Abstract
Aberrant glycosylation actively contributes to tumor progression and is a key hallmark of cancer. Most of the glycan moieties expressed on the surface of cancer cells are sialic acids that may modulate antitumor immune responses via binding to sialic acid–binding immunoglobulin-like lectins (Siglecs) expressed by immune cells. Here we show that Siglecs may decrease the bladder tumor immune response mediated by natural killer (NK) cells. We observed higher NK cell activity against desialylated bladder tumor cell lines. We therefore determined the expression of nine Siglecs on circulatory NK cells from healthy donors and patients with bladder cancer (BCa). NK cells from blood mainly express Siglec-7, which is highly upregulated in non–muscle-invasive BCa (NMIBC), as well as Siglec-6, albeit at a much lower level. However, both Siglecs are expressed by urinary NK cells from NMIBC patients undergoing bacillus Calmette-Guérin therapy. Ex vivo analysis of Siglec-6 and Siglec-7 expression levels on tumor-infiltrating NK cells (TINKs) from BCa patients showed that only Siglec-7 is expressed by TINKs. Finally, analyses for The Cancer Genome Atlas data set revealed that BCa patients with high expression levels of Siglec-7 have a poor survival rate. This work indicates that Siglec-7 may restrain NK-mediated antitumor immunity in BCa.
Patient summary
We investigated the expression of proteins called Siglecs in natural killer (NK) cells from patients with bladder cancer. We showed that levels of the protein Siglec-7 in blood, urine, and tumors from patients with bladder cancer are associated with poor clinical outcomes. Thus, Siglec-7 may be involved in the regulation of antitumor immunity mediated by NK cells in bladder cancer.
Keywords: Bladder cancer, Siglec-7, Natural killer cells, Immunoregulation, Immune checkpoint
Bladder cancer (BCa) is a highly prevalent disease associated with substantial morbidity [1]. BCa is classified as non–muscle-invasive (NMIBC) or muscle-invasive BCa (MIBC). After transurethral resection of bladder tumor (TURBT), bacillus Calmette-Guérin (BCG) intravesical immunotherapy is considered the standard of care for intermediate- to high-risk NMIBC [2], [3]. Unfortunately, recurrences after BCG therapy may occur. In these patients, radical cystectomy remains the standard therapy [3], [4]. The limited efficacy of treatment in many BCa patients underscores the importance of identifying new targetable immune checkpoints. Over the past decade, immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 or CTLA-4 axis have been a major breakthrough in cancer therapy, including BCa [5]. However, only a small fraction of patients respond to ICIs, suggesting that additional immunoregulatory mechanisms may be involved in antitumor immunity [6], [7]. Sialic acid–binding immunoglobulin-type lectins (Siglecs) are expressed on leukocytes and interact with sialic acids. Siglecs have been of particular interest in antitumor immunity owing to their regulatory role [8]. At homeostasis, the Siglec/sialic acid axis may help immune cells to sense self from non-self. However, tumor cells may exploit this feature to escape from antitumor surveillance by upregulating sialic acids at their surface and/or Siglecs on immune cells [8]. Although recent reports have highlighted the immunoregulatory role of Siglecs in the antitumor responses of T cells and natural killer (NK) cells [9], no data are available on the role of NK cells expressing Siglecs in BCa. Here we report on the first analysis of the expression and activity of Siglecs on NK cells from BCa patients.
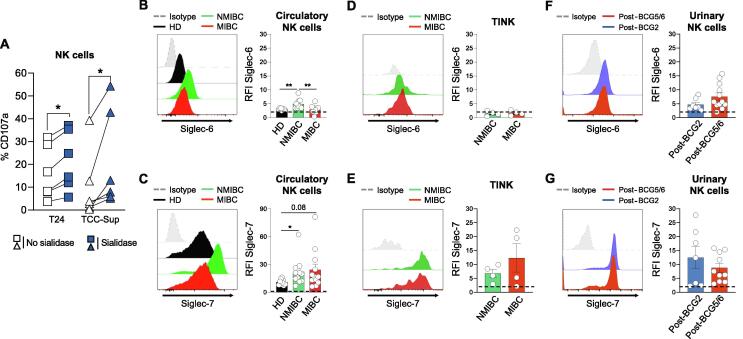
First, to determine whether the interaction between Siglecs and Siglec ligands may influence NK cell activity in the context of BCa, we co-cultured purified NK cells from healthy donors (HDs) and NMIBC patients with untreated or desialylated bladder tumor cells (T24 and/or TCC-Supp). Sialidase, a sialic acid–cleaving enzyme, removes sialic acids at the surface of tumor cells (Supplementary Fig. 1A), preventing subsequent intracellular signaling from interaction between Siglecs and Siglec ligands [8]. Importantly, NK cells co-cultured with sialidase-treated bladder tumor cells exhibited significant upregulation of the degranulation marker CD107a (Fig. 1A and Supplementary Fig. 1B,C), as well as IFN-γ and TNF-α (Supplementary Fig. 2A), which is not due to loss of HLA class I expression (Supplementary Fig. 2B) [10]. Overall, these results suggest that Siglec ligands may protect bladder tumor cells from recognition of Siglecs-expressing NK cells.
