Highlights
-
•
Ultrasound application in the preservation of fruits and vegetables was reviewed.
-
•
Ultrasound mechanism for inactivating microorganisms was described.
-
•
Ultrasound application can effectively remove microorganisms.
-
•
Ultrasound application can improve the quality of fruits and vegetables.
Keywords: Ultrasound, Microorganisms, Quality, Fruits and vegetables
Abstract
The eating safety and high quality of fruits and vegetables have always been concerned by consumers, so require a safe, non-toxic, environment-friendly technology for their preservation. The application of ultrasound is a potential technology in the preservation of fruits and vegetables. This paper describes the ultrasound mechanism for inactivating microorganisms, with the cavitation phenomena of ultrasound being considered as a main effect. Effect of ultrasound on microorganisms of fruits and vegetables was discussed. Ultrasound alone and its combined treatments can be an effective method to inactivate the spoilage and pathogenic microorganisms on the surface of fruit and vegetables. Effect of ultrasound on physicochemical quality of fruits and vegetables was reviewed. Ultrasound and its combined treatments reduced mass loss, decreased color change, maintained firmness, enhanced and inhibited enzyme activity as well as preserving nutritional components such as total phenolic, total flavonoids, anthocyanin, and ascorbic acid.
1. Introduction
Fruits and vegetables (FVs) have always been popular with consumers because of their beneficial nutrients for human health such as a rich variety of vitamins, dietary fibers and bioactive compounds [1]. However, postharvest FVs are living, so exhibit respiratory and metabolic activities, which easily causes water loss, microbial infection and quality deterioration during transportation and storage [2], [3], [4]. Thus, FVs have a short preservation time with great losses through decay, so methods are required to prolong their preservation time.
FVs are easily contaminated by microorganism during human handling, processing, transportation, and distribution [5]. Especially, Escherichia coli, Salmonella spp, and Listeria monocytogenes are the main pathogenic microorganisms, which may cause outbreaks of foodborne diseases [6], [7]. To reduce the loads of spoilage and pathogenic microorganism, washing with sanitizing agents for FVs is a critical step [8]. Chlorine is widely used as a sanitizer in the industry [9]. Nevertheless, the use of chlorine may induce the formation of trihalomethanes, endangering the health of consumers [10]. Additionally, long-time exposure to chlorine vapor may have an adverse effect on the worker’s skin and respiratory tract and environment [11]. Therefore, emerging technologies are required to improve the safety of FVs.
Emerging technologies, such as high pressure, pulsed electric field, ultraviolet light, gamma irradiation, ultrasound etc., have been applied for the preservation of FVs [12], [13], [14], [15], [16]. Among these technologies, ultrasound has unique advantages in the application process, such as low cost, environmental-friendliness and is non-toxic with no additives required [17]. Thus, ultrasound is widely applied in food processing, such as food drying [18], [19], [20], starch degradation [21], enzyme hydrolysis [22], freezing and thawing [23], [24], [25]. In this context, this paper aims to summarize research progress on the effects of ultrasound on inactivating microorganisms and the quality of FVs.
1.1. Ultrasound mechanism for inactivating microorganisms
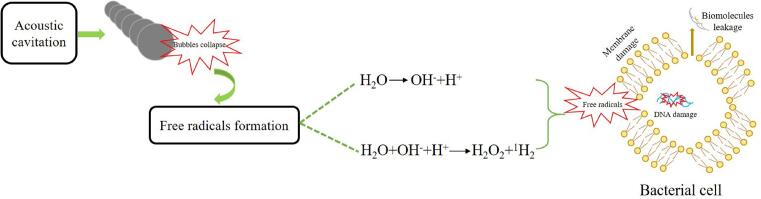
Ultrasound at 20–100 kHz can be applied to inactivate microorganisms in food processing due to acoustic cavitation. Cavitation causes the destruction of cell wall structure, increase of cell membrane permeability, cell membrane thinning, local high temperature and pressure and the production of free radicals, thereby inactivating microorganisms [26]. In the acoustic cavitation formation process, cavitation bubbles are generated in the liquid medium. These bubbles will produce periodic compression and rarefaction cycles. For the compression cycle, ultrasound waves compress liquid molecules. For the rarefaction cycle, ultrasound waves stretch the liquid molecules and generate negative pressure. When the negative pressure exceeds the force between the liquid molecules, the liquid molecules decompose, then form a cavity. During the periodic cycle, the cavity continues to grow as the ultrasound waves propagate in the liquid, thereby forming cavitation bubbles. Cavitation bubbles continue to grow, and then rupture instantaneously generating a local high temperature (5500 K) and pressure (50 MPa) [4]. Cavitation is divided into two types: stable cavitation and transient cavitation. Stable cavitation bubbles through the vibration of ultrasound waves in the liquid form tiny bubbles. Bubbles increase in size through many periodic cycles of ultrasound waves to form a microstreaming phenomenon. Shear force generated by the microstreaming can destroy the cell wall structure. Transient cavitation bubbles are unstable, and quickly burst in a short time then produce a high temperature and pressure, which cause the damage of cell structure, resulting in the leakage of biomolecules [27].
The acoustic cavitation causes an increase in local temperature and pressure when the bubbles burst, which helps the decomposition of water to form free radicals such as hydrogen atom (H+) and hydroxyl radical (OH−). Additionally, acoustic cavitation also causes single electron transfer in the cooling stage to recombine with hydrogen atom and hydroxyl radical to generate hydrogen peroxide (H2O2), which has a vital bactericidal effect. Furthermore, H+, OH− and H2O2 react with the DNA strands of microorganisms to break the DNA replication (Fig. 1), thereby inactivating them [28].
Fig. 1.
Microbial inactivation of free radicals from acoustic cavitation.
