Abstract
Background
Colistin is used against multi-drug resistant pathogens, yet resistance emerges through dissemination of plasmid-mediated genes (mcr) or chromosomal mutation of genes involved in lipopolysaccharide synthesis (i.e. mgrB, phoPQ, pmrCAB). Phenotypic susceptibility testing is challenging due to poor diffusion of colistin in agar media, leading to an underestimation of resistance. Performance of five phenotypic approaches was compared in the context of different molecular mechanisms of resistance. We evaluated Vitek 2® (bioMérieux, AST N242), Colistin MIC Test Strip (Liofilchem Diagnostici), UMIC (Biocentric), and Rapid Polymyxin™ NP test (ELITechGroup) against the standard broth microdilution (BMD) method. We used whole genome sequencing (WGS) to infer molecular resistance mechanisms. We analysed 97 Enterobacterales and non-fermenting bacterial isolates, largely clinical isolates collected up to 2018. Data was analysed by comparing susceptibility categories (susceptible or resistant) and minimal inhibitory concentrations (MIC). Susceptibility category concordance is the percentage of test results sharing the same category to BMD. MIC concordance was calculated similarly but considering ±1 MIC titre error range. We determined genomic diversity by core genome multi locus sequencing typing (cgMLST) and identified putative antimicrobial resistance genes using NCBI and CARD databases, and manual annotation.
Results
Of 97 isolates, 54 (56%) were resistant with standard BMD. Highest susceptibility category concordance was achieved by Rapid Polymyxin™ NP (98.8%) followed by UMIC (97.9%), Colistin E-test MIC strip (96.9%) and Vitek 2® (95.6%). Highest MIC concordance was achieved by UMIC (80.4%), followed by Vitek 2® (72.5%) and Colistin E-test MIC strip (62.9%). Among resistant isolates, 23/54 (43%) were intrinsically resistant to colistin, whereas 31/54 (57%) isolates had acquired colistin resistance. Of these, mcr-1 was detected in four isolates and mcr-2 in one isolate. Non-synonymous mutations in mgrB, phoQ, pmrA, pmrB, and pmrC genes were encountered in Klebsiella pneumoniae, Escherichia coli, and Acinetobacter bereziniae resistant isolates. Mutations found in mgrB and pmrB were only identified in isolates exhibiting MICs of ≥16 mg/L.
Conclusions
The Rapid Polymyxin™ NP test showed highest categorical concordance and the UMIC test provided MIC values with high concordance to BMD. We found colistin resistance in diverse species occurred predominantly through spontaneous chromosomal mutation rather than plasmid-mediated resistance.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-021-02388-8.
Keywords: Colistin, Resistance, Antimicrobial susceptibility testing, WGS, Antimicrobial resistance genes
Background
Colistin is an antimicrobial agent of the polymyxin class. Although still widely used in veterinary medicine, colistin usage in human medicine was initially restricted to topical administrations due to its nephrotoxic and neurotoxic properties if given systemically [1]. However, due to the recent dissemination of multidrug resistant (MDR) bacteria around the world, colistin has been increasingly used as a last resort antimicrobial for treatment of difficult-to-treat infections caused by MDR Gram-negative pathogens [1–3].
Colistin is a cationic polypeptide containing an acylated tripeptide chain at its N-terminus responsible for the toxicity of colistin. The mechanism of action relies on the interaction of the hydrophobic region of the fatty acid and phosphate groups of the lipid A of the lipopolysaccharide (LPS). This interaction displaces the divalent cations that naturally stabilize the outer bacterial membranes leading to leakage of cellular compounds and, ultimately, cell death [4–6]. Although this is the main mode of action, other mechanisms have been described such as inhibition of respiratory enzymes NDH-2 [5, 7, 8] and neutralization of the LPS, which may help prevent septic shock [9]. Due to its mechanism of action, colistin is highly effective against most Enterobacterales species and non-fermenting Gram-negative bacteria such as Acinetobacter baumannii and Pseudomonas aeruginosa. Conversely, colistin is not active against Gram-positive bacteria, Gram-negative cocci, and anaerobic bacteria. Some Enterobacterales species are intrinsically resistant to colistin, such as Serratia marcescens, Morganella morganii, Proteus mirabilis, and Burkholderia spp. due to the constitutive expression of genes (i.e. eptB) that lead to the modification of the LPS and an increase in its charge [4, 5, 10–14]. Hafnia spp. have also been suggested to be intrinsically resistant [14, 15].
Bacteria have developed resistance mechanisms against colistin mainly through the modification of the LPS. This is achieved by the addition of 4-amino-4-deoxy-L-arabinose (L-Ara-4 N) or phosphoethanolamine (pEtN) to lipid A that increases the positive charge of LPS and thus reduces its affinity to colistin [5, 6, 16]. The synthesis and addition of L-Ara-4 N and pEtN is mediated by the PmrAB and PhoPQ two-component system genes and its regulators genes (i.e. mgrB) but also through plasmid-mediated genes like mobile colistin resistance (mcr) [5, 16–18]. Colistin resistance by alteration of LPS has been widely described in several species like K. pneumoniae and E. coli [4, 5, 10, 19–22]. Other species, like A. baumannii acquire resistance due to the complete loss of LPS by inactivation of the lipid A biosynthesis genes (lpxA, lpxC and lpxD) [5, 23], or by alteration the expression of genes related to LPS synthesis or genes related to electrostatic modifications of the cell surface (pmrAB, adeRS) [24, 25].
In view of concerns around the emergence of resistance, it is of critical importance that reliable tools for susceptibility testing are available. However, susceptibility testing is a challenge due to the cationic nature of colistin, which causes it to adhere to the negatively charged polystyrene surfaces used in routine laboratory plates [26], but also due to its poor diffusion in agar because of its large molecular size. In 2016 EUCAST warned about the difficulties of colistin testing using disk diffusion and gradient test, as these methods seem to underestimate resistance. EUCAST recommends broth microdilution (BMD) as the only valid method, and that it should be performed with sulphate salts of polymyxins in cation-adjusted Mueller-Hinton broth, without any additives like polysorbate-80 (P-80) in trays made of polystyrene [27]. Nevertheless, this method may be difficult to implement in a routine diagnostic laboratory since other assays, such as disk diffusion or automated testing like Vitek 2® or BD Phoenix™, are commonly used for colistin susceptibility testing and are often part of laboratory automation.
The goal of our study was to first compare the performance of four different diagnostic assays for colistin susceptibility testing against BMD: Vitek 2® (bioMérieux, AST N242), Colistin E-Test MIC Strip (Liofilchem Diagnostici), UMIC (Biocentric), and Rapid Polymyxin™ NP test (ELITechGroup). We aimed to find the most accurate, robust and easy-to-perform assay suitable for the daily usage in the routine microbiology laboratory. Our second aim was to determine the underlying genetic mechanisms of resistance using whole genome sequencing and compare this against the minimal inhibitory concentrations (MICs).