Fig. 1.
Expression and function of Siglecs in NK cells from healthy donors and patients with bladder cancer. (A) Flow cytometry measurement of CD107a on NK cells from PBMCs from HDs after co-culture with untreated or desialylated T24 or TCC-Sup bladder tumor cells at an E/T ratio of 1:4. Representative FACS histograms of (B) Siglec-6 and (C) Siglec-7 expression levels and their quantification in NK cells from peripheral blood of HDs (black line; n = 9), NMIBC patients (green line; n = 12), and MIBC patients (red line; n = 12). Representative FACS histograms of (D) Siglec-6 and (E) or Siglec-7 expression levels and their quantification in tumor-infiltrating NK cells from NMIBC patients (green line; n = 4) and MIBC patients (red line; n = 4). Representative FACS histograms of (F) Siglec-6 and or (G) Siglec-7 expression levels and their quantification in in vitro expanded urinary NK cells from NMIBC patients undergoing BCG therapy at post-BCG2 (light blue; n = 7) and post-BCG5/6 (light red; n = 10–12). Data are presented as the mean ± standard error of the mean. Values are expressed as the ratio of mean fluorescence intensity for specific staining versus the isotype Ig control (RFI). The dotted line represents the detection limit. BCG = bacillus Calmette-Guérin; E/T = effector/target; FACS = fluorescent-activated cell sorting; HD = healthy donor; MIBC = muscle-invasive bladder cancer; NK = natural killer; NMIBC = non–muscle-invasive bladder cancer; PBMC = peripheral blood mononuclear cell; TINK = tumor-infiltrating NK cell; Siglec = sialic acid–binding immunoglobulin-like lectin. * p < 0.05; ** p < 0.01.
Then we characterized the expression of nine Siglecs (Siglec-2, -3, -5/-14, -6, -7, -8, -9, and -10) on circulatory NK cells from HDs and NMIBC and MIBC patients (Fig. 1B,C and Supplementary Fig. 3). Similar to what we recently found for T cells from BCa patients [11], Siglec-6 is weakly expressed by circulating NK cells from HDs and MIBC patients, and slightly upregulated in NMIBC patients (Fig. 1B). In addition, we observed that Siglec-7 expression is higher on circulatory NK cells from NMIBC and MIBC patients (albeit not significantly for the latter) in comparison to HDs (Fig. 1C). Of note, other Siglecs were not detectable or were very poorly expressed (Supplementary Fig. 3B). Knowing that Siglec-6 ligands are poorly expressed by bladder tumor cells [11], these results indicate that the weakening of NK cell cytotoxic activity observed in Figure 1A is mainly due to Siglec-7.
We next assessed Siglec-6 and Siglec-7 expression levels on tumor-infiltrating NK cells (TINKs) from tumor tissue collected during TURBT or cystectomy. Whereas Siglec-6 was not or very poorly expressed, TINKs from NMIBC and MIBC patients expressed Siglec-7 at similar levels (Fig. 1D,E). Next, we questioned whether BCG might influence Siglec-6 and Siglec-7 expression on urinary NK cells (Fig. 1F,G), as we recently reported for Siglec-6 on CD8+ T cells [11]. We therefore determined the expression of Siglec-6 and Siglec-7 on urinary NK cells from NMIBC patients after the second and the fifth or sixth BCG instillations (post-BCG2 and post-BCG5/6). Urinary NK cells express both Siglec-6 and Siglec-7, with no difference between post-BCG2 and post-BCG5/6, suggesting that the course of the BCG therapy did not modulate their expression (Fig. 1F,G).
We recently reported that bladder tumor cells, including T24 and TCC-Sup, have high expression of Siglec-7 ligands but poor expression of Siglec-6 ligands [11]. Furthermore, Siglec-6 is very poorly expressed by TINKs, in contrast to Siglec-7 (Fig. 1D,E). Taken together, our data suggest that Siglec-7 and its ligands may be the main Siglec/sialic acid axis involved in NK cell impairment in bladder tumors.
