FRE behaves as a downstream activating sequence of endogenous Ty1 elements, as suggested by previous experiments which showed that a region encompassing Ty1 FRE is required to activate transcription of a Ty1 element carried on a plasmid (28). Thus, Ste12 and Tec1 can activate transcription, when recruited to FRE sites located upstream or downstream of a TATA box.
In addition to the FRE site, other downstream activating sequences have been identified in Ty1 (57). Downstream repressing and activating sequences are also present in Ty2 (27, 37). Since sequences regulating Ty transcription should be located within the retrotransposon to be kept upon retrotransposition, the presence of regulatory sequences downstream of the TATA box of Ty1 and Ty2 retrotransposons suggests that this particular location could reflect an adaptation to size restraints of the LTR on these elements. The existence of such restraints is supported by the observation that increasing the length of the LTRs inhibits Ty1 retrotransposition (35). Since the 5′ LTR needs to be an active promoter unlike the 3′ LTR, the presence of regulatory sequences downstream from the 5′ LTR may also be the best way to specify promoter activity to this LTR.
A second piece of evidence that the invasive-filamentous signaling pathway regulates Ty1 transcription is that the constitutive STE11-4 allele, shown to activate both filamentation and FRE-dependent gene expression (42) induces TY1A-lacZ transcription in diploid cells (Fig. 5A). This result is supported by the two- and fivefold increases in Ty1 and Ty1his3AI-270 mRNA levels, respectively, observed in diploid cells in the presence of STE11-4 (Fig. 6). The Ste11 MEKK acts upstream of Fus3 and Kss1 MAPK to regulate both the mating and invasive-filamentous pathways (41). Activation of Ty1 transcription in diploid cells depends exclusively on the invasive-filamentous pathway since activation of TY1A-lacZ expression by STE11-4 requires Tec1 and Kss1 (Fig. 5B), which are specific to this pathway, and also because FUS3 is not expressed in diploid cells (23, 29). In agreement with the activation of Ty1 transcription by the filamentous pathway but not by the mating pathway, TY1A-lacZ transcription was activated in diploid cells upon nitrogen starvation, which has been shown to induce filamentous growth (Fig. 7A).
The Kss1 invasive-filamentous pathway activates Ty1 transposition.
In diploid cells, constitutive activation of the invasive-filamentous pathway by STE11-4 causes not only Ty1 transcription but also transposition of the endogenous Ty1his3AI-270 and Ty1his3AI-242 elements (Table 3). Activation of this pathway by nitrogen starvation also induces Ty1his3AI-270 retrotransposition (Fig. 7B). The activation of Ty1 retrotransposition is probably the direct consequence of Ty1 transcriptional activation by the invasive-filamentous pathway, since previous results suggest that increased Ty1 mRNA levels cause an increase in transposition frequency (16, 17). However, it remains possible that STE11-4 also activates Ty1 retrotransposition at both transcriptional and posttranscriptional steps in diploid cells.
The presence of STE11-4 enhances Ty1his3AI-270 retrotransposition frequency in haploid cells as well (Table 3). This enhancement may a priori be due to the activation of either the mating or the invasive-filamentous pathway or both. Two results seem to exclude the involvement of the mating pathway. First, activation of the mating pathway by pheromones inhibits Ty1 retrotransposition (56). Second, Fus3, the MAPK of the mating pathway, was also shown to repress Ty1 retrotransposition (13). Interestingly, both inhibitions occur at the posttranscriptional step. Therefore, we speculate that STE11-4 activates Ty1 retrotransposition via the Kss1 invasive-filamentous pathway in haploid cells, as in diploid cells. Our results show that STE11-4 stimulates Ty1his3AI-270 retrotransposition 15-fold (Table 3) in haploid cells. Under these conditions, Ty1his3AI-270 mRNA levels increase 1.6-fold (Fig. 6), and transcription of some TY1A-lacZ fusions are 2-fold higher (Morillon and Lesage, unpublished data). The lack of proportionality between the increase in Ty1his3AI-270 transcription and transposition might indicate that STE11-4 acts at a posttranscriptional level to activate Ty1 retrotransposition in haploid cells, unless a limited increase in transcription might be sufficient to observe larger effects on transposition. Posttranscriptional controls of Ty1 retrotransposition have already been demonstrated for the Fus3 MAPK and the nucleotide excision repair-transcription factor TFIIH subunits SSL2 and RAD3 (13, 36).