Results
Phenotypic colistin resistance in the strain collection
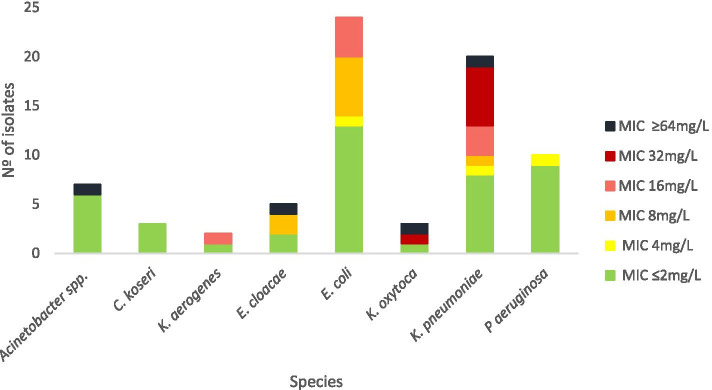
Of the 97 isolates tested, 54 (56%) were resistant to colistin by the standard BMD testing and 43 (44%) were susceptible. Among the resistant isolates, 23 (43%) belonged to the bacterial species P. vulgaris, P. mirabilis, S. marcescens and H. alvei and thus possess intrinsic resistance. Whereas 31 (57%) belonged to Acinetobacter spp., E. cloacae, E. coli, K. aerogenes, K. pneumoniae, K. oxytoca, and P. aeruginosa which display various mechanisms of acquired resistance. Among these latter non-intrinsically resistant isolates, three (10%) had a MIC value of 4 mg/L, nine (29%) had a MIC of 8 mg/L, eight (26%) had a MIC of 16 mg/L, seven (23%) had a MIC value of 32 mg/L, and four (13%) isolates displayed a MIC of ≥64 mg/L (Fig. 1). A total of 19 (20%) isolates had a MIC value ≥16 mg/L, belonging to Acinetobacter spp. (n = 1), E. cloacae (n = 1), E. coli (n = 4), K. aerogenes (n = 1), K. oxytoca (n = 2), and K. pneumoniae (n = 10). Due to the high number of isolates exhibiting a MIC between 4 and 8 mg/L compared to the few isolates with higher levels of resistance, we considered ≥16 mg/L to be higher MIC values in this study. Additionally, these isolates exhibited specific genomic traits explained in more detail below.
Fig. 1.
Colistin resistance in non-intrinsically resistant bacteria. MICs were determined by broth microdilution methods (BMD) and interpreted according to EUCAST breakpoints (Version 10.0, 2020)
Rapid Polymyxin™ NP test shows highest concordance with susceptibility category and UMIC with MIC measurements
Susceptibility category concordance (susceptible or resistant) between the BMD gold standard and the test protocols (Vitek 2®, Colistin MIC Test Strip, UMIC, and Rapid Polymyxin™ NP test) is shown in Table 1. The highest overall susceptibility category concordance was achieved with Rapid Polymyxin™ NP test (98.8%), followed by UMIC (97.9%), Colistin E-test MIC strip (96.9%), and Vitek 2® (95.6%). We calculated the sensitivity and specificity for the susceptibility category according to the BMD reference standard in all isolates. The highest sensitivity was shown for the Rapid Polymyxin™ NP test (98.8%) with a 100% specificity.
Table 1.
Susceptibility category (susceptible or resistant) concordance of different assays compared to reference BMD method
| Method | BMD | Vitek 2®a | Rapid PolymyxinTM NPb | UMIC | Colistin E-test MIC strip | ||||
|---|---|---|---|---|---|---|---|---|---|
| Species | S/R | S/R | % concordance | S/R | % concordance | S/R | % concordance | S/R | % concordance |
| Acinetobacter spp. | 6/1 | 4/0 | 100 | 1/1 | 50 | 6/1 | 100 | 6/1 | 100 |
| C. koseri | 3/0 | 3/0 | 100 | 3/0 | 100 | 3/0 | 100 | 3/0 | 100 |
| K. aerogenes | 1/1 | 1/1 | 100 | 1/1 | 100 | 1/1 | 100 | 1/1 | 100 |
| E. cloacae | 2/3 | 3/2 | 80 | 2/3 | 100 | 2/3 | 100 | 3/2 | 80 |
| E. coli | 13/11 | 13/11 | 100 | 13/11 | 100 | 13/11 | 100 | 13/11 | 100 |
| Hafnia spp. | 0/15 | 1/14 | 93.3 | 0/15 | 100 | 0/15 | 100 | 0/15 | 100 |
| K. oxytoca | 1/2 | 1/2 | 100 | 1/2 | 100 | 1/2 | 100 | 1/2 | 100 |
| K. pneumoniae | 8/12 | 9/10 | 94.7 | 9/11 | 95 | 9/11 | 95 | 9/11 | 95 |
| M. morganii | 0/3 | 0/3 | 100 | 0/3 | 100 | 0/3 | 100 | 0/3 | 100 |
| P. mirabilis | 0/2 | 0/2 | 100 | 0/2 | 100 | 0/2 | 100 | 0/2 | 100 |
| P. vulgaris | 0/1 | 0/1 | 100 | 0/1 | 100 | 0/1 | 100 | 0/1 | 100 |
| P. aeruginosa | 9/1 | 8/0 | 87.5 | NA | NA | 10/0 | 90 | 10/0 | 90 |
| S. marcescens | 0/2 | 0/2 | 100 | 0/2 | 100 | 0/2 | 100 | 0/2 | 100 |
| Total | 43/54 | 43/48 | 95.6 | 30/52 | 98.8 | 45/52 | 97.9 | 46/51 | 96.9 |
BMD Broth microdilution, S Susceptible, R Resistant, NA Not applicable
aA total of 91 isolates were tested by Vitek 2® method. Isolates not tested by Vitek 2® test was due to low growth, but were excluded from the comparison analysis with BMD
bA total of 82 were tested by the Rapid PolymyxinTM NP method. Isolates not tested by Rapid PolymyxinTM NP test were excluded from the comparison analysis with gold standard
Exploring subsets of bacterial isolates, the test that performed better compared to the gold standard in Enterobacterales was the UMIC test, with a concordance of 98.7%. The Rapid Polymyxin™ NP test also achieved a high concordance level (98.5%), whereas the Vitek 2® and the Colistin E-test MIC strip were concordant only in 96.2% of the tested isolates in both tests. In susceptibility testing for non-fermenting bacteria, the highest concordance to BMD was the UMIC (94.1%) and Colistin E-test MIC strip (94.4%) followed by Vitek 2® (90.9%). The Rapid Polymyxin™ NP test is specifically designed to detect polymyxin resistance among Enterobacterales [28]. The susceptibility concordance with BMD was high for the subset of Enterobacterales isolates included in this study (98.5%). However, and as expected, the performance for non-fermenting bacteria was poor, reaching a concordance percentage of only 50% in the Acinetobacter spp. No differences were observed in the capability to detect resistant isolates in non-fermenting and fermenting Enterobacterales between the different assays used in this study (Additional file 1).
MIC concordance of the different assays compared to BMD is shown in Table 2. Concordance was established as the same MIC value or ± 1 titre difference as that of the gold standard. All intrinsic resistant species were excluded from the analysis since no MIC value was obtained from the reference standard method as they were automatically considered as resistant isolates. The highest concordance was achieved with UMIC test (80.4%), followed by Vitek 2® (72.5%). The Colistin E-test MIC strip had the lowest concordance (62.9%) to BMD.
Table 2.