1.2. Effect of ultrasound on microorganisms of FVs
FVs undergo washing to remove the surface microorganisms, ensuring the safety of products at the time of distribution and sale [28]. Fully removing microorganisms on the surface of FVs remains a challenge in the food industry [29]. Ultrasound application is recommended for the washing of FVs in food industry as it causes no damage to environmental sustainability [26]. The researchers have confirmed that ultrasound alone and its combined methods can effectively remove or inactivate spoilage and pathogenic microorganisms on the surface of FVs.
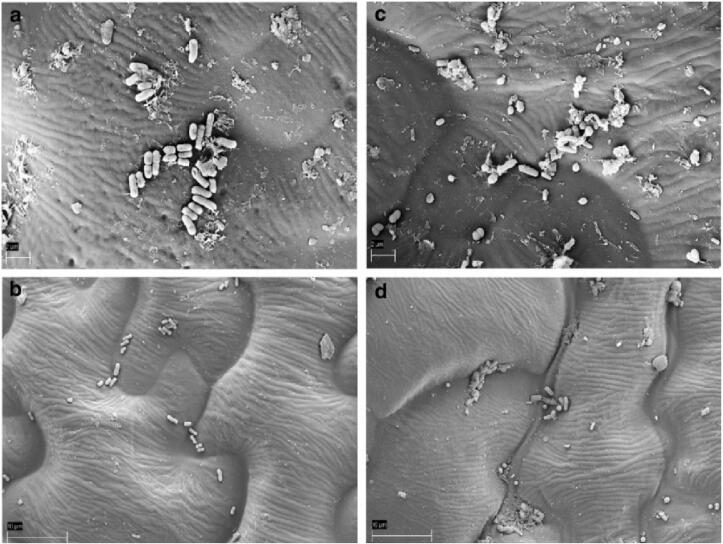
Some researchers have studied the effectiveness of ultrasound alone on FVs to inactivate microorganisms as seen in Table 1. Cao et al. [30] found that ultrasound (40 kHz) at optimal conditions (250 W, 9.8 min) noticeably suppressed an increase in microbial populations of strawberry, indicating that total bacteria, mould and yeast counts at the optimal parameters presented a reduction of 1.28, 0.7 lg CFU g−1, respectively. Similarly, Alexandre et al. [31] reported that ultrasound (120 W, 35 kHz, 2 min) alone on strawberry reduced total mesophiles by 0.6 lg CFU g−1, mould and yeast by 1.4 lg CFU g−1. Pinheiro et al. [32] observed that ultrasound (45 kHz) treated tomato at power level of 80% for 15 min and 100% for 19 min achieved a 2.55 and 2.95 lg CFU g−1 reduction of initial mesophilic load, respectively. For mould and yeast, there were no significant difference between the treatments that achieved a below 1 lg CFU g−1. According to Millan-Sango et al. [33], they evaluated the effectiveness of ultrasound (200 W, 26 kHz, 5 min) and oregano essential oil assisted ultrasound for Escherichia coli O157:H7 on inoculated Romaine lettuce, indicating that ultrasound treated lettuce had no surface damage in comparison to fresh lettuce. In addition, microbial cells on ultrasound treated lettuce had no cellular lysis and their morphology was intact (as shown in Fig. 2). This demonstrated that cavitation effect produced by ultrasound was beneficial in removing microbial cells to replace damage. However, ultrasound alone cannot completely remove microorganisms from FVs. Hence, ultrasound needs to be combined with other methods to enhance its disinfection effectiveness.
Table 1.
The application of ultrasound alone in microbial reduction of fruits and vegetables.
| Material | Conditions | Microbial reduction | References |
|---|---|---|---|
| Strawberry | 350 W, 40 kHz, 10 min, 20 °C | Mesophilic aerobic bacteria: 0.66 lg CFU g−1; Mould and yeast: 0.54 lg CFU g−1 | [49] |
| Strawberry | 60 W, 33 kHz, 40 min, 25 °C | Mesophilic aerobic bacteria: 2 lg CFU g−1; Mould and yeast: 1.22 lg CFU g−1 | [44] |
| Truffles | 35 kHz, 10 min, 4 °C | Mesophilic aerobic bacteria: 1 lg CFU g−1; Pseudomonads: 1.6 lg CFU g−1; Enterobacteriaceae: 1.5 lg CFU g−1; Lactic acid bacteria: 0.9 lg CFU g−1 | [57] |
| Iceberg lettuce | 280 W L-1, 20 kHz, 53 min | Escherichia coli O157:H7: 4.39 lg CFU mL−1 | [58] |
| Romaine lettuce | 30 W L-1, 37 kHz, 30 min, 25 °C | Escherichia coli: 2.3 lg CFU g−1; Staphylococcus aureus: 1.71 lg CFU g−1; Salmonella Enteritidis: 5.72 lg CFU g−1; Listeria innocua: 1.88 lg CFU g−1 | [59] |
| Romaine lettuce | 100 W, 42 kHz, 10 min, 20 °C | Escherichia coli O157:H7: 2.61 lg CFU cm−2; Listeria innocua: 2.23 lg CFU cm−2; Pseudomonas fluorescens: 1.1 lg CFU cm−2 | [60] |
| Cherry tomato | 45 kHz, 10 min, 20 °C | Mesophilic aerobic bacteria: 1 lg CFU g−1; Salmonella Typhimurium: 0.83 lg CFU g−1 | [29] |
| Cherry tomato | 106.19 W L-1, 20 kHz, 8 min, 25 °C | Mesophilic aerobic bacteria: 0.86 lg CFU g−1; Mould and yeast: 0.7 lg CFU g−1 | [17] |
| Red bell pepper | 120 W, 35 kHz, 2 min, 15 °C | Listeria innocua: 1.98 lg CFU g−1 | [61] |
| Chinese cabbage | 125.45 W L-1, 40 kHz, 15 min, 25 °C | Escherichia coli: 5.6 lg CFU g−1; Listeria innocua: 4.7 lg CFU g−1 | [62] |
| Blueberries | 500 W, 20 kHz, 10 min, 22 °C | Listeria innocua: 2.7 lg CFU g−1 | [63] |
| Cauliflower | 50 W L-1, 20 kHz, 5 min, 25 °C | Mesophilic aerobic bacteria: 0.51 lg CFU g−1; Mould and yeast: 0.52 lg CFU g−1; Coliform: 0.6 lg CFU g−1 | [56] |
Fig. 2.
