Abstract
Background
Chronic diseases are the leading cause of death worldwide. It is estimated that 20% of adults with chronic physical diseases experience concomitant depression, increasing their risk of morbidity and mortality. Low intensity psychosocial interventions, such as self-management, are part of recommended treatment; however, no systematic review has evaluated the effects of depression self-management interventions for this population. The primary objective was to examine the effect of self-management interventions on reducing depressive symptomatology in adults with chronic disease(s) and co-occurring depressive symptoms. Secondary objectives were to evaluate the effect of these interventions on improving other psychosocial and physiological outcomes (e.g., anxiety, glycemic control) and to assess potential differential effect based on key participant and intervention characteristics (e.g., chronic disease, provider).
Methods
Studies comparing depression self-management interventions to a control group were identified through a) systematic searches of databases to June 2018 [MEDLINE (1946 -), EMBASE (1996 -), PsycINFO (1967 -), CINAHL (1984 -)] and b) secondary ‘snowball’ search strategies. The methodological quality of included studies was critically reviewed. Screening of all titles, abstracts, and full texts for eligibility was assessed independently by two authors. Data were extracted by one author and verified by a second.
Results
Fifteen studies were retained: 12 for meta-analysis and three for descriptive review. In total, these trials included 2064 participants and most commonly evaluated interventions for people with cancer (n = 7) or diabetes (n = 4). From baseline to < 6-months (T1), the pooled mean effect size was − 0.47 [95% CI −0.73, − 0.21] as compared to control groups for the primary outcome of depression and − 0.53 [95% CI −0.91, − 0.15] at ≥ 6-months (T2). Results were also significant for anxiety (T1) and glycemic control (T2). Self-management skills of decision-making and taking action were significant moderators of depression at T1.
Conclusion
Self-management interventions show promise in improving depression and anxiety in those with concomitant chronic physical disease. The findings may contribute to the development of future Self-management interventions and delivering evidence-based care to this population. Further high-quality RCTs are needed to identify sources of heterogeneity and investigate key intervention components.
Keywords: Self-management, Chronic disease, Depression, Anxiety, Decision-making, Systematic review
Background
Prevalence and impact of depression in adults with chronic disease(s)
Depression affects 300 million people and is currently the leading cause of mortality worldwide substantially impacting social and occupational functioning [1–4]. Prevalence of depression is more common among individuals with chronic physical diseases [2, 3, 5]. Estimates indicate that approximately 20% of people with chronic physical diseases experience depression, at least twice the rate found in the general population [5–7].
Chronic diseases are increasingly prevalent and are currently estimated to account for 60% of all deaths worldwide [8, 9]. Studies have demonstrated that depression has a significant impact on the course and health outcomes of concomitant chronic physical diseases and complicates treatment [5, 6]. For example, depression has been shown to amplify somatic symptoms and diminish self-efficacy of health-related behaviours [6, 10, 11]. Furthermore, in addition to major depressive disorder, sub-threshold depression is associated with negative outcomes including increased morbidity in those with co-occurring physical diseases, and is also a risk factor for major depression [12–14].
Self-management interventions for depression
It is recognized that timely treatment of depression is likely to improve functional ability, quality of life, and will considerably reduce the burden of chronic physical disease on the care recipient [15, 16]. However, worldwide less than half of those affected by depression receive adequate treatment, as there is often limited care available or barriers to accessing it, particularly high cost and lack of mental health professionals [12, 17, 18]. In response to the need for more depression support, one cost-efficient option that has shown promise is self-management interventions. Self-management interventions for depression have been formally incorporated into many healthcare systems and are recommended in best practice guidelines [19–21]. The only guidelines specifically targeting the treatment of depression in those with concomitant chronic physical disease(s), recommend self-management interventions for the treatment of mild to moderate depressive symptoms or as adjunctive therapy in the case of more severe symptoms [22]. However, to our knowledge, no systematic review has evaluated the effects of depression self-management interventions across chronic physical disease populations.
Self-management refers to the tasks an individual must undertake to live well with their chronic disease(s) [23, 24]. Generally, these tasks involve: a) medical management; b) maintaining, changing, or developing new meaningful behaviours; and c) dealing with the emotional impacts of the disease(s) [25]. In self-management, the individual’s role in their own care is considered central and the individual takes on the responsibility, as much as possible, of developing the necessary skills to manage their symptoms.
Self-management includes more than providing health or disease-related information, rather it is focused on behaviour change through the development of the skills and confidence needed for successful self-management of chronic disease [26, 27]. This is usually accomplished through the use of psychoeducational or behaviour strategies [28]. Learning these self-management skills can be done independently or in collaboration with health care professionals (HCPs) or a non-professional, often peer, support person [25, 29].
Arguably the most robust conceptualization of self-management is that articulated by Drs. Lorig and Holman based on nearly 25 years of work in the area [25, 30, 31]. This theoretically informed research indicates that self-management involves core skills (e.g., problem-solving, decision-making) that can be applied across chronic diseases [25, 32, 33]. From this perspective, self-management is not considered disease specific, but is focused on the development of broad skills, and self-management programs may therefore include people with a variety of chronic diseases [32, 33]. Additional skills may also be targeted when supporting the learned management of particular diseases. Aligned with this, based on existing literature, self-management skills specific to depression have been identified including behavioural activation (e.g., increasing positive activities) and social support (see Appendix A for details and references).
Self-management, self-help, cognitive behavioural therapy, and self-care
A number of terms are often incorrectly used interchangeably with self-management, including self-help, cognitive behavioural therapy (CBT), and self-care. In comparison to self-management self-help encompasses a much broader range of interventions that are primarily self-directed (e.g., books, smartphone application). Self-help interventions are predominantly designed to limit contact with HCPs [34]. On the other hand, self-management interventions focus on specific skills and are often conducted in close collaboration with HCPs, although some may be self-directed [25]. Though many self-management interventions use principles of cognitive behavioural therapy (CBT), these are also distinct [29]. Self-management is one of the essential elements of the Chronic Care Model (CCM), an evidence-based guide to chronic disease management in primary care [26, 35]. Self-management interventions employ psychoeducational and/or behavioural strategies to assist care recipients and families develop the skills and confidence necessary to live well with a chronic disease (e.g., learning to locate resources, forming partnerships with HCPs) [25, 36]. Self-management is not a form of psychotherapy and rather than delivered by a therapist, self-management can be delivered by a variety of HCPs (e.g., it not a protected act) or as a self-directed intervention.
Finally, self-care is also frequently used interchangeably with self-management; however, they may be delineated on several fronts. Self-care involves managing one’s health, with or without a chronic condition. It also encompasses a broad range of strategies including ‘doing nothing.’ Another marked difference is that self-care does not usually involve HCP support and focuses primarily on health promotion or the prevention of disease or accident [37]. In contrast, as an element of the CCM, self-management was developed specifically for people with chronic disease and centres on the development of evidence-based skills [25, 28, 37].
Aims of this Review
Accounting for the issues outlined above, the primary objective of this review is to determine the effects of self-management interventions on reducing depressive symptomatology among adults with chronic physical disease(s) and co-occurring depressive symptoms. The secondary objectives are:
To determine whether the effects of self-management interventions on the primary outcome of depressive symptoms vary depending on the participants’ co-occurring chronic physical disease and baseline level of depressive symptoms (e.g., mild, moderate).
To assess whether the interventions reviewed have differential effects on the primary outcome of depressive symptoms depending on intervention characteristics and content: the type and number of self-management skills targeted, mode of delivery (e.g., face-to-face, online), type and intensity of guidance, intervention provider, format (e.g., group or individual), and duration (minutes of participation) and length of time over which the intervention was delivered.
To assess the effects of self-management interventions on other physiological and psychosocial outcomes (e.g., quality of life, fatigue) among the target population.
Methods
Methodological framework
The methods for this review were developed following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [38] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [39]. The protocol was registered with the prospective register of systematic reviews (PROSPERO CRD42019132215).
Criteria for considering studies for this review
Types of studies
Published or in press peer-reviewed full-text randomized controlled trials (RCTs) as well as cross-over trials and studies using a quasi-experimental design (e.g., controlled trials without randomization, but with a comparison group) were considered for inclusion in this review. To be eligible, the primary outcome (depressive symptoms) had to be measured pre- and post-intervention and only English and French language articles were included. Conference abstract and theses were excluded.
Types of participants and settings
Based on Lorig and Holman’s [25] conceptualization of self-management, participants with various chronic diseases were included. The target population for this review was adults (over age 18) with chronic physical disease(s) experiencing at least mild depressive symptoms according to a validated scale or clinical interview. The following psychometrically validated questionnaires and cut-off scores indicating at least mild depressive symptoms were eligible for inclusion:
Beck Depression Inventory-II (scores ≥13) [42]
Centre for Epidemiological Studies – Depression (scores ≥16) [43, 44]
Centre for Epidemiological Studies – Depression 10-item version (scores ≥10) [45, 46]
Hamilton Depression Rating Scale – Depression subscale (scores ≥8) [47]
Hospital Anxiety and Depression Scale – Depression (scores ≥8) [48]
Patient Health Questionnaire (scores ≥5) [49]
Geriatric Depression Scale (scores ≥11) [50]
In terms of clinical interviews, the DSM-III (Diagnostic and Statistical Manual of Mental Disorders) and above [51–55] as well as the ICD-9 (International classification of diseases) and above [56, 57] were considered eligible.
Studies including participants with one or multiple chronic physical diseases as defined by the Public Health Agency of Canada (excluding dementia) and World Health Organization were included [9, 58]. Participants taking anti-depressant medication were eligible, if pharmacotherapy was not administered as part of the intervention being evaluated. In terms of co-morbidities, studies including participants with a diagnosis of bipolar disorder, post-partum depression, seasonal affective disorder, or post-traumatic stress disorder were excluded as the etiology, disease course, and treatment recommendations for these conditions differ from depression [59–62]. No limits were placed on the setting in which the study was conducted.
Types of interventions
Experimental interventions.
Studies evaluating self-management interventions for depressive symptoms were included. Interventions were eligible if they incorporated at least one of the key self-management skills described in Appendix A. These skills were derived from the theoretical literature as well as from existing self-management interventions from reputable (peer-reviewed or government issued) sources [25, 29, 63–65]. Further, the intervention had to be administered to the individual directly. Interventions in which the person with the chronic disease participated with someone else (e.g., family member) were excluded due to the confounding effects of social support [66].
Multi-component interventions were eligible as a long as the above self-management criteria were met and at least one component targeted mood, distress, or depression. To avoid confounding effects, studies evaluating interventions including a component in which pharmacotherapy was administered were excluded. All formats of interventions were eligible (e.g., workbooks, online modules). Level of guidance by HCPs was not an exclusion criterion; self-directed, minimal contact, and HCP administered interventions were all eligible.
Comparator interventions.
Eligible control comparisons were no treatment, treatment as usual, waitlist, and attention control groups, as long as the participants were not receiving active components of the interventions (e.g., psychological therapy or the self-management skills outlined in Appendix A).
Outcome measures
The primary outcome was depressive symptoms. The secondary outcomes were a) improvement in psychosocial outcomes such as quality of life, anxiety, self-management skills, and self-efficacy; b) improvement in physical health measures; c) improvement in alcohol and drug consumption; and/or d) decrease in health care utilization.
Information sources and study selection
Search strategies
Eligible studies were primarily identified through searches of electronic bibliographic databases. Secondary search strategies consisted of verifying the reference lists of the included full-texts and using the PubMed ‘find similar’ function. Unpublished findings were also sought through the Cochrane library as well as the national and international trial registries outlined in the Cochrane Handbook for Systematic Reviews [38]. These were searched for relevant trial protocols and, if published findings of these trials could not be found, authors were contacted directly for further information.
Database searches
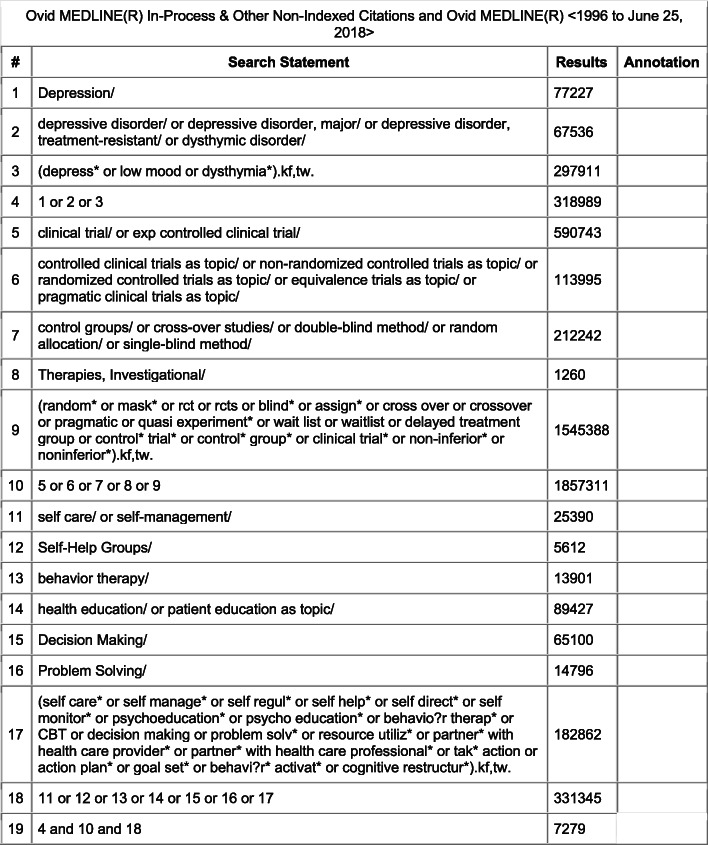
Eligible studies were identified by searching the following databases: MEDLINE (1946-), EMBASE (1996 -), PsycINFO (1967 -), and Cumulative Index to Nursing & Allied Health (CINAHL) (1984 -). The search was conducted in June 2018 and no limits were applied. All databases were searched using a combination of keywords and subject headings across three concepts: a) self-management, b) depression, and c) trial design (RCT or quasi-experimental). The search strategy was developed in consultation with a health sciences librarian and were assessed using the Peer Review for Electronic Search Strategies (PRESS) guidelines [67]. The full primary electronic search for Ovid Medline is included in Appendix B. All titles and abstracts were downloaded to a citation manager, EndNote, and screened using Rayyan online software. Duplicates were removed according to the procedures outlined in Bramer, Giustini [68].