MIC concordance of different assays compared to the reference BMD method
| Method | BMD | Vitek 2® | UMIC | Colistin E-test MIC strip | |||
|---|---|---|---|---|---|---|---|
| Specie | No. Isolates tested | No. Isolates tested | No. of concordant isolates [%]a | No. Isolates tested | No. of concordant isolates [%]a | No. Isolates tested | No. of concordant isolates [%]a |
| Acinetobacter spp. | 7 | 4 | 4 [100.0] | 7 | 7 [100.0] | 7 | 6 [85.7] |
| C. koseri | 3 | 3 | 3 [100.0] | 3 | 2 [66.7] | 3 | 2 [66.7] |
| K. aerogenes | 2 | 2 | 1 [50.0] | 2 | 1 [50.0] | 2 | 2 [100.0] |
| E. cloacae | 5 | 5 | 3 [60.0] | 5 | 5 [100.0] | 5 | 3 [60.0] |
| E. coli | 24 | 24 | 17 [70.8] | 24 | 19 [79.2] | 24 | 13 [54.2] |
| Hafnia spp. | 15 | 15 | 14 [93.3] | 15 | 14 [93.3] | 15 | 13 [86.7] |
| K. oxytoca | 3 | 3 | 1 [33.3] | 3 | 3 [100.0] | 3 | 1 [33.3] |
| K. pneumoniae | 20 | 19 | 16 [84.2] | 20 | 17 [85.0] | 20 | 13 [65.0] |
| P. aeruginosa | 10 | 8 | 7 [87.5] | 10 | 10 [100.0] | 10 | 8 [80.0] |
| Total | 97 | 91 | 66 [72.5] | 97 | 78 [80.4] | 97 | 61 [62.9] |
BMD Broth microdilution method
aConcordance was considered as the same MIC value or as one titre difference to that of the reference value obtained by BMD
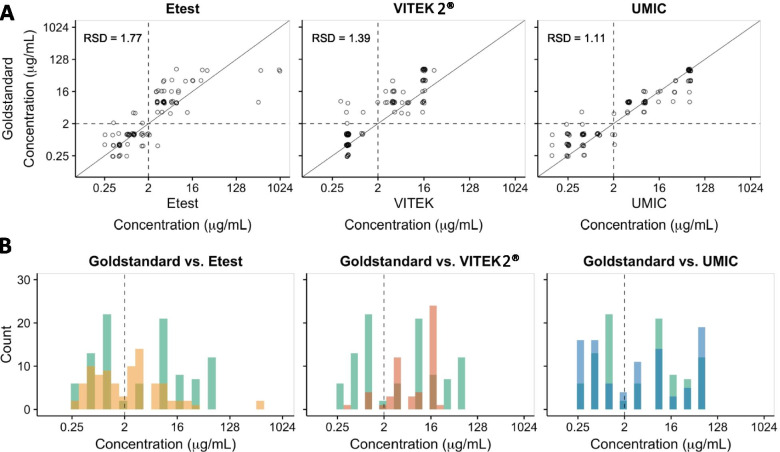
Additionally, for Enterobacterales species the most concordant test to the gold standard was UMIC (76.25%), followed by Vitek 2® (69.62%) and Colistin E-test MIC strip (58.75%). Similarly, the highest concordance for non-fermenting bacteria was found in the UMIC test (100%). The Vitek 2® test and the Colistin E-test MIC strip achieved a concordance to the gold standard of 91.67 and 82.35%, respectively. Figure 2 shows the MIC distribution of the BMD vs. each method and the MIC distribution for all isolates.
Fig. 2.
Distribution of MICs in BMD vs. respective phenotypic test. A MIC correlation of Colistin E-test MIC strip, Vitek 2®, and UMIC against the reference BMD. RSD, relative standard deviation. B Number of isolates per MIC tested by Colistin E-test MIC strip (yellow bars), Vitek 2® (orange bars), and UMIC (blue bars) compared to the number of isolates per MIC tested by the reference BMD. Dark coloured bars indicate concordant results
Heterogenous molecular causes of colistin resistance
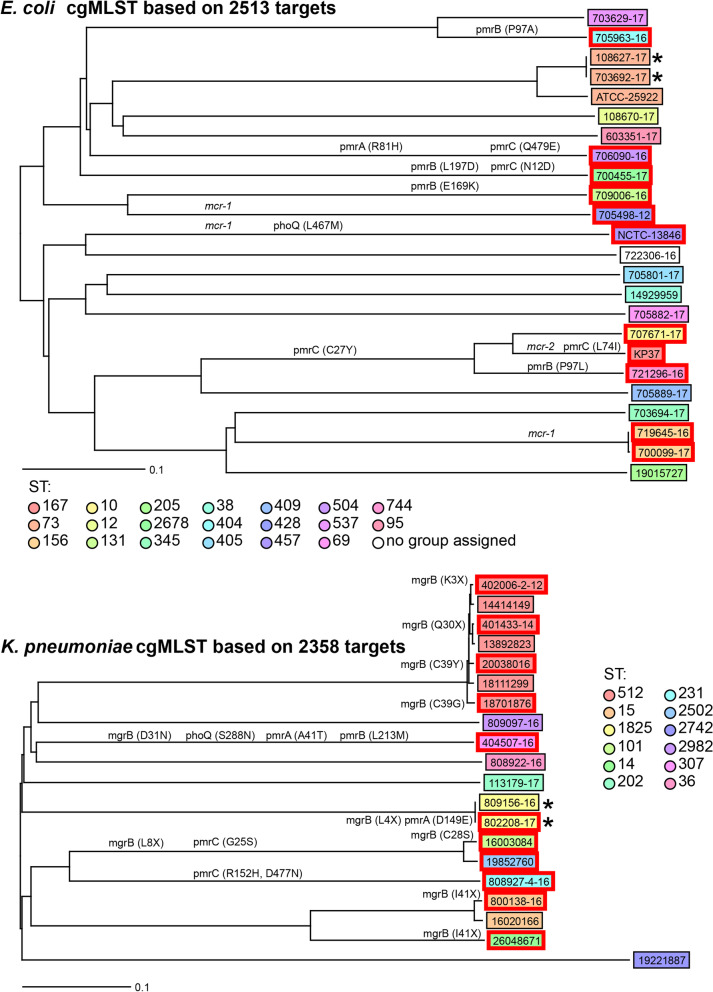
MLST sequence type (ST) designation and core genome MLST (cgMLST) analyses were used to explore the genetic diversity between bacterial isolates. The STs of isolates for which species MLST schemes exist was determined (Additional file 2). cgMLST comparison could only be performed on species with more than two isolates. The diversity within E. coli and K. pneumoniae are shown in Fig. 3. Isolates were genomically diverse in the cgMLST, with the exception of isolates from the same patients (indicated with * in the figures): in some cases multiple isolates belonged to the same ST, such as K. pneumoniae ST512 (n = 7), K. pneumoniae ST1825 (n = 2), E. coli ST73 (n = 3), and E. coli ST156 (n = 2).
Fig. 3.
Core genome MLST Neighbour Joining trees of E. coli and K. pneumoniae. MLST sequence types are shown. Scale bar indicates the number of variant alleles relative to the total number of targets for that species. * indicates isolates from the same patient. Red boxes around isolate names indicate colistin resistance. Mutations associated with colistin resistance are shown on the relevant branches. Acquired plasmid-mediated genes associated with resistance are also shown on the relevant branches in italics
Genes encoding colistin resistance were identified first by comparing genome assemblies against known databases. This identified mcr-1 in four isolates (NCTC-13846 as the control, 700,099-17, 719,645-16 and 705,498-12) and mcr-2 in one isolate (KP-37 as expected), but these results did not explain all the phenotypic resistance.