SEM images of Escherichia coli O157:H7 inoculated on Romaine lettuce: (a) No ultrasound treatment; (b) Pulse ultrasound (10 s on /6 s off); (c) Continuous ultrasound + 0.018% oregano essential oil; (D) Pulsed ultrasound (2 s on /8 s off) + 0.025% oregano essential oil [33]
Ultrasound combined with physical (heat, high pressure, pulsed electric fields, ultraviolet radiation etc.) or chemical methods were generally useful for cleaning and inactivating microorganisms. However, heat and pressure easily altered cell structure and nutrients for FVs [26]. Ultrasound combined with chemical methods such as organic acids, sanitizers, ozone, natural antimicrobials were suitable for washing and microbial inactivation of FVs as seen in Table 2. São José and Vanetti [34] evaluated the effectiveness of ultrasound combined with chemical sanitizers for the decontamination of watercress, parsley and strawberries, indicating that sodium dichloroisocyanurate (20 and 200 mg L-1), hydrogen peroxide (5%), chlorine dioxide (10 mg L-1) and peracetic acid (40 mg L-1) combined with ultrasound (45 kHz, 10 min) on watercress, parsley and strawberries reduced aerobic mesophiles by 0.9–6.5, 0.9–6.3 and 0.7–4.0 lg CFU g−1, respectively. Particularly, ultrasound combined with peracetic acid presented the highest microbial reduction. Millan-Sango et al. [35] found that ultrasound (200 W, 26 kHz, 5 min) combined with oregano and thyme essential oils (0.018%) notably reduced Salmonella enterica on lettuce, and achieved a above 3 log CFU cm−2 reduction. Millan-Sango et al. [36] assessed antimicrobial effect of ultrasound (200 W, 26 kHz) combined with aqueous chlorine dioxide (3 mg L-1) for Escherchia coli and Salmonella enteritidis on alfalfa and mung bean sprouts, and observed a synergistic effect in the reduction of both Escherchia coli and Salmonella enteritidis, up to above 2 lg CFU g−1 reduction. Similarly, Mustapha et al. [37] investigated the efficacy of dual-frequency ultrasound (20/40 kHz, 10 min) and chemical sanitizers on natural microbes of cherry tomato. Ultrasound combined with sodium dichloroisocyanurate (200 mg L-1), peracetic acid (40 mg L-1), peracetic acid (40 mg L-1) with H2O2 (5%), hydrogen peroxide (5%) reduced aerobic mesophiles, mould and yeast by 0.29–3.1 lg CFU g−1. Especially, combined treatment of ultrasound and peracetic acid with H2O2 showed a 3.07–3.1 lg CFU g−1 reduction. The above results showed that ultrasound combined with chemical methods can enhance microbial reduction on various products. Therefore, ultrasound combined treatments are promising to ensure microbial safety of FVs.
Table 2.
Ultrasound combined chemical methods application in microbial reduction of fruits and vegetables
| Material | Conditions | Microbial reduction | References |
|---|---|---|---|
| Organic lettuce | Ultrasound (30 W L-1, 40 kHz, 5 min, 20 °C) + malic acid, lactic acid, and citric acid (2%) | Escherchia coli: 2.75 lg CFU g−1; Salmonella Typhimurium: 3.18 lg CFU g−1; Listeria monocytogenes: 2.87 lg CFU g−1 | [5] |
| Romaine lettuce | Ultrasound (200 W, 26 kHz, 5 min, <45 °C) + thyme essential oil (0.018%) | Salmonella enterica: 3 lg CFU cm−2 | [35] |
| Plum fruit | Ultrasound (100 W, 40 kHz, 10 min, 20 °C) + aqueous chlorine dioxide (40 mg L-1) | Aerobic mesophilic bacteria: 3 lg CFU g−1; Aerobic psychrotrophic bacteria: 2.9 lg CFU g−1; Mould and yeast: 2 lg CFU g−1 | [50] |
| Carrots | Ultrasound (30 W L-1, 40 kHz, 5 min, 20 °C) + Tween 20 (0.1%) | Bacillus cereus spores: 2.22 lg CFU g−1 | [64] |
| Spinach | Ultrasound (400 W L-1, 40 kHz, 3 min, 23 °C) + slightly acidic electrolyzed water + water wash | Total bacterial count: 2.08 lg CFU g−1; Escherichia coli O157:H7: 2.41 lg CFU g−1; Listeria monocytogenes: 2.49 lg CFU g−1 | [65] |
| Kiwifruit | Ultrasound (368 W cm−2, 40 kHz, 8 min, 25 °C) + sodium hypochlorite (30 mg L-1) | Bacteria: 3.48 lg CFU g−1; Mould and yeast: 2.32 lg CFU g−1 | [66] |
| Strawberry | Ultrasound (500 W, 40 kHz, 5 min, 7 °C) + peracetic acid (40 mg L-1) | Mould and yeast: 1.8 lg CFU g−1; Mesophilic aerobic bacteria: 2 lg CFU g−1; Salmonella enterica: 2.1 lg CFU g−1 | [67] |
| Green asparagus | Ultrasound (360 W, 40 kHz, 10 min) + acetic acid (2%) + gibberellic acid (50 mg kg−1) | Bacteria: 2 lg CFU g−1; Mould and yeast: 2 lg CFU g−1 | [38] |
| Chinese cabbage | Ultrasound (120 W L-1, 40 kHz, 10 min, 30 °C) + sodium hypochlorite (100 mg L-1) | Listeria innocua: 3.35 lg CFU g−1 | [68] |
| Cherry tomato | Ultrasound (300 W, 20/40 kHz, 10 min, 25 °C) + aqueous ozone (0.85 mg L-1) | Mesophilic acteria: >3.00 lg CFU g−1; Mould and yeast: >3 lg CFU g−1 | [69] |
| Blueberries | Ultrasound (500 W, 20 kHz, 15 min, 18–22 °C) + citral (10 mM) | Listeria innocua: >2 lg CFU mL−1 | [70] |
1.3. Effect of ultrasound on physicochemical quality of FVs
FVs after harvest continue to conduct metabolic activity, which is susceptible to senescence and spoilage during storage and transportation due to mechanical injury, microbial infection and physiological change, leading to quality deterioration [38]. Hence, it is necessary to assess the effect of ultrasound on the quality of FVs. Studies have shown that ultrasound can effectively maintain the physicochemical quality of FVs during storage. These quality attributes of interest include mass loss, color, firmness, enzyme activity, nutritional components.