We also noticed that the transposition frequency of Ty1his3AI-270 in diploid cells activated by STE11-4 and in unactivated haploid cells was similar, while Ty1his3AI-270 mRNA levels were higher in haploid cells (Table 3 and Fig. 6). This lack of proportionality between Ty1 mRNA levels and transposition frequency may be due to the existence of additional negative posttranscriptional control(s) in haploid cells that are not effective in diploid cells. Since FUS3 is not expressed in diploid cells, the inhibition of Ty1 retrotransposition by Fus3 is specific to haploid cells and may be responsible for this difference (13, 23, 29). Alternatively, this difference may simply reflect the fact that the potential targets for retrotransposition are twice as abundant in diploid cells as in haploid cells.
Conte et al. have proposed that Fus3 negatively regulates the Ty1 life cycle in haploid cells by destabilizing Ty1 virus-like particles (13). More recent results from the same authors showed that, in addition to posttranscriptional effects, deletion of FUS3 leads to a 2.5-fold increase in Ty1 mRNA (14). We also found that the expression of some TY1A-lacZ fusion was twofold stimulated in fus3Δ strains (Morillon and Lesage, unpublished data). Given that fus3 mutants are hyperinvasive and result in an increase of FRE driven expression (42), we propose that FUS3 also inhibits Ty1 retrotransposition by limiting the activation of Ty1 transcription by the Kss1 MAPK invasive-filamentous pathway.
Activation of Ty1 retrotransposition by the invasive-filamentous pathway may be an adaptive response to stress.
Our results provide the first evidence that Ty1 retrotransposition can be activated by environmental signals that affect cellular differentiation, such as nitrogen starvation. We propose that activation of Ty1 retrotransposition by these signals is an adaptive response to stress. Upon nutrient limitation, diploid cells form filaments, which enable them to search for nutrients away from their colonization site. The Kss1 MAPK signaling pathway involved in the induction of this differentiation process also activates Ty1 transcription and increases Ty1 transposition to new sites. In some cases, Ty1 integration will lead to cis activation of cellular genes by Tec1 and Ste12, which might confer a selective advantage to the cell. This adaptive response to stress could be highly relevant, since diploid cells are predominant in nature. To strengthen this hypothesis, we found that Ty1 elements can provide the signals required for activation by the invasive-filamentous pathway to adjacent genes in diploid cells (Fig. 8).
Activation of Ty1 transcription and retrotransposition has already been observed in haploid cells exposed to DNA-damaging agents, which represent another kind of stress (8). Similarly, UV irradiation of Escherichia coli cells stimulates intermolecular transposition of IS10 in an SOS-stress-response-dependent process (20). In plants, retrotransposons that are largely quiescent during development are also activated by stress-like wounding or pathogen attacks (53). Identification of retrotransposon-derived sequences flanking plant and mammalian genes suggest that some host gene expression may depend on transposon signals (54). Activation of retrotransposition by stress might thus be a general way for genomes to evolve.
ACKNOWLEDGMENTS
We are very grateful to C. Condon, S. Gangloff, J. Smith, and P. Stragier for critical reading of the manuscript. We thank members of our laboratory for stimulating discussions and especially C. Sacerdot for providing strain LV150. We thank J. Curcio, B. Dujon, G. Faye, and F. Winston for kindly providing strains. Our thanks also go to J. Boeke, G. Eriani, G. Fink, and G. Sprague for sending us plasmids.
This work was supported by a grant from the CNRS (UPR 9073). A.M. was a recipient of a Docteur Ingénieur fellowship from the CNRS.