Individual genomic analysis to determine the underlying colistin resistance mechanisms was performed, looking at genes previously described as being involved in colistin resistance. These genes were extracted from the genome and compared between sensitive and resistant isolates. This could only be performed on isolates within species with sufficient numbers of each, namely K. pneumoniae (n = 20) and E. coli (n = 24). The nucleotide sequences and derived protein sequences from phoPQ and pmrCAB in both species, and additionally mgrB in K. pneumoniae isolates were compared between resistant and susceptible isolates (Additional files 3 and 4). Variations unique to the resistant isolates are described in Table 3.
Table 3.
Mutations in associated colistin-resistance proteins in E. coli, K. pneumoniae and A. bereziniae resistant isolates
| Species | Isolate | MIC (mg/L)a | Amino acid change | Plasmid mediated resistance | |||||
|---|---|---|---|---|---|---|---|---|---|
| MgrB | PmrB | PmrA | PmrC | PhoP | PhoQ | mcr | |||
| K. pneumoniae | 404,507-16 | ≥64 | D31N | L213M | A41T | S288N | |||
| 4,002,006-2 | 32 | K3* | |||||||
| 16,003,084 | 32 | C28S | A217V | G25S | |||||
| 20,038,016 | 32 | C39Y | |||||||
| 808,927-16 | 32 | L8* | R152H | ||||||
| D477N | |||||||||
| 26,048,671 | 32 | I41* (ISKpn26)b | |||||||
| 800,138-16 | 32 | I41* (ISEcp1)b | |||||||
| 401,433-14 | 16 | Q30* | |||||||
| 802,208-17 | 16 | L4* | D149E | ||||||
| 187,701,876 | 16 | C39G | |||||||
| 19,852,760 | 8 | A217V | G25S | ||||||
| 809,156-16 | 4 | D149E | |||||||
| E. coli | 721,296-16 | 16 | P97L | C27Y | |||||
| 700,455-17 | 16 | L197D | N12D | ||||||
| 709,006-16 | 16 | E169K | |||||||
| 705,963-16 | 16 | P97A | |||||||
| 700,099-17 | 8 | mcr 1.1 | |||||||
| 706,090-16 | 8 | R81H | Q479E | ||||||
| NCTC-13846 | 8 | L467M | mcr 1.1 | ||||||
| KP-37-MCR-2-18 | 8 | C27Y | mcr 2 | ||||||
| L74I | |||||||||
| 707,671-17 | 8 | C27Y | |||||||
| 719,645-16 | 8 | mcr 1.1 | |||||||
| 705,498-12 | 4 | mcr 1.1 | |||||||
| A. bereziniae | 502,814-14 | ≥64 | Q242R | ||||||
aMIC values obtained by the reference broth microdilution method
bNucleotide sequence interrupted by insertion sequence. * indicates premature stop codons or termination in the amino acid sequence indicates premature stop codons or termination in the amino acid sequence
None of the analysed K. pneumoniae isolates were carriers of mcr genes. A key finding within K. pneumoniae isolates was the presence of mutations in mgrB causing amino acid substitutions, premature stop codons, or termination resulting from insertion sequences ISEcp1 (IS138 family) and ISkpn26 (IS5 family). This applied to all resistant isolates with MIC values of ≥16 mg/L, whereas sensitive isolates have intact versions of mgrB. Further mutations leading to amino acid substitutions were found in other genes: the isolate with the highest MIC (≥64 mg/L) possesses additional amino acid substitutions in the PhoQ, PmrA, and PmrB proteins compared to those from sensitive isolates. Similarly, two isolates with MIC of 32 mg/L have amino acid substitutions in the PmrA and/or PmrC proteins in addition to MgrB. Two resistant isolates with MICs of 4 mg/L and 8 mg/L have an unaltered mgrB gene but possesses non-synonymous mutations in the pmrA and/or pmrC genes.
Only four of the resistant E. coli isolates, with MICs between 4 and 8 mg/L were carriers of the mcr-1.1 gene. Gene comparisons within E. coli showed that those with a MIC of 16 mg/L have amino acid changes in the PmrB protein sequence, whereas the isolates with a lower MIC (4-8 mg/L) possess PmrB identical to those in sensitive isolates. Two isolates with a MIC of 8 mg/L that did not harbour mcr genes had an unaltered PmrB protein sequence compared to the susceptible isolates but had amino acid substitutions in the protein sequences of PmrA or PmrC.
All the A. bereziniae isolates (n = 4) were isolated from the same patient, and were subjected to analysis of pmrAB and phoPQ genes, as well as lpxA, lpxD, lpxC genes since mutations in the latter genes produce a total loss of lipid A leading to colistin resistance in Acinetobacter spp. [5]. The single resistant isolate (MIC 64 mg/L) had a mutation causing amino acid change Q242R in PmrB (Additional file 5). No mutations were found in the other genes assessed.
Discussion
We compared the performance of four commercial assays for colistin susceptibility testing to the BMD gold standard. We used a panel of different bacterial species to reflect common situations found in routine diagnostics.
Numerous comparative studies dealing with colistin susceptibility testing have been published. Several studies find the highest rate of very major errors (VMEs), considered as discrepancy in the susceptibility category between a commercial kit and the reference method, with Colistin E-test MIC strip colistin [6, 29, 30], which was also confirmed with our study. Vitek 2® has been reported as reliable in some studies, but not in others [6, 31, 32]. In our study, the Rapid Polymyxin™ NP test showed the highest concordance with the gold standard in the susceptibility category agreement. However, in our experience, the main drawback is lack of MIC values and difficulties in interpretation of the colorimetric test. Additionally, the Rapid Polymyxin™ NP test showed a low concordance (50%) to the reference method when assessing the susceptibility of P.aeruginosa and Acinetobacter spp., but a high concordance for Enterobacterales. The Rapid Polymyxin™ NP test used in this study was clinically validated only with the most representative species of Enterobacterales [33]. Here we show that the concordance was low for the P.aeruginosa (n = 10) and Acinetobacter spp. (n = 7) isolates included in this study and, therefore, it would be interesting to analyse the performance of this test with a larger subset of isolates. The UMIC test was easy to perform and showed highest categorical and MIC agreement. This has, however, not been confirmed in other studies. Due to the high performance of the UMIC assay, this assay was established into routine diagnostics at the University Hospital Basel.
For this study we included 54 (56%) colistin resistant isolates, of which 23 (43%) were intrinsically resistant whereas 31 (57%) had an acquired resistance mechanism. All the isolates were collected up to 2018 but the susceptibility or resistance to colistin was determined following the EUCAST Version 10.02020 [23]. The breakpoints for Enterobacterales in the 2020 EUCAST version remained unaltered compared to previous versions from before 2018 [34] that applied when the isolates were collected, whereas the breakpoint for Pseudomonas spp. changed to lower MIC values (S ≤ 2 mg/L, R > 2 mg/L). However, these cut off changes did not affect the percentage of resistant isolates included in this study. Four E. coli isolates were carriers of the mcr-1 gene, and one isolate was positive for mcr-2. As expected, none of the mcr-positive isolates displayed a high MIC value, considered in this study as ≥16 mg/L [35, 36].
Several chromosomal mutations have been reported to be linked to colistin resistance in various species [4, 5, 16, 37, 38]. Most of the reported mutations have been found in genes involved in signalling pathways that lead to modification or loss of lipid A from the LPS. Mutations in genes related to efflux pumps have also been described [39, 40]. The most commonly reported colistin-related mutations are encountered in the pmrA/pmrB and phoP/phoQ genes encoding two-component systems in several Gram-negative bacteria such as E. coli, K. pneumoniae, P. aeruginosa and A. baumannii [5]. The mgrB gene encodes a negative regulator of the PhoPQ system. mgrB inactivation or disruption have been associated with colistin resistance in K. pneumoniae [19, 41]. Although these are the most commonly described genes the chromosomal mechanisms leading to resistance are highly diverse and involve numerous and different mutations and genes [5].