Study selection
Two authors independently assessed the eligibility of all retrieved titles, abstracts, and full texts to confirm inclusion or exclusion. Two authors also separately examined the reference lists of included full texts. Finally, searches were conducted using the ‘find similar’ function of PubMed. Any disagreements were discussed with a third author until consensus was established. Multiple reports relating to the same study were aggregated so that findings reflected each study rather than each report.
Data extraction
Data were extracted using a standardized Microsoft Excel form that was developed based on the Cochrane Handbook for Systematic Reviews [38]. The form was adapted from one used in a previous systematic review conducted by team members [69, 70]. Data were extracted by one author and confirmed by at least one other. Disagreements were discussed with a third author until resolved.
The following data on study characteristics were extracted: citation details, country of origin, study design, aims, theoretical framework, population (age, diagnosis, gender, depressive symptoms), sample size, setting (e.g., hospital, community), summary of intervention and control groups, self-management skills in the intervention, format of the intervention, intervention provider, level of guidance, duration of the intervention (number of minutes of participation), length of intervention (time period over which the intervention was delivered, e.g., 2-months of sessions), monitoring of fidelity and adherence, outcomes, timing of measurement, and attrition [38]. Outcomes were grouped into two time periods: T1 from baseline < 6-months post-baseline and T2 ≥ 6-months post-baseline. If any data were missing or unclear, the authors of the manuscript were contacted for further information.
Methodological quality
Two authors (combination of the first, seventh, and eight authors) independently assessed the risk of bias of each study based on the criteria outlined in the Cochrane Risk of Bias tool [71]. Again, disagreements were discussed with a third author until consensus was reached. The risk of bias was evaluated according to the following criteria: a) inclusion criteria specified, b) pre-specified primary outcome(s), c) psychometric properties of primary outcomes provided, d) explicit power calculation, e) target sample size reached, f) appropriate randomization procedures and allocation concealment, g) discussion of potential co-interventions, h) baseline characteristics of all groups provided, i) blinding of outcome assessors, participants, and interventionists, j) adherence to intervention (> 75%), k) fidelity monitoring, l) management of missing data (intention-to-treat analysis), m) participant retention (> 80%), and n) reasons for attrition stated.
Each potential source of bias was evaluated as having been met (score 1) or not met (score 0). If the information was not specified in the manuscript the authors were contacted. If the information could not be clarified, the item was deemed not to have been met. Studies were considered to be of high methodological quality if 13–17 of the criteria were met, moderate quality if 8–12 were met, and low fewer than 8 were met. Direct quotes from each study as well as supporting comments were included in the evaluation of each. For evidence of selective reporting, study protocols or trial registration of included articles, when available, were compared to the published findings and unexplained discrepancies were noted [71].
Data analysis
Effect sizes (Hedge’s adjusted g) were calculated using outcome scores at post-intervention assessment between treatment and control conditions for the T1 and T2 time periods [38, 72]. Initially analyses were planned for three time periods: T1< 3 months post-baseline, T2 - 3 to < 6 months post-baseline, and T3 ≥ 6 months post-baseline. Due to the limited sample size, T1 and T2 were combined. Hedge’s adjusted g was selected to reduce potential bias due to small sample sizes [73]. The magnitude of the effect size can be interpreted according to the benchmarks outlined by Cohen (1988) [74]; namely, small (0.2), moderate (0.5), and large (0.8).
Pooled mean effect sizes were calculated to obtain a summary statistic for the T1 and T2 periods. If a study reported both per protocol and intention-to-treat analyses, per protocol data were included in the meta-analysis. When outcome data were collected at more than one time point within the timeframe, as most studies reported data at 6-months, the data collection closest to this point was used (e.g., if 6 and 12 month post-baseline data were available, 6 month data were entered into the meta-analysis). The Higgin’s statistic (I2) was calculated to measure the heterogeneity across studies and interpreted as 0% indicating no heterogeneity, 25% low, 50% moderate, and > 75% high heterogeneity [71]. It was anticipated that there would be considerable heterogeneity across studies arising from differences in the interventions delivered, sample characteristics, and study designs. As such, a random effects model was used for meta-analysis calculations. As the number of studies was small, in addition to the DerSimonian–Laird approach, the Knapp-Hartung approach was used to make small-sample adjustments to the variance estimates of any outcomes that were statistically significant [75]. This more conservative approach produces a wider confidence limit appropriate when the sample size is small [75]. All tests were two-sided, and the significance level was set at p < 0.05. These analyses were conducted using RevMan 5.3.
Publication bias was evaluated through inspection of funnel plots of the primary outcome variable of depression [38]. The effect of potential moderators on the primary outcomes was also assessed [76]. The prespecified moderators, participant and intervention characteristics, are detailed in Table 1. Studies were separated into sub-groups based on these variables and meta-regressions were performed to identify whether there was a statistically significant difference in outcomes between the sub-groups. There is no consensus on the number of studies required to run a meta-regression; these were performed when sub-groups included 4 or more studies (p-value < 0.05 was set to establish significance) [77]. As meta-regressions cannot be performed using the Revman 5.3 software, these were calculated using the ‘metareg’ program in STATA (version 15.1). If needed information for any of the above calculations was not reported in the publication, authors were contacted for further details. If data required for inclusion in the meta-analysis were not available, the study was included for descriptive review only. Due to the substantial diversity in reported outcomes, only outcomes reported at least three times at one time point (T1 or T2) were included for review.
Table 1.
Descriptive summary of included studies
| Author, Year, Country, Quality Assessment Score (QAS) (/17) | Aim(s) | Demographics | Intervention and control conditions and assessments | Outcome(s) [Primary (P), Secondary (S), Unspecified (O)] |
|---|---|---|---|---|
|
United Kingdom Pilot RCT (2 groups) QAS:11 |
To explore the acceptability and feasibility of procedures to inform a definitive RCT of a practice nurse-led personalised care intervention for CHD patients with at least probable depression and chest pain. |
Symptomatic chronic heart disease (with active chest pain) N = 81 (T = 41, C = 40) Mean age: 65 (SD = 11) % female = 35.8 Race/ethnicity: 83% white Mean HADS-D score: T = 12 (SD = 3), C = 11 (SD = 3) |
T: Nine sessions (one face-to-face assessment + 15-min follow-up phone calls) with nurse focused on identifying problems contributing to depression, providing support resources, devising personal health plan, goal setting, and building self-efficacy. C: Usual care. Format: Individual. Mode of delivery: Face-to-face and telephone. Interventionist: Nurse. Intervention duration: Mean 203 min (SD 100) of nurse time (mean 78min SD 19 for face-to-face assessment; mean 125min SD 91 in follow-up telephone calls). Intervention length: 6-months. Level of guidance: Guided Timing of measures: 1-, 6-, and 12-months post baseline. |
P: T1 = C for depression T2 = C depression S: T1 = C for anxiety T1 = C for MCS and PCS T2 = C for anxiety, MCS, and PCS |
|
Netherlands RCT (3 groups) QAS: 10 |
To decrease depressive symptoms using low-intensity guided self-help based on problem-solving therapy delivered online to increase accessibility and decrease barriers to accessing mental health care. |
Glioma (CNS cancer) N = 115 (T = 45, C1 = 26, C2 = 44) Mean age: T = 43.6 (SD = 11.7), C1 = 52.8 (SD = 9.3), C2 = 46.4 (SD = 12.3) % female: T = 57.8, C1 = 65.4, C2 = 59.1 Most common diagnosis in C1: non-Hodgkin lymphoma (46.2%) Mean CES-D score: T = 21.5 (SD = 6.1), C1 = 25.1 (SD = 6.7), C2 = 24.1 (SD = 6.6) |
T = Guided self-help course based on problem-solving therapy including disease specific information. Five modules and exercises. Online support and feedback on exercises provided by coach. C1 = Non-CNS cancer control group. Received intervention. C2 = Glioma 12-week waitlist control group (WLC). Format: Individual. Mode of delivery: Online Interventionist: Psychologist, nurse, or psychology student (coaches). Intervention duration: n/a Intervention length: 5 weeks. Level of guidance: Guided self-directed. Timing of measures: 1.5-, 3-, 12-months post-baseline (last outcome measure not included for analysis as WLC group had completed intervention). |
P: T1 = C2 for depression S: T1 > C2 for MCS (ES: 0.87) T1 = C2 for PCS |
|
Espahbodi et al., 2015 [82] Iran Quasi-experimental (randomized matched design) QAS: 6 |
To investigate the impacts of education on psychological symptoms (anxiety and depression) in patients undergoing dialysis. |
Renal Failure (receiving dialysis) N = 55 (T = 27, C = 28) Mean age: T = 49.1 (SD = 14.5), C = 52.3 (SD = 15.6) % female: T = 52, C = 50 Mean HADS-D: T = 10.2 (SD = 3.4), C = 10.1 (SD = 3.4) |
T: Psychoeducational intervention (3 sessions × 60 min) focused on disease-specific information (e.g., physiology, causes, treatments) as well as problem-solving, stress management, adaptive responses, and muscle relaxation. C: Usual care. Format: Group Mode of delivery: Face-to-face. Interventionist: Unspecified. In collaboration with a nephrologist and psychiatrist. Intervention duration: 180 min. Intervention length: Approximately 5 days. Level of guidance: Guided. Timing of measures: 1-month post-baseline. |
P: T1 = C for depression and anxiety |
|
Fischer et al., 2015 [83] Germany RCT (2 groups) QAS: 9 |
To evaluate the feasibility and efficacy of a fully automated internet-based CBT program to reduce depressive symptoms in patients with multiple sclerosis (MS). |
Multiple sclerosis N = 90 (T = 45, C = 45) Mean age: T = 45.4 (SD = 12.6), C = 4524 (SD = 10.6) % female: T = 76, C = 80 Mean BDI score: T = 19.4 (SD = 9.0), C = 18.4 (SD = 8.2) |
T = Ten online modules using simulated dialogue and tailored based on participant response. Content draws on: 1) behavioral activation, 2) cognitive modification, 3) mindfulness and acceptance, 4) interpersonal skills, 5) relaxation, physical exercise and lifestyle modification, 6) problem solving, 7) childhood experiences and early schemas, 8) positive psychology interventions, 9) dreamwork and emotion-focused interventions, and 10) psychoeducation. C = 2.25-month WLC Format: Individual. Mode of delivery: Online. Interventionist: Self-directed. Intervention duration: Self-directed. Mean use: 332 min (range 50–905 min). Length of intervention: 2.25 months. Level of guidance: Self-directed. Timing of measures: 2.25- and 8.25-months post-baseline (last outcome measure not included for analysis as WLC group had completed intervention). |
P: T1 = C for depression S: T = C for fatigue |
|
Netherlands RCT (2 groups) QAS: 12 |
To evaluate the effectiveness of a nurse-administered minimal psychological intervention in reducing depressive symptoms in elderly primary care patients with type II diabetes or COPD with co-morbid non-severe depression and examine whether type of chronic illness modified the effects of the intervention. |
Type II diabetes, COPD N = 361 (T = 183, C = 178) Mean age: T = 70.8 (SD = 6.5), C = 70.6 (SD = 6.8) % female: T = 46.4, C = 46.6 Primary diagnosis: T: 49.7% diabetes, 50.3% COPD C: 52.8% diabetes, 47.2% COPD Mean BDI score: T = 17.1 (SD = 7.2) C = 17.7 (SD = 8.0) |
