Abstract
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that can be activated by structurally diverse compounds arising from the environment and the microbiota and host metabolism. Expanding evidence has been shown that the modulation of the canonical pathway of AHR occurs during several chronic diseases and that its abrogation might be of clinical interest for metabolic and inflammatory pathological processes. However, most of the evidence on the pharmacological abrogation of the AHR-CYP1A1 axis has been reported in vitro, and therefore, guidance for in vivo studies is needed. In this review, we cover the state-of-the-art of the pharmacodynamic and pharmacokinetic properties of AHR antagonists and CYP1A1 inhibitors in different in vivo rodent (mouse or rat) models of disease. This review will serve as a road map for those researchers embracing this emerging therapeutic area targeting the AHR. Moreover, it is a timely opportunity as the first AHR antagonists have recently entered the clinical stage of drug development.
Keywords: Aryl hydrocarbon receptor, CYP1A1, Metabolism, In vivo, Inflammation, Oxidative stress
Introductory note to the aryl hydrocarbon receptor (AHR)
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that belongs to the basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) family with important functions in sensing and incorporating environmental and outer stimuli (light–dark, O2 alterations, xenobiotic exposure, and microbiota metabolites) into cellular adaptive responses (reviewed by [1]). Other members of this family are the CLOCK-BMAL1 (key components of the circadian clock), the hypoxia-inducible factors (HIFs), and the aryl hydrocarbon receptor translocator (ARNT, also named HIF-1β), and interesting evidence on the cross-play among them have been reported (reviewed by [2]). In the present review, a brief introduction will be given to the AHR-CYP1A1 axis, but we would like to invite the reader to find more information about these druggable targets in the excellent reviews suggested throughout this introductory note.
As a cytosolic protein, the AHR exists in an inactive state bound to the chaperones heat shock protein 90 (HSP90), HSP90-associated co-chaperone p23, AHR-interacting protein (AIP), also called hepatitis B virus X-associated protein 2 (XAP2), and tyrosine kinase c-Src. This chaperone complex maintains the proper folding and assures the ligand-binding competency and, overall, the transcriptional effectiveness of AHR (reviewed by [3, 4]).
AHR ligands come from the environment and from the microbiota and cellular metabolism. There is a wide variety of compounds with different chemical properties, structure, and binding affinities, which have been recognized as AHR ligands.
Among the exogenous compounds, it may be referred environmental contaminants including the halogenated aromatic hydrocarbons (HAH), such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 2,3,7,8-tetrachlorodibenzofuran (TCDF), and 3,4,3′,4,′5-pentachlorobiphenyl (PCB), and the polycyclic aromatic hydrocarbons (PAH), such as benzo[a]pyrene (B[a]P) and 3-methylcholanthrene (3-MC). In addition, some dietary substances have been also described including polyphenols (quercetin, resveratrol, curcumin, indole-3-carbinol) (reviewed by [5, 6]).
Among the endogenous ligands, some ultraviolet photoproducts of tryptophan have been described as the 6-formylindolo[3,2-b]carbazole (FICZ); the indigoids indigo and indirubin; the kynurenine (Kyn) and its metabolites including kynurenic acid; the metabolites of arachidonic acid like lipoxin 4A, prostaglandin G2, and hydroxyeicosatetraenoic acid; and cystine [7] and the tetrapyrroles derived from heme, biliverdin, and bilirubin (reviewed by [8, 9]). This vast ligand promiscuity converges in the fact that according to the ligand, AHR might be activated in a diverse manner and in a cell- and tissue-dependent way [3, 5].
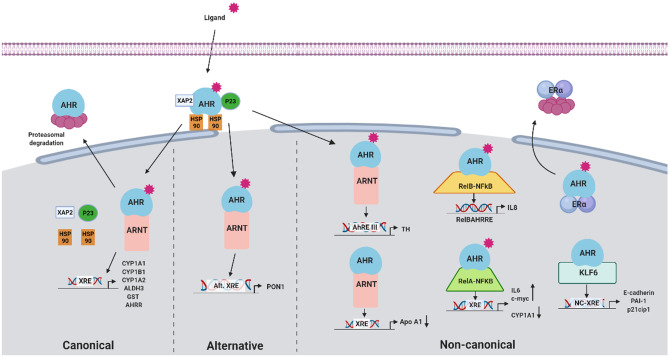
AHR has long been associated with an adaptive response to environmental contaminants, most of which are man-made and not related to human physiology. This response activates the AHR canonical or adaptive pathway, which involves AHR-ARNT heterodimer binding DNA at xenobiotic response elements (XRE), including xenobiotic enzymes and transporters that allow environmental contaminants detoxification (reviewed by [4, 10, 11]). The CYP1A1 expression is a sensitive marker of the activation of this route [12]. Moreover, a negative regulatory feedback mechanism is present, with the induction of AHR repressor (AHRR) gene, capable of competing with AHR for ARNT, originating a transcriptionally inactive heterodimer and thus repressing AHR transcriptional activity [13]. Alternatively, several exogenous ligands (e.g., polyphenols as resveratrol or quercetin) activate the AHR alternative pathway, triggering the expression of the antioxidant and anti-inflammatory paraoxonase 1 (PON-1) [11]. Besides ARNT, the AHR also has non-canonical dimerization partners that will activate the transcription of non-consensus xenobiotic response elements (NC-XRE). One of these novel proteins is the tumor suppressor Kruppel-like factor 6 (KLF6) that is involved in the regulation of cellular differentiation, proliferation, and apoptosis [14]. Also, the AHR can interplay with other proteins, most notably with NF-kB subunits and ERα. At final steps, the AHR undergoes proteasomal degradation (Fig. 1).
Fig. 1.
Representations of ligand-dependent AHR activation pathways. Inactive AHR is localized at the cytoplasm complexed to some chaperones and other elements (HSP90, XAP2, p23, c-Src). Upon ligand binding, conformational changes allow AHR to translocate to the nucleus, where it dissociates from its chaperone complex. Depending on the ligand, AHR can follow either ARNT-dependent or ARNT-independent pathways. In the ARNT-dependent pathways, the AHR-ligand complex dimerizes with its binding partner ARNT. The ligand-AHR-ARNT complex will activate or repress the expression of different genes depending on the type of ligand. Canonical pathway (left side). AHR binding to Kyn, HAH (e.g., dioxin), or PAH (e.g., B[a]P) activates the canonical pathway; the dimer AHR-ARNT binds to xenobiotic response elements (XRE) and drives the expression of xenobiotic metabolizing enzymes such as CYP1A1, the prototypical target gene of AHR. The AHR-ARNT complex promotes gene expression by recruiting several components of the transcriptional machinery and fulfilled this function; AHR activity ends by the dissociation of the complex from the DRE to be exported from the nucleus, where it enrolls in ubiquitin-mediated proteasomal degradation [26]. Moreover, a negative regulatory feedback mechanism is present, with the induction of AHR repressor (AHRR) gene, capable of competing with AHR for ARNT, originating a transcriptionally inactive heterodimer, thus repressing AHR transcriptional activity [13]. Alternative pathway (center). AHR binding to polyphenols such as resveratrol or quercetin activates the alternative pathway by binding to alternative xenobiotic response elements such as the antioxidant and anti-inflammatory paraoxonase 1 (PON-1). Other non-canonical pathways (right side) include alternative REs of alternative AHR-ARNT related transcriptional responses also observed in tyrosine hydroxylase [27], a precursor enzyme in the synthesis of dopamine and catecholamines, Bax, an apoptosis regulator gene [28], and in TGF-β [29]. Also, the AHR can bind to other non-ARNT binding partners, like the Kruppel-like factor 6 (KLF6) or the NF-kB subunits RelA or RelB. ALHD3, aldehyde dehydrogenase 3; AHR, aryl hydrocarbon receptor; AHRE, aryl hydrocarbon response element; AHRR, AHR repressor; ARNT, aryl hydrocarbon receptor nuclear translocator; B[a]P, Benzo[a]pyrene; CYP1A1, cytochrome P450, family 1, subfamily A, polypeptide 1; CYP1A2, cytochrome P450, family 1, subfamily A, polypeptide 2; CYP1B1, cytochrome P450, family 1, subfamily B, polypeptide 1; GST, glutathione S-transferase; HAH, halogenated aromatic hydrocarbon; HSP90, heat shock protein 90; KLF6, Kruppel-like factor 6; Kyn, kynurenine; NC-XRE, non-consensus xenobiotic responsive element; p21cip1, cyclin-dependent kinase inhibitor 1; p23, HSP90-associated co-chaperone; PAI-1, plasminogen activator inhibitor 1; PAH, polycyclic aromatic hydrocarbon; PON1, paraoxonase 1; RelA, nuclear factor NF-kappa-B P65 subunit; RelB, RELB proto-oncogene, NF-kB subunit; TH, tyrosine hydroxylase; XAP2, hepatitis B virus X-associated protein 2 or aryl hydrocarbon receptor-interacting protein (AIP); XRE, xenobiotic responsive element
To add more complexity, several lines of research have documented ligand-independent AHR activation, namely modulation by cAMP [15], oxidized LDL [16], vascular shear stress [17], or even reactive oxygen species (ROS) [18].
The mechanism by which AHR ligands affect physiological processes appears to involve multiple interactions between AHR and other signaling pathways (reviewed extensively by [3, 4, 10, 11]) or may be a consequence of a change in the activity of metabolic enzymes and transporters, which are AHR target genes that modify the availability and disposition of endogenous metabolites (e.g., estrogen, arachidonic acid, melatonin) [19–21]. AHR target genes include several drug-metabolizing enzymes belonging to the cytochrome P450, like cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1), CYP1A2 and CYP1B1, aldehyde dehydrogenase 3 (ALDH3), and UDP glucuronosyltransferase family 1 member A1 (UGT1A1) [22–24] and transporters such as the ATP binding cassette subfamily B member 1 (ABCB1) [25].
Pharmacological targeting of AHR-CYP1A1 axis in vivo
In this review, we aim to compile the state-of-the-art concerning AHR antagonists and CYP1A1 inhibitors, seeking consensus for their use in pharmacological in vivo studies. The role of the AHR axis in pathophysiology is expanding fast; however, many of these compounds are non-selective, and their pharmacology is poorly characterized. We summarized the pharmacodynamic and pharmacokinetic characteristics of AHR antagonists and CYP1A1 inhibitors that were tested in mouse (Mus musculus) and rat (Rattus norvegicus), which represent fundamental animal models of disease in translational research studies. While the blockade of AHR has been consistently described in cardiovascular diseases [30, 31], it must be mentioned that in other fields, including cancer, the AHR signaling is dichotomous (i.e., whereas in some type of cancer, the activity of AHR contributes to growth and progression, in other types of tumors, it may suppress them) [32].
For the accomplishment of this review, a search in PubMed was performed using the following keywords: aryl hydrocarbon receptor antagonist; AHR antagonist; CYP1A1 inhibitor; mice; rat; rodents; and in vivo until the end of June of 2021. Herein, for the sake of a better characterization for in vivo usage, we included all studies that reported any pharmacological effect of these drugs, even without a detailed description on the role of AHR-CYP1A1 in the underlying mechanism of disease. For all eligible studies, we provided a summing up of the conclusion in the main text of the manuscript and reserved the details on experimental approach and outcomes for Tables 1 and 2.
Table 1.
Summary of AHR antagonists used in animal models of disease
| Drug | Disease model | Study Design |
Animal Model Sex; strain; age |
Drug administration Route; dose; duration |
Pharmacodynamic outcomes | Ref |
|---|---|---|---|---|---|---|
|
3'M4'NF IC50 = 2.27 × 10−9 M |
Genotoxicity induced by B[a]P | P | M; WT and AHR null C57Bl/6 J mice; 7–8 wo | IP; 20 mg/kg; 4 h before, with, and 4 h after B[a]P | ↓ CYP1A1/2 activity (zoxazolamine paralysis test); ↓ B[a]P-induced micronucleated reticulocytes (higher decrease in AHR null than WT mice) | [51] |
| P | M; C57Bl/6 J and AHR null mice; 7–8 wo | IP; 0.2, 2, 20 or 40 mg/kg; 4 h before, with, and 4 h after B[a]P, 3d | ↓ CYP1A1/2 activity (zoxazolamine paralysis test) at 20 and 40 mg/kg; ↓ B[a]P and CSC-induced micronucleated reticulocytes; splenic toxicity at 20 mg/kg | [52] | ||
| Immunosuppression induced by UVR | R | F; C57BL/6 mice; 7–8 wo | IP; 20 μM | ↓ UVR-induced immunosuppression and Treg induction | [53] | |
| α-NF | Ovarian toxicity induced by VCD | P | F; F344 rat, C57BL/6 mice and AHR-KO mice; 3 wo | IP; 20 or 80 mg/kg; 2.3w | ↓ ovarian follicle depletion (dose-dependent) (rats); no effects in mice | [60] |
| Teratogenicity induced by TCDD | P | F; C57BL/6 J mice; 12 wo | PO; 50 μg/kg or 5 mg/kg; single dose or 6d | ↓ incidence of cleft palate and severity of renal malformations; no differences between single and multiple dosing | [61] | |
| Obesity induced by HFD | P | M + F; C57BL/6 J mice; 3–4 wo | PO (diet); 3 mg/kg; OD, 5w | ↓ body mass and fat mass gain; ↓ liver steatosis; hepatomegaly | [62] | |
| P | M + F; C57BL/6 J mice and B6.D2N-Ahrd/J mice; 3–4 wo | PO (diet); 90 mg/kg; OD, 26w | ↓ body mass gain in both sexes and strains (females and B6.D2N-Ahrd/J were less responsive); ↓ fat mass/total mass (female C57BL/6 J were the most responsive); ↓ liver steatosis; hepatomegaly present in all α-NF treated mice (particularly in female groups) | [63] | ||
| NAFLD induced by HFD | P | M; C57 mice; 7–9 wo | PO; 80 or 160 mg/kg; OD, 4w | ↓ AHR, CYP1A1, and TNFα protein & mRNA; ↓ steatosis, hepatocytes defects, interstitial expansion, serum AST, ALT, TG, and cholesterol (dose-dependent); ↓ MDA and ↑ SOD, CAT and GSH; attenuation of insulin resistance | [64] | |
| Psoriasis induced by aldara | P | F; R26Cyp1a1 C57Bl/6 mice; 8 wo | IP; 5 mg/kg; 1 w | ↓ CYP1A1 activity (EROD); ↑ K10 mRNA; ↓ Il-17a, Cxcl1 and S100a7a mRNA in tail and whole skin tissues; ↓ epidermis thickness | [65] | |
|
CB7993113 IC50 = 3.3 × 10−7 M |
Myelosuppression induced by DMBA | P | M; C57BL/6 J mice; 6–8 wo | IP/PO; 50 mg/kg; 2 doses | ↓ Cyp1a1 mRNA and bone marrow ablation | [66] |
|
CH-223191 IC50 = 0.03 M |
Interstitial cystitis—AOAH deficiency | R | F; Aoah−/; mice; 12 wo | PO; 10 mg/kg; OD, 2w | ↑ Voiding frequency (using the void spot assay) | [67] |
| Myelosuppression induced by DMBA | P | M; C57BL/6 J mice; 6–8 wo | IP; 50 mg/kg; 2 doses | ↓ Cyp1a1 mRNA in the liver; inhibition of acute bone marrow cell ablation | [66] | |
| Liver damage induced by TCDD | P | M; ICR mice; 6 wo | PO; 10 mg/kg; 3.6w | ↓ Cyp1a1 mRNA (30-fold-change); ↓ liver steatosis; ↓ AST, ALT; no agonist activity, cytotoxicity or effects on estrogen receptor | [68] | |
| Intestinal fibrosis induced by TNBS | R | F; Balb/c mice; 8 wo | IP; 10 μg; once every 2d, 8w | ↓ AHR activity and ↑ fibrotic markers (Col1A1, Col3A1 and α-SMA) and fibrosis severity | [70] | |
| Ulcerative colitis induced by DSS | P | M; C57BL/6 mice; 6–8 wo | IP; 10 mg/kg, OD, 10 days | ↓ amelioration of colitis symptoms associated to baicalein (↑ weight loss, worsening of disease activity index, ↓ colon length and histopathological changes like epithelial damage and inflammatory cells infiltration); disbalance between Th17 and Treg cells in mesenteric lymph nodes and colonic lamina propria; ↑ serum levels of proinflammatory cytokines (TNF-ɑ, IL-6 and IL-17) and ↓ anti-inflammatory cytokines (TGFβ, IL-10, IL-22) | [71] | |