Mass loss
FVs are prone to weight loss during storage. The increase in mass loss is due to the accelerated transpiration of FVs resulting in serious water loss. Water loss of FVs during storage will accelerate quality deterioration [39]. Various studies have reported the effect of ultrasound on mass loss of FVs. Li et al. [40] reported that ultrasound (300 W, 40 kHz) for 10 min combined with 95% relative humidity treated straw mushroom kept minimum mass loss of 28.83% of initial mass, which attributed to the restriction of hydrogen bonds on water molecules, thereby decreasing water loss. Similar results were found by Fan et al. [41], who reported that ultrasound for 10 min treated cucumber led to the lowest mass loss of 8.63% of initial mass during modified atmosphere packaging storage, which was attributed to the restriction of hydrogen bonds on water molecules, thereby decreasing water loss. Wang and Fan [38] also found that ultrasound (360 W 40 kHz, 10 min) combined with acetic acid and gibberellic acid treated green asparagus exhibited a minimum mass loss of 1.83%, which was significantly lower than untreated samples stored for the same period. Fan et al. [42] observed that the combination of ultrasound (23 W L-1, 20 kHz, 10 min) and ε-polylysine (0.4 g L-1) reduced the mass loss of lettuce with a lower value (1.42%) compared with untreated samples during storage. The above results demonstrated that ultrasound treatment helps control the mass loss of fresh products.
1.3.1. Color
Color is a critical index for consumer buying choice. Color change of FVs is assisted with the degradation of natural pigments such as chlorophylls, anthocyanins, carotenoids, flavonoids during postharvest storage. Thus, color change also reflects the maturity and shelf life of FVs [43]. Some studies have reported effect of ultrasound on color of FVs. Gani et al. [44] found that treatment with ultrasound (33 W) for 30 min (11.89%) and 40 min (11.87%) on strawberry had a lower L* (lightness) value compared with untreated samples (39.12%), and an increased a* (redness/greenness) value after 12 d of storage. In addition, total color difference (ΔE) of strawberry treated by ultrasound for 10–30 min had minimum change, indicating that ultrasound for 10–30 min was suitable for retaining the color of strawberry. Similarly, Muzaffar et al. [45] found that ultrasound (60 W, 33 kHz) treatment for 20 min was the least increase in ΔE and effectively kept the bright red color of cherry as compared to other treatments. Hashemi [46] also reported that pulsed ultrasound treatment for 60 min on Mirabelle plums produced the lowest ΔE compared with other ultrasound treatment times during storage. Alenyorege et al. [47] observed that sequential multi-frequency ultrasound treatments on Chinese cabbage had no significant effect on L*, a* and b* (yellowness/blueness). However, dual frequency ultrasound (20/40, 20/60, 40/60 kHz) treatments had lower ΔE compared with triple frequency ultrasound (20/40/60 kHz) treatment. These results illustrated that ultrasound treatment can reduce changes in color during storage.
1.3.2. Firmness
Firmness is a key quality attribute for consumers to accept FVs [4]. Firmness affects the structural and mechanical properties of fresh produce and has a great impact on people’s senses. In addition, external shape and touch of FVs also affect their sales and aesthetics [48]. Numerous studies have reported the effect of ultrasound on firmness of FVs. Cao et al. [49] found that ultrasound treatment (40 kHz) observably suppressed the softening of strawberry and maintained their firmness during storage. Chen and Zhu [50] studied the influence of ultrasound on the firmness of plum fruit during storage, and also observed that ultrasound at 100 W for 10 min effectively inhibited softening and maintained the firmness of plum. Additionally, Sagong, Lee, Chang, Heu et al. [5] reported that ultrasound (40 kHz, 5 min) combined with organic acids (malic/lactic/citric acids) had no significant effect on texture of lettuce during storage, indicating that ultrasound combined treatment had no influence on the quality of lettuce. Xu et al. [51] observed that the combination of ultrasound and 1-methylcyclopropene increased the firmness of apples by 19.4% compared with the untreated samples after 60 d of storage. As described above, studies indicated that firmness retention of FVs was improved by ultrasound treatment.
1.3.3. Enzyme activity
Enzyme activity can reflect the quality change in produce. Abnormal activities of enzymes can result in physiological metabolic disorders of FVs [4]. The researchers have reported the effect of ultrasound on enzyme activity of FVs. Yang et al. [52] found that the combination of ultrasound (40 kHz, 10 min) and salicylic acid (0.05 mM) effectively inhibited blue mold decay caused by Penicillium expansum in peach, and observed that combined treatment enhanced the activities of defense enzymes (chitinase, β-1,3-glucanase, phenylalanine ammonia-lyase, polyphenol oxidase and peroxidase), which the combined treatment is used as an effective preservation method to control the disease resistance and quality of peach fruit. Wei and Ye [53] studied the effect of ultrasound treatment (20 kHz, 20 min) combined with 6-benzylaminopurine (20 μg g−1) on enzyme activities of asparagus, indicating that ultrasound assisted 6-benzylaminopurine treatment increased catalase, polyphenol oxidase, ascorbate peroxidase activities and inhibiting the phenylalanine ammonia lyase and peroxidase activities of asparagus. Similarly, Xu et al. [51] observed that the combination of ultrasound and 1-methylcyclopropene increased the peroxidase, superoxide dismutase, catalase activities of apple. Mustapha and Zhou [54] also observed that ultrasound (20/40 kHz, 10 min) combined with and aqueous ozone (0.85 mg L-1) increased the peroxidase and polyphenol oxidase activities of cherry tomato. These studies suggested that ultrasound application is useful to enhance and inhibit the activities of enzymes in FVs.