T = Tailored intervention with variable number of sessions (2–10) based on principles of self-management and CBT and includes 5 phases: 1) exploring feelings, cognitions, and behaviours, 2) mood, symptom, and behaviourmonitoring, 3) linking mood to behaviour, 4) action planning, and 5) evaluation of progress in achieving goals. C = Usual care. Format: Individual. Mode of delivery: Face-to-face. Interventionist: Nurse. Intervention duration: Mean 240 min. Intervention length: Tailored up to 3-months. Level of guidance: Guided. Timing of measures: Approximately 3.25-, 6-, and 12-months post-baseline (assuming 3-month intervention period). |
P: T1 = C for depression T2 = C for depression S: T1 = C for MCS and PCS T2 = C for MCS and PCS COPD sub-group* T1 = C for MCS T2 = C for MCS DMII sub-group* T1 = C for MCS T2 = C for MCS (note: T>C for MCS at 9-months post intervention completion)* |
|
Lamers et al. 2010b [86] Note: Subgroup analysis of Lamers 2010a Netherlands RCT (2 groups) |
To evaluate the effect of a nurse-administered minimal psychological intervention on disease specific quality of life, depression, and anxiety in elderly primary care patients with COPD with co-morbid non-severe depression. |
COPD N = 187 (T = 96, C = 91) Mean age: T = 70.5 (SD = 6.3), C = 71.5 (SD = 7.1) % female: T = 38.5, C = 41.8 Mean BDI score: T = 17.1 (SD = 6.5), C = 18.3 (SD = 7.2) |
See Lamers et al., 2010a |
P: T1 = C for depression* T2 = C for depression* (Note: T>C for depression at 9-months post intervention completion).* S: T1=C for anxiety* T2=C for anxiety* (Note: T>C for anxiety at 9-months post intervention completion)* |
|
Lamers et al., 2011 [87] Note: Subgroup analysis of Lamers 2010a Netherlands RCT (2 groups) |
To evaluate whether a nurse-administered minimal psychological intervention based on CBT and self-management principles improves disease-specific quality of life and glycemic control in patients with type II diabetes and co-morbid non-severe depression. |
Type II diabetes N = 208 (T = 105, C = 103) Mean age: T = 70.7 (SD = 6.6), C = 69.7 (SD = 6.6) % female: T = 51.4, C = 50.4 Depression level: Not specified. Participants underwent Mini International Neuropsychiatric review. Those with minor depression, mild-to-moderate major depression or dysthymia were included. |
See Lamers et al., 2010a |
S: T1 = C for glycemic control (HbA1c) T2 = C for glycemic control (HbA1c) |
|
Lee et al., 2014 [88] Republic of Korea Quasi-RCT – group allocation based on consent date (2 groups) QAS: 12 |
To evaluate the effectiveness of a tablet PC-based single session psychoeducation intervention for cancer patients reporting significant levels of distress. |
Type II diabetes *per group data not available N = 36 (T = 19, C = 17) Median age: 57.5 (range 34–71) % female: 55.6 Mean HADS-D score: T = 12.0 (SD = 3.7), C = 12.7 (SD = 1.5) |
T = Twenty-minute psychoeducation video clip. Content consisted of distress education, cancer survivor interview, coping strategies and stress management, and psychological services. C = Control movie clip of scenic images and relaxing music. Format: Individual. Mode of delivery: Video presented on computer tablet. Interventionist: n/a Intervention duration: 20-min. Length of intervention: 20-min. Level of guidance: Self-directed Timing of measures: 1-day (post-intervention same day as baseline measures) and 2–4 weeks post-baseline. |
P: T1 > C for depression (ES: − 1.13) and MCS (ES: 1.08) T1 = C for anxiety |
|
Moncrieft et al., 2016 [89] United States RCT (2 groups) QAS: 9 |
To determine the effect of a multicomponent behavourial intervention on weight, glycemic control, renal function, and depressive symptoms in adults with DMII and depressive symptoms. |
Cancer patients receiving chemotherapy treatment N = 111 (T = 57, C = 54) Mean age: T = 54.8 (SD = 8.3), C = 54.8 (SD = 6.3) % female: T = 64.9, C = 77.8 Mean BDI-II score: T = 19.3 (SD = 7.1) C = 21.2 (SD = 7.1) |
T: Structured lifestyle intervention (17 sessions × 1.5–2 h). Two individual sessions followed by two weekly, four bi-weekly, and nine monthly group sessions. Intervention components focused on diet and physical activity, including a weight loss, exercise, and caloric intake goals, combined with cognitive behavioural and social learning approaches to managing depression. C: Usual care + brief educational booklet on diabetes management. Format: Individual and group. Mode of delivery: Face-to-face. Interventionist: Therapists. Intervention duration: 1530 to 2040 min. Intervention length: 12-months. Level of guidance: Guided. Timing of measures: 6- and 12-months post-baseline. |
P: T2 > C for depression (ES: − 0.62) T2 = C for glycemic control (HbA1c) |
|
Penckofer et al., 2012 [90] United States RCT (2 groups) QAS: 12 |
To examine the effects of a nurse-delivered psychoeducation intervention on depression, anxiety, and anger among women with type II diabetes. |
Type II diabetes N = 74 (T = 38, C = 36) Mean age: T = 54.8 (SD = 8.8), C = 54.0 (SD = 8.4) % female: 100 Mean CES-D score: T = 27.7 (SD = 9.3) C = 28.9 (SD = 9.5) |
T = Sessions [(8 weekly sessions + 2 booster sessions) × 1 h/session] focused on recognizing signs and symptoms of depression, relationship between mood, metabolic control, and self-care behaviours, the management of depression, anxiety, and anger using CBT. Includes elements from existing interventions such as CBT program for depression, progressive muscle relaxation CD, and system for management of anger including workbook and video. C = Usual care. Format: Group. Mode of delivery: Face-to-face. Interventionist: Nurse. Intervention duration: 600 min Intervention length: 6-months Level of guidance: Guided. Timing of measures: 3- and 6-months post-baseline. |
P: T1 > C for depression (ES: − 0.78) T1 = C for trait anxiety T1 = C for state anxiety T2 > C for depression (ES: − 0.94) and trait anxiety (ES: − 0.62) T2 = C for state anxiety (ES: − 0.74) S: T1 = C for MCS, PCS, and glycemic control (HbA1c) T2 = C for PCS and glycemic control (HbA1c) T2 > C for MCS (ES: 0.60) |
|
Rees et al., 2017 [91] Australia Pilot RCT (2 groups) QAS: 13 |
To provide preliminary evidence for the impact of problem-solving therapy for diabetes in adults with diabetic retinopathy and diabetes distress. |
Type II diabetes and diabetic retinopathy N = 40 (T = 21, C = 19) Mean age: T = 60.1 (SD = 7.0), C = 59.6 (SD = 8.8) % female: T = 33.3, C = 31.6 Mean PHQ-9 score: T = 10.5 (SD = 5.2) C = 10.2 (SD = 5.7) |
T: Provided publicly available information on diabetes + problem solving therapy for diabetes, which consisted of weekly sessions (8 × 45–60 min) in which participants identified problems related to diabetes and were guided through a problem-solving process. Participants were also asked to make plans to engage in enjoyable activities. C: Usual care + same publicly available brochures on diabetes as T group. Format: Individual Mode of delivery: Phone or in-person (based on preference and availability). Interventionist: Research assistant supervised by clinical psychologist. Intervention duration: 360–480 min. Intervention length: 2-months Level of guidance: Guided. Timing of measures: 3- and 6-months post-baseline. |
S: T1 = C for depression and glycemic control (HbA1c) T2 = C for depression and glycemic control (HbA1c) |
|
Schroder et al., 2014 [92] Germany RCT (2 groups) QAS: 10 |
To evaluate the feasibility and efficacy of an online program for depression in individuals with epilepsy and co-morbid depressive symptoms. |
Epilepsy N = 78 (T = 38, C = 40) Mean age: T = 35.0 (SD = 10.0), C = 40.0 (SD = 11.9) % female: T=67.5, C=84.2 Mean BDI score: T = 22.2 (SD = 10.4) C = 19.4 (SD = 9.8) |
T: Ten online modules (10–60 min each) comprised mostly of CBT elements (cognitive restructuring, behavioural activation) and mindfulness and acceptance exercises. C: 9-week WLC Format: Individual Mode of delivery: Online. Interventionist: Self-directed. Intervention duration: 100–600 min. Intervention length: 2.25 months. Level of guidance: Self-directed. Timing of measures: 2.25 months post-baseline. |
P: T1 = C for depression |
|
Sharpe et al., 2004 [93] United Kingdom (Scotland) Non-randomized matched control group design (2 groups) QAS: 12 |
To perform preliminary evaluation of the feasibility and efficacy of a nurse-led intervention with oncology outpatients. |
Cancer (outpatients with breast, gynaecological, bladder, prostate, testicular and colorectal) N = 60 (T = 30, C = 30) Mean age: T = 58.0 (SD = 10.6), C = 56.0 (SD = 10.5) % female: T = 93.3, C = 93.3 Mean HADS-D score: T = 10.4 (SD = 3.6) C = 10.3 (SD = 4.0) |
T: The intervention consisted of up to 10 weekly problem-solving therapy sessions (30 min each) to help with a positive and systematic approach to tackling problems, education about depression, encouragement to speak with their general practitioner about anti-depressant medication, and coordination and monitoring of the participant’s depression treatment. Participants could contact the nurse for further booster sessions. C: Usual care. Format: Individual. Mode of delivery: Face-to-face or phone. Interventionist: Nurse supervised by psychiatrist. Intervention duration: Nurse spent mean of 360 min with participants. Intervention length: Ranged from 0.5–4 months (with 6 participants requesting booster sessions). Level of guidance: Guided. Timing of measures: 3- and 6-months post-baseline. |
P: T1 > C for depression (ES: − 0.87) T2 > C for depression (ES: − 0.58) S: T1 > C for anxiety (ES: − 1.25) T2 > C for anxiety (ES: − 0.88) |
|
Sharpe et al., 2014 [94] (SMaRT Oncology-2) United Kingdom (Scotland) RCT (2 groups) QAS: 14 |
To compare the effectiveness of an integrated treatment programme for major depression in patients with cancer with usual care for patients with cancer who have co-morbid major depression and a survival prognosis of at least a year. |
Cancer with prognosis of survival over 12-months. N = 500 (T = 253, C = 247) Mean age: T = 56.6 (SD = 10.0), C = 56.1 (SD = 10.2) % female: T = 90, C = 90 Mean SLC-20 score: T = 2.10 (SD = 0.62) C = 2.11 (SD = 0.56) |
T: Based on Strong et al. (2008). Primary care physician and oncologist informed of major depression disorder diagnosis + multicomponent treatment program integrated into cancer care in which participants form relationships with nurses who provide information about depression, deliver problem-solving therapy, and monitor progress (up to 10 sessions X 45 min and additional sessions available for those not meeting treatment targets). C: Usual care + primary care physician and oncologist informed of major depression diagnosis + participant encouraged to consult their primary care physician to obtain treatment. Format: Individual. Mode of delivery: Primarily face-to-face, sometimes telephone. Interventionist: Oncology nurses supervised by a psychiatrist. Intervention duration: 405 min. Median number of sessions: 9 (range 0–10). Intervention length: 4-months for initial sessions and further sessions for those who are not meeting treatment targets. Level of guidance: Guided. Timing of measures: 3-, 6-, 9-, and 12-months post-baseline. |
P: T1 > C for depression (ES: − 0.87) T2 > C for depression (ES: − 1.03) S: T1 > C for anxiety (ES: − 0.61) and fatigue (ES: − 0.41) T2 > C for anxiety (ES: − 0.71) and fatigue (ES: − 0.60) |
|
Strong et al., 2008 [95] (SMaRT oncology 1) United Kingdom (Scotland) RCT (2 groups) QAS: 14 |
To assess the efficacy and cost of a nurse-delivered complex intervention designed to treat major depressive disorder in patients with cancer. |
Cancer N = 200 (T = 101, C = 99) Mean age: T = 56.6 (SD = 11.4), C = 56.6 (SD = 12.3) % female: T = 69, C = 72 Median SCL-20 score (IQR): T = 2.35 (2.05–2.75), C = 2.25 (1.95–2.75) |
T: Maximum of 10 session (45-min each) over 3-months followed by monthly monitoring of symptoms in the next 3-months and. optional 1–2 sessions for those whose depression scores increased. The intervention included education about depression and treatment, problem-solving treatment, and communicating with the participant’s primary care physician and oncologist about their depression diagnosis. C: Usual care + informed primary care physician and oncologist of depression diagnosis and, if requested, provided advice regarding choice of antidepressant medication. Format: Individual. Mode of delivery: Primarily in-person, some by telephone if needed. Interventionist: Oncology nurse supervised by a psychiatrist. Intervention duration: Mean of 315 min based on mean of 7 sessions (range 2–10). Intervention length: 6-months. Level of guidance: Guided. Timing of measures: 3-, 6-, and 12-months post-baseline |