REFERENCES
- 1.Adams A, Gottschling D E, Kaiser C A, Stearns T. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- 2.Baur M, Esch R K, Errede B. Cooperative binding interactions required for function of the Ty1 sterile responsive element. Mol Cell Biol. 1997;17:4330–4337. doi: 10.1128/mcb.17.8.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benard L, Carroll K, Valle R C, Wickner R B. Ski6p is a homolog of RNA-processing enzymes that affects translation of non-poly(A) mRNAs and 60S ribosomal subunit biogenesis. Mol Cell Biol. 1998;18:2688–2696. doi: 10.1128/mcb.18.5.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeke J D, Eichinger D, Castrillon D, Fink G R. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol Cell Biol. 1988;8:1432–1442. doi: 10.1128/mcb.8.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeke J D, Garfinkel D J, Styles C A, Fink G R. Ty elements transpose through an RNA intermediate. Cell. 1985;40:491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- 6.Boeke J D, Sandmeyer S B. Yeast transposable elements. In: Broach J R, Pringle J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 193–261. [Google Scholar]
- 7.Boeke J D, Styles C A, Fink G R. Saccharomyces cerevisiae SPT3 gene is required for transposition and transpositional recombination of chromosomal Ty elements. Mol Cell Biol. 1986;6:3575–3581. doi: 10.1128/mcb.6.11.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradshaw V A, McEntee K. DNA damage activates transcription and transposition of yeast Ty retrotransposons. Mol Gen Genet. 1989;218:465–474. doi: 10.1007/BF00332411. [DOI] [PubMed] [Google Scholar]
- 9.Christianson T W, Sikorski R S, Dante M, Shero J H, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 10.Ciriacy M, Freidel K, Lohning C. Characterization of trans-acting mutations affecting Ty and Ty-mediated transcription in Saccharomyces cerevisiae. Curr Genet. 1991;20:441–448. doi: 10.1007/BF00334769. [DOI] [PubMed] [Google Scholar]
- 11.Company M, Adler C, Errede B. Identification of a Ty1 regulatory sequence responsive to STE7 and STE12. Mol Cell Biol. 1988;8:2545–2554. doi: 10.1128/mcb.8.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Company M, Errede B. Cell-type-dependent gene activation by yeast transposon Ty1 involves multiple regulatory determinants. Mol Cell Biol. 1987;7:3205–3211. doi: 10.1128/mcb.7.9.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conte D J, Barber E, Banerjee M, Garfinkel D J, Curcio M J. Posttranslational regulation of Ty1 retrotransposition by mitogen-activated protein kinase Fus3. Mol Cell Biol. 1998;18:2502–2513. doi: 10.1128/mcb.18.5.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conte D J, Curcio J M. Fus3 controls Ty1 transpositional dormancy through the invasive growth MAPK pathway. Mol Cell Biol. 2000;35:415–427. doi: 10.1046/j.1365-2958.2000.01710.x. [DOI] [PubMed] [Google Scholar]
- 15.Cook J G, Bardwell L, Thorner J. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signaling pathway. Nature. 1997;390:85–88. doi: 10.1038/36355. [DOI] [PubMed] [Google Scholar]
- 16.Curcio M J, Garfinkel D J. Single-step selection for Ty1 element retrotransposition. Proc Natl Acad Sci USA. 1991;88:936–940. doi: 10.1073/pnas.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curcio M J, Hedge A M, Boeke J D, Garfinkel D J. Ty RNA levels determine the spectrum of retrotransposition events that activate gene expression in Saccharomyces cerevisiae. Mol Gen Genet. 1990;220:213–221. doi: 10.1007/BF00260484. [DOI] [PubMed] [Google Scholar]
- 18.Devine S E, Boeke J D. Integration of the yeast retrotransposon Ty1 is targeted to regions upstream of genes transcribed by RNA-polymerase III. Genes Dev. 1996;10:620–633. doi: 10.1101/gad.10.5.620. [DOI] [PubMed] [Google Scholar]