| Ischemic stroke induced by MCAO | R | M; C57BL/6 J mice; 10–12 wo | IP; 10 mg/kg; single dose | ↓ infarct size and neurologic damage severity | [72] | |
| P | M and F pregnant; ICR mice | IP; 10 mg/kg; 1 dose 1 h before MCAO | ↓ mRNA of AHR-CYP1A1 and proinflammatory markers (TNF-ɑ, IL-1β and COX-2) in cortex and striatum; ↓ infarct size and neurologic severity score; ↓ lipid peroxidation (TBARS) in the brain cortex | [73] | ||
| Obesity induced by HFD | P | M; C57BL/6 J mice | PO (diet); 10 mg/kg; 5w | ↓ body mass and fat mass gain; no liver steatosis | [62] | |
| P | M + F; C57BL/6 J mice | PO (diet); 10 mg/kg; 5w | ↓ hepatic Scd1 and CYP1B1; ↓ body mass and fat mass gain; no liver steatosis | [63] | ||
| Systemic HTN induced by CIH | P + R | M; Wistar rat; 8–12 wo | PO; 5 mg/kg; 2w | ↓ SBP and DBP | [30] | |
| Pulmonary HTN induced by Sugen 5416 + CH | R | F; Wistar rat; 9 wo | PO; 8 mg/kg; OD, 2w | Normalized lung ARNT, AHR and CYP1A1 expression; reversion of pulmonary HTN (no changes in systemic BP and HR) | [74] | |
| Smoke-induced airway inflammation | P | F and M; C57BL/6 mice | IP; 50 μg per mouse in DMSO and PBS; single dose, 1 h before smoke exposure | ↑ total number of cells and neutrophils in bronchoalveolar lavage upon smoke exposure, while AHR activation by FICZ attenuated smoke-induced inflammation and neutrophilia | [76] | |
| Rheumatoid arthritis induced by collagen | R | F; Wistar rat | PO; 5 mg/kg; 2w | ↓ CYP1A1 expression and ARNT; ↑ osteoclastogenesis and bone destruction | [77] | |
| Skin sclerosis induced by Bleomycin | P | F; Balb/c mice; 6 wo | SC; 10 μg in PBS; once every 2d, 4w | ↑ dermal thickness (scleroderma fibrosis); ↑ α-SMA compared to PBS-treated mice | [80] | |
| Experimental autoimmune encephalomyelitis induced by MOG | P | F; C57BL/6 mice; 6–8 wo | IV; 5 mg/kg, OD, 30 days | Worsening of the clinical score, ↑ proinflammatory cytokines production (TNF-ɑ, IL-6 IL-1β, IL-17A and IFN-γ) and ↑ Th17 and Th1 cells proportions, when compared to DIM | [81] | |
| Inflammation induced by BSO | P | M; C57BL/6 mice; 9–12 wo | IP; 20 μg/kg in DMSO (1%) and olive oil | ↓ mRNA of redox-related genes (HO-1, GCLM, GCLC) in splenocytes; influence on the fate of subpopulations of T cells: ↑ Treg, ↑Th2 and Th17 cells in co-administration with NAC and FICZ, ↑ Th1 and Th17 cells in co-administration with BSO and FICZ | [82] | |
| Polycystic ovary syndrome induced by DHEA | R | F; BALB/c mice | SC; 10 mg/kg in sesame oil; OD, 12 days | Restoration of estrous cyclicity and ovarian morphology improvement; ↓ AHR-CYP1B1 protein expression in granulosa cells | [83] | |
| Neurotoxicity induced by MDMA | P | M; Dark Agouti rats | IP; 10 mg/kg in solution of DMSO and Tween 80, 12 h, 30 min and 30 min after MDMA | ↑ serotonergic neurotoxicity (↓ density of serotonin transporters in hippocampus, measured by autoradiographic assay) | [84] | |
| Pulmonary fungal infection induced by Paracoccidioides brasiliensis | P | M; C57BL/6 mice; 6–8 wo | IP; 400 μg/mouse in DMSO + PBS, alternate days after infection up to 2 weeks | ↑ fungal load in the lungs; ↑ activated lung myeloid cells, ↑cells expressing TNF-α and ↓ IL-12, IL-1β, and IL-6 levels in lung CD11c + myeloid cells; ↓ Th1 and Treg cells and ↑ Th17 in the lungs | [88] | |
| Pulmonary fungal infection induced by Paracoccidioides brasiliensis | P | M; C57BL6/J mice; 8–12 wo | IP; 20 mg/kg in corn oil; three times a week for 2 weeks after infection | ↑ fungal load in the lungs; ↑ CD11c + leukocytes expressing IAb, CD40, CD80 and CD86, ↓ CD11c + cells expressing IDO-1 or AHR in the lungs; ↑ T17 cells and ↓T1, T22 and Foxp3 + Treg cells in the lungs. Similar results were obtained in infected AHR−/− mice | [87] | |
| Zika virus infection | R | Pregnant F; SJL mice; 8–10 wo | IP; 5 mg/kg (in 100 μl DMSO); OD from ED13 to birth | ↓ viral load in brain and spleen, ↓ fetal brain pathology and microcephaly; ↓ expression of genes linked to apoptosis, tissue damage and autophagy; ↑ NF-kB and IFN-1 levels in the brain | [89] | |
| COVID-19 infection | R | F; ICR and hACE2-transgenic mice; 6–8 wo and 6–11 mo (transgenic) | ICR (Intratracheal); 10 mg/kg, OD, 4 days. hACE2-transgenic mice: IV; 10 mg/kg; 5 days | ↓ expression of mucins in the lungs of IFN-treated mice and hACE2-transgenic mice infected with SARS-CoV-2; lung disease amelioration and ↑ respiratory function | [90] | |
| Resveratrol | Lung cancer induced by B[a]P | P | BALB/c mice | SC; 50 mg/kg; OW, 5w | Suppression DNA adducts of BPDE and ↓ apoptosis at the lung; abrogation of the induction of CYP1A1 | [97] |
| Thymic atrophy induced by TCDD | R | F; C57BL/6 (H-2b) mice | PO; 10 mg/kg; OD, 5d | ↓ CYP1A1 expression in thymus; ↓ thymic atrophy and apoptosis | [98] | |
| Male infertility induced by B[a]P | P | M; BALB/c mice | SC; 50 mg/kg; OW, 5w | ↓ germ cell apoptosis; CYP1A1 staining (Immunohistochemistry) similar to controls; ↓ BPDE DNA-adducts | [99] | |
| P | M; Wistar rats; 8 wo | PO; 50 mg/kg; 8.6w | ↓ CYP1A1 mRNA and protein in testes; restoration of sperm count, cell motility and testosterone levels; amelioration of testicular apoptotic index (twofold ↓); restoration of antioxidant defense mechanisms (GSH, catalase, MDA and GPx) | [100] | ||
| P | M; Wistar rats; 8 wo | PO; 50 mg/kg; 8.6w | restoration of sperm count, motility and testosterone levels; ↓ germ cell apoptosis; ↓ caspase 8 and 9 activation; ↓ Bax/Bcl2 ratio; ↑ phosphorylated Akt levels; ↓ testicular ROS generation; ↓ MDA levels in testicular cells; ↑ GSH/GSSG ratio; ↓ Cyp1a1 and Ahr mRNA and protein in testes | [101] | ||
| Testicular dysfunction induced by TCDD | P | F; SD rats; 8.6 wo | PO; 20 μg/kg; Gestational day 10 to 21 | ↑ sertoli cells and proportion of the seminiferous cord compartment in adults and neonates | [102] | |
| Prostate Development induced by TCDD | P | F; Wistar rats; 12 wo | PO; 20 mg/kg; gestational day 10 to PND 21 | Restoration of prostatic buds number (PND1 – immediate effects); ↑ AHRR, AR and NRF2 protein in ventral prostate (PND90 – latter effects) | [103] | |
| Pancreatitis induced by B[a]P, TCDD | P | M + F; WT, Cyp1b1−/− mice; 6–8 wo | PO; 20 mg/kg; 3 or 6d |
B[a]P + resveratrol. ↓ CYP1A1 and CYP1B1 mRNA and protein (in WT) and CYP1A2 (in CYP1b1−/−) in pancreatic tissue; ↑ mitochondrial respiration (in WT and Cyp1b1−/−); ↓ α-amylase levels (only tested in WT) and IL-6, IL-12, IL-1b, TNF-α and CCR5 mRNA (in WT and Cyp1b1−/−) TCDD + resveratrol. ↑ mitochondrial respiration, ↓ α-amylase levels (only tested in WT) |
[104] | |
| Pancreatitis induced by TCDD | P | M; C57Bl/6 J mice; 7–8 wo | IP; 20 mg/kg; OD | ↓ MALAT1 protein, H3K27me3 activity and miR-200b mRNA in pancreatic tissues | [105] | |
| Renal damage induced by gentamicin | P | M; Wistar rats | IP; 10 mg/kg; OD, 2.4w | ↑ AHR protein in kidney; ↓ KIM1, NGAL and CD-68 serum levels; ↓ urinary albumin/creatinine ratio | [106] | |
| Endometriosis induced by human endometrial xenograft | P | F; RAG-2 g(c) knockout mice | SC; 6, 30, and 60 mg; OD, 3w | ↓ ESR1 and Ki-67 in endometrial epithelial cells (E2 + resveratrol 60 mg); no differences in AHR, CYP1A1, and CYP1B1 mRNA | [107] | |
| Breast cancer induced by TCDD | P | F; SD rats | PO (diet); 7 ppm; From gestational day 7 until the end of pregnancy | ↑ BRCA-1 and AHRR, ↓ CDK4, cyclin D1 and CYP1A1 mRNA in mammary tissue; ↓ proliferative terminal ducts and terminal end buds in mammary tissue; ↓ BRCA-1 promoter CpG methylation and occupancy of BRCA-1 genes by DNMT-1 | [108] | |
| Renal damage induced by aristolochic acid | R | M; C57BL/6 mice; 6–7 wo | IP; 50 mg/kg; 3d | ↓ CYP1A1/CYP1A2 mRNA and protein in liver | [109] | |
| Liver Damage induced by BNF | P | M; WT, Cyp1a1/1a2(−/−) and Cyp1a1/1a2/1b1(−/−) mice; 6–8 wo | IP; 20 mg/kg; 7d | ↓ CYP1A1 and CYP1A2 mRNA and protein in liver mitochondria; ↑ OCR and NADH: ubiquinone oxidoreductase and Cco activities and Cco protein in WT mice | [110] | |
| Bone fracture induced by surgery | P | M; SD rats; 8–10 wo | PO; 400 mg/kg; OD, 6d | ↓ CYP1B1 protein in bone marrow mesenchymal stem cells; ↑ mineralized callus | [111] | |
| Bone damage induced by chronic kidney disease | R | M; BALB/c mice; 6 wo | IP; 30 mg/kg/day; 4w | ↑ Runx2 protein, trabecular number and thickness in bone tissue | [112] | |
| Diabetes induced by PCB-77 | P | M; C57BL/6 J mice; 8–9 wo | PO (diet); 0.1%; OD, 3d | Improved glycemia, insulin tolerance and ↑ NQO1 and restored insulin-stimulated levels of phosphorylated Akt in adipose tissue | [116] | |
| Congenital hypertension induced by TCDD, dexamethasone | P | F; SD rats; 12–16 wo | PO; 0.05%; during pregnancy and lactation | ↓ mRNA of Ahrr, Ren, Ace and Agtr1a in kidney; ↓ SBP, ↓ oxidative damage in the kidney (by Immunohistochemistry staining of 8-OHdG); ↓ asymmetric and symmetric dimethylarginine plasma levels | [117] | |
| Obesity induced by HFD | R | M; C57BL/6 mice; 6–8 wo | PO (diet); 0.03 or 0.06%; 13w | ↑ Ahr, Cyp1a1, Nqo1 and Ugt1a1 mRNA (0.06% dose) and ↓ malondialdehyde levels in spleen; dose-dependent ↓ IL-1, IL-6, and TNF-α and ↑ IL-10 in spleen and plasma; ↑ FoxP3 and FoxP3 related genes, ↑ PPARγ, ↓ MMP-9 mRNA in spleen | [118] | |
| Congenital hypertension induced by BPA, high-fat high-sucrose diet | P | F; SD rats; 12–16 wo | PO; 2.5 mg/kg; OD, During pregnancy and lactation | ↓ Ahrr, Cyp1a1 and Arnt mRNA, AHR protein in kidney; ↓ SBP; restoration of NO bioavailability and ↓ oxidative stress | [119] | |
|
TMF IC50 = 9 × 10–7 M |
Ischemic stroke induced by MCAO | R | M; C57BL/6 J mice; 10–12 wo | IP; 5 mg/kg; single dose | Block of L-Kyn-induced Cyp1a1 mRNA expression in cortical neurons; ↓ infarct size and neurologic severity. More pronounced effects than with CH-223191 | [72] |
| R | M; C57BL/6 J mice; 8–10 wo | IP; 5 mg/kg; single dose | ↓ AHR protein in brain; amelioration of ischemic brain infarction, sensorimotor deficits and nonspatial working memory; ↓ astrogliosis and microglial infiltration | [121] | ||
| Cerebral ischemic-reperfusion injury induced by transient MCAO and reperfusion | R | M; SD rats; 8 wo | IP; 5 mg/kg; OD, 10 or 50 min after ischemia | Inhibition of AHR nuclear translocation in brain tissue; amelioration ischemia-induced brain damage, ↑ relative apparent diffusion coefficient (10 min after ischemia), ↓ relative T2 values (magnetic resonance imaging) in the ischemic core and peri-infarct region (24 h after ischemia), ↓ infarct volume, neuronal loss and apoptosis | [122] | |
| 5,7,30,40,50 pentahydroxy-flavanone | Chronic kidney disease induced by 5/6 nephrectomy | R | M; SD rats, C57Bl/6 mice; 6–8 wo | PO; 10 mg/kg; 3w | ↓ Ahr and Cyp1a1 mRNA; ↓ fibronectin, vimentin and FSP1 levels; ↑ E-cadherin levels in whole kidney tissue; ↓ serum creatinine, urea and proteinuria | [123] |
| Barleriside A | Chronic kidney disease induced by 5/6 nephrectomy | R | M; SD rats, C57Bl/6 mice; 6–8 wo | PO; 10 mg/kg; 3w | ↓ Ahr, Cyp1a1 and Cyp1a2 mRNA; ↓ fibronectin, vimentin and FSP1 levels; ↑ E-cadherin levels in whole kidney tissue; ↓ serum creatinine, urea and proteinuria | [123] |
| HP163 | Zika virus infection | R | Pregnant F; SJL mice; 8–10 wo | PO; 2.5 mg/kg (in 100 μl DMSO); TD from ED13 to birth | ↓ viral load in brain, eyes, spleen and maternal placenta; ↓ fetal brain pathology and microcephaly, ↓ number of hippocampal infected cells, ↓ microglial activation | [88] |
| Clofazimine | Multiple myeloma—xenograft model (MM.1S and 8226 cells) | R | F; SCID mice; 6 wo | IP; 10 mg/kg; OD; 20–30 days | ↓ CYP1A1 protein in tumors; ↓ tumor growth | [124] |
| KYN-101 | Melanoma and colorectal cancer (B16-F10, CT26 models) | R |
F; B16-F10 melanoma model WT, overexpressing IDO or TDO; C57Bl/6 mice; 6–8 wo F; CT26 colorectal cancer model; Balb/C mice; 6–8 wo |
PO; 3 or 10 mg/kg; OD; 12 days | ↓ tumor volume in B16-F10 models overexpressing IDO or TDO and CT26 model (similar results obtained with CH223191 (PO, 50 mg/mg, OD, 14 days) and clofazimine (IP, 10 mg/kg, OD, 14 days)) | [125] |
↓: decreased; ↑: increase; α-NF: alpha naphthoflavone; α-SMA: alpha smooth muscle actin; ACE: angiotensin converting enzyme; AHR: aryl hydrocarbon receptor; AHRR: AHR repressor; Akt: protein kinase B; AOAH: acyloxyacyl hydrolase; AR: androgen receptor; ARNT: AHR nuclear translocator; ALT: alanine aminotransferase; AST: aspartate aminotransferase; B[a]P: benzo[a]pyrene; BNF: beta-naphthoflavone; BP: blood pressure; BPA: bisphenol A; BPDE: B[a]P diol epoxidation; BRCA-1: breast cancer type 1; BSO: buthionine sulfoximine; CAT: catalase; Cco: cytochrome c oxidase; CCR5: C–C chemokine receptor type 5; CD-68: cluster of differentiation 68; CDK4: cyclin-dependent kinase 4; CH: chronic hypoxia; CIH: chronic intermittent hypoxia; Col1A1: collagen 1A1; Col1A3: collagen 1A3; COVID-19: coronavirus disease 2019; COX-2: ciclooxigenase 2; CSC: cigarette smoke condensate administration; Cxcl1: CXC motif chemokine ligand 1; CYP1A1: cytochrome P450, family 1, subfamily A, polypeptide 1; CYP1B1: cytochrome P450, family 1, subfamily A, polypeptide 1; DBP: diastolic blood pressure; DHEA: dehydroepiandrosterone; DIM: 3,3’-diindolylmetheane; DMBA: 7,12-dimetilbenz[a]antraceno; DMSO: dimethyl sulfoxide; DNMT-1: DNA Methyltransferase 1; DSS: dextran sulfate sodium; E2: estradiol; ED: embrionic day; ESR1: estrogen receptor 1; EROD: ethoxyresorufin-O-deethylation; F: female; FICZ: 6-formylindolo[3,2-b]carbazole; FoxP3: forkhead box protein P3; GCLC: glutamate-cysteine ligase catalytic subunit; GCLM: glutamate-cysteine ligase modifier subunit; GSH: glutathione; h: hour; H3K27me3: tri-methylation of lysine 27 on histone H3 protein; hACE2: human angiotensin I-converting enzyme 2; HFD: high-fat diet; HO-1: heme oxygenase 1; HR: heart rate; HTN: hypertension; ICR: Institute of Cancer Research; IDO: indoleamine 2,3 dioxygenase; IFN-γ: interferon gamma; IL: interleukin; IP: intraperitoneal; IV: intravenous; K10: keratin 10; KIM1: Kidney Injury Molecule-1; Kyn: kynurenine; M: male; MALAT1: metastasis-associated lung adenocarcinoma transcript 1; MCAO: middle cerebral artery occlusion; MDA: malondialdehyde; MDMA: 3,4-methylenedioxymethamphetamine; MMP-9: matrix metallopeptidase 9; mo: months old; MOG: myelin oligodendrocyte glycoprotein; NAFLD: non-alcoholic fatty liver disease; NGAL: neutrophil gelatinase-associated lipocalin; NQO1: NAD(P)H:quinone oxidoreductase 1; OCR: oxygen consumption rate; OD: once a day; OW: once a week; P: prevention; PBS: phosphate-buffered saline; PCB-77: 3,3′,4,4′-tetrachlorobiphenyl; PPARγ: peroxisome proliferator-activated receptor gamma; PND: post-natal day; PO: Per os; R: reversion; Ren: renin; ROS: reactive oxygen species; S100a7a: S100 calcium binding protein A7A; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SBP: systolic blood pressure; SC: subcutaneous; Scd1: stearoyl-CoA desaturase-1; SD: Sprague–Dawley; SOD: superoxide dismutase; TBARS: thiobarbituric acid reactive substances; TDO: tryptophan 2,3 dioxygenase; TG: triglycerides; TGF-β: transforming growth factor β; Th1: type 1 T helper cells; Th17: type 17 T helper cells; Treg: regulatory T cells; TCDD: 2,3,7,8-tetraclorodibenzo-p-dioxina; TNBS: 2,4,6-trinitrobenzenesulfonic acid; TNF-α: tumor necrosis factor alpha; UGT1A1: UDP glucuronosyltransferase family 1, subfamily A, polypeptide 1; UVR: ultraviolet radiation; VCD: 4-vinylcyclohexene diepoxide; w: week; wo: weeks old; WT: wild type
Table 2.