1.3.4. Nutritional components
Nutritional components are a key indicator to determine the quality of FVs. Changes in the contents of nutritional components such as total phenolic, total flavonoids, anthocyanin, ascorbic acid occur during processing, transportation and storage. Some researchers have investigated the effect of ultrasound on the contents of bioactive nutritional components of FVs. Chen et al. [55] found that ultrasound (120 W, 10 min) application delayed the reduction in the contents of total phenolic and anthocyanins of litchi during storage. Alexandre et al. [31] reported that ultrasound treatment maintained the high anthocyanin and ascorbic acid contents of strawberry compared with sodium hypochlorite and hydrogen peroxide sanitizers. Wang et al. [17] studied the influence of ultrasound on the nutritional quality of cherry tomato, indicating that 106.19 W L-1 ultrasound enhanced total phenolics, total flavonoid, ascorbic acid contents. Similarly, Zhang et al. [56] reported that ultrasound (20/28 kHz, 100 W L-1) combined with 0.5% zinc acetate delayed the reduction of the total phenolics, total flavonoid, ascorbic acid contents of cauliflower during storage. As mentioned above, ultrasound application can retain bioactive components to avoid the reduction of antioxidant properties of FVs
2. Conclusions
Ultrasound application can effectively improve the microbial safety and physicochemical quality of fruits and vegetables. Ultrasound and its combined applications are effective to inactive spoilage and pathogenic microorganisms on the surface of fruit and vegetables, which ensure the safety of fresh produce. Furthermore, ultrasound and its combined applications in fruits and vegetables exhibited a good effect on the decrease of mass loss, the conservation of color, the maintenance of firmness, the control of enzyme activity as well as the retention of nutritional components such as total phenolic, total flavonoids, anthocyanin, and ascorbic acid. In summary, ultrasound and its combined applications are useful for extending the storage time of fruits and vegetables. Nevertheless, the preservation of ultrasound on fruits and vegetables is restricted by several factors such as combined treatments for the type and concentration of antimicrobial agent, contact time and frequency. Thus, the application of ultrasound in the food industry is still a challenge. Further research is required to find low cost, environmentally-friendly, high efficiency ultrasound combined technologies for the commercial preservation of fruits and vegetables.
CRediT authorship contribution statement
Kai Fan: Writing – review & editing. Jiaxin Wu: Writing – original draft. Libing Chen: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the support from Scientific Research and Development Fund Project of Yangtze University (No. 8021002902).
References
- 1.More A.S., Ranadheera C.S., Fang Z., Warner R., Ajlouni S. Biomarkers associated with quality and safety of fresh-cut produce. Food Bioscience. 2020;34 [Google Scholar]
- 2.Zhang M., Meng X., Bhandari B., Fang Z., Chen H. Recent Application of Modified Atmosphere Packaging (MAP) in Fresh and Fresh-Cut Foods. Food Rev. Int. 2015;31(2):172–193. [Google Scholar]
- 3.Yousuf B., Qadri O.S., Srivastava A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review, LWT- Food Sci. Technol. 2018;89:198–209. [Google Scholar]
- 4.Jiang Q., Zhang M., Xu B. Application of ultrasonic technology in postharvested fruits and vegetables storage: A review. Ultrason. Sonochem. 2020;69:105261. doi: 10.1016/j.ultsonch.2020.105261. [DOI] [PubMed] [Google Scholar]
- 5.Sagong H.-G., Lee S.-Y., Chang P.-S., Heu S., Ryu S., Choi Y.-J., Kang D.-H. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. Int. J. Food Microbiol. 2011;145:287–292. doi: 10.1016/j.ijfoodmicro.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Ramos B., Miller F.A., Brandão T.R.S., Teixeira P., Silva C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013;20:1–15. [Google Scholar]
- 7.Tango C.N., Khan I., Ngnitcho Kounkeu P.-F., Momna R., Hussain M.S., Oh D.-H. Slightly acidic electrolyzed water combined with chemical and physical treatments to decontaminate bacteria on fresh fruits. Food Microbiol. 2017;67:97–105. doi: 10.1016/j.fm.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Van Haute S., Tryland I., Escudero C., Vanneste M., Sampers I. Chlorine dioxide as water disinfectant during fresh-cut iceberg lettuce washing: Disinfectant demand, disinfection efficiency, and chlorite formation, LWT- Food Sci. Technol. 2017;75:301–304. [Google Scholar]
- 9.Luo Y., Zhou B., Van Haute S., Nou X., Zhang B., Teng Z.i., Turner E.R., Wang Q., Millner P.D. Association between bacterial survival and free chlorine concentration during commercial fresh-cut produce wash operation. Food Microbiol. 2018;70:120–128. doi: 10.1016/j.fm.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Gómez-López V.M., Marín A., Medina-Martínez M.S., Gil M.I., Allende A. Generation of trihalomethanes with chlorine-based sanitizers and impact on microbial, nutritional and sensory quality of baby spinach. Postharvest Biol. Technol. 2013;85:210–217. [Google Scholar]
- 11.Oliveira M., Abadias M., Usall J., Torres R., Teixidó N., Viñas I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables – A review. Trends Food Sci. Technol. 2015;46(1):13–26. [Google Scholar]