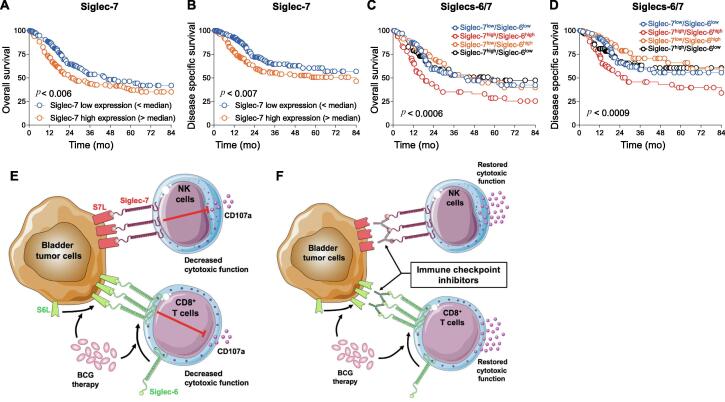
Finally, to investigate whether Siglec-6 and/or Siglec-7 might influence BCa patient survival, we performed a large-scale analysis of bladder urothelial cancer data from The Cancer Genome Atlas (TCGA) [12]. We recently showed that high Siglec-6 expression is significantly associated with lower overall and disease-specific survival in localized and locally advanced BCa [11]. Similarly, high Siglec-7 expression is associated with poor overall and disease-specific survival for BCa patients (Fig. 2A,B). Siglec-7 preferentially binds to α2,8-linked disialic acids on b-series gangliosides or on O-linked glycans, with binding mainly driven by two enzymes: ST8SIA I and VI [13], [14], [15]. Interestingly, high expression of ST8SIA I and VI is associated with poor survival in the TCGA BCa data set (Supplementary Fig.4), supporting the detrimental role of Siglec-7/sialic acids in BCa immunity. Finally, when evaluating Siglec-6 and Siglec-7 at the same time, high combined expression of Siglec-6 and Siglec-7 was significantly associated with poorer overall and disease-specific survival for BCa patients (Fig. 2C,D). However, low expression of both Siglecs does not seem to be more beneficial. This might be because of other regulatory mechanisms occurring during BCa development [5]. Of note, expression levels of Siglec-2, -3, -5, -8, -9, -10, and -14 do not influence BCa outcomes (Supplementary Fig. 5). These results suggest that in addition to Siglec-6 [11], Siglec-7 might be a potentially targetable immune checkpoint in BCa.
Fig. 2.
TCGA analysis for MIBC patients and a putative model of a suppressive axis mediated by Siglec-6 and Siglec-7. (A–D) Overall survival (n = 403; A,C) and disease-specific survival (n = 390; B,D) analyses by (A,B) Siglec-7 and (C,D) both Siglec-6 and Siglec-7 tumor mRNA expression obtained from TCGA data for patients with MIBC. (C,D) Patients were first segregated on the basis of Siglec-7 levels (below and above the median value) and the two groups were subsequently divided into two subgroups on the basis of Siglec-6 levels (below and above the respective median values in the subgroups). (E,F) Putative model of a suppressive axis mediated by Siglec-6 and Siglec-7 in bladder cancer. (E) Bladder tumor cells weakly express ligands for Siglec-6 (S6L), which is increased by BCG therapy, and high level of Siglec-7 ligands (S7L). Intratumoral CD8+ T cells express Siglec-6 at low levels, which is upregulated by BCG therapy. Siglec-7 and its ligands are highly expressed on intratumoral NK cells and in bladder tumor, respectively. Siglec-6/S6L and Siglec-7/S7L interactions restrict cytotoxic functions of effector CD8+ T and NK cells, respectively. (F) Immune checkpoint inhibitors targeting Siglec-6 and Siglec-7 may restore the function of intratumoral CD8+ T and NK cells. BCG = bacillus Calmette-Guérin; MIBC = muscle-invasive bladder cancer; NK = natural killer; Siglec = sialic acid–binding immunoglobulin-like lectin; SL = Siglec ligand. * p < 0.05.
The emergence of ICIs over the past decade has been a major breakthrough for cancer treatment. ICIs target key immune regulators such as the PD-1/PD-L1 axis and CTLA-4 that help tumor cells to escape antitumor immunity. However, a high rate of ICI failure indicates the need to find new targets for the development of novel promising ICIs. Our investigations revealed that Siglec-7 might be a potential immune checkpoint in BCa that can decrease the cytotoxic activity of NK cells against bladder cancer cells. We recently showed that Siglec-6 is the principal Siglec expressed by circulatory, urinary, and intratumoral CD8+ T cells, which may reduce their cytotoxic activity [11]. Moreover, we reported that Siglec-6 ligands, which are weakly expressed by bladder tumor cells, and Siglec-6 itself are upregulated on BCG infection [11]. Thus, our studies suggest that Siglec-6 on CD8+ T cells and Siglec-7 on NK cells may act in concert in situ to suppress antitumor immunity, even during BCG therapy, leading to BCa recurrence and progression (Fig. 2E). It has recently been shown in humanized immunocompetent mouse models that Siglec-7/-9 blockade reduces tumor burden in vivo, supporting the use of anti-Siglec antibodies to boost antitumor immunity [16]. Thus, although further validation in vivo is warranted, ICI targeting of Siglec-6 and Siglec-7 might be a promising therapeutic combinatorial approach in BCa (Fig. 2F).