- 19.Dubois E, Jacobs E, Jauniaux J C. Expression of the ROAM mutations in Saccharomyces cerevisiae: involvement of trans-acting regulatory elements and relation with the Ty1 transcription. EMBO J. 1982;1:1133–1139. doi: 10.1002/j.1460-2075.1982.tb01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichenbaum Z, Livneh Z. UV light induces IS10 transposition in Escherichia coli. Genetics. 1998;149:1173–1181. doi: 10.1093/genetics/149.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elder R T, St. John T P, Stinchcomb D T, Davis R W, Scherer S, Davis R W. Studies on the transposable element Ty1 of yeast. Cold Spring Harb Symp Quant Biol. 1981;45:581–591. doi: 10.1101/sqb.1981.045.01.075. [DOI] [PubMed] [Google Scholar]
- 22.Elion E A, Brill J A, Fink G R. FUS3 represses CLN1 and CLN2 and in concert with KSS1 promotes signal transduction. Proc Natl Acad Sci USA. 1991;88:9392–9396. doi: 10.1073/pnas.88.21.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elion E A, Grisafi P L, Fink G R. FUS3 encodes a cdc2+/CDC28-related kinase required for the transition from mitosis into conjugation. Cell. 1990;60:649–664. doi: 10.1016/0092-8674(90)90668-5. [DOI] [PubMed] [Google Scholar]
- 24.Errede B, Cardillo T S, Sherman F, Dubois E, Deschamps J, Wiame J M. Mating signals control expression of mutations resulting from insertion of a transposable repetitive element adjacent to diverse yeast genes. Cell. 1980;22:427–436. doi: 10.1016/0092-8674(80)90353-0. [DOI] [PubMed] [Google Scholar]
- 25.Errede B, Cardillo T S, Wever G, Sherman F, Stiles J I, Friedman L R, Sherman F. Studies on transposable elements in yeast. Cold Spring Harb Symp Quant Biol. 1981;45:593–607. [PubMed] [Google Scholar]
- 26.Errede B, Company M, Hutchison C A D. Ty1 sequence with enhancer and mating-type-dependent regulatory activities. Mol Cell Biol. 1987;7:258–265. doi: 10.1128/mcb.7.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farabaugh P J, Vimaladithan A, Turkel S, Johnson R, Zhao H. Three downstream sites repress transcription of a Ty2 retrotransposon in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:2081–2090. doi: 10.1128/mcb.13.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulton A M, Rathjen P D, Kingsman S M, Kingsman A J. Upstream and downstream transcriptional control signals in the yeast retrotransposon, TY. Nucleic Acids Res. 1988;16:5439–5458. doi: 10.1093/nar/16.12.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galitski T, Saldanha A J, Styles C A, Lander E S, Fink G R. Ploidy regulation of gene expression. Science. 1999;285:251–254. doi: 10.1126/science.285.5425.251. [DOI] [PubMed] [Google Scholar]
- 30.Gimeno C J, Ljungdahl P O, Styles C A, Fink G R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 31.Goffeau A, Barrell B G, Bussey H, Davis R W, Dujon B, Feldmann H, Galibert F, Hoheisel J D, Jacq C, Johnston M, Louis E J, Mewes H W, Murakami Y, Philipsen P, Tettelin H, Oliver S G. Life with 6000 genes. Science. 1996;274:546–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 32.Hagen D C, McCaffrey G, Sprague G F., Jr Pheromone response elements are necessary and sufficient for basal and pheromone-induced transcription of the FUS1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:2952–2961. doi: 10.1128/mcb.11.6.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J M, Vanguri S, Boeke J D, Gabriel A, Voytas D F. Transposable elements and genome organization: a comprehensive survey of retrotransposons revealed by the complete Saccharomyces cerevisiae genome sequence. Genome Res. 1998;8:464–478. doi: 10.1101/gr.8.5.464. [DOI] [PubMed] [Google Scholar]
- 34.Laloux I, Dubois E, Dewerchin M, Jacobs E. TEC1, a gene involved in the activation of Ty1 and Ty1-mediated gene expression in Saccharomyces cerevisiae: cloning and molecular analysis. Mol Cell Biol. 1990;10:3541–3550. doi: 10.1128/mcb.10.7.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lauermann V, Hermankova M, Boeke J D. Increased length of long terminal repeats inhibits Ty1 transposition and leads to the formation of tandem multimers. Genetics. 1997;145:911–922. doi: 10.1093/genetics/145.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee B S, Lichtenstein C P, Faiola B, Rinckel L A, Wysock W, Curcio M J, Garfinkel D J. Posttranslational inhibition of Ty1 retrotransposition by nucleotide excision repair/transcription factor TFIIH subunits Ssl2p and Rad3p. Genetics. 