- 12.Fernandez M.V., Denoya G.I., Jagus R.J., Vaudagna S.R., Agüero M.V. Microbiological, antioxidant and physicochemical stability of a fruit and vegetable smoothie treated by high pressure processing and stored at room temperature, LWT- Food Sci. Technol. 2019;105:206–210. [Google Scholar]
- 13.Timmermans R.A.H., Mastwijk H.C., Berendsen L.B.J.M., Nederhoff A.L., Matser A.M., Van Boekel M.A.J.S., Nierop Groot M.N. Moderate intensity Pulsed Electric Fields (PEF) as alternative mild preservation technology for fruit juice. Int. J. Food Microbiol. 2019;298:63–73. doi: 10.1016/j.ijfoodmicro.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Li L., Li C., Sun J., Xin M., Yi P., He X., Sheng J., Zhou Z., Ling D., Zheng F., Li J., Liu G., Li Z., Tang Y., Yang Y., Tang J. Synergistic effects of ultraviolet light irradiation and high-oxygen modified atmosphere packaging on physiological quality, microbial growth and lignification metabolism of fresh-cut carrots. Postharvest Biol. Technol. 2021;173 [Google Scholar]
- 15.Ben-Fadhel Y., Cingolani M.C., Li L., Chazot G., Salmieri S., Horak C., Lacroix M. Effect of γ-irradiation and the use of combined treatments with edible bioactive coating on carrot preservation. Food Packaging Shelf Life. 2021;28 [Google Scholar]
- 16.Pelissari E.M.R., Covre K.V., do Rosario D.K.A., de São José J.F.B. Application of chemometrics to assess the influence of ultrasound and chemical sanitizers on vegetables: Impact on natural microbiota, Salmonella Enteritidis and physicochemical nutritional quality, LWT- Food Sci. Technol. 2021;148 [Google Scholar]
- 17.Wang W., Ma X., Zou M., Jiang P., Hu W., Li J., Zhi Z., Chen J., Li S., Ding T., Ye X., Liu D. Effects of ultrasound on spoilage microorganisms, quality, and antioxidant capacity of postharvest cherry tomatoes. J. Food Sci. 2015;80(10):C2117–C2126. doi: 10.1111/1750-3841.12955. [DOI] [PubMed] [Google Scholar]
- 18.Xu B., Chen J., Sylvain Tiliwa E., Yan W., Roknul Azam S.M., Yuan J., Wei B., Zhou C., Ma H. Effect of multi-mode dual-frequency ultrasound pretreatment on the vacuum freeze-drying process and quality attributes of the strawberry slices. Ultrason. Sonochem. 2021;78 doi: 10.1016/j.ultsonch.2021.105714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Xu B., Wei B., Zeng R. Low frequency ultrasound pretreatment of carrot slices: Effect on the moisture migration and quality attributes by intermediate-wave infrared radiation drying. Ultrason. Sonochem. 2018;40:619–628. doi: 10.1016/j.ultsonch.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Xu B., Sylvain Tiliwa E., Yan W., Roknul Azam S.M., Wei B., Zhou C., Ma H., Bhandari B. Recent development in high quality drying of fruits and vegetables assisted by ultrasound: A review. Food Res. Int. 2021:110744. doi: 10.1016/j.foodres.2021.110744. [DOI] [PubMed] [Google Scholar]
- 21.Xu B., Ren A., Chen J., Li H., Wei B., Wang J., Azam S.M.R., Bhandari B., Zhou C., Ma H. Effect of multi-mode dual-frequency ultrasound irradiation on the degradation of waxy corn starch in a gelatinized state. Food Hydrocolloids. 2021;113 [Google Scholar]
- 22.Xu B., Yuan J., Wang L., Lu F., Wei B., Azam R.S.M., Ren X., Zhou C., Ma H., Bhandari B. Effect of multi-frequency power ultrasound (MFPU) treatment on enzyme hydrolysis of casein. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104930. [DOI] [PubMed] [Google Scholar]
- 23.Xu B.-guo., Zhang M., Bhandari B., Cheng X.-feng., Islam M.N. Effect of ultrasound-assisted freezing on the physico-chemical properties and volatile compounds of red radish. Ultrason. Sonochem. 2015;27:316–324. doi: 10.1016/j.ultsonch.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Xu B., Chen J., Yuan J., Azam SM.R., Zhang M. Effect of different thawing methods on the efficiency and quality attributes of frozen red radish. J. Sci. Food Agric. 2021;101(8):3237–3245. doi: 10.1002/jsfa.10953. [DOI] [PubMed] [Google Scholar]
- 25.Fan K., Zhang M., Wang W., Bhandari B. A novel method of osmotic-dehydrofreezing with ultrasound enhancement to improve water status and physicochemical properties of kiwifruit. Int. J. Refrig. 2020;113:49–57. [Google Scholar]
- 26.São José J.F.B.de., Andrade Nélio.José.de., Ramos A.M., Vanetti M.C.D., Stringheta P.César., Chaves José.Benício.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control. 2014;45:36–50. [Google Scholar]
- 27.Dolas R., Saravanan C., Kaur B.P. Emergence and era of ultrasonic’s in fruit juice preservation: A review. Ultrason. Sonochem. 2019;58:104609. doi: 10.1016/j.ultsonch.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Bilek S.E., Turantaş F. Decontamination efficiency of high power ultrasound in the fruit and vegetable industry, a review. Int. J. Food Microbiol. 2013;166(1):155–162. doi: 10.1016/j.ijfoodmicro.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Brilhante São José J.F., Dantas Vanetti M.C. Effect of ultrasound and commercial sanitizers in removing natural contaminants and Salmonella enterica Typhimurium on cherry tomatoes. Food Control. 2012;24(1-2):95–99. [Google Scholar]
- 30.Cao S., Hu Z., Pang B. Optimization of postharvest ultrasonic treatment of strawberry fruit. Postharvest Biol. Technol. 2010;55(3):150–153. [Google Scholar]
- 31.Alexandre E.M.C., Brandão T.R.S., Silva C.L.M. Efficacy of non-thermal technologies and sanitizer solutions on microbial load reduction and quality retention of strawberries. J. Food Eng. 2012;108(3):417–426. [Google Scholar]