Author contributions: Laurent Derré had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Benmerzoug, Chevalier, Derré.
Acquisition of data: Benmerzoug, Chevalier, Schneider, Cesson, Villier, Nguyen.
Analysis and interpretation of data: Benmerzoug, Derré.
Drafting of the manuscript: Benmerzoug, Derré.
Critical revision of the manuscript for important intellectual content: Chevalier, Nardelli-Haefliger, Jichlinski, Roth, Schneider, Nguyen, Lucca, Dartiguenave, Rodrigues-Dias.
Statistical analysis: Benmerzoug, Derré.
Obtaining funding: Jichlinski, Roth, Derré.
Administrative, technical, or material support: Cesson, Dartiguenave, Rodrigues-Dias, Lucca, Derré.
Supervision: Derré.
Other: None.
Financial disclosures: Laurent Derré certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This work was funded by grants from the Swiss National Foundation (32003B_146638 and 32003B_175559), a Ferring Innovation Grant, and Fondation pour la lutte contre le cancer (#SKB434). The sponsors played no direct role in the study.
Acknowledgments: We are obliged to all the patients for their dedicated collaboration and to the healthy blood donors. We also thank Mr. Dany Labes from the Flow Cytometry Facility at University of Lausanne for his contribution to data analysis and helpful discussions.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2021.10.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sanli O., Dobruch J., Knowles M.A., et al. Bladder cancer. Nat Rev Dis Primers. 2017;3:17022. doi: 10.1038/nrdp.2017.22. [DOI] [PubMed] [Google Scholar]
- 2.Guallar-Garrido S., Julian E. Bacillus Calmette-Guerin (BCG) therapy for bladder cancer: an update. Immunotargets Ther. 2020;9:1–11. doi: 10.2147/ITT.S202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babjuk M., Bohle A., Burger M., et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71:447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Kamat A.M., Sylvester R.J., Bohle A., et al. Definitions, end points, and clinical trial designs for non–muscle-invasive bladder cancer: recommendations from the International Bladder Cancer Group. J Clin Oncol. 2016;34:1935–1944. doi: 10.1200/JCO.2015.64.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider A.K., Chevalier M.F., Derre L. The multifaceted immune regulation of bladder cancer. Nat Rev Urol. 2019;16:613–630. doi: 10.1038/s41585-019-0226-y. [DOI] [PubMed] [Google Scholar]
- 6.Vaddepally R.K., Kharel P., Pandey R., Garje R., Chandra A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12:738. doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell J.S., Long G.V., Scolyer R.A., Teng M.W., Smyth M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev. 2017;52:71–81. doi: 10.1016/j.ctrv.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 8.van de Wall S., Santegoets K.C.M., van Houtum E.J.H., Bull C., Adema G.J. Sialoglycans and Siglecs can shape the tumor immune microenvironment. Trends Immunol. 2020;41:274–285. doi: 10.1016/j.it.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Duan S., Paulson J.C. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. 2020;38:365–395. doi: 10.1146/annurev-immunol-102419-035900. [DOI] [PubMed] [Google Scholar]
- 10.Moretta A., Bottino C., Vitale M., et al. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 11.Benmerzoug S., Chevalier M.F., Verardo M., et al. Siglec-6 as a new potential immune checkpoint for bladder cancer patients. Eur Urol Focus. 2021 doi: 10.1016/j.euf.2021.06.001. [In press] [DOI] [PubMed] [Google Scholar]
- 12.Robertson A.G., Kim J., Al-Ahmadie H., et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171 doi: 10.1016/j.cell.2017.09.007. 540 56.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman D.J., Crotts S.B., Shapiro M.J., et al. ST8Sia6 promotes tumor growth in mice by inhibiting immune responses. Cancer Immunol Res. 2021;9:952–966. doi: 10.1158/2326-6066.CIR-20-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teintenier-Lelievre M., Julien S., Juliant S., et al. Molecular cloning and expression of a human hST8Sia VI (α2,8-sialyltransferase) responsible for the synthesis of the diSia motif on O-glycosylproteins. Biochem J. 2005;392:665–674. doi: 10.1042/BJ20051120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicoll G., Avril T., Lock K., Furukawa K., Bovin N., Crocker P.R. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur J Immunol. 2003;33:1642–1648. doi: 10.1002/eji.200323693. [DOI] [PubMed] [Google Scholar]
- 16.Ibarlucea-Benitez I., Weitzenfeld P., Smith P., Ravetch J.V. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2107424118. e2107424118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.