Summary of CYP1A1 inhibitors tested in rodents and their major outcomes
| Drug | Disease | Study design |
Animal Model Sex; strain; age |
Drug administration Route; dose; duration |
Study outcomes | Ref |
|---|---|---|---|---|---|---|
|
Alizarin IC50 = 6.2 × 10−6 M |
Hepatotoxicity induced by bromobenzene | P | M; Kunming mice | PO; 5 mM/animal; single dose | ↓ hepatic lipid peroxidation and serum ALT | [127] |
| Mutagenesis induced by MeIQx | P | M; C57BL/6 N mice; 5 wo | PO (diet); 30 mg/animal; 3 or 6 d | Unchanged CYP1A1 (EROD assay); antimutagenic effect (↓ MeIQx-DNA adducts) | [128] | |
| Diabetes induced by alloxan | R | M; Kunming mice | 24, 48 or 96 mg/kg; 10 d | ↓ fasting blood glucose (dose-dependent) | [129] | |
|
Ellipticine IC50 = 1.1 × 10−7 M |
Lung cancer—xenograft model (A549 cells) | R | Balb/c nude mice; 5 wo | IP; 11.25 mg/kg; 14 d | ↓ tumor size | [134] |
| Septic shock model induced by lipopolysaccharide | P | F; C57BL/6 mice; 8–12 wo | IP; 10 or 20 mg/kg; single dose | ↓ TNF-α and IL-6 (time-dependent) in serum | [135] | |
|
Pterostilbene IC50 = 3.6 × 10−6 M |
Inflammation induced by carrageenan | P | M; C57Bl/6 mice | IP; 30 mg/kg; single dose | ↓ paw edema; ↓ IL-6 and MCP-1 protein in the paw tissue | [138] |
|
Purpurin IC50 = 5.5 × 10−6 M |
Mutagenesis induced by MelQx | P | M; C57BL/6 N mice; 5 wo | PO (diet); 30 mg/animal; 3 or 6 d | ↓ CYP1A1 activity (EROD assay); ↓ adduct formation in lungs and kidney and marginally in liver | [128] |
| Obesity induced by HFD | P | M; C57BL/6 mice; 6–8 wo | PO (diet); 40 or 80 mg/kg;10 w | ↓ in weight gain (dose-dependent) | [139] | |
|
Rhapontigenin IC50 = 4 × 10−4 M |
Myocardial infarction related to isoproterenol | P | M; SD rats | IV; 1, 2.5 or 5 mg/kg/day; OD, 8 d | ↓ infarct size, heart/body weight index, CK, LD and CTT; ↓ TNF-α, IL-6, MD, SOD, p38 and iNOS protein (5 mg/kg/day) in serum and heart tissue | [145] |
| Rutaecarpine | Myocardial infarction—coronary artery occlusion | P | M; Wistar rats | IV; 100 or 300 μg/kg; single dose | ↓ infarct size and creatine kinase activity; ↑ plasma CGRP (dose-dependent) | [153] |
| Systemic Hypertension—2 kidneys-1 clip | R | M; SD rats | PO; 10, 20 or 40 mg/kg; bid, 4 w | ↓ SBP and mesenteric arteries AngII content (dose-dependent); ↑ plasma CGRP and α- and β-CGRP mRNA (20 and 40 mg/kg) | [155] | |
| Systemic Hypertension—SHR | P | M; SHR and WKY rats; 16 wo | PO; 10, 20 or 40 mg/kg; bid, 18 d | ↓ SBP and platelet aggregation (20 and 40 mg/kg); ↑plasma CGRP and α- and β-CGRP mRNA (20 and 40 mg/kg) | [156] | |
| P | M; SHR and WKY rats; 16 wo | PO; 20 or 40 mg/kg/day; bid, 18 d | ↓ SBP and CK activity (dose-dependent); ↑plasma CGRP; improved vasorelaxation to Ach in aortic rings (ex vivo) | [157] | ||
| P | M; SHR and WKY rats 16 wo | PO; 10, 20 or 40 mg/kg/day; bid, 14 d | ↓ SBP (dose-dependent); ↑ plasma CGRP and α-CGRP mRNA; ↑ telomerase activity and ↓ EPC senescence | [158] | ||
| Atherosclerosis—HFD | P | F; C57Bl/6 ApoE−/− mice; 8 wo | PO; 10, 20 or 40 mg/kg; daily, 8 w | ↓ face lesions on the aorta (dose-dependent); ↓ TC and TG; ↑ ABCA1 and SR-BI protein and mRNA | [159] | |
| Right ventricular remodeling—Hypoxia | P | M; SD rats; 6–8 wo | PO; 20 or 40 mg/kg; OD, for 3 w | ↓ ventricular SBP (dose-dependent); ↓ cardiac ANP, BNP, α-SMA, collagen-I, collagen-III, eIF3a and TGF-β1 mRNA; ↓ TGF-β1 and ↑ CGRP in plasma | [154] | |
| Obesity—HFD + streptozotocin | R | M; SD rats | PO; 25 mg/kg; OD, 7 w | ↓ TC, TG, LDL-C, blood glucose; ↓ serum CRP, MCP-1, TNF-α and IL-6; ↓ liver NF-kB protein; ↑ HDL-C and insulin sensitivity | [161] | |
| Renal Injury—ischemia- reperfusion injury | P | M; SD rats; 6–8 wo | IP; 30 or 60 mg/kg; single dose | ↓ serum Cr, BUN, NGAL (dose-dependent); ↓ NF-kB, TNF-α, IL-6, ICAM-1 mRNA and protein; ↓ MD and ↑ SOD content in renal tissue | [162] | |
| Vascular disease—carotid balloon-injury | R | M; SD rats | PO; 25, 50 or 75 mg/kg; OD, 14 d | ↑ NO protein and eNOS mRNA (dose-dependent); ↑ plasma cGMP (highest dose); ↓ c-myc, ERK2 and PCNA mRNA (50 and 75 mg/kg); ↑ MKP-1 (highest dose) and p-ERK2 (50 and 75 mg/kg) protein | [160] |
-/-: null; α-SMA: α-smooth muscle actin; ABCA1: ATP binding cassette transporter A1; ACh: acetylcholine; ALT: alanine aminotransferase; AngII: angiotensin II; ANP: atrial natriuretic peptide; Bid: twice a day; BNP: B-type natriuretic peptide; BUN: blood urea nitrogen; cGMP: cyclic guanosine 3',5'-monophosphate; CGRP: calcitonin gene-related peptide; CK: creatinine kinase; Cr: creatinine; CRP: C-reactive protein; CTT: cardiac troponin-T; CYP1A1: cytochrome P450, family 1, subfamily A, polypeptide 1; d: days; eNOS: endothelial nitric oxide synthase; EPC: endothelial progenitor cell; ERK2: extracellular signal-regulated kinase 2; EROD: ethoxyresorufin-O-deethylase; F: Female; HDL-C: high density lipoprotein-cholesterol; HFD: high-fat diet; ICAM-1: intercellular adhesion molecule-1; IL: interleukin; iNOS: inducible nitric oxide synthase; IP: intraperitoneal; IV: intravenous; LD: lactate dehydrogenase; LDL-C: low density lipoprotein-cholesterol; M: Male; MeIQx: 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; MCP-1: monocyte chemotactic protein-1; MD: malondialdehyde; MKP-1: MAPK phosphatase-1; NF-kB: nuclear factor kappa B; NGAL: neutrophil gelatinase-associated lipocalin; NO: nitric oxide; OD: once a day; P: prevention; PCNA: proliferating cell nuclear antigen; PO: Per os; R: reversion; SBP: systolic blood pressure; SD: Sprague–Dawley; SHR: spontaneously hypertensive rats; SOD: superoxide dismutase; SR-BI: scavenger receptor class B type I; TC: total cholesterol; TG: triglycerides; TGF-β1: transforming growth factor beta 1; TNF-α: tumor necrosis factor-α; w: weeks; wo: weeks old; WKY: Wistar Kyoto
AHR antagonists
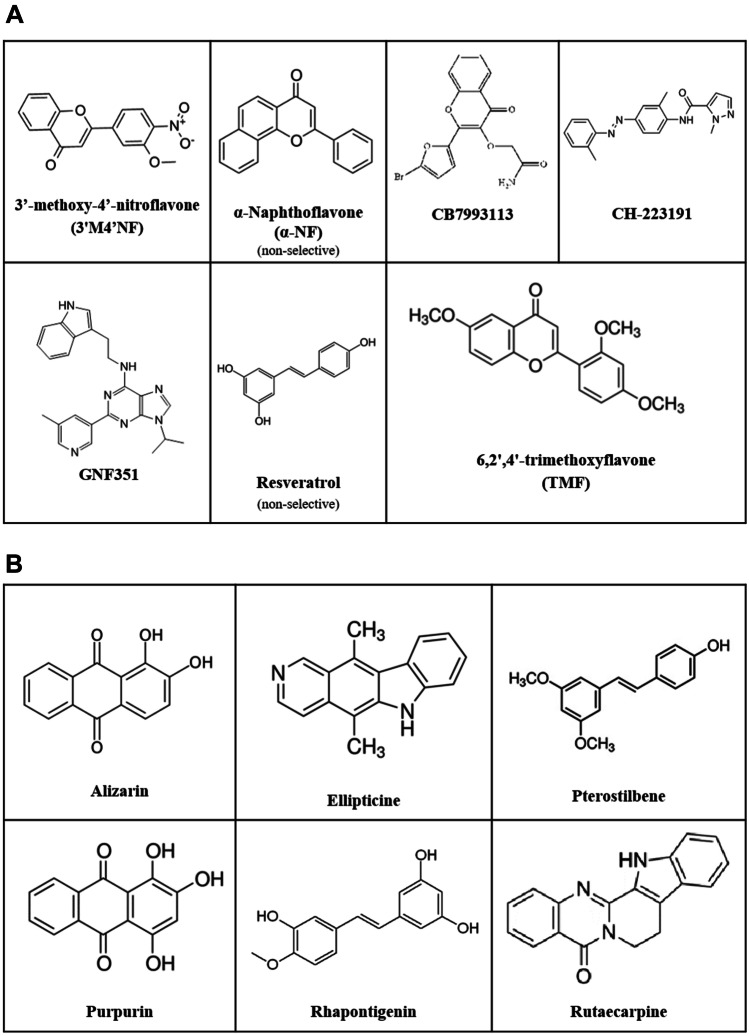
AHR might activate different signaling pathways (Fig. 1). Herein, we will focus on the main one, the canonical AHR-CYP1A1 axis, and particularly on the putative pharmacological properties of compounds that abrogate its ligand-dependent activation. These compounds have been evaluated in several models of disease. We annotated the animal species (strain, sex, and age) and the chemical or biological agents used as model of disease, together with the pharmacological properties of these compounds: drug administration route, frequency and duration of the treatment, pharmacokinetic properties, effects on the AHR axis related genes (AHR, CYP1A1, ARNT, AHRR), and drug response observed in the animal model investigated. The details on the eligible studies of the AHR antagonists are presented at Table 1. In vitro competitive binding assays, using labeled TCDD or labeled PAL (2-azido-3-[(125)I]iodo-7,8-dibromodibenzo-p-dioxin) as AHR agonists, proved that most molecules target directly the AhR and the IC50 values are presented in Table 1. Despite the same pharmacological target, AHR antagonists are compounds with diverse chemical structures (Fig. 2A), which in addition to different pharmacokinetic profiles (e.g., organ disposition) may account for pharmacodynamic differences. The mostly used and best described AHR antagonist is the CH-223191, which is considered a pure AHR antagonist [33]. Other AHR antagonists might present organ-dependent responses [34] or reduce AHR activation through a partial agonist effect [34–36]. Other compounds, which is the case of resveratrol, have the capability to abrogate the canonical pathway and allow the activation of the alternative pathway (Fig. 1), for which the AHR target gene is the paraoxonase 1 (PON-1) [37].
Fig. 2.
AHR antagonists and CYP1A1 inhibitors used in vivo. Chemical structures of AHR antagonists (A) and non-selective CYP1A1 inhibitors (B)
CYP1A1 inhibitors
Cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1) is a xenobiotic metabolizing enzyme involved in the phase I metabolism of several exogenous (e.g., Sudan I, caffeine, B[a]P) and endogenous (estrogens, estradiol, progesterone, testosterone, pregnenolone, melatonin arachidonic acid) compounds [38, 39]. CYP1A1 is responsible for ROS production (e.g., superoxide anion, hydrogen peroxide, and hydroxyl radical derived from the oxygen and electron transfers that occur during the CYP reaction cycle) [40] and for the metabolic activation of procarcinogens (e.g., B[a]P, estrogens) [41–45]. AHR signaling is constitutively activated, and the expression of CYP1A1 gene is almost exclusively regulated by AHR [46], allowing this gene to be considered the hallmark of its activation. Thus, CYP1A1 gene is a good indicator to associate AHR pathway to disease state and to evaluate the efficacy of the AHR antagonists abrogating this pathway. In addition, CYP1A1 inhibitors (Fig. 2B) represent important tools to clarify the deleterious effects of an increased activation of AHR-adaptive/canonical pathway. Unfortunately, there are no selective inhibitors for this CYP450 isoenzyme up to date. Importantly, most of the presented compounds were classified as CYP1A1 inhibitors in vitro, particularly using the ethoxyresorufin-O-deethylation (EROD) assay (measures CYP1A1/A2 activity) with recombinant CYP1A1 and NADPH fractions [47] (IC50 values in Table 2). However, when tested in vivo, some of these compounds revealed AHR agonist activity, acting as CYP1A1 inducers and allowing an increase in CYP1A1 transcription and activity. This is the case of rutaecarpine (see section of CYP1A1 inhibitors). A plausible explanation is that not all in vitro models are suitable to study enzyme induction [48, 49]; EROD activity does not measure only CYP1A1 activity [49]; and most of the compounds are not highly selective for CYP1A1 (see section of CYP1A1 inhibitors). Moreover, enzyme inhibition effects by direct binding to the enzyme are more rapid and short than induction effects. Induction effects take longer to be observed and are long lasting compared to enzyme inhibition. Another possibility relies on the fact that some CYP1A1 substrates (like FICZ) can also be AHR ligands. As such, the inhibition of the metabolism of CYP1A1 can lead to an increase of the concentrations of these compounds and, therefore, to an increase in AHR-mediated CYP1A1 activation. This may be also dependent on the dose and the number of doses administered and might justify differences among studies. Thus, with increased evidence that is becoming available, it is plausible that some compounds are, in fact, not direct AHR ligands but indirect AHR modulators (e.g., CYP1A1 inhibitors). We decided to include the compounds that have been classified in vitro as CYP1A1 inhibitors and to discuss in vitro/in vivo controversies in literature, highlighting the need for more in vivo evidence and particularly to understand the underlying mechanisms of AHR-CYP1A1 modulation in vivo by CYP1A1 inhibitors.
Pharmacological effects of the antagonists of AHR canonical pathway
3′-methoxy-4′-nitroflavone
3′-methoxy-4′-nitroflavone (3′M4′NF) impairs AHR nuclear translocation, plausibly by hindering the dissociation of HSP90 from the chaperone complex [50]. Nazarenko and collaborators (2001) used transgenic C57Bl/6 J male mice that express β-galactosidase in response to AHR agonists (DRE-lacZ mice) and showed that TCDD-induced AHR activation was abrogated by 3′M4′NF at the liver but not at the lung, highlighting an organ-specific difference in 3′M4′NF effects. In addition, CYP1A1 protein levels at the lungs showed a moderate increase in mice treated exclusively with 3′M4′NF, which might be suggestive of a partial agonist action for this compound [34]. This compound has been used in vivo to investigate the relation of AHR to genotoxicity by environmental pollutants [51, 52] and ultraviolet radiation-induced immunosuppression [53] (Table 1).
α-Naphthoflavone
α-Naphthoflavone (α-NF), also known as 7,8-benzoflavone or 2-phenyl-4H-benzo[h]chromen-4-one, is a synthetic flavone considered a putative chemopreventive agent due to its non-selective activities as inhibitor of aromatase (CYP19A1 [54]) and also as modulator of AHR and of several CYP450 enzymes [35, 55]. α-NF has been described as a partial agonist/antagonist of AHR [36] and as a competitive inhibitor of CYP1 family, namely of CYP1A1 [56], CYP1A2 [57] and CYP1B1 [58], rendering its common use in xenobiotic biotransformation studies. Moreover, α-NF is an allosteric activator of CYP3A4 [59]. The preventive effect of this compound was investigated on chemical exposure (ovotoxicity and teratogenicity) [60, 61], metabolic (obesity and non-alcoholic fatty liver disease) [62–64], and autoimmune diseases (psoriasis) [65] (Table 1).
Cardiometabolic diseases
Evidence suggested dose- and sex-dependent effects of α-NF in obesity-related features (liver status and fat mass) [62–64] (Table 1).
A preventive protocol showed that α-NF (3 mg/kg/day) impacted high-fat diet (HFD)-induced obesity in male mice [62]: it reduced the gain/increase in body mass, fat mass, triglycerides, and polyunsaturated fatty acids. In addition, α-NF ameliorated liver steatosis (assessed by a histologic decrease in fat vesicles) but caused hepatomegaly.