- 32.Pinheiro J., Alegria C., Abreu M., Gonçalves E.M., Silva C.L.M. Influence of postharvest ultrasounds treatments on tomato (Solanum lycopersicum, cv. Zinac) quality and microbial load during storage. Ultrason. Sonochem. 2015;27:552–559. doi: 10.1016/j.ultsonch.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Millan-Sango D., McElhatton A., Valdramidis V.P. Determination of the efficacy of ultrasound in combination with essential oil of oregano for the decontamination of Escherichia coli on inoculated lettuce leaves. Food Res. Int. 2015;67:145–154. [Google Scholar]
- 34.São José J.F.B.de., Vanetti M.C.D. Application of ultrasound and chemical sanitizers to watercress, parsley and strawberry: Microbiological and physicochemical quality. LWT - Food Sci. Technol. 2015;63(2):946–952. [Google Scholar]
- 35.Millan-Sango D., Garroni E., Farrugia C., Van Impe J.F.M., Valdramidis V.P. Determination of the efficacy of ultrasound combined with essential oils on the decontamination of Salmonella inoculated lettuce leaves, LWT- Food Sci. Technol. 2016;73:80–87. [Google Scholar]
- 36.Millan-Sango D., Sammut E., Van Impe J.F., Valdramidis V.P. Decontamination of alfalfa and mung bean sprouts by ultrasound and aqueous chlorine dioxide, LWT- Food Sci. Technol. 2017;78:90–96. [Google Scholar]
- 37.Mustapha A.T., Zhou C., Amanor-Atiemoh R., Ali T.A.A., Wahia H., Ma H., Sun Y. Efficacy of dual-frequency ultrasound and sanitizers washing treatments on quality retention of cherry tomato. Innov. Food Sci. Emerg. Technol. 2020;62 [Google Scholar]
- 38.Wang J., Fan L. Effect of ultrasound treatment on microbial inhibition and quality maintenance of green asparagus during cold storage. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104631. [DOI] [PubMed] [Google Scholar]
- 39.Maghoumi M., Gómez P.A., Mostofi Y., Zamani Z., Artés-Hernández F., Artés F. Combined effect of heat treatment, UV-C and superatmospheric oxygen packing on phenolics and browning related enzymes of fresh-cut pomegranate arils, LWT- Food Sci. Technol. 2013;54(2):389–396. doi: 10.1002/jsfa.5868. [DOI] [PubMed] [Google Scholar]
- 40.Li N., Chen F., Cui F., Sun W., Zhang J., Qian L., Yang Y., Wu D., Dong Y., Jiang J., Yang H. Improved postharvest quality and respiratory activity of straw mushroom (Volvariella volvacea) with ultrasound treatment and controlled relative humidity. Sci. Hortic. 2017;225:56–64. [Google Scholar]
- 41.Fan K., Zhang M., Jiang F. Ultrasound treatment to modified atmospheric packaged fresh-cut cucumber: Influence on microbial inhibition and storage quality. Ultrason. Sonochem. 2019;54:162–170. doi: 10.1016/j.ultsonch.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Fan K., Zhang M., Bhandari B., Jiang F. A combination treatment of ultrasound and ε-polylysine to improve microorganisms and storage quality of fresh-cut lettuce. LWT- Food Sci. Technol. 2019;113 [Google Scholar]
- 43.Fagundes C., Moraes K., Pérez-Gago M.B., Palou L., Maraschin M., Monteiro A.R. Effect of active modified atmosphere and cold storage on the postharvest quality of cherry tomatoes. Postharvest Biol. Technol. 2015;109:73–81. [Google Scholar]
- 44.Gani A., Baba W.N., Ahmad M., Shah U., Khan A.A., Wani I.A., Masoodi F.A., Gani A. Effect of ultrasound treatment on physico-chemical, nutraceutical and microbial quality of strawberry. LWT - Food Sci. Technol. 2016;66:496–502. [Google Scholar]
- 45.Muzaffar S., Ahmad M., Wani S.M., Gani A., Baba W.N., Shah U., Khan A.A., Masoodi F.A., Gani A., Wani T.A. Ultrasound treatment: effect on physicochemical, microbial and antioxidant properties of cherry (Prunus avium) J. Food Sci. Technol. 2016;53:2752–2759. doi: 10.1007/s13197-016-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashemi S.M.B. Effect of pulsed ultrasound treatment compared to continuous mode on microbiological and quality of Mirabelle plum during postharvest storage. Int. J. Food Sci. Tech. 2018;53(3):564–570. [Google Scholar]
- 47.Alenyorege E.A., Ma H., Aheto J.H., Agyekum A.A., Zhou C. Effect of sequential multi-frequency ultrasound washing processes on quality attributes and volatile compounds profiling of fresh-cut Chinese cabbage. LWT- Food Sci. Technol. 2020;117 [Google Scholar]
- 48.Belay Z.A., Caleb O.J., Opara U.L. Influence of initial gas modification on physicochemical quality attributes and molecular changes in fresh and fresh-cut fruit during modified atmosphere packaging. Food Packaging Shelf Life. 2019;21 [Google Scholar]
- 49.Cao S., Hu Z., Pang B., Wang H., Xie H., Wu F. Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control. 2010;21(4):529–532. [Google Scholar]
- 50.Chen Z., Zhu C. Combined effects of aqueous chlorine dioxide and ultrasonic treatments on postharvest storage quality of plum fruit (Prunus salicina L.) Postharvest Biol. Technol. 2011;61(2-3):117–123. [Google Scholar]
- 51.Xu F., Liu S., Xiao Z., Fu L. Effect of ultrasonic treatment combined with 1-methylcyclopropene (1-MCP) on storage quality and ethylene receptors gene expression in harvested apple fruit. J. Food Biochem. 2019;43(8) doi: 10.1111/jfbc.v43.810.1111/jfbc.12967. [DOI] [PubMed] [Google Scholar]
- 52.Yang Z., Cao S., Cai Y., Zheng Y. Combination of salicylic acid and ultrasound to control postharvest blue mold caused by Penicillium expansum in peach fruit. Innov. Food Sci. Emerg. Technol. 2011;12(3):310–314. [Google Scholar]
- 53.YUNXIAO WEI XINGQIAN YE Effect OF 6-benzylaminopurine combined with ultrasound as pre-treatment on quality and enzyme activity of green asparagus 35 5 2011 587 595.