P: T1 > C for depression* T2 > C for depression* S: T1 > C for anxiety and fatigue* T2 > C for anxiety and fatigue* |
|
Thorton et al., 2009 [96] United States RCT (2 groups) Secondary analysis QAS: 11 |
To test experimentally whether a psychological intervention reduces depression-related symptoms and markers of inflammation among cancer patients. |
Breast cancer (Stage II/III, surgically treated, and waiting for adjuvant therapies) N = 45 (T = 23, C = 22) Mean age: T = 50.0 (SD = 8.6), C = 50.0 (SD = 11.6) % female: 100 Mean CES-D Iowa short-form score not reported. All participants included in the secondary analysis scored ≥10 as part of inclusion criteria. |
T: Group sessions of 8–12 patients for 90 min for 18 weekly sessions followed by 8 monthly sessions. Topics included stress management, emotional distress, social adjustment, health behaviours (e.g., diet, exercise), and adherence to treatment. C: Usual care. Format: Group. Mode of delivery: Face-to-face (some telephone contact to catch up on information if sessions were missed). Interventionist: Psychologists. Intervention duration: 2340 min. Intervention length: 12-months. Level of guidance: Guided. Timing of measures: 4-, 8-, 12-months post-baseline. |
P: T1 > C for depression* T2 > C for depression* S: T1 > C for fatigue* T2 > C for fatigue* |
|
Walker et al., 2014 [97] (SMaRT Oncology-3) United Kingdom (Scotland) RCT (2 groups) QAS: 13 |
To assess the efficacy of an integrated treatment program for major depressive disorder in patients with lung cancer compared with usual care. |
Lung cancer N = 142 (T = 68, C = 74) Mean age: T = 63.6 (SD = 8.8), C = 63.9 (SD = 8.7) % female: T = 65, C = 65 Mean SCL-20 score: T = 1.90 (SD 0.52), C = 1.98 (0.58) |
T: Adapted from Sharpe et al. (2014). Maximum of 10 sessions (30–45 min) over 4-months followed by monitoring of symptoms and optional additional sessions for participants who did not meet treatment target. Nurses establish therapeutic relationship, provide information about depression, delivey problem-solving therapy and behavioural activation and monitor progress. Psychiatrists supervise treatment, advise primary care physicians, and provide direct consultation to participants not progressing. C: Usual care + primary care physician and oncologist informed of the diagnosis of major depression and participant encouraged to see primary care physician to obtain treatment. Format: Individual. Mode of delivery: Primarily face-to-face, some telephone contact. Interventionist: Nurse and psychiatrist. Intervention duration: 240–360 min (median number of sessions 8 IQR 7–10). Intervention length: 8-months. Level of guidance: Guided. Timing of measures: 1-, 2-, 3-, 4-, 5-, 6-, 7-, and 8-months post-baseline. Outcomes averaged over the participants time in the trial (up to 8-months). |
Outcomes averaged over the participants time in the trial (up to 8-months). P: T > C for depression* S: T > C for anxiety* T = C for fatigue* |
Notes: Only post-intervention primary and secondary outcomes of interest in this review reported across at least 3 studies within one time period (T1 and/or T2) included. T1- baseline to < 6 months post-baseline; T2 ≥ 6 months post-baseline. T = treatment condition; C = control condition; T > C = treatment significantly superior to control; T < C = control superior to treatment; T = C = no significant differences between. ES = Effect size (Hedge’s g calculated at 95% confidence level); Intervention duration = number of minutes spent participating in intervention based on reported participation or expected duration; Intervention length= length of time over which intervention was delivered; *Indicates that insufficient data available to calculate effect size so outcome is as reported by authors; sign of effect size based on negative orientation of scale (as intervention always compared with control – scales in which decreased scores indicate improvement are negative); Duration of the intervention based on reported mean or median adherence (in minutes) multiplied by the number of sessions, or, if not available, amount of time authors reported intervention would take (e.g., 4 sessions X 60 min = 240 min). If the range of individual sessions was provided (e.g., 15 to 30 min per session), the midpoint (e.g., 22.5) was multiplied by the number of sessions.; IQR interquartile range; CHD coronary heart disease, CNS central nervous system, PST problem-solving therapy; WLC wait list control group; MS multiple sclerosis; COPD chronic obstructive pulmonary disorder; ER emergency room; QoL quality of life; CBT cognitive behavioural therapy; BDI Beck Depression Inventory [40]; BDI-II Beck Depression Inventory-II [42]; CES-D Centre for Epidemiological Studies-Depression [44]; HADS-D Hospital Anxiety and Depression Scale-Depression [48]; PHQ-9 Patient Health Questionnaire [49]; HRQoL Health related Quality of Life and includes: PCS physical health composite scale; MCS mental health composite scale
Results
Study selection
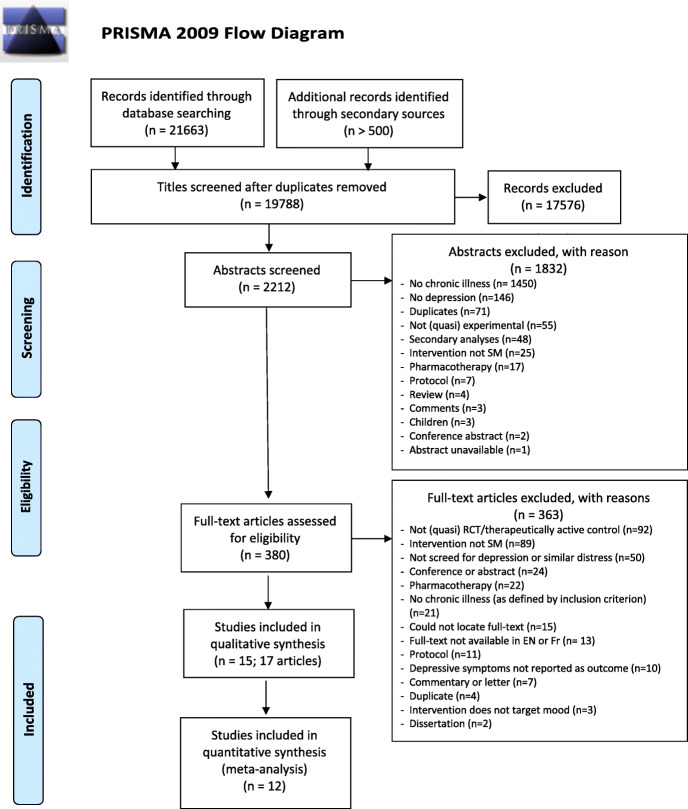
In total, 21,663 titles were retrieved through database searches and over 500 titles were screened through secondary searches. After removing duplicates, 19,788 titles remained. Screening of these resulted in the inclusion of 2212 abstracts, 1832 of which were excluded, leaving 380 full texts to be reviewed. Seventeen manuscripts reporting on 15 studies were retained: 12 for inclusion in the meta-analysis and three for descriptive review only. Flow of studies and reasons for exclusion are detailed in Fig. 1.
Fig. 1.
PRISMA 2009 Flow Diagram
Description of studies
Characteristics of included studies are described in Table 2 and there was no indication of publication bias (Appendix C). Studies were conducted in the United Kingdom (n = 5), United States (n = 3), the Netherlands (n = 2), Germany (n = 2), Iran (n = 1), Republic of Korea (n = 1), and Australia (n = 1). Eleven studies used a 2-group RCT design (including two pilot trials), two studies used a 2-group quasi-experimental design, and one used a 3-group RCT design. The intervention groups were compared to usual care (n = 6), attention control groups (n = 6) (e.g., provided publicly available health information), or waitlist control (n = 3).
Table 2.
Effect sizes for T1 and T2 for secondary outcomes
| Timepoints | ||||||
|---|---|---|---|---|---|---|
| T1 | T2 | |||||
| Secondary Outcomes | # of studies | SMD (95% CI) | I2 (%) | # of studies | SMD (95% CI) | I2 (%) |
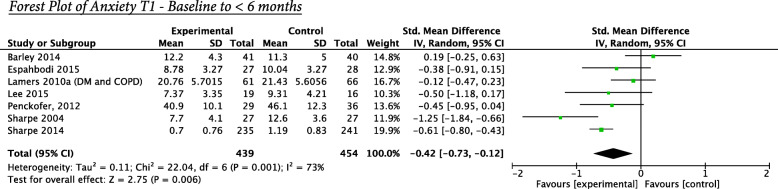
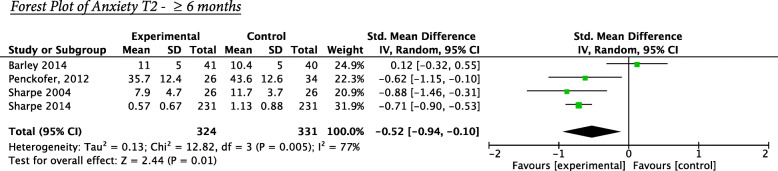
| Anxiety | 7 | -0.42 [− 0.73, − 0.12] | 73 | 4 | − 0.52 [− 0.94, − 0.10] | 77 |
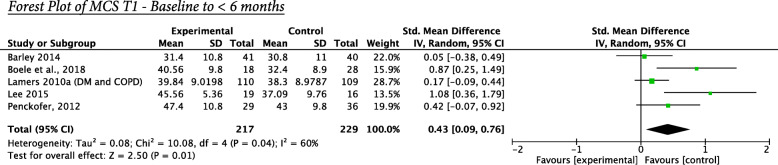
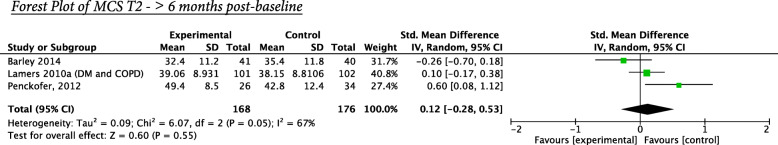
| Mental Component Score (HRQoL) | 5 | 0.43 [0.09, 0.76] | 60 | 3 | 0.12 [− 0.28, 0.53] | 67 |
| Physical Component Score (HRQoL) | 5 | 0.01 [−0.18, 0.20] | 0 | 3 | 0.03 [−0.18, 0.24] | 0 |
| Fatigue | 3 | −0.36 [− 0.67, − 0.06] | 50 | 1 | −0.60 [− 0.78, − 0.41] | |
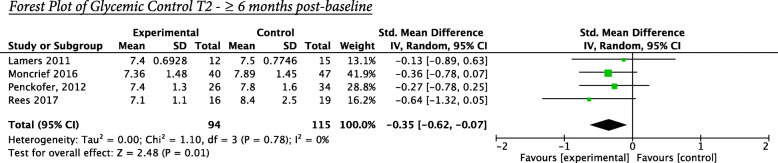
| Glycemic Control (HbA1c) | 3 | − 0.08 [− 0.57, 0.41] | 49 | 4 | −0.35 [− 0.62, − 0.07] | 0 |
Note: T1 baseline to < 6-months; T2 ≥ 6-months. HRQoL Health-related Quality of Life, I2 Higgin’s I2 statistic, CI confidence interval
Participants
In total, 2064 participants were included in this review, with study sample sizes ranging from 40 [91] to 500 [94]. Most studies (n = 12) included more women than men and two studies included only women [90, 96]. Participants’ primary diagnoses were cancer (n = 7) [80, 89, 93–97], diabetes type II (n = 4) [84, 88, 90, 91], chronic heart disease (n = 1) [78], chronic obstructive pulmonary disorder (n = 1) [84], multiple sclerosis (MS) (n = 1) [83], epilepsy (n = 1) [92], and chronic kidney disease (n = 1) [82] (non-exclusive categories as some studies focused on two disease groups). Mean reported age in the sample ranged from 35.0 to 70.8 years. Depressive symptomatology across study groups ranged from mild to severe, with the mean reported symptoms most often in the moderate range [41–44, 47–49, 98, 99].
Interventions
Included interventions are described in Table 2. Six of the 15 studies evaluated the same or similar interventions in different populations (4 one type of intervention and 2 another) [83, 92–95, 97]. Time spent participating in interventions (duration) ranged from 20 [88] to 2340 min [96] (n = 14, mean = 552.9, SD = 662.9). The length of time over which interventions were delivered ranged from one session [88] to two 12 month programs [89, 96].
The primary format of the interventions was individual (n = 11) [78, 80, 83, 84, 88, 91–95, 97]; however, three studies used a group format [82, 90, 96], and one included both group and individual sessions [89]. In terms of mode of delivery, seven studies used a combination of face-to-face and telephone contact (most favoured face-to-face contact with telephone follow-up only if needed) [78, 91, 93–97], four were delivered entirely face-to-face [82, 84, 89, 90], three were online [80, 83, 92], and one was a video on a computer tablet [88].
In terms of level of guidance, most were led by an interventionist (n = 11) [78, 82, 84, 89–91, 93–97], three were self-directed (two online programs and one video) [83, 88, 92], and one intervention was guided self-directed (participants independently worked through the intervention with feedback on exercises) [80]. The interventionists were all HCPs, other than one provided by a trained research assistant supervised by a psychologist [91]. Four of the interventions were delivered by nurses supervised or supported by psychiatrists [93–95, 97], three were delivered solely by nurses [78, 84, 90], one by psychologists [96], and the remaining two interventions were delivered by interdisciplinary HCPs [80, 82].
The content of the interventions focused on a combination of structured problem-solving (n = 12) [78, 80, 84, 89, 91–97], providing disease specific health information (n = 13; it was an optional component in two of the 13 interventions) [78, 82, 88–97], relaxation and stress management (n = 7) [82, 83, 88–90, 92, 96], using CBT principles (e.g., challenging negative self-talk) (n = 5) [83, 84, 89, 90, 92], care coordination (e.g., communicating the depressive symptoms to HCP team members) (n = 4) [93–95, 97], and finding health services (n = 2) [78, 88].
Eleven interventions were coded for a possible 13 depression self-management skills (interventions that contained the same content were combined). The skills identified for each intervention are summarized in Appendix A. Across the sample, the mean number of skills was 6.2 (SD = 2.4, range 2–11). The most frequently included skill was problem-solving (n = 9), followed by decision-making (n = 8), taking action (n = 7), social support (n = 7), and self-tailoring (n = 7). Less frequently addressed skills were social support (n = 1), resource utilization (n = 2), and forming partnerships with HCPs (n = 2).
Methodological quality
Quality assessment scores are included in Table 2 and detailed scoring is available in Appendix D. The mean quality assessment score across the sample was 11.2 (SD 2.14) out of a possible 17 indicating that on average the studies were of moderate methodological quality. Scores ranged from 6 to 14 with four studies assessed as being of high methodological quality [91, 94, 95, 97]. The least met criteria related to blinding.
Outcomes: Descriptive and meta-analysis
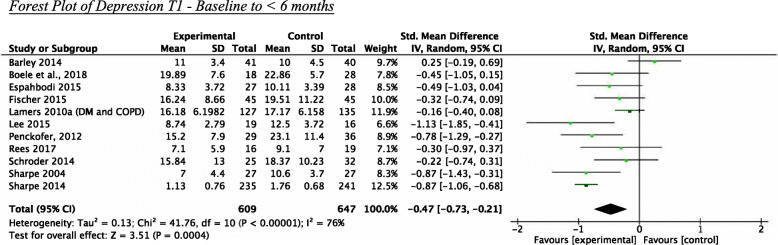
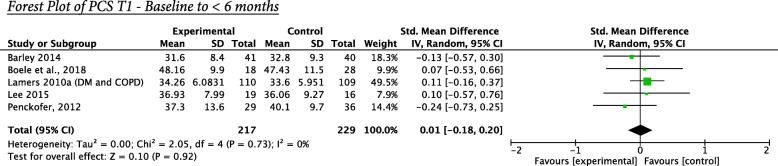
Primary outcome: Depression
Eleven studies were included in the meta-analysis for the T1 period (see Fig. 2). The pooled effect size of − 0.47 [95% CI -0.73, − 0.21] was significant with high heterogeneity I2 = 76% and favoured the interventions over the control conditions. The results remained significant after the Knapp-Hartung (KH) conversion [95% CI -0.74, − 0.02]. Statistically significant effect sizes ranged from − 0.78 [90] to − 1.13 [88].
Fig. 2.