A higher dose of α-NF (90 mg/kg/day) was administered to male and female mice of two strains, the C57Bl/6 B6 and the C57Bl/6.D2, which express a ligand-binding domain of AHR with higher and lower affinity, respectively. In addition to dose-dependent effects, it was possible to observe sex- and AHR genotype-dependent differences with long-term α-NF administration. Both AHR genotypes and sexes presented better fat mat-related outcomes upon α-NF treatment, although there were differences in the degree of response, dependent on the strain and sex [63]. α-NF has also reduced steatosis in both sexes and genotypes. However, hepatomegaly was present in all α-NF mice treated independently of the diet, especially in the female groups [63].
In addition, in male mice on a HFD-induced non-alcoholic fatty liver disease (NAFLD), the administration of α-NF reduced liver damage, attenuated oxidative stress, and diminished insulin resistance in a dose- and AHR-dependent manner [64].
Autoimmune diseases
Using a mice model of skin inflammation (Aldara-induced psoriasis), Kyoreva and colleagues (2021) investigated the α-NF effects and observed decreased CYP1A1 activity, epidermis thickness, and levels of several proinflammatory mediators, together with an increase in keratinocyte differentiation markers [65].
CB7993113
Parks and collaborators (2014) discovered CB7993113 (2-((2-(5-bromofuran-2-yl)-4-oxo-4H-chromen-3-yl)oxy)acetamide) by ligand shape–based virtual modeling techniques. The compound showed no partial AHR agonist effect [66]. CB7993113 is a competitive antagonist of AHR (upon activation by β-naphthoflavone (BNF) or TCDD) and prevents AHR nuclear translocation as its primary mechanism of action. This compound is slightly less effective than CH-223191 (see CH-223191) in blocking the AHR-CYP1A1 activation in the liver by TCDD [66].
CB7993113 prevented bone marrow cell ablation (Table 1), in 7,12-dimetilbenz[a]antraceno (DMBA)-induced myelosuppression in mice [66].
In pharmacokinetic experiments in mice, CB79993113 was administered intraperitoneally or orally (single dose of 50 mg/kg), and serum was collected 4, 8, and 16 h after treatment. Pharmacokinetic analyses revealed that this antagonist was readily absorbed 1 h after both oral and intraperitoneal administration. However, serum concentrations were twice higher 1 h after intraperitoneal than oral administration, revealing a high oral first-pass effect. The compound presented a half-life of 4 h [66].
CH-223191
CH-223191 (1-methyl-N-[2-methyl-4-[2-(2-methylphenyl)diazenyl]phenyl-1H-pyrazole-5-carboxamide) is the most well-known AHR antagonist [33], and its effects have been widely investigated in diverse models of disease (Table 1), including interstitial cystitis [67]. CH-223191 does not stimulate AHR-dependent transcription even at higher doses, thus being described as a pure antagonist. This compound also displays no affinity for the estrogen receptor, as some other AHR antagonists do, and displays no cytotoxic properties [68]. Like CB7993113, this compound prevented DMBA-induced bone marrow cell ablation [66].
CH-223191 preferentially avoids AHR activation by TCDD and other related HAHs than BNF, PAHs, flavonoids, or indirubin. Other CH-223191 derivatives presented similar antagonistic properties at blocking TCDD-induced AHR nuclear translocation and similar affinity to AHR, but no other pharmacological properties for these derivatives were evaluated [69]. To the best of our knowledge, there are no pharmacokinetic studies of CH-223191 in wild-type animals nor in models of disease. Moreover, long-term studies using CH-223191 are also limited, plausibly due to the elevated cost of this drug as referred elsewhere [62].
Gastrointestinal tract diseases
Gastrointestinal toxicity of CH-223191 might be anticipated [70]. In fact, CH-223191 worsened the intestinal fibrosis induced by trinitrobenzene-sulfonic acid (TNBS) [70]. CH-223191 administration promoted an upregulation of the fibrosis markers collagen 1A1 (Col1A1) and 3A1 (Col3A1) and alpha smooth muscle actin (α-SMA), in opposition to the effect of the administration of the AHR agonist FICZ. These results suggest the role of AHR activation as a negative regulator of profibrotic signals in the gut [70].
Accordingly, other studies have shown that CH-223191 might worsen colitis in a murine model. Ulcerative colitis was induced in mice by drinking dextran sulfate sodium (DSS), whereas the AHR agonist baicalein displayed an effective anti-colitis effect. This effect was associated with an anti-inflammatory effect promoted by regulatory T cells (Treg) cell differentiation and Type 17 T helper—Th17/Treg balance. CH-223191 administration avoided the amelioration of symptoms of colitis associated with baicalein and abrogated the baicalein-dependent restoration in the balance between Th17 and Treg cells. Moreover, CH-223191 prevented the anti-inflammatory effect of AHR activation by precluding the decrease of the proinflammatory cytokines tumor necrosis factor ɑ (TNF-ɑ) and interleukins 6 (Il-6) and 17 (Il-17) and the increase of anti-inflammatory cytokines (transforming growth factor—TGF-β and IL-10) [71].
Cardiometabolic diseases
In vivo studies showed CH-223191 pharmacodynamic properties in models of ischemic stroke [72, 73], obesity [62, 63], and arterial hypertension related to obstructive sleep apnea [30], among others (Table 1).
The cardiovascular and neuroprotective effects of CH-223191 were shown in an ischemic stroke model of middle cerebral artery occlusion. Upon this procedure, mice presented increased activation of the AHR-CYP1A1 axis and increased AHR protein in neurons of the damaged area. Intraperitoneal administration of the AHR antagonist CH-223191 (10 mg/kg) decreased infarct size and neurologic damage severity [72]. Similar results were obtained in a more recent study. Administration of CH223191 (intraperitoneal, 10 mg/kg) 1 h before middle cerebral artery occlusion prevented AHR and CYP1A1 overexpression in brain cortex and striatum (mRNA levels). Moreover, neuroinflammation was decreased, namely by suppressing the upregulation of TNF-ɑ, IL-1β, and cyclooxygenase 2 (COX-2) in those tissues. Lipid peroxidation was also reduced in the cortex (thiobarbituric acid-reactive substance assay). Consequently, CH-223191 reduced vasogenic edema, infarct size, and neurological severity [73].
Using a model of obesity induced by a HFD (same study presented in “Cardiometabolic diseases” [62]), CH-223191 reduced body mass gain and fat mass and ameliorated liver steatosis (as assessed by a histologic decrease in fat vesicles). Importantly, and in contrast to α-NF, animals treated with CH-223191 showed no hepatomegaly [62]. Diet-induced obesity was also prevented by the use of the same dose of CH-223191 in female mice [63].
Coelho and his team (2020) established a mechanistic link between AHR and systemic hypertension induced by chronic intermittent hypoxia. Chronic intermittent hypoxia is a pivotal clinical feature present in obstructive sleep apnea, being responsible for most of its comorbidities, namely arterial hypertension that is often resistant among these patients. Using a moderate paradigm of chronic intermittent hypoxia mimicking mild obstructive sleep apnea, the authors found an increased activation of AHR-CYP1A1 axis (increased Ahr and Cyp1a1 mRNA), particularly in kidney when hypertension was already established. Moreover, AHR pharmacological blockade by CH-223191 (5 mg/kg) was able not only to prevent, but specially to revert fully established hypertension [30].
Lung diseases
This AHR antagonist has been studied in pulmonary diseases as well. Oral administration of CH-223191 (8 mg/kg) to Wistar rats reversed pulmonary hypertension induced by the combination of a vascular endothelial growth factor receptor antagonist and AHR agonist (Sugen 5416) and chronic sustained hypoxia, without effects in systemic blood pressure and heart rate [74]. CH-223191 totally reverted the increase in ARNT levels in diseased lungs and partly reverted AHR and CYP1A1 pulmonary overexpression [74], pointing to AHR inhibition as a potential pulmonary hypertension treatment (Table 1).
It is widely known that cigarette smoking contains AHR ligands [75], making its link plausible with exacerbated inflammatory responses associated with smoking. However, AHR activation seems to attenuate smoke-induced pulmonary inflammatory responses, in particular neutrophilia. When mice were exposed to cigarette smoke, the group of animals receiving CH-223191 intraperitoneally (50 μg) contained an increased number of total cells and particularly neutrophils in the bronchoalveolar fluid, compared to the vehicle (DMSO) group. This pattern was also observed when exposing AHR−/− mice to cigarette smoke, thus highlighting that AHR activation by smoking is important to limit smoke-induced neutrophilia. Nevertheless, most of the cytokines produced by neutrophils, macrophages, and lymphocytes were increased upon exposure to the cigarette smoking, although no significant differences between the group treated with CH-223191 and the vehicle group were observed, meaning an independent effect of AHR activation occurring in the increased cytokine production of the bronchoalveolar fluid [76].
Immune-mediated diseases
The role of the AHR in inflammatory and autoimmune diseases, such as rheumatological diseases, has been increasingly studied, in particular using CH-223191 as a proof-of-concept antagonist (Table 1). Undesirable effects of CH-223191 in the bone tissue might be anticipated. In a collagen-induced arthritis model in rats, the AHR agonists tetrandrine and 3,3′-diindolylmetheane (DIM) inhibited osteoclastogenesis and bone destruction. AHR blocking by CH-223191 (oral, 5 mg/kg) almost abolished the protective effect of these AHR ligands, thus emphasizing the need of more studies to better characterize the pharmacology of AHR antagonists in bone [77].
Systemic sclerosis is an autoimmune disease characterized by the presence of fibrosis in several organs for which there is no effective treatment. AHR signaling has been implicated in fibrotic processes in multiple tissues, such as liver, dermis, and vascular structures, although the exact extent of this implication is yet unclear [78, 79]. In a murine model of skin sclerosis induced with bleomycin, the administration of the AHR agonist FICZ reduced collagen induction (ɑ-SMA protein) and dermal thickness. These protective effects were inhibited by CH-223191 subcutaneous administration (10 μg), hence showing that AHR signaling controls scleroderma fibrosis [80].
Another group employed the indole DIM, a dietary ligand of AHR, and CH-223191 to assess immunomodulatory functions of the AHR in a murine model of experimental autoimmune encephalomyelitis (EAE). DIM improved the clinical scores of EAE and suppressed the production of proinflammatory cytokines, whereas CH-223191 (intravenous) abolished that effect, aggravating the clinical score when compared to DIM-treated animals, and promoting the production of TNF-ɑ, IL-6, IL-1β, IL-17A and IFN-γ cytokines, while decreasing IL-10 and TGF-β. Animals treated with CH-223191 also showed a great proportion of Th17 and Type 1 T helper (Th1) cells, suggesting an immunomodulatory effect AHR-dependent on regulatory T cells [81].
The modulatory effect of AHR over T cells, namely CD4 + T cells, may rely on redox homeostasis. Mice were treated with FICZ, buthionine sulfoximine (BSO), an inhibitor of the synthesis of the antioxidant glutathione, N-acetylcysteine, an antioxidant, or CH-223191. The expression of redox-related genes was assessed in splenocytes. FICZ and BSO increased the mRNA expression of several redox-related genes, such as heme oxygenase 1 (HO-1), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM), whereas CH-223191 + FICZ co-treatment or NAC prevented those overexpressions. The AHR agonist FICZ increased the total CD4 + cells count and Th1 cells, while a dose-dependent decrease in Treg cells was determined. Nevertheless, the simultaneous administration of FICZ + CH-223191 + NAC was responsible for enhancing Treg production, together with Th1 and Th17 cells with a high dose, and for decreasing Treg and increasing Th2 and Th17 cells in a lower dose. Replacing NAC for BSO in the latter scenario increased Th1 and Th17 cells. Such results led to the conclusion that AHR activation interferes with the fate of T cells, influenced by redox alterations [82].
Hormonal disorders
A recent work by a Japanese group aimed to study how the relationship between endoplasmic reticulum stress in granulosa cells from the ovary and AHR could contribute to polycystic ovary syndrome (PCOS). Using a murine model of PCOS induced by dehydroepiandrosterone (DHEA), they observed no cyclicity in the estrous cycle and alteration in ovarian morphology. CH-223191 (10 mg/kg) subcutaneous administration restored the loss of cyclicity and ovarian morphology with a decrease in atretic antral follicles, which occurred simultaneously with a downregulation in the AHR-CYP1B1 axis in the granulosa cells [83].
Brain injury
Apart from the aforementioned applications of CH-223191 in the context of ischemic stroke, this antagonist has also been used to investigate mechanisms of brain diseases and neurotoxicity. To assess how 3,4-methylenedioxymethamphetamine (MDMA), a psychostimulant drug used for recreational purposes, could interfere with Kyn and AHR activity and hence impact on serotonergic neurotoxicity, a group of investigators treated rats with MDMA and CH-223191. MDMA was able to increase Kyn levels and AHR activity, in the short term, in the hippocampus. CH-223191 (10 mg/kg) intraperitoneal treatment potentiated the MDMA-induced serotonergic neurotoxicity as shown by a lower density of serotonin transporter in the co-treated group (MDMA + CH-223191), while DIM (AHR agonist) treatment showed an opposite scenario of partial prevention on the MDMA-associated serotonergic damage. Therefore, it appears that AHR and Kyn may play a role dampening MDMA-induced neurotoxicity [84].
Infectious diseases
The multiplicity of actions attributed to CH-223191 treatment involves microbial diseases as well. Host defenses against fungal infections like paracoccidioidomycosis (PCM) rely on the immunoprotective Th1 and Th17 cells, while Th2/Th9 cell predominance is implicated in infection’s progression [85]. Among several players implicated in this immune network, AHR is gaining prominent attention, namely due to its influence on Th17 and Treg cells [86]. In fact, in a murine model of PCM induced by intratracheal infection of the fungus Paracoccidioides brasiliensis, administration of AHR agonists, either FICZ or Kyn, decreased pulmonary and hepatic fungal loads and the number of activated lung myeloid cells (CD11c +). However, CH-223191 treatment showed the opposite scenario, increasing fungal load (only in the lungs) and the number of the pulmonary CD11c + cells, both at short and long term after infection (96 h and 2 weeks). In addition, CH-223191 led to a reduced number of CD11c + cells expressing intracellular cytokines (IL-12, IL-1β, IL-6, and TGF-β) and also indoleamine 2, 3-dioxygenase 1 (IDO-1) and AHR, together with an increase in cells expressing TNF-α. The number of CD11c + cells expressing membrane (IAb, CD80, CD86) markers was also increased. CH-223191 also augmented the migration of myeloid dendritic cells to the lungs and promoted the expansion of Th17 lymphocytes associated with concomitant reduction of Treg cells, Th1, and Th22 [87, 88]. The results strongly suggest that fungal infections can be modulated by AHR signaling, unveiling new perspectives to treat these infections.
The implications of CH-223191 and AHR pathway are also seen in viral diseases. A study concerning Zika virus classified AHR as a host-enabling replication factor for the virus. Indeed, AHR signaling is activated upon Zika virus infection, via Kyn production. SJL pregnant mice were infected with Zika, whose consequences mimic several features of congenital Zika, especially microcephaly and cortical brain lesions. CH-223191 (5 mg/kg) was administered, via intraperitoneal, in a nanoliposomal formulation, enabling an increase in its solubility and biodistribution. The antagonist suppressed AHR signaling, decreasing CYP1B1 expression. CH-223191 ameliorated fetal intrauterine growth restriction, microcephaly, and reduced fetal brain pathology (thicker cortical plates and reduced ventricle sizes) while reducing brain and splenic viral load. Sustaining these structural observations, molecular analysis using RNA sequencing and polymerase chain reaction (PCR) analysis observed a decrease in genes associated with apoptosis, tissue damage, and autophagy upon CH-223191 treatment, simultaneously with an upregulation of inflammatory pathways like NF-kB and IFN-1, suggesting that AHR blocking in the context of Zika virus enhances immune and inflammatory mechanisms to control viral replication and pathogenesis and unraveling a potential antiviral approach [89].
The effectiveness of the antiviral effect of CH-223191 has also been investigated in COVID-19 (coronavirus disease 2019), the pandemic disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). AHR was activated by either IFN-β or IFN-γ, in an IDO-Kyn fashion, in the presence of SARS-CoV-2 infection. Consequently, mucins were upregulated in alveolar pulmonary epithelial cells, promoting a pro-hypoxic state, thus aggravating COVID-19-associated respiratory disease. The intratracheal administration of CH-223191 ameliorated the IFN-induced impairment of respiratory function and prevented the expression of mucins in the lungs of IFN-treated mice. Furthermore, upon infection of a transgenic murine line (human angiotensin-converting enzyme 2—hACE2 transgenic) with SARS-CoV-2, CH-223191 intravenous treatment reduced the expression of several types of mucins and the disease severity in the lungs, highlighting the potential of CH-223191 as an effective strategy against COVID-19 [90].
GNF-351
GNF-351 (N-(2-(3H-Indol-3-yl)ethyl)-9-isopropyl-2-(5-methyl-3-pyridyl)-7H-purin-6-amine) is an AHR antagonist without partial agonist activity that has been used to characterize AHR signaling and AHR tissue specificities in vitro [91]. While no studies addressing its pharmacological actions in in vivo models of disease could be found, we herein included an in vivo study dedicated to GNF-351 pharmacokinetics [92]. Upon oral administration in mice (5 mg/kg of body weight), GNF-351 was detected in serum at several time points up to 6 h after drug administration. At 24 h post-administration, the compound was found in feces, but not in urine. Reduced intestinal absorption and extensive intestinal biotransformation of GNF-351 were supported by several phase I metabolites of GNF-351 exclusively detected in feces. Accordingly, this drug prevented the AHR-agonist BNF-induced mRNA expression of Cyp1a1 at the ileum and colon, but not at the liver. These results emphasize the relevance of in vivo studies to better understand absorption, tissue distribution, and impact of AHR-CYP1A1 activation in different tissues. Likewise, this study suggests that GNF-351 might be a useful inhibitor of the AHR signaling in the distal intestinal tract [92] with a minor systemic impact. However, GNF-351 effects remain to be investigated in a disease model.