- 54.Mustapha A.T., Zhou C. Novel assisted/unassisted ultrasound treatment: Effect on respiration rate, ethylene production, enzymes activity, volatile composition, and odor of cherry tomato. LWT- Food Sci. Technol. 2021;149 [Google Scholar]
- 55.YULONG CHEN YUEMING JIANG SHAOYU YANG EN YANG BAO YANG K. NAGENDRA PRASAD Effects of ultrasonic treatment on pericarp browning of postharvest litchi fruit 36 5 2012 613 620.
- 56.Zhang L., Yu X., Yagoub A.E.A., Owusu-Ansah P., Wahia H., Ma H., Zhou C. Effects of low frequency multi-mode ultrasound and it’s washing solution’s interface properties on freshly cut cauliflower. Food Chem. 2022;366 doi: 10.1016/j.foodchem.2021.130683. [DOI] [PubMed] [Google Scholar]
- 57.Susana Rivera C., Venturini María.E., Oria R., Blanco D. Selection of a decontamination treatment for fresh Tuber aestivum and Tuber melanosporum truffles packaged in modified atmospheres. Food Control. 2011;22(3-4):626–632. [Google Scholar]
- 58.Elizaquível P., Sánchez G., Selma M.V., Aznar R. Application of propidium monoazide-qPCR to evaluate the ultrasonic inactivation of Escherichia coli O157:H7 in fresh-cut vegetable wash water. Food Microbiol. 2012;30:316–320. doi: 10.1016/j.fm.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Birmpa A., Sfika V., Vantarakis A. Ultraviolet light and Ultrasound as non-thermal treatments for the inactivation of microorganisms in fresh ready-to-eat foods. Int. J. Food Microbiol. 2013;167(1):96–102. doi: 10.1016/j.ijfoodmicro.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Huang K., Wrenn S., Tikekar R., Nitin N. Efficacy of decontamination and a reduced risk of cross-contamination during ultrasound-assisted washing of fresh produce. J. Food Eng. 2018;224:95–104. [Google Scholar]
- 61.Alexandre E.M.C., Brandão T.R.S., Silva C.L.M. Impact of non-thermal technologies and sanitizer solutions on microbial load reduction and quality factor retention of frozen red bell peppers. Innov. Food Sci. Emerg. Technol. 2013;17:99–105. [Google Scholar]
- 62.Alenyorege E.A., Ma H., Aheto J.H., Ayim I., Chikari F., Osae R., Zhou C. Response surface methodology centred optimization of mono-frequency ultrasound reduction of bacteria in fresh-cut Chinese cabbage and its effect on quality. LWT- Food Sci. Technol. 2020;122 [Google Scholar]
- 63.Zhang H., Tsai S., Tikekar R.V. Inactivation of Listeria innocua on blueberries by novel ultrasound washing processes and their impact on quality during storage. Food Control. 2021;121 [Google Scholar]
- 64.Sagong H.-G., Cheon H.-L., Kim S.-O., Lee S.-Y., Park K.-H., Chung M.-S., Choi Y.-J., Kang D.-H. Combined effects of ultrasound and surfactants to reduce Bacillus cereus spores on lettuce and carrots. Int. J. Food Microbiol. 2013;160(3):367–372. doi: 10.1016/j.ijfoodmicro.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 65.Forghani F., Oh D.-H. Hurdle enhancement of slightly acidic electrolyzed water antimicrobial efficacy on Chinese cabbage, lettuce, sesame leaf and spinach using ultrasonication and water wash. Food Microbiol. 2013;36(1):40–45. doi: 10.1016/j.fm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Vivek K., Subbarao K.V., Srivastava B. Optimization of postharvest ultrasonic treatment of kiwifruit using RSM. Ultrason. Sonochem. 2016;32:328–335. doi: 10.1016/j.ultsonch.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 67.do Rosário D.K.A., da Silva Mutz Y., Peixoto J.M.C., Oliveira S.B.S., de Carvalho R.V., Carneiro J.C.S., de São José J.F.B., Bernardes Patrícia.C. Ultrasound improves chemical reduction of natural contaminant microbiota and Salmonella enterica subsp. enterica on strawberries. Int. J. Food Microbiol. 2017;241:23–29. doi: 10.1016/j.ijfoodmicro.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Alenyorege E.A., Ma H., Ayim I., Aheto J.H., Hong C., Zhou C. Reduction of Listeria innocua in fresh-cut Chinese cabbage by a combined washing treatment of sweeping frequency ultrasound and sodium hypochlorite, LWT- Food Sci. Technol. 2019;101:410–418. [Google Scholar]
- 69.Mustapha A.T., Zhou C., Wahia H., Amanor-Atiemoh R., Otu P., Qudus A., Abiola Fakayode O., Ma H. Sonozonation: Enhancing the antimicrobial efficiency of aqueous ozone washing techniques on cherry tomato. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.105059. [DOI] [PubMed] [Google Scholar]
- 70.Zhang H., Wang S., Goon K., Gilbert A., Nguyen Huu C., Walsh M., Nitin N., Wrenn S., Tikekar R.V. Inactivation of foodborne pathogens based on synergistic effects of ultrasound and natural compounds during fresh produce washing. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104983. [DOI] [PubMed] [Google Scholar]