Forest Plot of Depression T1 - Baseline to < 6 months
When examining potential sources of heterogeneity, three studies reported pharmacological co-intervention that was significantly imbalanced between the intervention and control groups (participants in the intervention group were more likely to begin pharmacotherapy than those in the control group) [78, 93, 94]. When these studies were removed from the meta-analysis, the pooled effect size with 8 studies was of − 0.41 [95% CI − 0.61, − 0.20] with I2 = 32%. Using both the DerSimonian–Laird and the more conservative KH approach and examining potential sources of heterogeneity, the findings of all analyses indicated a statistically significant moderate effect of interventions as compared to control conditions. The two studies not included in the meta-analysis measuring depression outcomes reporting findings in line with those of the meta-analysis [95, 96].
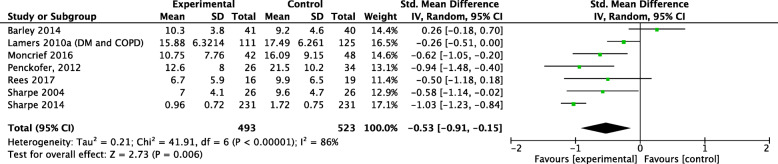
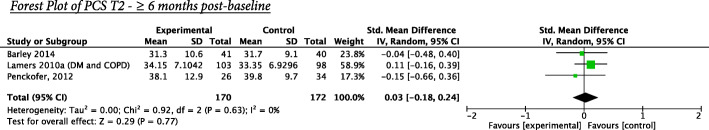
In the T2 time period, 7 studies were included in the meta-analysis (see Fig. 3). The pooled effect size of − 0.53 [95% CI -0.91, − 0.15] was statistically significant with high heterogeneity, I2 = 86% favouring the interventions. The results remained significant after KH conversion [95% CI -95, − 0.13]. Excluding the same three studies as in T1 [78, 93, 94] from the meta-analysis resulted in a pooled effect size, with 4 studies, of − 0.53 [95% CI -0.84, − 0.21] with moderate heterogeneity, I2 = 50%. The one study [97] not entered into the meta-analysis at T2 also favored the intervention over control group.
Fig. 3.
Forest Plot Depression T2 ≥ 6 months post-baseline
Secondary outcomes
A summary of results for secondary outcomes is presented in Table 3. Forest plots of meta-analysis results at the T1 and T2 periods for secondary outcomes are in Appendix E.
Table 3.
Moderator analyses outcomes T1
| Variables | # of studies | Pooled ES | L95 | U95 | P-value | I2 | Meta-regression p-value |
|---|---|---|---|---|---|---|---|
| Overall | 8 | − 0.41 | − 0.61 | − 0.20 | < 0.001 | 32% | |
| Disease | |||||||
| Cancer | 1 | −0.45 | − 1.05 | 0.15 | |||
| Other | 7 | −0.41 | − 0.65 | − 0.18 | 0.001 | 41% | |
| Baseline depression level | 0.926 | ||||||
| Mild to moderate | 5 | −0.42 | − 0.72 | − 0.11 | 0.007 | 44% | |
| Moderately severe to severe | 3 | −0.43 | −0.75 | − 0.11 | 0.009 | 26% | |
| Level of guidance | 0.840 | ||||||
| Guided | 5 | −0.37 | −0.62 | − 0.13 | 0.002 | 26% | |
| Self-directed | 3 | −0.49 | − 0.96 | − 0.02 | 0.042 | 56% | |
| Mode of delivery | 0.840 | ||||||
| Face to face | 5 | −0.37 | −0.62 | − 0.13 | 0.002 | 26% | |
| Not face to face | 3 | −0.49 | − 0.96 | − 0.02 | 0.042 | 56% | |
| Provider | 0.840 | ||||||
| Professional | 5 | −0.38 | −0.63 | − 0.14 | 0.002 | 28% | |
| Self-directed | 3 | −0.5 | − 0.98 | − 0.02 | 0.042 | 58% | |
| Format | |||||||
| Individual | 6 | −0.33 | − 0.55 | − 0.11 | 0.004 | 25% | |
| Group | 2 | −0.65 | −1.01 | − 0.28 | 0.001 | 0% | |
| Duration of Intervention | 1.000 | ||||||
| < 300 min | 4 | −0.47 | − 0.85 | − 0.09 | 0.016 | 58% | |
| ≥ 300 | 4 | −0.41 | − 0.66 | − 0.16 | 0.002 | 0% | |
| Control Group | |||||||
| Active | 2 | − 0.70 | −1.52 | 0.11 | 0.09 | 64% | |
| Not active | 6 | −0.33 | − 0.51 | − 0.15 | < 0.001 | 10% | |
| Methodological Quality | |||||||
| Low | 1 | −0.49 | −1.03 | 0.04 | 0.072 | ||
| Moderate | 7 | −0.41 | − 0.64 | − 0.17 | 0.001 | 40% | |
| Length of Intervention | 0.858 | ||||||
| < 3 months | 5 | − 0.43 | − 0.70 | − 0.17 | 0.001 | 15% | |
| ≥ 3 months | 3 | −0.41 | −0.81 | − 0.02 | 0.042 | 60% | |
| Depression Self-Management Skills | |||||||
| Decision-making | 0.016* | ||||||
| No | 3 | −0.75 | −1.08 | − 0.42 | < 0.001 | 0% | |
| Yes | 5 | −0.23 | −0.41 | − 0.05 | 0.011 | 0% | |
| Problem-solving | |||||||
| No | 2 | −0.90 | −1.31 | − 0.48 | < 0.001 | 0% | |
| Yes | 6 | −0.26 | −0.43 | − 0.09 | 0.003 | 0% | |
| Resource Utilization | |||||||
| No | 7 | −0.31 | − 0.47 | − 0.15 | < 0.001 | 0% | |
| Yes | 1 | −1.13 | −1.85 | −0.41 | 0.002 | ||
| Partnerships with HCPs | |||||||
| No | 7 | −0.49 | −0.70 | − 0.28 | < 0.001 | 4% | |
| Yes | 1 | −0.16 | − 0.4 | 0.08 | 0.197 | ||
| Taking Action | 0.020* | ||||||
| No | 3 | −0.75 | −1.08 | − 0.42 | < 0.001 | 0% | |
| Yes | 5 | −0.23 | −0.41 | − 0.05 | 0.011 | 0% | |
| Behavioural Activation | 1.000 | ||||||
| No | 4 | −0.47 | −0.85 | − 0.09 | 0.016 | 58% | |
| Yes | 4 | −0.41 | −0.66 | − 0.16 | 0.002 | 0% | |
| Cognitive Restructuring | 0.297 | ||||||
| No | 3 | −0.61 | −1.06 | − 0.16 | 0.008 | 34% | |
| Yes | 5 | −0.32 | −0.53 | − 0.11 | 0.003 | 21% | |
| Self-monitoring | 0.694 | ||||||
| No | 5 | −0.45 | −0.71 | − 0.19 | 0.001 | 12% | |
| Yes | 3 | −0.38 | −0.78 | 0.02 | 0.065 | 57% | |
| Health Habits | 0.283 | ||||||
| No | 4 | −0.25 | −0.45 | −0.05 | 0.014 | 0% | |
| Yes | 4 | −0.56 | −0.93 | − 0.19 | 0.003 | 49% | |
| Communicating about Depression | |||||||
| No | 7 | −0.34 | − 0.53 | −0.14 | 0.001 | 17% | |
| Yes | 1 | −0.78 | −1.29 | −0.27 | 0.003 | ||
| Social Support | 0.279 | ||||||
| No | 3 | −0.23 | −0.43 | −0.02 | 0.036 | 0% | |
| Yes | 5 | −0.53 | −0.82 | − 0.23 | < 0.001 | 33% | |
| Relaxation | 0.212 | ||||||
| No | 3 | −0.21 | − 0.42 | 0.00 | 0.053 | 0% | |
| Yes | 5 | −0.53 | − 0.82 | − 0.25 | < 0.001 | 32% | |
| Self-tailoring | 0.747 | ||||||
| No | 4 | −0.47 | − 0.80 | − 0.14 | 0.005 | 34% | |
| Yes | 4 | −0.37 | −0.67 | − 0.07 | 0.015 | 40% | |
| Number of Skills | 0.311 | ||||||
| 1–6 | 4 | −0.56 | −0.88 | − 0.24 | 0.001 | 6% | |
| 7–13 | 4 | −0.32 | −0.57 | − 0.07 | 0.013 | 37% | |
Random effect model was used to compute the pooled effect size.
*p < 0.05 indicating statistical significance
Anxiety.
In the T1 period, 7 studies were entered in the meta-analysis [78, 82, 86, 88, 90, 93, 94]. The pooled effect size was − 0.42 [95% CI -0.73, − 0.12] with heterogeneity of I2 = 73% in favour of the interventions. This finding remained significant after HK conversion [95% CI -0.82, − 0.02]. Removing the same three studies as identified for depression due to high pharmacological co-intervention [78, 93, 94], the pooled effect size was significant, − 0.29 [95% CI -0.53, − 0.06], with no heterogeneity, I2 = 0%. One study [95] was not entered into the meta-analysis and favoured the intervention.
For the T2 time period, 4 studies were included in the meta-analysis [78, 90, 93, 94]. The pooled effect size was − 0.52 [95% CI -0.94, − 0.10] with high heterogeneity, I2 = 77%. This was not significant after HK conversion. The one study [97] not entered into the meta-analysis reported a significant improvement in anxiety in the intervention group as compared to the control.
Health-related quality of life.
Mental component score (MCS) of health-related quality of life. Five studies were included in the meta-analysis in the T1 period [78, 80, 86, 88, 90]. The pooled effect size was statistically significant with moderate heterogeneity, 0.43 [0.09, 0.76] with I2 = 60%. However, it was not significant after HK conversion. Removing the results reported by Barley et al., 2014 [78] did not improve heterogeneity. For T2, the 3 studies were entered in the meta-analysis and did not result in significant pooled effect sizes [84, 90, 91].
Physical component score. (PCS) of health-related quality of life Five studies were entered into the meta-analysis for the T1 period [78, 80, 84, 88, 90] and 3 studies for the T2 time period [78, 84, 90]. The pooled effect sizes were not significant at either time point.
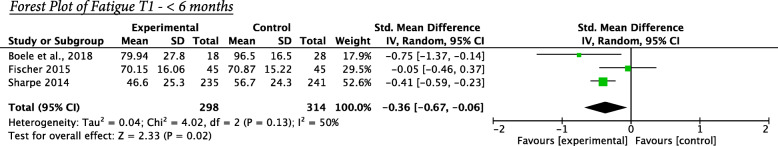
Fatigue.
For the T1 period, three studies were included in the meta-analysis [80, 83, 94] and resulted in a significant pooled effect size of − 0.36 [− 0.67, − 0.06] with moderate heterogeneity, I2 = 50%. The results were not significant if Sharpe, Walker [94] was removed from the analysis. For T2, only one study reported the needed data for meta-analysis [94] and the results were statistically significant with an effect size of − 0.60 [− 0.78, − 0.41] in favour of the intervention. Of the three studies not included in the meta-analysis, two reported in favour of the intervention at the T1 and T2 time periods [95, 96], and the remaining study reported no significant effect on this outcome [97].
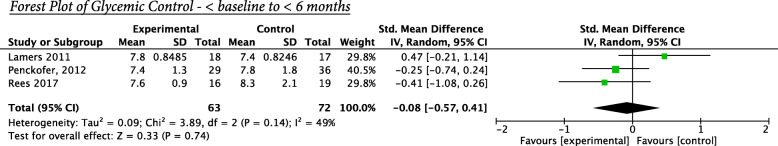
Glycemic control (HbA1c).
Three studies were entered into the meta-analysis in the T1 period [87, 90, 91] and the results of the pooled effect size were not significant. At T2, 4 studies were included in the meta-analysis [87, 89–91] and the results were significant with an effect size of − 0.35 [CI 95% -0.62, − 0.07] and no heterogeneity, I2 = 0%.
Moderator analyses
The results of the moderator analyses for the T1 time period are presented in Table 3. The three studies found to be outliers [78, 93, 94] due to imbalanced pharmacological co-intervention were not included in these analyses. There was not enough data to perform meta-regression for the T2 period (4 studies total); however, 8 studies were included at T1. Meta-regressions were performed for the following 5 moderators, as there were 4 studies in each sub-group: duration of the intervention (< 300 min or ≥ 300 min), behavioural activation (yes/no), health habits (yes/no), self-tailoring (yes/no), and number of self-management skills included in the intervention [1–6 or 7–13]. None were found to be significant. Additional meta-regressions were run for the following 9 moderators including a minimum of 3 studies per sub-group: baseline depression level of the study sample (mild to moderate/moderately severe to severe), level of guidance (guided/self-directed), intervention provider (professional/self-directed), length of the intervention (< 3 months/ ≥ 3 months), decision-making (yes/no), taking action (yes/no), cognitive restructuring (yes/no), self-monitoring (yes/no), and relaxation (yes/no). Of these, the results were significant only for the two self-management skills of decision-making (p = 0.020) and taking action (p = 0.017).
Discussion
To our knowledge, this is the first systematic review to examine the effect of self-management interventions on reducing depressive symptoms among adults with chronic physical disease(s) and co-occurring depression. The results were drawn from the findings of 15 studies. Meta-analysis was conducted for two time periods for the primary outcome of depression as well as the secondary outcomes of anxiety, health-related quality of life (mental component and physical component), fatigue, and glycemic control. Analyses of potential moderators of intervention effect on the primary outcome were performed to identify active elements. Overall, the findings support: a) an effect of interventions on improving depression and anxiety as well as glycemic control at ≥ 6-months post-baseline; b) intervention duration and intervention length were not found to impact effect; and c) some moderators merit further attention in future studies, including level of guidance and type of self-management skills included.