In this study, we found that all the K. pneumoniae isolates displaying a high MIC (≥16 mg/L) had a disrupted or altered mgrB gene and an altered protein sequence, whereas isolates with MICs ≤8 mg/L displayed a wild type mgrB. Some of the amino acid alterations encountered in MgrB during this study (truncations at K3 and Q30, substitutions at C28S, C39Y/G and disruption of the gene by ISKpn26 and ISEcp1) have been described in other studies [41, 42]. However, to our best knowledge, the truncations at L4 and L8, and the D31N amino acid change are novel to this study. Noteworthy, the cgMLST analysis identified several closely related K. pneumoniae isolates (ST512) with different susceptibilities to colistin. Within this ST type, all resistant isolates carried mutations in the mgrB gene, leading to altered MgrB protein sequences, and in no other colistin resistance-related protein sequences we analysed. However, these alterations were unique in each isolate and appear to have occurred independently. Additionally, we identified further mutations in the mgrB gene in isolates from other STs, which also led to high MIC values. These results suggest that mutations of mgrB in any of these locations can lead to high colistin resistance in K. pneumoniae and that there is not a unique mgrB mutation associated with a specific ST type. The single K. pneumoniae isolate with a MIC ≥64 mg/L also carried altered pmrB and phoQ in addition to an mgrB mutant (causing D31N). The mutation in pmrB (causing L213M) has already been associated with colistin resistance in K. pneumoniae [41]. This may indicate that association between pmrB and/or phoQ mutations with mgrB mutations confer a higher resistance than these mutations on their own. Together this data suggests that mutation or inactivation of the mgrB gene leads to MICs ≥16 mg/L, with further mutations in pathway genes able to synergistically increase MICs further.
Similarly, all the resistant E. coli isolates with MIC of 16 mg/L carried amino acid changes in PmrB. Of these amino acid substitutions (P97L, P97A, E169K and L197D), only that at position 97 has been reported previously, in a clinical colistin resistant isolate from a Lebanese hospital, also displaying a MIC of 16 mg/L [43], although the amino acid change in this position was different to the ones in our study. As far as we are aware, the other two PmrB alterations have not been described, although other alterations within the same domains, namely the HAMP domain (covering residues 92-144) and histidine-kinase domain (residues 145-205) have been reported [5, 10, 37]. Further amino acid changes were found in PmrA, PmrC and PhoQ in isolates with MIC between 4 and 8 mg/L, suggesting that alterations in these proteins may confer a lower level of resistance. Of these, only the amino acid change at position 81 in PmrA from E. coli has been previously characterized, in an isolate from swine origin and with a colistin MIC of 4 mg/L [22]. In this published case, it was not determined whether the mutation in PmrA was the sole cause of colistin resistance, as other mutations were also identified within this isolate.
Total loss of lipid A of the LPS by alteration of lxpA, lxpC and lpxD genes has been described as a colistin resistance mechanism in Acinetobacter baumannii [5]. Mutations in pmrAB also lead to colistin resistance in this species [23, 44, 45]. A published comparison between susceptible and resistant A. baumannii isolates after in vivo exposure in three different patients found different but unique mutations in pmrB that led to resistance levels of 16 mg/L [45]. Similarly, we found that the only difference between the susceptible and resistant A. bereziniae isolates from the same patient was a single amino acid change (Q242R) in the PmrB protein. Interestingly, the resistant isolate was highly resistant to colistin (MIC ≥64 mg/L). Detection of an alteration at this same position has so far not been reported. This may suggest that alterations in PmrB in Acinetobacter spp. and/or the specific PmrB alteration encountered in this study (Q242R) are putative mechanisms that confer high levels colistin resistance.
Typing isolates shows that resistance can occur in diverse isolates within the species we analysed. Our data also suggests the impact of selective pressure, with stochastic presence of resistance throughout the phylogenies, and resistant isolates of A. bereziniae and K. pneumoniae (ST512) closely related to sensitive isolates.
This study has several limitations. Only small number of isolates (n = 10) showed MICs close to the breakpoint. Future studies should include more isolates close to the breakpoint. Similarly, a lower number of non-fermenting bacterial isolates were analysed in this study compared to the number of Enterobacterales members included in this study. This may have affected the real performance and concordance to BMD. Again, studies including a higher number of non-fermenting bacteria should be carried out to better evaluate the performance of these tests. Secondly, we were not able to investigate putative resistance mechanisms in all species due to the low number of isolates. Thirdly, we have investigated putative resistance mechanisms in diverse clinical isolates and have not confirmed the effects of the observed mutations in isogenic backgrounds.
Conclusions
In summary, in our clinical setup MIC values provide important information. The UMIC assay provided the highest concordance on MIC values with the reference method. For a categorical assessment the Rapid Polymyxin™ NP test provided highly concordant results. Our genetic study identified highly heterogenous putative causes of resistance. Whereas some resistance assays may cause only small differences in MICs determined, sensitive and precise phenotypic assays are important in routine diagnostics.
Materials and methods
Ethical statement
All strains were collected as part of quality control purposes and establishment of new diagnostic assays. All strains were used in anonymized way and no clinical data was collected. For these quality control studies no ethical approval is necessary according to the Human Research Act in Switzerland.
Clinical isolates and culture conditions
We used 97 isolates from the Enterobacterales order and non-fermenting bacteria: 93 from clinical samples in the period from 2008 to 2018 at the clinical microbiology laboratories of the University Hospital Basel, Switzerland; Cantonal Hospital Lucerne, Switzerland and laboratory Viollier in Allschwil, Switzerland (Additional file 6), and four reference strains: E. coli ATCC-25922, E. coli NCTC-13846 (mcr-1), P. mirabilis ATCC-25933 and E. coli KP-37 (mcr-2) [46]. All colistin resistant isolates tested with Vitek 2® (bioMérieux, Marcy’l Etoile, France) from the University Hospital Basel were included in the study. The strain collection included 43 colistin sensitive isolates.
Species identification was performed at the time of diagnosis with matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS; Bruker, Bremen, Germany) by using the mass-spectrum library and the MALDI Biotyper 3 software (OC 3.1, Bruker Daltonics) at standard conditions. All bacterial isolates were frozen at − 70 °C in cryogenic Microbank™ vials (Pro-Lab Diagnostics, Birkenhead, UK). Prior to testing, the strains were cultured on Columbia agar supplemented with 5% sheep blood (BD Diagnostic Systems, Allschwil, Switzerland) with subsequent subculture after 24 h.