Resveratrol
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) is a naturally occurring polyphenol, commonly found in the skin of grapes, with antioxidant activity and free-radical scavenging properties (reviewed by [93]). Many mechanisms of action independent of AHR-CYP1A1 axis [94, 95] have been reported for resveratrol and several review papers were dedicated to the broad applications of resveratrol in pre-clinical and clinical contexts. For the sake of the scope of this review, we considered those studies that linked the pharmacological effects of resveratrol with the AHR canonical pathway. Although resveratrol inhibits the adaptive pathway of AHR, it also allows its nuclear localization and the binding to alternative xenobiotic response elements. The activation of this alternative pathway of AHR by resveratrol upregulates another set of genes associated with anti-inflammatory and antioxidant properties [37] (Fig. 1). In addition, resveratrol has also been described as a weak CYP1A1 inhibitor [96].
Resveratrol has been reported to protect from toxic effects on lung [97], thymus [98], testis [99–102], prostate [103], and pancreas [104, 105] related to environmental contaminants that are AHR activators. Resveratrol effect in drug iatrogenic effects [106], female related diseases [107, 108], kidney [109], liver [110], and bone disease [111, 112] was also evaluated (Table 1).
Regarding the pharmacokinetic properties (reviewed by Wenzel and Somoza [113]), resveratrol undergoes rapid first-pass metabolism, and it is mainly metabolized to its glucuronide and sulfate metabolites [114].
Cardiometabolic diseases
Polychlorinated biphenyls (PCBs) are persistent organic pollutants that have been associated with the development of type 2 diabetes. In vivo studies have demonstrated that PCBs accumulate in adipose tissue leading to its inflammation and impaired glucose homeostasis [115]. Resveratrol supplementation in the diet was able to prevent PCB-77-induced impairment of glucose and insulin tolerance in adipose tissue of mice. Additionally, resveratrol increased the mRNA expression of NAD(P)H quinone dehydrogenase 1 (NQO1) and restored insulin-stimulated levels of phosphorylated protein kinase B (Akt) in adipose tissue [116].
Female pregnant Sprague–Dawley rats were given the AHR activators dexamethasone (from gestational day 16 to 22) and/or TCDD (on gestational day 14 and 21 and postnatal day 7 and 14). When resveratrol was administered during pregnancy and lactation periods, the adult male offspring had a reduction in systolic blood pressure (SBP) of 20 mmHg at 16 weeks of age, compared with the TCDD and dexamethasone maternal exposure groups. This effect was accompanied by less oxidative damage in the kidney and a decrease in renal AHRR expression. In addition, a blockade of renal renin-angiotensin system (RAS) was observed, including a decrease in expression of renin (Ren), angiotensin-converting enzyme (Ace), and angiotensin II receptor type 1a (Agtr1a) as well as increased nitric oxide (NO) bioavailability [117].
Other studies have been investigating the beneficial effects of resveratrol in models of obesity [118] and arterial hypertension programmed by maternal exposure [119]. Hsu and his team investigated the ability of resveratrol to prevent arterial hypertension programmed by maternal exposure to bisphenol A (BPA) with or without high-fat high-sucrose diet during the entire period of pregnancy. Resveratrol reduced SBP (10 mmHg) in the groups that also received BPA with and without high-fat high-sucrose diet. Immunohistochemistry staining 8-hydroxydeoxyguanosine in the kidney, used as an index of oxidative stress-derived DNA damage, showed that resveratrol therapy prevented the synergistic effect of high-fat high-sucrose diet and BPA exposure on oxidative stress damage. This compound also restored nitric oxide (NO) bioavailability through the increase of plasmatic L-arginine levels and protein levels of endothelial NO synthase (eNOS) and neuronal NO synthase (nNOS). Additionally, resveratrol decreased renal mRNA expression of Ahrr, Cyp1a1, and Arnt in the group receiving high-fat high-sucrose diet plus BPA [119].
6,2′,4′-trimethoxyflavone (TMF)
TMF (6,2′,4′-trimethoxyflavone) has been described as a pure and selective AHR antagonist that effectively competes with TCDD [120]. Although there are several in vitro studies about the pharmacodynamic properties of TMF, its use in vivo is much less frequent (Table 1).
Cardiometabolic and cardiovascular diseases
TMF was used in a mice model of stroke induced by middle cerebral artery occlusion (same model described with CH-223191 [72]). TMF shared with CH-223191 neuroprotective properties but allowed a more pronounced reduction in infarct size and neurological severity in comparison with CH-223191. More recently, Chen and collaborators (2019), using the same mice model of ischemic stroke, showed that AHR inactivation, through either TMF or conditional AHR knockout, reduced brain infarction. Similar effects were observed between TMF or AHR knockout: a decrease in astrogliosis and increase in neural progenitor cells. TMF reduced brain inflammatory markers after stroke (IL-1β, IL-6 and IFN-γ) and ameliorated animal’s sensorimotor deficits and nonspatial working memory [121]. Later, Kwon and collaborators (2020) investigated the neuroprotective effects of TMF on cerebral ischemia–reperfusion injury. TMF was administered 10 or 50 min after ischemia but before reperfusion in a rat model of stroke induced by transient middle cerebral artery occlusion and reperfusion. At 24 h after ischemia, neuronal lesion was lower in the ischemic core and peri-infarct region (higher relative apparent diffusion coefficient and lower relative T2 values measured with magnetic resonance imaging) in the group that received TMF 10 min after ischemia. TMF administered 10 and 50 min after ischemia also reduced total infarct volume (magnetic resonance imaging) and the number of apoptotic cells (TUNEL staining). Immunofluorescence showed that TMF groups had lower AHR activity in the peri-infarct region [122].
Novel AHR antagonists
As the involvement of the AHR in the mechanism of a multiplicity of diseases has been increasingly advocated, the search for novel AHR antagonists has emerged as a hot topic. In fact, other compounds have been recently evaluated for their potential to antagonize the AHR signaling pathway (Table 1).
The flavonoids 5,7,30,40,50-pentahydroxy flavanone and barleriside A were recently evaluated in a CKD model. Both compounds decreased Ahr, Cyp1a1, and Cyp1a2 mRNA levels in the kidney and reduced serum creatinine, urea, and proteinuria besides reducing fibronectin, vimentin, and FSP1 levels and increasing E-cadherin levels in the kidney [123].
HP163 is a monoalkylated amide of CB7993113. The intraperitoneal administration of HP163 (2.5 mg/kg) was evaluated against Zika infection. Similarly, to the observed results with CH-223191, HP1763 reduced viral replication of Zika virus in mice, together with an amelioration in several clinical features associated with this infection, namely a reduction in microcephaly, in ventricular dilation and in cortical thinning [89].
Clofazimine is an antimycobacterial drug that showed anti-AHR activity in vitro (luciferase AHR reporter cell assay) [124]. The drug was tested in mice models of multiple myeloma. After tumor development, clofazimine (10 mg/kg, daily intraperitoneal injections) reduced tumor growth in a similar way as the antineoplastic drug bortezomib (1 mg/kg, biweekly intraperitoneal injections). Moreover, clofazimine decreased CYP1A1 protein levels in the tumor tissue [124].
KYN-101 (IC50 of 22 nM in human HepG2 DRE-luciferase reporter assay) was tested in a melanoma model with physiological levels of IDO and tryptophan 2,3 dioxygenase (TDO) (B16WT) or overexpressing IDO (B16IDO) or TDO (B16TDO) and a colorectal cancer model overexpressing IDO in mice (CT26). KYN-101 (3 or 10 mg/kg) led to tumor growth inhibition in B16IDO, B16TDO, and CT26 models but not in the B16WT model. Interestingly, similar results were obtained with the AHR antagonists CH-223191 (50 mg/mg) and clofazimine (IP, 10 mg/kg) [125].
Pharmacological effects of CYP1A1 antagonists
Alizarin
The anthraquinone derivative alizarin (1,2-dihydroxyanthraquinone) is a food pigment described as a strong competitive inhibitor of CYP1A1 and CYP1A2 in vitro [126]. Alizarin also inhibits CYP1B1 and to a lesser extent CYP2A6 and CYP2E1 in vitro [126]. The antioxidant activity of alizarin was tested in a mice model of hepatotoxicity induced by bromobenzene. Pre-treatment with alizarin reduced the hepatic lipid peroxidation (thiobarbituric acid reactive substances (TBARS) assay) and serum aspartate aminotransferase (AST) levels [127]. CYP1A1 was not investigated.
Takahashi and collaborators (2007) evaluated the preventive effect of alizarin on MeIQx (amine 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline)-induced DNA adducts. C57BL/6 N male mice (5-weeks-old) were administered with the anthraquinone for 3 days followed by MeIQx alone or in combination with alizarin for 3 days. Pre-treatment with alizarin showed only a marginal reduction in the amount of adducts present in the lung and kidney. Moreover, the EROD assay (CYP1A activity) remained unchanged [128].
Cardiometabolic and cardiovascular diseases
Although the association with CYP1A1 was not investigated, Xu and co-workers (2019) showed that alizarin significantly decreased the levels of blood glucose, ameliorated lipid metabolism abnormalities, and decreased oxidative stress in a diabetic mice model upon administration of alizarin to male Kunming mice for 10 days [129].
Ellipticine
Ellipticine (5,11-dimethyl-6H-pyrido[4,3-b] carbazole) is a polyaromatic alkaloid that inhibits DNA topoisomerase II and forms covalent adducts with DNA. Ellipticine has been associated with anti-tumoral activity in vitro [130]. Ellipticine is metabolized mainly by CYP1A1/2 and CYP3A4 in vitro [131]. Although in vitro studies classify ellipticine as an inhibitor of CYP1A1, CYP2B, and CYP3A [132], in vivo evidence shifts to different conclusions. In a dose–response study, where male and female Wistar rats were treated with a single dose of 4, 40, or 80 mg/kg of ellipticine, Aimová and collaborators (2007) found sex differences in CYP1A1 content in the liver upon ellipticine administration, with males being more responsive to this compound. The lowest dose of ellipticine (4 mg/kg) originated a 26-fold increase in CYP1A1 induction in males, differently to the moderate fivefold induction observed in females. Also, while in males, CYP1A1 induction correlated positively with ellipticine dose, in females, the maximum induction of CYP1A1 was attained with the intermediate dose (40 mg/kg). CYP1A1 protein and mRNA levels and activity were assessed in the lung, liver, and kidney of male rats, showing a clear induction of CYP1A1 in these organs. The middle dose induced the highest increase of mRNA levels in the lung (24-fold), followed by the kidney (tenfold) and the liver (fivefold) [133]. The effects of ellipticine on CYP1A1 activity were also evaluated in a time-response study. Animals were given a single dose of 80 mg/kg of ellipticine and sacrificed at four time points (2–224 days). CYP1A1 protein levels achieved their peak at day 2, returned to basal levels at day 14, and remained low until the end of follow-up [133].
More studies are needed to evaluate the effects of single and multiple dose administration of ellipticine on CYP1A1 in vivo. It also remains to be understood the mechanism by which ellipticine might induce CYP1A1 despite being described as a CYP1A1 inhibitor in vitro.
Cancer
Ellipticine reduced the tumor size and demonstrated its activity against cell proliferation in a model of non-small cell lung cancer [134] (Table 2).
Infection
Ellipticine effectively prevented inflammation in the endotoxic shock mouse model: there was a time-dependent reduction in the levels of TNF-α and IL-6 [135] (Table 2).
Pterostilbene
Pterostilbene is a cell-permeable stilbenoid, analog of resveratrol, originally derived from Pterocarpus marsupium. This compound has antioxidant, anti-proliferative, anti-inflammatory, and hypoglycemic effects and is a very potent inhibitor of CYP1A1 in vitro [136]. Pterostilbene also inhibits CYP2C8 and UGT1A6 enzyme activities in vitro [137].
Inflammation
Pterostilbene prevented edema and inflammatory markers in a carrageenan-induced inflammation mice model [138].
Purpurin
Like alizarin, purpurin is an anthraquinone derivative and a food supplement inhibitor of CYP1A1 and CYP1A2 in vitro [126]. Purpurin also inhibits CYP1B1 and to a lesser extent CYP2A6 and CYP2E1 in vitro [126].
Using a mice model of hepatotoxicity induced by bromobenzene (see Alizarin and Table 2), purpurin showed a time-dependent decrease in the activity of CYP1A1 and the formation of MelQx-DNA adducts at the lungs, kidney, and marginally at the liver. Purpurin displayed a stronger inhibitory capacity for CYP1A1 than alizarin [128].
Cardiometabolic and cardiovascular diseases
Although CYP1A1 activity was not evaluated, an anti-obesity effect has been described for this compound: a dose-dependent reduction in weight gain [139].
Rhapontigenin
Rhapontigenin (3, 3′, 5-trihydroxy-4′-methoxy-stilbene) is a hydroxystilbene derivative, with similar structure to resveratrol, derived from the roots of Rheum undulatum [140]. This compound proved to be a mechanistic based inhibitor of the human CYP1A1 in vitro [141]. Rhapontigenin also inhibits CYP3A4 and CYP2C9 [142]. Pharmacokinetic investigation in rats showed that rhapontigenin has a half-life of 3 h and is extensively glucuronidated and predominantly cleared by the liver [143, 144].
Cardiometabolic and cardiovascular diseases
While no relation with AHR signaling was investigated, rhapontigenin was associated with cardioprotective effects in a model of isoproterenol-induced myocardial infarction in male Sprague–Dawley rats [145]. Rhapontigenin pretreatment ameliorated infarct size and heart weight and reduced protein expression of cardiac markers such as creatine kinase (CK), cardiac troponin-T (CTT), and lactate dehydrogenase (LD). It also reduced protein expression of superoxide dismutase (SOD) and malonaldehyde (MD), IL-6, p38, inducible NO synthase (iNOS), and TNF-α [145].
Rutaecarpine
Rutaecarpine is a quinazolinocarboline alkaloid extracted from the fruit Evodia rutaecarpa and commonly found in herbal products [146]. Rutaecarpine is an example of a compound whose impact on CYP1A1 activation has been described with conflicting results in different studies. In vitro studies described the compound as a selective CYP1A1 inhibitor [147]. However, most of the in vivo evidence shows that rutaecarpine activates the AHR-CYP1A1 axis [148–151]. For instance, rutaecarpine increased EROD activity in the mice liver (sixfold) and hepatic CYP1A1 (western blot). This effect was not observed in the kidney, denoting organ differences in rutaecarpine modulation of CYP1A1 [148].
Cardiometabolic and cardiovascular diseases
The effects of rutaecarpine in vitro and in vivo that support its putative cardiovascular protective effect are reviewed elsewhere [152]. Rutaecarpine was investigated in in vivo models of myocardial ischemia–reperfusion injury [153], hypoxia-induced right ventricular remodeling [154], arterial hypertension [155–158], atherosclerosis [159], arterial remodeling [160], obesity [161], and renal ischemia–reperfusion injury [162]. Despite the beneficial cardiovascular effects observed, none of these works linked rutaecarpine action to the AHR-CYP1A1 axis (Table 2).
Overview of in vivo models’ data
This review highlights the paucity of in vivo data on the effects of AHR antagonists and CYP1A1 inhibitors in rodent models of disease, namely the compound´s pharmacodynamics and tissue specificities for the mechanism of action; dose–effect dependence, AHR target genes, long term, and toxicity use.
The experimental design of these studies relied mostly on prevention protocols. Most studies administered the compound orally, but pharmacokinetic properties and dose–response relationship were barely evaluated. The age of animals varied from infant to young adult ages (3 to 12 weeks for mice and 6 to 16 weeks for rats), and there was no study in elderly. Sex differences were also underrepresented, as only 40% of the studies with the AHR antagonists and 10% of studies with CYP1A1 inhibitors were performed in female animals. The mean time of drug administration was 3.6 ± 0.73 and 3.2 ± 0.44 weeks for mice and rats, respectively. The longer period of administration was investigated for α-NF, an AHR antagonist, that was used for 26 weeks in a mice model of obesity (Tables 1 and 2).
Clinical trials of AHR antagonists
Cancer
AHR is a prognostic marker for aggressive cancer progression. High AHR levels have been found in many solid cancer types, including glioblastoma, ovarian cancer, and lung cancer. AHR might also have a direct effect on cancer at various stages: cell proliferation, tissue invasion, angiogenesis, inflammation, and metastasis. Blocking AHR is a promising approach to cancer immunotherapy (reviewed in [163–165]).
BAY2416964
BAY2416964 represents an orally active antagonist of AHR with IC50 of 341 nM. An ongoing clinical trial with this small molecule is being conducted in patients with advanced cancer. By blocking AHR, it is expected that the oral administration of this small molecule will activate immune response against the tumor cells. This open-label, phase 1, first-in-human, dose-escalation, and dose-expansion study will evaluate the safety, tolerability, maximum tolerated or administered dose, pharmacokinetics, pharmacodynamics, and tumor response profile in patients with non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), and colorectal cancer microsatellite stable (MSS). The compound was extracted from patent WO2018146010A1, and the study started in August 2019 and is at the recruiting phase [166].
IK-175
A phase 1, open-label, dose-escalation, and dose-expansion study of IK-175 is being conducted in patients with locally advanced or metastatic solid tumors and urothelial carcinoma.
This oral antagonist of AHR will be investigated in adult subjects diagnosed with any form of an advanced or metastatic solid tumor especially in patients who do not fully benefit from standard-of-care, including the checkpoint inhibitors.
Safety and tolerability of IK-175, to determine the recommended phase 2 dose, will be assessed in addition to pharmacokinetics, pharmacodynamics, and biomarkers of response [167].
Duchenne muscular dystrophy
Ezutromid
Ezutromid is a clinical stage compound for Duchenne muscular dystrophy patients. This compound was developed aiming at an increased expression of utrophin to mimic the missing dystrophin in this condition [168]. Ezutromid is an orally administered antagonist of AHR [169]. The drug undergoes extensive first-pass metabolism leading to low oral bioavailability. Drug absorption increased with lipid-rich diet when compared with fasted conditions and the absorption profile manifested secondary peaks in some patients. Ezutromid followed a biphasic elimination [170]. Ezutromid was discontinued in 2018 after failing to show any benefit as a modifying disease drug in phase II trial [171].