Effect on participant outcomes
The results of the review indicate a moderate effect of self-management interventions on depression and a small effect on anxiety. These results are consistent with the broader literature on self-management for individuals with depression or chronic physical diseases. A systematic review by Houle et al. (2013) found that self-management interventions reduced depressive symptomatology in the general adult population and improved functioning, self-efficacy, and self-management behaviours [65]. Findings related to relapse of depression were mixed. The results of the present review could not shed further light on this issue, as only three studies reported intervention and control group data at 12 or more months [78, 89, 93]. Another recent systematic review assessing the effect of face-to-face self-management interventions for adults with a chronic diseases (not necessarily with co-morbid depression) found that efficacious interventions were more likely to include psychological coping or stress management strategies [27].
Results of the present review can also be compared to those of a review of psychotherapy for adults with depression (with or without chronic co-morbid chronic conditions) that found the interventions to have a larger but still moderate effect size (d = 0.68) in improving depressive symptoms [100]. Reviews of the effect of psychotherapy on co-morbid depression in adults with chronic physical diseases report between small and large effect sizes [101]. Due to the relatively small number of studies, strong comparisons or conclusions cannot be drawn; however, results suggest that depression self-management interventions for this population may have a similarly beneficial effect while generally being more cost-effective.
Moderator analyses
The analyses of moderators found no significant difference in intervention effect on depression based on intervention length or duration. These findings have important implications for the integration of such interventions into resource constrained clinical environments. Seeking evidence-based cost- and time-effective interventions is imperative for the sustainability of providing these in clinical practice [27]. As feasibility is a priority, further investigation of these intervention characteristics is warranted.
Self-directed interventions (no contact with an HCP or coach) did not have significantly lower effect sizes than guided ones. However, this must be interpreted with much caution as the sample size was very small. A number of reviews support the efficacy of minimally guided and self-directed interventions [102–105, 120]. A review of self-directed psychological interventions found a small significant effect of interventions (d = 0.23 at post-test, d = 0.28 from 4- to 12-months) on depression [121]. Only one trial to our knowledge has directly compared the effects of a guided (coached) and self-directed depression intervention for adults with chronic conditions [106]. This trial was excluded from our meta-analysis because of the active control group. The results indicated an overall significant benefit of coaching on depressive symptoms at 3 months; among those who were not receiving psychological treatment at study entry, the benefit was extended to 6 months. The increased effect is likely explained by the greater adherence to the self-care tools in the coached (guided) group [22, 103].
The results indicated that not all self-management skills may be equally beneficial in improving depressive symptoms. Of the 13 self-management skills examined, two of them, decision-making and taking action, were potentially significant moderators of the primary outcome of depression. Of the skills examined, six, drawn from the work of Lorig & Holman (2003), are considered “core” self-management skills that are applicable across chronic diseases. The remaining seven skills were drawn from the literature specifically on the self-management of depression. The findings of this study parallel those of a previous review of non-pharmacological depression interventions for caregivers, which found that problem-solving, decision-making, and taking action were significant moderators of depression [70]. It is notable that neither review identified depression specific skills as significant moderators. Interestingly, a review of self-management interventions in a different population, adults with low income or low health literacy, also found that problem-solving and taking action were more often included in efficacious interventions [69]. Due to limited data, it was not possible to examine problem-solving in this review. Together, the findings of these reviews suggest that developing core self-management skills to foster behaviour change might be more important than disease-specific self-management skills.
Methodological quality
The majority of studies were of moderate methodological quality, and none met all of the criteria. Due to insufficient sample size, it was not possible to examine the heterogeneity among studies of different methodological quality. Adherence rates were reported in nine of 15 studies, similar to a previous review of self-care interventions for anxiety or depression that found 55% of studies reported adherence measures [107]. A number of studies described the amount of time participants engaged in the intervention; however, no study indicated a minimal therapeutic dose or exposure to the intervention. More detailed standardized measures of adherence including activity or module completion, time spent, and active engagement have been proposed to address this issue [108]. The pre-established criteria, based on Cochrane Risk of Bias tool, were difficult to apply to self-directed interventions. For example, intervention fidelity becomes essentially synonymous with adherence in the case of self-directed interventions. In this case, blinding of participants to group allocation may be of greater importance as they are in essence the interventionists and frequently self-report their own outcomes.
If attrition was 20% or less across the sample, the criterion was assessed as being met [71, 109]. However, in six of the 15 studies, attrition rates were notably higher in the intervention group as compared to the control [78, 84, 89–92] and no studies reported greater attrition in the control group. This raises concern regarding the potential impact of attritional bias on study outcomes [110]. Though four of these studies used intention-to-treat analyses, this may not entirely mitigate the impacts of this missing data [111]. Reporting the baseline characteristics of participants who were and were not included in analyses is recommended to help address this [111]. Further, previous work has addressed predictors of attrition of participants with depression from pharmacological trials, but this has not been thoroughly addressed in psychosocial trials [112, 113].
Reported outcomes
There was substantial variety in the outcomes reported across studies and measurement instruments used. Of the 38 outcomes measured across studies, only five were reported at least three times at one time point. Further, six instruments were used to measure the primary outcome of depression. These instruments were also used to establish the presence of at least mild symptoms of depression across the sample, a criterion for inclusion in this review. This is notable as a recent co-calibration study examining the variations in five commonly used depression self-report instruments found that the cut-off scores across scales were not equivalent [114]. The impact of this on the primary outcome could have resulted in an under- or over-estimation of intervention effect.
Strengths and limitations
The study methods were guided by the Cochrane Handbook and the PRISMA statement, and the protocol was registered with PROSPERO [38, 39]. The methods were outlined in detail and are reproducible. Given some terminological ambiguity in the literature regarding what constitutes a self-management intervention, another strength of this review was the search terms applied were very inclusive. Interventions that were not self-described as self-management were included in the review based on the meeting the predetermined definition based on current self-management literature. This is in line with recommendations by Lorig and Holman (2003). However, due to the variations in definitions of self-management, it is possible that other teams would have identified different interventions for inclusion. Further, self-management interventions are recommended as part of a stepped care approach to depression management in which a sequence of treatments are provided based on an individual’s response [115]. Lower intensity interventions, such as self-management, are initially delivered and treatment is ‘stepped up’ to higher intensity psychological interventions (e.g., pharmacotherapy, psychotherapy) for those who do not benefit from the initial treatment [22, 105, 115, 116]. Though four such interventions were screened at the full text stage, none were included as they did not meet the a priori inclusion criterion of providing non-therapeutically active control group data. Future research could evaluate the role of self-management within the structure of stepped care approach to intervention delivery.
The results should be interpreted with caution as the sample size was small with substantial heterogeneity, though this was found to be largely attributable to the confounding impact of pharmacotherapy. The limited number of studies prevented further examination of sources of heterogeneity and analyses of moderators was also conducted with a very small sample. There was only sufficient data to examine outcomes up to 6 months; longer-term outcome data is needed. Further, most of the included studies were focused on those with cancer or diabetes. Though the findings offer potential future avenues for exploration, further evidence is required to investigate longer-term outcomes, sources of heterogeneity, and possible differences in chronic physical disease populations.
Conclusion
This is the first systematic review to examine the effect of self-management interventions on depression in adults with co-occurring chronic physical diseases. The findings indicate the interventions reduced depression with a moderate effect size and anxiety with a small effect size. Impact of the interventions on other psychosocial and physical health outcomes was mixed. Recommendations include further evaluation of the impact on the amount of guidance, length, and duration of interventions, as self-directed, shorter and thereby less resource intensive interventions may be effective. Including self-management skills of decision-making and taking action in future interventions is also recommended.
Acknowledgements
Not applicable.
Abbreviations
- BDI-II
Beck depression inventory-II
- BDI
Beck depression inventory
- C
Control condition
- CBT
Cognitive behavioural therapy
- CES-D
Centre for epidemiological studies-depression
- CHD
Coronary heart disease
- CI
Confidence interval
- CNS
Central nervous system
- COPD
Chronic obstructive pulmonary disease
- DSM
Diagnostic and statistical manual of mental disorders
- ER
Emergency room
- ES
Effect size (hedge’s g calculated at 95% confidence level)
- HADS-D
Hospital anxiety and depression scale-depression
- HbA1c
Hemoglobin A1c test for glycemic control
- HCP
Healthcare professional
- HRQoL
Health related quality of life and includes
- PCS
Physical health composite scale
- MCS
Mental health composite scale
- ICD
International classification of diseases
- IQR
Interquartile range
- KH
Knapp-Hartung
- MS
Multiple sclerosis
- PHQ-9
Patient health questionnaire
- PRESS
Peer Review for electronic search strategies
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- PST
Problem-solving therapy
- QoL
Quality of life
- RCT
Randomized controlled trial
- SD
Standard deviation
- T < C
control superior to treatment
- T = C
no significant differences between treatment and control conditions
- T > C
treatment significantly superior to control
- T
Treatment condition
- WLC
Waitlist control group
Appendix 1
Table 4.
Depression self-management skills included in the interventions
| Barley et al. (2014) [78] | Boele et al. (2018) [80] | Espahbodi et al. (2015) [82] | Fischer et al. (2015) [83] & Schroder et al. (2014)* [92] | Lamers et al. (2010) [86] | Lee et al. (2014) [88] | Moncrief et al. (2016) [89] | Penckofer et al. (2012) [90] | Rees et al. (2016) | Sharpe et al. (2004) [93], [93] Sharpe et al. (2014) [94], Strong et al. (2008) [95], & Walker et al. (2014)† [97] | Thorton et al. (2009) [96] | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Decision-Making Often occurs in the context of problem-solving and is based on having enough and appropriate information to meet common changes associated with chronic illness [25]. |
1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 8 |
|
Problem-solving Using a structured approach and learning skills such as problem definition, generating solutions, implementation, and evaluation of results to move towards a solution [25, 29]. |
1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 9 |
|
Resource Utilization Learning how to seek out many resources (using different sources) [25]. |
1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
|
Forming Partnerships with HCPs Learning how to provide disease-related feedback to HCPs and make informed treatment decisions and discuss with HCPs [25]. |
0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
|
Taking Action Making a plan and carrying it out, learning skills involved in behaviour change [25]. |
1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 7 |
|
Behavioural Activation Learning to gradually increase positive activities through effective goal setting [29] |
0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 4 |
|
Cognitive Restructuring Learning to identify depressive self-talk, challenge it, and come up with fair-realistic ways of evaluating situations [29]. |
0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 5 |
|
Self-Monitoring Monitoring depression symptoms and evaluating whether current strategies are working effectively and, when necessary, reassessing treatment plans [63]. |
1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 5 |
|
Health Habits Learning about the links between health habits (e.g., sleep, diet) and mental health. Learning how to enact helpful health related habits [64, 65]. |
0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 5 |
|
Communicating about Depression Learning to explain what it means to experience depression to family members, friends, and colleagues [117]. |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
|
Social Support Arranging instrumental and emotional support [117–119]. Involving close friends/family in treatment and support [117, 118]. |
1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 7 |
|
Relaxation Maintaining or developing activities related to relaxation (e.g., meditation, breathing exercises). |
0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 6 |
|
Self-tailoring Learning to use other self-management skills based on a personal evaluation of your own needs. |
1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 7 |
| Total | 7 | 6 | 2 | 8 | 7 | 4 | 11 | 8 | 6 | 4 | 5 |
*Fischer et al. (2015) and Schroder et al. (2014) delivered the same intervention to different disease populations. † Intervention delivered by Sharpe et al. (2004), Sharpe et al. (2014), Strong et al. (2008), & Walker et al. (2014) identically reported in terms of content related to self-management skills. Delivered to different oncology samples and change reported in the interventionists
Appendix 2
Fig. 4.
Sample Search Strategy from Ovid Medline conducted June 25th, 2018
Appendix 3
Funnel Plots for Depression (primary outcome)
Fig. 5.
Funnel plot of intervention effect estimates for individual studies for depression at T1 - Baseline to < 6 months
Fig. 6.
Funnel plot of intervention effect estimates for individual studies for depression at T2 - ≥ 6 months post-baseline
Appendix 4
Table 5.
Quality Assessment of Included Studies
| Barley et al., 2014 [78] | Boele et al., 2018 [80] | Espahbodi et al., 2015 [82] | Fischer et al., 2015 [83] | Lamers et al., 2010a b [88. 100]; 2011 [87] | Lee et al., 2014 [88] | Moncrief et al., 2016 [89] | Penckofer et al., 2012 [90] | Rees et al., 2017 [91] | Schröder et al., 2014 [92] | Sharpe et al., 2004 [93] | Sharpe et al., 2014 [94] | Strong et al., 2008 [95] | Thornton et al., 2009 [96] | Walker et al., 2014 [97] | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion criteria specified | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Pre-specified primary and secondary outcomes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Psychometric properties provided | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Explicit power calculation | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 11 |
| Target sample size reached | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 6 |
| Randomization method specified and truly random | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 11 |
| Randomization - Allocation concealed | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 8 |
| Potential co-interventions discussed | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 10 |
| Outcome assessors blind | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| Participants blind to treatment allocation | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Interventionists blind | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Adherence > 75% | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Intention-to-treat data analysis | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 10 |
| Overall > 80% of sample in primary data analysis | 1 | 0 | 1* | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 10 |
| Reason for attrition stated | 1 | 1 | 1* | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 11 |
| Baseline characteristics for both groups reported | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Fidelity monitoring | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 12 |
| Total (/17) | 11 | 10 | 6 | 9 | 12 | 12 | 9 | 12 | 13 | 10 | 12 | 14 | 14 | 11 | 13 |
Notes: Score of 1 if criterion met; 0 if not met or information unavailable. Pilot studies given automatic 0 for explicit power calculation as aim of pilot studies is not establish efficacy or effectiveness. Pilot studies given 1 for target sample size reached if rationale was provided for sample size and this was met. Thornton et al. (2009) was a sub-group analysis of a larger trial. A post-hoc power calculation was not included; as such, this study was scored as 0 for both power calculation and target sample size reached. Randomization- allocation concealment criterion was met if the person conducting randomization was independent from the research team (e.g., data collection, analysis, development of project aims). For self-directed interventions, participants themselves were considered to the be outcome assessors and interventionists; as such, if participants in self-directed interventions were aware of their group allocation, outcome assessors and interventionist were deemed not to be blind and given a 0 for these criteria. Criterion of overall > 80% of sample in primary data analysis was based on the stated primary data collection time point. If no primary time point was specified, the first data collection point was used. Reasons for attrition stated was based on dropouts after randomization. For self-directed interventions, if adherence criterion was met, fidelity criterion was also considered to be met. Studies were considered to be of high methodological quality if 13–17 criteria were met, moderate quality if 8–12 were met, and low fewer than 8 were met.*Espahbodi et al. (2015): Those who did not complete all intervention sessions were excluded from the study
Appendix 5
Forest plots of secondary outcomes
Fig. 7.