Assays for colistin susceptibility testing
Standard BMD was performed according to EUCAST recommendations by using 11 concentrations ranging from 0.06 to 64 mg/L including a growth control without colistin [47]. Colistin susceptibility testing with Vitek 2® (bioMérieux) was performed by using the AST N242 card. For calculation of colistin MIC, the following dilutions were tested: 4 mg/L, 16 mg/L and 32 mg/L. MIC determination was performed with Colistin E-test MIC Strip (Liofilchem Diagnostici, Roseto degli Abruzzi, Te, Italy) (Additional file 7). UMIC is a manual broth microdilution test and was performed according to the manufacturer’s instructions. Briefly, a 1:200 dilution of a 0.5 Mc Farland solution of bacteria in Mueller-Hinton II broth was inoculated in the UMIC strips and incubated in a humid atmosphere at 35-37 °C for 18 h. The MICs were read visually (turbid = growth, clear = no growth) (Additional file 7). Rapid Polymyxin™ NP test (ELITechGroup) is based on the colourimetric detection of rapid glucose metabolism associated with bacterial growth, through a pH indicator colour change from orange to yellow, in the presence of a defined concentration of colistin. The test was read after 2 and 3 h of incubation and the results were recorded as either colistin susceptible or colistin resistant (without MIC value) (Additional file 7). Reading of all assays was performed with two independent persons in a blinded fashion. If the results were discrepant, the testing was repeated. Susceptibility (susceptible or resistant) category concordance was considered if there was a categorical agreement to the standard BMD. The MIC variation of ±1 titre range compared to reference MIC was considered as concordant. All MICs were interpreted according to EUCAST Version 10.0, 2020 [48].
Whole genome sequencing and antimicrobial resistance gene detection
All isolates underwent whole genome sequencing on an Illumina Miseq 2x300bp or NextSeq 2x150bp after NexteraXT library preparation to mean coverage over 35x. All data is available under project number PRJEB47075. Assembly was performed, after trimming with trimmomatic v 0.38 [49], with Unicycler v0.3.0b [50] using standard settings. The assemblies were annotated with Prokka v1.13 [51] and ABRicate v0.8.10 [52] was used to search for antimicrobial resistance genes using the NCBI or CARD AMR gene databases. Species were identified using ribosomal MLST [53] and Average Nucleotide Identity (ANI) comparisons for Klebsiella spp. Where discrepancies in species classification between the original MALDI-TOF MS identification and ribosomal MLST identification from whole genome sequencing data occurred, the ribosomal MLST was taken as accurate. In particular this was important for the classification of isolates with known low resolution in MALDI-TOF MS such as Klebsiella oxytoca or Klebsiella michiganensis, and between species belonging to the E. cloacae complex- namely E. cloacae, E. homaechei and E. bugandensis. Assemblies were typed by core genome multi-locus sequencing typing (cgMLST) within Seqphere+ (Ridom, Münster, Germany) using relevant published schemes where available [54] or ad hoc schemes where unavailable for that species. Detailed searches of genome assemblies from resistant isolates and also from susceptible isolates as negative controls, were performed using visualization in Artemis v 18.1.0 [55], with alignments in Jalview v 2.11.1.0 [56]. Where assemblies required checking by remapping, this was done in CLC Genomics Workbench v20.0.2, using default mapping parameters. Insertion sequence families were determined using ISFinder [57].
Supplementary Information
Additional file 1. Susceptibility category (susceptible or resistant) concordance of different assays compared to the reference broth microdilution method in Enterobacterales species and non-fermenting bacteria.xlsx showing the percentage of concordance for different test in Enterobacterales and non-fermenting bacteria.
Additional file 2. Species identification by MALDI-TOF MS and rMLST from whole genome sequencing data.docx showing the difference in species identification by MALDI-TOF MS and rMLST as well as the ST types of every isolate included in this study.
Additional file 3. Klebsiella pneumoniae protein sequence alignments.pdf showing the protein sequences alignments of all colistin resistance-related proteins analyzed in this study.
Additional file 4. Escherichia coli protein sequence alignments.pdf showing the protein sequences alignments of all colistin resistance-related proteins analyzed in this study.
Additional file 5. Acinetobacter bereziniae protein sequence alignments.pdf showing the protein sequences alignments of all colistin resistance-related proteins analyzed in this study.
Additional file 6. Species distribution as determined by MALDI-TOF MS. A total of 97 isolates were investigated, including 93 collected collected from the Basel University Hospital (Basel, Switzerland), Cantonal Hospital Luzerne (Luzerne, Switzerland) and Laboratory Viollier (Allschwil, Switzerland)
Additional file 7. Different phenotypic assays used for colistin susceptibility testing: UMIC, Colistin E-Test MIC strip and Rapid Polymyxin NP Test.docx showing example images of the phenotypic assays using in this study.
Acknowledgements
We thank Aline Cuenod (University of Basel) for assistance with Klebsiella species identification. We thank Dr. Janina E. Linnik (ETH Zurich) for visualization of MIC distributions. We thank Christine Kiessling, Magdalena Schneider, Elisabeth Schultheiss, Clarisse Straub, and Rosa-Maria Vesco (University Hospital Basel) for excellent technical assistance in whole genome sequencing. Assemblies and searches were performed at sciCORE (http://scicore.unibas.ch/) scientific computing centre at the University of Basel. We thank Prof. Herbert Hächler (NENT, University of Zurich) for reference measurements of colistin MICs. We thank the Luzerner Kantonsspital for providing strains also included in this study.
Abbreviations
- LPS
Lipopolysaccharide
- L-Ara-4 N
4-amino-4-deoxy-L-arabinose
- pEtN
Phosphoethanolamine
- MIC
Minimum inhibitory concentration
- CPS
Capsular polysaccharide
- pmrA
Two-component regulator system response regulator PmrA
- pmrB
Two-component regulator system response regulator PmrB
- phoP
Response regulator in two-component regulatory system with PhoQ
- phoQ
Two-component system sensor histidine kinase PhoQ
- mgrB
PhoP/PhoQ regulator MgrB
- mcr
Mobilized colistin resistance
- lpxA
UDP-N-acetylglucosamine acyltransferase
- lpxC
UDP-3-O-acyl-N-acetylglucosamine deacetylase
- lpxD
UDP-3-O-(3-hydroxymyristoyl)glucosamine N-acyltransferase
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- BMD
Broth microdilution
- MLST
Multilocus sequence typing
- cgMLST
Core genome Multilocus sequence typing
- NCBI
National Center for Biotechnology Information
- CARD AMR
The Comprehensive Antibiotic Resistance Database
- ANI
Average Nucleotide Identity
- MALDI-TOF MS
Matrix-Assisted Laser Desorption Ionization T-Of-Flight Mass Spectrometry
- VME
Very major errors
- HAMP
Histidine kinases, adenylyl cyclases, methyl binding proteins, and phosphatases
Authors’ contributions
AE designed the study and supervised the phenotypical part. DAT and HMBSS performed whole genome sequencing data analysis and drafted the first version of the manuscript. All authors revised the manuscript and provided feedback. NJ performed the phenotypic analysis. CL, OD and MNI contributed by providing isolates for characterisation. VH designed and supervised the phenotypic analysis. The author(s) read and approved the final manuscript.
Funding
The study was financed by unrestricted funding from the University of Basel and University Hospital Basel. The funding bodies did not have a role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The dataset(s) supporting the conclusions of this article are included within the article and its additional files, or submitted to the ENA under project PRJEB47075.
Declarations
Ethics approval and consent to participate
This study was conducted as a quality control study and for assay development – no patient data was used. According to the Swiss Human Research this does not require a specific ethical approval. All strains used in this study were anonymized and no associated clinical data was collected. For this reason, no ethical approval was necessary.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Diana Albertos Torres and Helena M. B. Seth-Smith contributed equally to this work.