Conclusions
Over the past few years, a multiplicity of important functions of AHR has been found, surpassing its original role as a xenobiotic sensor and regulator of xenobiotic detoxification. In fact, AHR has been confirmed as an important signaling molecule regulating and maintaining homeostasis in different cells, tissues, and organs. Novel data highlights AHR-CYP1A1 axis activation in the mechanisms of disease, justifying the putative value of its therapeutic blockade. Thus, it is timely to investigate and better characterize the pharmacological properties of blockers of the AHR-CYP1A1 axis. AHR-CYP1A1 blockers might be useful in the treatment of chronic diseases including diabetes, hypertension, skeletal muscle disorders, and cancer, diseases known for being poorly controlled with the currently available drugs. For that, compiling evidence of pre-clinical pilot studies performed so far with AHR-CYP1A1 blockers is needed. Notably, many drugs identified in vitro demonstrated to be auto-inducers of its own metabolism in vivo, and this effect was tissue/organ specific. Most of the tested compounds ameliorated the disease although some potential adverse reactions might be also anticipated. Summing up, we believe the information herein compiled will be helpful to guide researchers when planning experiments with AHR-CYP1A1 blockers. Finally, and in view of the ongoing clinical studies using three AHR antagonists, we reinforce a call for further evidence on the pharmacological properties of AHR-CYP1A1 blockers.
Author’s contribution
EM and SAP had the idea for this review; NC, AP, MJC, TR, JM, and SAP contributed to the literature search and critical appraisal of the studies; all authors contributed to the writing and review of the manuscript.
Funding
This work was supported by the Fundação para Ciência e Tecnologia [PTDC/MED-TOX/30418/2017] and iNOVA4Health [UID/Multi/04462/2013]. NRC, MJC, and JM were supported by FCT [PhD grant PD/BD/114257/2016, PhD grant SFRH/BD/131331/2017 and Post-doctoral contract PTDC/MED-TOX/30418/2017, respectively].
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
NR Coelho and AB Pimpão have the same contribution. J Morello and SA Pereira have the same contribution.
References
- 1.Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta - Gen Subj. 2003;1619:263–268. doi: 10.1016/S0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 2.Nebert DW. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog Lipid Res. 2017;67:38–57. doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denison MS, Soshilov AA, He G, Degroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor, toxicol. Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larigot L, Juricek L, Dairou J, Coumoul X. AhR signaling pathways and regulatory functions. Biochim Open. 2018;7:1–9. doi: 10.1016/j.biopen.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degroot D, He G, Fraccalvieri D, Bonati L, Pandini AA, Denison MS (2011) AHR Ligands: promiscuity in Binding and Diversity in Response, in: AH Recept. Biol. Toxicol., John Wiley and Sons, Hoboken, NJ, USA, pp. 63–79. 10.1002/9781118140574.ch4
- 6.Yi T, Wang J, Zhu K, Tang Y, Huang S, Shui X, Ding Y, Chen C, Lei W (2018) Aryl Hydrocarbon receptor: a new player of pathogenesis and therapy in cardiovascular diseases. Biomed Res Int 2018. 10.1155/2018/6058784 [DOI] [PMC free article] [PubMed]
- 7.Correia MJ, Pimpão AB, Lopes-Coelho F, Sequeira CO, Coelho NR, Gonçalves-Dias C, Barouki R, Coumoul X, Serpa J, Morello J, Monteiro EC, Pereira SA (2021) Aryl hydrocarbon receptor and cysteine redox dynamics underlie (mal)adaptive mechanisms to chronic intermittent hypoxia in kidney cortex, antioxidants. 10:1484. 10.3390/ANTIOX10091484 [DOI] [PMC free article] [PubMed]
- 8.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: endogenous and dietary routes to ah receptor activation. Drug Metab Dispos. 2015;43:1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barouki R, Aggerbeck M, Aggerbeck L, Coumoul X. The aryl hydrocarbon receptor system. Drug Metabol Drug Interact. 2012;27:3–8. doi: 10.1515/dmdi-2011-0035. [DOI] [PubMed] [Google Scholar]
- 11.Guyot E, Chevallier A, Barouki R, Coumoul X. The AhR twist: ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discov Today. 2013;18:479–486. doi: 10.1016/j.drudis.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR. Induction of Cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol Pharmacol. 2007;71:1475–1486. doi: 10.1124/mol.106.032748. [DOI] [PubMed] [Google Scholar]
- 13.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13:20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson SR, Joshi AD, Elferink CJ. The tumor suppressor Kruppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. J Pharmacol Exp Ther. 2013;345:419–429. doi: 10.1124/jpet.113.203786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oesch-Bartlomowicz B, Huelster A, Wiss O, Antoniou-Lipfert P, Dietrich C, Arand M, Weiss C, Bockamp E, Oesch F (2005) Aryl hydrocarbon receptor activation by cAMP vs. dioxin: divergent signaling pathways. Proc Natl Acad Sci USA 102:9218–9223. 10.1073/pnas.0503488102 [DOI] [PMC free article] [PubMed]
- 16.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc Natl Acad Sci U S A. 2007;104:1412–1417. doi: 10.1073/pnas.0607296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han Z, Miwa Y, Obikane H, Mitsumata M, Takahashi-Yanaga F, Morimoto S, Sasaguri T. Aryl hydrocarbon receptor mediates laminar fluid shear stress-induced CYP1A1 activation and cell cycle arrest in vascular endothelial cells. Cardiovasc Res. 2008;77:809–818. doi: 10.1093/cvr/cvm095. [DOI] [PubMed] [Google Scholar]
- 18.Xiao W, Son J, Vorrink SU, Domann FE, Goswami PC. Ligand-independent activation of aryl hydrocarbon receptor signaling in PCB3-quinone treated HaCaT human keratinocytes. Toxicol Lett. 2015;233:258–266. doi: 10.1016/j.toxlet.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17β-estradiol and estrone formed by 15 selectively expressed human cytochrome P450 isoforms. Endocrinology. 2003;144:3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz D, Kisselev P, Ericksen SS, Szklarz GD, Chernogolov A, Honeck H, Schunck WH, Roots I. Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem Pharmacol. 2004;67:1445–1457. doi: 10.1016/j.bcp.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes P450. Drug Metab Dispos. 2005;33:489–494. doi: 10.1124/dmd.104.002410. [DOI] [PubMed] [Google Scholar]
- 22.Favreau LV, Pickett CB (1991) Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem 266:4556–4561. http://www.jbc.org/ (accessed June 15, 2020) [PubMed]
- 23.Watson AJ, Hankinson O. Dioxin-and Ah Receptor-dependent protein binding to xenobiotic responsive elements and G-rich DNA studied by in vivo footprinting. J Biol Chem. 1992;266:6874–6878. doi: 10.1016/S0021-9258(19)50509-9. [DOI] [PubMed] [Google Scholar]
- 24.Baba T, Mimura J, Gradin K, Kuroiwa A, Watanabe T, Matsuda Y, Inazawa J, Sogawa K, Fujii-Kuriyama Y. Structure and expression of the ah receptor repressor gene. J Biol Chem. 2001;276:33101–33110. doi: 10.1074/jbc.M011497200. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Hawkins BT, Miller DS. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J. 2011;25:644. doi: 10.1096/FJ.10-169227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davarinos NA, Pollenz RS. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. J Biol Chem. 1999;274:28708–28715. doi: 10.1074/jbc.274.40.28708. [DOI] [PubMed] [Google Scholar]
- 27.Akahoshi E, Yoshimura S, Uruno S, Ishihara-Sugano M. Effect of dioxins on regulation of tyrosine hydroxylase gene expression by aryl hydrocarbon receptor: a neurotoxicology study. Environ Heal A Glob Access Sci Source. 2009;8:24. doi: 10.1186/1476-069X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, Laine J, Sakai T, Korsmeyer SJ, Casper RF, Sherr DH, Tilly JL. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 29.Boutros PC, Moffat ID, Franc MA, Tijet N, Tuomisto J, Pohjanvirta R, Okey AB. Dioxin-responsive AHRE-II gene battery: identification by phylogenetic footprinting. Biochem Biophys Res Commun. 2004;321:707–715. doi: 10.1016/j.bbrc.2004.06.177. [DOI] [PubMed] [Google Scholar]
- 30.Coelho NR, Tomkiewicz C, Correia MJ, Gonçalves-Dias C, Barouki R, Pereira SA, Coumoul X, Monteiro EC. First evidence of aryl hydrocarbon receptor as a druggable target in hypertension induced by chronic intermittent hypoxia. Pharmacol Res. 2020;159:104869. doi: 10.1016/j.phrs.2020.104869. [DOI] [PubMed] [Google Scholar]
- 31.Coelho NR, Matos C, Pimpão AB, Correia MJ, Sequeira CO, Morello J, Pereira SA, Monteiro EC (2021) AHR canonical pathway: in vivo findings to support novel antihypertensive strategies. Pharmacol Res 105407. 10.1016/j.phrs.2020.105407 [DOI] [PubMed]
- 32.Bersten DC, Sullivan AE, Peet DJ, Whitelaw ML. BHLH-PAS proteins in cancer. Nat Rev Cancer. 2013;13:827–841. doi: 10.1038/nrc3621. [DOI] [PubMed] [Google Scholar]
- 33.Zhao B, Degroot DE, Hayashi A, He G, Denison MS. CH223191 Is a ligand-selective antagonist of the Ah (dioxin) receptor. Toxicol Sci. 2010;117:393–403. doi: 10.1093/toxsci/kfq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nazarenko DA, Dertinger SD, Gasiewicz TA. In vivo antagonism of AhR-mediated gene induction by 3-methoxy-4-nitroflavone in TCDD-responsive lacZ mice. Toxicol Sci. 2001;61:256–264. doi: 10.1093/toxsci/61.2.256. [DOI] [PubMed] [Google Scholar]
- 35.Datta A, Bhasin N, Kim H, Ranjan M, Rider B, Abd Elmageed ZY, Mondal D, Agrawal KC, Abdel-Mageed AB (2015) Selective targeting of FAK-Pyk2 axis by alpha-naphthoflavone abrogates doxorubicin resistance in breast cancer cells. Cancer Lett 362:25–35. 10.1016/j.canlet.2015.03.009 [DOI] [PubMed]
- 36.Timme-Laragy AR, Cockman CJ, Matson CW, Di Giulio RT. Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquat Toxicol. 2007;85:241–250. doi: 10.1016/j.aquatox.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gouédard C, Barouki R, Morel Y. Induction of the paraoxonase-1 gene expression by resveratrol, arterioscler. Thromb Vasc Biol. 2004;24:2378–2383. doi: 10.1161/01.ATV.0000146530.24736.ce. [DOI] [PubMed] [Google Scholar]
- 38.Stejskalova L, Pavek P. The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbon receptor (AhR) in the placenta. Curr Pharm Biotechnol. 2011;12:715–730. doi: 10.2174/138920111795470994. [DOI] [PubMed] [Google Scholar]
- 39.Mescher M, Haarmann-Stemmann T. Modulation of CYP1A1 metabolism: from adverse health effects to chemoprevention and therapeutic options. Pharmacol Ther. 2018;187:71–87. doi: 10.1016/j.pharmthera.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Schlezinger JJ, White RD, Stegeman JJ. Oxidative inactivation of cytochrome P-450 1A (CYP1A) stimulated by 3,3’,4,4’-tetrachlorobiphenyl: production of reactive oxygen by vertebrate CYP1As. Mol Pharmacol. 1999;56:588–597. doi: 10.1124/mol.56.3.588. [DOI] [PubMed] [Google Scholar]
- 41.Beresford AP. CYP1A1: Friend or foe? Drug Metab Rev. 1993;25:503–517. doi: 10.3109/03602539308993984. [DOI] [PubMed] [Google Scholar]
- 42.Eugster HP, Probst M, Würgler FE, Sengstag C (1993) Caffeine, estradiol, and progesterone interact with human CYP1A1 and CYP1A2. Evidence from cDNA-directed expression in Saccharomyces cerevisiae. Drug Metab Dispos 21:43LP–49. http://dmd.aspetjournals.org/content/21/1/43.abstract [PubMed]
- 43.Stiborová M, Martínek V, Rýdlová H, Koblas T, Hodek P. Expression of cytochrome P450 1A1 and its contribution to oxidation of a potential human carcinogen 1-phenylazo-2-naphthol (Sudan I) in human livers. Cancer Lett. 2005;220:145–154. doi: 10.1016/j.canlet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 44.Badal S, Delgoda R. Role of the modulation of CYP1A1 expression and activity in chemoprevention. J Appl Toxicol. 2014;34:743–753. doi: 10.1002/jat.2968. [DOI] [PubMed] [Google Scholar]
- 45.Go RE, Hwang KA, Choi KC. Cytochrome P450 1 family and cancers. J Steroid Biochem Mol Biol. 2015;147:24–30. doi: 10.1016/j.jsbmb.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Ma Q, Lu AYH. CYP1A induction and human risk assessment: An evolving tale of in vitro and in vivo studies. Drug Metab Dispos. 2007;35:1009–1016. doi: 10.1124/dmd.107.015826. [DOI] [PubMed] [Google Scholar]
- 47.Joshi P, McCann GJP, Sonawane VR, Vishwakarma RA, Chaudhuri B, Bharate SB. Identification of potent and selective CYP1A1 inhibitors via combined ligand and structure-based virtual screening and their in vitro validation in sacchrosomes and live human cells. J Chem Inf Model. 2017;57:1309–1320. doi: 10.1021/acs.jcim.7b00095. [DOI] [PubMed] [Google Scholar]
- 48.Marinho AT, Dias CG, Pinheiro PF, Lemos AR, Antunes AMM, Marques MM, Monteiro EC, Miranda JP, Pereira SA. Nevirapine modulation of paraoxonase-1 in the liver: an in vitro three-model approach. Eur J Pharm Sci. 2016;82:147–153. doi: 10.1016/J.EJPS.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Pinheiro PF, Pereira SA, Harjivan SG, Martins IL, Marinho AT, Cipriano M, Jacob CC, Oliveira NG, Castro MF, Marques MM, Antunes AMM, Miranda JP. Hepatocyte spheroids as a competent in vitro system for drug biotransformation studies: nevirapine as a bioactivation case study. Arch Toxicol. 2016;91:1199–1211. doi: 10.1007/s00204-016-1792-x. [DOI] [PubMed] [Google Scholar]
- 50.Lu YF, Santostefano M, Cunningham BDM, Threadgill MD, Safe S. Identification of 3’-methoxy-4’-nitroflavone as a pure aryl hydrocarbon (Ah) receptor antagonist and evidence for more than one form of the nuclear Ah receptor in MCF-7 human breast cancer cells. Arch Biochem Biophys. 1995;316:470–477. doi: 10.1006/abbi.1995.1062. [DOI] [PubMed] [Google Scholar]
- 51.Dertinger SD, Lantum HBM, Silverstone AE, Gasiewicz TA (2000) Effect of 3’-methoxy-4’-nitroflavone on benzo[a]pyrene toxicity. Aryl hydrocarbon receptor-dependent and -independent mechanisms. Biochem Pharmacol 60:189–196. 10.1016/S0006-2952(00)00314-2 [DOI] [PubMed]
- 52.Dertinger SD, Nazarenko DA, Silverstone AE, Gasiewicz TA. Aryl hydrocarbon receptor signaling plays a significant role in mediating benzo[a]pyrene-and cigarette smoke condensate-induced cytogenetic damage in vivo. Carcinogenesis. 2001;22:171–177. doi: 10.1093/carcin/22.1.171. [DOI] [PubMed] [Google Scholar]
- 53.Navid F, Bruhs A, Schuller W, Fritsche E, Krutmann J, Schwarz T, Schwarz A. The aryl hydrocarbon receptor is involved in UVR-Induced immunosuppression. J Invest Dermatol. 2013;133:2763–2770. doi: 10.1038/jid.2013.221. [DOI] [PubMed] [Google Scholar]
- 54.Campbell DR, Kurzer MS. Flavonoid inhibition of aromatase enzyme activity in human preadipocytes. J Steroid Biochem Mol Biol. 1993;46:381–388. doi: 10.1016/0960-0760(93)90228-O. [DOI] [PubMed] [Google Scholar]
- 55.Shimada T, Yamazaki H, Foroozesh M, Hopkins NE, Alworth WL, Guengerich FP. Selectivity of polycyclic inhibitors for human cytochrome P450s 1A1, 1A2, and 1B1. Chem Res Toxicol. 1998;11:1048–1056. doi: 10.1021/tx980090+. [DOI] [PubMed] [Google Scholar]
- 56.Walsh AA, Szklarz GD, Scott EE. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J Biol Chem. 2013;288:12932–12943. doi: 10.1074/jbc.M113.452953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sansen S, Yano JK, Reynald RL, Schoch GA, Griffin KJ, Stout CD, Johnson EF. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J Biol Chem. 2007;282:14348–14355. doi: 10.1074/jbc.M611692200. [DOI] [PubMed] [Google Scholar]