1998;148:1743–1761. doi: 10.1093/genetics/148.4.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao X-B, Clare J, Farabaugh P. The upstream activation site of a Ty2 element of yeast is necessary but not sufficient to promote maximal transcription of the element. Proc Natl Acad Sci USA. 1987;84:8520–8524. doi: 10.1073/pnas.84.23.8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Styles C A, Fink G R. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science. 1993;262:1741–1744. doi: 10.1126/science.8259520. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Styles C A, Fink G R. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madhani H D, Fink G R. Combinatorial control required for the specificity of yeast MAPK signaling. Science. 1997;275:1314–1317. doi: 10.1126/science.275.5304.1314. [DOI] [PubMed] [Google Scholar]
- 41.Madhani H D, Fink G R. The riddle of MAP kinase signaling specificity. Trends Genet. 1998;14:151–155. doi: 10.1016/s0168-9525(98)01425-5. [DOI] [PubMed] [Google Scholar]
- 42.Madhani H D, Styles C A, Fink G R. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell. 1997;91:673–684. doi: 10.1016/s0092-8674(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 43.Maftahi M, Gaillardin C, Nicaud J M. Sticky-end polymerase chain reaction method for systematic gene disruption in Saccharomyces cerevisiae. Yeast. 1996;12:859–68. doi: 10.1002/(SICI)1097-0061(199607)12:9%3C859::AID-YEA978%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 44.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 45.Myers A M, Tzagoloff A, Kinney D M, Lusty C J. Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene. 1986;45:299–310. doi: 10.1016/0378-1119(86)90028-4. [DOI] [PubMed] [Google Scholar]
- 46.Oehlen L, Cross F R. The mating factor response pathway regulates transcription of TEC1, a gene involved in pseudohyphal differentiation of Saccharomyces cerevisiae. FEBS Lett. 1998;429:83–88. doi: 10.1016/s0014-5793(98)00568-7. [DOI] [PubMed] [Google Scholar]
- 47.Pan X, Heitman J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4874–4887. doi: 10.1128/mcb.19.7.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian Z, Huang H, Hong J Y, Burck C L, Johnston S D, Berman J, Carol A, Liebman S W. Yeast Ty1 retrotransposition is stimulated by a synergistic interaction between mutations in chromatin assembly factor I and histone regulatory proteins. Mol Cell Biol. 1998;18:4783–4792. doi: 10.1128/mcb.18.8.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts R L, Fink G R. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- 50.Rose M, Botstein D. Construction and use of gene fusions to lacZ (beta-galactosidase) that are expressed in yeast. Methods Enzymol. 1983;101:167–180. doi: 10.1016/0076-6879(83)01012-5. [DOI] [PubMed] [Google Scholar]
- 51.Rupp S, Summers E, Lo H J, Madhani H, Fink G. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 1999;18:1257–1269. doi: 10.1093/emboj/18.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wessler S R. Plant retrotransposons—turned on by stress. Curr Biol. 1996;6:959–961. doi: 10.1016/s0960-9822(02)00638-3. [DOI] [PubMed] [Google Scholar]
- 54.White S E, Habera L F, Wessler S R. Retrotransposons in the flanking regions of normal plant genes: a role for copia-like elements in the evolution of gene structure and expression. Proc Natl Acad Sci USA. 1994;91:11792–11796. doi: 10.1073/pnas.91.25.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winston F, Dollard C, Ricupero-Hovasse S L. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 1995;11:53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]
- 56.Xu H, Boeke J D. Inhibition of Ty1 transposition by mating pheromones in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:2736–2743. doi: 10.1128/mcb.11.5.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu K, Elder R. A region internal to the coding sequence is essential for transcription of the yeast Ty1-D15 element. Mol Cell Biol. 1989;9:3667–3678. doi: 10.1128/mcb.9.9.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]