Forest Plot of Anxiety T1 - Baseline to < 6 months
Fig. 8.
Forest Plot of Anxiety T2 ≥ 6 months post-baseline
Fig. 9.
Forest Plot of MCS T1 - Baseline to < 6 months
Fig. 10.
Forest Plot of MCS T2 ≥ 6 months post-baseline
Fig. 11.
Forest Plot of PCS T1 - Baseline to < 6 months
Fig. 12.
Forest Plot of PCS T2 ≥ 6 months post-baseline
Fig. 13.
Forest Plot of Fatigue T1 - Baseline to < 6 months
Fig. 14.
Forest Plot of Glycemic Control T1 - Baseline to < 6 months
Fig. 15.
Forest Plot of Glycemic Control T2 ≥ 6 months post-baseline
Authors’ contributions
LOB: Conceptualized and designed the work, collected, analyzed, and interpreted data, and drafted the manuscript. SL: Conceptualized and designed the work, contributed to data collection, and assisted with interpreting data, and substantially revised the manuscript. NF: Assisted in designing the work, interpreting the data, and substantially revised the manuscript. CC: Conducted data collection and substantially revised the manuscript. JS: Conducted data collection and substantially revised the manuscript. JC: Contributed to conceptualizing and designing the work and substantially revised the manuscript. EEMM: Contributed to data analysis and interpretation and substantially revised the manuscript. JK: Contributed to data collection and substantially revised the manuscript. KK: Contributed to data collection. EB: Conducted some data analysis, assisted with data interpretation, and substantially revised manuscript. CG: Contributed to data interpretation and substantially revised manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Fonds de recherche santé Québec (FRQS), the Réseau de recherche en interventions en sciences infirmières (RRISIQ), Dr. Lambert’s Tier 2 Canada Research Chair and the McGill Nursing Collaborative for Education and Innovation in Patient and Family Centered Care. All bodies listed provided general funding to the named author to support their research; however, these bodies had no role or influence in the design of the study or collection, analysis, or interpretation of the data or writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lydia Ould Brahim, Email: lydia.oulbrahim@mail.mcgill.ca.
Eric Belzile, Email: eric.belzile@ssss.gouv.qc.ca.
References
- 1.World Health Organization. Depression Fact Sheet: World Health Organization; 2018 [Available from: http://www.who.int/mediacentre/factsheets/fs369/en/.
- 2.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34(1):119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. 2018;8(1):2861. doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Global Health estimates 2016: deaths by cause, age, sex, by country and by region, 2000–2016. Geneva: World Health Organization; 2018. [Google Scholar]
- 5.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet (London, England) 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 6.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13(1):7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Ge S, Greene B, Dunbar-Jacob J. Depression in the context of chronic diseases in the United States and China. Int J Nurs Sci. 2018;6(1):117–122. doi: 10.1016/j.ijnss.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization & Public Health Agency of Canada. Preventing chronic diseases: a vital investment. Geneva: World Health Organization; 2005. https://apps.who.int/iris/handle/10665/43314.
- 9.World Health Organization. Noncommunicable diseases 2018 [Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases. Accessed Dec 2021.
- 10.Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kravitz RL, Ford DE. Introduction: chronic medical conditions and depression--the view from primary care. Am J Med. 2008;121(11 Suppl 2):S1–S7. doi: 10.1016/j.amjmed.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Health and Clinical Excellence . Depression: the treatment and Management of Depression in adults (updated edition) Leicester (UK): British Psychological Society; 2010. [PubMed] [Google Scholar]
- 13.Cuijpers P, Smit F. Subclinical depression: a clinically relevant condition? Tijdschr Psychiatr. 2008;50(8):519–528. [PubMed] [Google Scholar]
- 14.Tuithof M, Ten Have M, van Dorsselaer S, Kleinjan M, Beekman A, de Graaf R. Course of subthreshold depression into a depressive disorder and its risk factors. J Affect Disord. 2018;241:206–215. doi: 10.1016/j.jad.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Clarke DM, Currie KC. Depression, anxiety and their relationship with chronic diseases: a review of the epidemiology, risk and treatment evidence. Med J Aust. 2009;190(S7):S54–S60. doi: 10.5694/j.1326-5377.2009.tb02471.x. [DOI] [PubMed] [Google Scholar]
- 16.Craven MA, Bland R. Depression in primary care: current and future challenges. Can J Psychiatry. 2013;58(8):442–448. doi: 10.1177/070674371305800802. [DOI] [PubMed] [Google Scholar]
- 17.Mojtabai R, Olfson M, Sampson NA, Jin R, Druss B, Wang PS, Wells KB, Pincus HA, Kessler RC. Barriers to mental health treatment: results from the National Comorbidity Survey Replication. Psychol Med. 2011;41(8):1751–1761. doi: 10.1017/S0033291710002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renn BN, Hoeft TJ, Lee HS, Bauer AM, Areán PA. Preference for in-person psychotherapy versus digital psychotherapy options for depression: survey of adults in the U.S. NPJ Digital Med. 2019;2(1):6. doi: 10.1038/s41746-019-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournier L, Roberge P, Brouillet H. Faire face à la dépression au Québec. Montréal: Protocole de soins à l’intention des intervenants de première ligne Centre de recherche du CHUM; 2012. [Google Scholar]
- 20.Patten SB, Kennedy SH, Lam RW, O'Donovan C, Filteau MJ, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. I. Classification, burden and principles of management. J Affect Disord, 14. 2009;117(Suppl 1):S5. [DOI] [PubMed]
- 21.Health Quality Ontario . Major Depression: Care for Adults and Adolescents. Toronto, ON: Health Quality Ontario; 2016. [Google Scholar]
- 22.National Institute for Health Care Excellence. Depression in adults with a chronic physical health problem: recognition and management In: Guideline C, editor. 2009.
- 23.Institute of Medicine Committee on the Crossing the Quality Chasm . Next Steps Toward a New Health Care S. In: Adams K, Greiner AC, Corrigan JM, editors. The 1st Annual Crossing the Quality Chasm Summit: A Focus on Communities. Washington (DC): National Academies Press (US); 2004. [PubMed] [Google Scholar]
- 24.McGowan P, Fredericks S, Beatty G. Strategies to Support Self-Management in Chronic Conditions: Collaboration with Clients. Toronto, ON: Registered Nurses Association of Ontario (RNAO); 2010. [Google Scholar]
- 25.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 26.Bodenheimer T, Wagner EH, Grumbach K. Improving primary Care for Patients with Chronic Illness. JAMA. 2002;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 27.Dineen-Griffin S, Garcia-Cardenas V, Williams K, Benrimoj SI. Helping patients help themselves: a systematic review of self-management support strategies in primary health care practice. PLoS One. 2019;14(8):e0220116. doi: 10.1371/journal.pone.0220116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. Jama. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 29.Bilsker D, Goldner EM, Anderson E. Supported self-management: a simple, effective way to improve depression care. Can J Psychiatry. 2012;57(4):203–209. doi: 10.1177/070674371205700402. [DOI] [PubMed] [Google Scholar]
- 30.Lorig K. Chronic Disease Self-Management Program: Insights from the Eye of the Storm. Front Public Health. 2015;2:253. doi: 10.3389/fpubh.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239–243. doi: 10.1016/j.phr.2004.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn S, Basu R, Smith ML, Jiang L, Lorig K, Whitelaw N, Ory MG. The impact of chronic disease self-management programs: healthcare savings through a community-based intervention. BMC Public Health. 2013;13(1):1141. doi: 10.1186/1471-2458-13-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Jr, Bandura A, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39(11):1217–1223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Bower P, Richards D, Lovell K. The clinical and cost-effectiveness of self-help treatments for anxiety and depressive disorders in primary care: a systematic review. Br J Gen Pract. 2001;51(471):838–845. [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff. 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 36.Adams K, Greiner AC, Corrigan JM. The 1st Annual Crossing the Quality Chasm Summit: A focus on communities. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 37.Jones MC, MacGillivray S, Kroll T, Zohoor AR, Connaghan J. A thematic analysis of the conceptualisation of self-care, self-management and self-management support in the long-term conditions management literature. J Nurs Healthc Chronic Illn. 2011;3(3):174–185. doi: 10.1111/j.1752-9824.2011.01096.x. [DOI] [Google Scholar]
- 38.Higgins JPT, Green SE. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 Higgins JPTG, S. (Eds). editor: The Cochrane Collabroation; 2011.
- 39.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 40.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- 41.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York, NY: Guilford Press; 1979. [Google Scholar]
- 42.Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 43.Vilagut G, Forero CG, Barbaglia G, Alonso J. Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES-D): a systematic review with Meta-analysis. PLoS One. 2016;11(5):e0155431. doi: 10.1371/journal.pone.0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 45.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 46.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. doi: 10.1016/S0749-3797(18)30622-6. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 49.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 51.American Psychiatric Association. Diagnostic Criteria from Dsm-Iii-R. Washington, DC: American Psychiatric Association; 1980.
- 52.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (3rd ed., revised). Washington, DC: American Psychiatric Association; 1987.
- 53.American Psychiatric Association. Diagnostic Criteria from Dsm-Iv. Washington, DC: American Psychiatric Association; 1994.
- 54.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., text revision). Washington, DC: American Psychiatric Association; 2000.
- 55.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association; 2013. 10.5555/appi.books.9780890425596.x00pre.
- 56.World Health Organization (WHO) International Classification of Disease (ICD-9) Geneva: World Health Organization; 1977. [Google Scholar]
- 57.World Health Organization . The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 58.Public Health Agency of Canada. The Canadian Chronic Disease Surveillance System – An Overview. Government of Canada; 2021. https://www.canada.ca/en/public-health/services/publications/canadian-chronic-disease-surveillance-system-factsheet.html.
- 59.Drug and Therapeutics Bulletin. Management of seasonal affective disorder. BMJ. 2010;340:c2135. [DOI] [PubMed]
- 60.Kurlansik SL, Ibay AD. Seasonal affective disorder. Am Fam Physician. 2012;86(11):1037–1041. [PubMed] [Google Scholar]
- 61.Muller-Oerlinghausen B, Berghofer A, Bauer M. Bipolar disorder. Lancet (London, England) 2002;359(9302):241–247. doi: 10.1016/S0140-6736(02)07450-0. [DOI] [PubMed] [Google Scholar]
- 62.O'Hara MW, Wisner KL. Perinatal mental illness: definition, description and aetiology. Best Pract Res Clin Obstet Gynaecol. 2014;28(1):3–12. doi: 10.1016/j.bpobgyn.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.British Colombia Partners for Mental Health and Addictions Information (BC Partners). Depression Toolkit: Information and Resources for Effective Self-Management of Depression. 2003. http://www.gpscbc.ca/sites/default/files/uploads/AMH_212.0_Depression_Toolkit_PR.pdf.
- 64.Bilsker D, Paterson R. Antidepressant skills workbook. Vancouver, BC: Simon Fraser University; 2005. [Google Scholar]
- 65.Houle J, Gascon-Depatie M, Bélanger-Dumontier G, Cardinal C. Depression self-management support: a systematic review. Patient Educ Couns. 2013;91(3):271–279. doi: 10.1016/j.pec.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 66.Regan T, Lambert SD, Kelly B. Uptake and attrition in couple-based interventions for cancer: perspectives from the literature. Psycho-Oncology. 2013;22(12):2639–2647. doi: 10.1002/pon.3342. [DOI] [PubMed] [Google Scholar]
- 67.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 68.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Library Assoc. 2016;104(3):240–243. doi: 10.3163/1536-5050.104.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaffler J, Leung K, Tremblay S, Merdsoy L, Belzile E, Lambrou A, Lambert SD. The effectiveness of self-management interventions for individuals with low health literacy and/or low income: a descriptive systematic review. J Gen Intern Med. 2018;33(4):510–523. doi: 10.1007/s11606-017-4265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lambert S, Ould Brahim L, McCusker J, Coumoundouros C, Audet L, Yaffe M, et al. Non-pharmacological interventions for caregivers with depression and caregivers of care recipients with co-morbid depression: A systematic review and meta-analysis. Manuscript submitted for publication. 2020. [DOI] [PMC free article] [PubMed]
- 71.Higgins JPT SJ, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials. 2016. In: Cochrane Database Syst Rev. [Suppl 1]. Available from: dx.doi.org/10.1002/14651858.CD201601.
- 72.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 73.Cooper HM, Hedges LV, Valentine JC. Handbook of research synthesis and meta-analysis. New York: Russell Sage Foundation; 2019. http://www.jstor.org/stable/10.7758/9781610448864. Available from: https://muse.jhu.edu/book/65827/. http://www.vlebooks.com/vleweb/product/openreader?id=none&isbn=9781610448864.