- 58.Wang A, Savas U, Stout CD, Johnson EF. Structural characterization of the complex between α-naphthoflavone and human cytochrome P450 1B1. J Biol Chem. 2011;286:5736–5743. doi: 10.1074/jbc.M110.204420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sineva EV, Rumfeldt JAO, Halpert JR, Davydov DR (2013) A large-scale allosteric transition in cytochrome P450 3A4 revealed by luminescence resonance energy transfer (LRET). PLoS One 8. 10.1371/journal.pone.0083898 [DOI] [PMC free article] [PubMed]
- 60.Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, Hoyer PB. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol Appl Pharmacol. 2005;203:114–123. doi: 10.1016/j.taap.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Jang JY, Shin S, il Choi B, Park D, Jeon JH, Hwang SY, Kim JC, Kim YB, Nahm SS (2007) Antiteratogenic effects of α-naphthoflavone on 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposed mice in utero. Reprod Toxicol 24:303–309. 10.1016/j.reprotox.2007.08.002 [DOI] [PubMed]
- 62.Moyer BJ, Rojas IY, Kerley-Hamilton JS, Hazlett HF, Nemani KV, Trask HW, West RJ, Lupien LE, Collins AJ, Ringelberg CS, Gimi B, Kinlaw WB, Tomlinson CR (2016) Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. Model for AHR activation by kynurenine via oxidized-LDL, TLR2/4, TGFβ, and IDO1. Toxicol Appl Pharmacol 300:13–24. 10.1016/j.taap.2016.03.011 [DOI] [PMC free article] [PubMed]
- 63.Moyer BJ, Rojas IY, Kerley-Hamilton JS, Nemani KV, Trask HW, Ringelberg CS, Gimi B, Demidenko E, Tomlinson CR. Obesity and fatty liver are prevented by inhibition of the aryl hydrocarbon receptor in both female and male mice. Nutr Res. 2017;44:38–50. doi: 10.1016/j.nutres.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia H, Zhu X, Zhang X, Jiang H, Li B, Wang Z, Li D, Jin Y. Alpha-naphthoflavone attenuates non-alcoholic fatty liver disease in oleic acid-treated HepG2 hepatocytes and in high fat diet-fed mice. Biomed Pharmacother. 2019;118:109287. doi: 10.1016/j.biopha.2019.109287. [DOI] [PubMed] [Google Scholar]
- 65.Kyoreva M, Li Y, Hoosenally M, Hardman-Smart J, Morrison K, Tosi I, Tolaini M, Barinaga G, Stockinger B, Mrowietz U, Nestle FO, Smith CH, Barker JN, Di Meglio P. CYP1A1 Enzymatic activity influences skin inflammation via regulation of the AHR pathway. J Invest Dermatol. 2021;141:1563. doi: 10.1016/J.JID.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parks AJ, Pollastri MP, Hahn ME, Stanford EA, Novikov O, Franks DG, Haigh SE, Narasimhan S, Ashton TD, Hopper TG, Kozakov D, Beglov D, Vajda S, Schlezinger JJ, Sherr DH. In silico identification of an aryl hydrocarbon receptor antagonist with biological activity in vitro and in vivo. Mol Pharmacol. 2014;86:593–608. doi: 10.1124/mol.114.093369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aguiniga LM, Searl TJ, Rahman-Enyart A, Yaggie RE, Yang W, Schaeffer AJ, Klumpp DJ. Acyloxyacyl hydrolase regulates voiding activity. Am J Physiol. 2019;317:289–300. doi: 10.1152/AJPRENAL.00442.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SH, Henry EC, Kim DK, Kim YH, Kum JS, Myoung SH, Lee TG, Kang JK, Gasiewicz TA, Sung HR, Suh PG. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o- tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69:1871–1878. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- 69.Choi EY, Lee H, Dingle RWC, Kim KB, Swanson HI. Development of novel CH223191-based antagonists of the aryl hydrocarbon receptor. Mol Pharmacol. 2012;81:3–11. doi: 10.1124/mol.111.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monteleone I, Zorzi F, Marafini I, Di Fusco D, Dinallo V, Caruso R, Izzo R, Franzè E, Colantoni A, Pallone F, Monteleone G. Aryl hydrocarbon receptor-driven signals inhibit collagen synthesis in the gut. Eur J Immunol. 2016;46:1047–1057. doi: 10.1002/eji.201445228. [DOI] [PubMed] [Google Scholar]
- 71.Liu C, Li Y, Chen Y, Huang S, Wang X, Luo S, Su Y, Zhou L, Luo X. Baicalein Restores the Balance of Th17/Treg cells via aryl hydrocarbon receptor to attenuate colitis. Mediators Inflamm. 2020 doi: 10.1155/2020/5918587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cuartero MI, Ballesteros I, De La Parra J, Harkin AL, Abautret-Daly A, Sherwin E, Fernández-Salguero P, Corbí ÁL, Lizasoain I, Moro MA. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation. 2014;130:2040–2051. doi: 10.1161/CIRCULATIONAHA.114.011394. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka M, Fujikawa M, Oguro A, Itoh K, Vogel CFA, Ishihara Y. Involvement of the microglial aryl hydrocarbon receptor in neuroinflammation and vasogenic edema after ischemic stroke. Cells. 2021;10:718. doi: 10.3390/CELLS10040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dean A, Gregorc T, Docherty CK, Harvey KY, Nilsen M, Morrell NW, MacLean MR. Role of the aryl hydrocarbon receptor in sugen 5416-induced experimental pulmonary hypertension. Am J Respir Cell Mol Biol. 2017;58:320–330. doi: 10.1165/rcmb.2017-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Löfroth G, Rannug A. Ah receptor ligands in tobacco smoke. Toxicol Lett. 1988;42:131–136. doi: 10.1016/0378-4274(88)90070-7. [DOI] [PubMed] [Google Scholar]
- 76.Rico de Souza A, Traboulsi H, Wang X, Fritz JH, Eidelman DH, Baglole CJ (2021) The aryl hydrocarbon receptor attenuates acute cigarette smoke-induced airway neutrophilia independent of the dioxin response element. Front Immunol 0:274. 10.3389/FIMMU.2021.630427 [DOI] [PMC free article] [PubMed]
- 77.Jia Y, Tao Y, Lv C, Xia Y, Wei Z, Dai Y (2019) Tetrandrine enhances the ubiquitination and degradation of Syk through an AhR-c-src-c-Cbl pathway and consequently inhibits osteoclastogenesis and bone destruction in arthritis. Cell Death Dis 10. 10.1038/s41419-018-1286-2 [DOI] [PMC free article] [PubMed]
- 78.Pierre S, Chevallier A, Teixeira-Clerc F, Ambolet-Camoit A, Bui L-C, Bats A-S, Fournet J-C, Fernandez-Salguero P, Aggerbeck M, Lotersztajn S, Barouki R, Coumoul X. Aryl hydrocarbon receptor–dependent induction of liver fibrosis by dioxin. Toxicol Sci. 2014;137:114–124. doi: 10.1093/TOXSCI/KFT236. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 80.Shi Y, Tang B, Yu J, Luo Y, Xiao Y, Pi Z, Tang R, Wang Y, Kanekura T, Zeng Z, Xiao R. Aryl hydrocarbon receptor signaling activation in systemic sclerosis attenuates collagen production and is a potential antifibrotic target. Int Immunopharmacol. 2020;88:106886. doi: 10.1016/J.INTIMP.2020.106886. [DOI] [PubMed] [Google Scholar]
- 81.Yang S, Tan L, Chen Y, Liu A, Hong M, Peng Z. DIM mitigates the development of experimental autoimmune encephalomyelitis by maintaining the stability and suppressive function of regulatory T cells. Cell Immunol. 2020;358:104238. doi: 10.1016/J.CELLIMM.2020.104238. [DOI] [PubMed] [Google Scholar]
- 82.Mohammadi H, Daryabor G, Bahraman AG, Keshavarzi M, Kalantar K, Mohammadi-Bardbori A. Aryl hydrocarbon receptor engagement during redox alteration determines the fate of CD4+ T cells in C57BL/6 mice. J Biochem Mol Toxicol. 2021;35:e22821. doi: 10.1002/JBT.22821. [DOI] [PubMed] [Google Scholar]
- 83.Kunitomi C, Harada M, Kusamoto A, Azhary JM, Nose E, Koike H, Xu Z, Urata Y, Takahashi N, Wada-Hiraike O, Hirota Y, Koga K, Fujii T, Osuga Y (2021) Induction of aryl hydrocarbon receptor in granulosa cells by endoplasmic reticulum stress contributes to pathology of polycystic ovary syndrome. Mol Hum Reprod 27. 10.1093/MOLEHR/GAAB003 [DOI] [PubMed]
- 84.Abuin-Martínez C, Vidal R, Gutiérrez-López MD, Pérez-Hernández M, Giménez-Gómez P, Morales-Puerto N, O’Shea E, Colado MI. Increased kynurenine concentration attenuates serotonergic neurotoxicity induced by 3,4-methylenedioxymethamphetamine (MDMA) in rats through activation of aryl hydrocarbon receptor. Neuropharmacology. 2021;187:108490. doi: 10.1016/J.NEUROPHARM.2021.108490. [DOI] [PubMed] [Google Scholar]
- 85.de Castro LF, Ferreira MC, da Silva RM, de Blotta MHSL, L.N.A. Longhi, Mamoni RL (2013) Characterization of the immune response in human paracoccidioidomycosis. J Infect 67:470–485. 10.1016/J.JINF.2013.07.019 [DOI] [PubMed]
- 86.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 87.de Araújo EF, Preite NW, Veldhoen M, Loures FV, Calich VLG. Pulmonary paracoccidioidomycosis in AhR deficient hosts is severe and associated with defective Treg and Th22 responses. Sci Rep. 2020;10:1–16. doi: 10.1038/s41598-020-68322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Araújo EF, Loures FV, Preite NW, Feriotti C, AL Galdino N, Costa TA, Calich VLG (2021) AhR Ligands modulate the differentiation of innate lymphoid cells and t helper cell subsets that control the severity of a pulmonary fungal infection. Front Immunol 1277. 10.3389/FIMMU.2021.630938 [DOI] [PMC free article] [PubMed]
- 89.Giovannoni F, Bosch I, Polonio CM, Torti MF, Wheeler MA, Li Z, Romorini L, Rodriguez Varela MS, Rothhammer V, Barroso A, Tjon EC, Sanmarco LM, Takenaka MC, Modaresi SMS, Gutiérrez-Vázquez C, Zanluqui NG, dos Santos NB, Munhoz CD, Wang Z, Damonte EB, Sherr D, Gehrke L, Peron JPS, Garcia CC, Quintana FJ (2020) AHR is a Zika virus host factor and a candidate target for antiviral therapy. Nat Neurosci 23:939–951. 10.1038/s41593-020-0664-0 [DOI] [PMC free article] [PubMed]
- 90.Liu Y, Lv J, Liu J, Li M, Xie J, Lv Q, Deng W, Zhou N, Zhou Y, Song J, Wang P, Qin C, Tong W-M, Huang B. Mucus production stimulated by IFN-AhR signaling triggers hypoxia of COVID-19. Cell Res. 2020;30:1078–1087. doi: 10.1038/s41422-020-00435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith KJ, Murray IA, Tanos R, Tellew J, Boitano AE, Bisson WH, Kolluri SK, Cooke MP, Perdew GH. Identification of a high-affinity ligand that exhibits complete aryl hydrocarbon receptor antagonism. J Pharmacol Exp Ther. 2011;338:318–327. doi: 10.1124/jpet.110.178392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fang Z-Z, Krausz KW, Nagaoka K, Tanaka N, Gowda K, Amin SG, Perdew GH, Gonzalez FJ. In vivo effects of the pure aryl hydrocarbon receptor antagonist GNF-351 after oral administration are limited to the gastrointestinal tract. Br J Pharmacol. 2014;171:1735–1746. doi: 10.1111/bph.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bonnefont-Rousselot D (2016) Resveratrol and cardiovascular diseases. Nutrients 8. 10.3390/nu8050250 [DOI] [PMC free article] [PubMed]
- 94.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 95.Marti N, Bouchoucha N, Sauter KS, Flück CE (2017) Resveratrol inhibits androgen production of human adrenocortical H295R cells by lowering CYP17 and CYP21 expression and activities. PLoS One 12. 10.1371/journal.pone.0174224 [DOI] [PMC free article] [PubMed]
- 96.Beedanagari SR, Bebenek I, Bui P, Hankinson O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci. 2009;110:61–67. doi: 10.1093/toxsci/kfp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Revel A, Raanani H, Younglai E, Xu J, Rogers I, Han R, Savouret JF, Casper RF. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J Appl Toxicol. 2003;23:255–261. doi: 10.1002/jat.916. [DOI] [PubMed] [Google Scholar]
- 98.Singh NP, Singh US, Nagarkatti M, Nagarkatti PS. Resveratrol (3,5,4’-trihydroxystilbene) protects pregnant mother and fetus from the immunotoxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Nutr Food Res. 2011;55:209–219. doi: 10.1002/mnfr.201000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Revel A, Raanani H, Younglai E, Xu J, Han R, Savouret JF, Casper RF. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects sperm from DNA damage and apoptosis caused by benzo(a)pyrene. Reprod Toxicol. 2001;15:479–486. doi: 10.1016/S0890-6238(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 100.Banerjee B, Nandi P, Chakraborty S, Raha S, Sen PC, Jana K. Resveratrol ameliorates benzo(a)pyrene-induced testicular dysfunction and apoptosis: Involvement of p38 MAPK/ATF2/iNOS signaling. J Nutr Biochem. 2016;34:17–29. doi: 10.1016/j.jnutbio.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Banerjee B, Chakraborty S, Ghosh D, Raha S, Sen PC (2016) Jana K, Benzo(a)pyrene induced p53 mediated male germ cell apoptosis: synergistic protective effects of curcumin and resveratrol. Front Pharmacol 7. 10.3389/fphar.2016.00245 [DOI] [PMC free article] [PubMed]
- 102.Erthal RP, Siervo GEML, Silveira LTR, Scarano WR, Fernandes GSA. Can resveratrol attenuate testicular damage in neonatal and adult rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin during gestation? Reprod Fertil Dev. 2018;30:442–450. doi: 10.1071/RD17180. [DOI] [PubMed] [Google Scholar]
- 103.Silveira LTR, de Mello Santos T, Camora LF, Pinho CF, Anselmo-Franci JA, Domeniconi RF, Justulin LA, Barbisan LF, Scarano WR (2018) Protective effect of resveratrol on urogenital sinus and prostate development in rats exposed in utero to TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin). Reprod Toxicol 83:82–92. 10.1016/j.reprotox.2018.06.012 [DOI] [PubMed]
- 104.Ghosh J, Chowdhury AR, Srinivasan S, Chattopadhyay M, Bose M, Bhattacharya S, Raza H, Fuchs SY, Rustgi AK, Gonzalez FJ, Avadhani NG. Cigarette smoke toxins-induced mitochondrial dysfunction and pancreatitis involves aryl hydrocarbon receptor mediated Cyp1 gene expression: protective effects of resveratrol. Toxicol Sci. 2018;166:428–440. doi: 10.1093/toxsci/kfy206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.eun Lee J, Cho SG, Ko SG, Ahrmad SA, Puga A, Kim K (2020) Regulation of a long noncoding RNA MALAT1 by aryl hydrocarbon receptor in pancreatic cancer cells and tissues. Biochem Biophys Res Commun 532:563–569. 10.1016/J.BBRC.2020.08.053 [DOI] [PMC free article] [PubMed]
- 106.Mokhtar MM, Khidr EG, Shaban HM, Allam S, Elsadek BEM, Salama SA, Ali SS. The effect of aryl hydrocarbon receptor ligands on gentamicin-induced nephrotoxicity in rats. Environ Sci Pollut Res. 2020;27:16189–16202. doi: 10.1007/s11356-020-08073-z. [DOI] [PubMed] [Google Scholar]
- 107.Amaya SC, Savaris RF, Filipovic CJ, Wise JD, Hestermann E, Young SL, Lessey BA. Resveratrol and endometrium: a closer look at an active ingredient of red wine using in vivo and in vitro models. Reprod Sci. 2014;21:1362–1369. doi: 10.1177/1933719114525271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Papoutsis AJ, Selmin OI, Borg JL, Romagnolo DF. Gestational exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter hypermethylation and reduces BRCA-1 expression in mammary tissue of rat offspring: Preventive effects of resveratrol. Mol Carcinog. 2015;54:261–269. doi: 10.1002/mc.22095. [DOI] [PubMed] [Google Scholar]
- 109.Wang K, Feng C, Li C, Yao J, Xie X, Gong L, Luan Y, Xing G, Zhu X, Qi X, Ren J. Baicalin protects mice from aristolochic acid I-induced kidney injury by induction of CYP1A through the aromatic hydrocarbon receptor. Int J Mol Sci. 2015;16:16454–16468. doi: 10.3390/ijms160716454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anandasadagopan SK, Singh NM, Raza H, Bansal S, Selvaraj V, Singh S, Chowdhury AR, Leu NA, Avadhani NG (2017) β-naphthoflavone-induced mitochondrial respiratory damage in Cyp1 knockout mouse and in cell culture systems: attenuation by resveratrol treatment. Oxid Med Cell Longev 2017. 10.1155/2017/5213186 [DOI] [PMC free article] [PubMed]
- 111.Zhou Y, Jiang R, An L, Wang H, Cheng S, Qiong S, Weng Y. Benzo[a]pyrene impedes self-renewal and differentiation of mesenchymal stem cells and influences fracture healing. Sci Total Environ. 2017;587–588:305–315. doi: 10.1016/j.scitotenv.2017.02.152. [DOI] [PubMed] [Google Scholar]
- 112.Liu W-C, Shyu J-F, Lin Y-F, Chiu H-W, Lim PS, Lu C-L, Zhen C-M, Hou Y-C, Chen P-H, Lu K-C (2020) Resveratrol rescue indoxyl sulfate-induced deterioration of osteoblastogenesis via the aryl hydrocarbon receptor /MAPK pathway. Int J Mol Sci 21:7483. 10.3390/IJMS21207483 [DOI] [PMC free article] [PubMed]