References
- 1.Medicines Agency E . Updated advice on the use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health. 2016. [Google Scholar]
- 2.Ecdc . PRESS RELEASE Resistance to last-line antibiotics continues to cause concern in Europe. 2014. [Google Scholar]
- 3.Nordmann P, Naas T, Poirel L. Global spread of carbapenemase producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghapour Z, Gholizadeh P, Ganbarov K, Bialvaei AZ, Mahmood SS, Tanomand A, et al. Molecular mechanisms related to colistin resistance in enterobacteriaceae. Infect Drug Resist. 2019;12:965–975. doi: 10.2147/IDR.S199844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirel L, Jayol A, Nordmanna P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew KL, Van LM, Lin RTP, Teo JWP. Colistin and polymyxin B susceptibility testing for carbapenem-resistant and mcr-positive enterobacteriaceae: comparison of Sensititre, MicroScan, Vitek 2, and Etest with broth microdilution. J Clin Microbiol. 2017;55(9):2609–2616. doi: 10.1128/JCM.00268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deris ZZ, Akter J, Sivanesan S, Roberts KD, Thompson PE, Nation RL, et al. A secondary mode of action of polymyxins against Gram-negative bacteria involves the inhibition of NADH-quinone oxidoreductase activity. Available from: http://www.nature.com/authors/editorial_policies/license.html#terms. Cited 2020 May 12. [DOI] [PMC free article] [PubMed]
- 8.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 9.El-Sayed Ahmed MAE-G, Zhong L-L, Shen C, Yang Y, Doi Y, Tian G-B. Colistin and its role in the Era of antibiotic resistance: an extended review (2000–2019) Emerg Microbes Infect. 2020;9(1):868–885. doi: 10.1080/22221751.2020.1754133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol. 2014;5(NOV):1–18. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scoffone VC, Chiarelli LR, Trespidi G, Mentasti M, Riccardi G, Buroni S. Burkholderia cenocepacia infections in cystic fibrosis patients: drug resistance and therapeutic approaches. Front Microbiol. 2017;0(AUG):1592. doi: 10.3389/fmicb.2017.01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SA L, MA V. Extreme antimicrobial peptide and polymyxin B resistance in the genus Burkholderia. Front Microbiol 2011;2. Available from: https://pubmed.ncbi.nlm.nih.gov/21811491/. Cited 2021 Aug 9. [DOI] [PMC free article] [PubMed]
- 13.Kumar M, Gupta A, Sahoo RK, Jena J, Debata NK, Subudhi E. Functional genome screening to elucidate the colistin resistance mechanism. Sci Rep. 2016;6(March):1–7. doi: 10.1038/srep23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EUCAST Expert Rules version 2. Available from: http://www.eucast.org/expert_rules_and_intrinsic_resistance/. Cited 2021 Aug 16.
- 15.Jayol A, Saly M, Nordmann P, Ménard A, Poirel L, Dubois V. Hafnia, an enterobacterial genus naturally resistant to colistin revealed by three susceptibility testing methods. J Antimicrob Chemother. 2017;72(9):2507–2511. doi: 10.1093/jac/dkx154. [DOI] [PubMed] [Google Scholar]
- 16.Mlynarcik P, Kolar M. Molecular mechanisms of polymyxin resistance and detection of mcr genes. Biomed Pap. 2019;163(1):28–38. doi: 10.5507/bp.2018.070. [DOI] [PubMed] [Google Scholar]
- 17.Gharaibeh MH, Shatnawi SQ. An overview of colistin resistance, mobilized colistin resistance genes dissemination, global responses, and the alternatives to colistin: a review. Vet World. 2019;12(11):1735–1746. doi: 10.14202/vetworld.2019.1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colistin M, Genes R. Global burden of colistin-resistant bacteria: mobilized colistin resistance genes study. 2019. pp. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirel L, Jayol A, Bontron S, Villegas MV, Ozdamar M, Türkoglu S, et al. The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70(1):75–80. doi: 10.1093/jac/dku323. [DOI] [PubMed] [Google Scholar]
- 20.De Majumdar S, Yu J, Fookes M, McAteer SP, Llobet E, Finn S, et al. Elucidation of the RamA Regulon in Klebsiella pneumoniae reveals a role in LPS regulation. PLoS Pathog. 2015;11(1):1–22. doi: 10.1371/journal.ppat.1004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castanheira M, Castanheira M, Doyle T, Davis AP, Deshpande LM, Mendes RE. Disruption of mgrB and alterations on pmrB are most common resistance mechanisms among colistin-resistance among Klebsiella pneumoniae from a global surveillance program. Available from: http://www.eucast.org/fileadmin/src/media. Cited 2020 May 19.
- 22.Quesada A, Concepción Porrero M, Téllez S, Palomo G, García M, Domínguez L. Polymorphism of genes encoding PmrAB in colistin-resistant strains of Escherichia coli and Salmonella enterica isolated from poultry and swine. J Antimicrob Chemother. 2015;70(1):71–74. doi: 10.1093/jac/dku320. [DOI] [PubMed] [Google Scholar]
- 23.Boinett CJ, Cain AK, Hawkey J, Do Hoang NT, Khanh NNT, Thanh DP, et al. Clinical and laboratory-induced colistin-resistance mechanisms in Acinetobacter baumannii. Microb Genomics. 2019;5(2):e000246. doi: 10.1099/mgen.0.000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cafiso V, Stracquadanio S, Lo Verde F, Gabriele G, Mezzatesta ML, Caio C, et al. Colistin resistant A. baumannii: genomic and transcriptomic traits acquired under colistin therapy. Front Microbiol. 2019;0:3195. doi: 10.3389/fmicb.2018.03195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park YK, Lee JY, Ko KS. Transcriptomic analysis of colistin-susceptible and colistin-resistant isolates identifies genes associated with colistin resistance in Acinetobacter baumannii. Clin Microbiol Infect. 2015;21(8):765.e1–765.e7. doi: 10.1016/j.cmi.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Turlej-Rogacka A, Xavier BB, Janssens L, Lammens C, Zarkotou O, Pournaras S, et al. Evaluation of colistin stability in agar and comparison of four methods for MIC testing of colistin. Eur J Clin Microbiol Infect Dis. 2018;37(2):345–353. doi: 10.1007/s10096-017-3140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EUCAST. Guidance documents. Available from: https://www.eucast.org/ast_of_bacteria/guidance_documents/. Cited 2020 May 19.
- 28.Rapid Polymyxin™ NP – ELITechGroup. In vitro diagnostic equipment & reagents. Available from: https://www.elitechgroup.com/product/rapid-polymyxinnp#tab-features. Cited 2021 Apr 23.
- 29.Arroyo LA, García-Curiel A, Pachón-Ibañez ME, Llanos AC, Ruiz M, Pachón J, et al. Reliability of the E-test method for detection of colistin resistance in clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 2005;43(2):903–905. doi: 10.1128/JCM.43.2.903-905.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matuschek E, Åhman J, Webster C, Kahlmeter G. Antimicrobial susceptibility testing of colistin – evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin Microbiol Infect. 2018;24(8):865–870. doi: 10.1016/j.cmi.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Dafopoulou K, Zarkotou O, Dimitroulia E, Hadjichristodoulou C, Gennimata V, Pournaras S, et al. Comparative evaluation of colistin susceptibility testing methods among carbapenem-nonsusceptible Klebsiella pneumoniae and Acinetobacter baumannii clinical isolates. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Angelis G, Posteraro B, Menchinelli G, Marzia Liotti F, Spanu T, Sanguinetti M. Antimicrobial susceptibility testing of pathogens isolated from blood culture: a performance comparison of Accelerate Pheno TM and VITEK V R 2 systems with the broth microdilution method. J Antimicrob Chemother. 2019;74(Suppl 1):i24–i31. 10.1093/jac/dky532. [DOI] [PMC free article] [PubMed]
- 33.Jayol A, Kieffer N, Poirel L, Guérin F, Güneser D, Cattoir V, et al. Evaluation of the rapid Polymyxin NP test and its industrial version for the detection of polymyxin-resistant Enterobacteriaceae. Diagn Microbiol Infect Dis. 2018;92(2):90–94. doi: 10.1016/j.diagmicrobio.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 34.EUCAST. Previous versions of documents. Available from: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/. Cited 2021 Mar 31.