- 74.Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition ed. New York, NY: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 75.Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, Goodman SN. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160(4):267–270. doi: 10.7326/M13-2886. [DOI] [PubMed] [Google Scholar]
- 76.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 77.Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, Griffith L, Oremus M, Raina P, Ismaila A, Santaguida P, Lau J, Trikalinos TA. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the effective health care program. J Clin Epidemiol. 2011;64(11):1187–1197. doi: 10.1016/j.jclinepi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 78.Barley EA, Walters P, Haddad M, Phillips R, Achilla E, McCrone P, van Marwijk H, Mann A, Tylee A. The UPBEAT nurse-delivered personalized care intervention for people with coronary heart disease who report current chest pain and depression: a randomised controlled pilot study. PLoS One. 2014;9(6):e98704. doi: 10.1371/journal.pone.0098704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tylee A, Barley EA, Walters P, Achilla E, Borschmann R, Leese M, et al. Programme Grants for applied research. UPBEAT-UK: a programme of research into the relationship between coronary heart disease and depression in primary care patients. Southampton (UK): NIHR Journals Library; 2016. [PubMed] [Google Scholar]
- 80.Boele FW, Klein M, Verdonck-de Leeuw IM, Cuijpers P, Heimans JJ, Snijders TJ, Vos M, Bosma I, Tijssen CC, Reijneveld JC, On behalf of the Dutch Society for Neuro-Oncology (LWNO) Internet-based guided self-help for glioma patients with depressive symptoms: a randomized controlled trial. J Neuro-Oncol. 2018;137(1):191–203. doi: 10.1007/s11060-017-2712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boele FW, Verdonck-de Leeuw IM, Cuijpers P, Reijneveld JC, Heimans JJ, Klein M. Internet-based guided self-help for glioma patients with depressive symptoms: design of a randomized controlled trial. BMC Neurol. 2014;14(1):81. doi: 10.1186/1471-2377-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Espahbodi F, Hosseini H, Mirzade MM, Shafaat AB. Effect of psycho education on depression and anxiety symptoms in patients on hemodialysis. Iran J Psychiatry Behav Sci. 2015;9(1):e227-e. [DOI] [PMC free article] [PubMed]
- 83.Fischer A, Schröder J, Vettorazzi E, Wolf OT, Pöttgen J, Lau S, Heesen C, Moritz S, Gold SM. An online programme to reduce depression in patients with multiple sclerosis: a randomised controlled trial. Lancet Psychiatry. 2015;2(3):217–223. doi: 10.1016/S2215-0366(14)00049-2. [DOI] [PubMed] [Google Scholar]
- 84.Lamers F, Jonkers CC, Bosma H, Kempen GI, Meijer JA, Penninx BW, et al. A minimal psychological intervention in chronically ill elderly patients with depression: a randomized trial. Psychother Psychosom. 2010;79(4):217–226. doi: 10.1159/000313690. [DOI] [PubMed] [Google Scholar]
- 85.Lamers F, Jonkers CC, Bosma H, Diederiks JP, van Eijk JT. Effectiveness and cost-effectiveness of a minimal psychological intervention to reduce non-severe depression in chronically ill elderly patients: the design of a randomised controlled trial [ISRCTN92331982] BMC Public Health. 2006;6(1):161. doi: 10.1186/1471-2458-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamers F, Jonkers CC, Bosma H, Chavannes NH, Knottnerus JA, van Eijk JT. Improving quality of life in depressed COPD patients: effectiveness of a minimal psychological intervention. Copd. 2010;7(5):315–322. doi: 10.3109/15412555.2010.510156. [DOI] [PubMed] [Google Scholar]
- 87.Lamers F, Jonkers CC, Bosma H, Knottnerus JA, van Eijk JT. Treating depression in diabetes patients: does a nurse-administered minimal psychological intervention affect diabetes-specific quality of life and glycaemic control? A randomized controlled trial. J Adv Nurs. 2011;67(4):788–799. doi: 10.1111/j.1365-2648.2010.05540.x. [DOI] [PubMed] [Google Scholar]
- 88.Lee JY, Park HY, Jung D, Moon M, Keam B, Hahm BJ. Effect of brief psychoeducation using a tablet PC on distress and quality of life in cancer patients undergoing chemotherapy: a pilot study. Psychooncology. 2014;23(8):928–935. doi: 10.1002/pon.3503. [DOI] [PubMed] [Google Scholar]
- 89.Moncrieft AE, Llabre MM, McCalla JR, Gutt M, Mendez AJ, Gellman MD, Goldberg RB, Schneiderman N. Effects of a multicomponent life-style intervention on weight, glycemic control, depressive symptoms, and renal function in low-income, minority patients with type 2 diabetes: results of the community approach to lifestyle modification for diabetes randomized controlled trial. Psychosom Med. 2016;78(7):851–860. doi: 10.1097/PSY.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Penckofer SM, Ferrans C, Mumby P, Byrn M, Emanuele MA, Harrison PR, Durazo-Arvizu RA, Lustman P. A psychoeducational intervention (SWEEP) for depressed women with diabetes. Ann Behav Med. 2012;44(2):192–206. doi: 10.1007/s12160-012-9377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rees G, O'Hare F, Saeed M, Sudholz B, Sturrock BA, Xie J, Speight J, Lamoureux EL. Problem-solving therapy for adults with diabetic retinopathy and diabetes-specific distress: a pilot randomized controlled trial. BMJ Open Diabetes Res Care. 2017;5(1):e000307. doi: 10.1136/bmjdrc-2016-000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schröder J, Brückner K, Fischer A, Lindenau M, Köther U, Vettorazzi E, Moritz S. Efficacy of a psychological online intervention for depression in people with epilepsy: a randomized controlled trial. Epilepsia. 2014;55(12):2069–2076. doi: 10.1111/epi.12833. [DOI] [PubMed] [Google Scholar]
- 93.Sharpe M, Strong V, Allen K, Rush R, Maguire P, House A et al. Management of major depression in outpatients attending a cancer Centre: a preliminary evaluation of a multicomponent cancer nurse-delivered intervention. Br J Cancer. 2004;90(2):310–313. doi: 10.1038/sj.bjc.6601546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharpe M, Walker J, Hansen CH, Martin P, Symeonides S, Gourley C, Wall L, Weller D, Murray G. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): a multicentre randomised controlled effectiveness trial. Lancet. 2014;384(9948):1099–1108. doi: 10.1016/S0140-6736(14)61231-9. [DOI] [PubMed] [Google Scholar]
- 95.Strong V, Waters R, Hibberd C, Murray G, Wall L, Walker J, McHugh G, Walker A, Sharpe M. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372(9632):40–48. doi: 10.1016/S0140-6736(08)60991-5. [DOI] [PubMed] [Google Scholar]
- 96.Thornton LM, Andersen BL, Schuler TA, Carson WE., 3rd A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: secondary analysis of a randomized controlled trial. Psychosom Med. 2009;71(7):715–724. doi: 10.1097/PSY.0b013e3181b0545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walker J, Hansen CH, Martin P, Symeonides S, Gourley C, Wall L, Weller D, Murray G, Sharpe M, SMaRT (Symptom Management Research Trials) Oncology-3 Team Integrated collaborative care for major depression comorbid with a poor prognosis cancer (SMaRT Oncology-3): a multicentre randomised controlled trial in patients with lung cancer. Lancet Oncol. 2014;15(10):1168–1176. doi: 10.1016/S1470-2045(14)70343-2. [DOI] [PubMed] [Google Scholar]
- 98.Derogatis LR. The SCL-90 manual I: scoring, administration and procedures for the SCL-90. Baltimore, MD: Clinical psychometric research; 1977. [Google Scholar]
- 99.O'Connor M, Butcher I, Hansen CH, Kleiboer A, Murray G, Sharma N, Thekkumpurath P, Walker J, Sharpe M. Measuring improvement in depression in cancer patients: a 50% drop on the self-rated SCL-20 compared with a diagnostic interview. Gen Hosp Psychiatry. 2010;32(3):334–336. doi: 10.1016/j.genhosppsych.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 100.Cuijpers P, van Straten A, Bohlmeijer E, Hollon S, Andersson G. The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]: Centre for Reviews and Dissemination (UK); 2010. [DOI] [PubMed]
- 101.Hunsley J, Elliott K, Therrien Z. The efficacy and effectiveness of psychological treatments for mood, anxiety, and related disorders. Can Psychol. 2014;55(3):161–176. doi: 10.1037/a0036933. [DOI] [Google Scholar]
- 102.Cuijpers P, Clignet F, van Meijel B, van Straten A, Li J, Andersson G. Psychological treatment of depression in inpatients: a systematic review and meta-analysis. Clin Psychol Rev. 2011;31(3):353–360. doi: 10.1016/j.cpr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 103.Cuijpers P, Kleiboer AM, DeRubeis RJ, Strunk DR. The Oxford handbook of mood disorders. Oxford: Oxford University Press; 2017. Self-directed approaches to the treatment of depression; pp. 469–477. [Google Scholar]
- 104.Van't Hof E, Cuijpers P, Stein DJ. Self-help and internet-guided interventions in depression and anxiety disorders: a systematic review of meta-analyses. CNS Spectr. 2009;14(2 Suppl 3):34–40. doi: 10.1017/S1092852900027279. [DOI] [PubMed] [Google Scholar]
- 105.Gyani A, Shafran R, Layard R, Clark DM. Enhancing recovery rates: lessons from year one of IAPT. Behav Res Ther. 2013;51(9):597–606. doi: 10.1016/j.brat.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCusker CMG. Yaffe M, Strumpf E, Sewitch M, Sussman T, et al. a randomized trial of a depression self-care toolkit with or without lay telephone coaching for primary care patients with chronic physical conditions. Gen Hosp Psychiatry. 2015;37(3):257–265. doi: 10.1016/j.genhosppsych.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 107.Simco R, McCusker J, Sewitch M. Adherence to self-care interventions for depression or anxiety: a systematic review. Health Educ J. 2014;73(6):714–730. doi: 10.1177/0017896913514738. [DOI] [Google Scholar]
- 108.Donkin L, Christensen H, Naismith SL, Neal B, Hickie IB, Glozier N. A systematic review of the impact of adherence on the effectiveness of e-therapies. J Med Internet Res. 2011;13(3):e52. doi: 10.2196/jmir.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet (London, England) 2002;359(9308):781–785. doi: 10.1016/S0140-6736(02)07882-0. [DOI] [PubMed] [Google Scholar]
- 110.Nunan D, Aronson J, Bankhead C. Catalogue of bias: attrition bias. Bmj Evid Based Med. 2018;23(1):21–22. doi: 10.1136/ebmed-2017-110883. [DOI] [PubMed] [Google Scholar]
- 111.Dumville JC, Torgerson DJ, Hewitt CE. Reporting attrition in randomised controlled trials. BMJ. 2006;332(7547):969–971. doi: 10.1136/bmj.332.7547.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Warden D, Rush AJ, Carmody TJ, Kashner TM, Biggs MM, Crismon ML, Trivedi MH. Predictors of attrition during one year of depression treatment: a roadmap to personalized intervention. J Psychiatr Pract. 2009;15(2):113–124. doi: 10.1097/01.pra.0000348364.88676.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Warden D, Trivedi MH, Wisniewski SR, Davis L, Nierenberg AA, Gaynes BN, et al. Predictors of attrition during initial (citalopram) treatment for depression: a STAR*D report. Am J Psychiatry. 2007;164(8):1189–1197. doi: 10.1176/appi.ajp.2007.06071225. [DOI] [PubMed] [Google Scholar]
- 114.Lambert SD, Clover K, Pallant JF, Britton B, King MT, Mitchell AJ, Carter G. Making sense of variations in prevalence estimates of depression in Cancer: a co-calibration of commonly used depression scales using Rasch analysis. J Natl Compr Cancer Netw. 2015;13(10):1203–1211. doi: 10.6004/jnccn.2015.0149. [DOI] [PubMed] [Google Scholar]
- 115.van Straten A, Seekles W, van't Veer-Tazelaar NJ, Beekman AT, Cuijpers P. Stepped care for depression in primary care: what should be offered and how? Med J Aust. 2010;192(11):S36. doi: 10.5694/j.1326-5377.2010.tb03691.x. [DOI] [PubMed] [Google Scholar]
- 116.Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency. Br J Psychiatry. 2005;186(1):11–17. doi: 10.1192/bjp.186.1.11. [DOI] [PubMed] [Google Scholar]
- 117.van Grieken RA, Kirkenier ACE, Koeter MWJ, Schene AH. Helpful self-management strategies to cope with enduring depression from the patients' point of view: a concept map study. BMC Psychiatry. 2014;14:331. doi: 10.1186/s12888-014-0331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Grieken RA, Kirkenier AC, Koeter MW, Nabitz UW, Schene AH. Patients' perspective on self-management in the recovery from depression. Health Expect. 2015;18(5):1339–1348. doi: 10.1111/hex.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived Behavioural determinants to behaviour change techniques. Appl Psychol. 2008;57(4):660–680. doi: 10.1111/j.1464-0597.2008.00341.x. [DOI] [Google Scholar]
- 120.Lambert SD, Beatty L, McElduff P, Levesque JV, Lawsin C, Jacobsen P, Turner J, Girgis A. A systematic review and meta-analysis of written self-administered psychosocial interventions among adults with a physical illness. Patient education and counseling. 2017;100(12):2200–17. 10.1016/j.pec.2017.06.039. [DOI] [PubMed]
- 121.Cuijpers P, Donker T, Johansson R, Mohr DC, van Straten A, et al. Self-Guided Psychological Treatment for Depressive Symptoms: A Meta-Analysis. PLOS ONE. 2011;6(6):e21274. 10.1371/journal.pone.0021274. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.