- 113.Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 114.Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2010;68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baker NA, Karounos M, English V, Fang J, Wei Y, Stromberg A, Sunkara M, Morris AJ, Swanson HI, Cassis LA. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ Health Perspect. 2013;121:105–110. doi: 10.1289/ehp.1205421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baker NA, English V, Sunkara M, Morris AJ, Pearson KJ, Cassis LA. Resveratrol protects against polychlorinated biphenyl-mediated impairment of glucose homeostasis in adipocytes. J Nutr Biochem. 2013;24:2168–2174. doi: 10.1016/j.jnutbio.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hsu CN, Lin YJ, Lu PC, Tain YL. Maternal resveratrol therapy protects male rat offspring against programmed hypertension induced by TCDD and dexamethasone exposures: Is it relevant to aryl hydrocarbon receptor? Int J Mol Sci. 2018;19:2459. doi: 10.3390/ijms19082459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang B, Sun J, Li X, Zhou Q, Bai J, Shi Y, Le G. Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr Res. 2013;33:971–981. doi: 10.1016/j.nutres.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 119.Hsu CN, Lin YJ, Tain YL. Maternal exposure to bisphenol a combined with high-fat diet-induced programmed hypertension in adult male rat offspring: effects of resveratrol. Int J Mol Sci. 2019;20:4382. doi: 10.3390/ijms20184382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Murray IA, Flaveny CA, DiNatale BC, Chairo CR, Schroeder JC, Kusnadi A, Perdew GH. Antagonism of aryl hydrocarbon receptor signaling by 6,2′,4′- trimethoxyflavone. J Pharmacol Exp Ther. 2010;332:135–144. doi: 10.1124/jpet.109.158261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen WC, Chang LH, Huang SS, Huang YJ, Chih CL, Kuo HC, Lee YH, Lee IH. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J Neuroinflammation. 2019;16:187. doi: 10.1186/s12974-019-1572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwon J-I, Heo H, Ham SJ, Chae YJ, Lee D-W, Kim ST, Min J, Sung YS, Kim KW, Choi Y, Woo DC, Woo C-W. Aryl hydrocarbon receptor antagonism before reperfusion attenuates cerebral ischaemia/reperfusion injury in rats. Sci Rep. 2020;10:14906. doi: 10.1038/s41598-020-72023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miao H, Cao G, Wu X-Q, Chen Y-Y, Chen D-Q, Chen L, Vaziri ND, Feng Y-L, Su W, Gao Y, Zhuang S, Yu X-Y, Zhang L, Guo Y, Zhao Y-Y. Identification of endogenous 1-aminopyrene as a novel mediator of progressive chronic kidney disease via aryl hydrocarbon receptor activation. Br J Pharmacol. 2020;177:3415–3435. doi: 10.1111/BPH.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bianchi-Smiraglia A, Bagati A, Fink EE, Affronti HC, Lipchick BC, Moparthy S, Long MD, Rosario SR, Lightman SM, Moparthy K, Wolff DW, Yun DH, Han Z, Polechetti A, Roll MV, Gitlin II, Leonova KI, Rowsam AM, Kandel ES, Gudkov AV, Bergsagel PL, Lee KP, Smiraglia DJ, Nikiforov MA. Inhibition of the aryl hydrocarbon receptor/polyamine biosynthesis axis suppresses multiple myeloma. J Clin Invest. 2018;128:4682–4696. doi: 10.1172/JCI70712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Campesato LF, Budhu S, Tchaicha J, Weng C-H, Gigoux M, Cohen IJ, Redmond D, Mangarin L, Pourpe S, Liu C, Zappasodi R, Zamarin D, Cavanaugh J, Castro AC, Manfredi MG, McGovern K, Merghoub T, Wolchok JD. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun. 2020;11:1–11. doi: 10.1038/s41467-020-17750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Takahashi E, Fujita KI, Kamataki T, Arimoto-Kobayashi S, Okamoto K, Negishi T. Inhibition of human cytochrome P450 1B1, 1A1 and 1A2 by antigenotoxic compounds, purpurin and alizarin. Mutat Res - Fundam Mol Mech Mutagen. 2002;508:147–156. doi: 10.1016/S0027-5107(02)00212-9. [DOI] [PubMed] [Google Scholar]
- 127.Zhang J, Shen X. Antioxidant activities of baicalin, green tea polyphenols and alizarin in vitro and in vivo. J Nutr Environ Med. 1997;7:79–90. doi: 10.1080/13590849762664. [DOI] [Google Scholar]
- 128.Takahashi E, Arimoto S, Okamoto K, Negishi T. Enhancement of phase II enzyme activity by purpurin resulting in the suppression of MeIQx-DNA-adduct formation in mice. Mutat Res - Genet Toxicol Environ Mutagen. 2007;626:128–134. doi: 10.1016/j.mrgentox.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 129.Xu L, Xing M, Xu X, Saadeldeen FS, Liu Z, Wei J, Kang W. Alizarin increase glucose uptake through PI3K/Akt signaling and improve alloxan-induced diabetic mice, Future. Med Chem. 2019;11:395–406. doi: 10.4155/fmc-2018-0515. [DOI] [PubMed] [Google Scholar]
- 130.Stiborová M, Bieler CA, Wiessler M, Frei E. The anticancer agent ellipticine on activation by cytochrome P450 forms covalent DNA adducts. Biochem Pharmacol. 2001;62:1675–1684. doi: 10.1016/S0006-2952(01)00806-1. [DOI] [PubMed] [Google Scholar]
- 131.Stiborová M, Sejbal J, Bořek-Dohalská L, Aimová D, Poljaková J, Forsterová K, Rupertová M, Wiesner J, Hudeček J, Wiessler M, Frei E. The anticancer drug ellipticine forms covalent DNA adducts, mediated by human cytochromes P450, through metabolism to 13-hydroxyellipticine and ellipticine N2-oxide. Cancer Res. 2004;64:8374–8380. doi: 10.1158/0008-5472.CAN-04-2202. [DOI] [PubMed] [Google Scholar]
- 132.Aimová D, Stiborová M. Antitumor drug ellipticine inhibits the activaties of rat hepatic cytochromes P450. Biomed Pap. 2005;149:437–440. doi: 10.5507/bp.2005.076. [DOI] [PubMed] [Google Scholar]
- 133.Aimová D, Svobodová L, Kotrbová V, Mrázová B, Hodek P, Hudeček J, Václavíková R, Frei E, Stiborová M. The anticancer drug ellipticine is a potent inducer of rat cytochromes P450 1A1 and 1A2, thereby modulating its own metabolism. Drug Metab Dispos. 2007;35:1926–1934. doi: 10.1124/dmd.107.016048. [DOI] [PubMed] [Google Scholar]
- 134.Wu Y, Sadatmousavi P, Wang R, Lu S, Yuan YF, Chen P (2012) Self-assembling peptide-based nanoparticles enhance anticancer effect of ellipticine in vitro and in vivo. Int J Nanomedicine 7:3221–3233. 10.2147/IJN.S31858 [DOI] [PMC free article] [PubMed]
- 135.Chen Q, Liu J, Zhuang Y, ping Bai L, Yuan Q, Zheng S, Liao K, Khan MA, Wu Q, Luo C, Liu L, Wang H, Li T (2019) Identification of an IKKβ inhibitor for inhibition of inflammation in vivo and in vitro. Pharmacol Res 149:104440. 10.1016/j.phrs.2019.104440 [DOI] [PubMed]
- 136.Mikstacka R, Przybylska D, Rimando AM, Baer-Dubowska W. Inhibition of human recombinant cytochromes P450 CYP1A1 and CYP1B1 by trans-resveratrol methyl ethers. Mol Nutr Food Res. 2007;51:517–524. doi: 10.1002/mnfr.200600135. [DOI] [PubMed] [Google Scholar]
- 137.Albassam AA, Frye RF. Effect of pterostilbene on in vitro drug metabolizing enzyme activity. Saudi Pharm J. 2019;27:406–412. doi: 10.1016/j.jsps.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eräsalo H, Hämäläinen M, Leppänen T, Mäki-Opas I, Laavola M, Haavikko R, Yli-Kauhaluoma J, Moilanen E. Natural stilbenoids have anti-inflammatory properties in vivo and down-regulate the production of inflammatory mediators NO, IL6, and MCP1 possibly in a PI3K/Akt-dependent manner. J Nat Prod. 2018;81:1131–1142. doi: 10.1021/acs.jnatprod.7b00384. [DOI] [PubMed] [Google Scholar]
- 139.Nam W, Nam SH, Kim SP, Levin C, Friedman M. Anti-adipogenic and anti-obesity activities of purpurin in 3T3-L1 preadipocyte cells and in mice fed a high-fat diet. BMC Complement Altern Med. 2019;19:364. doi: 10.1186/s12906-019-2756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim JK, Kim N, Lim YH. Evaluation of the antibacterial activity of rhapontigenin produced from rhapontin by biotransformation against Propionibacterium acnes. J Microbiol Biotechnol. 2010;20:82–87. doi: 10.4014/jmb.0907.07022. [DOI] [PubMed] [Google Scholar]
- 141.Chun YJ, Ryu SY, Jeong TC, Kim MY. Mechanism-based inhibition of human cytochrome P450 1A1 by rhapontigenin. Drug Metab Dispos. 2001;29:389–393. [PubMed] [Google Scholar]
- 142.Cieniak C, Liu R, Fottinger A, Smiley SAM, Guerrero-Analco JA, Bennett SAL, Haddad PS, Cuerrier A, Saleem A, Arnason JT, Foster BC. In vitro inhibition of metabolism but not transport of gliclazide and repaglinide by Cree medicinal plant extracts. J Ethnopharmacol. 2013;150:1087–1095. doi: 10.1016/j.jep.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 143.Roupe KA, Helms GL, Halls SC, Yáñez JA, Davies NMT (2005) Graduate program, preparative enzymatic synthesis and HPLC Analysis of rhapontigenin: applications to metabolism, pharmacokinetics and anti-cancer studies. J Pharm Pharm Sci 8:374–386. www.cspscanada.org (accessed July 9, 2020) [PubMed]
- 144.Roupe K, Remsberg C, Yanez J, Davies N. Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 145.Fan Y. Cardioprotective effect of rhapontigenin in isoproterenol-induced myocardial infarction in a rat model. Pharmacology. 2019;103:291–302. doi: 10.1159/000496800. [DOI] [PubMed] [Google Scholar]
- 146.Asahina Y, Kashiwaki K (1915) chemical constituents of the fruits of Evodia rutaecarpa. J Pharm Soc Jpn 1293
- 147.Ueng Y-F, Jan W-C, Lin L-C, Chen T-L, Guengerich FP, Chen C-F. The alkaloid rutaecarpine is a selective inhibitor of cytochrome P450 1A in mouse and human liver microsomes. Drug Metab Dispos. 2002;30:349–353. doi: 10.1124/DMD.30.3.349. [DOI] [PubMed] [Google Scholar]
- 148.Ueng YF, Wang JJ, Lin LC, Park SS, Chen CF. Induction of cytochrome P450-dependent monooxygenase in mouse liver and kidney by rutaecarpine, an alkaloid of the herbal drug Evodia rutaecarpa. Life Sci. 2001;70:207–217. doi: 10.1016/S0024-3205(01)01390-X. [DOI] [PubMed] [Google Scholar]
- 149.Ueng Y-F, Tsai T-H, Don M-J, Chen R-M, Chen T-L. Alteration of the pharmacokinetics of theophylline by rutaecarpine, an alkaloid of the medicinal herb Evodia rutaecarpa, in rats. J Pharm Pharmacol. 2005;57:227–232. doi: 10.1211/0022357055489. [DOI] [PubMed] [Google Scholar]
- 150.Haarmann-Stemmann T, Sendker J, Götz C, Krug N, Bothe H, Fritsche E, Proksch P, Abel J. Regulation of dioxin receptor function by different beta-carboline alkaloids. Arch Toxicol. 2010;84:619–629. doi: 10.1007/s00204-010-0548-2. [DOI] [PubMed] [Google Scholar]
- 151.Han EH, Kim HG, Im JH, Jeong TC, Jeong HG. Up-regulation of CYP1A1 by rutaecarpine is dependent on aryl hydrocarbon receptor and calcium. Toxicology. 2009;266:38–47. doi: 10.1016/j.tox.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 152.Tian KM, Li JJ, Xu SW (2019) Rutaecarpine: a promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol Res 141:541–550. 10.1016/j.phrs.2018.12.019 [DOI] [PubMed]
- 153.Hu CP, Li NS, Xiao L, Deng HW, Li YJ. Involvement of capsaicin-sensitive sensory nerves in cardioprotection of rutaecarpine in rats. Regul Pept. 2003;114:45–49. doi: 10.1016/S0167-0115(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 154.Li W-Q, Li X-H, Du J, Zhang W, Li D, Xiong X-M, Li Y-J. Rutaecarpine attenuates hypoxia-induced right ventricular remodeling in rats, Naunyn. Schmiedebergs. Arch Pharmacol. 2016;389:757–767. doi: 10.1007/s00210-016-1240-8. [DOI] [PubMed] [Google Scholar]
- 155.Qin XP, Zeng SY, Li D, Chen QQ, Luo D, Zhang Z, Hu GY, Deng HW, Li YJ. Calcitonin gene-related peptide-mediated depressor effect and inhibiting vascular hypertrophy of rutaecarpine in renovascular hypertensive rats. J Cardiovasc Pharmacol. 2007;50:654–659. doi: 10.1097/FJC.0b013e3181579e7e. [DOI] [PubMed] [Google Scholar]
- 156.Li D, Peng J, Xin HY, Luo D, Zhang YS, Zhou Z, Jiang DJ, Deng HW, Li YJ. Calcitonin gene-related peptide-mediated antihypertensive and anti-platelet effects by rutaecarpine in spontaneously hypertensive rats. Peptides. 2008;29:1781–1788. doi: 10.1016/j.peptides.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 157.Li D, Zhang XJ, Chen L, Yang Z, Deng HW, Peng J, Li YJ. Calcitonin gene-related peptide mediates the cardioprotective effects of rutaecarpine against ischaemia-reperfusion injury in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2009;36:662–667. doi: 10.1111/j.1440-1681.2008.05136.x. [DOI] [PubMed] [Google Scholar]
- 158.Zhou Z, Peng J, Wang CJ, Li D, Li TT, Hu CP, Chen XP, Li YJ. Accelerated senescence of endothelial progenitor cells in hypertension is related to the reduction of calcitonin gene-related peptide. J Hypertens. 2010;28:931–939. doi: 10.1097/HJH.0b013e3283399326. [DOI] [PubMed] [Google Scholar]
- 159.Xu Y, Liu Q, Xu Y, Liu C, Wang X, He X, Zhu N, Liu J, Wu Y, Li Y, Li N, Feng T, Lai F, Zhang M, Hong B, Jiang JD, Si S. Rutaecarpine suppresses atherosclerosis in ApoE-/- mice through upregulating ABCA1 and SR-BI within RCT. J Lipid Res. 2014;55:1634–1647. doi: 10.1194/jlr.M044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu Y, Chen XP, Zhang F, Hou HH, Zhang JY, Lin SX, Sun AS (2017) Rutaecarpine inhibits intimal hyperplasia in A balloon-injured rat artery model. Chin J Integr Med 24:429–435. 10.1007/s11655-017-2900-3 [DOI] [PubMed]
- 161.Nie XQ, Chen HH, Zhang JY, Zhang YJ, Yang JW, Pan HJ, Song WX, Murad F, He YQ, Bian K. Rutaecarpine ameliorates hyperlipidemia and hyperglycemia in fat-fed, streptozotocin-treated rats via regulating the IRS-1/PI3K/Akt and AMPK/ACC2 signaling pathways. Acta Pharmacol Sin. 2016;37:483–496. doi: 10.1038/aps.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang C, Hao Z, Zhou J, Zhang L, Sun Y, Liang C. Rutaecarpine alleviates renal ischemia reperfusion injury in rats by suppressing the JNK/p38 MAPK signaling pathway and interfering with the oxidative stress response. Mol Med Rep. 2017;16:922–928. doi: 10.3892/mmr.2017.6631. [DOI] [PubMed] [Google Scholar]
- 163.Wang Z, Monti S, Sherr DH. The diverse and important contributions of the AHR to cancer and cancer immunity. Curr Opin Toxicol. 2017;2:93–102. doi: 10.1016/j.cotox.2017.01.008. [DOI] [Google Scholar]
- 164.Gutiérrez-Vázquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xue P, Fu J, Zhou Y. The aryl hydrocarbon receptor and tumor immunity. Front Immunol. 2018;9:13. doi: 10.3389/fimmu.2018.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.A first-in-humans dose finding study for an aryl hydrocarbon receptor inhibitor (AhRi) in patients with advanced cancer (n.d.) https://clinicaltrials.gov/ct2/show/NCT04069026?term=aryl&draw=2&rank=2 (accessed 15 Dec 2020)
- 167.IK-175 in patients with advanced or metastatic solid tumors and urothelial carcinoma (n.d.) https://clinicaltrials.gov/ct2/show/NCT04200963?term=aryl&draw=2&rank=4 (accessed 15 Dec 2020)
- 168.Loro E, Sengupta K, Bogdanovich S, Whig K, Schultz DC, Huryn DM, Khurana TS. High-throughput identification of post-transcriptional utrophin up-regulators for Duchenne muscle dystrophy (DMD) therapy. Sci Rep. 2020;10:2143. doi: 10.1038/s41598-020-58737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wilkinson IVL, Perkins KJ, Dugdale H, Moir L, Vuorinen A, Chatzopoulou M, Squire SE, Monecke S, Lomow A, Geese M, Charles PD, Burch P, Tinsley JM, Wynne GM, Davies SG, Wilson FX, Rastinejad F, Mohammed S, Davies KE, Russell AJ. Chemical proteomics and phenotypic profiling identifies the aryl hydrocarbon receptor as a molecular target of the utrophin modulator ezutromid. Angew Chemie Int Ed. 2020;59:2420–2428. doi: 10.1002/anie.201912392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Muntoni F, Tejura B, Spinty S, Roper H, Hughes I, Layton G, Davies KE, Harriman S, Tinsley J. A phase 1b trial to assess the pharmacokinetics of ezutromid in pediatric Duchenne muscular dystrophy patients on a balanced diet. Clin Pharmacol Drug Dev. 2019;8:922–933. doi: 10.1002/cpdd.642. [DOI] [PubMed] [Google Scholar]
- 171.Proof of concept study to assess activity and safety of SMT C1100 (Ezutromid) in boys with Duchenne muscular dystrophy (DMD) (n.d.) https://clinicaltrials.gov/ct2/show/study/NCT02858362?term=PhaseOut+DMD&rank=1 (accessed 15 Dec 2020)