- 35.(No Title). Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf. Cited 2020 May 19.
- 36.Luo Q, Yu W, Zhou K, Guo L, Shen P, Lu H, Huang C, Xu H, Xu S, Xiao Y, Li L. Molecular epidemiology and colistin resistant mechanism of mcr-positive and mcr-negative clinical isolated Escherichia coli. Front Microbiol. 2017;8:2262. 10.3389/fmicb.2017.02262. [DOI] [PMC free article] [PubMed]
- 37.Bialvaei AZ, Samadi Kafil H. Colistin, mechanisms and prevalence of resistance. Curr Med Res Opin. 2015;31(4):707–721. doi: 10.1185/03007995.2015.1018989. [DOI] [PubMed] [Google Scholar]
- 38.Berglund B. Acquired resistance to colistin via chromosomal and plasmid-mediated mechanisms in Klebsiella pneumoniae. Infect Microbes Dis. 2019;1(1):10–19. [Google Scholar]
- 39.Sundaramoorthy NS, Suresh P, Selva Ganesan S, GaneshPrasad AK, Nagarajan S. Restoring colistin sensitivity in colistin-resistant E. coli: combinatorial use of MarR inhibitor with efflux pump inhibitor. Sci Rep. 2019;9(1) Available from: https://pubmed.ncbi.nlm.nih.gov/31882661/. Cited 2020 Jul 13. [DOI] [PMC free article] [PubMed]
- 40.Lin MF, Lin YY, Lan CY. Contribution of EmrAB efflux pumps to colistin resistance in Acinetobacter baumannii. J Microbiol. 2017;55(2):130–136. doi: 10.1007/s12275-017-6408-5. [DOI] [PubMed] [Google Scholar]
- 41.Castanheira M, Castanheira M, Doyle T, Davis AP, Deshpande LM, Mendes RE. Disruption of mgrB and alterations on pmrB are most common resistance mechanisms among colistin-resistance among Klebsiella pneumoniae from a global surveillance program. Available from: http://www.eucast.org/fileadmin/src/media. Cited 2020 May 14.
- 42.Jayol A, Nordmann P, Lehours P, Poirel L, Dubois V. Comparison of methods for detection of plasmid-mediated and chromosomally encoded colistin resistance in Enterobacteriaceae. Clin Microbiol Infect. 2018;24(2):175–179. doi: 10.1016/j.cmi.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Nawfal Dagher T, Al-Bayssari C, Chabou S, Baron S, Hadjadj L, Diene SM, Azar E, Rolain JM. Intestinal carriage of colistin resistant Enterobacteriaceae at Saint Georges Hospital in Lebanon. J Glob Antimicrob Resist. 2020;21:386–390. 10.1016/j.jgar.2019.12.001. [DOI] [PubMed]
- 44.Ko KS, Choi Y, Lee J-Y. Old drug, new findings: colistin resistance and dependence of Acinetobacter baumannii. Precis Futur Med. 2017;1(4):159–167. doi: 10.23838/pfm.2017.00184. [DOI] [Google Scholar]
- 45.Marano V, Marascio N, Pavia G, Lamberti AG, Quirino A, Musarella R, et al. Identification of pmrB mutations as putative mechanism for colistin resistance in A. baumannii strains isolated after in vivo colistin exposure. Microb Pathog. 2020;142(November 2019):104058. doi: 10.1016/j.micpath.2020.104058. [DOI] [PubMed] [Google Scholar]
- 46.Xavier BB, Lammens C, Ruhal R, Malhotra-Kumar S, Butaye P, Goossens H, et al. Identification of a novel plasmid-mediated colistinresistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Eurosurveillance. 2016;21(27) Available from: https://pubmed.ncbi.nlm.nih.gov/27416987/. Cited 2021 Mar 30. [DOI] [PubMed]
- 47.EUCAST . Recommendations for MIC determination of colistin ( polymyxin E ) as recommended by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group. Http://WwwEucastOrg; 2016. [Google Scholar]
- 48.EUCAST clinical breakpoints - bacteria v 5.0 (until Dec 31, 2015). Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_5.0_Breakpoint_Table_01.pdf. Cited 2020 Jul 13.
- 49.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. Phillippy AM, editor. PLoS Comput Biol. 2017;13(6):e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 52.GitHub - tseemann/abricate: mass screening of contigs for antimicrobial and virulence genes. Available from: https://github.com/tseemann/abricate. Cited 2020 May 27.
- 53.Ribosomal Multilocus Sequence Typing (rMLST) - PubMLST.org. Available from: https://pubmlst.org/rmlst/. Cited 2020 May 27.
- 54.cgMLST.org Nomenclature Server. Available from: https://www.cgmlst.org/ncs. Cited 2020 May 27.
- 55.Carver T, Harris SR, Berriman M, Parkhill J, McQuillan JA. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics. 2011;28(4):464–469. doi: 10.1093/bioinformatics/btr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalview Version 2--a multiple sequence alignment editor and analysis workbench - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/19151095/. Cited 2020 May 27. [DOI] [PMC free article] [PubMed]
- 57.Siguier P, Pérochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34(Database issue) Available from: https://pubmed.ncbi.nlm.nih.gov/16381877/. Cited 2021 Aug 20. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Susceptibility category (susceptible or resistant) concordance of different assays compared to the reference broth microdilution method in Enterobacterales species and non-fermenting bacteria.xlsx showing the percentage of concordance for different test in Enterobacterales and non-fermenting bacteria.
Additional file 2. Species identification by MALDI-TOF MS and rMLST from whole genome sequencing data.docx showing the difference in species identification by MALDI-TOF MS and rMLST as well as the ST types of every isolate included in this study.
Additional file 3. Klebsiella pneumoniae protein sequence alignments.pdf showing the protein sequences alignments of all colistin resistance-related proteins analyzed in this study.
Additional file 4. Escherichia coli protein sequence alignments.pdf showing the protein sequences alignments of all colistin resistance-related proteins analyzed in this study.
Additional file 5. Acinetobacter bereziniae protein sequence alignments.pdf showing the protein sequences alignments of all colistin resistance-related proteins analyzed in this study.
Additional file 6. Species distribution as determined by MALDI-TOF MS. A total of 97 isolates were investigated, including 93 collected collected from the Basel University Hospital (Basel, Switzerland), Cantonal Hospital Luzerne (Luzerne, Switzerland) and Laboratory Viollier (Allschwil, Switzerland)
Additional file 7. Different phenotypic assays used for colistin susceptibility testing: UMIC, Colistin E-Test MIC strip and Rapid Polymyxin NP Test.docx showing example images of the phenotypic assays using in this study.
Data Availability Statement
The dataset(s) supporting the conclusions of this article are included within the article and its additional files, or submitted to the ENA under project PRJEB47075.